Performance and mechanism of La-Fe modified vermiculite adsorbent for efficient phosphorus removal
-
摘要: 蛭石(VE)作为吸附剂具有很强的吸附性及离子交换能力,价格低廉、丰富易得,对环境无害。近年来,许多关于蛭石吸附的研究获得报道,但由于疏水性弱且缺乏活性位点使其在磷酸盐吸附方面表现不佳。本文制备了Le-Fe双金属改性蛭石提升对磷酸盐的吸附性能。结果表明:La、Fe原子比为1∶2时改性蛭石(La-Fe/VE-2)具有优异的磷吸附能力,最大吸附容量为185.09 mg P/g,且在pH=3~9的范围内能实现高效吸附。La-Fe/VE-2具有较强的抗阴离子干扰性能,在中低浓度磷酸盐溶液(50~100 mg P/L)条件下仍然保持较高的吸附率(>90%)。材料表征结果显示,La和Fe成功负载在蛭石上,静电吸附和配体交换是主要吸附机制。综上所述,双金属改性蛭石对磷酸盐亲和力强,是一种很有前途的除磷吸附剂。Abstract: Vermiculite (VE) as an adsorbent had strong adsorption and the capability of ion exchange, which was inexpensive, abundant, available and environmental friendly. In recent years, many studies on vermiculite adsorption have been reported, but the weak hydrophobicity and lack of active sites made it perform poorly in phosphate adsorption. In this paper, Le-Fe bimetallic modified vermiculite (La-Fe/VE) was prepared to enhance the adsorption performance of phosphate. The results shows that vermiculite at the atomic ratio of La and Fe of 1∶2 (La-Fe/VE-2) has excellent phosphorus adsorption capacity with a maximum adsorption capacity of 185.09 mg P/g and it can achieve highly efficient adsorption in the pH range of 3 to 9. La-Fe/VE-2 has highly resistant to interference from anions, and maintains high adsorption rate (>90%) at low and medium concentrations of phosphates (50-100 mg P/L). Characterization analyses of the materials conclusively establishes the successful deposition of La and Fe onto the vermiculite substrate. The primary adsorption mechanisms are identifies as electrostatic attraction and ligand exchange. In summary, bimetallic-modified vermiculite has a pronounced affinity for phosphate and is a promising adsorbent for phosphorus removal.
-
Key words:
- vermiculite /
- lanthanum /
- phosphate /
- adsorption /
- modified materials
-
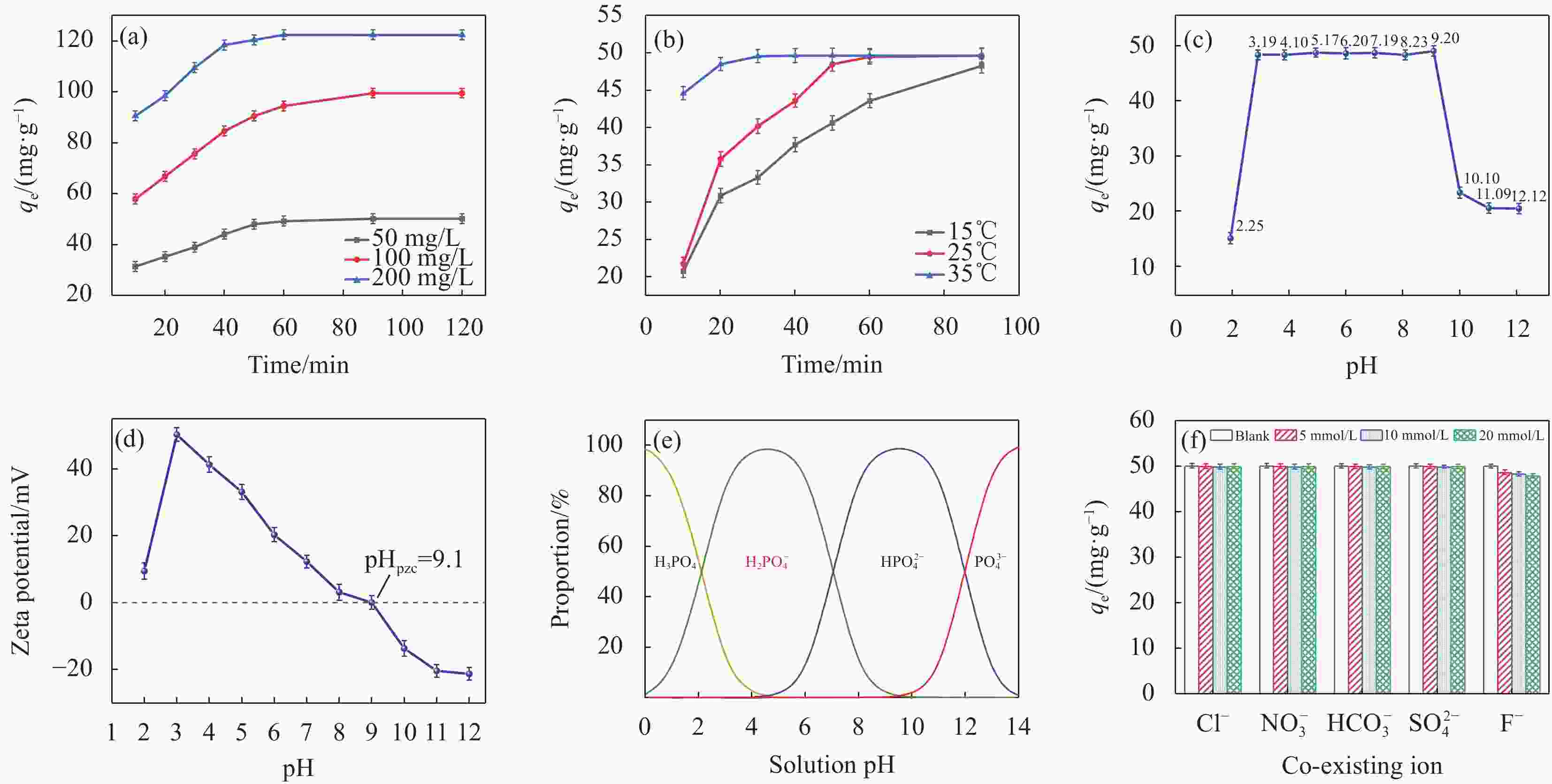
图 2 初始溶液(a)、温度(b)、pH值(c)对磷酸盐吸附的影响;(d) La-Fe/VE-2样品的 Zeta 电位分析;(e)磷酸盐在不同pH下的主要形态分布;(f) 共存离子对磷酸盐吸附的影响
Figure 2. Effect of initial solution (a), temperature (b), pH (c) on phosphate adsorption; (d) Zeta potential analysis of La-Fe/VE-2 sample; (e) Distribution of phosphate species at different pH; (f) Effect of co-existing ions on phosphate adsorption
pzc—Point of zero charge
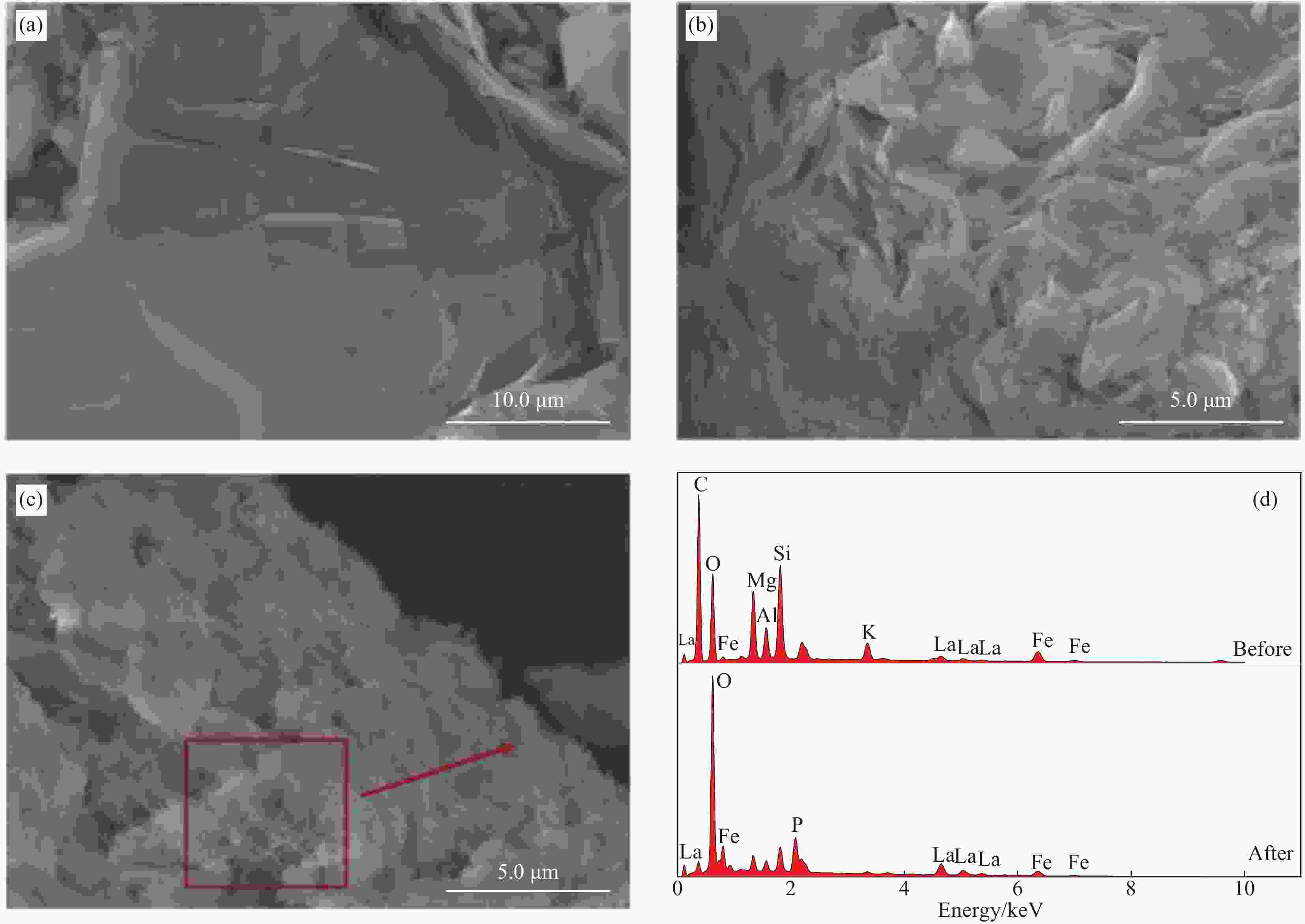
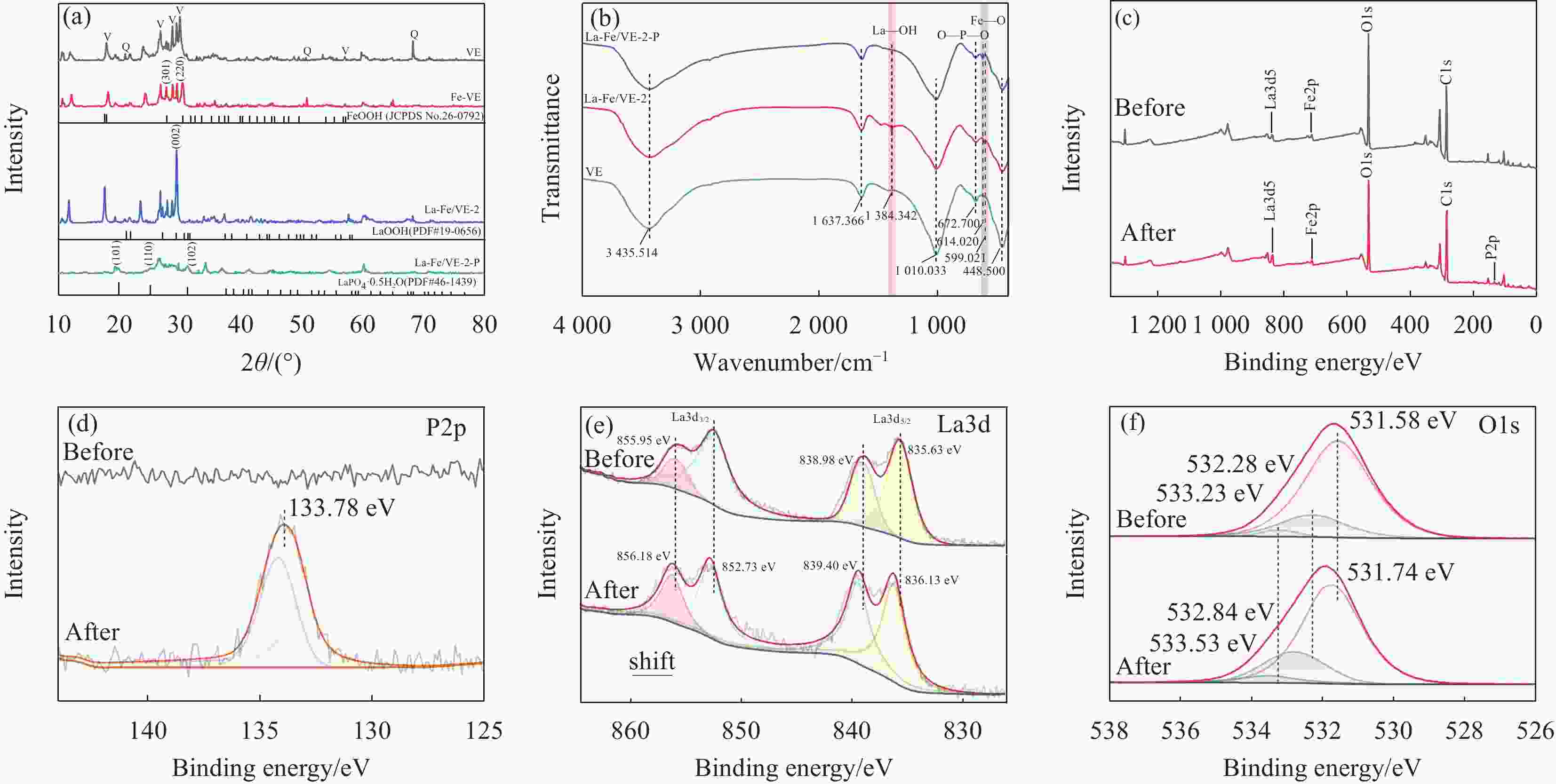
图 8 (a) VE、Fe-VE、La-Fe/VE-2、La-Fe/VE-2-P的XRD图谱;(b) VE、La-Fe/VE-2、La-Fe/VE-2-P的FTIR图谱;La-Fe/VE-2和La-Fe/VE-2-P的XPS 图谱:(c) 宽扫描;(d) P2p;(e) La3d5/2;(f) O1s
Figure 8. (a) XRD patterns of VE, Fe-VE, La-Fe/VE-2, La-Fe/VE-2-P; (b) FTIR spectra of VE, La-Fe/VE-2, La-Fe/VE-2-P; XPS spectra of La-Fe/VE-2 and La-Fe/VE-2-P: (c) Wide scan; (d) P2p; (e) La3d5/2; (f) O1s
表 1 蛭石化学成分
Table 1. Chemical composition of vermiculite
Oxide SiO2 Al2O3 K2O Na2O Fe2O3 FeO TiO2 MgO CaO MnO P2O5 H2O Content/wt% 37.46 14.19 1.83 0.25 13.07 0.8 1.51 11.42 3.47 0.14 0.043 7.00 表 2 La-Fe/VE复合材料的命名
Table 2. Naming of La-Fe/VE composites
Sample Atomic ratio of La and Fe La-Fe/VE-1 1∶1 La-Fe/VE-2 1∶2 La-Fe/VE-3 1∶3 La-Fe/VE-5 1∶4 La-Fe/VE-2-P 1∶2 (After phosphate adsorption) 表 3 La-Fe/VE-2上磷酸盐吸附的动力学参数
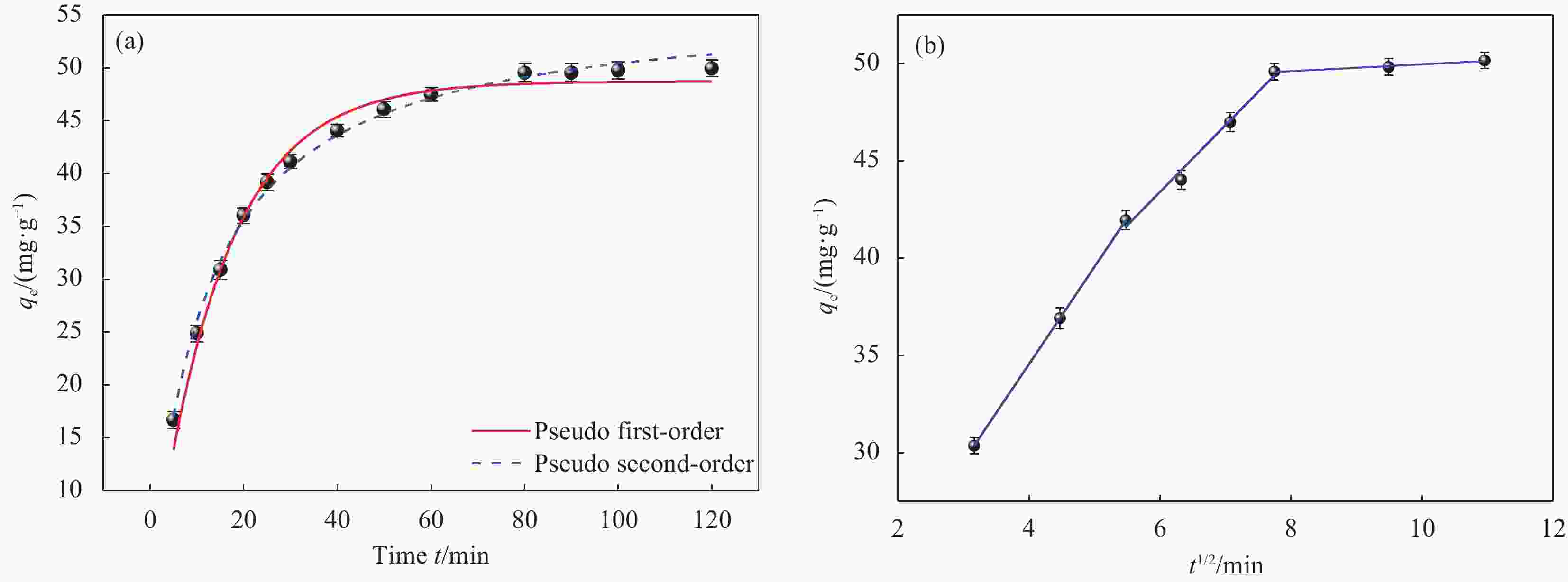
Table 3. Kinetic parameters of phosphate adsorption on La-Fe/VE-2
Kinetic equations qe/(mg·g−1) k1/k2 R2 Pseudo first-order 48.73 0.0668 0.985 Pseudo second-order 56.23 0.0015 0.995 Notes: qe―Phosphate adsorption capacity inequilibrium; k1― Seudo-first-order kinetic con stant (min−1); k2―Pseudo-second-order kinetic constant (g·mg−1·min−1); R2―Determination coefficient. 表 4 La-Fe/VE-2 粒子内扩散方程参数
Table 4. Parameters of the intra-particle diffusion equation for La-Fe/VE-2
kd1 R2 kd2 R2 kd3 R2 5.01 0.99 3.43 0.98 0.18 0.98 Note: kd1, kd2, kd3―Phase I, phase II, phase III intra-particle diffusion rate constant (mg·min0.5/g). 表 5 La-Fe/VE-2 的 Langmuir 和 Freundlich 拟合参数
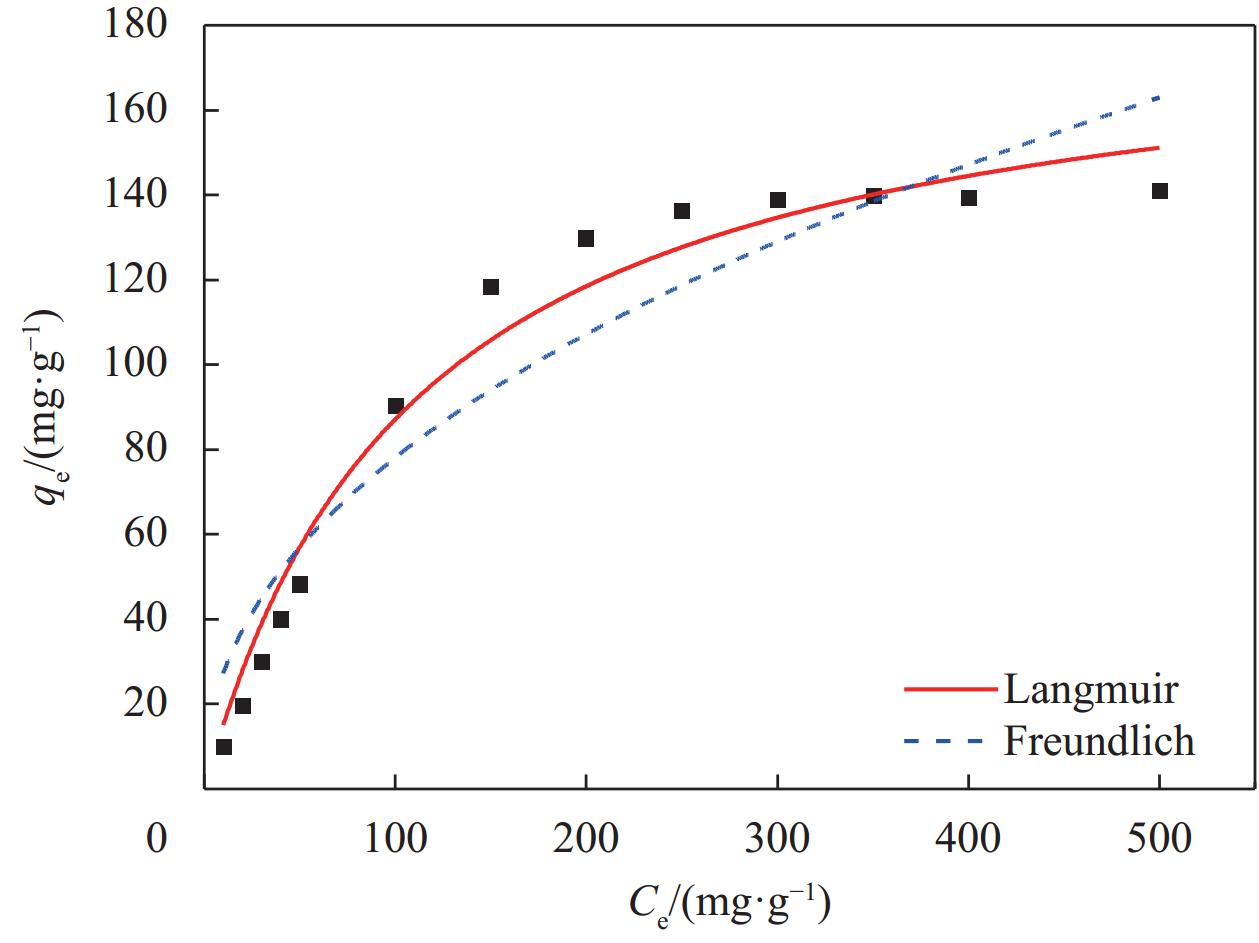
Table 5. Langmuir and Freundlich fitting parameters for La-Fe/VE-2
Langmuir Freundlich qm/(mg·g−1) KL/(L·mg−1) $ R^2$ KF/(L·mg−1) 1/n $R^2 $ 185.09 0.0089 0.977 9.53 0.456 0.907 Notes: qm―Maximum adsorption capacity; KL―Adsorption equilibrium constants of Langmuir; KF―Freundlich Adsorption equilibrium constant; n―Constant related to the adsorption capacity and affinity. 表 6 制备的 La-Fe/VE-2 与其他材料的性能比较
Table 6. Performance comparison of prepared La-Fe/VE-2 with other materials
Material Adsorption capacity/
(mg·g−1)pH Temperature/℃ Ref. Fe3O4/La-MOF 58.70 5-7 25 [28] Lanthanum modified natural zeolite (LZ)
Lanthanum-modified magnetic zeolite (LMZ)122.70
109.176 25 [29] La/Fe engineered bentonite (LFB) 82.02 2-6 20 [19] NaLa(CO3)2/Fe3O4 composites (MLC) 77.85 4-11 25 [27] La/bi-hydroxyl double salts (HDS) 168.12 2-12 25 [30] La-incorporated ternary (hydr)oxides nanocomposite (MALZ) 80.80 4-10 25 [25] Lanthanum/aluminum engineered bentonite (LAB) 93.61 3-6 25 [31] La(OH)3-modified exfoliated vermiculites 79.60 3-7 25 [32] Le/Fe bimetallic-modified vermiculite (LFV) 185.09 3-9 25 This work -
[1] LÜRLING M, MACKAY E, REITZEL K, et al. Editorial—A critical perspective on geo-engineering for eutrophication management in lakes[J]. Water Research, 2016, 97: 1-10. doi: 10.1016/j.watres.2016.03.035 [2] YOU K, YANG W, SONG P, et al. Lanthanum-modified magnetic oyster shell and its use for enhancing phosphate removal from water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 633: 127897. doi: 10.1016/j.colsurfa.2021.127897 [3] SHANG Y, GUO K, JIANG P, et al. Adsorption of phosphate by the cellulose-based biomaterial and its sustained release of laden phosphate in aqueous solution and soil[J]. International Journal of Biological Macromolecules, 2018, 109: 524-534. doi: 10.1016/j.ijbiomac.2017.12.118 [4] LIU X, ZHANG L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies[J]. Powder Technology, 2015, 277: 112-119. doi: 10.1016/j.powtec.2015.02.055 [5] KOH K Y, ZHANG S, PAUL C J. Hydrothermally synthesized lanthanum carbonate nanorod for adsorption of phosphorus: Material synthesis and optimization, and demonstration of excellent performance[J]. Chemical Engineering Journal, 2020, 380: 122153. doi: 10.1016/j.cej.2019.122153 [6] HUANG W, ZHANG Y, LI D. Adsorptive removal of phosphate from water using mesoporous materials: A review[J]. Journal of Environmental Management, 2017, 193: 470-482. [7] HAGHSERESHT F, WANG S, DO D D. A novel lanthanum-modified bentonite, phoslock, for phosphate removal from wastewaters[J]. Applied Clay Science, 2009, 46(4): 369-375. doi: 10.1016/j.clay.2009.09.009 [8] LIU M, LI S, TANG N, et al. Highly efficient capture of phosphate from water via cerium-doped metal-organic frameworks[J]. Journal of Cleaner Production, 2020, 265: 121782. doi: 10.1016/j.jclepro.2020.121782 [9] BUGARČIĆ M, LOPIČIĆ Z, ŠOŠTARIĆ T, et al. Vermiculite enriched by Fe(III) oxides as a novel adsorbent for toxic metals removal[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106020. doi: 10.1016/j.jece.2021.106020 [10] 苏小丽. 河北灵寿蛭石的结构与表面性质调控及其反应机理[D]. 广州: 中国科学院大学(中国科学院广州地球化学研究所), 2019.SU Xiaoli. Modification on structure and surface property of vermiculite from Linshou Hebei and the mechamism involved[D]. Guangzhou: Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 2019(in Chinese). [11] HAN L, WANG T, GONG J, et al. Multi-hydroxyl containing organo-vermiculites for enhanced adsorption of coexisting methyl blue and Pb(II) and their adsorption mechanisms[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 650: 129542. doi: 10.1016/j.colsurfa.2022.129542 [12] FANG L, WU B, CHAN J K M, et al. Lanthanum oxide nanorods for enhanced phosphate removal from sewage: A response surface methodology study[J]. Chemosphere, 2018, 192: 209-216. doi: 10.1016/j.chemosphere.2017.10.154 [13] LIU B, LOU S, ZENG Y, et al. High-efficiency adsorption of phosphate by Fe-Zr-La tri-metal oxide composite from aqueous media: Performance and mechanism[J]. Advanced Powder Technology, 2021, 32(12): 4587-4598. doi: 10.1016/j.apt.2021.10.011 [14] YUAN M, QIU S, LI M, et al. Enhancing phosphate removal performance in water using La-Ca/Fe-LDH: La loading alleviates ineffective stacking of laminates and increases the number of active adsorption sites[J]. Journal of Cleaner Production, 2023, 388: 135857. doi: 10.1016/j.jclepro.2023.135857 [15] 李含, 赵雨, 陈嘉超, 等. Ce-La双金属氧化物同步去除酸性废水中磷酸盐和氟的性能与机理[J]. 中国环境科学, 2023, 43(10): 5148-5156.LI Han, ZHAO Yu, CHEN Jiachao, et al. Simultaneous removal of phosphate and fluoride from acid wastewater by Ce-La bimetal oxides: Performance and mechanism[J]. China Environmental Science, 2023, 43(10): 5148-5156(in Chinese). [16] 刘晨阳, 王毅力, 李小林, 等. Fe3O4负载非晶态(碳酸)氧化锆复合材料对磷的吸附性能及机理[J]. 环境工程学报, 2022, 16(4): 1133-1144.LIU Chenyang, WANG Yili, LI Xiaolin, et al. Fe3O4 loaded amorphous zirconium (carbonate) oxides composite for phosphate adsorption: Performance, mechanism and treatment effect in real wastewater[J]. Chinese Journal of Environmental Engineering, 2022, 16(4): 1133-1144(in Chinese). [17] 徐硕, 夏夜, 邹本东, 等. 镧铝复合改性膨润土的制备及其对磷酸盐吸附性能的研究[J]. 稀土, 2023, 44(1): 198-206.XU Shuo, XIA Ye, ZOU Bendong, et al. Preparation and phosphate adsorption performance of lanthanum aluminum composite modified bentonite[J]. Chinese Rare Earths, 2023, 44(1): 198-206(in Chinese). [18] YU J, XIANG C, ZHANG G, et al. Activation of lattice oxygen in LaFe (oxy)hydroxides for efficient phosphorus removal[J]. Environmental Science & Technology, 2019, 53(15): 9073-9080. [19] WANG B, ZHANG H, HU X, et al. Efficient phosphate elimination from aqueous media by La/Fe bimetallic modified bentonite: Adsorption behavior and inner mechanism[J]. Chemosphere, 2023, 312: 137149. doi: 10.1016/j.chemosphere.2022.137149 [20] TANG N, NIU C, LI X, et al. Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino- and thiol-functionalized activated carbon: Isotherm and kinetics modeling[J]. Science of the Total Environment, 2018, 635: 1331-1344. doi: 10.1016/j.scitotenv.2018.04.236 [21] 中华人民共和国生态环境部. 污水综合排放标准: GB/T 8978—1996[S]. 北京: 中国环境科学出版社, 1997.Ministry of Ecology and Environment of the People's Republic of China. Integrated wastewater discharge standard: GB/T 8978—1996[S]. Beijing: China Environmental Science Press, 1997(in Chinese). [22] CHUBAR N I, KANIBOLOTSKYY V A, STRELKO V V, et al. Adsorption of phosphate ions on novel inorganic ion exchangers[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 255(1-3): 55-63. [23] XIE J, WANG Z, LU S, et al. Removal and recovery of phosphate from water by lanthanum hydroxide materials[J]. Chemical Engineering Journal, 2014, 254: 163-170. doi: 10.1016/j.cej.2014.05.113 [24] LIU J, ZHOU Q, CHEN J, et al. Phosphate adsorption on hydroxyl-iron-lanthanum doped activated carbon fiber[J]. Chemical Engineering Journal, 2013, 215-216: 859-867. doi: 10.1016/j.cej.2012.11.067 [25] SHI W, FU Y, JIANG W, et al. Enhanced phosphate removal by zeolite loaded with Mg-Al-La ternary (hydr)oxides from aqueous solutions: Performance and mechanism[J]. Chemical Engineering Journal, 2019, 357: 33-44. doi: 10.1016/j.cej.2018.08.003 [26] ZHANG W, OU J, WANG B, et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight[J]. Journal of Hazardous Materials, 2021, 418: 126358. doi: 10.1016/j.jhazmat.2021.126358 [27] HAO H, WANG Y, SHI B. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study[J]. Water Research, 2019, 155: 1-11. doi: 10.1016/j.watres.2019.01.049 [28] HE Q, ZHAO H, TENG Z, et al. Efficient recovery of phosphate by Fe3O4/La-MOF: An insight of adsorption performance and mechanism from electrochemical properties[J]. Separation and Purification Technology, 2023, 314: 123529. doi: 10.1016/j.seppur.2023.123529 [29] LUO Q, WEI J, GUO Z, et al. Adsorption and immobilization of phosphorus from water and sediments using a lanthanum-modified natural zeolite: Performance, mechanism and effect[J]. Separation and Purification Technology, 2024, 329: 125187. doi: 10.1016/j.seppur.2023.125187 [30] LU B, WANG G, ZHAO L, et al. Bimetallic capture sites on porous La/Bi hydroxyl double salts for efficient phosphate adsorption: Multiple active centers and excellent selective properties[J]. Chemosphere, 2023, 344: 140304. doi: 10.1016/j.chemosphere.2023.140304 [31] WANG B, ZHANG H, XU Z, et al. La/Al engineered bentonite composite for efficient phosphate separation from aqueous media: Preparation optimization, adsorptive behavior and mechanism insight[J]. Separation and Purification Technology, 2022, 290: 120894. doi: 10.1016/j.seppur.2022.120894 [32] HUANG W, LI D, LIU Z, et al. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents[J]. Chemical Engineering Journal, 2014, 236: 191-201. doi: 10.1016/j.cej.2013.09.077 [33] CHEN M, GUO Q, PEI F, et al. The role of Fe(III) in enhancement of interaction between chitosan and vermiculite for synergistic co-removal of Cr(VI) and Cd(II)[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 606: 125356. doi: 10.1016/j.colsurfa.2020.125356 [34] WANG G, YUE X, ZHANG S, et al. La(III) loaded Fe(III) cross-linked chitosan composites for efficient removal of phosphate from wastewater: Performance and mechanisms[J]. Journal of Cleaner Production, 2022, 379: 134833. doi: 10.1016/j.jclepro.2022.134833 [35] QIU H, LIANG C, YU J, et al. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration[J]. Chemical Engineering Journal, 2017, 315: 345-354. doi: 10.1016/j.cej.2017.01.043 [36] HE Y, LIN H, DONG Y, et al. Simultaneous removal of ammonium and phosphate by alkaline-activated and lanthanum-impregnated zeolite[J]. Chemosphere, 2016, 164: 387-395. doi: 10.1016/j.chemosphere.2016.08.110 [37] REN S, HUANG S, LIU B. Enhanced removal of ammonia nitrogen from rare earth wastewater by NaCl modified vermiculite: Performance and mechanism[J]. Chemosphere, 2022, 302: 134742. doi: 10.1016/j.chemosphere.2022.134742 [38] AKALIN H A, HIÇSÖNMEZ Ü, YILMAZ H, et al. Removal of cesium from aqueous solution by adsorption onto sivas-yildizeli (Türkiye) vermiculite: Equilibrium, kinetic and thermodynamic studies[J]. Journal of the Turkish Chemical Society, Section A: Chemistry, 2018, 5(1): 85-116. doi: 10.18596/jotcsa.317771 [39] KONG L, TIAN Y, PANG Z, et al. Needle-like Mg-La bimetal oxide nanocomposites derived from periclase and lanthanum for cost-effective phosphate and fluoride removal: Characterization, performance and mechanism[J]. Chemical Engineering Journal, 2020, 382: 122963. doi: 10.1016/j.cej.2019.122963 [40] AKRAM M, GAO B, PAN J, et al. Enhanced removal of phosphate using pomegranate peel-modified nickel-lanthanum hydroxide[J]. Science of the Total Environment, 2022, 809: 151181. doi: 10.1016/j.scitotenv.2021.151181 [41] ZHAO Y, SHAN X, AN Q, et al. Interfacial integration of zirconium components with amino-modified lignin for selective and efficient phosphate capture[J]. Chemical Engineering Journal, 2020, 398: 125561. doi: 10.1016/j.cej.2020.125561 -





 下载:
下载: