Advances in photoelectrocatalysis and artificial photosynthesis for the reduction of CO2
-
摘要: 随着工业化的不断发展,化石燃料的过度使用产生的CO2导致了温室效应等问题,已经引起国际社会的高度关注,并制定了一系列应对措施。因此,对大气中CO2的还原回收技术研发具有迫切性和重要意义。光电催化是目前可用于还原CO2的具有良好应用前景的技术之一,为了对该技术进行更深入的研究,推动其实际应用,本文首先阐述了光催化、电催化、光电催化还原CO2的基本原理和优缺点,并举例介绍了各类催化剂还原CO2的效率。由于光催化是光合作用中的重要步骤之一,接着重点分析了光合作用在还原CO2研究现状和前景,提出人工光合作用还原CO2可行性与潜力。本文旨在为人工光合作用还原CO2提供新思路和参考,为减少大气中CO2的积累和应对当前的环境挑战提供新的见解和视角。Abstract: With the continuous development of industrialization, CO2 produced by the excessive use of fossil fuels has led to problems such as the greenhouse effect, which has attracted great attention from the international community and a series of countermeasures have been formulated. Therefore, the research and development of technology for the reduction and recovery of CO2 from the atmosphere is urgent and important. Photoelectrocatalysis is one of the technologies with good application prospects that can be used to reduce CO2. In order to carry out a more in-depth research on this technology and promote its practical application, this paper firstly describes the basic principles and advantages and disadvantages of photocatalysis, electrocatalysis, and photoelectrocatalysis for CO2 reduction, and gives examples of the efficiency of various types of catalysts for CO2 reduction. Because photocatalysis is one of the important steps in photosynthesis, it then focuses on analyzing the current status and prospects of photosynthesis in reducing CO2 research, and proposes the feasibility and potential of artificial photosynthesis for CO2 reduction. The aim of this paper is to provide new ideas and references for the reduction of CO2 by artificial photosynthesis, and to provide new insights and perspectives for reducing the accumulation of CO2 in the atmosphere and addressing current environmental challenges.
-
Key words:
- solar energy /
- photosynthesis /
- CO2 reduction /
- catalyst /
- photocatalysis /
- electrocatalysis /
- photoelectrocatalysis
-
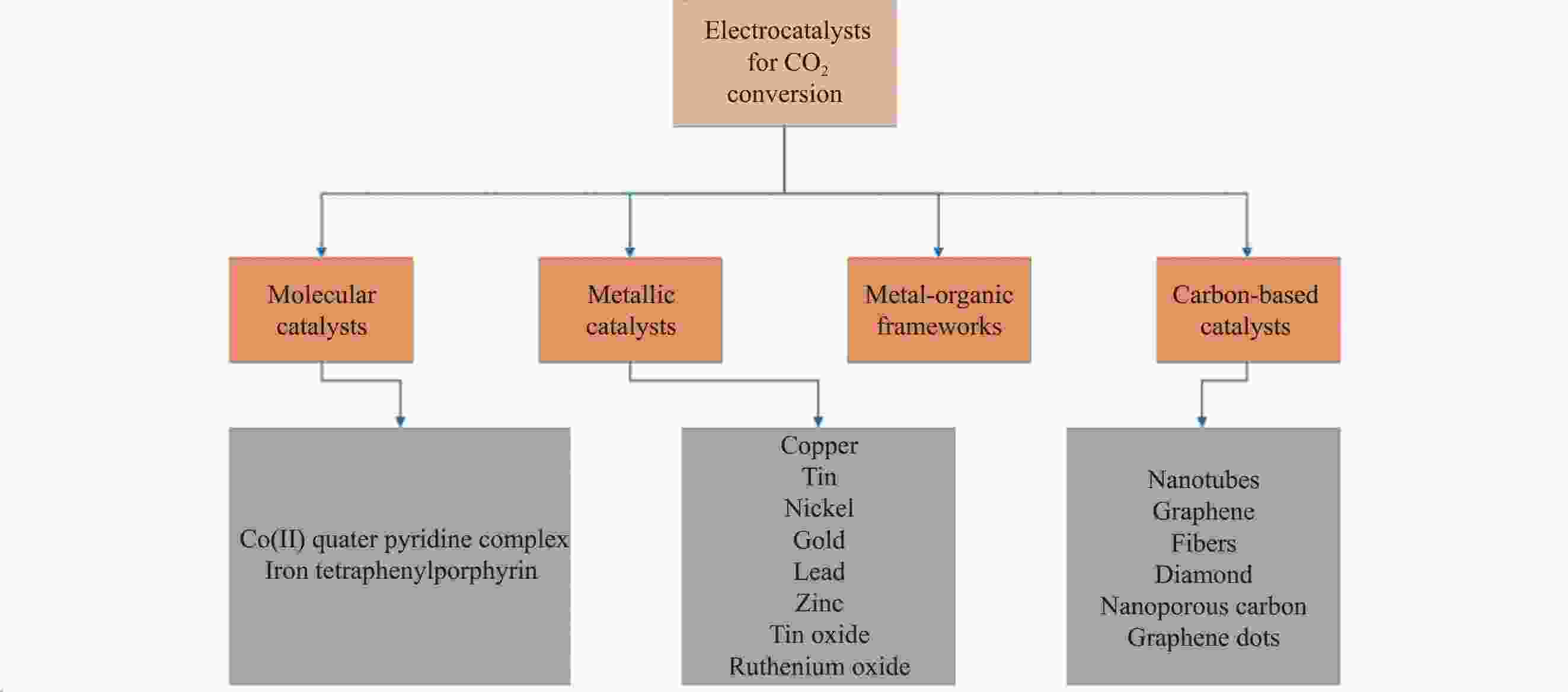
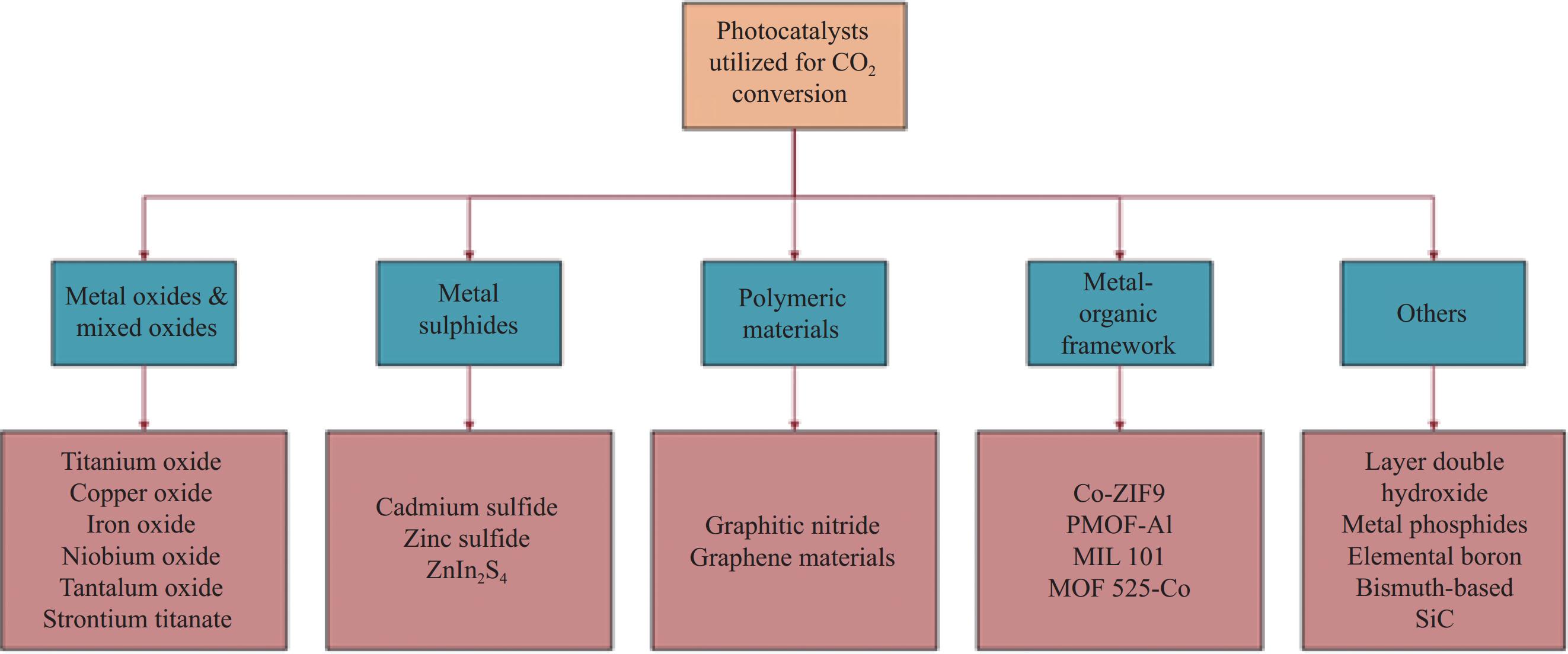
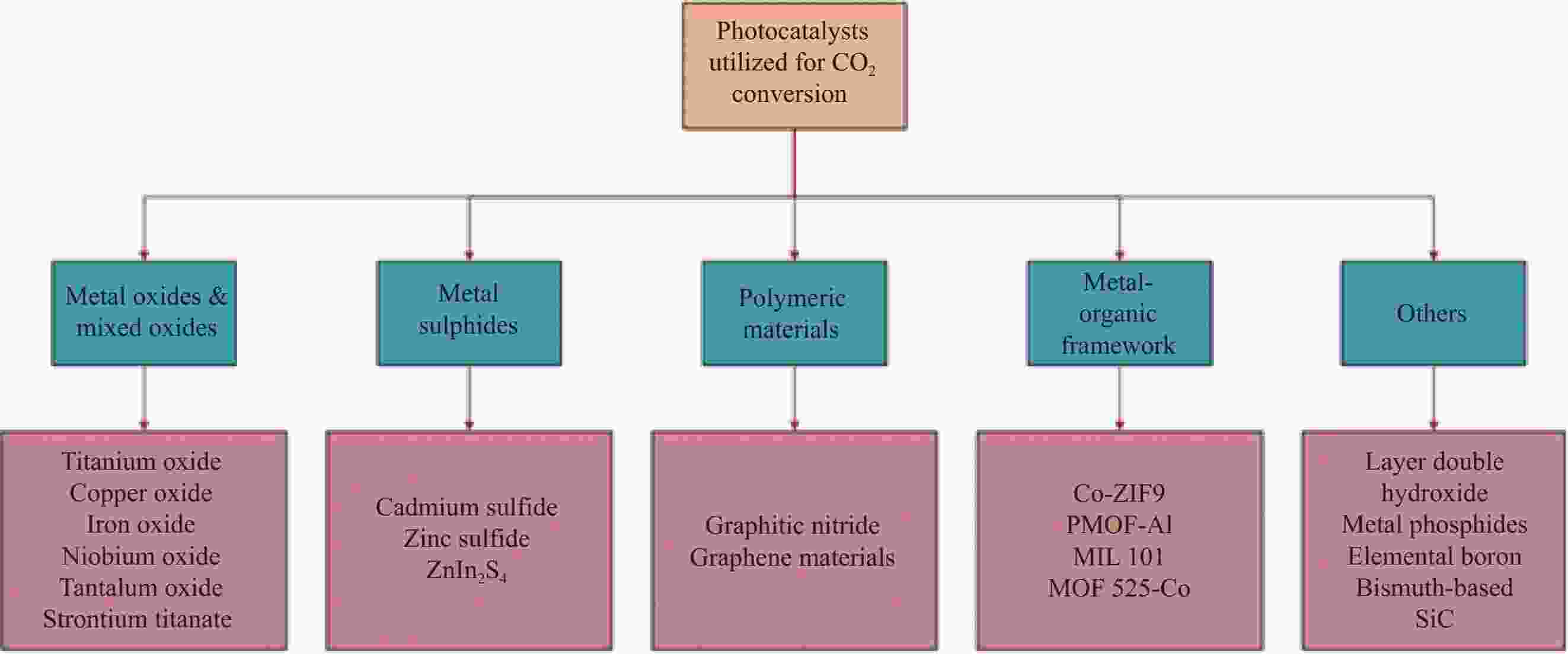
表 1 部分非金属或金属负载半导体光催化剂复合材料用于CO2还原
Table 1. Several non-metallic or metal supported semiconductor photocatalyst composites for CO2 reduction
Photocatalyst Main product Photocatalytic activity Ref. m-CeO2/g-C3N4 CH4 and CO CH4: 13.88 µmol·h−1·g−1; CO: 11.8 µmol·h−1·g−1 [54] SrCO3/SrTiO3 CO CO: 23.82 (100) μmol·h−1·g−1 [55] Fe2O3/Cu2O CO 5.0 μmol·g−1 catalyst [56] Cu2ZnSnS4-ZnO CH4 138.90 μmol·g−1·L−1 [57] TiO2-SiO2 CH4 2.42 μmol·g−1 [58] Cu/TiO2 CH3OH 1.8 μmol·cm−2·h−1 [59] Sulfur-doped g-C3N4 CH3OH 1.12 μmol·g−1 [60] ZnPc/TiO2 HCOOH 978.6 μmol·g−1 catalyst [61] GO-TiO2 CH3OH/C2H5OH 47.0 μmol·g−1·h−1/144.7 μmol·g−1·h−1 [62] Bi2S3 HC(O)OCH3 300.94 μmol·g−1 [63] Notes: m-CeO2—Mesoporous-CeO2; GO—Graphene oxide; ZnPc—Zinc-phthalocyanine. 表 2 一些用于CO2还原的选择性电催化剂
Table 2. Some selective electrocatalysts for CO2 reduction
Electrocatalyst Electrolyte Main product Corresponding overpotential Ref. Fe-N4O 0.1 mol/L KHCO3, pH=6.8 CO 470 mV [88] In(OH)3-Cu2O 0.7 mol/L KHCO3 CO 290 mV [89] BiOI 0.5 mol/L NaHCO3, pH=6 HCOOH −0.40 V [90] Pyridoxine modification
graphene oxide (GO-VB6-Cu)0.1 mol/L KHCO3, pH=6.8 CH3CH2OH 0.14 V [84] Graphite/carbon nanoparticle (NPs)/
Cu/polytetrafluoroethylene (PTFE)7 mol/L KOH, pH>14 C2H4 −0.63 V [91] Cu2O/ZnO/Graphene (GN) 0.5 mol/L NaHCO3 C3H7OH −0.90 V [92] Cu/TiO2/GN 0.2 mol/L KI, pH=6.62 C2H5OH 0.84 V [93] Bi nanosheet 0.1 mol/L KHCO3, pH=6.8 HCOOH 420 mV [94] Nanoporous Au-Sn (NPAS) 0.5 mol/L KHCO3, pH=7.2 CO 0.45 V [95] 表 3 CO2还原的主要产物及其对应电位(pH=7)
Table 3. Main products of CO2 reduction and their corresponding potentials (pH=7)
Reaction Eo(vs NHE)/V Product CO2 + e−→•$\text{CO}_{2}^{-} $ −1.90 •$\text{CO}_{2}^{-} $ anion radical 2CO2 + 2H+ + 2e− → H2C2O4 −0.87 Oxalate CO2 + 2H+ + 2e− → HCOOH −0.61 Formic acid CO2 + 2H+ + 2e− → CO + H2O −0.53 Carbon monoxide CO2 + 4H+ + 4e− → HCHO + H2O −0.48 Formaldehyde CO2 + 6H+ + 6e− → CH3OH + H2O −0.38 Methanol 2CO2 + 12H+ + 12e− → C2H5OH + 3H2O −0.33 Ethanol 2CO2 + 14H+ + 14e− → C2H6 + 4H2O −0.27 Ethane CO2 + 8H+ + 8e− → CH4 + 2H2O −0.24 Methane Notes: Eo—Standard electrode potential; NHE—Normal hydrogen electrode. 表 4 用于CO2还原的几种光电催化界面
Table 4. Several photoelectrocatalytic interfaces for CO2 reduction
Catalyst Number of electrons transferred Main product Yield/(μmol·gcat−1·h−1) Ref. Pt/TiO2 8 CH4 1361 [108] Cu@TiO2-Au 2 HCOOH N.A. [100] Au-ZnTe/ZnO 2 CO N.A. [109] Rh grain boundaries (GBs)/TiO2 12 C2H5OH 12.1 [110] NH3/g-C3N4 8, 6 CH4, CH3OH 1.39, 1.87 [111] NH2-C/Cu2O 2 HCOOH 138.65 [112] Co-ZIF9/g-C3N4 2 CO 495 [113] UiO-66/MoS2 8 CH3COOH 39 [114] Ni(II) MOF/g-C3N4 2, 8 CO, CH4 13.6 [115] Note: N.A.—Not available. -
[1] FU J W, JIANG K X, QIU X Q, et al. Product selectivity of photocatalytic CO2 reduction reactions[J]. Materials Today, 2020, 32: 222-243. doi: 10.1016/j.mattod.2019.06.009 [2] JIA C, DASTAFKAN K, ZHAO C. Key factors for designing single-atom metal-nitrogen-carbon catalysts for electrochemical CO2 reduction[J]. Current Opinion in Electrochemistry, 2022, 31: 100854. doi: 10.1016/j.coelec.2021.100854 [3] CHAND S S, WALSH K J E, CAMARGO S J, et al. Declining tropical cyclone frequency under global warming[J]. Nature Climate Change, 2022, 12(7): 655-661. doi: 10.1038/s41558-022-01388-4 [4] PRABHU P, JOSE V, LEE J M. Heterostructured catalysts for electrocatalytic and photocatalytic carbon dioxide reduction[J]. Advanced Functional Materials, 2020, 30(24): 1910768. doi: 10.1002/adfm.201910768 [5] SHANG Z A, FENG X T, CHEN G Z, et al. Recent advances on single-atom catalysts for photocatalytic CO2 reduction[J]. Small, 2023, 19(48): 2304975. doi: 10.1002/smll.202304975 [6] XIONG Z, WANG H B, XU N Y, et al. Photocatalytic reduction of CO2 on Pt2+-Pt0/TiO2 nanoparticles under UV/Vis light irradiation: A combination of Pt2+ doping and Pt nanoparticles deposition[J]. International Journal of Hydrogen Energy, 2015, 40(32): 10049-10062. doi: 10.1016/j.ijhydene.2015.06.075 [7] ALKHATIB I I, GARLISI C, PAGLIARO M, et al. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications[J]. Catalysis Today, 2020, 340: 209-224. doi: 10.1016/j.cattod.2018.09.032 [8] OCHEDI F O, LIU D J, YU J L, et al. Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: A review[J]. Environmental Chemistry Letters, 2021, 19(2): 941-967. doi: 10.1007/s10311-020-01131-5 [9] HIRAGOND C B, POWAR N S, LEE J, et al. Single-atom catalysts (SACs) for photocatalytic CO2 reduction with H2O: Activity, product selectivity, stability, and surface chemistry[J]. Small, 2022, 18(29): 2201428. doi: 10.1002/smll.202201428 [10] VU N N, KALIAGUINE S, DO T O. Critical aspects and recent advances in structural engineering of photocatalysts for sunlight-driven photocatalytic reduction of CO2 into fuels[J]. Advanced Functional Materials, 2019, 29(31): 1901825. doi: 10.1002/adfm.201901825 [11] CHANG P Y, TSENG I H. Photocatalytic conversion of gas phase carbon dioxide by graphitic carbon nitride decorated with cuprous oxide with various morphologies[J]. Journal of CO2 Utilization, 2018, 26: 511-521. doi: 10.1016/j.jcou.2018.06.009 [12] CHENG L, YUE X Y, FAN J J, et al. Site-specific electron-driving observations of CO2-to-CH4 photoreduction on Co-doped CeO2/crystalline carbon nitride S-scheme heterojunctions[J]. Advanced Materials, 2022, 34(27): 2200929. doi: 10.1002/adma.202200929 [13] RAMBABU Y, KUMAR U, SINGHAL N, et al. Photocatalytic reduction of carbon dioxide using graphene oxide wrapped TiO2 nanotubes[J]. Applied Surface Science, 2019, 485: 48-55. doi: 10.1016/j.apsusc.2019.04.041 [14] NUR N U M, AMIN N A S. Glucose precursor carbon-doped TiO2 heterojunctions for enhanced efficiency in photocatalytic reduction of carbon dioxide to methanol[J]. Journal of CO2 Utilization, 2019, 33: 372-383. doi: 10.1016/j.jcou.2019.07.002 [15] HOU W B, HUNG W H, PAVASKAR P, et al. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions[J]. ACS Catalysis, 2011, 1(8): 929-936. doi: 10.1021/cs2001434 [16] LEE B H, GONG E, KIM M, et al. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O[J]. Energy and Environmental Science, 2022, 15(2): 601-609. doi: 10.1039/D1EE01574E [17] TORRES J A, NOGUEIRA A E, SILVA G T S T, et al. Enhancing TiO2 activity for CO2 photoreduction through MgO decoration[J]. Journal of CO2 Utilization, 2020, 35: 106-114. doi: 10.1016/j.jcou.2019.09.008 [18] WANG J, GUO R T, BI Z X, et al. A review on TiO2− x-based materials for photocatalytic CO2 reduction[J]. Nanoscale, 2022, 14(32): 11512-11528. doi: 10.1039/D2NR05316K [19] ZHANG X D, ZHOU Y J, ZHANG H, et al. Tuning the electron structure enables the NiZn alloy for CO2 electroreduction to formate[J]. Journal of Energy Chemistry, 2021, 63: 625-632. doi: 10.1016/j.jechem.2021.08.060 [20] PARDO PÉREZ L C, CHALKLEY Z, WENDT R, et al. CO2 electroreduction activity and dynamic structural evolution of in situ reduced nickel-indium mixed oxides[J]. Journal of Materials Chemistry A, 2022, 10(38): 20593-20605. doi: 10.1039/D2TA05214H [21] JIANG X, LI X, KONG Y, et al. A hierarchically structured tin-cobalt composite with an enhanced electronic effect for high-performance CO2 electroreduction in a wide potential range[J]. Journal of Energy Chemistry, 2023, 76: 462-469. doi: 10.1016/j.jechem.2022.10.008 [22] FARID M A, IJAZ S, ASHIQ M N, et al. Synthesis of mesoporous zirconium manganese mixed metal oxide nanowires for photocatalytic reduction of CO2[J]. Journal of Materials Research, 2022, 37(2): 522-532. [23] DONG H, ZHANG X, LU Y, et al. Regulation of metal ions in smart metal-cluster nodes of metal-organic frameworks with open metal sites for improved photocatalytic CO2 reduction reaction[J]. Applied Catalysis B: Environmental, 2020, 276: 119173. doi: 10.1016/j.apcatb.2020.119173 [24] WANG W, DENG C Y, XIE S J, et al. Photocatalytic C-C coupling from carbon dioxide reduction on copper oxide with mixed-valence copper (I)/copper (II)[J]. Journal of the American Chemical Society, 2021, 143(7): 2984-2993. doi: 10.1021/jacs.1c00206 [25] JIANG Z, XU X H, MA Y H, et al. Filling metal-organic framework mesopores with TiO2 for CO2 photoreduction[J]. Nature, 2020, 586(7830): 549-554. doi: 10.1038/s41586-020-2738-2 [26] LIU Z L, SUN L K, ZHANG Q T, et al. TiO2-supported single-atom catalysts: Synthesis, structure, and application[J]. Chemical Research in Chinese Universities, 2022, 38(5): 1123-1138. doi: 10.1007/s40242-022-2224-5 [27] DONG G X, ZHANG W, MU Y F, et al. A halide perovskite as a catalyst to simultaneously achieve efficient photocatalytic CO2 reduction and methanol oxidation[J]. Chemical Communications, 2020, 56(34): 4664-4667. doi: 10.1039/D0CC01176B [28] IDRIS A M, ZHENG S, WU L, et al. A heterostructure of halide and oxide double perovskites Cs2AgBiBr6/Sr2FeNbO6 for boosting the charge separation toward high efficient photocatalytic CO2 reduction under visible-light irradiation[J]. Chemical Engineering Journal, 2022, 446: 137197. doi: 10.1016/j.cej.2022.137197 [29] TEH Y W, ER C C, KONG X Y, et al. Charge modulation at atomic-level through substitutional sulfur doping into atomically thin Bi2WO6 toward promoting photocatalytic CO2 reduction[J]. ChemSusChem, 2022, 15(14): 18. [30] XU F H, LI Z Z, ZHU R L, et al. Narrow band-gapped perovskite oxysulfide for CO2 photoreduction towards ethane[J]. Applied Catalysis B: Environmental, 2022, 316: 121615. doi: 10.1016/j.apcatb.2022.121615 [31] RAZIQ F, KHAN K, ALI S, et al. Accelerating CO2 reduction on novel double perovskite oxide with sulfur, carbon incorporation: Synergistic electronic and chemical engineering[J]. Chemical Engineering Journal, 2022, 446: 137161. doi: 10.1016/j.cej.2022.137161 [32] CAO Y H, GUO L, DAN M, et al. Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction[J]. Nature Communications, 2021, 12(1): 1675. doi: 10.1038/s41467-021-21925-7 [33] CAI S C, ZHANG M, LI J J, et al. Anchoring single-atom Ru on CdS with enhanced CO2 capture and charge accumulation for high selectivity of photothermocatalytic CO2 reduction to solar fuels[J]. Solar RRL, 2021, 5(2): 2000313. doi: 10.1002/solr.202000313 [34] BI Q Q, WANG J W, LYU J X, et al. Selective photocatalytic CO2 reduction in water by electrostatic assembly of CdS nanocrystals with a dinuclear cobalt catalyst[J]. ACS Catalysis, 2018, 8(12): 11815-11821. doi: 10.1021/acscatal.8b03457 [35] EZUGWU C I, LIU S W, LI C H, et al. Engineering metal-organic frameworks for efficient photocatalytic conversion of CO2 into solar fuels[J]. Coordination Chemistry Reviews, 2022, 450: 214245. doi: 10.1016/j.ccr.2021.214245 [36] DAO X Y, XIE X F, GUO J H, et al. Boosting photocatalytic CO2 reduction efficiency by heterostructures of NH2-MIL-101(Fe)/g-C3N4[J]. ACS Applied Energy Materials, 2020, 3(4): 3946-3954. doi: 10.1021/acsaem.0c00352 [37] GHOSH U, MAJUMDAR A, PAL A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104631. doi: 10.1016/j.jece.2020.104631 [38] HUANG P P, HUANG J H, PANTOVICH S A, et al. Selective CO2 reduction catalyzed by single cobalt sites on carbon nitride under visible-light irradiation[J]. Journal of the American Chemical Society, 2018, 140(47): 16042-16047. doi: 10.1021/jacs.8b10380 [39] WANG Y Y, QU Y, QU B H, et al. Construction of six-oxygen-coordinated single Ni sites on g-C3N4 with boron-oxo species for photocatalytic water-activation-induced CO2 reduction[J]. Advanced Materials, 2021, 33(48): 2105482. doi: 10.1002/adma.202105482 [40] PRASAD C, MADKHALI N, GOVINDA V, et al. Recent progress on the development of g-C3N4 based composite material and their photocatalytic application of CO2 reductions[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 109727. [41] ZHANG Q Q, DUAN Z Y, LI M, et al. Atomic cobalt catalysts for the oxygen evolution reaction[J]. Chemical Communications, 2020, 56(5): 794-797. doi: 10.1039/C9CC09007J [42] ZHAO L M, HAN X N, KONG W C, et al. Graphene supported single metal atom catalysts for the efficient hydrogen oxidation reaction in alkaline media[J]. Catalysis Science & Technology, 2022, 12(2): 530-541. [43] REN S, YU Q, YU X H, et al. Graphene-supported metal single-atom catalysts: A concise review[J]. Science China Materials, 2020, 63(6): 903-920. doi: 10.1007/s40843-019-1286-1 [44] LIU M L, LIU C, GOUSE PEERA S, et al. Catalytic oxidation mechanism of CO on FeN2-doped graphene[J]. Chemical Physics, 2022, 559: 111536. doi: 10.1016/j.chemphys.2022.111536 [45] ZHANG P P, ZHAN X N, XU L B, et al. Mass production of a single-atom cobalt photocatalyst for high-performance visible-light photocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2021, 9(46): 26286-26297. doi: 10.1039/D1TA07169F [46] LI Y X, WANG S Y, WANG X S, et al. Facile top-down strategy for direct metal atomization and coordination achieving a high turnover number in CO2 photoreduction[J]. Journal of the American Chemical Society, 2020, 142(45): 19259-19267. doi: 10.1021/jacs.0c09060 [47] GAO C, CHEN S M, WANG Y, et al. Heterogeneous single-atom catalyst for visible-light-driven high-turnover CO2 reduction: The role of electron transfer[J]. Advanced Materials, 2018, 30(13): 1704624. doi: 10.1002/adma.201704624 [48] ZHANG H B, WEI J, DONG J C, et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework[J]. Angewandte Chemie International Edition, 2016, 55(46): 14308-14312. doi: 10.1002/ange.201608597 [49] REN J T, ZHENG Y L, YUAN K, et al. Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal-organic framework for efficient visible-light photocatalytic CO2 reduction[J]. Nanoscale, 2020, 12(2): 755-762. doi: 10.1039/C9NR08669B [50] WANG L, WAN J W, ZHAO Y S, et al. Hollow multi-shelled structures of Co3O4 dodecahedron with unique crystal orientation for enhanced photocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2019, 141(6): 2238-2241. doi: 10.1021/jacs.8b13528 [51] LI J, HUANG H, XUE W, et al. Self-adaptive dual-metal-site pairs in metal-organic frameworks for selective CO2 photoreduction to CH4[J]. Nature Catalysis, 2021, 4(8): 719-729. doi: 10.1038/s41929-021-00665-3 [52] WANG R, HU Y S, DU J H, et al. Boosting the visible-light activity of ZrO2/g-C3N4 by controlling the crystal structure of ZrO2[J]. Journal of Materials Research, 2021, 36(15): 3086-3095. doi: 10.1557/s43578-021-00309-z [53] CHENG L, ZHANG D N, LIAO Y L, et al. Structural engineering of 3D hierarchical Cd0.8Zn0.2S for selective photocatalytic CO2 reduction[J]. Chinese Journal of Catalysis, 2021, 42(1): 131-140. doi: 10.1016/S1872-2067(20)63623-3 [54] GUO L N, YOU Y, HUANG H W, et al. Z-scheme g-C3N4/Bi2O2 [BO2(OH)] heterojunction for enhanced photocatalytic CO2 reduction[J]. Journal of Colloid and Interface Science, 2020, 568: 139-147. doi: 10.1016/j.jcis.2020.02.025 [55] LI Z L, ZHENG P Y, ZHANG W B, et al. Constructing SrCO3/SrTiO3 nanocomposites with highly selective photocatalytic CO2-to-CO reduction[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 650: 129686. doi: 10.1016/j.colsurfa.2022.129686 [56] WANG J C, ZHANG L, FANG W X, et al. Enhanced photoreduction CO2 activity over direct Z-scheme α-Fe2O3/Cu2O heterostructures under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2015, 7(16): 8631-8639. [57] ZUBAIR M, RAZZAQ A, GRIMES C A, et al. Cu2ZnSnS4 (CZTS)-ZnO: A noble metal-free hybrid Z-scheme photocatalyst for enhanced solar-spectrum photocatalytic conversion of CO2 to CH4[J]. Journal of CO2 Utilization, 2017, 20: 301-311. doi: 10.1016/j.jcou.2017.05.021 [58] DONG C Y, XING M Y, ZHANG J L. Economic hydrophobicity triggering of CO2 photoreduction for selective CH4 generation on noble-metal-free TiO2-SiO2[J]. The Journal of Physical Chemistry Letters, 2016, 7(15): 2962-2966. doi: 10.1021/acs.jpclett.6b01287 [59] LIU E Z, QI L L, BIAN J J, et al. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol[J]. Materials Research Bulletin, 2015, 68: 203-209. doi: 10.1016/j.materresbull.2015.03.064 [60] WANG K, LI Q, LIU B S, et al. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance[J]. Applied Catalysis B: Environmental, 2015, 176: 44-52. [61] ZHAO Z H, FAN J M, WANG Z Z. Photo-catalytic CO2 reduction using sol-gel derived titania-supported zinc-phthalocyanine[J]. Journal of Cleaner Production, 2007, 15(18): 1894-1897. doi: 10.1016/j.jclepro.2006.05.003 [62] PASTRANA-MARTÍNEZ L M, SILVA A M T, FONSECA N N C, et al. Photocatalytic reduction of CO2 with water into methanol and ethanol using graphene derivative-TiO2 composites: Effect of pH and copper (I) oxide[J]. Topics in Catalysis, 2016, 59(15): 1279-1291. doi: 10.1007/s11244-016-0655-2 [63] CHEN J S, QIN S Y, SONG G X, et al. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol[J]. Dalton Transactions, 2013, 42(42): 15133-15138. doi: 10.1039/c3dt51887f [64] BAGGER A, JU W, VARELA A S, et al. Electrochemical CO2 reduction: A classification problem[J]. ChemPhysChem, 2017, 18(22): 3266-3273. doi: 10.1002/cphc.201700736 [65] ZHANG W, HUANG C Q, XIAO Q, et al. A typical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2020, 142(26): 11417-11427. doi: 10.1021/jacs.0c01562 [66] YANG Y S, TAN Z H, WANG S, et al. Cu/Cu2O nanocrystals for electrocatalytic carbon dioxide reduction to multi-carbon products[J]. Chemical Communications, 2023, 59(17): 2445-2448. [67] WAN Q, ZHANG J L, ZHANG B X, et al. Boron-doped CuO nanobundles for electroreduction of carbon dioxide to ethylene[J]. Green Chemistry, 2020, 22(9): 2750-2754. doi: 10.1039/D0GC00730G [68] KIM C, CHO K M, PARK K, et al. Cu/Cu2O interconnected porous aerogel catalyst for highly productive electrosynthesis of ethanol from CO2[J]. Advanced Functional Materials, 2021, 31(32): 2102142. doi: 10.1002/adfm.202102142 [69] YUAN X T, CHEN S, CHENG D F, et al. Controllable Cu0-Cu+ sites for electrocatalytic reduction of carbon dioxide[J]. Angewandte Chemie International Edition, 2021, 60(28): 15344-15347. doi: 10.1002/ange.202105118 [70] ISMAIL A M, SAMU G F, BALOG A, et al. Composition-dependent electrocatalytic behavior of Au-Sn bimetallic nanoparticles in carbon dioxide reduction[J]. ACS Energy Letters, 2019, 4(1): 48-53. [71] TAN F, LIU T X, LIU E R, et al. On ZnAlCe-THs nanocomposites electrocatalysts for electrocatalytic carbon dioxide reduction to carbon monoxide[J]. Catalysis Letters, 2024,154(1):11-22. [72] GONGLACH S, PAUL S, HAAS M, et al. Molecular cobalt corrole complex for the heterogeneous electrocatalytic reduction of carbon dioxide[J]. Nature Communications, 2019, 10(1): 3864. doi: 10.1038/s41467-019-11868-5 [73] HUANG N, LEE K H, YUE Y, et al. A stable and conductive metallophthalocyanine framework for electrocatalytic carbon dioxide reduction in water[J]. Angewandte Chemie, 2020, 132(38): 16730-16736. doi: 10.1002/ange.202005274 [74] JIANG X L, CAI F, GAO D F, et al. Electrocatalytic reduction of carbon dioxide over reduced nanoporous zinc oxide[J]. Electrochemistry Communications, 2016, 68: 67-70. doi: 10.1016/j.elecom.2016.05.003 [75] ZHANG Y L, LAN J, XIE F, et al. Aligned InS nanorods for efficient electrocatalytic carbon dioxide reduction[J]. ACS Applied Materials & Interfaces, 2022, 14(22): 25257-25266. [76] WANG J J, LI G N, LI Z L, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. doi: 10.1126/sciadv.1701290 [77] ZHANG W Y, QIN Q, DAI L, et al. Electrochemical reduction of carbon dioxide to methanol on hierarchical Pd/SnO2 nanosheets with abundant Pd-O-Sn interfaces[J]. Angewandte Chemie International Edition, 2018, 57(30): 9475-9479. doi: 10.1002/anie.201804142 [78] XUAN X X, CHEN S Y, ZHAO S, et al. Carbon nanomaterials from metal-organic frameworks: A new material horizon for CO2 reduction[J]. Frontiers in Chemistry, 2020, 8: 573797. doi: 10.3389/fchem.2020.573797 [79] JIANG X, NIE X W, GUO X W, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. doi: 10.1021/acs.chemrev.9b00723 [80] ZHUO L L, CHEN P, ZHENG K, et al. Flexible cuprous triazolate frameworks as highly stable and efficient electrocatalysts for CO2 reduction with tunable C2H4/CH4 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(28): e202204967. doi: 10.1002/anie.202204967 [81] YI J D, XIE R K, XIE Z L, et al. Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: Stabilizing key intermediates with hydrogen bonding[J]. Angewandte Chemie International Edition, 2020, 59(52): 23641-23648. doi: 10.1002/anie.202010601 [82] ZHOU Y Z, CHEN S H, XI S B, et al. Spatial confinement in copper-porphyrin frameworks enhances carbon dioxide reduction to hydrocarbons[J]. Cell Reports Physical Science, 2020, 1(9): 100182. doi: 10.1016/j.xcrp.2020.100182 [83] ZHAO K, NIE X W, WANG H Z, et al. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon[J]. Nature Communications, 2020, 11(1): 2455. doi: 10.1038/s41467-020-16381-8 [84] YUAN J, YANG M P, ZHI W Y, et al. Efficient electrochemical reduction of CO2 to ethanol on Cu nanoparticles decorated on N-doped graphene oxide catalysts[J]. Journal of CO2 Utilization, 2019, 33: 452-460. doi: 10.1016/j.jcou.2019.07.014 [85] ZHANG B H, GUO Z H, ZUO Z, et al. The ensemble effect of nitrogen doping and ultrasmall SnO2 nanocrystals on graphene sheets for efficient electroreduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 239: 441-449. doi: 10.1016/j.apcatb.2018.08.044 [86] HUANG J Z, GUO X R, YUE G Q, et al. Boosting CH3OH production in electrocatalytic CO2 reduction over partially oxidized 5 nm cobalt nanoparticles dispersed on single-layer nitrogen-doped graphene[J]. ACS Applied Materials & Interfaces, 2018, 10(51): 44403-44414. [87] HU X M, HVAL H H, BJERGLUND E T, et al. Selective CO2 reduction to CO in water using earth-abundant metal and nitrogen-doped carbon electrocatalysts[J]. ACS Catalysis, 2018, 8(7): 6255-6264. doi: 10.1021/acscatal.8b01022 [88] WANG X S, PAN Y Y, NING H, et al. Hierarchically micro-and meso-porous Fe-N4O-doped carbon as robust electrocatalyst for CO2 reduction[J]. Applied Catalysis B: Environmental, 2020, 266: 118630. doi: 10.1016/j.apcatb.2020.118630 [89] LI T F, WEI H M, LIU T M, et al. Achieving efficient CO2 electrochemical reduction on tunable In(OH)3-coupled Cu2O-derived hybrid catalysts[J]. ACS Applied Materials & Interfaces, 2019, 11(25): 22346-22351. [90] HAN N, WANG Y, YANG H, et al. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate[J]. Nature Communications, 2018, 9(1): 1320. doi: 10.1038/s41467-018-03712-z [91] DINH C T, BURDYNY T, KIBRIA M G, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface[J]. Science, 2018, 360(6390): 783-787. doi: 10.1126/science.aas9100 [92] GEIOUSHY R A, KHALED M M, ALHOOSHANI K, et al. Graphene/ZnO/Cu2O electrocatalyst for selective conversion of CO2 into n-propanol[J]. Electrochimica Acta, 2017, 245: 456-462. doi: 10.1016/j.electacta.2017.05.185 [93] YUAN J, YANG M P, HU Q L, et al. Cu/TiO2 nanoparticles modified nitrogen-doped graphene as a highly efficient catalyst for the selective electroreduction of CO2 to different alcohols[J]. Journal of CO2 Utilization, 2018, 24: 334-340. doi: 10.1016/j.jcou.2018.01.021 [94] ZHAO M M, GU Y L, GAO W C, et al. Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction[J]. Applied Catalysis B: Environmental, 2020, 266: 118625. doi: 10.1016/j.apcatb.2020.118625 [95] LU X L, YU T S, WANG H L, et al. Nanoporous Au-Sn with solute strain for simultaneously enhanced selectivity and durability during electrochemical CO2 reduction[J]. Journal of Materials Science & Technology, 2020, 43: 154-160. [96] XU S H, SHEN Q, ZHENG J G, et al. Advances in biomimetic photoelectrocatalytic reduction of carbon dioxide[J]. Advanced Science, 2022, 9(31): 2203941. doi: 10.1002/advs.202203941 [97] ZHOU B W, KONG X H, VANKA S, et al. A GaN: Sn nanoarchitecture integrated on a silicon platform for converting CO2 to HCOOH by photoelectrocatalysis[J]. Energy & Environmental Science, 2019, 12(9): 2842-2848. [98] WEI W, YANG Z X, SONG W J, et al. Different CdSeTe structure determined photoelectrocatalytic reduction performance for carbon dioxide[J]. Journal of Colloid and Interface Science, 2017, 496: 327-333. doi: 10.1016/j.jcis.2016.11.054 [99] SHEN Q, HUANG X F, LIU J B, et al. Biomimetic photoelectrocatalytic conversion of greenhouse gas carbon dioxide: Two-electron reduction for efficient formate production[J]. Applied Catalysis B: Environmental, 2017, 201: 70-76. doi: 10.1016/j.apcatb.2016.08.008 [100] SHEN Q, MA J, HUANG X F, et al. Enhanced carbon dioxide conversion to formate on a multi-functional synergistic photoelectrocatalytic interface[J]. Applied Catalysis B: Environmental, 2017, 219: 45-52. doi: 10.1016/j.apcatb.2017.07.029 [101] YUAN J L, WANG X, GU C H, et al. Photoelectrocatalytic reduction of carbon dioxide to methanol at cuprous oxide foam cathode[J]. RSC Advances, 2017, 7(40): 24933-24939. doi: 10.1039/C7RA03347H [102] REZAUL KARIM K M, TAREK M, ONG H R, et al. Photoelectrocatalytic reduction of carbon dioxide to methanol using CuFe2O4 modified with graphene oxide under visible light irradiation[J]. Industrial & Engineering Chemistry Research, 2019, 58(2): 563-572. [103] WANG L C, QI G C, LIU X J. Ag/α-Fe2O3 nanowire arrays enable effectively photoelectrocatalytic reduction of carbon dioxide to methanol[J]. Journal of Power Sources, 2021, 507: 230272. doi: 10.1016/j.jpowsour.2021.230272 [104] KOBAYASHI K, LOU S N, TAKATSUJI Y, et al. Photoelectrochemical reduction of CO2 using a TiO2 photoanode and a gas diffusion electrode modified with a metal phthalocyanine catalyst[J]. Electrochimica Acta, 2020, 338: 135805. doi: 10.1016/j.electacta.2020.135805 [105] XIE L, JIANG Y J, ZHU W L, et al. Cu-based catalyst designs in CO2 electroreduction: Precise modulation of reaction intermediates for high-value chemical generation[J]. Chemical Science, 2023, 14(47): 13629-13660. doi: 10.1039/D3SC04353C [106] YUAN L, WAN Q Q, JIANG W X, et al. Converting CO2 to multi-carbon products at >1A/cm2 using gas diffusion electrode based on commercial materials via transfer process engineering[J]. Electrochimica Acta, 2024, 475: 143662. [107] ZHONG W F, HUANG W H, RUAN S H, et al. Electrocatalytic reduction of CO2 coupled with organic conversion to selectively synthesize high-value chemicals[J]. Chemistry—A European Journal, 2023, 29(20): e202203228. doi: 10.1002/chem.202203228 [108] WANG W N, AN W J, RAMALINGAM B, et al. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals[J]. Journal of the American Chemical Society, 2012, 134(27): 11276-11281. doi: 10.1021/ja304075b [109] JANG Y J, JANG J W, LEE J, et al. Selective CO production by Au coupled ZnTe/ZnO in the photoelectrochemical CO2 reduction system[J]. Energy & Environmental Science, 2015, 8(12): 3597-3604. [110] ZHU Y Z, XU Z X, LANG Q Q, et al. Grain boundary engineered metal nanowire cocatalysts for enhanced photocatalytic reduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2017, 206: 282-292. doi: 10.1016/j.apcatb.2017.01.035 [111] XIA P F, ZHU B C, YU J G, et al. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2017, 5(7): 3230-3238. doi: 10.1039/C6TA08310B [112] ZHU Q, CAO Y N, TAO Y, et al. CO2 reduction to formic acid via NH2-C@Cu2O photocatalyst in situ derived from amino modified Cu-MOF[J]. Journal of CO2 Utilization, 2021, 54: 101781. doi: 10.1016/j.jcou.2021.101781 [113] WANG S B, LIN J L, WANG X C. Semiconductor-redox catalysis promoted by metal-organic frameworks for CO2 reduction[J]. Physical Chemistry Chemical Physics, 2014, 16(28): 14656-14660. doi: 10.1039/c4cp02173h [114] YU F Y, JING X, WANG Y, et al. Hierarchically porous metal-organic framework/MoS2 interface for selective photocatalytic conversion of CO2 with H2O into CH3COOH[J]. Angewandte Chemie International Edition, 2021, 60(47): 24849-24853. doi: 10.1002/anie.202108892 [115] DES MARAIS D J. When did photosynthesis emerge on Earth?[J]. Science, 2000, 289(5485): 1703-1705. doi: 10.1126/science.289.5485.1703 [116] EL-KHOULY M E, EL-MOHSNAWY E, FUKUZUMI S. Solar energy conversion: From natural to artificial photosynthesis[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2017, 31: 36-83. doi: 10.1016/j.jphotochemrev.2017.02.001 [117] KUMAR A, HASIJA V, SUDHAIK A, et al. Artificial leaf for light-driven CO2 reduction: Basic concepts, advanced structures and selective solar-to-chemical products[J]. Chemical Engineering Journal, 2022, 430: 133031. doi: 10.1016/j.cej.2021.133031 [118] ZHAO L N, ZHAO Z L, LI Y X, et al. The synthesis of interface-modulated ultrathin Ni(ii) MOF/g-C3N4 heterojunctions as efficient photocatalysts for CO2 reduction[J]. Nanoscale, 2020, 12(18): 10010-10018. doi: 10.1039/D0NR02551H [119] YORIFUJI R, OBARA S. Economic design of artificial light plant factories based on the energy conversion efficiency of biomass[J]. Applied Energy, 2022, 305: 117850. doi: 10.1016/j.apenergy.2021.117850 [120] WHITE J L, BARUCH M F, PANDER III J E, et al. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes[J]. Chemical Reviews, 2015, 115(23): 12888-12935. doi: 10.1021/acs.chemrev.5b00370 [121] APPEL A M, BERCAW J E, BOCARSLY A B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chemical Reviews, 2013, 113(8): 6621-6658. doi: 10.1021/cr300463y [122] LIM R J, XIE M S, SK M A, et al. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts[J]. Catalysis Today, 2014, 233: 169-180. doi: 10.1016/j.cattod.2013.11.037 [123] XIE S J, ZHANG Q H, LIU G D, et al. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures[J]. Chemical Communications, 2016, 52(1): 35-59. doi: 10.1039/C5CC07613G [124] WON D I, LEE J S, JI J M, et al. Highly robust hybrid photocatalyst for carbon dioxide reduction: Tuning and optimization of catalytic activities of dye/TiO2/Re(I) organic-inorganic ternary systems[J]. Journal of the American Chemical Society, 2015, 137(42): 13679-13690. doi: 10.1021/jacs.5b08890 [125] QIU J, ZENG G T, HA M A, et al. Artificial photosynthesis on TiO2-passivated InP nanopillars[J]. Nano Letters, 2015, 15(9): 6177-6181. doi: 10.1021/acs.nanolett.5b02511 [126] KANG Q, WANG T, LI P, et al. Photocatalytic reduction of carbon dioxide by hydrous hydrazine over Au-Cu alloy nanoparticles supported on SrTiO3/TiO2 coaxial nanotube arrays[J]. Angewandte Chemie, 2015, 127(3): 855-859. doi: 10.1002/ange.201409183 [127] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials, 2009, 8(1): 76-80. doi: 10.1038/nmat2317 [128] LIU H, CHEN S, ZHANG Y, et al. An effective Z-scheme hybrid photocatalyst based on zinc porphyrin derivative and anatase titanium dioxide microsphere for carbon dioxide reduction[J]. Materials Today Sustainability, 2022, 19: 100164. doi: 10.1016/j.mtsust.2022.100164 [129] ZHANG S Q, WANG S Y, GUO L P, et al. An artificial photosynthesis system comprising a covalent triazine framework as an electron relay facilitator for photochemical carbon dioxide reduction[J]. Journal of Materials Chemistry C, 2020, 8(1): 192-200. doi: 10.1039/C9TC05297F [130] RAO H, SCHMIDT L C, BONIN J, et al. Visible-light-driven methane formation from CO2 with a molecular iron catalyst[J]. Nature, 2017, 548(7665): 74-77. doi: 10.1038/nature23016 [131] YUAN H Q, CHENG B G, LEI J X, et al. Promoting photocatalytic CO2 reduction with a molecular copper purpurin chromophore[J]. Nature Communications, 2021, 12(1): 1835. doi: 10.1038/s41467-021-21923-9 [132] 孙晓丽. 基于细菌叶绿素衍生物/Ti3C2Tx MXene复合物的光催化产氢研究[D]. 长春: 吉林大学, 2022.SUN Xiaoli. Photocatalytic hydrogen production based on bacterial chlorophyll derivatives/Ti3C2Tx MXene complexes[D]. Changchun: Jilin University, 2022(in Chinese). [133] WU T S, ZHU C, HAN D X, et al. Highly selective conversion of CO2 to C2H6 on graphene modified chlorophyll Cu through multi-electron process for artificial photosynthesis[J]. Nanoscale, 2019, 11(47): 22980-22988. doi: 10.1039/C9NR07824J [134] XIAO D M, JIANG M Y, LUO X F, et al. Sustainable carbon dot-based AIEgens: Promising light-harvesting materials for enhancing photosynthesis[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(11): 4139-4145. [135] LI D N, LI W, ZHANG H R, et al. Far-red carbon dots as efficient light-harvesting agents for enhanced photosynthesis[J]. ACS Applied Materials & Interfaces, 2020, 12(18): 21009-21019. [136] WU T S, ZOU L Y, HAN D X, et al. A carbon-based photocatalyst efficiently converts CO2 to CH4 and C2H2 under visible light[J]. Green Chemistry, 2014, 16(4): 2142-2146. doi: 10.1039/C3GC42454E [137] PIAO M H, LIU N, WANG Y S, et al. Efficiently converting CO2 into C2H4 using a porphyrin–graphene composite photocatalyst[J]. Australian Journal of Chemistry, 2015, 69(1): 27-32. [138] ZHANG C, DUAN S N, ZHOU M, et al. Electropolymerized chlorophyll derivative biopolymers for supercapacitors[J]. Chemical Engineering Journal, 2022, 450: 138000. doi: 10.1016/j.cej.2022.138000 [139] GAUT N J, ADAMALA K P. Toward artificial photosynthesis[J]. Science, 2020, 368(6491): 587-588. doi: 10.1126/science.abc1226 [140] SCHMERMUND L, JURKAŠV, ÖZGEN F F, et al. Photo-biocatalysis: Biotransformations in the presence of light[J]. ACS Catalysis, 2019, 9(5): 4115-4144. doi: 10.1021/acscatal.9b00656 [141] JACOBS M, LOPEZ-GARCIA M, PHRATHEP O P, et al. Photonic multilayer structure of Begonia chloroplasts enhances photosynthetic efficiency[J]. Nature Plants, 2016, 2(11): 16162. [142] ZHOU J, LI J, KAN L, et al. Linking oxidative and reductive clusters to prepare crystalline porous catalysts for photocatalytic CO2 reduction with H2O[J]. Nature Communications, 2022, 13(1): 4681. doi: 10.1038/s41467-022-32449-z [143] BUKHANOV E, SHABANOV A V, VOLOCHAEV M N, et al. The role of periodic structures in light harvesting[J]. Plants, 2021, 10(9): 1967. doi: 10.3390/plants10091967 [144] LIU J, ZHAO H, WU M, et al. Slow photons for photocatalysis and photovoltaics[J]. Advanced Materials, 2017, 29(17): 1605349. doi: 10.1002/adma.201605349 [145] WU S L, YANG W X, MENG Z P, et al. Photonic crystals assembled by SiO2@Ni/TiO2 for photocatalytic reduction of CO2[J]. Catalysis Letters, 2020, 150(12): 3598-3607. doi: 10.1007/s10562-020-03263-3 [146] LIN H C, LIU Y, YANG C H, et al. Microfluidic artificial photosynthetic system for continuous NADH regeneration and l-glutamate synthesis[J]. Catalysis Science & Technology, 2022, 12(12): 4057-4065. [147] ZHAO Y J, LIU H, WU C Y, et al. Fully conjugated two-dimensional sp2-carbon covalent organic frameworks as artificial photosystem I with high efficiency[J]. Angewandte Chemie International Edition, 2019, 58(16): 5376-5381. doi: 10.1002/anie.201901194 [148] WANG Y H, LIU J L, WANG Y F, et al. Efficient solar-driven electrocatalytic CO2 reduction in a redox-medium-assisted system[J]. Nature Communications, 2018, 9(1): 5003. doi: 10.1038/s41467-018-07380-x [149] SON E J, LEE Y W, KO J W, et al. Amorphous carbon nitride as a robust photocatalyst for biocatalytic solar-to-chemical conversion[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(2): 2545-2552. [150] YU S Z, HOU Y C, JIN Q J, et al. Biomimetic chlorophyll derivatives-based photocatalytic fabric for highly efficient O2 production via CO2 and H2O photoreaction[J]. Chemical Engineering Journal, 2023, 472: 145103. doi: 10.1016/j.cej.2023.145103 [151] MILLER T E, BENEYTON T, SCHWANDER T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. doi: 10.1126/science.aaz6802 -





 下载:
下载: