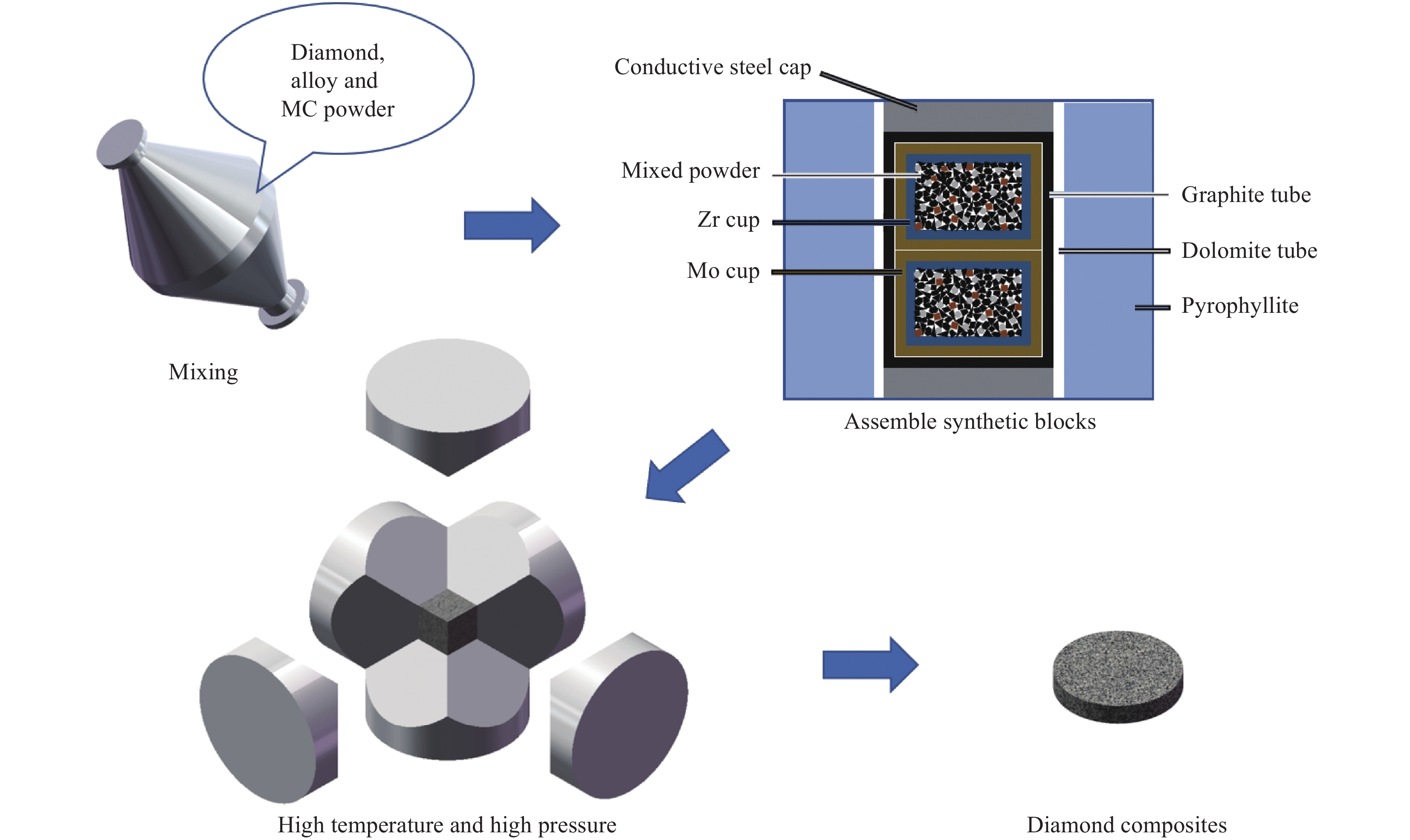
Effect of multi-element alloy-carbide bonding phase on the microstructure of diamond composites
-
摘要: 金刚石复合材料在加工、钻探等领域应用广泛,提高材料中金刚石骨架的结合强度是重要研究方向。以Co50Ni40Fe10多元合金-碳化物替代纯Co作为粘结相,在高温高压条件下制备了金刚石复合材料,结合热力学计算研究了多元合金和碳化物对材料组织的影响。结果表明:相比于Co,Co50Ni40Fe10多元合金具有更强的促进C原子迁移和扩散的能力,能加快金刚石骨架的形成。高温高压条件下,WC中C含量轻微增加,对金刚石骨架的形成影响不大;TiC轻微失C,能在一定程度上促进金刚石骨架的形成;Cr3C2分解产生的C能促进金刚石骨架的形成。Abstract: Diamond composites are widely used in processing, drilling and other fields, and improving the bonding strength of the diamond skeleton is an important research direction. In this work, Co50Ni40Fe10 multi-element alloy-carbide was used as the binder instead of Co to prepare diamond composites under high temperature and high pressure, and the influence of multi-element alloys and carbides on the microstructure of composites was studied by experiments and thermodynamic calculations. The results show that, compared with Co, Co50Ni40Fe10 multi-element alloy has stronger ability to promote the migration and diffusion of C atoms, which can accelerate the formation of diamond skeleton. Under the condition of high temperature and high pressure, the carbon content in WC increased slightly, which has little effect on the formation of diamond skeleton; TiC lost C slightly, which can promote the formation of diamond skeleton to a certain extent; The C produced by Cr3C2 decomposition can promote the formation of diamond skeleton.
-
Key words:
- multi-element alloy /
- carbide /
- thermodynamics /
- polycrystalline diamond /
- diamond skeleton
-
表 1 金刚石复合材料的命名及成分组成
Table 1. Nomenclature and compositions of diamond composites
Name Composition WC-Comp. (Co50Ni40Fe10)-5vol%WC-75vol%Dia. Cr3C2-Comp. (Co50Ni40Fe10)-5vol%Cr3C2-75vol%Dia. TiC-Comp. (Co50Ni40Fe10)-5vol%TiC-75vol%Dia. CoNiFe-Comp. (Co50Ni40Fe10)-75vol%Dia. Co-Comp. Co-75vol%Dia. Notes: Comp.—Composites; Dia.—Diamond. Spot Phase Co Ni Fe W/Cr/Ti C 1 Diamond — — — — 100.00 2 Alloy in WC-Comp. 46.30±1.62 36.04±1.39 9.47±0.74 0.96±0.76(W) 7.23±2.27 3 WC 3.63±0.77 3.17±0.85 1.26±0.47 38.68±3.79(W) 53.25±7.74 4 Cr-rich phase 29.95±2.05 9.05±1.97 6.92±1.06 35.31±1.75(Cr) 18.77±2.97 5 Ni-rich phase 42.10±4.68 38.72±3.87 8.14±1.46 4.30±0.67(Cr) 6.64±1.65 6 TiC 1.00±1.46 0.78±1.12 0.33±0.59 62.13±4.58(Ti) 35.78±6.99 7 Alloy in TiC-Comp. 44.38±7.55 32.39±4.24 9.22±1.48 0.65±0.44(Ti) 13.35±10.32 表 3 式(3)、(4)和(10)中涉及物质的物理性质参数
Table 3. Physical parameters of the substances involved in the reaction (3), (4) and (10)
Substance Volume expansion coefficient γ/K−1 $ {B}_{0} $/GPa ${B'}_{0}$ Diamond[27] −2.013×10−6+2.4×10−8T−9.219×10−12T2+
1.237×10−15T3443.0 4.0 W[28-29] (1+4.40×10−6)3−1 309.2 6.6 WC[30-31] (1+6.25×10−6)3−1 389.6 4.3 Cr (1+6.20×10−6)3−1 161.5 4.26 Cr3C2[32] (1+10.3×10−6)3−1 329.0 4.0 Ti (1+10.1×10−6)3−1 96.5 3.65 TiC[28] (1+7.7×10−6)3−1 253.0 4.1 Notes: B0—Bulk modulus; $ {B'}_{0} $—Derivative of B0; $ {B}_{0} $ and $ {B'}_{0} $ of Cr and Ti are obtained by the author through first-principles calculations. 表 4 Akira Takeuchi和Akihisa Inoue计算的组元i和j的二元混合焓(
$ {\Delta H}_{i,j}^{\mathrm{m}\mathrm{i}\mathrm{x}} $ )的值 [34]Table 4. Values of binary mixing enthalpy of components i, j (
$ {\Delta H}_{i,j}^{\mathrm{m}\mathrm{i}\mathrm{x}} $ ) calculated by Akira Takeuchi and Akihisa Inoue [34]$ {\Delta H}_{i,j}^{\mathrm{m}\mathrm{i}\mathrm{x}} $/(kJ·mol) Ni Fe Cr Co 0 −1 −4 Ni — −2 −7 Fe — — −1 -
[1] KUNUKU S, SANKARAN K J, TSAI C Y, et al. Investigations on diamond nanostructuring of different morphologies by the reactive-ion etching process and their potential applications[J]. ACS Applied Materials & Interfaces,2013,5(15):7439-7449. doi: 10.1021/am401753h [2] FURBERG A, FRANSSON K, ZACKRISSON M, et al. Environmental and resource aspects of substituting cemented carbide with polycrystalline diamond: The case of machining tools[J]. Journal of Cleaner Production,2020,277:123577. doi: 10.1016/j.jclepro.2020.123577 [3] GAO K, LI M, DONG B, et al. Bionic coupling polycrystalline diamond composite bit[J]. Petroleum Exploration and Development,2014,41(4):533-537. doi: 10.1016/S1876-3804(14)60063-X [4] TANG H, YUAN X H, CHENG Y, et al. Synthesis of paracrystalline diamond[J]. Nature,2021,599:605-610. doi: 10.1038/s41586-021-04122-w [5] ZENG Z D, YANG L X, ZENG Q S, et al. Synthesis of quenchable amorphous diamond[J]. Nature Communications,2017,8(1):1-7. doi: 10.1038/s41467-016-0009-6 [6] EGGERT J H, HICKS D G, CELLIERS P M, et al. Melting temperature of diamond at ultrahigh pressure[J]. Nature Physics,2010,6(1):40-43. doi: 10.1038/nphys1438 [7] TANIGAKI K, OGI H, SUMIYA H, et al. Observation of higher stiffness in nanopolycrystal diamond than monocrystal diamond[J]. Nature Communications,2013,4(1):1-7. [8] XIAO J W, WEN B, XU B, et al. Intersectional nanotwinned diamond-the hardest polycrystalline diamond by design[J]. npj Computational Materials,2020,6(1):1-7. doi: 10.1038/s41524-019-0267-z [9] HUANG Q, YU D L, BO X, et al. Nanotwinned diamond with unprecedented hardness and stability[J]. Nature,2014,510(7504):250-253. doi: 10.1038/nature13381 [10] LI Q, ZHAN G D, LI D, et al. Ultrastrong catalyst-free polycrystalline diamond[J]. Scientific Reports, 2020, 10(1): 1-10. [11] ZHAO B, ZHANG S Y, DUAN S, et al. Enhanced strength of nano-polycrystalline diamond by introducing boron carbide interlayers at the grain boundaries[J]. Nanoscale Advances,2020,2(2):691-698. doi: 10.1039/C9NA00699K [12] 邓福铭, 赵国刚, 王振廷, 等. 聚晶金刚石复合体超高压液相烧结理论研究[J]. 高压物理学报, 2004, 18(3):252-260. doi: 10.3969/j.issn.1000-5773.2004.03.010DENG Fuming, ZHAO Guogang, WANG Zhenting, et al. Theoretical study on high pressure liquid sintering of polycrystalline diamond compact[J]. Chinese Journal of High Pressure Physics,2004,18(3):252-260(in Chinese). doi: 10.3969/j.issn.1000-5773.2004.03.010 [13] MALLIKA K, DEVRIES R C, KOMANDURI R. On the low pressure transformation of graphite to diamond in the presence of a 'catalyst-solvent'[J]. Thin Solid Films,1999,339(1-2):19-33. doi: 10.1016/S0040-6090(98)00978-X [14] CHEN N, MA H G, FANG C, et al. Synthesis and characterization of IIa-type S-doped diamond in FeNi catalyst under high pressure and high temperature conditions[J]. International Journal of Refractory Metals and Hard Materials,2017,66:122-126. doi: 10.1016/j.ijrmhm.2017.03.006 [15] 胡强, 贾晓鹏, 李尚升, 等. 高压熔渗生长法制备金刚石聚晶中碳的转化机制研究[J]. 物理学报, 2016, 65(6):068101. doi: 10.7498/aps.65.068101HU Qiang, JIA Xiaopeng, LI Shangsheng, et al. Research on mechanism of carbon transformation in the preparation of polycrystalline diamond by melt infiltration and growth method under high pressures[J]. Acta Physica Sinica,2016,65(6):068101(in Chinese). doi: 10.7498/aps.65.068101 [16] MASHHADIKARIMI M, MEDEIROS R B D, BARRETO L P P, et al. Development of a novel triple-layer polycrystalline diamond compact[J]. Diamond and Related Materials,2021,111:108182. doi: 10.1016/j.diamond.2020.108182 [17] GUO Z H, DENG F M, ZHANG L, et al. The novel and facile electrolysis method for removing the cobalt binder phase from large diameter polycrystalline diamond compacts[J]. Ceramics International,2022,48(3):3125-3132. doi: 10.1016/j.ceramint.2021.10.086 [18] CHEN F, XU G, MA C D, et al. Thermal residual stress of polycrystalline diamond compacts[J]. Transactions of Nonferrous Metals Society of China,2010,20(2):227-232. doi: 10.1016/S1003-6326(09)60126-6 [19] JIA H S, JIA X P, MA H A, et al. Synthesis of growth-type polycrystalline diamond compact (PDC) using the solvent Fe55Ni29Co16 alloy under HPHT[J]. Science China: Physics, Mechanics & Astronomy,2012,55(8):1394-1398. [20] LI C, TENG J W, YANG B B, et al. Correlation between microstructure and mechanical properties of novel Co-Ni-based powder metallurgy superalloy[J]. Materials Characterization,2021,181:111480. doi: 10.1016/j.matchar.2021.111480 [21] 刘咏, 曹远奎, 吴文倩, 等. 粉末冶金高熵合金研究进展[J]. 中国有色金属学报, 2019, 29(9):2155-2184. doi: 10.19476/j.ysxb.1004.0609.2019.09.16LIU Yong, CAO Yuankui, WU Wenqian, et al. Progress of powder metallurgical high entropy alloys[J]. The Chinese Journal of Nonferrous Metals,2019,29(9):2155-2184(in Chinese). doi: 10.19476/j.ysxb.1004.0609.2019.09.16 [22] FERRARI A C, BASKO D M. Raman spectroscopy as a versatile tool for studying the properties of graphene[J]. Nature Nanotechnology,2013,8(4):235-246. doi: 10.1038/nnano.2013.46 [23] 朱瑞华, 刘金龙, 陈良贤, 等. 金刚石自支撑膜拉曼光谱1420 cm−1特征峰研究[J]. 人工晶体学报, 2015, 44(4):867-871. doi: 10.3969/j.issn.1000-985X.2015.04.003ZHU Ruihua, LIU Jinlong, CHEN Liangxian, et al. Research on 1420 cm−1 characteristic peak of free-standing diamond films in raman spectrum[J]. Journal of Synthetic Crystals,2015,44(4):867-871(in Chinese). doi: 10.3969/j.issn.1000-985X.2015.04.003 [24] BERMAN R, SIMON F. On the graphite-diamond equilibrium[J]. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie,1955,59(5):333-338. doi: 10.1002/bbpc.19550590503 [25] KENNEDY C S, KENNEDY G C. The equilibrium boundary between graphite and diamond[J]. Journal of Geophysical Research,1976,81(14):2467-2470. doi: 10.1029/JB081i014p02467 [26] 叶大伦, 胡建华. 实用无机热力学数据手册[M]. 北京: 冶金工业出版社, 2002: 1-5.YE Dalun, HU Jianhua. The thermodynamic data manual of practical inorganic materials[M]. Beijing: Metallurgical Industry Press, 2002: 1-5(in Chinese). [27] MOUNET N, MARZARI N. High-accuracy fist-principles determination of the structural, vibrational and thermodynamical properties of diamond, graphite, and derivatives[J]. Physical Review B,2005,71(20):1-17. doi: 10.1103/PhysRevB.71.205214 [28] CONNÉTABLE D. First-principles study of transition metal carbides[J]. Materials Research Express,2016,3(12):126502. doi: 10.1088/2053-1591/3/12/126502 [29] JIANG D, ZHONG S, XIAO W, et al. Structural, mechanical, electronic, and thermodynamic properties of pure tungsten metal under different pressures: A first-principles study[J]. International Journal of Quantum Chemistry,2020,120(13):e26231. doi: 10.1002/qua.26231 [30] GOLOVCHAN V T. On the thermal residual micro-stresses in WC-Co hard metals[J]. International Journal of Refractory Metals and Hard Materials,2007,25(4):341-344. doi: 10.1016/j.ijrmhm.2006.08.002 [31] LI X, ZHANG X, QIN J, et al. First-principles calculations of structural stability and mechanical properties of tungsten carbide under high pressure[J]. Journal of Physics & Chemistry of Solids,2014,75(11):1234-1239. doi: 10.1016/j.jpcs.2014.06.011 [32] JIANG C. First-principles study of structural, elastic, and electronic properties of chromium carbides[J]. Applied Physics Letters,2008,92(4):041909. doi: 10.1063/1.2838345 [33] TAKEUCHI A, INOUE A. Calculations of mixing enthalpy and mismatch entropy for ternary amorphous alloys[J]. Materials Transactions,2000,41(11):1372-1378. doi: 10.2320/matertrans1989.41.1372 [34] TAKEUCHI A, INOUE A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element[J]. Materials Transactions,2005,46(12):2817-2829. doi: 10.2320/matertrans.46.2817 -






 下载:
下载: