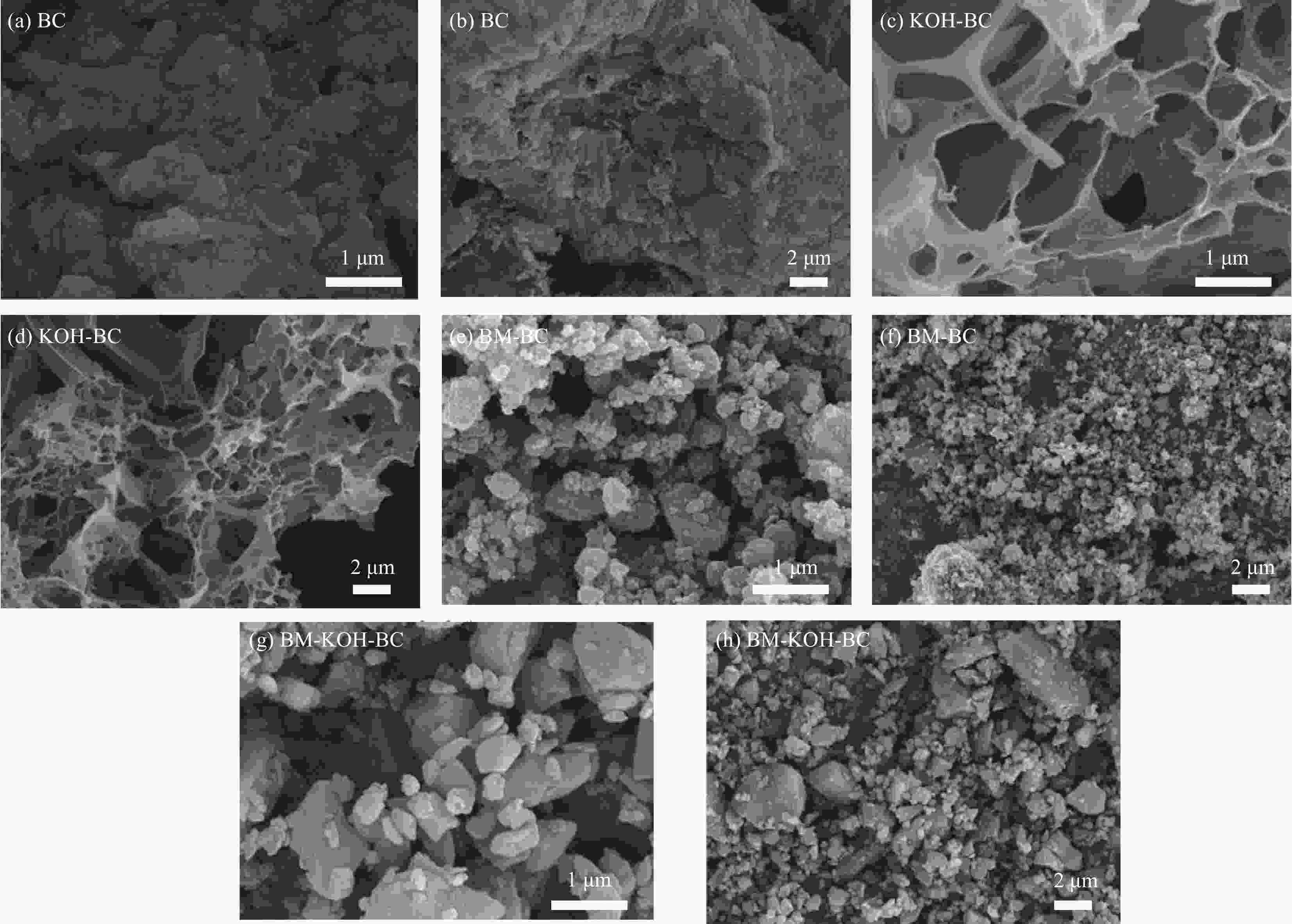
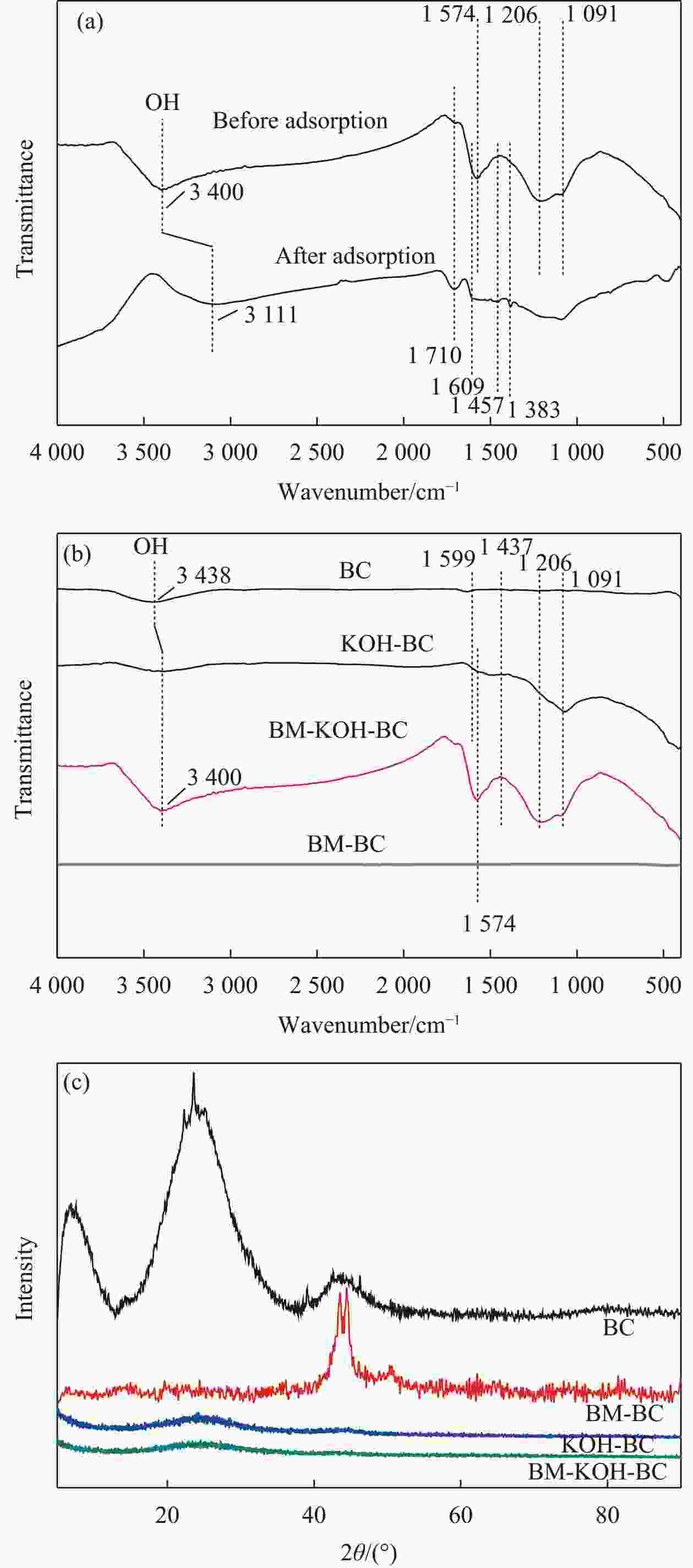
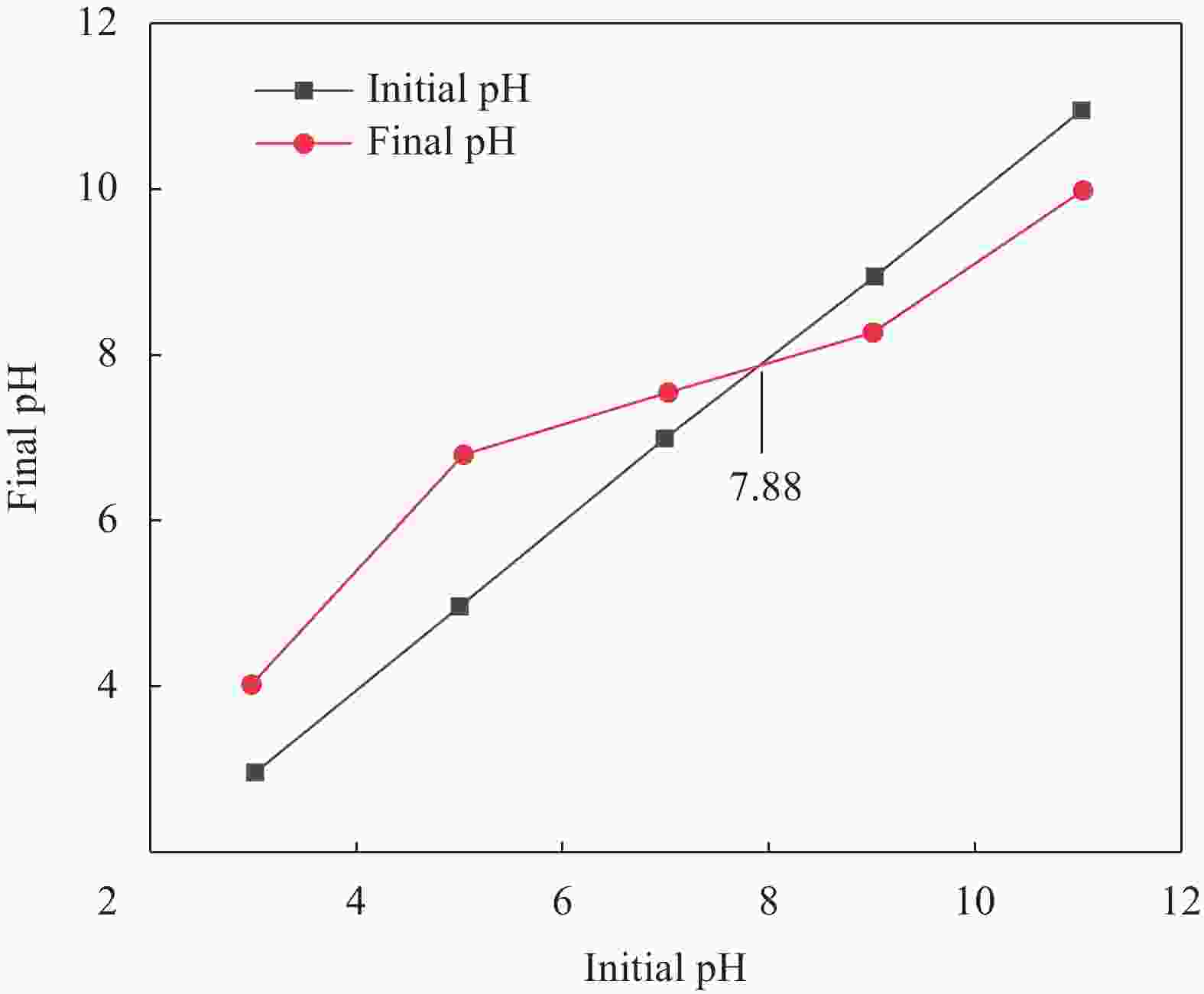
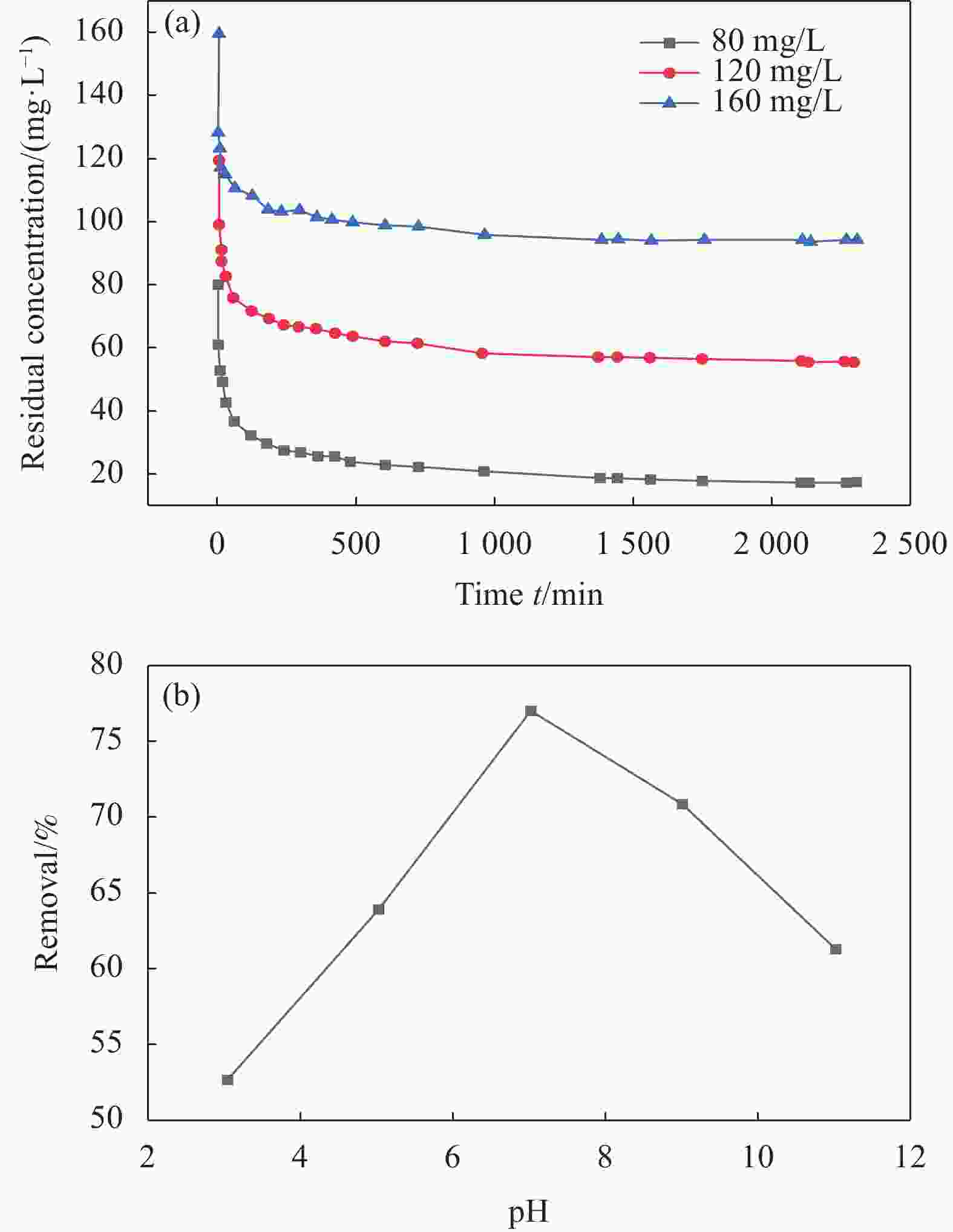
Modification of coconut shell biochar by ball milling with an alkali for enrofloxacin adsorption
-
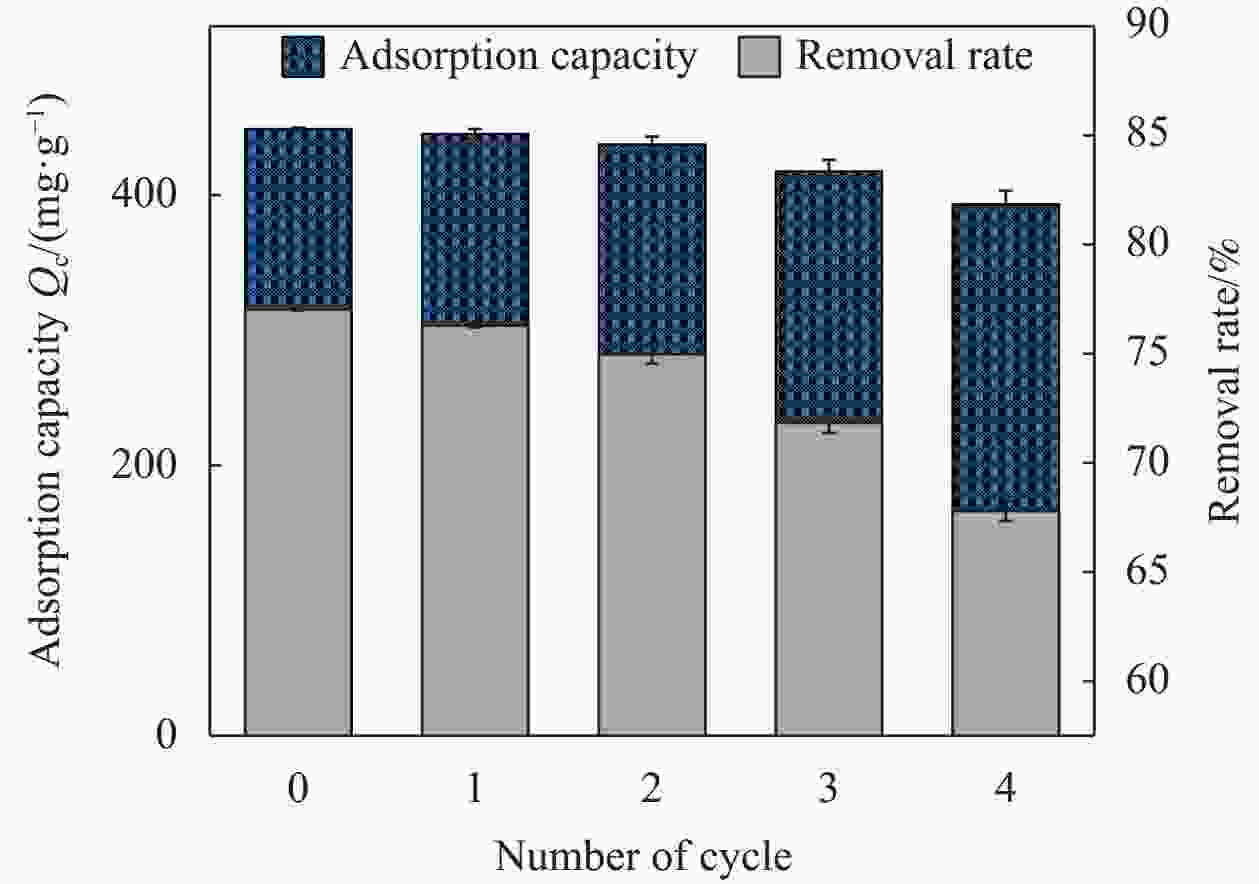
摘要: 为了高效吸附水中的恩诺沙星(EFA),本文通过球磨法和KOH活化对椰壳进行改性制备椰壳生物炭(BM-KOH-BC),并在吸附EFA方面进行深入研究。通过扫描电子显微镜、傅里叶变换红外光谱和X射线衍射等方法对BM-KOH-BC进行表征,结果揭示了KOH活化和球磨改性显著提高了BM-KOH-BC的孔隙结构和比表面积。在优化条件下(初始EFA浓度为80 mg·L−1时,在pH值为7,温度为25℃,吸附剂剂量为0.14 g·mg·L−1,搅拌速度为200 r/min、接触时间为35 h的条件下,BM-KOH-BC表现出良好的吸附性能,去除率达77.4%,最大吸附容量为481.1 mg·g−1。吸附过程符合二级动力学模型和Freundlich等温线模型。此外,BM-KOH-BC在5次吸附-解吸循环后仍保持高效的EFA去除率。这一低成本、高效吸附和可循环利用的特性使BM-KOH-BC在处理水体中的EFA方面展现出潜在的应用前景。Abstract: In order to efficiently adsorb enrofloxacin (EFA) in water, coconut shell biochar (BM-KOH-BC) was prepared by modifying coconut shell through ball milling and KOH activation, and in-depth research was conducted on the adsorption of EFA. BM-KOH-BC was characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, and X-ray diffraction. The results revealed that KOH activation and ball milling modification significantly improved the pore structure and specific surface area of BM-KOH-BC. Under optimized conditions (Initial EFA concentration is 80 mg·L−1, pH value is 7, temperature is 25℃, adsorbent dosage is 0.14 g·mg·L−1, stirring speed is 200 r/min, contact time under the conditions of 35 h), BM-KOH-BC showed good adsorption performance, with a removal rate of 77.4% and a maximum adsorption capacity of 481.1 mg·g−1. The adsorption process is consistent with the second-order kinetic model and Freundlich isotherm model. In addition, BM-KOH-BC still maintains efficient EFA removal rate after 5 adsorption-desorption cycles. This low-cost, efficient adsorption and recyclability feature makes BM-KOH-BC show potential application prospects in treating EFA in water bodies.
-
Key words:
- ball-milling /
- biochar /
- coconut shell /
- enrofloxacin /
- adsorption
-
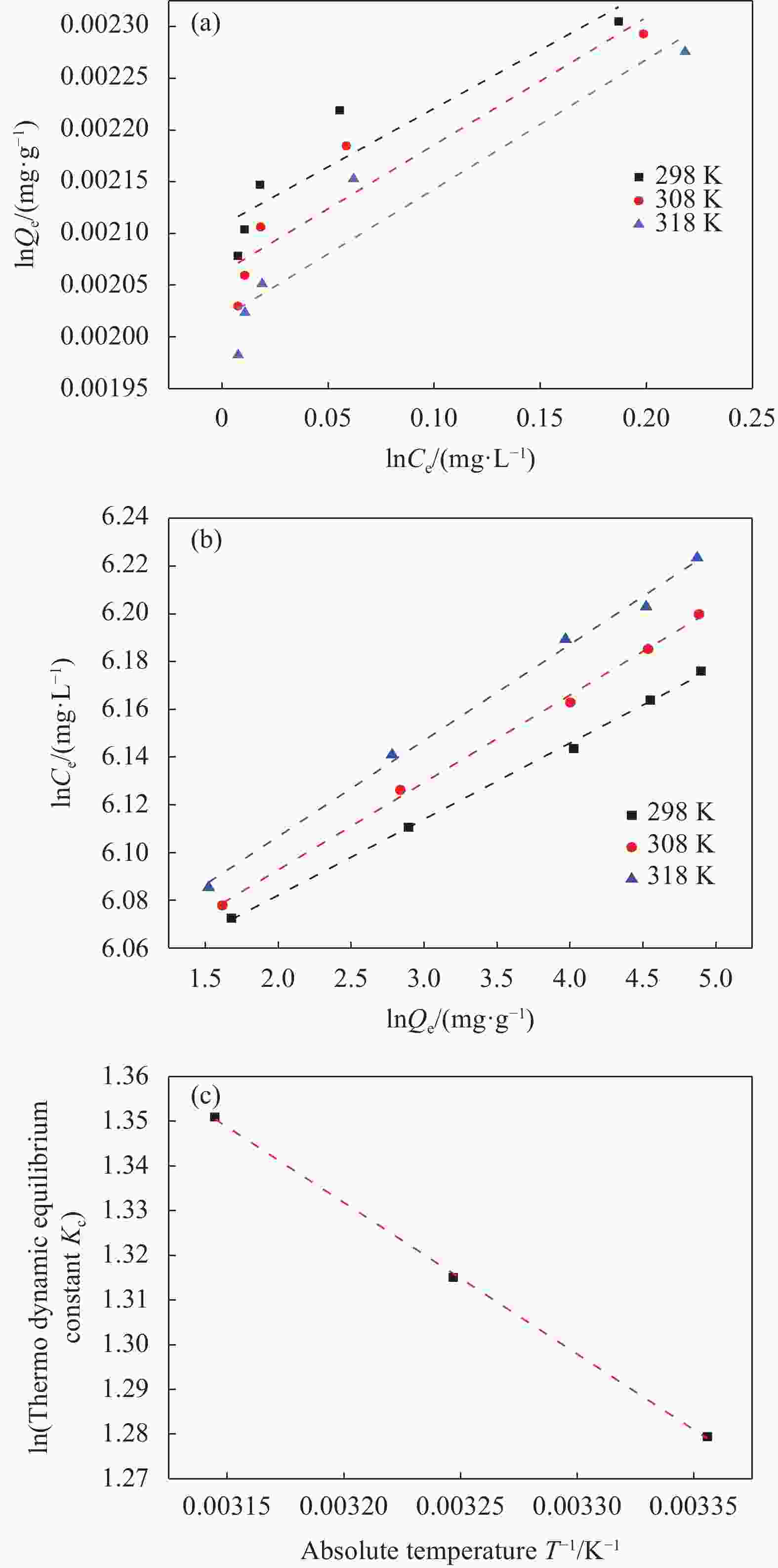
图 6 BM-KOH-BC去除EFA的吸附等温线:(a) Langmuir;(b) Freundlich;(c) EFA吸附的Van't Hoff图
Ce—Equilibrium concentration; Qe—Amount of adsorbate adsorbed per unit mass of adsorbent at equilibrium during the adsorption process
Figure 6. Adsorption isotherms of EFA removal by BM-KOH-BC:(a) Langmuir; (b) Freundlich; (c) Van’t Hoff plot of EFA adsorption
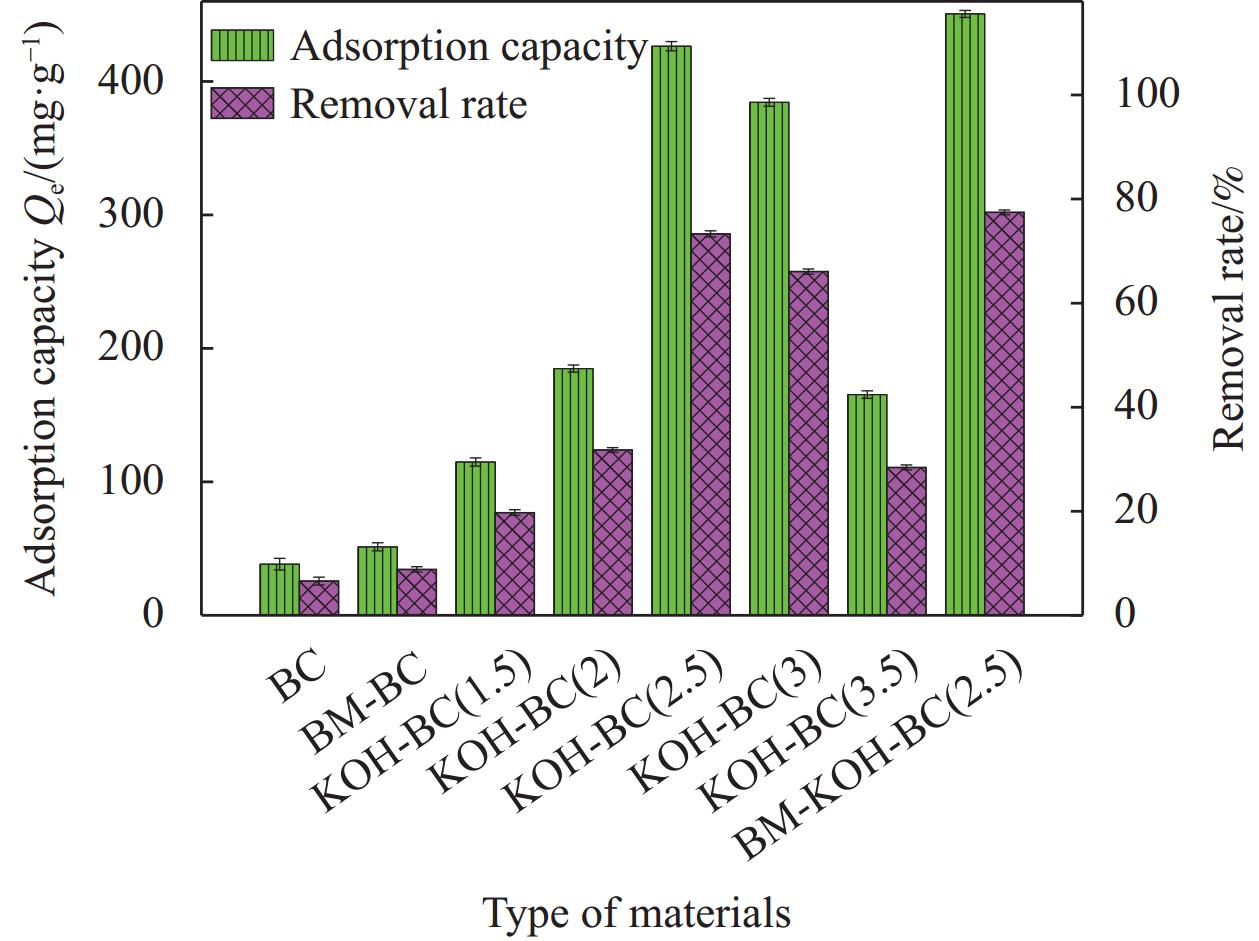
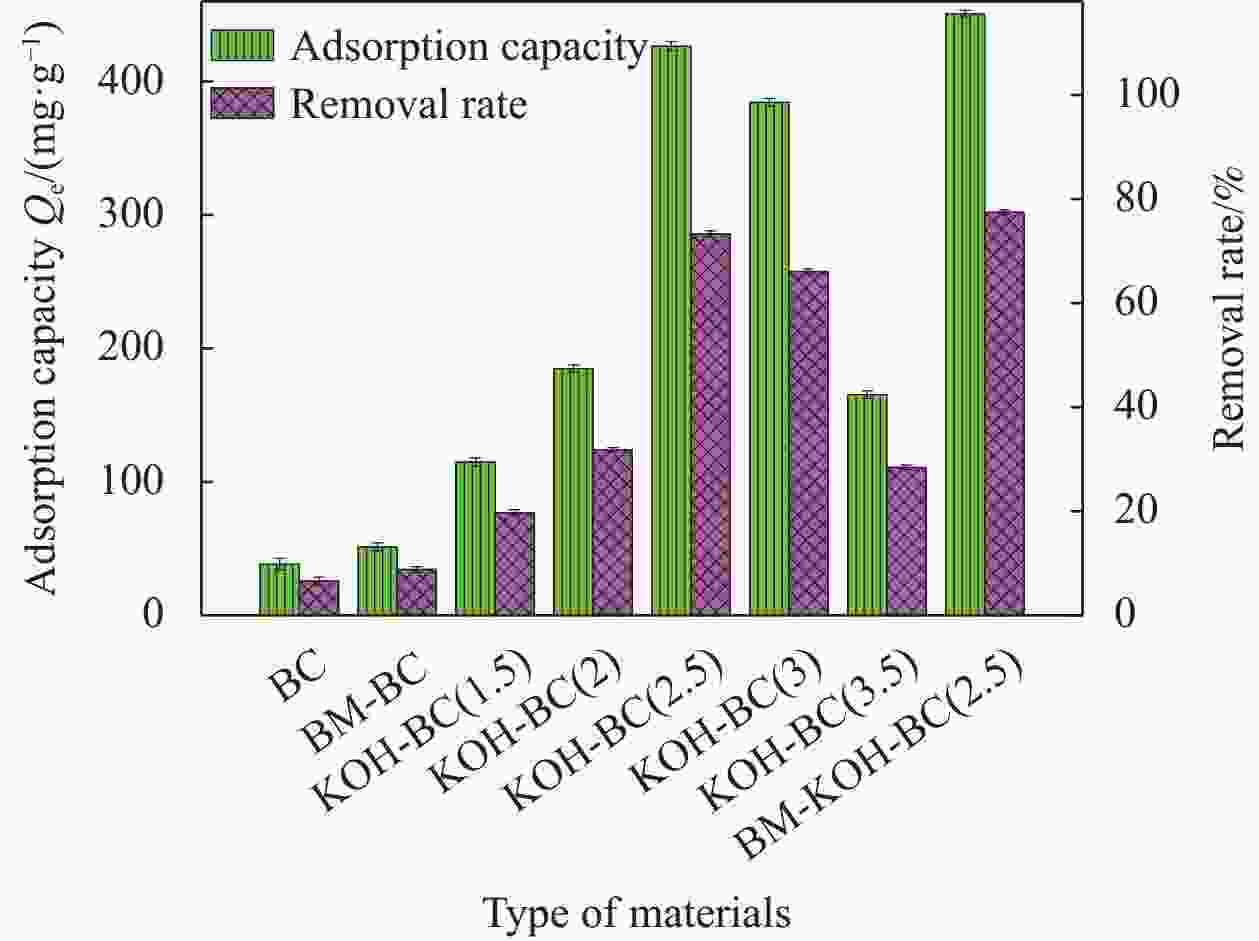
表 1 生物炭(BC)、碱活化生物炭(KOH-BC)的不同质量比分析
Table 1. Analysis of different mass ratios of biochar (BC) and alkali-activated biochar (KOH-BC)
Sample Mass of BC and KOH BC 1∶0 KOH-BC (1.5) 1∶1.5 KOH-BC (2) 1∶2 KOH-BC (2.5) 1∶2.5 KOH-BC (3) 1∶3 KOH-BC (3.5) 1∶3.5 表 2 BC、KOH-BC 和BM-KOH-BC 的 BET 分析
Table 2. BET analysis of BC, KOH-BC and BM-KOH-BC
Material BET surface area/(m2·g−1) Pore volume/(cm3·g−1) Element mass fraction/wt% C O H N BC 775.99±2.53 0.424163±0.008 79.78±3.53 9.85±0.42 1.34±0.35 0.31±0.02 BM-BC 1559.12±1.83 0.604902±0.005 71.09±2.47 18.29±0.28 1.79±0.33 0.56±0.01 KOH-BC 2277.28±1.02** 1.238911±0.002** 60.85±2.53 28.04±0.45 3.37±0.28 0.58±0.03 BM-KOH-BC 2620.49±0.21* 1.433558±0.001* 60.51±2.38 29.89±0.57 3.53±0.21 0.65±0.03 Notes: *—P< 0.05; **—P< 0.01; P-value indicates stronger evidence against the null hypothesis, suggesting that the observed results are less likely to be due to chance. 表 3 BM-KOH-BC吸附 EFA 的 Langmuir 和 Freundlich 吸附等温线参数
Table 3. Langmuir and Freundlich adsorption isotherm parameters for EFA adsorption by BM-KOH-BC
T/K Langmuir Freundlich Qmax,exp/(mg·g−1) Qmax,cal/(mg·g−1) KL/(mg·g−1) R2 1/n KF/(mg·g−1) R2 298 481.1 476.1 2.1 0.8644 0.0318 410.9617 0.9982 308 492.6 476.1 1.75 0.8850 0.0366 411.4551 0.9978 318 504.4 500 1.5384 0.8979 0.0402 414.2211 0.9957 Notes:T—Temperature; Qmax—Maximum adsorption capacity; KL—Adsorptive constant of Langmuir model; 1/n—Empirical parameter varied with the degree of heterogeneity of adsorbing sites; KF—Adsorptive constant of Freundlich model; R2—Correlation coefficient of Langmuir and Freundlich models. 表 4 不同吸附剂对EFA的吸附容量
Table 4. EFA adsorption capacities of different adsorbents
Adsorbent SBET/ (m2·g−1) Qmax/(mg·g−1) Ref. Montmorillonite 32 239.7 [29] Illite 22 81.6 [29] Kaolinite 10 7.2 [29] Ca-montmorillonite ND 144.4 [30] Na-montmorillonite ND 163.4 [30] Ligno-cellulosic substrate from wheat bran 11±3 3.56 [31] Chemically activated carbon derived from industrial paper sludge 4514 44.4 [32] Chemically activated carbon developed from green coconut shell 1005.76 12.3 [17] Ball milling-alkali activated green coconut shell biochar 2620.49 481.1 This work Notes:Qmax—Maximum sorption capacity;SBET—BET surface area; ND—Not detected. 表 5 不同EFA初始浓度下BM-KOH-BC吸附EFA的动力学参数
Table 5. Kinetic parameters for EFA adsorption by BM-KOH-BC at different initial EFA concentrations
Initial concentration/(mg·L−1) Qe,exp/(mg·g−1) Pseudo-first-order Pseudo-second-order k1 $ Q\mathrm{_{e,cal}} $ R2 k2 $ Q\mathrm{_{e,cal}} $ R2 80 2.50 0.0031 0.98 0.86470 0.0094 2.54 0.9994 120 2.56 0.0031 1.07 0.88360 0.0080 2.60 0.9992 160 2.61 0.0034 0.86 0.90536 0.0119 2.65 0.9996 Notes:Qe,exp—Equilibrium sorption capacity obtained from experiment; k1—First-order apparent sorption rate constants; Qe,cal— Equilibrium sorption capacity calculated by pseudo-first order kinetics or pseudo-second order kinetics; k2—Second-order apparent sorption rate constants; R2—Correlation coefficient of pseudo-first order kinetics or pseudo-second order kinetics. 表 6 BM-KOH-BC吸附EFA的热力学参数
Table 6. Thermodynamic parameters of EFA adsorption by BM-KOH-BC
Temperature/℃ ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(kJ·mol−1) 25 −3.1012 2.8176 0.02 35 −3.2946 45 −3.4944 Notes:ΔG—Gibbs free energy; ΔH—Enthalpy; ΔS—Entropy. -
[1] GRENNI P, ANCONA V, BARRA CARACCIOLO A. Ecological effects of antibiotics on natural ecosystems: A review[J]. Microchemical Journal, 2018, 136: 25-39. doi: 10.1016/j.microc.2017.02.006 [2] FATTA D, ACHILLEOS, NIKOLAOUA A, et al. Analytical methods for tracing pharmaceutical residues in water and wastewater[J]. Trac Trends in Analytical Chemistry, 2007, 26(6): 515-533. doi: 10.1016/j.trac.2007.02.001 [3] SIB E, VOIGT A M, WILBRING G, et al. Antibiotic resistant bacteria and resistance genes in biofilms in clinical wastewater networks[J]. International Journal of Hygiene and Environmental Health, 2019, 222(4): 655-662. doi: 10.1016/j.ijheh.2019.03.006 [4] WALKER R, STEIN G, HAUPTMAN J, et al. Pharmacokinetic evaluation of enrofloxacin administered orally to healthy dogs[J]. American Journal of Veterinary Research, 1993, 53: 2315-2319. [5] KEMPER N. Antibiotics in the aquatic and terrestrial environment[J]. Ecological Indicators, 2008, 8(1): 1-13. [6] ZHU T T, SU Z X, LAI W X, et al. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology[J]. Science of the Total Environment, 2021, 776: 145906. doi: 10.1016/j.scitotenv.2021.145906 [7] BHATTACHARYA S, YADAV J. Microbial P450 enzymes in bioremediation and drug discovery: Emerging potentials and challenges[J]. Current Protein & Peptide Science, 2018, 191: 75-86. [8] SAVEV S, ORTENBACH M, GUILLON E. Sportive removal of enrofloxacin antibiotic from aqueous solution using a lingo-cellulosic substrate from wheat bran[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 5820-5829. doi: 10.1016/j.jece.2018.08.012 [9] CHUA S F, NOURI A, ANG W L, et al. The emergence of multifunctional adsorbents and their role in environmental remediation[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104793. doi: 10.1016/j.jece.2020.104793 [10] ZHAO L, ZHANG Y, WANG L, et al. Effective removal of Hg(II) and Me Hg from aqueous environment by ball milling aided thiol-modification of biochars: Effect of different pyrolysis temperatures[J]. Chemosphere, 2022, 294: 133820. [11] LYU H H, GAO B, HE F, et al. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue[J]. Chemical Engineering Journal, 2017, 335: 110-119. [12] HUANG J, ZIMMERMAN A R, CHEN H, et al. Ball milled biochar effectively removes sulfamethoxazole and sulfa pyridine antibiotics from water and wastewater[J]. Environmental Pollution, 2019, 258: 113809. [13] WU J, WANG T, LIU Y, et al. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar[J]. Chemosphere, 2022, 303: 135264. [14] SUN Y, ZHENG L, ZHENG X, et al. Adsorption of sulfonamides in aqueous solution on reusable coconut-shell biochar modified by alkaline activation and magnetization[J]. Frontiers in Chemistry, 2021, 9: 814647. [15] WANG S W, XIAO D, ZHENG X, et al. Halloysite and coconut shell biochar magnetic composites for the sorption of Pb(II) in wastewater: Synthesis, characterization and mechanism investigation[J]. Journal of Environmental Chemical Engineering, 2021, 10(2): 106865. [16] DIPA D, SAMAIL D, MEIKAP B C. Preparation of activated carbon from green coconut shell and its characterization[J]. Journal of Chemical Engineering & Process Technology, 2015, 6: 1000248. [17] CHAKRABORTY P, BANERJEE S, KUMAR S, et al. Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: Isotherm, kinetics, thermodynamics and cost estimation[J]. Process Safety and Environmental Protection, 2018, 118: 10-23. doi: 10.1016/j.psep.2018.06.015 [18] XIAO Y, LYU H H, TANG J C, et al. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms[J]. Chemical Engineering Journal, 2020, 384: 123311. doi: 10.1016/j.cej.2019.123311 [19] GRAOUER-BACART M, SAYEN S, GUILLON E. Macroscopic and molecular approaches of enrofloxacin retention in soils in presence of Cu(II)[J]. Journal of Colloid and Interface Science, 2013, 408: 191-199. doi: 10.1016/j.jcis.2013.07.035 [20] ZHANG A, LI X, XING J, et al. Adsorption of potentially toxic elements in water by modified biochar: A review[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 104196. doi: 10.1016/j.jece.2020.104196 [21] DASSHARMA D, SAMANTA S. A mechanistic insight into enrofloxacin sorptive affinity of chemically activated carbon engineered from green coconut shell[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104140. doi: 10.1016/j.jece.2020.104140 [22] ZHAO H, CHENG Y, LYU H, et al. A novel hierarchically porous magnetic carbon derived from biomass for strong lightweight microwave absorption[J]. Carbon, 2019, 142: 245-253. doi: 10.1016/j.carbon.2018.10.027 [23] YAO X, JI L, GUO J, et al. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption[J]. Bioresource Technology, 2020, 302: 122842. doi: 10.1016/j.biortech.2020.122842 [24] XIE H J, LIU W F, ZHANG J, et al. Sorption of norfloxacin from aqueous solutions by activated carbon developed from Trapa natans husk[J]. Science China Chemistry, 2011, 54(5): 835-843. [25] KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. [26] GUPTA V K, JAIN R, SIDDIQUI M, et al. Equilibrium and thermodynamic studies on the adsorption of the dye Rhodamine-B onto mustard cake and activated carbon[J]. Journal of Chemical Engineering Data, 2010, 55: 5225-5229. doi: 10.1021/je1007857 [27] GERCEL Ö, GERCEL H F. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida[J]. Chemical Engineering Journal, 2007, 132(1): 289-297. [28] SANKARANARAYANAN S, LAKSHMI D, VIVEKANANDHAN S, et al. Biocarbons as emerging and sustainable hydrophobic/oleophilic sorbent materials for oil/water separation[J]. Sustainable Materials and Technologies, 2021, 28: 100268. [29] WAN M, LI Z, HONG H, et al. Enrofloxacin uptake and retention on different types of clays[J]. Journal of Asian Earth Sciences, 2013, 77(15): 287-294. [30] ELISA R, ANNA P, MICHELA S, et al. Clay minerals for adsorption of veterinary FQs: Behavior and modeling[J]. Journal of Environmental Chemical Engineering, 2014, 91: 22-31. [31] STEPHANIE S, MARTA O L, EMMANUEL G. Sportive removal of enrofloxacin antibiotic from aqueous solution using a lingo-cellulosic substrate from wheat bran[J]. Journal of Environmental Chemical Engineering, 2018, 6(11): 5820-5829. [32] SOMNATH C, JAYA S, MANDAL T, et al. Comprehensive analysis on sorptive uptake of enrofloxacin by activated carbon derived from industrial paper sludge[J]. The Science of the Total Environment, 2019, 665: 438-452. [33] MAZIARZ P, MATUSIK J, RADZISZEWSKA A. Halloysite-zero-valent iron nanocomposites for removal of Pb(II)/Cd(II) and As(V)/Cr(VI): Competitive effects, regeneration possibilities and mechanisms[J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 103507. doi: 10.1016/j.jece.2019.103507 -





 下载:
下载: