Pyrolysis kinetics of cabin panel materials for civil aircraft at low ambient pressure
-
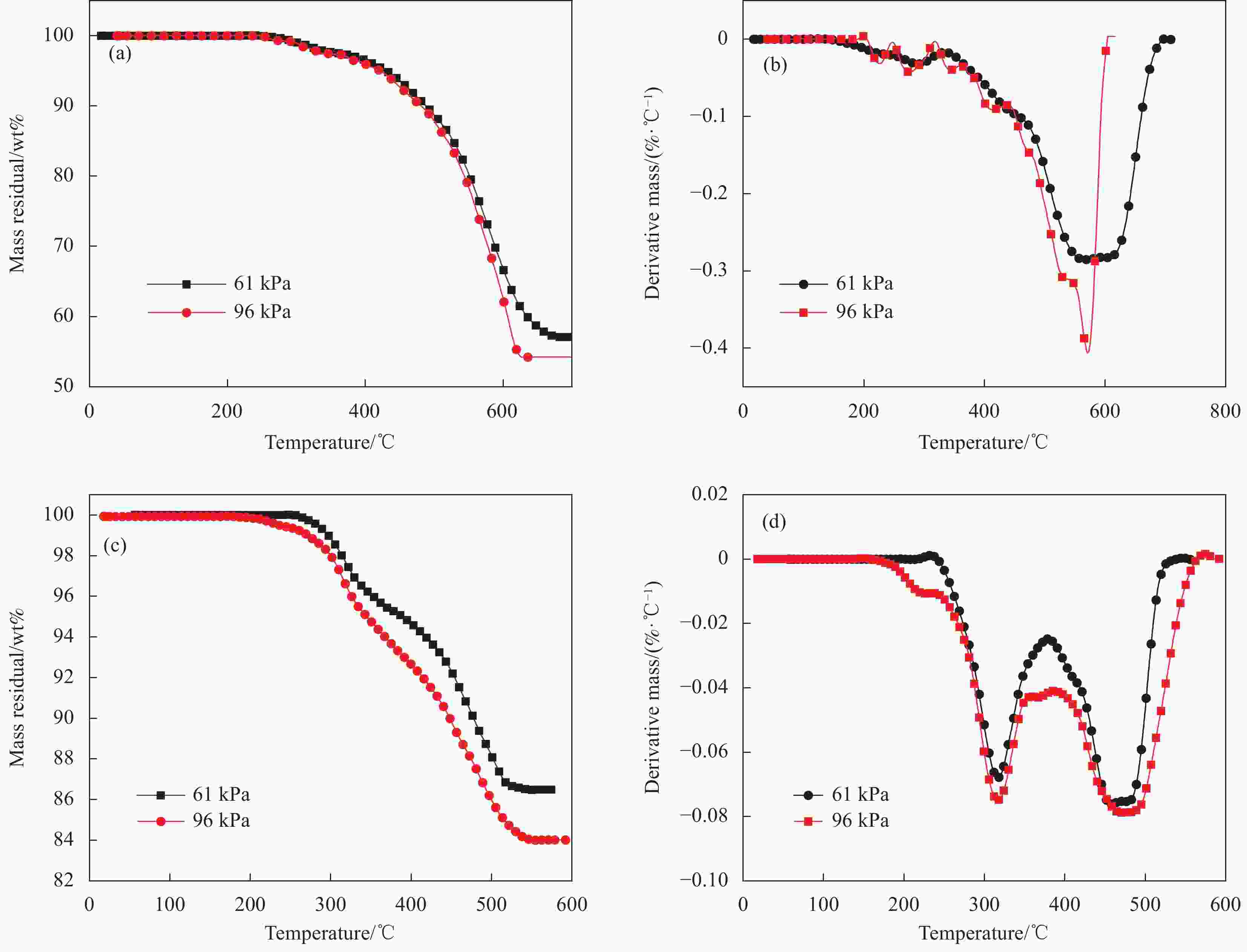
摘要: 航空运输环境为低气压环境,低气压会对火灾的发生发展产生重大影响。为了探究低环境压力下民航客机机舱壁板材料的热解特性,利用热重分析仪对其热解特性进行研究。选取空客某型飞机玻璃纤维/酚醛树脂夹层板结构壁板材料(A壁板)和玻璃纤维/酚醛树脂层压板结构壁板材料(B壁板)作为研究对象,分别在四川广汉(96 kPa)和四川康定(61 kPa)进行研究。结果表明:随着压力的降低和升温速率的升高,A壁板、B壁板热分解反应的初始反应温度、终止反应温度及最大质量损失速率温度均略向高温方向移动。在15℃/min升温速率下,A壁板的上下树脂基面板由两个热解阶段组成,芳纶蜂窝芯只有一个热解阶段,且树脂基面板的初始分解温度约182℃,明显小于芳纶蜂窝芯分解温度413℃;B壁板热解分为两个阶段,初始热解温度约258℃。采用Kissinger法、Flynn-Wall-Ozawa (FWO)法、Starink法和KAS法进行热解动力学分析,FWO法、Starink法和KAS法得到的表观活化能相近,低压下A壁板、B壁板表观活化能相对于常压下分别提高了大约10.4%和28.5%。而且96 kPa环境下A壁板和B壁板的化学反应速率大约是61 kPa环境下的1.9倍和1.2倍。Abstract: The air transport environment is a low pressure environment, which will have a significant impact on the occurrence and development of fire. In order to explore the pyrolysis characteristics of civil aircraft cabin panel materials under low environmental pressure, the pyrolysis characteristics of civil aircraft cabin panels were studied by the thermogravimetric analyzer. Selecting the glass fiber/phenolic resin sandwich panel structure panel material (A panel) and the glass fiber/phenolic resin laminated panel structure panel material (B panel) of a certain type of Airbus aircraft as the research objects, and studied in Guanghan (96 kPa) and Kangding (61 kPa) of Sichuan province, respectively. The results show that the initial reaction temperature, termination temperature and maximum mass loss rate temperature of thermal decomposition of A and B panels move slightly to high temperature with the decrease of pressure and the increase of heating rate. At the heating rate of 15℃/min, the upper and lower resin base panel of A panel consists of two pyrolysis stages, and there is only one pyrolysis stage of aramid honeycomb core, and the initial decomposition temperature of the resin base panel is about 182℃, which is obviously lower than that of the aramid honeycomb core, while the pyrolysis temperature of B panel is divided into two stages and the initial pyrolysis temperature is about 258℃. The pyrolysis kinetics was analyzed by the Kissinger method, the Flynn-Wall-Ozawa method, the Starink method and the KAS method. The apparent activation energy obtained by the Flynn-Wall-Ozawa method, the Starink method and the KAS method is similar, and the apparent activation energy of A and B panels under low pressure increase by approximately 10.4% and 28.5% relative to that under normal pressure, respectively. And the chemical reaction rates of A panel and B panel in 96 kPa environment are about 1.9 and 1.2 times higher than that in 61 kPa environment.
-
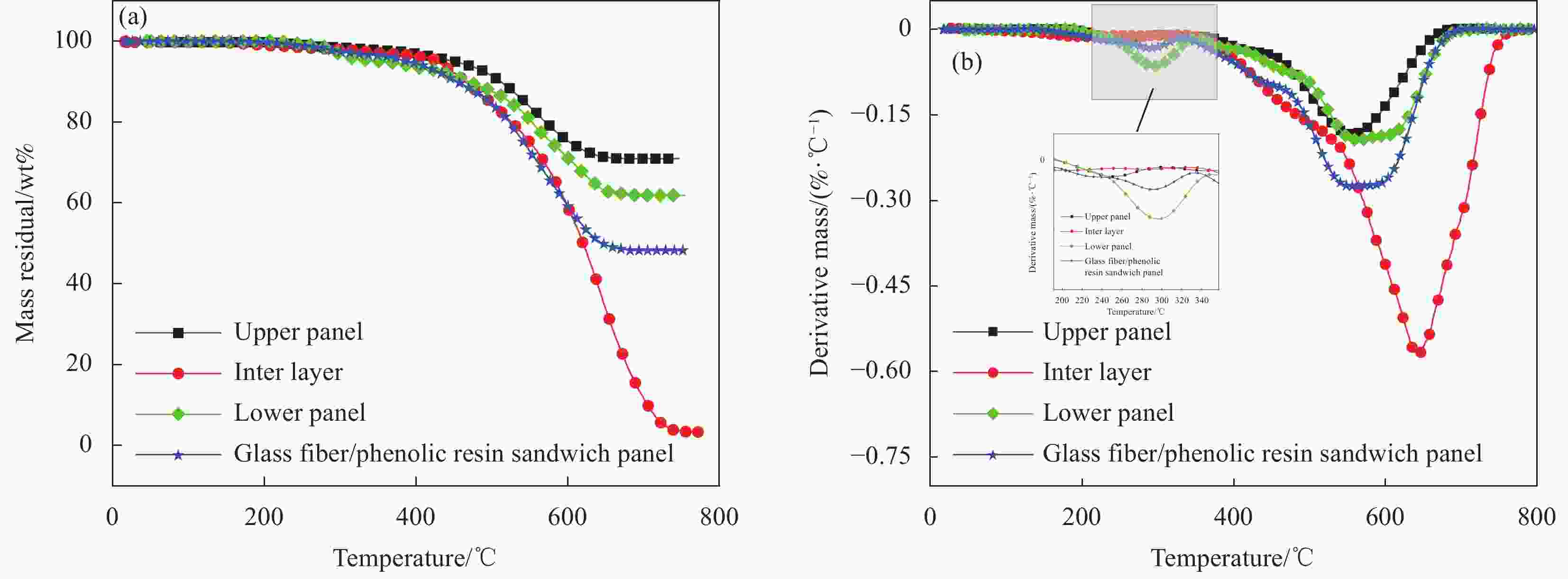
表 1 玻璃纤维/酚醛树脂夹层板和层压板热解参数
Table 1. Pyrolysis parameters of the glass fiber/phenolic resin sandwich panels and laminated panels
Material Temperature scope of thermal
decomposition/℃Temperature of maximum
mass loss rate/℃First stage Second stage First stage Second stage Upper panel 195-340 340-655 247 550 Aramid honeycomb core 413-768 — 612 — Lower panel 183-350 350-702 295 567 Glass fiber/phenolic resin sandwich panel 182-331 331-760 295 578 Glass fiber/phenolic resin laminated panel 258-450 450-616 365 528 表 2 不同压力环境下玻璃纤维/酚醛树脂夹层板和层压板的热解参数
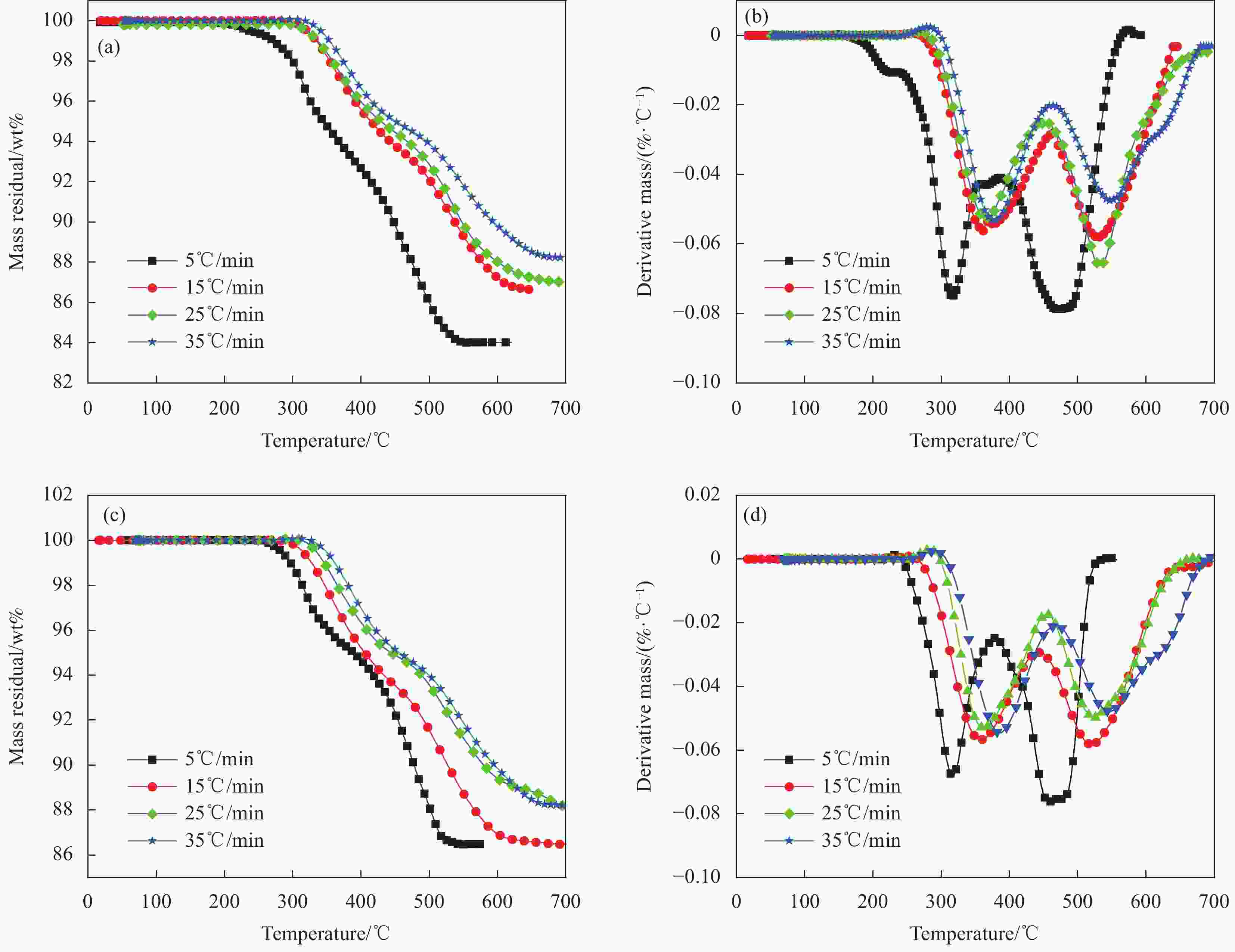
Table 2. Pyrolysis parameters of the glass fiber/phenolic resin sandwich panel and laminated panel under different pressure environments
Material Pressure/
kPaHeating rate/
(℃·min−1)Temperature scope of thermal
decomposition/℃Temperature of maximum mass
loss rate/℃First stage Second stage Third stage First stage Second stage Third stage
Glass fiber/
phenolic resin
sandwich panel96 5
15
25
35153-221
181-245
184-256
—221-304
245-314
256-372
262-382304-580
314-610
372-718
382-763187
225
235
—258
280
304
320522
572
634
67761 5
15
25
35157-225
182-331
291-378
—225-320
—
—
—320-657
331-706
378-731
368-738199
—
—
—256
295
334
—535
578
633
639
Glass fiber/
phenolic resin
laminated panel96 5
15
25
35167-374
215-457
272-457
282-461374-581
457-612
457-676
461-686—
—
—
—317
361
363
377475
527
531
547—
—
—
—61 5
15
25
35231-380
258-450
281-459
291-471380-545
450-616
459-668
471-694—
—
—
—321
365
368
393481
528
532
551—
—
—
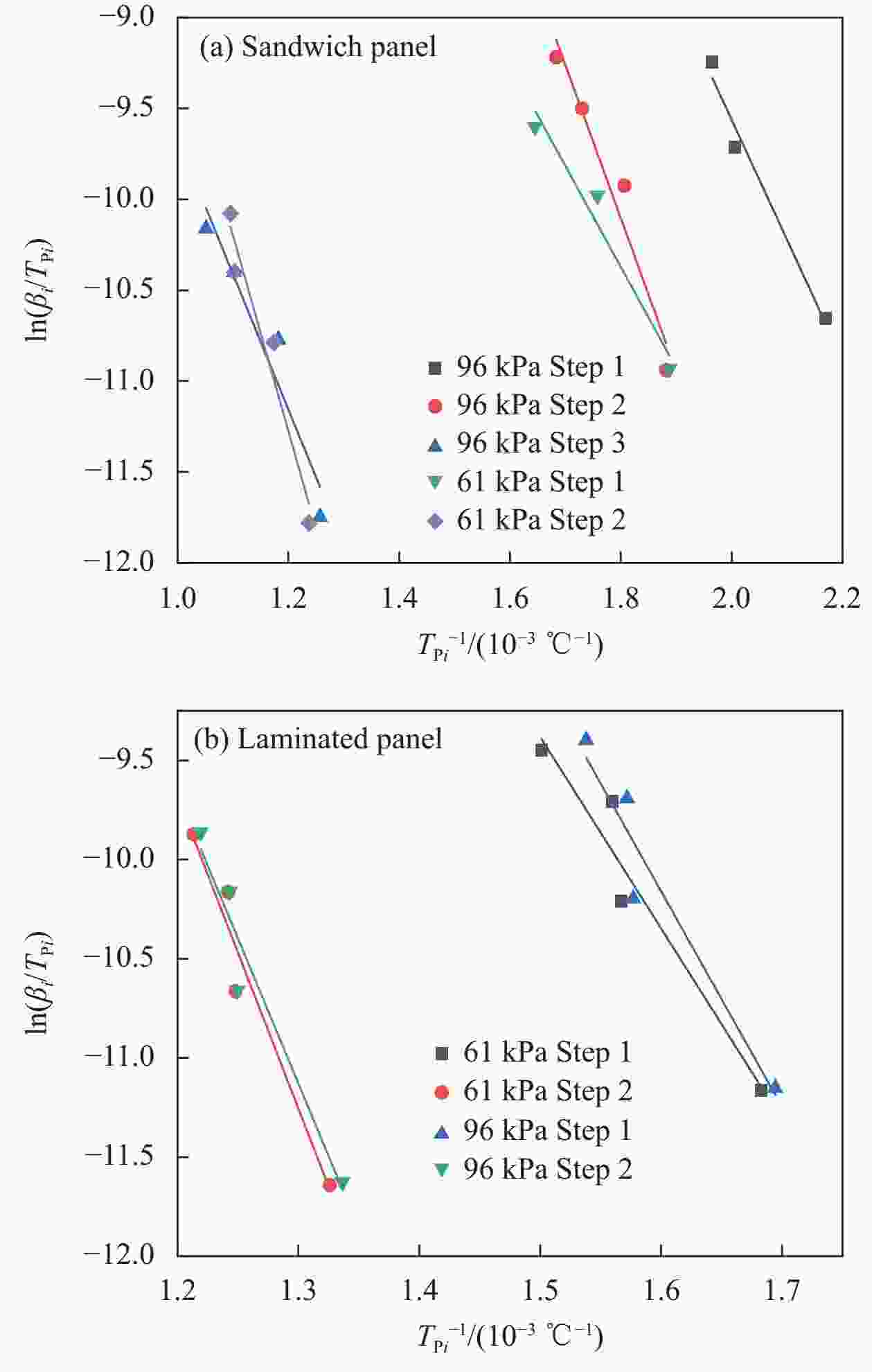
—表 3 Kissinger法计算的动力学参数
Table 3. Kinetic parameters calculated by Kissinger method
Material Pressure/
kPaSlope k=E/R E/
(kJ·mol−1)lnA Glass fiber/phenolic resin sandwich panel 96 6.6
8.4
7.454.9
70.0
61.95.5
7.2
−0.2Ave 62.3 61 5.6
10.846.2
89.51.3
4.0Ave 67.9
Glass fiber/phenolic resin laminated panel96 10.9
14.689.8
121.49.6
11.9Ave 105.6 61 9.7
15.780.6
130.57.4
10.6Ave 105.6 Notes: $k$—Slope of the curve fitted to Fig.5; E—Activation energy; R—Molar gas constants; A—Apparent pre-exponential factor; Ave—Average of E. 表 4 Flynn-Wall-Ozawa、Starink和KAS方法所求表观活化能
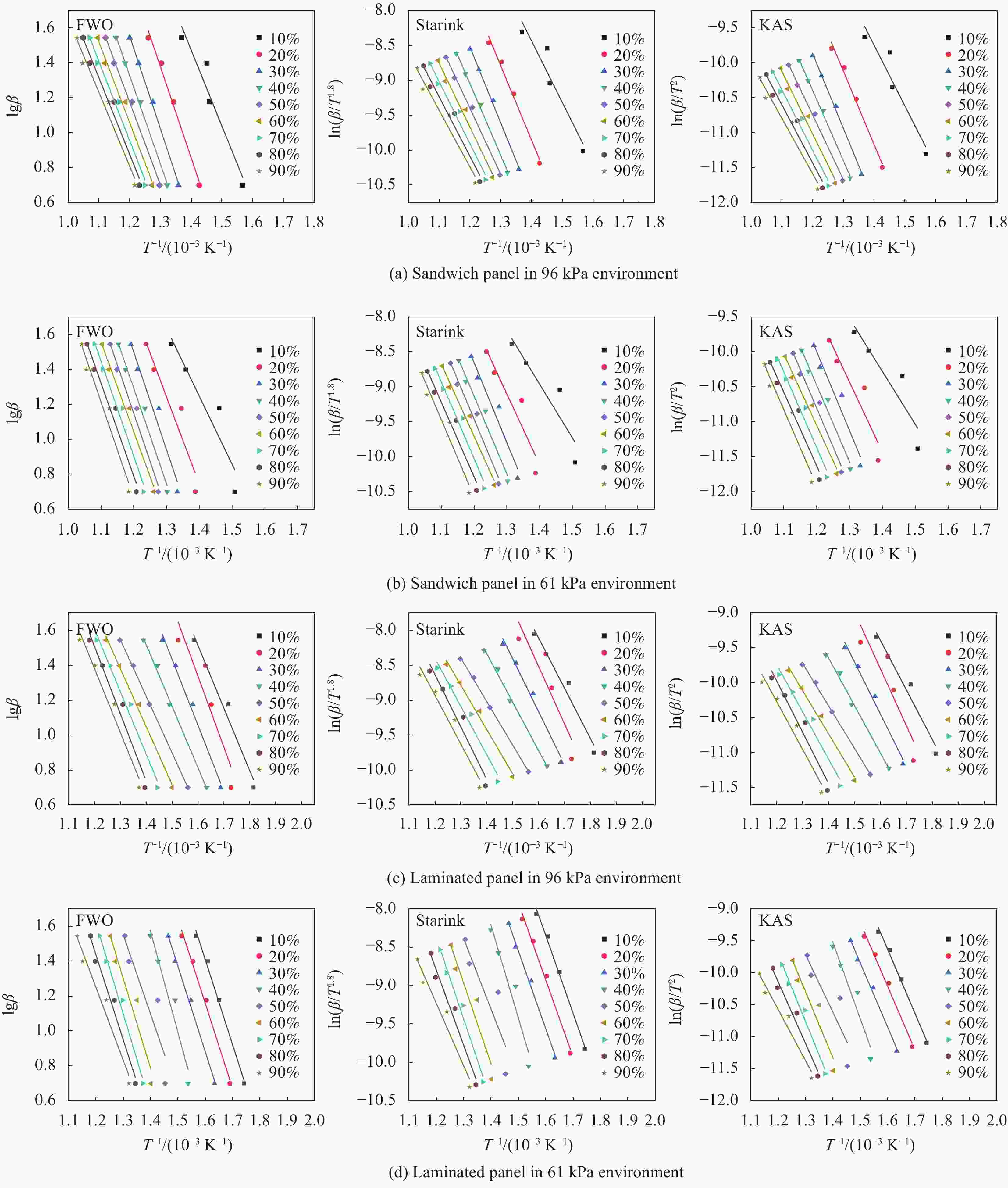
Table 4. Apparent activation energy obtained by Flynn-Wall-Ozawa, Starink and KAS methods
α/% FWO Starink KAS Glass fiber/phenolic resin sandwich
panelGlass fiber/phenolic resin laminated
panelGlass fiber/phenolic resin sandwich
panelGlass fiber/phenolic resin laminated
panelGlass fiber/phenolic resin sandwich
panelGlass fiber/phenolic resin laminated
panel96 kPa 61 kPa 96 kPa 61 kPa 96 kPa 61 kPa 96 kPa 61 kPa 96 kPa 61 kPa 96 kPa 61 kPa 10 79.3 70.9 65.3 88.6 73.0 63.7 59.6 83.9 72.1 62.8 58.9 83.1 20 94.9 91.0 74.0 88.4 88.3 83.9 68.3 83.3 87.4 83.0 67.5 82.6 30 96.9 101.9 69.0 89.3 89.9 94.9 62.7 83.9 89.0 94.0 61.9 83.1 40 92.4 99.5 61.1 104.9 84.8 92.1 57.3 99.8 83.8 91.1 56.5 99.0 50 86.4 100.8 59.0 82.7 78.2 93.1 51.4 75.8 77.2 92.0 50.5 74.8 60 84.2 93.9 60.4 93.6 75.6 85.7 52.4 86.8 74.5 84.7 51.4 85.9 70 82.3 99.2 65.4 95.1 70.8 91.0 57.2 88.0 72.2 90.0 56.2 87.1 80 79.8 97.1 70.3 88.5 70.5 88.6 62.0 80.9 69.3 87.5 60.9 79.9 90 76.9 100.0 66.7 75.9 67.2 91.4 58.0 67.4 66.0 90.2 56.9 66.3 Ave 85.9 94.9 66.0 89.7 77.6 87.2 58.8 83.3 76.8 86.1 57.9 82.4 Note:α—Conversion. -
[1] Federal Aviation Administration. Department of transportation federal aviation administration regulations with fire: FAR 25.855[S]. Washington: Federal Aviation Administration, 2014. [2] 中国民用航空局政策法规司. 中国民用航空规章 第25部 运输类飞机适航标准: CCAR 25-R4[S]. 北京: 中国民用航空局, 2011.Department of Policies and Regulations, Civil Aviation Administration of China. Civil aviation regulations of China: Part 25: Airworthiness standards for transport aircraft: CCAR 25-R4[S]. Beijing: Civil Aviation Administration of China, 2011(in Chinese). [3] QUANG D D, LUCHE J, RICHARD F, et al. Determination of characteristic parameters for the thermal decomposition of epoxy resin/carbon fibre composites in cone calorimeter[J]. International Journal of Hydrogen Energy, 2013, 38(19): 8167-8178. [4] EIBL S. Influence of carbon fibre orientation on reaction-to-fire properties of polymer matrix composites[J]. Fire and Materials, 2012, 36(4): 309-324. doi: 10.1002/fam.1112 [5] RÉGNIER N, FONTAINE S. Determination of the thermal degradation kinetic parameters of carbon fibre reinforced epoxy using TG[J]. Journal of Thermal Analysis and Calorimetry, 2001, 64(2): 789-799. [6] 张颖, 王志, 徐艳英, 等. 高强玻璃纤维复合材料热解动力学研究[J]. 消防科学与技术, 2017, 36(2): 149-152.ZHANG Ying, WANG Zhi, XU Yanying, et al. Study on pyrolysis kinetics of high-strength glass fiber/epoxy resin composites[J]. Fire Science and Technology, 2017, 36(2): 149-152(in Chinese). [7] 朱倩, 刘全义, 马凯庆, 等. ABS材料的热解燃烧特性研究[J]. 塑料科技, 2022, 50(10): 17-22.ZHU Qian, LIU Quanyi, MA Kaiqing, et al. Study on pyrolytic and combustion characteristics of ABS materials[J]. Plastics Science and Technology, 2022, 50(10): 17-22(in Chinese). [8] 杨扬, 徐艳英, 张旭, 等. 不同铺层方式碳纤维层合板热解动力学研究[J]. 消防科学与技术, 2018, 37(2): 167-171. doi: 10.3969/j.issn.1009-0029.2018.02.006YANG Yang, XU Yanying, ZHANG Xu, et al. Thermogravimetric analysis on the carbon fiber laminates with different layer structure[J]. Fire Science and Technology, 2018, 37(2): 167-171(in Chinese). doi: 10.3969/j.issn.1009-0029.2018.02.006 [9] 刘全义, 马凯庆, 朱倩, 等. 民航客机货舱侧壁板材料的热解特性[J]. 塑料工业, 2023, 51(1): 108-112, 136. doi: 10.3969/j.issn.1005-5770.2023.01.018LIU Quanyi, MA Kaiqing, ZHU Qian, et al. Study of pyrolysis characteristics of cargo hold wallpanels materials for civil aviation passenger aircraft[J]. China Plastics Industry, 2023, 51(1): 108-112, 136(in Chinese). doi: 10.3969/j.issn.1005-5770.2023.01.018 [10] KIM Y S, KIM Y S, KIM K M, et al. Thermal decomposition kinetics of polymeric wastes using a nonisothermal thermogravimetric method[J]. Journal of Industrial and Engineering Chemistry, 2003, 9(3): 219-224. [11] DEMIRBAS A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons[J]. Journal of Analytical and Applied Pyrolysis, 2004, 72(1): 97-102. doi: 10.1016/j.jaap.2004.03.001 [12] SYGUŁA E, ŚWIECHOWSKI K, HEJNA M, et al. Municipal solid waste thermal analysis—Pyrolysis kinetics and decomposition reactions[J]. Energies, 2021, 14(15): 4510. doi: 10.3390/en14154510 [13] DUBDUB I, AL-YAARI M. Thermal behavior of mixed plastics at different heating rates: I. Pyrolysis kinetics[J]. Polymers, 2021, 13(19): 3413. doi: 10.3390/polym13193413 [14] PATNAIK S, KUMAR S, PANDA A K. Thermal degradation of eco-friendly alternative plastics: Kinetics and thermodynamics analysis[J]. Environmental Science and Pollution Research, 2020, 27(13): 14991-15000. doi: 10.1007/s11356-020-07919-w [15] SINGH S, PRASAD CHAKRABORTY J, KUMAR MONDAL M. Intrinsic kinetics, thermodynamic parameters and reaction mechanism of non-isothermal degradation of torrefied Acacia nilotica using isoconversional methods[J]. Fuel, 2020, 259: 116263. doi: 10.1016/j.fuel.2019.116263 [16] 徐圣. 含磷双金属氢氧化物材料的制备及其阻燃聚丙烯性能[D]. 湘潭: 湘潭大学, 2016.XU Sheng. Preparation of phosphorus layered double hydroxides and their flame resistant property in polypropylene[D]. Xiangtan: Xiangtan University, 2016(in Chinese). [17] 池铁, 齐会民, 黄发荣, 等. 含硅芳炔树脂的热氧化降解动力学研究[J]. 复合材料科学与工程, 2013(230): 34-38, 12. doi: 10.3969/j.issn.1003-0999.2013.02.007CHI Tie, QI Huimin, HUANG Farong, et al. Thermal oxiadative degradation kinetics of silicon-arylacetylene-containing resin[J]. Composites Science and Engineering, 2013(230): 34-38, 12(in Chinese). doi: 10.3969/j.issn.1003-0999.2013.02.007 [18] 胡炳涛, 李志健. 基于TG-FT-IR的关中麦秆热解特性及动力学研究[J]. 生物质化学工程, 2016, 50(2): 6-12. doi: 10.3969/j.issn.1673-5854.2016.02.002HU Bingtao, LI Zhijian. Pyrolysis characteristics and kinetics of Guanzhong wheat straw using TG-FT-IR[J]. Biomass Chemical Engineering, 2016, 50(2): 6-12(in Chinese). doi: 10.3969/j.issn.1673-5854.2016.02.002 [19] 段一航, 高宁博, 全翠. 水热处理对含油污泥热解特性及动力学影响[J]. 化工进展, 2023, 42(2): 603-613. doi: 10.16085/j.issn.1000-6613.2022-1208DUAN Yihang, GAO Ningbo, QUAN Cui. Effect of hydrothermal treatment on pyrolysis characteristics and kinetics of oily sludge[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 603-613(in Chinese). doi: 10.16085/j.issn.1000-6613.2022-1208 [20] MOSQUERA M E G, JAMOND M, MARTINEZ-ALONSO A, et al. Thermal transformations of Kevlar aramid fibers during pyrolysis: Infrared and thermal analysis studies[J]. Chemistry of Materials, 1994, 6(11): 1918-1924. doi: 10.1021/cm00047a006 [21] ZHENG F J, REN Z Y, XU B, et al. Elucidating multiple-scale reaction behaviors of phenolic resin pyrolysis via TG-FTIR and Reax FF molecular dynamics simulations[J]. Journal of Analytical and Applied Pyrolysis, 2021, 157: 105222. doi: 10.1016/j.jaap.2021.105222 [22] 张美云, 王茹楠, 陆赵情, 等. 对位芳纶纸热稳定性及其热分解动力学研究[J]. 中国造纸, 2016, 35(5): 6-10.ZHANG Meiyun, WANG Runan, LU Zhaoqing, et al. Study on the thermal stability and thermal decomposition kinetics of para-aramid paper based composite[J]. China Pulp & Paper, 2016, 35(5): 6-10(in Chinese). [23] WANG X W, HU Z M, LIU Z F. Thermal degradation of meta- and para-aramid fibers in different atmospheres[J]. International Polymer Processing, 2008, 23(1): 81-87. [24] KISSINGER H E. Reaction kinetics in the differential thermal analysis[J]. Analytical Chemistry, 1957, 29(11): 1702-1706. doi: 10.1021/ac60131a045 [25] 胡荣祖, 高胜利, 赵凤起, 等. 热分析动力学[M]. 北京: 科学出版社, 2008.HU Rongzu, GAO Shengli, ZHAO Fengqi, et al. Thermal analysis kinetics[M]. Beijing: Science Press, 2008(in Chinese). [26] 骆强, 曲芳, 姚志鹏, 等. 典型航空电缆的热解动力学研究[J]. 华南师范大学学报(自然科学版), 2021, 53(5): 30-36.LUO Qiang, QU Fang, YAO Zhipeng, et al. Research on pyrolysis kinetics of typical aviation cable[J]. Journal of South China Normal University (Natural Science Edition), 2021, 53(5): 30-36(in Chinese). [27] 徐艳英, 张颖, 王志, 等. 典型碳纤维编织布的热解动力学[J]. 材料研究学报, 2017, 31(1): 57-64. doi: 10.11901/1005.3093.2016.158XU Yanying, ZHANG Ying, WANG Zhi, et al. Study on pyrolysis kinetics of typical carbon fiber bidirectional sheet[J]. Chinese Journal of Materials Research, 2017, 31(1): 57-64(in Chinese). doi: 10.11901/1005.3093.2016.158 [28] CHEN R Y, XU X K, LU S X, et al. Pyrolysis study of waste phenolic fiber-reinforced plastic by thermogravimetry/Fourier transform infrared/mass spectrometry analysis[J]. Energy Conversion and Management, 2018, 165: 555-566. [29] 吕雪. HMMM交联剂与两种树脂复配固化成膜条件及性能研究[D]. 重庆: 重庆大学, 2021.LYU Xue. Study on curing conditions and performance of film formed by cross linking agent HMMM with two resins[D]. Chongqing: Chongqing University, 2021(in Chinese). [30] 李少能, 张蔚男, 吴嵘泰, 等. 生物质理化特性与热解动力学参数相关性研究[J]. 广州化工, 2022, 50(22): 138-142 doi: 10.3969/j.issn.1001-9677.2022.22.042LI Shaoneng, ZHANG Weinan, WU Rongtai, et al. Study on correlation between physicochemical properties and kinetic parameters of biomass pyrolysis[J]. Guangzhou Chemical Industry, 2022, 50(22): 138-142(in Chinese). doi: 10.3969/j.issn.1001-9677.2022.22.042 [31] XING X, NIU X, LIU Y, et al. In-depth understanding on the early stage of phenolic resin thermal pyrolysis through ReaxFF-molecular dynamics simulation[J]. Polymer Degradation and Stability, 2021, 186: 109534. [32] 陈华, 沈哲炎, 黄在青, 等. 阿伦尼乌斯方程在全钢载重子午线轮胎硫化计算中的应用研究[J]. 橡胶工业, 2021, 68(6): 409-414. doi: 10.12136/j.issn.1000-890X.2021.06.0409CHEN Hua, SHEN Zheyan, HUANG Zaiqing, et al. Study on application of arrhenius equation in vulcanization calculation of TBR tire[J]. China Rubber Industry, 2021, 68(6): 409-414(in Chinese). doi: 10.12136/j.issn.1000-890X.2021.06.0409 [33] SAHA D, SINHA A, PATTANAYAK S, et al. Pyrolysis kinetics and thermodynamic parameters of plastic grocery bag based on thermogravimetric data using iso-conversional methods[J]. International Journal of Environmental Science and Technology, 2022, 19(1): 391-406. doi: 10.1007/s13762-020-03106-z -





 下载:
下载: