Research progress of organic-inorganic composite electrolytes for solid-statelithium batteries
-
摘要: 相比于传统液态锂电池,固态锂电池兼具高安全性和高比能量,在学术界和工业界引起了广泛关注。发展具备优异力学性能、高离子电导率和宽电化学窗口的有机-无机复合固态电解质是开发高性能固态锂电池的有效途径之一。近年来,基于聚合物电解质与无机材料的复合型固态电解质成为了研究的热点。基于此,本文回顾了有机-无机复合固态电解质的研究进展,综述了改善固态电解质离子电导率的研究策略,梳理了有机-无机复合固态电解质在固态锂金属电池、固态锂-硫电池和固态锂-空气电池等领域的应用,并对固态锂电池用有机-无机复合固态电解质存在的挑战和未来的发展趋势进行了展望。Abstract: Compared to traditional liquid-state lithium batteries, solid-state lithium batteries have distinct advantages such as high safety and high specific energy, and have attracted widespread attention in both academia and industry. Exploring organic-inorganic composite solid electrolytes that combine excellent mechanical properties, high ion conductivity, and large electrochemical windows is a feasible solution to developing high-performance solid-state lithium batteries. In recent years, composite solid-state electrolytes based on polymer electrolytes and inorganic materials have become a hot topic. In this tutorial review, we focus on recent advances in various classes of organic-inorganic composite electrolytes and summarize the state-of-the-art strategies for improving the performance (Especially the ionic conductivity) of solid-state electrolytes. This is followed by detailed discussions on the implementation of composite solid-electrolytes in various energy storage systems, including solid-state lithium-metal batteries, solid-state lithium-sulfur batteries and solid-state lithium-air batteries, and the current challenges and future opportunities of organic-inorganic composite solid-state electrolytes for lithium batteries are also provided.
-
随着人类社会的不断进步与发展,化石能源的供需矛盾日益严重,人们亟需发展一种低碳、高效的能源体系,如锂离子电池、超级电容器、太阳能电池和燃料电池等[1-3]。其中,锂离子电池因其高能量密度、使用寿命长、工作温度范围广等优点成为当前最受欢迎的电化学能源技术之一。早在上世纪90年代,锂离子电池已实现商品化,为便携式电子产品(如手机和笔记本电脑)迭代和电动汽车动力电源应用提供了保障。为此,2019年诺贝尔化学奖授予了为电池领域发展做出卓越贡献的3位科学家John B. Goodenough、M. Stanley Whittingham和Akira Yoshino,这也肯定了锂离子电池的划时代意义。然而,商业锂离子电池主要采用高挥发、可燃性有机电解液,当电池短路、过充时,会引发燃烧、爆炸等安全事故[4-6]。为了解决这一难题,发展固态电池(包括固态锂离子电池和固态锂金属电池)是行之有效的途径之一。其中,固态锂金属电池是当下最火热、最被寄予厚望的“超级电池”研发技术路径。固态锂金属电池采用高安全的固态电解质代替易燃易爆炸的有机电解液和隔膜,使用金属锂(能量密度为3860 mA·h·g−1)代替石墨负极(372 mA·h·g−1),能大幅度提升电池单体能量密度、延长电池使用寿命。2020年,国务院颁布了《新能源汽车产业发展规划(2021—2035年)》,明确提出要加快固态动力电池技术的研究[7]。在此背景下,工业界涌现出一批致力于固态电池产业化的中坚力量。在学术界,更多科研人员也将目光聚焦在研发安全可靠的高比能固态锂电池。
固态电解质作为固态锂电池的核心组分,应该具备以下特点:高离子电导率(>10−4 S·cm−1)、宽电位窗口(~ 5.0 V vs Li+/Li)、良好固-固界面兼容、制备工艺简单及价格低廉等[8]。固态电解质主要包括有机电解质(即聚合物)和无机电解质[9]。其中,无机固态电解质(又称陶瓷电解质)主要包括氮化物(如Li3N、LiPON)[10],硫化物(如Li10GeP2S12 (LGPS))[11]和氧化物(如钙钛矿型ATiO3(A=Ca、Sr、Ba)[12],LISICON型Li14ZnGeO4[13],NASICON型LiM2(PO4)3 (M=Ti、Ge)[14]和石榴石型LixLa3M2O12 (M=Ta、Nb、Zr)[15]。氮化物主要有Li3N和LiPON,两者对锂金属负极都非常稳定,但多晶Li3N电化学稳定性差,而LiPON电解质的离子电导率相对较低。此外,氮化物固态电解质的研究工作相对较少,本综述不展开讨论。至于硫化物,虽然少数硫化物电解质具有与液态电解质相当的离子电导率,但多数硫化物电解质缺点明显,对水/氧极为敏感,存在制备和存储等难题。在氧化物电解质中,石榴石型和NASICON型综合优势显著,然而电解质-电极界面物理接触需要加以改善。与无机固态电解质相比,聚合物-锂盐组成的固态电解质(如聚环氧乙烷-双三氟甲烷磺酰亚胺锂(PEO-LiTFSI)[16-17]、聚偏氟乙烯(PVDF)-LiTFSI)[18-19]质地柔软、易成型加工,能很好地与电极界面兼容。然而,聚合物电解质存在室温电导率低(10−5~10−7 S·cm−1)和机械强度差等问题。针对于此,研究人员曾尝试许多方法来提高聚合物电解质的离子电导率,包括对聚合物进行嵌段和接枝[9],或者添加其他组分(无机填料[20]、离子液体[21]或塑晶分子[22])等。其中,在聚合物中添加无机填料可以削弱聚合物与Li+的相互作用,促进锂盐的解离和破坏聚合物链的规整性,增加聚合物的自由体积,提高链段的运动能力。无机填料的添加可以在不牺牲聚合物柔性和可加工性能的前提下,提升聚合物基电解质的离子迁移数、机械性能、热稳定性和化学/电化学稳定性。因此,发展兼具优异力学性能、高离子电导率和宽电化学窗口的有机-无机复合固态电解质,将是开发高性能固态锂电池的有效途径之一。
一般来说,无机填料分为活性填料和惰性填料[23-24]。活性填料则包括LGPS、Li1+xAlxTi2−x(PO4)3 (LATP)和Li7La3Zr2O12 (LLZO)等,其本身可作为固态电解质,与聚合物复合后,锂离子可以在有机相、无机相和两相界面处传导,固态电解质的离子电导率会得到明显改善。惰性填料主要为不含锂无机材料,在复合体系中能够降低聚合物宿主的结晶度,有助于分子间链段运动,有利于Li+快速传导[25]。此外,无机填料/聚合物两相界面会为锂离子提供额外的传输路径。无机填料的种类、尺寸、添加比例、分散程度和复合方式对聚合物电解质的性能会有不同程度的影响。本文回顾了有机-无机复合固态电解质的研究工作,阐述了提升固态电解质的离子电导率研究策略,梳理了有机-无机复合固态电解质在固态锂金属电池、固态锂-硫电池和固态锂-空气电池等领域的应用研究,并对固态锂电池用有机-无机复合固态电解质存在的挑战和未来的发展趋势进行了展望。
1. 聚合物-氧化物复合固态电解质
1.1 聚合物-石榴石型陶瓷复合固态电解质
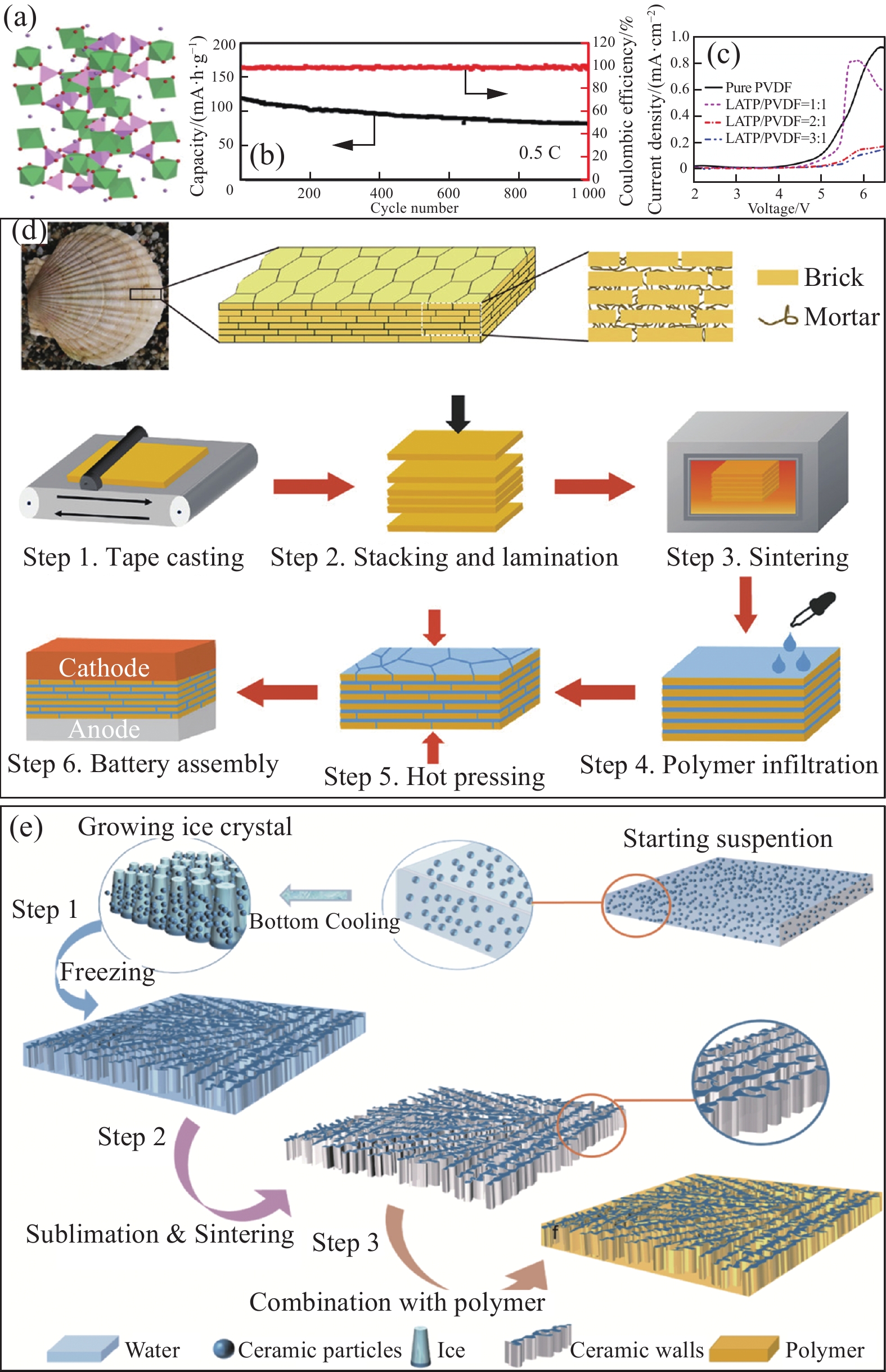
石榴石型氧化物(LLZO,图1(a))[26]具有较高的离子电导率(10−4~10−3 S·cm−1)、较宽的电化学稳定性窗口和较好的化学稳定性,与金属锂负极具有较好的界面稳定性,是一类极具发展前景的锂离子导电氧化物。自2007年首次用作电池固态电解质以来,石榴石型氧化物受到了极大的关注[27]。然而,基于石榴石型电解质的固态锂电池应用仍然面临着一些问题,比如:(1) 在空气中稳定性较差,其表面容易形成离子绝缘性的碳酸锂薄层;(2) 虽然石榴石型电解质对锂金属具有良好化学稳定性,但在正负极间的固-固界面问题仍然是一个顽疾。鉴于聚合物电解质能与电极界面兼容,将石榴石型陶瓷与聚合物电解质复合会改善与正负极接触时固-固界面问题。此外,与无机固态电解质相比,复合电解质具有更高的机械强度和电化学稳定性。Zhang等[28]在PVDF电解质中添加10wt%含量的Li6.75La3Zr1.75Ta0.25O12 (LLZTO)纳米颗粒(图1(b)),制备出机械强度高和热稳定性好的柔性固态薄膜。LLZTO中的La3+可以络合溶剂(如N, N-二甲基甲酰胺上的N原子和羰基)而具有高电子密度态,进而诱导PVDF脱氢氟化改善电极界面兼容性,增强PVDF、LLZTO颗粒和锂盐之间相互作用,最终室温锂离子电导率高达5×10−4 S·cm−1。
![]() 图 1 (a) 石榴石构型Li7La3Zr2O12 (LLZO)[26];(b) 聚偏氟乙烯 (PVDF)/ Li6.75La3Zr1.75Ta0.25O12 (LLZTO)复合固态电解质的结构模型[28];PVDF-LLZO纳米纤维固态电解质形貌((c), (d))和对称电池测试(e)[29];三维珊瑚状LLZO-PVDF复合电解质制备示意图(f)及其SEM图像((g), (h))[30]Figure 1. (a) Garnet-type Li7La3Zr2O12 (LLZO)[26]; (b) Possible complex structures in the poly(vinylidene fluoride) (PVDF)/Li6.75La3Zr1.75Ta0.25O12 (LLZTO)[28]; SEM images ((c), (d)) of PVDF-LLTO nanofibers solid-state electrolyte and symmetrical battery test (e)[29]; Schematic illustration (f) for the preparation procedures of the coral-like LLZO/PVDF electrolyte and SEM images ((g), (h))[30]NFs—Nanofibers; RT—Room temperature
图 1 (a) 石榴石构型Li7La3Zr2O12 (LLZO)[26];(b) 聚偏氟乙烯 (PVDF)/ Li6.75La3Zr1.75Ta0.25O12 (LLZTO)复合固态电解质的结构模型[28];PVDF-LLZO纳米纤维固态电解质形貌((c), (d))和对称电池测试(e)[29];三维珊瑚状LLZO-PVDF复合电解质制备示意图(f)及其SEM图像((g), (h))[30]Figure 1. (a) Garnet-type Li7La3Zr2O12 (LLZO)[26]; (b) Possible complex structures in the poly(vinylidene fluoride) (PVDF)/Li6.75La3Zr1.75Ta0.25O12 (LLZTO)[28]; SEM images ((c), (d)) of PVDF-LLTO nanofibers solid-state electrolyte and symmetrical battery test (e)[29]; Schematic illustration (f) for the preparation procedures of the coral-like LLZO/PVDF electrolyte and SEM images ((g), (h))[30]NFs—Nanofibers; RT—Room temperature一般来说,分散均匀的纳米颗粒可以提高聚合物电解质的力学性能、热稳定性、电化学稳定性和离子电导率。然而,纳米颗粒表面的高能性和不稳定性会导致纳米颗粒团聚,进而影响Li+的有效传输,造成电解质膜的机械性能下降,严重损害电池的循环性能。相较于纳米颗粒,纳米线具有高长径比,与聚合物具有更大的接触面积,因此可以创建出连续的离子传输路径,进而提高电解质的离子电导率。Zhao等[29]利用静电纺丝法在PVDF中引入LLZO纳米纤维(图1(c)、图1(d)),构筑了Li+快速传导的纳米结构。该复合电解质在室温下Li+电导率高达1.2×10−4 S·cm−1,基于此电解质构筑的固态Li//Li对称电池在锂沉积/剥离过程中能稳定循环700 h (图1(e))。如图1(f)所示,Wu等[30]设计出珊瑚状三维互连的LLZO (图1(g)),并将其与PVDF复合(图1(h))。珊瑚状LLZO的三维互连结构在聚合物中会提供连续的Li+传导路径和结构增强,从而获得较高室温离子导电率(1.5×10−4 S·cm−1)和机械柔性。此外,珊瑚状三维互连结构能够为Li+提供连续的传导网络,基于该固态电解质的磷酸铁锂(LFP)//Li电池表现出长循环稳定性(1 C下200次循环后容量保持率为95.2%)。
早在2019年,南策文院士在《Joule》期刊发文,认为LLZO在典型固态电解质中综合性能最优,极具应用潜力[31]。表1为不同形态(纳米颗粒、纳米线、三维结构)石榴石型陶瓷/聚合物复合固态电解质的Li+电导率及其电池性能[28-30, 32-33]。此外,填料的添加量和分散程度对复合电解质的性能会有不同程度的影响,相关机制也需要进一步探究。
表 1 聚合物-石榴石型陶瓷复合电解质性能Table 1. Performance of polymer-garnet ceramic electrolyteComposite electrolyte Conductivity/(S·cm−1) Li//Li cells Solid-state cells Ref. PVDF-LLZTO nanoparticles 5.0×10−4 — 140 mA·h·g−1 at 1.2 C; LCO//Li cell [28] PVDF-LLZTO (3D) 1.5×10−4 0.1 mA·cm−2, 200 h 168 mA·h·g−1 at 0.1 C; LFP//Li cell [29] PVDF-LLZTO nanofibers 1.2×10−4 0.5 mA·cm−2, 700 h 213 mA·h·g−1 at 0.2 C; NCA//Li cell [30] PVDF-LLZTO nanoparticles 2.1×10−4 3.0 mA·cm−2, 700 h 141 mA·h·g−1 at 0.1 C; LFP//Li cell [32] PVDF-LLTO nanowires 2.4×10−4 — — [33] Notes: LLTO—Li0.33La0.557TiO3; LCO—LiCoO2; LFP—LiFePO4; NCA—LiNi0.8Co0.15Al0.05O2. 1.2 聚合物-NASICON型陶瓷复合固态电解质
如图2(a)所示,NASICON型[26]材料的化学式为LiM2(PO4)3,其中M位可以被Ti、Ge或Zr占据,所占离子的价态元素不同会导致复合固态电解质表现出不同的电化学性能[34-35]。NASICON型陶瓷电解质具有环境稳定和室温离子导电性高等特点,其中磷酸盐电解质是NASICON型材料的典型代表,主要形式有LATP[36-37]和Li1+xAlxGe2−x(PO4)3 (LAGP)[38-39]。Yu等[40]将LATP纳米颗粒超声分散在PEO/LiTFSI聚合物,通过浆料浇铸法制备出复合固态电解质。该复合电解质的锂离子电导率较好(60℃时1.6×10−4 S·cm−1),能够有效地抑制锂枝晶的生长。基于该复合电解质的固态电池可以稳定循环1000次,单次循环的容量衰减率<0.03% (图2(b))。Zhao等[41]研究了添加不同含量的LAGP填料对PEO聚合物电解质的影响。当添加20wt%的LAGP时,该复合电解质的离子电导率最高,在60℃时高达6.8×10−4 S·cm−1。Shi等[42]浇铸制备出LATP纳米填料-PVDF复合电解质膜,并研究不同LATP-PVDF摩尔比的固态电解质在结构形貌、热稳定性、离子电导率和电化学窗口等方面的差异。研究发现,随着纳米填料含量增加,复合电解质的热稳定性和电化学窗口也会增加(图2(c))。当LATP-PVDF摩尔比为2∶1时,复合固态电解质的室温Li+电导率高达9.6×10−4 S·cm−1。综上,在聚合物中添加纳米颗粒,可以促使聚合物链段重构,增加聚合物中非晶态区域,提高Li+浓度,有利于Li+传导。
![]() 图 2 (a) NASICON构型Li1+xAlxGe2−x(PO4)3 (LAGP)[26];(b) 聚环氧乙烷(PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)的循环稳定测试[40];(c) PVDF-LATP的电化学窗口测试[42];(d) 贝壳启发下 LAGP陶瓷-聚合物复合电解质的制备[43];(e) 冰模板法制备LAGP-PEO复合电解质流程图[44]Figure 2. (a) NASICON-type Li1+xAlxGe2−x(PO4)3 (LAGP)[26]; (b) Cycle stability of polyethylene oxide (PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)[40]; (c) Electrochemical stability windows of PVDF-LATP[42]; (d) Design and fabrication of LAGP ceramic-polymer composite electrolyte[43]; (e) Schematic of preparation process of the ice-templated LAGP-PEO composite electrolyte[44]
图 2 (a) NASICON构型Li1+xAlxGe2−x(PO4)3 (LAGP)[26];(b) 聚环氧乙烷(PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)的循环稳定测试[40];(c) PVDF-LATP的电化学窗口测试[42];(d) 贝壳启发下 LAGP陶瓷-聚合物复合电解质的制备[43];(e) 冰模板法制备LAGP-PEO复合电解质流程图[44]Figure 2. (a) NASICON-type Li1+xAlxGe2−x(PO4)3 (LAGP)[26]; (b) Cycle stability of polyethylene oxide (PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)[40]; (c) Electrochemical stability windows of PVDF-LATP[42]; (d) Design and fabrication of LAGP ceramic-polymer composite electrolyte[43]; (e) Schematic of preparation process of the ice-templated LAGP-PEO composite electrolyte[44]考虑到纳米颗粒在聚合物电解质中容易团聚等局限性,Li等[43]受贝壳启发设计出具有“砖+砂浆”结构的LAGP陶瓷-聚合物复合固态电解质(图2(d))。二维LAGP陶瓷(砖)与聚合物(砂浆)间存在更大的接触面积,能够极大地增加电解质的室温离子电导率(1.3×10−4 S·cm−1)。在施加外力时,二维砖块在聚合物砂浆间更倾向于产生滑移,表现出强劲的力学性能(弯曲模量高达7.8 GPa),能够很好地抑制锂枝晶的生长。Wang等[44]利用冰模板法制备垂直排列的三维多孔LAGP膜,随后在多孔陶瓷中填充PEO聚合物(图2(e))。具体来说,首先将LAGP-PEO悬浮液均匀涂覆在Al2O3基底,迅速冻结后冰晶从悬浮液底部生长,并将LAGP纳米颗粒溅射到侧面形成垂直壁。随后通过真空干燥去除冰,获得垂直排列的LAGP多孔膜。将多孔膜在800℃下进一步退火5 h使膜致密化,LAGP纳米颗粒之间会形成良好连接的通道,这有利于锂离子的传输。将PEO聚合物浇注到多孔膜上,待其充分浸透到LAGP膜的孔隙。LAGP多孔膜可以提供良好的机械支撑,聚合物填充物则会降低LAGP颗粒间的界面阻抗。三维LAGP骨架能够提供连续的锂离子传输通道,Li+可以通过聚合物、LAGP骨架及两相界面进行传导,因此复合电解质的室温电导率可达1.67×10−4 S·cm−1。
表2总结了近年来NASICON型陶瓷-聚合物复合电解质的性能[37, 39-40, 43-50]。NASICON型陶瓷与聚合物电解质复合后,会明显改善Li+电导率和电化学稳定窗口,有望匹配高压正极材料,实现高比能固态电池。然而,复合电解质中不同组分之间的界面Li+传导机制仍然需要展开深入研究。
表 2 聚合物-NASICON型陶瓷复合电解质性能Table 2. Performance of polymer-NASICON ceramic electrolyteComposite electrolyte Conductivity/
(S·cm−1)Electrochemical window Ref. PEG-CA/LATP/
LiClO4>10−4 at 60℃ ~5.0 V [37] PVDF-HFP/LAGP/
EMITFSI7.6×10−4 at 25℃ 4.8 V [39] PEO/LATP/LiClO4 1.6×10−4 at 60℃ — [40] LAGP-PEA/LiTFSI 1.3×10−4 at 25℃ — [43] PEO-PEG/LAGP/
LiClO41.7×10−4 at 25℃ — [44] PVDF/LATP/LiTFSI 3.3×10−4 at 20℃ — [45] PEO/LiTFSI+PAN/
LATP6.5×10−4 at 60℃ 4.2 V [46] PEO/LiTFSI+LATP/ PAN/LiTFSI 6.3×10−4 at 60℃ ~5.0 V [47] PEO-SN/LiTFSI+PAN/
LATP/LiTFSI1.3×10−4 at 25℃ 5.0 V [48] PET-PIL/LAGP/
LiTFSI7.8×10−5 at 30℃ 4.55 V [49] PEGDA/LiTFSI+
PAN/LAGP/LiTFSI3.7×10−4 at 25℃ 5.0 V [50] Notes: PEG-CA—Polyethylene glycol-cellulose acetate; HFP—
Hexafluoropropylene; PEO—Polyethylene oxide; PEA—Poly (ether-acrylate); PET—Polyethylene terephthalate; PIL—Polyme
rized ionic liquid; PEGDA—Polyethylene glycol diacrylate; LiTFSI—Lithium bis (trifluoromethanesulphonyl) imide; PAN—Polyacrylonitrile; SN—Succinonitrile; EMITFSI—1-ethyl-3-methylimidazolium triluoromethanesufonate.1.3 聚合物-钙钛矿型陶瓷复合固态电解质
钙钛矿型材料具有立方相结构(图3(a)),结构公式为ABO3(A=La、Sr或Ca;B=Al或Ti)[26]。钙钛矿型固态电解质具有较高离子电导率、宽电化学稳定窗口和力学性能好等优点,是一种备受关注的电解质材料。然而,刚性的无机固态电解质与电极的非良性接触会导致电池内部存在很大的Li+迁移阻力,将钙钛矿型陶瓷与聚合物电解质进行复合在一定程度上能够解决上述难题。Liu等[33]研究了Li0.33La0.557TiO3 (LLTO)纳米线和纳米颗粒分别与聚合物电解质复合后对锂离子电导率的影响(图3(b)、图3(c))。研究发现,纳米线填料可以作为一维导离子通路连续地传输Li+,其与聚合物电解质复合后,其离子电导率是LLTO纳米颗粒-聚合物复合电解质的10倍。与此同时,Zhu等[51]构建了一维LLTO纳米纤维-PEO聚合物复合后,也实现了高离子电导率(2.4×10−4 S·cm−1)和宽电化学窗口(达5.0 V)。为了更好地理解纳米线分布对复合电解质性能的影响,Liu等[52]对聚合物中纳米线的分布取向进行了0°~90°的系统探究,发现当纳米线在聚合物电解质中正、负电极之间垂直分布时具有更低的Li+迁移能垒。无机纳米纤维填料可降低聚合物的结晶程度,破坏聚合物链的规整性,增加聚合物的自由体积,提高链段的运动能力。
一般来说,锂离子可以在活性填料/聚合物界面处进行传导[53],纳米线可以提供相对连续的Li+运输路径,但是纳米线-聚合物界面接触面积有限,这也限制了固态复合电解质性能的进一步提升。因此,使用二维纳米片或三维骨架替代一维纳米线,可以提供连续而有效的Li+传导界面。如图4(a)所示,Bae等[54]将PEO与三维LLTO框架进行复合,多维界面层可为Li+提供快捷的传导途径(图4(b))。从光学照片和扫描电镜图(图4(b)、图4(c))可以看出PEO与三维LLZO框架间的紧密接触界面。PEO-三维LLTO骨架复合固态电解质的离子传导率明显高于PEO-LLTO纳米颗粒,可达8.8×10−5 S·cm−1。
![]() 图 4 PEO-LLTO合成示意图(a)、复合电解质中Li+传导机制(b)、扫描电镜图(c)、Arrhenius曲线(d)[54]Figure 4. Schematic representation of the synthesis of PEO-LLTO composite electrolytes (a), the possible conduction mechanism in composite electrolytes (b), top view (left) and cross-section (right) SEM images (c) of the composite electrolyte, Arrhenius plots (d)[54]PVA—Polyvinyl alcohol; GA—Glutaraldehyde
图 4 PEO-LLTO合成示意图(a)、复合电解质中Li+传导机制(b)、扫描电镜图(c)、Arrhenius曲线(d)[54]Figure 4. Schematic representation of the synthesis of PEO-LLTO composite electrolytes (a), the possible conduction mechanism in composite electrolytes (b), top view (left) and cross-section (right) SEM images (c) of the composite electrolyte, Arrhenius plots (d)[54]PVA—Polyvinyl alcohol; GA—Glutaraldehyde表3总结了近年来钙钛矿型陶瓷/聚合物复合电解质一些研究工作[54-61]。钙钛矿型电解质中Ti4+容易被还原为Ti3+,导致总电导率比其晶粒电导率低约两个数量级。除了考虑形态、尺寸、添加比例和界面传导机制外,如何规避Ti4+还原,进一步提高复合电解质的离子电导率也是将来需要研究的重点。
表 3 聚合物-钙钛矿型陶瓷复合电解质性能Table 3. Performance of polymer-perovskite ceramic electrolyteComposite electrolyte Conductivity/
(S·cm−1)Electrochemical window/V Ref. PEO/LLTO/LiTFSI 8.8×10−4 at 25℃ 4.5 [54] PEO/LLTO/LiTFSI 1.8×10−4 at 25℃ 4.5 [55] PEO/LLTO/LiClO4 2.3×10−4 at 60℃ 4.5 [56] PVDF/LLTO/LiTFSI 5.3×10−4 at 60℃ 5.1 [57] PAN/LLTO/LiTFSI 2.2×10−3 at 30℃ 5.1 [58] PEO/LLTO/LiTFSI 1.3×10−2 at 24℃ 3.8 [59] PVDF-HFP/LLTO/
LiTFSI1.2×10−4 at 25℃ 4.7 [60] PVDF/LLTO/LiClO4 5.8×10−4 at 25℃ 5.2 [61] 2. 聚合物-硫化物复合电解质
硫化物电解质(图5(a)[26])具有更好的Li+传导,离子电导率可高达10−3~10−4 S·cm−1[62-63],被视为氧化物的替代品。然而硫化物材料自身存在许多问题,比如热稳定性差、对锂金属负极不稳定、分解产物会产生阻抗层、对空气也极为敏感及原料昂贵等。为了避免上述缺点,更好地发挥出硫化物本征高电导特性,近些年硫化物电解质研究方向包括复合、掺杂、包覆和纳米结构等[64]。其中,硫化物-聚合物复合型固态电解质能够在空气中稳定存在,也能与锂金属负极良好接触。
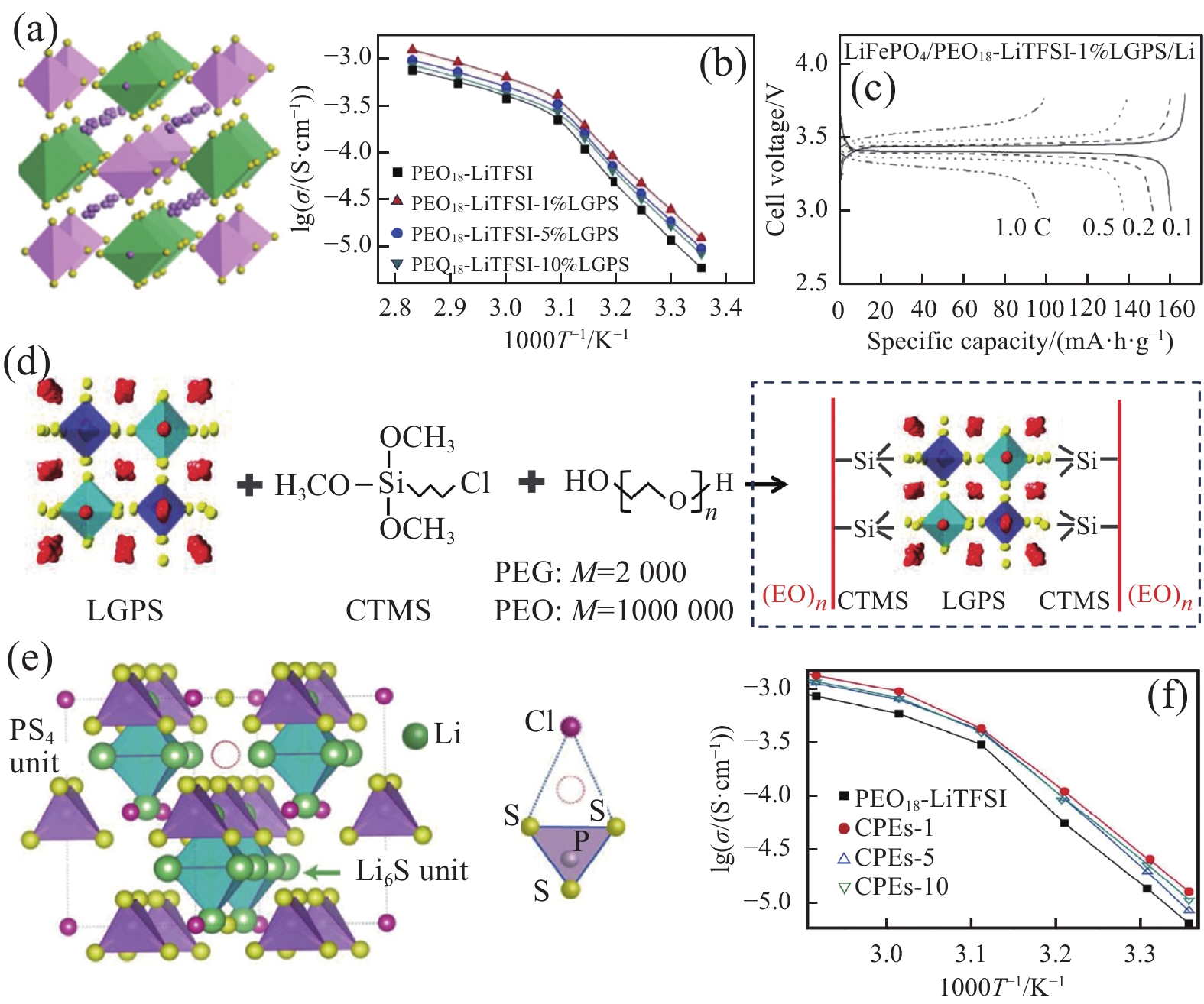
![]() 图 5 (a) 硫化物构型Li10GeP2S12 (LGPS)[26];Arrhenius曲线(b)和充放电曲线(c)[65];(d) PEO-LGPS原位合成示意图[66];Li6.25PS5.25Cl0.75晶格(e)和Arrhenius曲线(f)[67]Figure 5. (a) Sulfide-type Li10GeP2S12 (LGPS)[26]; Arrhenius plots (b) and charge/discharge curves (c) for the ionic conductivities of the composite electrolytes[65]; (d) Schematic illustration of the in-situ synthesis of PEO-LGPS solid electrolyte[66]; Lattice model (e) and Arrhenius curves (f) of sulfide electrolyte Li6.25PS5.25Cl0.75[67]CTMS—(3-chloropropyl) trimethoxysilane; CPEs—Composite polymer electrolytes; M—Molecular weight
图 5 (a) 硫化物构型Li10GeP2S12 (LGPS)[26];Arrhenius曲线(b)和充放电曲线(c)[65];(d) PEO-LGPS原位合成示意图[66];Li6.25PS5.25Cl0.75晶格(e)和Arrhenius曲线(f)[67]Figure 5. (a) Sulfide-type Li10GeP2S12 (LGPS)[26]; Arrhenius plots (b) and charge/discharge curves (c) for the ionic conductivities of the composite electrolytes[65]; (d) Schematic illustration of the in-situ synthesis of PEO-LGPS solid electrolyte[66]; Lattice model (e) and Arrhenius curves (f) of sulfide electrolyte Li6.25PS5.25Cl0.75[67]CTMS—(3-chloropropyl) trimethoxysilane; CPEs—Composite polymer electrolytes; M—Molecular weightZhao等[65]将LGPS微粒作为活性填料与PEO-LiTFSI电解质复合,并研究LGPS添加量对聚合物体系离子电导率的影响。结果表明:LGPS的最佳添加含量为1wt%,离子电导率最高为1.2×10−3 S·cm−1 (图5(b))。基于复合电解质的固态电池在0.1 C时,比容量为158 mA·h·g−1 (图5(c))。Pan等[66]将PEO与LGPS进行原位复合(图5(d)),LGPS-聚合物之间的强化学键能够有效消除两相界面问题,在界面处形成锂离子快速传导的通道,室温离子电导率可达9.83×10−4 S·cm−1,Li+迁移数为0.68。Li等[67]制备出一种与Li6PS5Cl不同的新型硫化物Li6.25PS5.25Cl0.75 (图5(e)),该硫化物中Li2S稍过量而LiCl不足,在与PEO复合后室温离子电导率高达10−2 S·cm−1 (图5(f))。通过小角X射线衍射研究硫化物添加量对PEO晶态的影响。随着硫化物添加量增加,PEO聚合物中非晶态区域增加,这有利于聚合物链段蠕动,从而促进锂离子传导。Zheng等[68]通过同位素交换法研究LGPS-PEO-LiTFSI中离子传导机制,证明了聚合物非晶相和聚合物-LGPS界面相都有利于Li+快速传导。
近些年来,聚合物-硫化物复合电解质在固态锂电池的应用方面取得了很好的进展。但在电池内部,硫化物电解质存在许多问题,比如正极-硫化物电解质界面会发生电化学分解和机械降解行为等。如何合理设计聚合物-硫化物复合电解质,有效解决上述存在的问题是将来研究的重点。
3. 聚合物-惰性填料复合电解质
惰性氧化物填料比如Al2O3、TiO2、SiO2和MgO等[69-72],早已被报道用于固态聚合物-惰性填料复合电解质。1982年,Weston等[73]将10vol%的Al2O3颗粒添加到PEO基聚合物。随着Al2O3含量增加,颗粒团聚在复合电解质中形成大的惰性粒子,会阻碍Li+传输。Dissanayake等[74]发现随着Al2O3颗粒纳米尺寸减小,复合电解质的电导率会逐渐提高,由于小颗粒具有更大比表面积,表面—OH基团会与锂离子相互作用形成有效的离子传导通路。
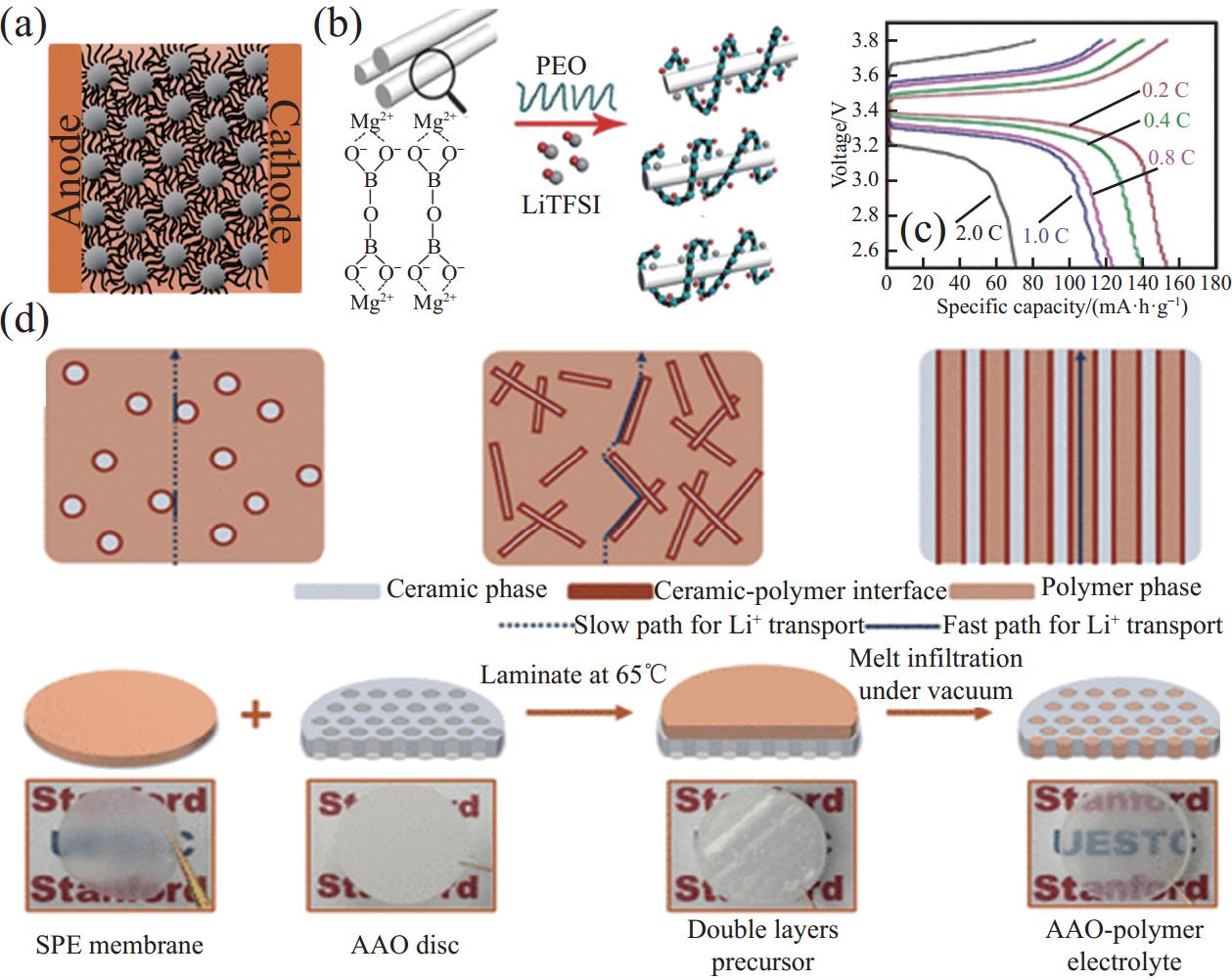
无机填料的种类、尺寸和分布会影响复合电解质的性能,填料表面的物化性质起着重要的作用。TiO2作为路易斯酸会与PEO相互作用,Li+从TiO2粒子表面迁移,形成高Li+转移数和传导率[75]。增强有效的无机填料-聚合物界面兼容,是当前研究固态复合电解质的重要思路。Choudhury等[76]将SiO2纳米颗粒嫁接在PEO主链(图6(a)),当PEO/Li+摩尔比为20时,复合电解质的离子电导率最高。SiO2纳米颗粒在PEO基体中均匀分布,大而连续的路易斯酸中心与TFSI−相互作用,释放出更多Li+,大量锂离子自由迁移可提高离子电导率。基于此,Sheng等[77]将低成本阻燃的Mg2B2O5纳米线添加到PEO电解质(图6(b)),基于复合电解质的LFP固态电池在0.2 C时,比容量为150 mA·h·g−1(图6(c))。虽然纳米颗粒可以增强锂离子传导,但传输路径是孤立的、短距离的。因此,高长径比的纳米线具有较长离子传输路径,更有利于锂离子传输。Zhang等[78]将PEO电解质浇筑到Al2O3纳米通道中(图6(d)),Li+能够在填料-聚合物两相界面处传导,室温锂离子电导率为1.79×10−4 S·cm−1。
![]() 图 6 (a) SiO2纳米颗粒嫁接在PEO主链卡通图[76];Li+在PEO-Mg2B2O5复合电解质中的迁移示意图(b)和充放电曲线图(c)[77];(d) 3种填料-聚合物界面几何结构图(上图)和PEO-Al2O3复合电解质合成示意图[78]Figure 6. (a) Cartoon showing the SiO2 nanoparticles grafted onto the PEO backbone[76]; Schematics of Li+ migration in PEO-Mg2B2O5 composite electrolytes (b) and charge/discharge curves (c)[77]; (d) Schematics of composite solid polymer electrolyte with three types of geometrical structures of ceramic-polymer interface (upper image) and schematics of fabrication procedures of polymer-Al2O3 composite electrolyte(below image)[78]SPE—Solid polymer electrolytes; AAO—Anodized aluminum oxide
图 6 (a) SiO2纳米颗粒嫁接在PEO主链卡通图[76];Li+在PEO-Mg2B2O5复合电解质中的迁移示意图(b)和充放电曲线图(c)[77];(d) 3种填料-聚合物界面几何结构图(上图)和PEO-Al2O3复合电解质合成示意图[78]Figure 6. (a) Cartoon showing the SiO2 nanoparticles grafted onto the PEO backbone[76]; Schematics of Li+ migration in PEO-Mg2B2O5 composite electrolytes (b) and charge/discharge curves (c)[77]; (d) Schematics of composite solid polymer electrolyte with three types of geometrical structures of ceramic-polymer interface (upper image) and schematics of fabrication procedures of polymer-Al2O3 composite electrolyte(below image)[78]SPE—Solid polymer electrolytes; AAO—Anodized aluminum oxide虽然惰性填料本身不能提供离子导电性,但仍在复合电解质中扮演着重要的角色,主要包括:(1) 无机填料可以削弱聚合物与Li离子的相互作用,促进锂盐的解离;(2) 无机填料/聚合物两相界面能为锂离子提供了额外的传输路径;(3) 惰性填料的存在,会破坏聚合物链的规整性,增加局部非晶区域,促进Li离子传导;(4) 惰性填料能支撑聚合物基体,提高杨氏模量和抗拉强度等力学性能。
4. 有机-无机固态电解质在锂电池中的应用
固态锂电池是由正负极和固态电解质组成的电池,具有强力学性能、高能量密度和安全可靠等优点,是下一代重要的储能技术之一。固态电解质不仅能够传导锂离子,也可以当作电池隔膜[79]。全固态锂离子电池的正极材料(锂过渡金属氧化物、锂过渡金属硫化物等[80-82])和负极材料(金属锂、硅材料、石墨烯和钛酸锂等[83-85])。固态锂电池能够实现循环稳定和安全可靠等性能,但也伴随着一些重要难题,如固-固界面阻抗大、活性材料低载量和较差循环性能等。通常,当固态锂电池的主流正极材料为LFP和三元材料时,电池能量密度已然接近理论极限,这难以满足国家重大发展的能源需求。鉴于此,提高电池的能量密度是固态锂电池进一步发展的主要目标。国内外电池领域学者普遍认为,高比能固态锂电池的研究应该先聚焦固态锂金属电池、其次是固态锂-硫电池,最终目标是固态锂-空气电池(图7)[86]。
4.1 全固态锂金属电池
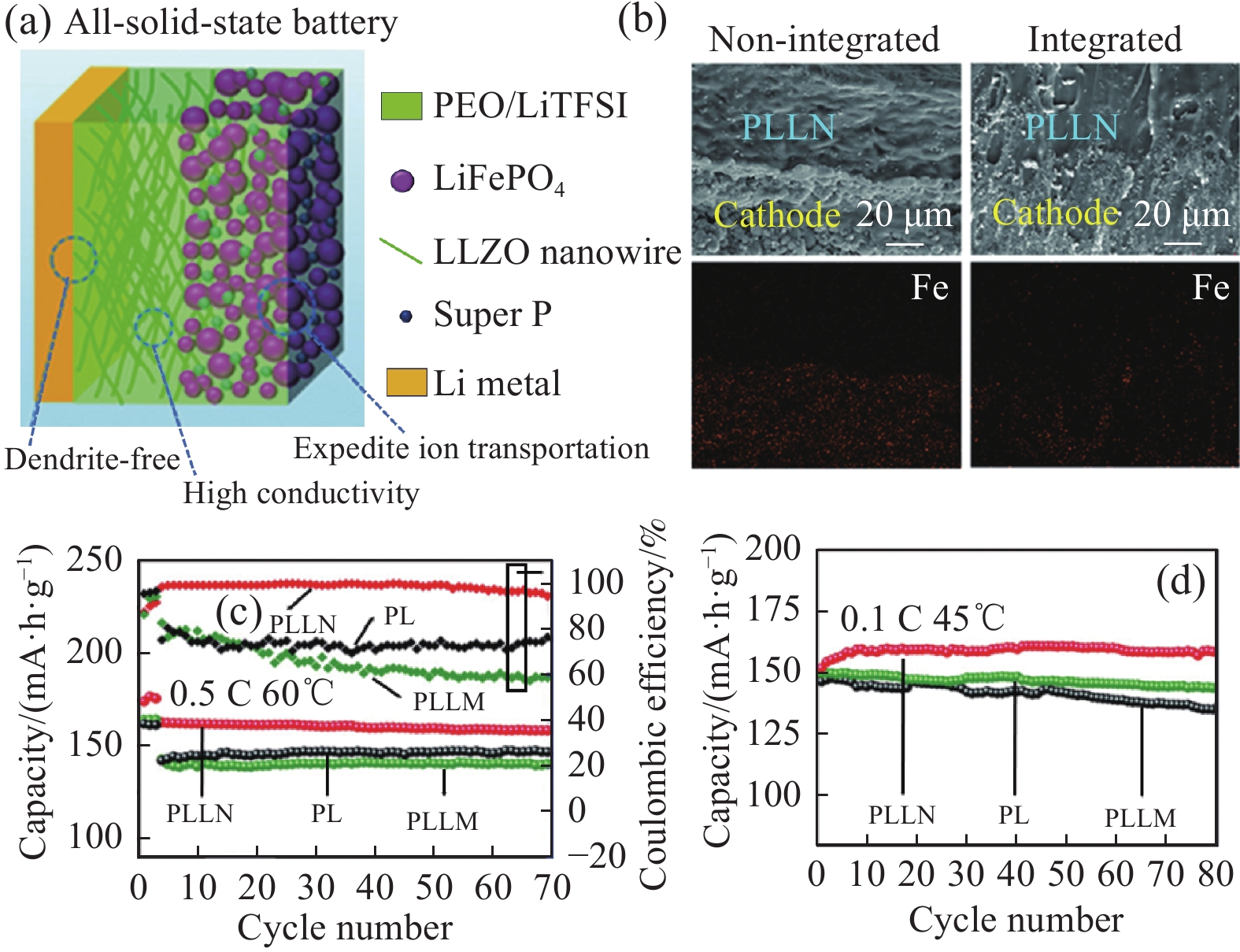
锂金属是下一代高比能锂电池负极材料的理想候选者,具有高理论比容量(3860 mA·h·g−1)、低电化学电位(−3.04 V)和低密度(0.59 g·cm−3)等特点。固态锂金属电池是最具潜力替代现有高能量密度锂电池的候选技术,其能量密度是现有锂电池的2~5倍,循环性和服役寿命更长,倍率性能更高,可从本质上解决液态锂电池的安全性问题。Wan等[87]将LLZO纳米线分散到PEO中得到复合固态电解质,与LFP正极在高温下熔合形成一体化结构(图8(a)、图8(b))。一体化结构可以适应电极体积变化,增强充放电循环过程中正极与固态电解质的界面亲和性和稳定性,还可以增加材料内部的离子电导率。该固态LFP//Li电池在60℃下、电流密度为0.5 C时循环70次后,放电容量达到158.8 mA·h·g−1 (图8(c))。当工作温度为45℃时,在0.1 C下循环80次后,LFP电池放电容量仍可达158.7 mA·h·g−1 (图8(d))。
![]() 图 8 (a) 固态LiFPO4 (LFP)/LLZO纳米线(PLLN)/Li电池示意图;(b) 熔合前后LFP与PLLN电解质横截面积及Fe元素mapping图;0.5 C/60℃ (c)和0.1 C/45℃ (d)下的循环性能[87]Figure 8. (a) Schematic illustration of LiFPO4 (LFP)/LLZO nanowire (PLLN)/Li battery; (b) Cross-sectional images of LFP and PLLN electrolyte and the corresponding EDS mapping of Fe before and after the fused; Cycling performances at 0.5 C/60℃ (c) and 0.1 C/45℃ (d)[87]PL—LiTFSI; PLLM—LLZO microparticles
图 8 (a) 固态LiFPO4 (LFP)/LLZO纳米线(PLLN)/Li电池示意图;(b) 熔合前后LFP与PLLN电解质横截面积及Fe元素mapping图;0.5 C/60℃ (c)和0.1 C/45℃ (d)下的循环性能[87]Figure 8. (a) Schematic illustration of LiFPO4 (LFP)/LLZO nanowire (PLLN)/Li battery; (b) Cross-sectional images of LFP and PLLN electrolyte and the corresponding EDS mapping of Fe before and after the fused; Cycling performances at 0.5 C/60℃ (c) and 0.1 C/45℃ (d)[87]PL—LiTFSI; PLLM—LLZO microparticles钴酸锂(LCO)具有理论容量高(274 mA·h·g−1),一直是电池电极材料的研究前沿。然而在相对高的电压工作时,LCO材料会发生不可逆地表面降解和破坏性相变引起的结构损坏、晶格氧的损失和钴离子的溶解等,从而造成容量严重衰减[88]。有效的解决方法就是在电极材料表面构建具有保护性作用的钝化层,从而优化表面结构、控制界面响应和增强电池动力学。Qiu等[89]分别用陶瓷电解质导体Li1.4Al0.4Ti1.6(PO4)3和LiMn0.7Fe0.3PO4作为LCO表/界面保护涂层,然后匹配PEO聚合物电解质,固态电池在4.2 V电压下可以实现长寿命循环性能。
三元材料(LiNi0.8Co0.1Mn0.1O2,NCM811)具有高理论容量和宽电化学窗口,是实现高比能锂电池的关键正极材料。当固态电解质与NCM材料进行匹配时,既要考虑界面物理接触,也要考虑高电压下电化学氧化等因素。Yu等[48]提出了一种多层结构的双聚合物/聚合物-陶瓷材料复合电解质的设计方法,该复合电解质能够承受高电压,并在锂金属负极下保持稳定。在该复合电解质中,耐氧化的聚丙烯腈/LATP复合电解质层贴近正极一侧,负极一侧则为锂金属负极稳定的PEO-琥珀腈(SN)-LiTFSI (图9(a)),双层复合电解质的室温离子电导率为1.31×10−4 S·cm−1。当与NCM811匹配时,在0.05 C时能发挥出200 mA·h·g−1的高容量(图9(b))。此外,该固态NCM//Li电池能在0.2 C时能稳定循环300圈(图9(c))。
4.2 全固态锂-硫(Li-S)电池
在Li-S电池中,硫正极和锂金属负极的理论容量分别为~ 1675 mA·h·g−1和~ 3800 mA·h·g−1,Li-S电池具有~ 2600 W·h·kg−1的超高能量密度。此外,丰富、廉价和环保的硫降低了使用成本,高能Li-S电池或许可以在包括便携式电子设备、电动汽车和电网等储能领域取代传统锂电池[90-92]。近些年来,尽管液态Li-S电池在能量密度、功率密度和循环寿命等方面取得了巨大进步,但仍存在许多亟待解决的问题[93-94]:(1) 溶解的多硫化物会迁移至负极而造成自放电现象;(2) 有机电解液具有挥发、易燃等隐患;(3) 锂金属负极在充放电过程中枝晶生长;(4) 硫正极本身不导电和体积膨胀严重等问题。鉴于此,国内外众多科研单位对Li-S电池开展了大量研究工作。研究内容从正极材料到电解质,从隔膜到锂负极,Li-S电池中各个关键材料都进行了相应的优化。其中,开发适用的固态电解质能在很大程度上解决传统Li-S电池面临的诸多问题,既能缓减多硫化物的穿梭效应,也能抑制锂枝晶的无规律生长,还能避免有机电解质漏液、燃烧等问题,可以助力高比能高安全Li-S电池的发展。
Tao等[95]利用溶胶凝胶法制备LLZO纳米颗粒修饰的泡沫碳,将其作为硫载体。同时制备LLZO-PEO-LiClO4复合固态电解质,该固态电解质结合了聚合物电解质和陶瓷电解质的优点。由于固态电解质与正极材料的材质相似(图10(a)),界面表现出良好的兼容性,固态电解质离子传导和硫正极电子导电性也得到了改善。全固态锂-硫电池在50℃时,比容量>1210 mA·h·g−1,表现出高库仑效率和稳定循环性能(图10(b))。Zhu等[56]分别将三维碳纳米纤维/硫(CNF/S)与LLTO纳米线-PEO固体复合电解质作为室温全固态锂-硫电池正极和电解质(图10(c))。稳定的双层结构设计减少界面阻力和增强电极/电解液界面稳定性,LLTO纳米线确保了锂离子长径传导,三维结构CNF/S正极则提供了电子快速传输的通路。双层结构既能适应连续充放电过程中硫的体积变化,也有助于抑制锂枝晶的形成。基于此,该固态Li-S电池在室温下循环50次后,容量仍然保持为415 mA·h·g−1,库仑效率高达99%,具有稳定的循环性能(图10(d)、图10(e))。
![]() 图 10 全固态Li-S电池示意图(a)及其在0.1 mA·cm–2/50℃时充放电曲线(b)[95];碳纳米纤维(CNF)/S-PEO/LLTO双层结构设计示意图(c)、不同倍率下充放电曲线(d)和0.1 C/45℃下的循环性能(e)[56]Figure 10. Schematic illustration (a) of Li-S battery and the corresponding discharge-charge curves at 0.1 mA·cm–2/50℃ (b)[95]; Schematic illustration of the carbon nanofiber (CNF)/S-PEO/LLTO bilayer structure design (c), the discharge-charge curves at various current densities (d), and cycling performance at 0.1 C/45℃ (e)[56]SE—Solid-state electrolyte
图 10 全固态Li-S电池示意图(a)及其在0.1 mA·cm–2/50℃时充放电曲线(b)[95];碳纳米纤维(CNF)/S-PEO/LLTO双层结构设计示意图(c)、不同倍率下充放电曲线(d)和0.1 C/45℃下的循环性能(e)[56]Figure 10. Schematic illustration (a) of Li-S battery and the corresponding discharge-charge curves at 0.1 mA·cm–2/50℃ (b)[95]; Schematic illustration of the carbon nanofiber (CNF)/S-PEO/LLTO bilayer structure design (c), the discharge-charge curves at various current densities (d), and cycling performance at 0.1 C/45℃ (e)[56]SE—Solid-state electrolyte4.3 全固态锂-空气电池
锂-空气电池是基于锂金属负极和空气正极的新型储能体系,如图11(a)所示[96],具有极高的理论能量密度(~ 11680 W·h·kg−1),足以与汽油(13000 W·h·kg−1)媲美[97-98]。除了超高的能量密度外,锂-空气电池还具有环境友好、造价低廉等优点。锂-空气电池虽然经过长期发展取得了长足的进步,但仍面临着诸多问题和挑战[99],比如:(1) 电解液的稳定性,由于空气电极充电电位高,电解液在高电压下会发生复杂的副反应,可能会导致电池失效;(2) 锂负极的保护,锂金属会与溶于电解质的空气发生反应,进而造成锂负极粉化。此外,锂枝晶会穿透隔膜致使电池短路。固态电解质在高电位下稳定性好,又能隔绝空气、抑制锂枝晶,还可以有效地防止电池漏液,能从根本上解决上述问题,并推动锂-空气电池的进一步发展。
![]() 图 11 (a) 锂-空气电池示意图[96];(b) PEO/LATP复合电解质基锂-空气电池[100];三维LLZO框架/聚合物制备流程(c)、LLZO颗粒(d)和LLZO/聚合物(e)的SEM图像及Arrhenius曲线(f)[101]Figure 11. (a) Schematic representation of the Li-air battery[96]; (b) Schematic diagram of the PEO/LATP composite electrolyte based solid-state lithium-air battery[100]; Preparation procedure for the composite polymer electrolyte with 3D LLZO network (c), SEM images of 3D LLZO network grains (d) and PEO-LLZO hybrid electrolyte (e), and Arrhenius curves (f)[101]
图 11 (a) 锂-空气电池示意图[96];(b) PEO/LATP复合电解质基锂-空气电池[100];三维LLZO框架/聚合物制备流程(c)、LLZO颗粒(d)和LLZO/聚合物(e)的SEM图像及Arrhenius曲线(f)[101]Figure 11. (a) Schematic representation of the Li-air battery[96]; (b) Schematic diagram of the PEO/LATP composite electrolyte based solid-state lithium-air battery[100]; Preparation procedure for the composite polymer electrolyte with 3D LLZO network (c), SEM images of 3D LLZO network grains (d) and PEO-LLZO hybrid electrolyte (e), and Arrhenius curves (f)[101]锂-空气电池中的固态电解质分为陶瓷电解质和聚合物电解质两大类。在陶瓷电解质中,NASICON结构氧化物具有较好的室温电导率和较低的烧结温度,但与锂负极接触会发生不稳定的副反应,导致界面阻抗增加。Kitaura等[100]使用PEO聚合物等作为保护层来避免金属锂与LATP固体电解质的直接接触,将Li1+x+yAlx(Ti, Ge)2−xSiyP3−yO12/PEO复合物作为固态电解质,以碳纳米管/固态电解质作为空气正极,组装成全固态锂-空气电池(图11(b))。该固态锂-空气电池在10 mA·g−1的电流密度下,容量达到400 mA·h·g−1。石榴石结构固态电解质(LLZO)对锂的电化学稳定性更高,可作为锂-空气电池固态电解质。有机聚合物电解质中,PEO聚合物对锂负极相对稳定,而且塑形性较好,易于加工改性,这些特点使其成为固态锂-空气电池研究的热点。但是研究发现,PEO容易与锂-氧电池产物反应,会丧失导锂活性。Song等[101]成功制备出三维互连的LLZO无机陶瓷(图11(c)~11(f)),随后在LLZO网络中均匀的大孔结构中容纳PEO聚合物,该复合电解质对锂金属负极具有很好的保护效果。此外,锂离子既可以沿三维LLZO网络进行传导,又可以在聚合物/陶瓷间连续界面处传导,在室温下表现出9.2×10−5 S·cm−1的高离子电导率。
5. 结论与展望
固态电池能够有效避免液态电池的诸多安全隐患,因而在储能领域备受关注。本综述围绕有机-无机复合固态电解质的研究工作进行了回顾,并详述了有机-无机复合电解质在组分、结构、形态和界面改性等方面的工作内容,最后讨论了复合电解质在固态锂金属电池、固态锂-硫电池和固态锂-空气电池等领域的应用研究。固态锂电池是当前电池领域研发的热点,也是未来商用电池的发展方向之一。有机-无机复合电解质兼具优异力学性能、高离子电导率和宽电化学窗口,在全固态锂电池中的研究取得了长足的进步,未来的研究重点应该围绕以下几个方面进行:
(1) 离子电导率。在室温下,复合固态电解质的锂离子电导率一般可达10−4 S·cm−1,但仍不能与有机液体电解质(~10−2 S·cm−1)媲美。无机填料在有机-无机复合固态电解质中具有重要的作用,无机填料的尺寸、含量、分散程度和微观结构会影响复合电解质的力学性能和锂离子电导率。因此,需要通过无机填料结构设计和组分调控来改善锂离子电导率。此外,研究表明Li+倾向于在填料/聚合物两相界面处传导。因此,需要设计新型功能化(具有氧空位、微/纳结构等)无机填料,从纳米颗粒到纳米线再到三维纳米结构,构建长程连续的Li+传导快捷通道;
(2) 界面问题。电解质与正/负极的界面及聚合物/填料的物理接触都是复杂而棘手的问题。电解质与正/负极的非良性物理接触,会导致界面处不稳定的物理化学/电化学反应,电池阻抗会明显增大,不利于Li+的快速传导。因此,对于复合电解质固态锂电池,多角度全方位的界面改性技术至关重要;
(3) 力学强度、柔韧性。较薄的固态电解质有利于电池能量密度的增加,力学强劲、足够柔韧的电解质薄膜一直是高性能固态锂电池的首选。先进的方法,比如原子/分子层沉积、溅射沉积和液相层沉积有望实现制备高Li+电导薄膜电解质;
(4) 机制研究。聚合物-无机复合固态电解质中离子传输、亚分子尺度和原子水平上填料-聚合物界面的相互作用需进一步深入了解。此外,需要研究固态电池中的副反应(聚合物-无机复合固态电解质分解产物的化学组分等)。可以通过实验表征(如TEM、计算机断层(CT)扫描、拉曼、核磁共振等)和理论计算多尺度模拟(密度泛函理论、分子动力学等)等方法相结合进行系统研究,此外还需要从电化学、物理、材料科学、纳米科学和纳米工程等不同学科角度加深固态锂电池基础原理的理解。
-
图 1 (a) 石榴石构型Li7La3Zr2O12 (LLZO)[26];(b) 聚偏氟乙烯 (PVDF)/ Li6.75La3Zr1.75Ta0.25O12 (LLZTO)复合固态电解质的结构模型[28];PVDF-LLZO纳米纤维固态电解质形貌((c), (d))和对称电池测试(e)[29];三维珊瑚状LLZO-PVDF复合电解质制备示意图(f)及其SEM图像((g), (h))[30]
Figure 1. (a) Garnet-type Li7La3Zr2O12 (LLZO)[26]; (b) Possible complex structures in the poly(vinylidene fluoride) (PVDF)/Li6.75La3Zr1.75Ta0.25O12 (LLZTO)[28]; SEM images ((c), (d)) of PVDF-LLTO nanofibers solid-state electrolyte and symmetrical battery test (e)[29]; Schematic illustration (f) for the preparation procedures of the coral-like LLZO/PVDF electrolyte and SEM images ((g), (h))[30]
NFs—Nanofibers; RT—Room temperature
图 2 (a) NASICON构型Li1+xAlxGe2−x(PO4)3 (LAGP)[26];(b) 聚环氧乙烷(PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)的循环稳定测试[40];(c) PVDF-LATP的电化学窗口测试[42];(d) 贝壳启发下 LAGP陶瓷-聚合物复合电解质的制备[43];(e) 冰模板法制备LAGP-PEO复合电解质流程图[44]
Figure 2. (a) NASICON-type Li1+xAlxGe2−x(PO4)3 (LAGP)[26]; (b) Cycle stability of polyethylene oxide (PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)[40]; (c) Electrochemical stability windows of PVDF-LATP[42]; (d) Design and fabrication of LAGP ceramic-polymer composite electrolyte[43]; (e) Schematic of preparation process of the ice-templated LAGP-PEO composite electrolyte[44]
图 3 (a)钙钛矿构型LLTO[26];(b) 纳米线/纳米颗粒-聚合物固态电解质中Li+传导路径对比;(c) PAN-LLTO的Arrhenius曲线[33]
Figure 3. (a) Perovskite-type LLTO[26]; (b) Comparison of possible lithium-ion conduction pathway in nanowire-filled and nanoparticle-filled composite electrolytes; (c) Arrhenius plots of PAN-LLTO[33]
σ—Conductivity; T—Temperature
图 4 PEO-LLTO合成示意图(a)、复合电解质中Li+传导机制(b)、扫描电镜图(c)、Arrhenius曲线(d)[54]
Figure 4. Schematic representation of the synthesis of PEO-LLTO composite electrolytes (a), the possible conduction mechanism in composite electrolytes (b), top view (left) and cross-section (right) SEM images (c) of the composite electrolyte, Arrhenius plots (d)[54]
PVA—Polyvinyl alcohol; GA—Glutaraldehyde
图 5 (a) 硫化物构型Li10GeP2S12 (LGPS)[26];Arrhenius曲线(b)和充放电曲线(c)[65];(d) PEO-LGPS原位合成示意图[66];Li6.25PS5.25Cl0.75晶格(e)和Arrhenius曲线(f)[67]
Figure 5. (a) Sulfide-type Li10GeP2S12 (LGPS)[26]; Arrhenius plots (b) and charge/discharge curves (c) for the ionic conductivities of the composite electrolytes[65]; (d) Schematic illustration of the in-situ synthesis of PEO-LGPS solid electrolyte[66]; Lattice model (e) and Arrhenius curves (f) of sulfide electrolyte Li6.25PS5.25Cl0.75[67]
CTMS—(3-chloropropyl) trimethoxysilane; CPEs—Composite polymer electrolytes; M—Molecular weight
图 6 (a) SiO2纳米颗粒嫁接在PEO主链卡通图[76];Li+在PEO-Mg2B2O5复合电解质中的迁移示意图(b)和充放电曲线图(c)[77];(d) 3种填料-聚合物界面几何结构图(上图)和PEO-Al2O3复合电解质合成示意图[78]
Figure 6. (a) Cartoon showing the SiO2 nanoparticles grafted onto the PEO backbone[76]; Schematics of Li+ migration in PEO-Mg2B2O5 composite electrolytes (b) and charge/discharge curves (c)[77]; (d) Schematics of composite solid polymer electrolyte with three types of geometrical structures of ceramic-polymer interface (upper image) and schematics of fabrication procedures of polymer-Al2O3 composite electrolyte(below image)[78]
SPE—Solid polymer electrolytes; AAO—Anodized aluminum oxide
图 8 (a) 固态LiFPO4 (LFP)/LLZO纳米线(PLLN)/Li电池示意图;(b) 熔合前后LFP与PLLN电解质横截面积及Fe元素mapping图;0.5 C/60℃ (c)和0.1 C/45℃ (d)下的循环性能[87]
Figure 8. (a) Schematic illustration of LiFPO4 (LFP)/LLZO nanowire (PLLN)/Li battery; (b) Cross-sectional images of LFP and PLLN electrolyte and the corresponding EDS mapping of Fe before and after the fused; Cycling performances at 0.5 C/60℃ (c) and 0.1 C/45℃ (d)[87]
PL—LiTFSI; PLLM—LLZO microparticles
图 10 全固态Li-S电池示意图(a)及其在0.1 mA·cm–2/50℃时充放电曲线(b)[95];碳纳米纤维(CNF)/S-PEO/LLTO双层结构设计示意图(c)、不同倍率下充放电曲线(d)和0.1 C/45℃下的循环性能(e)[56]
Figure 10. Schematic illustration (a) of Li-S battery and the corresponding discharge-charge curves at 0.1 mA·cm–2/50℃ (b)[95]; Schematic illustration of the carbon nanofiber (CNF)/S-PEO/LLTO bilayer structure design (c), the discharge-charge curves at various current densities (d), and cycling performance at 0.1 C/45℃ (e)[56]
SE—Solid-state electrolyte
图 11 (a) 锂-空气电池示意图[96];(b) PEO/LATP复合电解质基锂-空气电池[100];三维LLZO框架/聚合物制备流程(c)、LLZO颗粒(d)和LLZO/聚合物(e)的SEM图像及Arrhenius曲线(f)[101]
Figure 11. (a) Schematic representation of the Li-air battery[96]; (b) Schematic diagram of the PEO/LATP composite electrolyte based solid-state lithium-air battery[100]; Preparation procedure for the composite polymer electrolyte with 3D LLZO network (c), SEM images of 3D LLZO network grains (d) and PEO-LLZO hybrid electrolyte (e), and Arrhenius curves (f)[101]
表 1 聚合物-石榴石型陶瓷复合电解质性能
Table 1 Performance of polymer-garnet ceramic electrolyte
Composite electrolyte Conductivity/(S·cm−1) Li//Li cells Solid-state cells Ref. PVDF-LLZTO nanoparticles 5.0×10−4 — 140 mA·h·g−1 at 1.2 C; LCO//Li cell [28] PVDF-LLZTO (3D) 1.5×10−4 0.1 mA·cm−2, 200 h 168 mA·h·g−1 at 0.1 C; LFP//Li cell [29] PVDF-LLZTO nanofibers 1.2×10−4 0.5 mA·cm−2, 700 h 213 mA·h·g−1 at 0.2 C; NCA//Li cell [30] PVDF-LLZTO nanoparticles 2.1×10−4 3.0 mA·cm−2, 700 h 141 mA·h·g−1 at 0.1 C; LFP//Li cell [32] PVDF-LLTO nanowires 2.4×10−4 — — [33] Notes: LLTO—Li0.33La0.557TiO3; LCO—LiCoO2; LFP—LiFePO4; NCA—LiNi0.8Co0.15Al0.05O2. 表 2 聚合物-NASICON型陶瓷复合电解质性能
Table 2 Performance of polymer-NASICON ceramic electrolyte
Composite electrolyte Conductivity/
(S·cm−1)Electrochemical window Ref. PEG-CA/LATP/
LiClO4>10−4 at 60℃ ~5.0 V [37] PVDF-HFP/LAGP/
EMITFSI7.6×10−4 at 25℃ 4.8 V [39] PEO/LATP/LiClO4 1.6×10−4 at 60℃ — [40] LAGP-PEA/LiTFSI 1.3×10−4 at 25℃ — [43] PEO-PEG/LAGP/
LiClO41.7×10−4 at 25℃ — [44] PVDF/LATP/LiTFSI 3.3×10−4 at 20℃ — [45] PEO/LiTFSI+PAN/
LATP6.5×10−4 at 60℃ 4.2 V [46] PEO/LiTFSI+LATP/ PAN/LiTFSI 6.3×10−4 at 60℃ ~5.0 V [47] PEO-SN/LiTFSI+PAN/
LATP/LiTFSI1.3×10−4 at 25℃ 5.0 V [48] PET-PIL/LAGP/
LiTFSI7.8×10−5 at 30℃ 4.55 V [49] PEGDA/LiTFSI+
PAN/LAGP/LiTFSI3.7×10−4 at 25℃ 5.0 V [50] Notes: PEG-CA—Polyethylene glycol-cellulose acetate; HFP—
Hexafluoropropylene; PEO—Polyethylene oxide; PEA—Poly (ether-acrylate); PET—Polyethylene terephthalate; PIL—Polyme
rized ionic liquid; PEGDA—Polyethylene glycol diacrylate; LiTFSI—Lithium bis (trifluoromethanesulphonyl) imide; PAN—Polyacrylonitrile; SN—Succinonitrile; EMITFSI—1-ethyl-3-methylimidazolium triluoromethanesufonate.表 3 聚合物-钙钛矿型陶瓷复合电解质性能
Table 3 Performance of polymer-perovskite ceramic electrolyte
Composite electrolyte Conductivity/
(S·cm−1)Electrochemical window/V Ref. PEO/LLTO/LiTFSI 8.8×10−4 at 25℃ 4.5 [54] PEO/LLTO/LiTFSI 1.8×10−4 at 25℃ 4.5 [55] PEO/LLTO/LiClO4 2.3×10−4 at 60℃ 4.5 [56] PVDF/LLTO/LiTFSI 5.3×10−4 at 60℃ 5.1 [57] PAN/LLTO/LiTFSI 2.2×10−3 at 30℃ 5.1 [58] PEO/LLTO/LiTFSI 1.3×10−2 at 24℃ 3.8 [59] PVDF-HFP/LLTO/
LiTFSI1.2×10−4 at 25℃ 4.7 [60] PVDF/LLTO/LiClO4 5.8×10−4 at 25℃ 5.2 [61] -
[1] LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. DOI: 10.1038/nchem.2085
[2] YAGHOOBNEJAD H, MANTHIRAM A. Towards sustainable batteries[J]. Nature Sustainability, 2021, 5(4): 379-380.
[3] LI W, LIU J, ZHAO D. Mesoporous materials for energy conversion and storage devices[J]. Nature Reviews Materials, 2016, 1(6): 1-17.
[4] HOU J, LU L, WANG L, et al. Thermal runaway of lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes[J]. Nature Communications, 2020, 11(1): 5100. DOI: 10.1038/s41467-020-18868-w
[5] FENG X, REN D, HE X, et al. Mitigating thermal runaway of lithium-ion batteries[J]. Joule, 2020, 4(4): 743-770. DOI: 10.1016/j.joule.2020.02.010
[6] WANG J, YAMADA Y, SODETAMA K, et al. Fire-extinguishing organic electrolytes for safe batteries[J]. Nature Energy, 2018, 3(1): 22-29.
[7] 程俊, 黄蕊, 雷惊雷, 等. 电化学能源核心技术的关键科学问题[J]. 中国科学基金, 2020, 5(34): 350-357. DOI: 10.16262/j.cnki.1000-8217.2020.03.021 CHENG Jun, HUANG Rui, LEI Jinglei, et al. Key scientific questions in the core technologies of electrochemical energy[J]. National Natural Science Foundation of China, 2020, 5(34): 350-357. DOI: 10.16262/j.cnki.1000-8217.2020.03.021
[8] ZHAO Q, STALIN S, ZHAO C, et al. Designing solid-state electrolytes for safe, energy-dense batteries[J]. Nature Reviews Materials, 2020, 5(3): 229-252. DOI: 10.1038/s41578-019-0165-5
[9] GUO B, FU Y, WANG J, et al. Strategies and characterization methods for achieving high performance PEO-based solid-state lithium-ion batteries[J]. Chemical Communications, 2022, 58(59): 8182-8193. DOI: 10.1039/D2CC02306G
[10] SONG X, YU W, ZHOU S, et al. Enhancement of Mn-doped LiPON electrolyte for higher performance of all-solid-state thin film lithium battery[J]. Materials Today Physics, 2023, 33: 101037. DOI: 10.1016/j.mtphys.2023.101037
[11] TAO B, REN C, LI H, et al. Thio-/LISICON and LGPS-type solid electrolytes for all-solid-state lithium-ion batteries[J]. Advance Functional Materials, 2022, 32(34): 2203551. DOI: 10.1002/adfm.202203551
[12] ZHANG Y, HUANG J, SAITO N, et al. Layered perovskite lithium yttrium titanate as a low-potential and ultrahigh-rate anode for lithium-ion batteries[J]. Advanced Energy Materials, 2022, 12(31): 2200922. DOI: 10.1002/aenm.202200922
[13] WOO S, KANG B. Superior compatibilities of a LISICON-type oxide solid electrolyte enable high energy density all-solid-state batteries[J]. Journal of Material Chemistry A, 2022, 10(43): 23185-23194. DOI: 10.1039/D2TA05948G
[14] LI Y, LI M, SUN Z, et al. Recent advance on NASICON electrolyte in solid-state sodium metal batteries[J]. Energy Storage Materials, 2023, 56(58): 582-599.
[15] SUN F, YANG Y, ZHAO S, et al. Local Li+ framework regulation of a garnet-type solid-state electrolyte[J]. ACS Energy Letter, 2022, 7(8): 2835-2844. DOI: 10.1021/acsenergylett.2c01432
[16] ZHAO S, WU Q, MA W, et al. Polyethylene oxide-based composites as solid-state polymer electrolytes for lithium metal batteries: A mini review[J]. Frontiers in Chemistry, 2020, 8: 640. DOI: 10.3389/fchem.2020.00640
[17] LIANG J, CHEN D, ADAIR K, et al. Insight into prolonged cycling life of 4 V all-solid-state polymer batteries by a high-voltage stable binder[J]. Advanced Energy Materials, 2021, 11(1): 2002455. DOI: 10.1002/aenm.202002455
[18] HUANG Y, ZENG J, LI S, et al. Conformational regulation of dielectric poly(vinylidene fluoride)-based solid-state electrolytes for efficient lithium salt dissociation and lithium-ion transportation[J]. Advanced Energy Materials, 2023, 13(15): 2203888. DOI: 10.1002/aenm.202203888
[19] MI J, MA J, CHEN L, et al. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling high-voltage lithium metal solid-state batteries[J]. Energy Storage Materials, 2022, 48: 375-383. DOI: 10.1016/j.ensm.2022.02.048
[20] FU Y, YANG K, XUE S, et al. Surface defects reinforced polymer-ceramic interfacial anchoring for high-rate flexible solid-state batteries[J]. Advanced Functional Materials, 2023, 33(10): 2210845. DOI: 10.1002/adfm.202210845
[21] FU C, HOMANN G, GRISSA R, et al. A polymerized-ionic-liquid-based polymer electrolyte with high oxidative stability for 4 and 5 V class solid-state lithium metal batteries[J]. Advanced Energy Materials, 2022, 12(27): 2200412. DOI: 10.1002/aenm.202200412
[22] LIU Y, ZHAO Y, LU W, et al. PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries[J]. Nano Energy, 2021, 88: 106205. DOI: 10.1016/j.nanoen.2021.106205
[23] ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Review, 2020, 49(23): 8790-8839. DOI: 10.1039/D0CS00305K
[24] CHENG Z, LIU T, ZHAO B, et al. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries[J]. Energy Storage Materials, 2021, 34: 388-416. DOI: 10.1016/j.ensm.2020.09.016
[25] YU Q, JIANG K, YU C, et al. Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries[J]. Chinese Chemical Letters, 2021, 32(9): 2659-2678. DOI: 10.1016/j.cclet.2021.03.032
[26] LIU Y, HE P, ZHOU H. Rechargeable solid-sate Li-air and Li-S batteries: Materials, construction, and challenges[J]. Advanced Energy Materials, 2018, 8(4): 1701602. DOI: 10.1002/aenm.201701602
[27] KRAVCHYK K, ZHANG H, OKUR F, et al. Li-garnet solid-state batteries with LLZO scaffolds[J]. Account of Materials Research, 2022, 3(4): 411-415. DOI: 10.1021/accountsmr.2c00004
[28] ZHANG X, LIU T, ZHANG S, et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes[J]. Journal of the American Chemical Society, 2017, 139(39): 13779-13785. DOI: 10.1021/jacs.7b06364
[29] ZHAO Y, YAN J, CAI W, et al. Elastic and well-aligned ceramic LLZO nanofiber-based electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 23: 306-313. DOI: 10.1016/j.ensm.2019.04.043
[30] WU M, LIU D, QU D, et al. 3D coral-like LLZO/PVDF composite electrolytes with enhanced ionic conductivity and mechanical flexibility for solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(47): 52652-52659.
[31] ZHAO N, KHOKHAR W, BI Z, et al. Solid garnet batteries[J]. Joule, 2019, 3(5): 1190-1199. DOI: 10.1016/j.joule.2019.03.019
[32] ZHANG J, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. DOI: 10.1016/j.nanoen.2016.09.002
[33] LIU W, LIU N, SUN J, et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers[J]. Nano Letter, 2015, 15(4): 2740-2745. DOI: 10.1021/acs.nanolett.5b00600
[34] XI G, XIAO M, WANG S, et al. Polymer-based solid electrolytes: Material selection, design, and application[J]. Advanced Functional Materials, 2021, 31(9): 2007598. DOI: 10.1002/adfm.202007598
[35] ZENG Y, OUYANG B, LIU J, et al. High-entropy mechanism to boost ionic conductivity[J]. Science, 2022, 378(6626): 1320-1324. DOI: 10.1126/science.abq1346
[36] YANG K, CHEN L, MA J, et al. Progress and perspective of Li1+ x Al x Ti2− x (PO4)3 ceramic electrolyte in lithium batteries[J]. InfoMat, 2021, 3(11): 1195-1217. DOI: 10.1002/inf2.12222
[37] MA Q, ZENG X, YUE J, et al. Viscoelastic and nonflammable interface design-enabled dendrite-free and safe solid lithium metal batteries[J]. Advanced Energy Materials, 2019, 9(13): 1803854. DOI: 10.1002/aenm.201803854
[38] MOUSAVI T, SLATTERY I, JAGGER B, et al. Development of sputtered nitrogen-doped Li1+ x Al x Ge2− x (PO4)3 thin films for solid state batteries[J]. Solid State Ionics, 2021, 364: 115613. DOI: 10.1016/j.ssi.2021.115613
[39] GUO Q, HAN Y, WANG H, et al. Flame retardant and stable Li1.5Al0.5Ge1.5(PO4)3-supported ionic liquid gel polymer electrolytes for high safety rechargeable solid-state lithium metal batteries[J]. The Journal of Physical Chemistry C, 2018, 122(19): 10334-10342. DOI: 10.1021/acs.jpcc.8b02693
[40] YU X, MANTHIRAM A. A long cycle life, all-solid-state lithium battery with a ceramic-polymer composite electrolyte[J]. ACS Applied Energy Materials, 2020, 3(3): 2916-2924.
[41] ZHAO Y, HUANG Z, CHEN S, et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries[J]. Solid State Ionics, 2016, 295: 65-71.
[42] SHI X, MA N, WU Y, et al. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery[J]. Solid State Ionics, 2018, 325: 112-119. DOI: 10.1016/j.ssi.2018.08.010
[43] LI A, LIAO X, ZHANG H, et al. Nacre-inspired composite electrolytes for load-bearing solid-state lithium-metal batteries[J]. Advanced Materials, 2020, 32(2): 1905517. DOI: 10.1002/adma.201905517
[44] WANG X, ZHAI H, QIE B, et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte[J]. Nano Energy, 2019, 60: 205-212. DOI: 10.1016/j.nanoen.2019.03.051
[45] XIA Y, WANG X, XIA X, et al. A newly designed composite gel polymer electrolyte based on poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for enhanced solid-state lithium-sulfur batteries[J]. Chemistry European Journal, 2017, 23(60): 15203-15209. DOI: 10.1002/chem.201703464
[46] LI D, CHEN L, WANG T, et al. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(8): 7069-7078.
[47] LIANG J, ZENG X, ZHANG X, et al. Engineering janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries[J]. Journal of the American Chemical Society, 2019, 141(23): 9165-9169. DOI: 10.1021/jacs.9b03517
[48] YU X, LI J, MANTHIRAM A. Rational design of a laminated dual-polymer/polymer-ceramic composite electrolyte for high-voltage all-solid-state lithium batteries[J]. ACS Materials Letter, 2020, 2(4): 317-324. DOI: 10.1021/acsmaterialslett.9b00535
[49] MA F, ZHANG Z, YAN W, et al. Solid polymer electrolyte based on polymerized ionic liquid for high performance all-solid-state lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 4675-4683.
[50] DUAN H, FAN M, CHEN W, et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries[J]. Advanced Materials, 2019, 31(12): 1807789. DOI: 10.1002/adma.201807789
[51] ZHU P, YAN C, ZHANG X, et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2018, 6(10): 4279-4285. DOI: 10.1039/C7TA10517G
[52] LIU W, LEE S, LIN D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires[J]. Nature Energy, 2017, 2(5): 17035. DOI: 10.1038/nenergy.2017.35
[53] YANG T, ZHENG J, CHENG Q, Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: Mechanism of conductivity enhancement and role of doping and morphology[J]. ACS Applied Materials & Interfaces, 2017, 9(26): 21773-21780.
[54] BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte[J]. Angewandte Chemie International Edition, 2018, 57(8): 2096-2100. DOI: 10.1002/anie.201710841
[55] WANG X, ZHANG Y, ZHANG X, et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24791-24798.
[56] ZHU P, YAN C, ZHU J, et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries[J]. Energy Storage Materials, 2019, 17: 220-225. DOI: 10.1016/j.ensm.2018.11.009
[57] LI B, SU Q, YU L, et al. Li0.35La0.55TiO3 nanofibers enhanced poly(vinylidene fluoride)-based composite polymer electrolytes for all-solid-state batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(45): 42206-42213.
[58] BI J, MU D, WU B, et al. A hybrid solid electrolyte Li0.33La0.557TiO3/PAN membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries[J]. Journal of Materials Chemistry A, 2020, 8(17): 706-713.
[59] LIU K, WU M, WEI L, et al. A composite solid electrolyte with a framework of vertically aligned perovskite for all-solid-state Li-metal batteries[J]. Journal of Membrane Science, 2020, 610: 118265. DOI: 10.1016/j.memsci.2020.118265
[60] LI J, ZHU L, ZHANG J, et al. Approaching high performance PVDF-HFP based solid composite electrolytes with LLTO nanorods for solid-state lithium-ion batteries[J]. International Journal of Energy Research, 2021, 45(5): 7663-7674. DOI: 10.1002/er.6347
[61] LI B, SU Q, YU L, et al. Biomimetic PVDF/LLTO composite polymer electrolyte enables excellent interface contact and enhanced ionic conductivity[J]. Applied Surface Science, 2021, 541: 148434. DOI: 10.1016/j.apsusc.2020.148434
[62] WU J, LIU S, HAN F, et al. Lithium/sulfide all-solid-state batteries using sulfide electrolytes[J]. Advanced Materials, 2021, 33(6): 2000751. DOI: 10.1002/adma.202000751
[63] JANG S, TATEYAMA Y, JALEM R, et al. High-throughput data-driven prediction of stable high-performance Na-ion sulfide solid electrolytes[J]. Advanced Functional Materials, 2022, 32(48): 2206036. DOI: 10.1002/adfm.202206036
[64] PENG J, WANG X, LI H, et al. High-capacity, long-life iron fluoride all-solid-state lithium battery with sulfide solid electrolyte[J]. Advanced Energy Materials, 2023, 13(23): 2300706. DOI: 10.1002/aenm.202300706
[65] ZHAO Y, WU C, PENG G, et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries[J]. Journal of Power Sources, 2016, 301: 47-53. DOI: 10.1016/j.jpowsour.2015.09.111
[66] PAN K, ZHANG L, QIAN W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. DOI: 10.1002/adma.202000399
[67] LI D, CAO L, SHAO G, et al. A designer fast Li-ion conductor Li6.25PS5.25Cl0.75 and its contribution to the polyethylene oxide-based electrolyte[J]. Applied Surface Science, 2019, 493: 1326-1333. DOI: 10.1016/j.apsusc.2019.07.041
[68] ZHENG J, WANG P, LIU H, et al. Interface-enabled ion conduction in Li10GeP2S12-poly(ethylene oxide) hybrid electrolytes[J]. ACS Applied Energy Materials, 2019, 2(2): 1452-1459. DOI: 10.1021/acsaem.8b02008
[69] LIU S, LIU W, BA D, et al. Filler-integrated composite polymer electrolyte for solid-state lithium batteries[J]. Advanced Materials, 2023, 35(2): 2110423. DOI: 10.1002/adma.202110423
[70] ZHANG Z, WANG X, LI X, et al. Review on composite solid electrolytes for solid-state lithium-ion batteries[J]. Materials Today Sustainability, 2023, 21: 100316. DOI: 10.1016/j.mtsust.2023.100316
[71] FAN L, HE H, NAN C. Tailoring inorganic-polymer composites for the mass production of solid-state batteries[J]. Nature Reviews Materials, 2021, 6(11): 1003-1019. DOI: 10.1038/s41578-021-00320-0
[72] LIANG H, WANG A, SONG Y, et al. Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: A review[J]. Nano-Micro Letter, 2023, 15(1): 42. DOI: 10.1007/s40820-022-00996-1
[73] WESTON J E, STEELE B C H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes[J]. Solid State Ionics, 1982, 7(1): 75-79.
[74] DISSANAYAKE M, JAYATHILAKA P, BAKALAWALA R, et al. Effect of concentration and grain size of alumina filler on the ionic conductivity enhancement of the (PEO)9LiCF3SO3:Al2O3 composite polymer electrolyte[J]. Journal of Power Sources, 2003, 119: 409-414.
[75] CHUNG S, WANG Y, PERSI L, et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides[J]. Journal of Power Sources, 2001, 97: 644-648.
[76] CHOUDHURY S, STALIN S, DENG Y, et al. Soft colloidal glasses as solid-state electrolytes[J]. Chemistry of Materials, 2018, 30(17): 5996-6004. DOI: 10.1021/acs.chemmater.8b02227
[77] SHENG O, JIN C, LUO J, et al. Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance[J]. Nano Letter, 2018, 18(5): 3104-3112. DOI: 10.1021/acs.nanolett.8b00659
[78] ZHANG X, XIE J, SHI F, et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity[J]. Nano Letter, 2018, 18(6): 3829-3838. DOI: 10.1021/acs.nanolett.8b01111
[79] KIM S, CHART Y, NARAYANAN S, et al. Thin solid electrolyte separators for solid-state lithium-sulfur batteries[J]. Nano Letter, 2022, 22(24): 10176-10183. DOI: 10.1021/acs.nanolett.2c04216
[80] LI Q, LI H, XIA Q, et al. Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry[J]. Nature Materials, 2021, 20(1): 76-83. DOI: 10.1038/s41563-020-0756-y
[81] WANG S, QU C, WEN J, et al. Progress of transition metal sulfides used as lithium-ion battery anodes[J]. Materials Chemistry Frontiers, 2023, 7: 2779-2808. DOI: 10.1039/D2QM01200F
[82] HOU T, LIU B, SUN X, et al. Covalent coupling-stabilized transition-metal sulfide/carbon nanotube composites for lithium/sodium-ion batteries[J]. ACS Nano, 2021, 15(4): 6735-6746. DOI: 10.1021/acsnano.0c10121
[83] SUN L, LIU Y, SHAO R, et al. Recent progress and future perspective on practical silicon anode-based lithium-ion batteries[J]. Energy Storage Materials, 2022, 46: 482-502. DOI: 10.1016/j.ensm.2022.01.042
[84] YU S, GUO B, ZENG T, et al. Graphene-based lithium-ion battery anode materials manufactured by mechanochemical ball milling process: A review and perspective[J]. Composites Part B: Engineering, 2022, 246: 110232. DOI: 10.1016/j.compositesb.2022.110232
[85] ZHAO T, LIU C, MENG T, et al. Vacancy-clusters in-situ induced via microwave-irradiation enable high-durability and capacitor-level rate Li-ion storage[J]. Chemical Engineering Journal, 2023, 466: 143053. DOI: 10.1016/j.cej.2023.143053
[86] CHEN S, ZHANG J, NIE L, et al. All-solid-state batteries with a limited lithium metal anode at room temperature using a garnet-based electrolyte[J]. Advanced Material, 2021, 33(1): 2002325. DOI: 10.1002/adma.202002325
[87] WAN Z, LEI D, HE Y, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials, 2019, 29(1): 1805301. DOI: 10.1002/adfm.201805301
[88] SHALEV O, LEIFER N, ROSY D, et al. Molecular layer deposition of alucone thin film on LiCoO2 to enable high voltage operation[J]. Batteries & Supercaps, 2021, 4(11): 1-11.
[89] QIU J, LIU X, CHEN R, et al. Enabling stable cycling of 4.2 V high-voltage all-solid-state batteries with PEO-based solid electrolyte[J]. Advanced Functional Materials, 2020, 30(22): 1909392. DOI: 10.1002/adfm.201909392
[90] HUANG Y, LIN L, ZHANG C, et al. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li-S batteries[J]. Advanced Science, 2022, 9(12): 2106004. DOI: 10.1002/advs.202106004
[91] HAN Z, GAO R, JIA Y, et al. Catalytic effect in Li-S batteries: From band theory to practical application[J]. Materials Today, 2022, 57: 84-120. DOI: 10.1016/j.mattod.2022.05.017
[92] XING C, CHEN H, QIAN S, et al. Regulating liquid and solid-state electrolytes for solid-phase conversion in Li-S batteries[J]. Chem, 2022, 8(5): 1201-1230. DOI: 10.1016/j.chempr.2022.01.002
[93] WU J, YE T, WANG Y, et al. Understanding the catalytic kinetics of polysulfide redox reactions on transition metal compounds in Li-S batteries[J]. ACS Nano, 2022, 16(10): 15734-15759. DOI: 10.1021/acsnano.2c08581
[94] XIA J, HUA W, WANG L, et al. Boosting catalytic activity by seeding nanocatalysts onto interlayers to inhibit polysulfide shuttling in Li-S batteries[J]. Advanced Functional Materials, 2021, 31(26): 2101980. DOI: 10.1002/adfm.202101980
[95] TAO X, LIU Y, CUI Y, et al. Solid-state lithium-sulfur batteries operated at 37℃ with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer[J]. Nano Letter, 2017, 17(5): 2967-2972. DOI: 10.1021/acs.nanolett.7b00221
[96] HE P, ZHANG T, JIANG J, et al. Lithium-air batteries with hybrid electrolytes[J]. The Journal of Physical Chemistry Letters, 2016, 7(7): 1267-1280. DOI: 10.1021/acs.jpclett.6b00080
[97] AHMED G, AWAN Z, BUTT F, et al. The study of different redox mediators for competent Li-air batteries[J]. Journal of Power Sources, 2022, 538(538): 231379.
[98] PATHAK A, ADHIKARI P, CHOI W. Lithium-CO2 batteries and beyond[J]. Frontiers in Energy Research, 2023, 11: 1150737. DOI: 10.3389/fenrg.2023.1150737
[99] WANG H, WANG X, LI F, et al. Fundamental understanding and construction of solid-state Li-air batteries[J]. International Journal of Energy Research, 2022, 2(5): 2200005.
[100] KITAURA H, ZHOU H. Electrochemical performance of solid-state lithium-air batteries using carbon nanotube catalyst in the air electrode[J]. Advanced Energy Materials, 2012, 2(7): 889-894. DOI: 10.1002/aenm.201100789
[101] SONG S, QIN X, RUAN Y, et al. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte[J]. Journal of Power Sources, 2020, 461: 228146. DOI: 10.1016/j.jpowsour.2020.228146
-





 下载:
下载: