Photodeposition Pt composite graphitic carbon nitride realizes efficient photocatalytic hydrogen production
-
摘要: 贵金属作为助催化剂,可以提高石墨相氮化碳(g-C3N4)光催化产氢的性能,引起了人们的广泛关注。但是,由于贵金属的不可再生性和高价格,“更少的贵金属,更好的性能”始终是目标。为了实现这一目标,通过光沉积还原法成功制备了一系列不同铂负载量氮化碳复合材料(Pt/CN),并用于光催化产H2。结果表明:不同Pt负载量的Pt/CN复合材料都表现出优异的光催化产氢性能。并发现当Pt的负载量为0.5wt%时, Pt/CN复合材料具有最优异的光催化产氢活性,产氢量为409.2 μmol/g,是纯CN (17.8 μmol/g)的23倍,同时证实了Pt和CN二者之间形成了肖特基势垒,使导带的电子快速迁移到Pt上,降低了CN的电子-空穴复合速率。并且Pt作为光催化分解水的活性位点,促进水中的绝大部分氢质子快速吸附到Pt位点,得到电子被还原为H2,实现了高效光催化产氢。Abstract: Noble-metal, as co-catalysts, can improve the photocatalytic hydrogen production performance of graphitic carbon nitride (g-C3N4). It has been paid extensive attention, however, due to the non-renewability and high expense of noble-metal, "less noble-metal, better performance" is always the goal. To achieve this goal, composite CN with different Pt loadings (Pt/CN) was successfully prepared by photoreduction deposition and used for photocatalytic hydrogen production. The results show that Pt/CN with different Pt loadings can improved photocatalytic hydrogen production performance than CN. It is found that Pt/CN loaded with 0.5wt%Pt have the best photocatalytic hydrogen production activity, with a hydrogen production rate of 409.2 μmol/g, which is 23 times higher than that of CN (17.8 μmol/g). At the same time, it is confirmed that a Schottky barrier is formed between Pt and CN, which makes the electrons of the conduction band migrate rapidly to Pt, which reduces the electron-hole recombination rate of CN. Moreover, Pt is used as the active site of photocatalytic water splitting, which promotes the rapid adsorption of most of the hydrogen protons in the water to the Pt site, and the electrons are reduced to hydrogen, realizing efficient photocatalytic hydrogen production.
-
Keywords:
- graphitic carbon nitride /
- photocatalytic hydrogen production /
- Pt /
- photodeposition /
- compound rad
-
氢能源是一种安全、清洁、可再生的能源,其作为化石燃料的替代能源而备受关注。光催化已被确定为将太阳能转化为氢能的最有前途的战略之一。石墨相氮化碳(g-C3N4,CN)由于其带隙合适(2.7 eV) [1]、化学稳定性高、无毒性和持久性等优点,在可见光光催化水分解和有机污染物降解方面得到了广泛的关注。
但纯CN表现出很高的光诱导电子与空穴的复合率、催化剂表面活性位点少等缺陷。目前常用到的改性方法有很多,如形貌控制、元素掺杂、缺陷工程、金属掺杂、半导体复合等[2-7],可有效弥补CN的不足。例如,Wu等[8]通过化学气相沉积法合成大面积石墨氮化碳纳米片;Ding等[9]通过在B掺杂氮化碳中原位形成纳米1T相MoS2,实现高效的可见光驱动H2生产;Jiang等[10]使用掺Ga氮化碳,实现了高效产氢。由于其性能可以通过改性得到极大的提高,因此这方面的探索从未停止。
众所周知,光催化剂负载贵金属具有优异的产氢性能。这得益于贵金属良好的稳定性和选择性,贵金属可从以下两个方面起作用,促进光生电荷的分离、作为反应位点。例如,Liu等[11]在石墨氮化碳上单分散Pt纳米颗粒在水分解中具有最高的光催化活性,产氢速率为2.02 mmol·g−1·h−1。Peng等[12]使用钌离子络合石墨相氮化碳纳米片来获得更好的性能。但是,由于贵金属的不可再生性和高昂的价格[13],很难大规模使用。因此,我们需要提高贵金属的利用率。
本文采用光沉积还原法成功制备了一系列不同铂负载量氮化碳复合材料(Pt/CN)[14-18]。通过XRD、TEM、PL、EIS 等测试发现,负载Pt可以提高光催化产氢性能,探索了Pt的最佳负载量,并对其过程有了更深入的了解。
1. 实验部分
1.1 原材料
实验所用试剂均为分析纯,无需进一步纯化。所有实验均使用去离子水。尿素、氯化铵、硫酸钠、无水乙醇、三乙醇胺、溴化钾,天津市永大化学试剂有限公司;三聚氰胺、氯铂酸、全氟化树脂Nafion溶液,上海麦克林生化科技有限公司。
1.2 CN和Pt/CN的制备
图1为CN和Pt/CN的制备过程。如图1(a)所示,以尿素、三聚氰胺和氯化铵作为前驱体,通过水热与热缩聚合两步法制备了CN材料。将前驱体按照一定计量比混合溶解在去离子水中,超声30 min形成均匀的溶液。再将混合均匀的溶液置于反应釜中,180℃保温24 h,待冷却至室温时,将混合物抽滤干燥,得到干燥的固体粉末。将固体粉末转移至带盖的氧化铝坩埚中,并在马弗炉(KJ-M1200-IC,郑州科佳电炉有限公司)内550℃煅烧2 h,得到石墨相氮化碳(g-C3N4)固体粉末,记为CN。
采用光还原法制备Pt复合的CN材料,具体实验工艺如图1(b)所示:将CN粉末分散在去离子水中,用超声波粉碎机(JV920-IIN,宁波新芝生物科技股份有限公司)超声30 min,得到均匀的CN悬浮溶液。将H2PtCl6·6H2O水溶液滴加到上述悬浮液中,在氙灯光源(300 W 氙灯,北京泊菲莱科技有限公司)下照射沉积1 h后,冷冻干燥12 h。最终产物被命名为wPt/CN (w=0.1wt%、0.3wt%、0.5wt%、0.7wt%、0.9wt%,其中w代表样品中Pt的理论质量分数)。其他条件不变,将CN悬浮液进行冷冻干燥,与之形成对照组,研究样品的光催化产H2性能。
1.3 催化剂的表征
通过铜Kα辐射(波长λ=0.15403 nm)的X射线衍射仪(XRD,D/MAX2500 PC,日本理学株式会社)测试了样品的晶体结构。由傅里叶变换红外光谱仪(FTIR,VERTEX70,德国布鲁克公司)观察材料表面的官能团。使用透射电子显微镜(TEM,JEM-2800,日本电子株式会社)观察样品形貌及元素分布。通过比表面积测试(BET,ASAP 2460,美国麦克仪器公司)测量孔的大小和数量。
1.4 光电化学测试
通过紫外-可见漫反射光谱仪(UV-vis DRS,Lambda750,美PerkinElmer公司)测试样品对光的吸收性能,扫描范围为200~800 nm。光致发光光谱仪(PL,F-7000 FL,日本株式会社日立制作所)用激发波长为357 nm的分光光度计对样品进行了测量。
电化学测试采用电化学工作站(CHI660C,上海晨华仪器有限公司)以常规三电极实验系统进行。Ag/AgCl和Pt分别用作参比电极和对电极。氟掺杂氧化锡(F-doped tin oxide,FTO)玻璃作为工作电极,0.5 mol/L Na2SO4水溶液作为电解质,300 W Xe灯(滤光片λ>420 nm)作为可见光源。工作电极的制备如下:将光催化剂与Nafion溶液和N, N二甲基甲酰胺按一定比例混合,形成均匀的溶液。再涂在FTO玻璃上,再将获得的电极在干燥箱(DH6-907385-III,天津市实验仪器厂)中60℃加热1 h至干燥。
1.5 光催化析氢测试
光催化析氢反应在封闭的气密循环体系中进行。采用300 W Xe灯和420 nm截止滤光片作为可见光激发源,模拟自然光照过程。通过水分解生成H2来评价样品的光催化性能。对于每个反应,将50 mg光催化剂均匀分散在含有去离子水和三乙醇胺(体积比为9∶1)的100 mL混合溶剂中。反应前,将整个反应体系用氮气抽真空数次,确保反应液和体系中的空气排出,整个实验过程中温度保持在5℃以下。在光催化反应过程中,每隔1 h收集排出的气体,并用N2作为载气通过气相色谱法(GC9790II (PLF-01),浙江福立分析仪器有限公司)进行分析。
2. 结果与讨论
2.1 Pt负载量对CN结构形貌的影响
为研究不同负载量下Pt/CN样品的物相组成和官能团类别,分别进行了XRD和FTIR测试,如图2所示。图2(a)为不同Pt负载量下CN光催化材料的XRD图谱。在2θ为12.9° ((001)面)和27.3° ((002)面)处的衍射峰属于CN (PDF#87-1526)。对于Pt/CN样品,清楚地观察到12.9°和27.5°的峰,这表明Pt的负载保持了CN的基本结构。(002)晶面的峰值从27.3°到27.5°的轻微移动意味着CN层间堆叠距离的减小。12.9°处的(100)面处的峰不明显,这表明Pt原子填充在CN的面内空穴中[19]。然而,在XRD图谱中没有分辨出Pt的特征衍射峰,这可能是由于Pt纳米粒子的分散性非常好且复合材料Pt含量相对较低,使其难以被观察到。通过FTIR图谱进一步表征了CN和Pt/CN样品的结构特征,如图2(b)所示, Pt/CN样品的FTIR图谱显示了与CN相似的峰,这意味着Pt单个原子或颗粒的负载没有改变CN的化学结构。
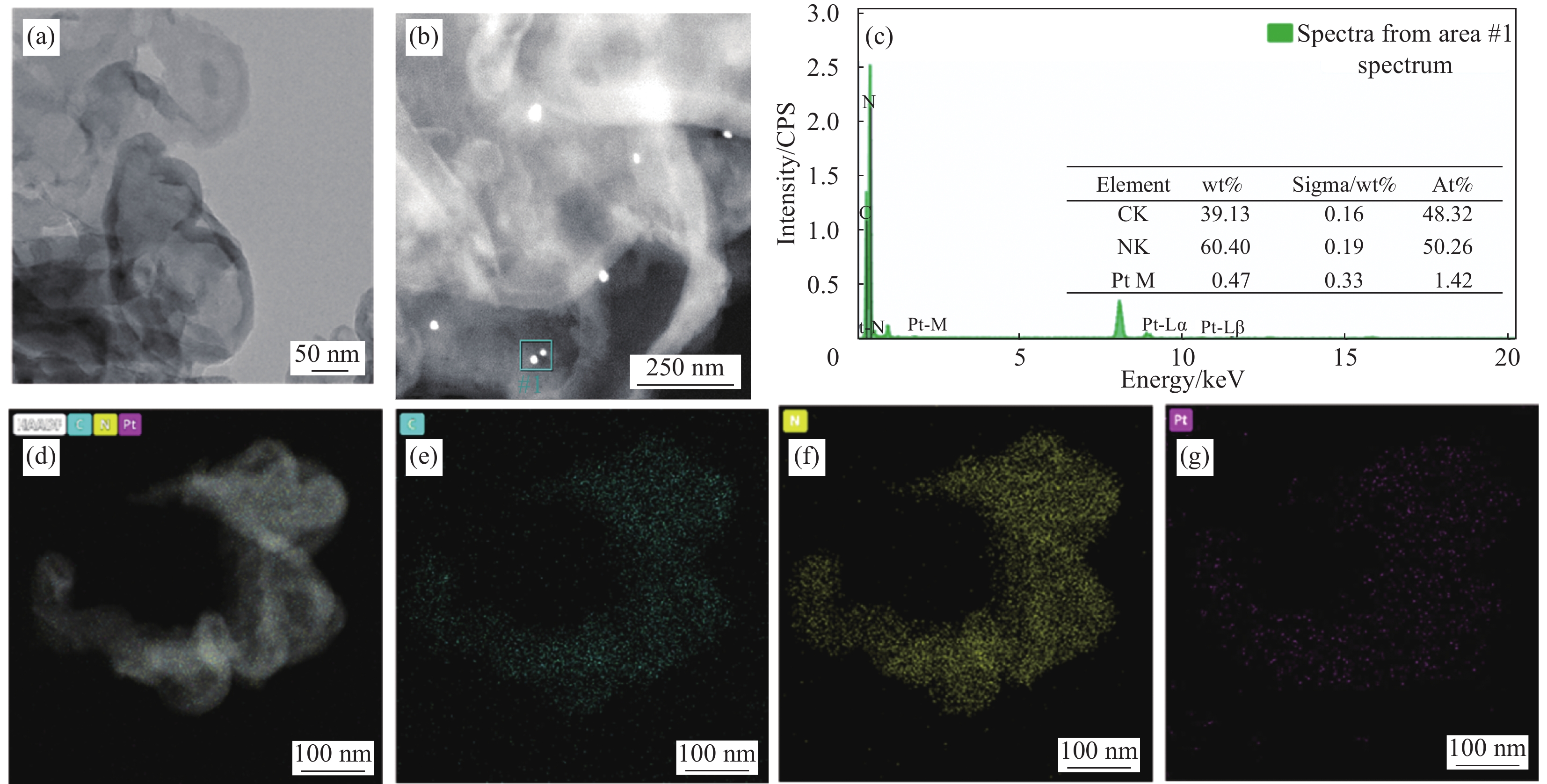
为了验证制备的光催化材料的元素分布,对Pt负载量为0.5wt%的Pt/CN样品进行TEM测试,如图3所示。图3(a)显示0.5wt%Pt/CN具有层状形态不规则的结构,并且可以从图3(b)中看出Pt以纳米粒子的形式分布在CN上,尺寸为2 nm左右。如图3(d)~3(g)所示,可以清楚地观察到C、N和Pt元素均匀分布,进一步表明Pt在CN表面上的均匀分散,清楚地证明制备的复合材料中Pt的存在。从图3(c)插表可知ZAF法无标定量分析结果证实0.5wt%Pt/CN复合材料的有效制成。
为研究不同负载量下Pt/CN样品的比表面积变化,进行了BET测试。如图4所示,样品的氮气吸附-脱附等温线出现了很明显 H3滞后环,均属于典型的IV型。氮气吸附-脱附曲线说明CN和0.5wt%Pt/CN几乎无小孔吸附,是具有片层结构的介孔材料。这一结果与孔径分布图一致,两种材料的孔径在10~50 nm,属于介孔范围。0.5wt%Pt/CN样品显示出与CN相似的等温线,这意味着Pt原子的负载没有改变CN的微观结构。用BET法计算0.5wt%Pt/CN比表面积为45.9 m²/g,与比表面积为69.1 m²/g 的CN相比,比表面积减小是由于 Pt的负载导致表面积的减少,Pt堵塞了CN层间的孔隙。
2.2 Pt负载量对CN光电化学性能的影响
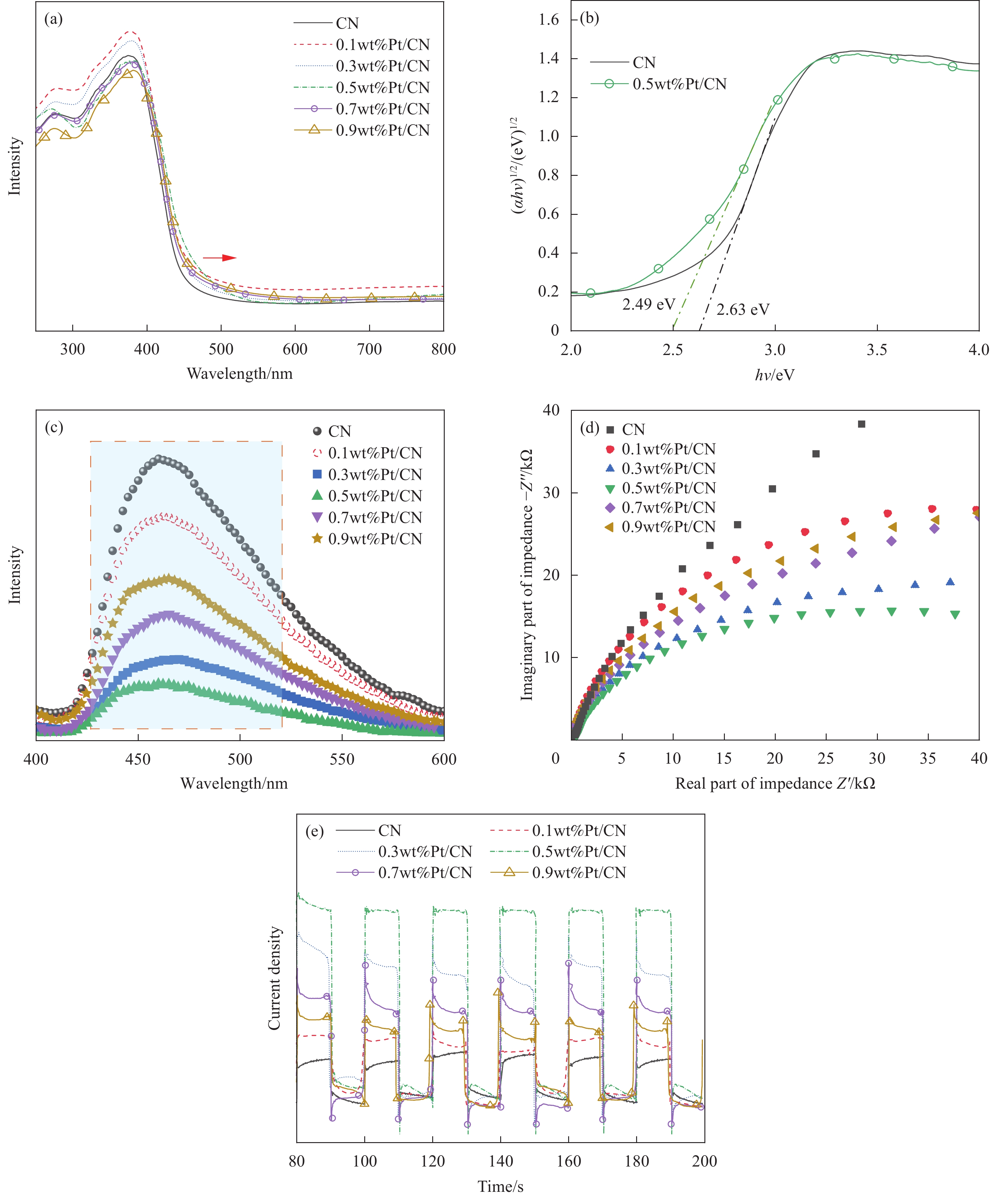
为了研究不同负载量的Pt/CN样品对光的吸收利用情况,进行了UV-vis DRS表征分析,如图5(a)~5(c)所示。由图5(a)可知,对于Pt/CN复合材料,吸收能带边缘轻微红移,这会产生电子-空穴对,样品带隙变窄,增强了对可见光的吸收,从而提高光催化性能。又通过PL测试研究不同负载量的Pt/CN的光生电子和空穴的分离效果。如图5(b)所示,较低的光致发光强度表明光激发电子-空穴对的复合效率较低。从5(c)可以清楚地注意到,所有Pt/CN样品都显示出强发射峰,主要发射峰都集中在大约470 nm处。Pt的掺入没有改变峰的位置,但减弱了发射强度。发射峰强度依次为CN>0.1wt%Pt/CN>0.9wt%Pt/CN>0.7wt%Pt/CN>0.3wt%Pt/CN>0.5wt%Pt/CN,这表明光诱导的e–/h+ 对的复合受到抑制,光生载流子在Pt纳米粒子沉积后被有效分离和传输。
为了进一步研究不同负载量的Pt/CN样品的电荷分离和传输效率,对样品进行了电化学性能的测试。图5(d)显示了纯CN和Pt/CN样品电化学交流阻抗(EIS)图谱,以阐明接触电阻对光催化性能的影响。所有样品在测试频率范围内均显示半圆形曲线,主要反映活性材料的电荷转移阻抗[20]。0.5wt%Pt/CN的电荷转移电阻低于具有较高Pt负载量的其他Pt/CN样品;此外,CN显示出高得多的阻抗,表明通过用Pt修饰CN而大大提高了电子电导率。这一结果表明,Pt助催化剂大大提高了催化剂中的电子传输速度,提升其光催化性能。图5(e)为在开-关循环可见光照射下,在0 V (vs SCE)[21]下进行光电流响应。Pt/CN复合材料的光电流高于纯CN的光电流,这表明Pt可以增强光生e–/h+对的分离,并防止其在Pt原子或团簇与CN之间的界面处复合。这表明在光激发下,Pt掺入后光生电子和空穴分离效果提高,有利于光催化性能的提升。但是过量的Pt负载增加了在CN到Pt界面产生的肖特基势垒[22]的高度,并进一步降低了光催化活性。
2.3 Pt负载量对CN光催化产H2的影响
根据上述分析的结果,证实了在CN上均匀分布的Pt有效地提高了光催化性能。为了研究CN和0.5wt%Pt/CN样品的光催化活性,进行了光催化分解水产H2的实验,如图6所示。在可见光下,以三乙醇胺为牺牲剂,研究了CN和0.5wt% Pt/CN复合材料的光催化产H2性能(图6(a))。CN呈现了很低的析H2量,这是由于CN样品的光生电子和空穴之间的快速复合,并加速了反向过程。相比之下,在沉积Pt纳米粒子之后,复合材料在可见光下表现出了优异的产氢性能。0.5wt%Pt/CN样品表现出显著的析H2量,为409.2 μmol/g,约为CN的23倍,这种活性增强可归因于Pt纳米颗粒在CN表面沉积,成为产氢的活性位点。如图6(b)所示,测量0.5wt%Pt/CN复合材料的光催化稳定性。可以清楚地看到,随着光照时间的延长,产H2量相对稳定的增加。在12 h的3次循环实验后,没有观察到活性的明显降低,表明所获得的0.5wt%Pt/CN复合材料在所研究的条件下对产H2表现出良好的光催化稳定性。为了进一步研究0.5wt%Pt/CN样品的稳定性,对反应前后的样品进行了XRD和FTIR测试。由图6(c)、图6(d)可以看出,即使在光催化12 h后仍保持相同的晶体结构和官能团结构,表明0.5wt%Pt/CN具有极好的稳定性和可重复使用性。
![]() 图 6 (a)不同Pt负载量下Pt/CN的光解水产H2曲线;(b) 0.5wt%Pt/CN的循环光催化测试;反应前后0.5wt%Pt/CN的XRD图谱(c)和FTIR图谱(d)Figure 6. (a) Photolyzed aquatic H2 curves of Pt/CN under different Pt doping amounts; (b) Cycling photocatalytic test of 0.5wt%Pt/CN; XRD patterns (c) and FTIR spectra (d) of 0.5wt%Pt/CN before and after reaction
图 6 (a)不同Pt负载量下Pt/CN的光解水产H2曲线;(b) 0.5wt%Pt/CN的循环光催化测试;反应前后0.5wt%Pt/CN的XRD图谱(c)和FTIR图谱(d)Figure 6. (a) Photolyzed aquatic H2 curves of Pt/CN under different Pt doping amounts; (b) Cycling photocatalytic test of 0.5wt%Pt/CN; XRD patterns (c) and FTIR spectra (d) of 0.5wt%Pt/CN before and after reaction2.4 Pt/CN复合光催化产氢过程
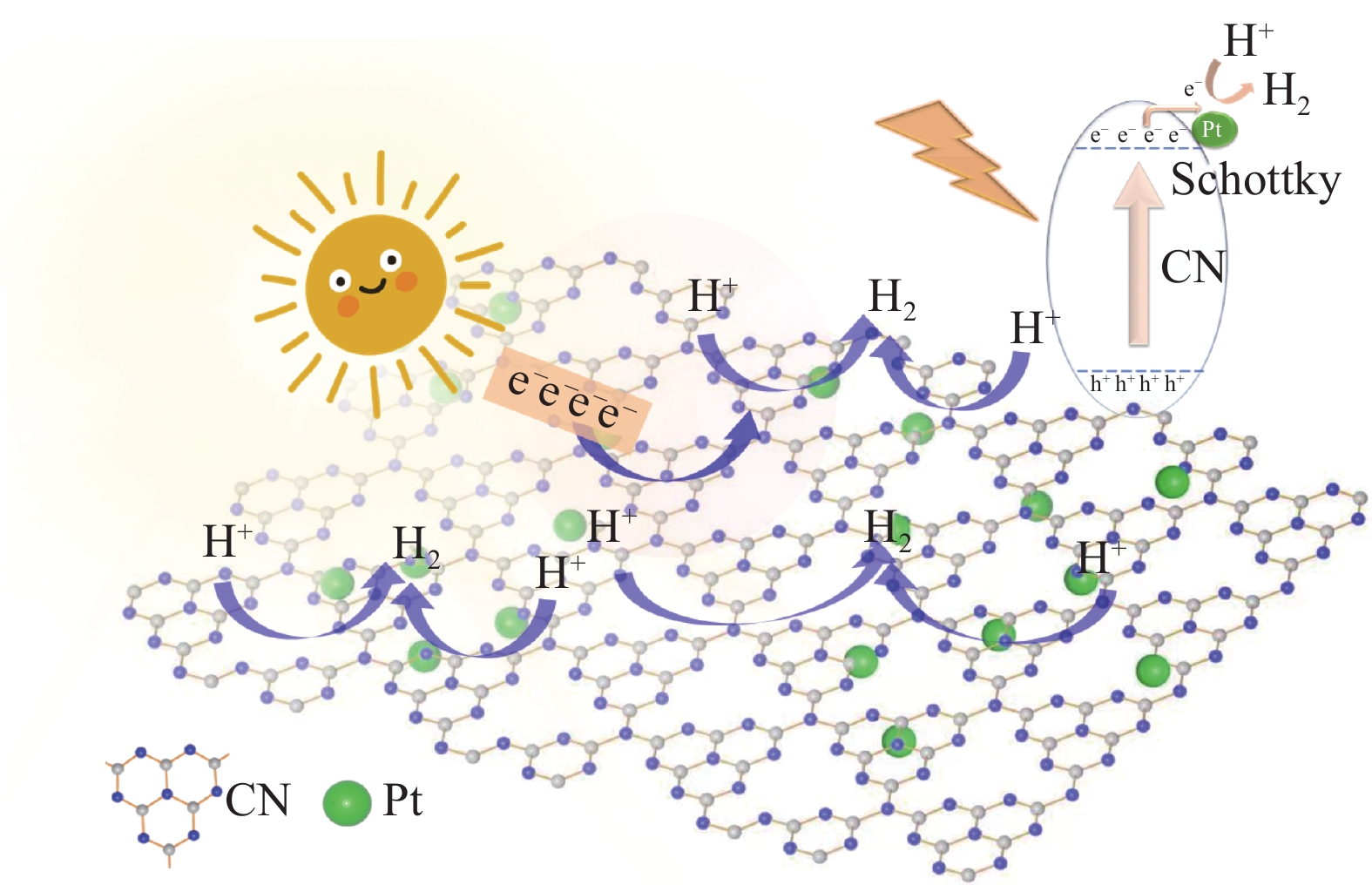
本实验成功制备了Pt/CN复合材料,其中Pt以纳米粒子的形式存在,从其光电化学性能测试中得出Pt和CN二者之间形成了肖特基势垒,如图7所示,当光照射到催化剂表面,价带上的电子吸收大于其禁带宽度的光子跃迁到导带,由于Pt 的存在使导带的电子快速迁移到Pt上,降低了CN的电子-空穴复合速率。并且Pt作为光催化分解水的活性位点,促进水中的绝大部分氢质子快速吸附到Pt位点,得到电子被还原为H2,进一步加快了产氢的速率,还有一少部分的氢质直接在CN上被还原为H2。
3. 结 论
(1) 对于Pt/石墨相氮化碳(CN)复合光催化剂,Pt的最佳负载量为0.5wt%,其电流密度大于其他比例下的复合物,PL光谱与Uv-vis DRS光谱的结果也证实了0.5wt%Pt/CN有较好的光吸收性能与光电化学性能,有利于光催化产H2的进行,0.5wt% Pt/CN材料4 h产H2量为409.2 μmol/g,约为CN的23倍。
(2) 根据测试结果分析,Pt是通过和CN之间形成肖特基势垒,使导带的电子快速迁移到Pt上,降低了CN的电子-空穴复合速率。并且Pt作为光催化分解水的活性位点,促进水中的绝大部分氢质子快速吸附到Pt位点,得到电子被还原为H2,进一步加快了产氢的速率,从而实现了高效光催化产氢。
-
图 6 (a)不同Pt负载量下Pt/CN的光解水产H2曲线;(b) 0.5wt%Pt/CN的循环光催化测试;反应前后0.5wt%Pt/CN的XRD图谱(c)和FTIR图谱(d)
Figure 6. (a) Photolyzed aquatic H2 curves of Pt/CN under different Pt doping amounts; (b) Cycling photocatalytic test of 0.5wt%Pt/CN; XRD patterns (c) and FTIR spectra (d) of 0.5wt%Pt/CN before and after reaction
-
[1] KARTHIKEYAN C, ARUNACHALAM P, RAMACHANDRAN K, et al. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications[J]. Journal of Alloys and Compounds,2020,828:154281. DOI: 10.1016/j.jallcom.2020.154281
[2] ZHANG S, WANG K, LI F, et al. Structure-mechanism relationship for enhancing photocatalytic H2 production[J]. International Journal of Hydrogen Energy,2022,47(88):37517-37530. DOI: 10.1016/j.ijhydene.2021.10.139
[3] XIONG S, TANG R, GONG D, et al. Environmentally-friendly carbon nanomaterials for photocatalytic hydrogen production[J]. Chinese Journal of Catalysis,2022,43(7):1719-1748. DOI: 10.1016/S1872-2067(21)63994-3
[4] MUN S J, PARK S J. Graphitic carbon nitride materials for photocatalytic hydrogen production via water splitting: A short review[J]. Catalysts,2019,9(10):805. DOI: 10.3390/catal9100805
[5] ZHOU Y Z, ZHANG L X, QIN L X, et al. A mixed phase lanthanum vanadate in situ induced by graphene oxide/graphite carbon nitride for efficient photocatalytic hydrogen generation[J]. International Journal of Hydrogen Energy,2021,46(54):27495-27505. DOI: 10.1016/j.ijhydene.2021.05.213
[6] XUE F, CHEN C, FU W L, et al. Interfacial and dimensional effects of Pd co-catalyst for efficient photocatalytic hydrogen generation[J]. The Journal of Physical Chemistry C,2018,122(44):25165-25173. DOI: 10.1021/acs.jpcc.8b06943
[7] LI K, LIN Y Z, WANG K, et al. Rational design of cocatalyst system for improving the photocatalytic hydrogen evolution activity of graphite carbon nitride[J]. Applied Catalysis B: Environmental,2020,268:118402. DOI: 10.1016/j.apcatb.2019.118402
[8] WU Q K, JEONG T, KIM S H, et al. Synthesis of large area graphitic carbon nitride nanosheet by chemical vapor deposition[J]. Journal of Alloys and Compounds,2022,900:163310. DOI: 10.1016/j.jallcom.2021.163310
[9] DING L, QI F, LI Y F, et al. In-situ formation of nanosized 1T-phase MoS2 in B-doped carbon nitride for high efficient visible-light-driven H2 production[J]. Journal of Colloid and Interface Science,2022,614:92-101. DOI: 10.1016/j.jcis.2022.01.100
[10] JIANG W S, ZHAO Y J, ZONG X P, et al. Photocatalyst for high-performance H2 production: Ga-doped polymeric carbon nitride[J]. Angewandte Chemie International Edition,2021,60(11):6124-6129. DOI: 10.1002/anie.202015779
[11] LIU Y, GAO M Y, YANG W W, et al. Facile synthesis of monodisperse Pt nanoparticles on graphitic carbon Nitride for high-performance photocatalytic H2 evolution[J]. ChemistrySelect,2022,7(9):e202103882.
[12] PENG Y, LU B Z, CHEN L M, et al. Hydrogen evolution reaction catalyzed by ruthenium ion-complexed graphitic carbon nitride nanosheets[J]. Journal of Materials Chemistry A,2017,5(34):18261-18269. DOI: 10.1039/C7TA03826G
[13] WU J E, ZHANG Y Y, ZHANG B, et al. Zn-doped CoS2 nanoarrays for an efficient oxygen evolution reaction: Understanding the doping effect for a precatalyst[J]. ACS Applied Materials & Interfaces,2022,14(12):14235-14242.
[14] HUANG L, LIU X, WU H C, et al. Surface state modulation for size-controllable photodeposition of noble metal nanoparticles on semiconductors[J]. Journal of Materials Chemistry A,2020,8(40):21094-21102. DOI: 10.1039/C9TA14181B
[15] BAI L, LI Y J, ZHAO J, et al. Highly efficient utilization of precious metals for hydrogen evolution reaction with photo-assisted electro-deposited urchin-like Te nano- structure as a template[J]. ChemCatChem,2019,11(9):2283-2287. DOI: 10.1002/cctc.201900125
[16] WU H C, LIU Y D, CHEN G L, et al. Surface-confined photodeposition of noble metal nanoclusters on TiO2 in a fluidized bed for the catalytic oxidation of formaldehyde[J]. ACS Applied Nano Materials,2022,5(9):13100-13111. DOI: 10.1021/acsanm.2c02886
[17] KARÁCSONVI É, BAIA L, DONBI A, et al. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitecture obtained by selective photodeposition[J]. Catalysis Today,2013,208:19-27. DOI: 10.1016/j.cattod.2012.09.038
[18] LIU Y D, NASERI A, LI T, et al. Shape-controlled photochemical synthesis of noble metal nanocrystals based on reduced graphene oxide[J]. ACS Applied Materials & Interfaces,2022,14(14):16527-16537.
[19] ONG W J, TAN L L, CHAI S P, et al. Heterojunction engineering of graphitic carbon nitride (g-C3N4) via Pt loading with improved daylight-induced photocatalytic reduction of carbon dioxide to methane[J]. Dalton Transactions,2015,44(3):1249-1257. DOI: 10.1039/C4DT02940B
[20] LIU Z, HUO P, LU Z, et al. Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator[J]. Chemical Engineering Journal,2018,331:615-625. DOI: 10.1016/j.cej.2017.08.131
[21] 孟培媛, 郭明媛, 乔勋. WS2/g-C3N4异质结光催化分解水制氢性能及机制[J]. 复合材料学报, 2021, 38(2):591-600. MENG Peiyuan, GUO Mingyuan, QIAO Xun. H2 production performance of photocatalyst and mechanism of WS2/g-C3N4 heterojunction[J]. Acta Materiae Compositae Sinica,2021,38(2):591-600(in Chinese).
[22] 孙术博, 于海瀚, 李强, 等. NaNbO3@g-C3N4复合材料的可控构筑及其压电光催化性能[J]. 复合材料学报, 2023, 40(3):1534-1540. SUN Shubo, YU Haihan, LI Qiang, et al. Controlled construction of NaNbO3@g-C3N4 composites and their piezo-photocatalytic properties[J]. Acta Materiae Compositae Sinica,2023,40(3):1534-1540(in Chinese).
-
期刊类型引用(3)
1. 孙树旺,张明星,解晓敏. ZnWO_4/ZnO异质结的制备及其在石化废水处理中的应用. 化学工程师. 2025(02): 8-12+33 .  百度学术
百度学术
2. 张雪婷,高晓红,杨旭礼,包育闻,王梁宇. GO@P-g-C_3N_4复合光催化材料的制备及其抗菌性能. 复合材料学报. 2025(02): 900-911 .  本站查看
本站查看
3. 王丽苹,李仁星,余莹,阮玉娴,陈琳,姜帅. 增强UiO-66-NH_2光催化水分解制氢性能的研究进展. 化学通报. 2024(07): 785-801 .  百度学术
百度学术
其他类型引用(0)
-
-
石墨相氮化碳(CN)由于其带隙合适(2.7 eV)、化学稳定性高、无毒性和持久性等优点,在可见光光催化水分解和有机污染物降解方面得到了广泛的关注。但纯CN表现出很高的光诱导电子与空穴的复合率、催化剂表面活性位点少等缺陷。贵金属作为助催化剂,可以提高石墨相氮化碳光催化产氢的性能。其优异的性能引起了人们的关注。但是,由于贵金属的不可再生性和高价格,“更少的贵金属,更好的性能”始终是目标。
本工作通过光还原方式制备Pt负载氮化碳,研究了一系列负载量下Pt/CN材料的光催化水分解析氢性能。从光响应能力和载流子寿命方面分析了0.5wt % Pt/CN材料活性提升的内在原因,并提出Pt的原位负载导致了肖特基势垒的形成。这是光催化领域相关研究人员感兴趣的课题。并发现当Pt的负载量为0.5wt %时, Pt/CN具有最优异的光催化产氢活性,产氢量为409.2 μmol/g,是纯CN(17.8 μmol/g)的23倍。是现有文献中性能较好的样品之一。

不同负载量下Pt/CN的光解水产H2曲线 (a)、0.5wt % Pt/CN循环性能图 (b)、Pt/CN复合光催化产氢过程 (c)





 下载:
下载: