Modification strategy and application of cobalt-based electrode materials
-
摘要: 钴基材料作为非贵金属材料中重要的一员,因其具有较高理论容量、良好的催化活性及出色的热/化学稳定性,被广泛应用在超级电容器(SCs)和电催化等电化学能源储存与转化领域中。然而目前在钴基材料的应用中还存在诸多缺陷,如导电性偏低,活性位点暴露的不充分,测试过程中活性组分易团聚、分解,结构稳定性较差等。近年来,许多研究报道了改性钴基材料来提升其电化学性能,基于此,本综述详细介绍了近几年对钴基材料的改性研究,主要包括形貌调控、元素掺杂、构筑异质结、缺陷工程及与载体材料复合。然后,对其在SCs、电催化氧还原反应(ORR)、析氧反应(OER)及析氢反应(HER)中的应用进行系统性的总结。最后,提出钴基材料当前存在的问题和未来的发展方向。Abstract: As an important member of non-precious metal materials, cobalt-based materials have been widely used in electrochemical energy storage and conversion fields such as supercapacitors and electrocatalysis due to their high theoretical capacity, good catalytic activity, and excellent thermal/chemical stability. However, cobalt-based materials have also many shortcomings, such as low conductivity, insufficient exposure of active sites, easy agglomeration and decomposition of active components during testing, poor structural stability, etc. In recent years, many studies have reported the modification of cobalt-based materials to improve their electrochemical performance. Based on this, this review introduces the modification research of cobalt-based materials in recent years in detail, mainly including morphology control, elemental doping, heterostructure construction, defect engineering and composite with specific supports materials, etc. Then, their electrochemistry applications including supercapacitors (SCs), electrocatalytic oxygen reduction reaction (ORR), oxygen evolution reaction (OER), and hydrogen evolution reaction (HER) is systematically summarized. Finally, the current problems and future development directions of cobalt-based materials are proposed.
-
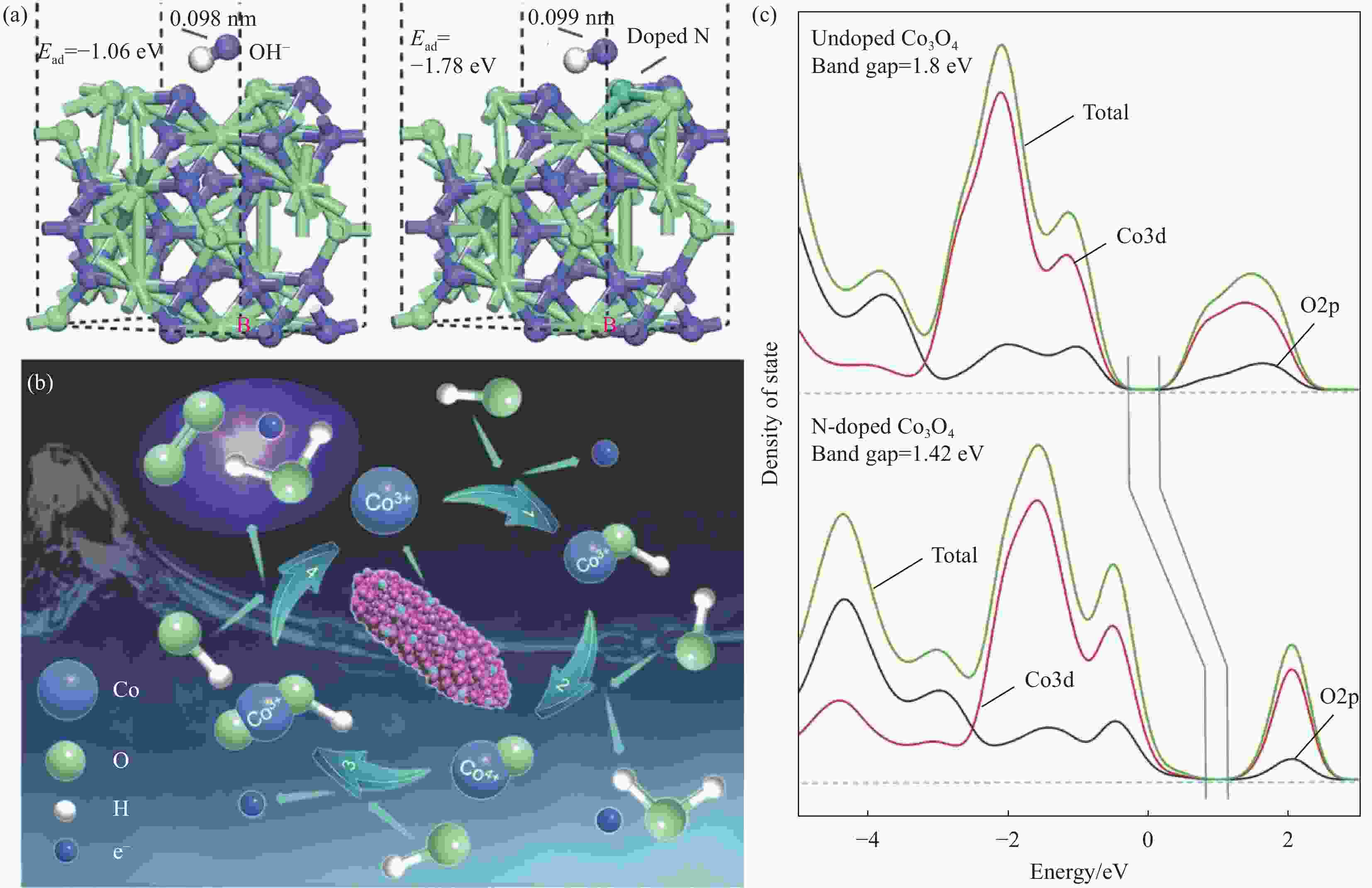
图 3 (a) 未掺杂和N掺Co3O4(110)表面的计算结构和OH–吸附能(Ead);(b) 析氧反应(OER)循环的示意图;(c) 未掺杂Co3O4和N掺杂Co3O4的总态密度和预计态密度[31]
Figure 3. (a) Calculated structures and OH− adsorption energies (Ead) of the undoped and N-doped Co3O4 (110) surfaces; (b) Schematic illustration of the oxygen evolution reaction (OER) cycle; (c) Total density of states and projected densities of states of undoped Co3O4 and N-doped Co3O4[31]
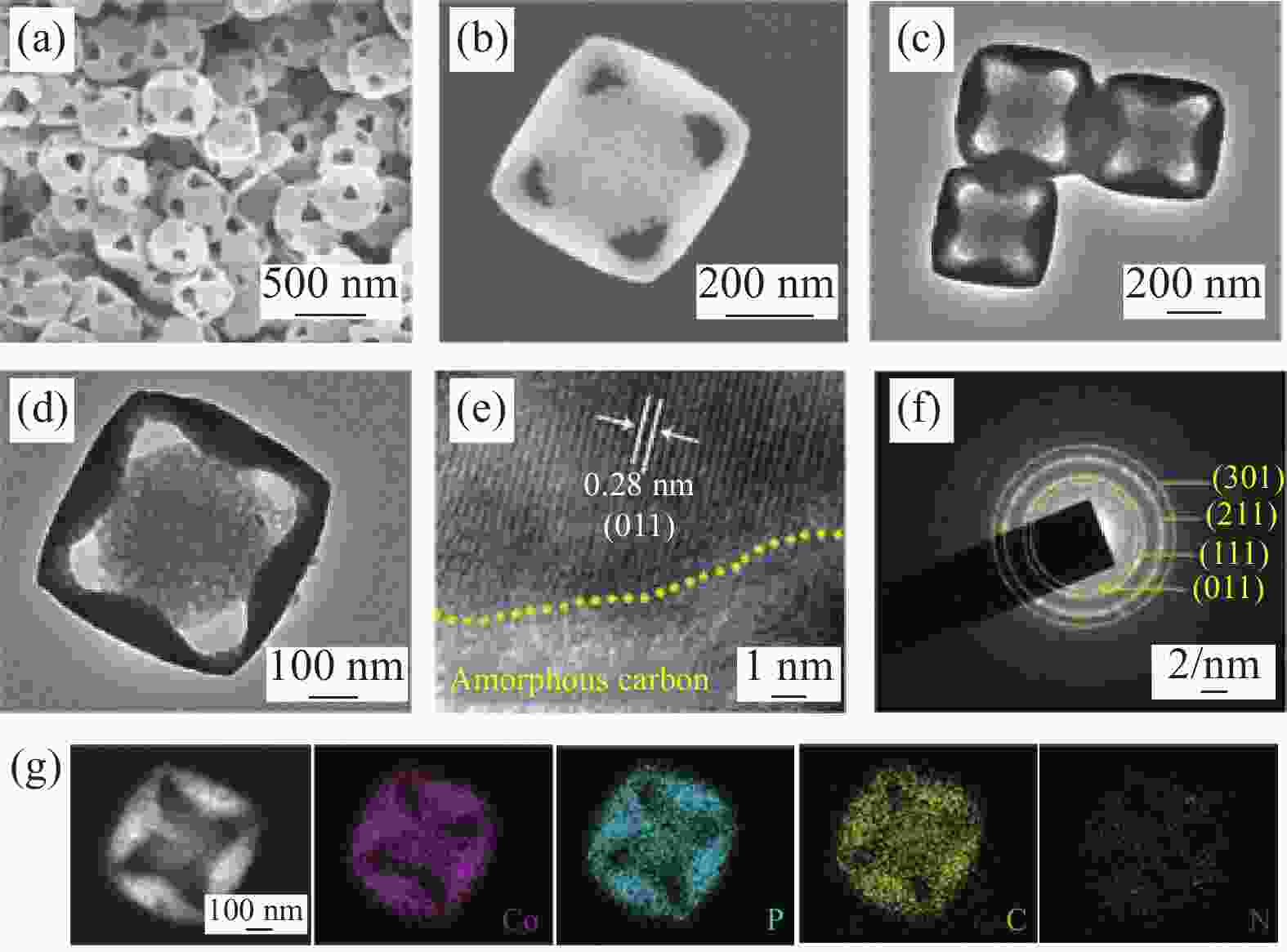
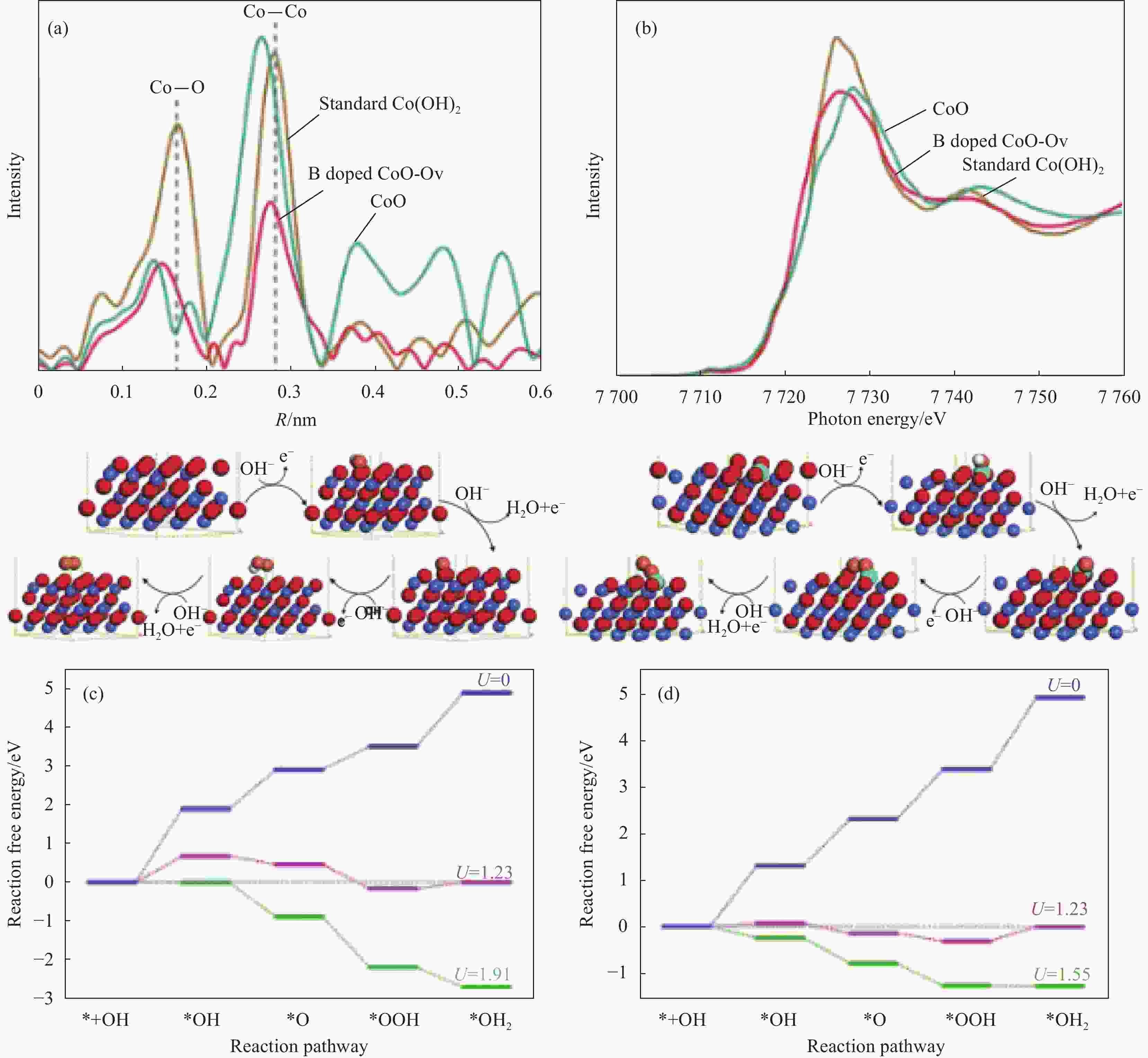
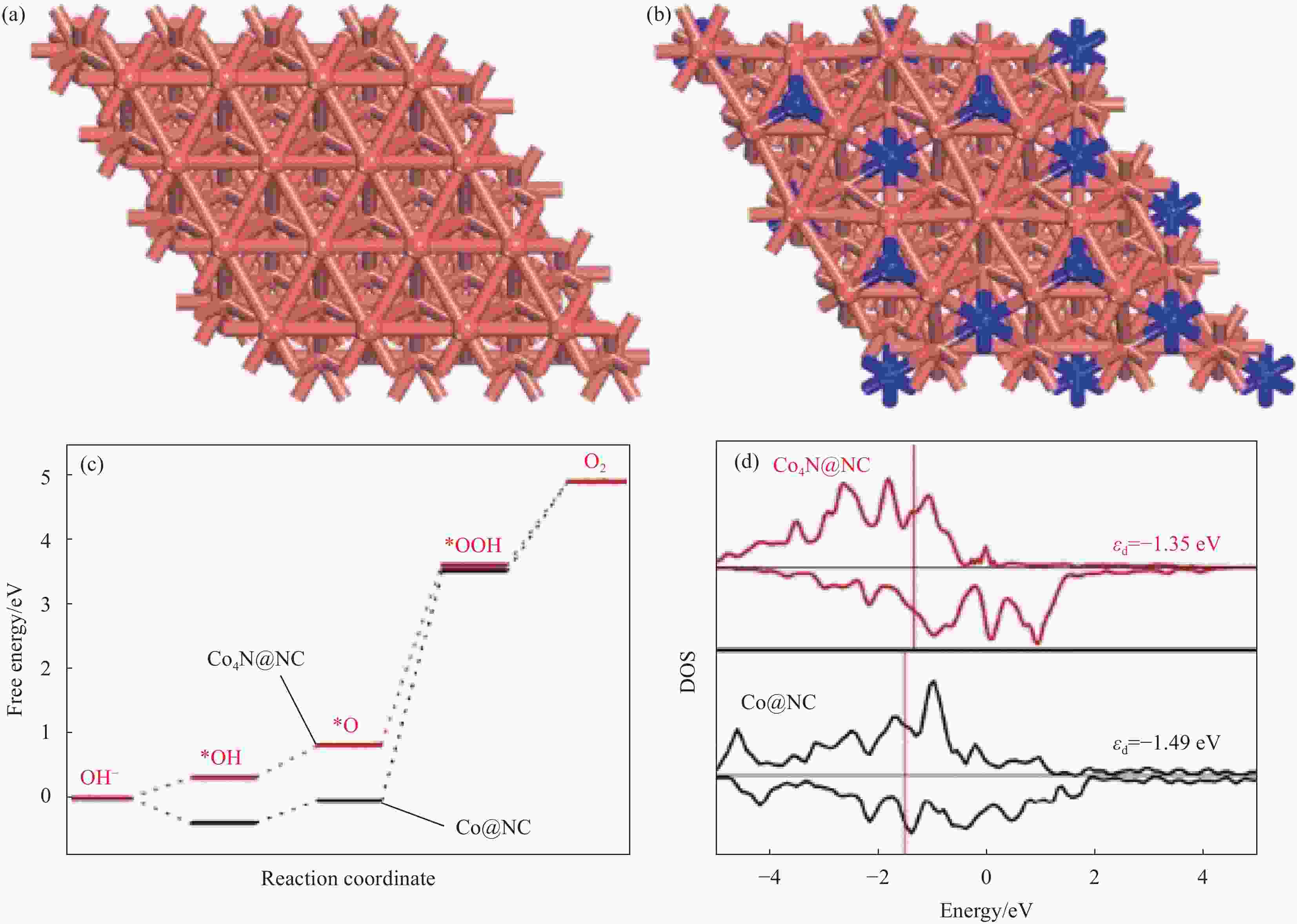
图 4 (a) B掺杂具有氧空位的CoO纳米线(CoO-Ov)、CoO和标准Co(OH)2在Co K边缘的傅里叶变换光谱;(b) X射线吸收近边缘结构(XANES)光谱;通过氧缔合机制在CoO(111)表面(c)和B掺杂的CoO-Ov(111)(d)表面上不同电极电位下OER的自由能图[45]
U—Electrode potential; R—Radial distance
Figure 4. (a) Fourier transform spectra at the Co K-edge for vacancies in CoO nanowires by B doping (B doped CoO-Ov) , CoO and standard Co(OH)2; (b) Overlaid X-ray absorption near edge structure (XANES) spectra; Free energy diagrams for OER at different electrode potential on CoO(111) surface (c) and B doped CoO-Ov (111) surface (d) through oxygen associative mechanism[45]
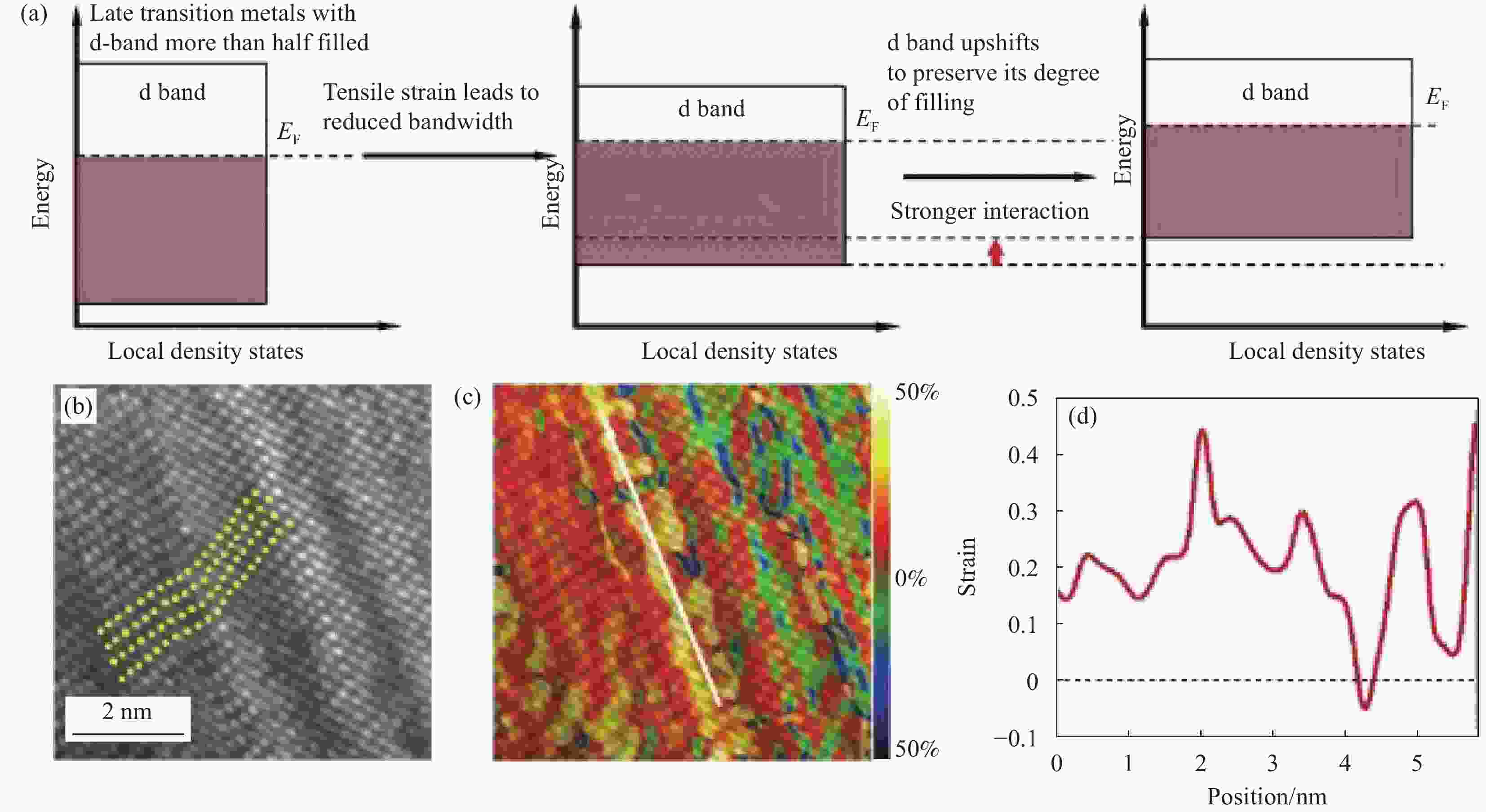
图 5 (a) 拉伸应变对后期过渡金属d带位置影响的能量图;(b) 沿xx方向计算的应变图;(c) HRTEM图像;(d) 沿图5(c)中白线的应变线剖面[47]
EF—Fermi level
Figure 5. (a) Energy diagrams explaining the effect of tensile strain on the d-band position of late transition metals; (b) Strain map taken along the xx direction calculated; (c) HRTEM image; (d) Line profiles of strain along the white line in Fig.5(c)[47]
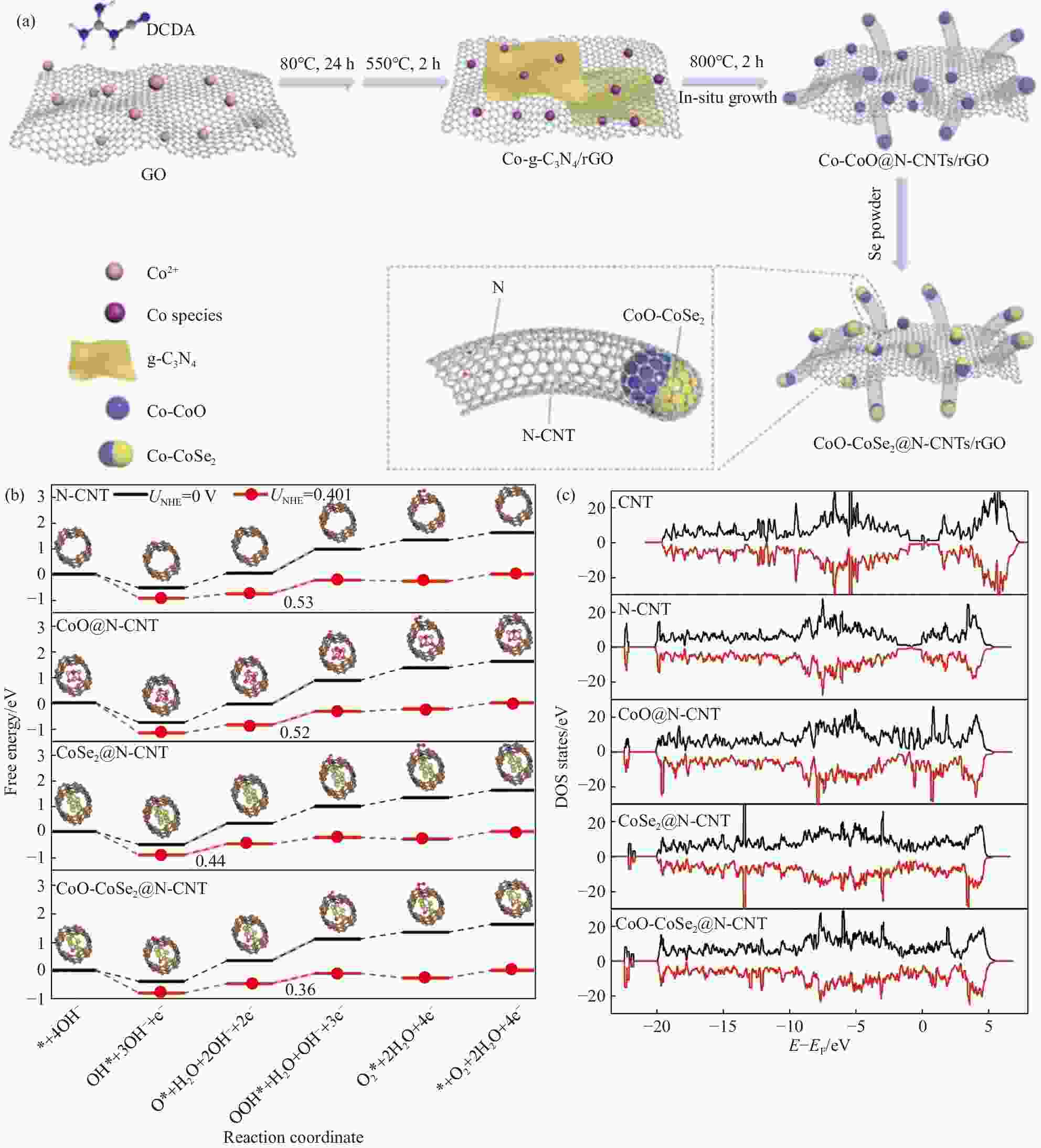
图 6 (a) CoO-CoSe2@N-CNTs/rGO的制备过程示意图;(b) OER的自由能图;(c)态密度(DOS)图[49]
DCDA—Dicyandiamide; GO—Graphene oxide; rGO—Reduced graphene oxide; N-CNT—N doping carbon nanotube; DOS—Density of states; UNHE—Potential vs normal hydrogen electrode
Figure 6. (a) Schematic illustration of the preparation process of CoO-CoSe2@N-CNTs/rGO; (b) Free energy diagram of OER; (c) Density of state (DOS) patterns[49]
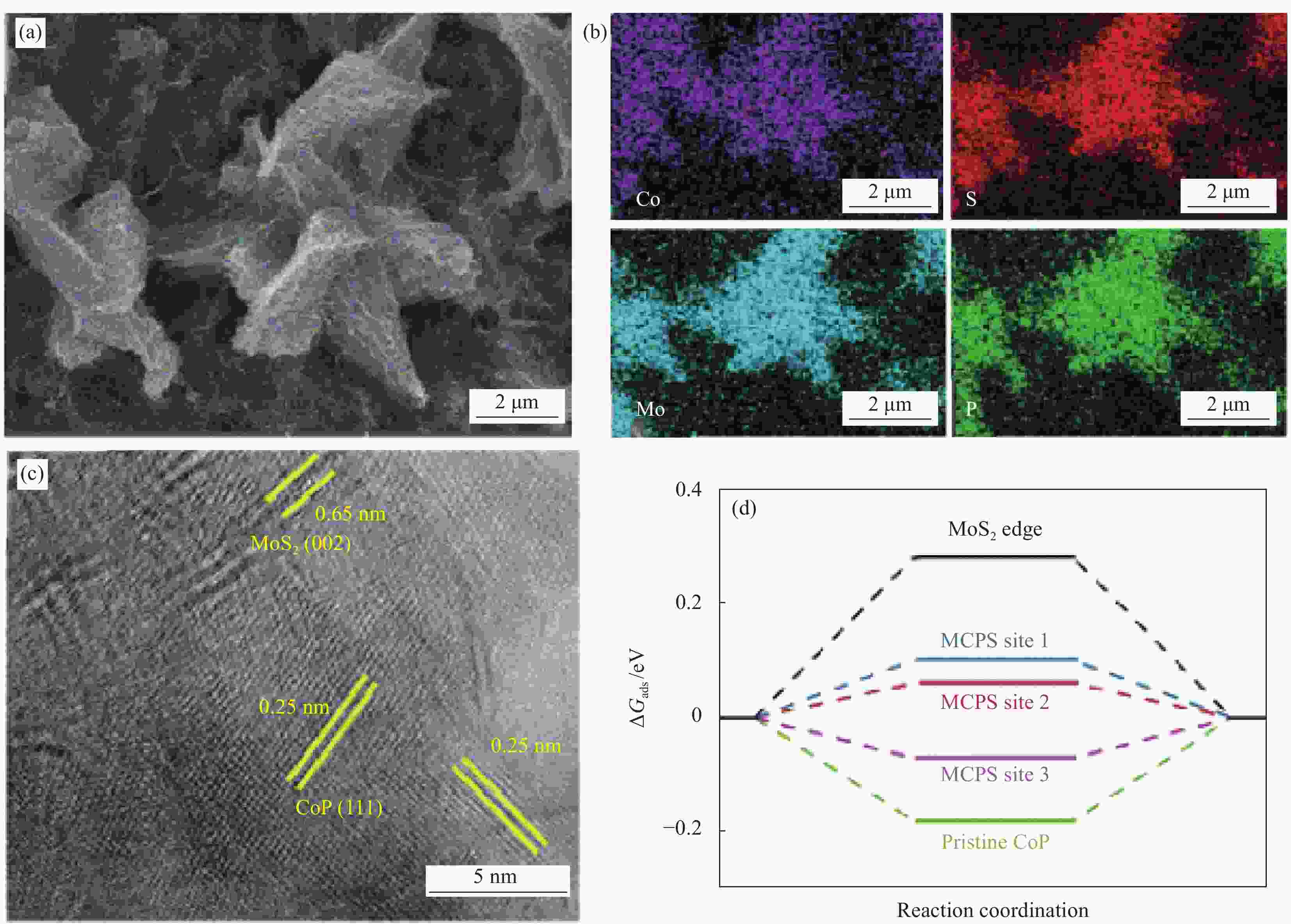
图 7 (a) 纳米片MoS2修饰的空心纳米片CoP异质结复合材料(MCPS)的SEM图像;(b) 元素面扫图像;(c) HRTEM图像;(d) 在不同活性位点的氢吸附能的DFT计算[51]
ΔGads—Gibbs free energy of reactive adsorption intermediates
Figure 7. (a) SEM image of MoS2 nanosheets arrays on CoP hollow structure (MCPS); (b) Element mapping images; (c) HRTEM image; (d) DFT calculation of hydrogen adsorption energy on different sites[51]
表 1 不同元素掺杂钴基电极材料的电化学性能
Table 1. Electrochemical performance of cobalt-based electrode materials with doped different elements
Electrode material Electrolyte/Reaction Specific capacitance ORR
E1/2/VOER/HER
E10/mVTafel slop value/
(mV·dec−1)Ref. Ni-CoP3 1.0 mol·L−1 KOH/SCs 0.7 mA·h·cm−2 at 2.5 mA·cm−2 − − − [28] Zn-Co3O4 1.0 mol·L−1 KOH/OER − − 151 − [29] Fe-CoP UNSs/NF 1.0 mol·L−1 KOH/HER − − 67 66.22 [30] N-Co3O4 1.0 mol·L−1 KOH/OER − − 190 29.8 [31] S-CoSe2 0.5 mol·L−1 H2SO4/HER − − 88 50 [32] Co-I-N/G 1.0 mol·L−1 KOH/HER − − 52 56.1 [33] Mo-CoP 1.0 mol·L−1 KOH/OER/HER − − 305/40 56/65 [34] Fe-CoP 1.0 mol·L−1 KOH/OER/HER − − 310/78 67/75 [35] Bi-CoP 0.1/1.0 KOH/ORR/OER − 0.81 370 − [36] Mn-ZnO 2.0 mol·L−1 KOH/SCs 515 F·g−1 at 2 mA·g−1 − − − [37] NCO 0.1/1.0 KOH/ORR/OER − 0.65 275 74/54 [38] NCoHPOF-450 1.0/3.0 KOH/OER/SCs 206.3 F·g−1 at 1 A·g−1 − 276 57.11 [39] N-doped NiCo2O4 0.1/0.1 KOH/ORR/OER − 0.63 419 113/74 [40] Fe, Mn-Co3S4 6.0 mol·L−1 KOH/SCs 390 mA·h·g−1 at 5 A·g−1 − − − [41] N-CoP 0.5 mol·L−1 H2SO4/HER − − 42 41.2 [42] Notes: SCs—Supercapacitors; OER—Oxygen evolution reaction; HER—Hydrogen evolution reaction; ORR—Oxygen reduction reaction; E1/2—Limiting current density; E10—Overpotential at current density of 10 mA·cm−2; Fe-CoP UNSs/NF—Iron doped cobalt phosphide ultrathin nanosheets (Fe-CoP UNSs) with a 2.3 nm thickness on a nickel foam (NF) substrate; NCO—Atomically-thin nickel-doped spinel cobalt oxide; Co−I−N/G—Doping the carbon substrate with iodine atoms can effectively modulate the electronic structure of the atomically dispersed Co sites in a Co−N−C catalyst; NCoHPOF—Monoclinic-phase cobalt-phosphates, NH4Co3(HPO4)2(H2PO4)F2; NCoHPOF-450—Monoclinic-phase cobalt-phosphates was calcined at 450℃. -
[1] 王立伟. 美国能源信息署发布《国际能源展望2019》报告[J]. 天然气地球科学, 2019, 30(11): 1628.WANG Liwei. The US energy information administration releases the international energy outlook 2019 report[J]. Gas Geoscience, 2019, 30(11): 1628(in Chinese). [2] 赵鹬, 周飞, 张伟伟, 等. 磷化钴材料在电化学能源领域的研究进展[J]. 化工进展, 2021(4):2188-2205. doi: 10.16085/j.issn.1000-6613.2020-1045ZHAO Yu, ZHOU Fei, ZHANG Weiwei, et al. Research progress of cobalt phosphide materials in the field of electrochemical energy[J]. Chemical Industry and Engineering Progress,2021(4):2188-2205(in Chinese). doi: 10.16085/j.issn.1000-6613.2020-1045 [3] 刘虎, 杨东辉, 王许云, 等. 金属-有机框架衍生的中空碳材料及其在电化学能源存储与氧还原领域中的应用[J]. 无机化学学报, 2019, 35(11):1921-1933. doi: 10.11862/CJIC.2019.237LIU Hu, YANG Donghui, WANG Xuyun, et al. Metal-orga-nic framework-derived hollow carbon materials for electrochemical energy storage and oxygen reduction reaction[J]. Chinese Journal of Inorganic Chemistry,2019,35(11):1921-1933(in Chinese). doi: 10.11862/CJIC.2019.237 [4] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature,2001,414(6861):359-367. doi: 10.1038/35104644 [5] XU H M, CI S Q, DING Y C, et al. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems[J]. Journal of Materials Chemistry A,2019,7(14):8006-8029. doi: 10.1039/C9TA00833K [6] CAVALIERE S, SUBIANTO S, SAVYCH I, et al. Electrospinning: Designed architectures for energy conversion and storage devices[J]. Energy Environmental Science,2011,4:4761-4785. doi: 10.1039/c1ee02201f [7] FAN L Z, HU Y S, MAIER J, et al. High electroactivity of polyaniline in supercapacitors by using a hierarchically porous carbon monolith as a support[J]. Advanced Function Materials,2007,17(16):3083-3087. doi: 10.1002/adfm.200700518 [8] SHAO M H, CHANG Q W, DODELET J P, et al. Recent advances in electrocatalysts for oxygen reduction reaction[J]. Chemical Reviews,2016,116(6):3594-3657. doi: 10.1021/acs.chemrev.5b00462 [9] TAHIR M, PAN L, IDREES F, et al. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review[J]. Nano Energy,2017,37:136-157. doi: 10.1016/j.nanoen.2017.05.022 [10] VARGHESE B, HOONG T C, YANWU Z, et al. CO3O4 nanostructures with different morphologies and their field-emission properties[J]. Advanced Function Materials,2007,17(12):1932-1939. doi: 10.1002/adfm.200700038 [11] LI W Y, ZHANG B J. A dendritic nickel cobalt sulfide nanostructure for alkaline battery electrodes[J]. Advanced Functional Materials,2018,28(23):1705937. doi: 10.1002/adfm.201705937 [12] WANG X, TIAN W, ZHAI T Y, et al. Cobalt(II, III) oxide hollow structures: Fabrication, properties and applications[J]. Journal of Materials Chemistry,2012,22:23310-23326. doi: 10.1039/c2jm33940d [13] SUN H, ANG H M, TADE M O, et al. Co3O4 nanocrystals with predominantly exposed facets: Synthesis, environmental and energy applications[J]. Journal of Materials Chemistry A,2013,1:14427-14442. doi: 10.1039/c3ta12960h [14] QIU H J, LIU L, MU Y P, et al. Designed synthesis of cobalt-oxide-based nanomaterials for superior electrochemical energy storage devices[J]. Nano Research,2015,8:321-339. doi: 10.1007/s12274-014-0589-6 [15] ZHU Y P, GUO C, ZHENG Y, et al. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes[J]. Accounts of Chemical Research,2017,50:915-923. doi: 10.1021/acs.accounts.6b00635 [16] YAO Y F, ZHU Y H, HUANG J F, et al. Porous CoS nanosheets coated by N and S doped carbon shell on graphene foams for free-standing and flexible lithium ion battery anodes: Influence of void spaces, shell and porous nanosheet[J]. Electrochimica Acta,2018,271:242-251. doi: 10.1016/j.electacta.2018.03.144 [17] ZHAO J, HE Y, WANG J J, et al. Regulating metal active sites of atomically-thin nickel-doped spinel cobalt oxide toward enhanced oxygen electrocatalysis[J]. Chemical Engineering Journal,2022,435:134261-134268. doi: 10.1016/j.cej.2021.134261 [18] KANG T, KIM J. Optimal cobalt-based catalyst containing high-ratio of oxygen vacancy synthesized from metal-organic-framework (MOF) for oxygen evolution reaction (OER) enhancement[J]. Applied Surface Science,2021,560:150035-150043. doi: 10.1016/j.apsusc.2021.150035 [19] ZANG Y, YANG B P, LI A, et al. Tuning interfacial active sites over porous Mo2N-supported cobalt sulfides for efficient hydrogen evolution reactions in acid and alkaline electrolytes[J]. ACS Applied Materials Interfaces,2021,13(35):41573-41583. doi: 10.1021/acsami.1c10060 [20] WANG Y H, ZHANG Y Y, PENG Y Y, et al. Physical confinement and chemical adsorption of porous C/CNT micro/nano-spheres for CoS and Co9S8 as advanced lithium batteries anodes[J]. Electrochimica Acta,2019,299:489-499. doi: 10.1016/j.electacta.2018.11.138 [21] YANG X F, WANG A Q, QIAO B T, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research,2013,46(8):1740-1748. doi: 10.1021/ar300361m [22] JI L L, WANG J Y, TENG X, et al. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting[J]. ACS Catalysis,2019,10(1):412-419. [23] WANG J Q, QUAN Y L, WANG G X, et al. 3D hollow cage copper cobalt sulfide derived from metal-organic frameworks for high-performance asymmetric super-capacitors[J]. CrystEngComm,2021,23:7385-7396. doi: 10.1039/D1CE00884F [24] PARK H, OH S, LEE S, et al. Cobalt- and nitrogen-codoped porous carbon catalyst made from core-shell type hybrid metal-organic framework (ZIF-L@ZIF-67) and its efficient oxygen reduction reaction (ORR) activity[J]. Applied Catalysis B: Environmental,2019,246:322-329. doi: 10.1016/j.apcatb.2019.01.083 [25] WANG H, LI J M, LI K, et al. Transition metal nitrides for electrochemical energy application[J]. Chemical Society Reviews,2021,50:1354-1390. doi: 10.1039/D0CS00415D [26] WANG X D, ZHOU H P, ZHANG D K, et al. Mn-doped NiP2 nanosheets as an efficient electrocatalyst for enhanced hydrogen evolution reaction at all pH values[J]. Journal of Power Sources,2018,387(31):1-8. [27] 王敏, 谢元华, 由美雁, 等. 两步法制备B-Mo共掺杂BiVO4及光催化活性研究[J]. 材料导报, 2016, 30(28):248-252.WANG Min, XIE Yuanhua, YOU Meiyan, et al. Synthesis and photocatalytic performance of B-Mo co-doped BiVO4 photocatalyst through two steps[J]. Materials Reports,2016,30(28):248-252(in Chinese). [28] JIANG J, LI Z P, HE X R, et al. Novel skutterudite CoP3-based asymmetric supercapacitor with super high energy density[J]. Small,2020,16(31):2000180. doi: 10.1002/smll.202000180 [29] LIU X J, XI W, LI C, et al. Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting[J]. Nano Energy,2018,44:371-377. doi: 10.1016/j.nanoen.2017.12.016 [30] LI Y, LI F M, ZHAO Y, et al. Iron doped cobalt phosphide ultrathin nanosheets on nickel foam for overall water splitting[J]. Journal of Materials Chemistry A,2019,7(36):20658-20666. doi: 10.1039/C9TA07289F [31] LI X R, WEI J L, LI Q, et al. Nitrogen-doped cobalt oxide nanostructures derived from cobalt-alanine complexes for high-performance oxygen evolution reactions[J]. Advanced Functional Materials,2018,28(23):1800886. doi: 10.1002/adfm.201800886 [32] XUE N, LIN Z, LI P K, et al. Sulfur-doped CoSe2 porous nanosheets as efficient electrocatalysts for the hydrogen evolution reaction[J]. ACS Applied Materials Interfaces,2020,12(25):28288-28297. doi: 10.1021/acsami.0c07088 [33] LIU J B, WANG D S, HUANG K, et al. Iodine-doping-induced electronic structure tuning of atomic cobalt for enhanced hydrogen evolution electrocatalysis[J]. ACS Nano,2021,15(11):18125-18134. doi: 10.1021/acsnano.1c06796 [34] GUAN C, XIAO W, WU H J, et al. Hollow Mo-doped CoP nanoarrays for efficient overall water splitting[J]. Nano Energy,2018,48:73-80. doi: 10.1016/j.nanoen.2018.03.034 [35] CHUN T, RONG Z, LU W B, et al. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation[J]. Advanced Materials,2017,29(2):1602441. doi: 10.1002/adma.201602441 [36] CHEN J P, NI B Q, HU J G, et al. Defective graphene aerogel-supported Bi-CoP nanoparticles as a high potential air cathode for rechargeable Zn-air batteries[J]. Journal of Materials Chemistry A,2019,7(39):22507-22513. doi: 10.1039/C9TA07669G [37] RASHID A R, ABID A G, SUMAIRA M, et al. Inductive effect in Mn-doped ZnO nanoribon arrays grown on Ni foam: A promising key for boosted capacitive and high specific energy supercapacitors[J]. Ceramics International,2021,47(20):28338-28347. doi: 10.1016/j.ceramint.2021.06.251 [38] ZHAO J, HE Y, WANG J J, et al. Regulating metal active sites of atomically-thin nickel-doped spinel cobalt oxide toward enhanced oxygen electrocatalysis[J]. Chemical Engineering Journal,2022,435:34261-34268. [39] PAN D S, GUO Z H, LI J K, et al. Rational construction of a N, F co-doped mesoporous cobalt phosphate with rich-oxygen vacancies for oxygen evolution reaction and supercapacitors[J]. Chemistry-A European Journal,2021,27:7731-7737. doi: 10.1002/chem.202100383 [40] BIAN J J, CHENG X P, MENG X Y, et al. Nitrogen-doped NiCo2O4 microsphere as an efficient catalyst for flexible rechargeable zinc-air batteries and self-charging power system[J]. ACS Applied Energy Materials,2019,2(3):2296-2304. doi: 10.1021/acsaem.9b00120 [41] LU W, YANG Y, ZHANG T Y, et al. Synergistic effects of Fe and Mn dual-doping in Co3S4 ultrathin nanosheets for high-performance hybrid supercapacitors[J]. Journal of Colloid and Interface Science,2021,590(15):226-237. [42] ZHOU Q W, SHEN Z H, ZHU C, et al. Nitrogen-doped CoP electrocatalysts for coupled hydrogen evolution and sulfur generation with low energy consumption[J]. Advanced Materials,2018,30(27):1800140. doi: 10.1002/adma.201800140 [43] LIN Y, CHEN X M, TUO Y X, et al. In-situ doping-induced lattice strain of NiCoP/S nanocrystals for robust wide pH hydrogen evolution electrocatalysis and super-capacitor[J]. Journal of Energy Chemistry,2022,70:27-35. doi: 10.1016/j.jechem.2022.02.024 [44] 向阳, 熊昆, 张海东, 等. 电催化尿素氧化的镍基催化剂表界面调控[J]. 材料导报, 2022, 36(10):104-111. doi: 10.11896/cldb.20080297XIANG Yang, XIONG Kun, ZHANG Haidong, et al. Surface interface regulation of nickel-based catalysts for electrocatalytic urea oxidation[J]. Materials Reports,2022,36(10):104-111(in Chinese). doi: 10.11896/cldb.20080297 [45] ZHANG K, ZHANG G, QU J H, et al. Disordering the atomic structure of Co(II) oxide via B-doping: An efficient oxygen vacancy introduction approach for high oxygen evolution reaction electrocatalysts[J]. Small,2018,14(41):1802760-1802768. [46] XING M, GAO A M, LIANG Y S, et al. Defect-engineered 3D cross-network Co3O4-xNx nanostructure for high-performance solid-state asymmetric supercapacitors[J]. ACS Applied Energy Materials,2021,4(1):888-898. doi: 10.1021/acsaem.0c02779 [47] LIU H M, JIN M M, ZHAN D, et al. Stacking faults triggered strain engineering of ZIF-67 derived Ni-Co bimetal phosphide for enhanced overall water splitting[J]. Applied Catalysis B: Environmental,2020,272:118951-118959. doi: 10.1016/j.apcatb.2020.118951 [48] 林海, 苏玮韬, 朱玉, 等. WO3纳米花的热处理晶格调控及WO3/CdS/α-S异质结的构筑[J]. 无机材料学报, 2020, 35(12):1349-1359. doi: 10.15541/jim20200023LIN Hai, SU Weitao, ZHU Yu, et al. Lattice control of WO3 nanoflowers by heat treatment and construction of WO3/CdS/α-S heterojuntion[J]. Journal of Inorganic Materials,2020,35(12):1349-1359(in Chinese). doi: 10.15541/jim20200023 [49] XU D Y, LONG X D, XIAO J X, et al. Rationally constructing CoO and CoSe2 hybrid with CNTs-graphene for impres-sively enhanced oxygen evolution and DFT calculations[J]. Chemical Engineering Journal,2021,422:129982. doi: 10.1016/j.cej.2021.129982 [50] LI F Z, CHEN Z, ZHANG D, et al. Neoteric hollow tubular MnS/Co3S4 hybrids as high-performance electrode materials for supercapacitors[J]. Electrochimica Acta,2021,390(10):138893-138901. [51] GE Y C, CHU H, CHEN J Y, et al. Ultrathin MoS2 nanosheets decorated hollow CoP heterostructures for enhanced hydrogen evolution reaction[J]. ACS Sustainable Chemistry & Engineering,2019,7(11):10105-10111. [52] 华丽. 催化剂制备与应用[M]. 黄德奇. 北京: 化学工业出版社, 2018: 1-10.HUA Li. Preparation and application of catalyst[M]. HUANG Deqi. Beijing: Chemical Industry Press, 2018: 1-10(in Chinese). [53] 郝策, 刘自若, 刘炜, 等. 用于氧还原反应的碳基负载金属单原子催化剂研究进展[J]. 无机材料学报, 2021, 36(8):820-834. doi: 10.15541/jim20200582HAO Ce, LIU Ziruo, LIU Wei, et al. Research progress of carbon-supported metal single atom catalysts for oxygen reduction reaction[J]. Journal of Inorganic Materials,2021,36(8):820-834(in Chinese). doi: 10.15541/jim20200582 [54] 李亚东, 李伟平, 王琴, 等, 碳纤维支撑柔性碳硫复合电极的制备、物性及电池性能研究[J]. 无机材料学报, 2019, 34(4): 373-378.LI Yadong, LI Weiping, WANG Qin, et al. Flexible carbon-fiber supported carbon-sulfur electrode: Preparation, physical property and electrochemical performance[J]. Journal of Inorganic Materials, 2019, 34(4): 373-378(in Chinese). [55] PU Z H, LIU Q, JIANG P, et al. CoP nanosheet arrays supported on a Ti plate: An efficient cathode for electrochemical hydrogen evolution[J]. Chemistry of Materials,2014,26(15):4326-4329. doi: 10.1021/cm501273s [56] WANG J, LI K, ZHONG H X, et al. Synergistic effect between metal-nitrogen-carbon sheets and NiO nanoparticles for enhanced electrochemical water-oxidation performance[J]. Angewandte Chemie International Edition,2015,54(36):10530-10534. doi: 10.1002/anie.201504358 [57] DENG J, REN P J, DENG D H, et al. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction[J]. Angewandte Chemie International Edition,2015,54:2100-2104. doi: 10.1002/anie.201409524 [58] SUBBARAMAN R, TRIPKOVIC D, STRMCNIK D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces[J]. Science,2011,334:1256-1260. doi: 10.1126/science.1211934 [59] 贾少培, 宗泳吉, 黄权, 等. 蛋白质衍生氮掺杂碳用作电化学能源材料的研究进展[J]. 材料导报, 2023, 37(15):21100210-21100234.JIA Shaopei, ZONG Yongji, HUANG Quan, et al. Research progress of protein-derived nitrogen-doped carbon materials as electrochemical energy materials[J]. Materials Reports,2023,37(15):21100210-21100234(in Chinese). [60] 俞彬, 谌小燕, 赵越, 等. 氧化石墨烯基钴卟啉复合材料的电催化析氢反应[J]. 高等学校化学学报, 2022, 43(2):20210549-20210556.YU Bing, CHEN Xiaoyan, ZHAO Yue, et al. Graphene oxide based cobalt porphyrin composites for electrocatalytic hydrogen evolution reaction[J]. Chemical Journal of Chinese Universities,2022,43(2):20210549-20210556(in Chinese). [61] ABBAS Q, SIYAL S H, MATEEN A, et al. Hydrothermal synthesis of binder-free metallic NiCo2O4 nano-needles supported on carbon cloth as an advanced electrode for supercapacitor applications[J]. Materials,2022,15:4499-4510. doi: 10.3390/ma15134499 [62] 薛李静, 费星, 刘江淋, 等. 木质素基碳材料催化剂的制备及应用研究进展[J]. 化工进展, 2022, 41(5):2441-2450.XUE Lijing, FEI Xing, LIU Jianglin, et al. Research progress on the preparation and application of lignin-based carbon catalysts[J]. Chemical Industry and Engineering Progress,2022,41(5):2441-2450(in Chinese). [63] 古铭岚, 苏永庆, 代灵英, 等. 碳材料和硫、硒掺杂金属材料电化学还原CO2[J]. 应用化工, 2021, 50(10):2817-2821. doi: 10.3969/j.issn.1671-3206.2021.10.041GU Minglan, SU Yongqing, DAI Lingying, et al. Electrochemical reduction of CO2 with carbon materials, sulfur and selenium doped metal materials[J]. Applied Chemi-cal Industry,2021,50(10):2817-2821(in Chinese). doi: 10.3969/j.issn.1671-3206.2021.10.041 [64] LIU S, LI L, AHNB H S, et al. Delineating the roles of Co3O4 and N-doped carbon nanoweb (CNW) in bifunctional Co3O4/CNW catalysts for oxygen reduction and oxygen evolution reactions[J]. Journal of Materials Chemistry A,2015,3(21):11615-11623. doi: 10.1039/C5TA00661A [65] JIA X D, GAO S J, LIU T Y, et al. Controllable synthesis and bi-functional electrocatalytic performance towards oxygen electrode reactions of Co3O4/NRGO composites[J]. Electrochimica Acta,2017,226(1):104-112. [66] MIAO C X, XIAO X H, GONG Y, et al. Facile synthesis of metal-organic framework-derived CoSe2 nanoparticles embedded in the N-doped carbon nanosheet array and application for supercapacitors[J]. ACS Applied Materials Interfaces,2020,12(8):9365-9375. doi: 10.1021/acsami.9b22606 [67] GE H Y, LI G D, SHEN J X, et al. Co4N nanoparticles encapsulated in N-doped carbon box as tri-functional catalyst for Zn-air battery and overall water splitting[J]. Applied Catalysis B: Environmental,2020,275:119104-119115. doi: 10.1016/j.apcatb.2020.119104 [68] PARK S W, SHIN H J, KIM D W. S, N co-doped reduced graphene oxide sheets with cobalt hydroxide nanocrystals for highly active and stable bifunctional oxygen catalysts[J]. Inorganic Chemistry Frontiers,2019,6:3501-3509. doi: 10.1039/C9QI01108K [69] 赵文誉, 王振祥, 郑玉婴, 等. NiS2/三维多孔石墨烯复合材料作为超级电容器电极材料的电化学性能[J]. 复合材料学报, 2020, 37(2):422-431.ZHAO Wenyu, WANG Zhenxiang, ZHENG Yuying, et al. Electrochemical performance of NiS2 3D porous reduce graphene oxide composite as electrode materials for supercapacitors[J]. Acta Materiae Compositae Sinica,2020,37(2):422-431(in Chinese). [70] LOU X. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors[J]. Energy Environmental Science,2012,5:7883-7887. doi: 10.1039/c2ee21745g [71] LI D X, YUE C M, HU B Y, et al. Amorphous CoSx nanoparticles anchoring onto N-doped carbon nanotubes as high-performance electrodes for hybrid super-capacitors[J]. Journal of Alloys and Compounds,2021,865:158756-158765. doi: 10.1016/j.jallcom.2021.158756 [72] ABDEL-SALAM A I, ATTIA S Y, EL-HOSINY F I, et al. Facile one-step hydrothermal method for NiCo2S4/rGO nanocomposite synthesis for efficient hybrid supercapacitor electrodes[J]. Materials Chemistry and Physics,2022,277:125554-125564. doi: 10.1016/j.matchemphys.2021.125554 [73] QU G M, LI C L, HOU P Y, et al. Hierarchically hollow structured NiCo2S4@NiS for high-performance flexible hybrid supercapacitors[J]. Nanoscale,2020,12(7):4686-4694. doi: 10.1039/C9NR09991C [74] FANG W G, ZHAO J J, ZHANG W, et al. Recent progress and future perspectives of flexible Zn-air batteries[J]. Journal of Alloys and Compounds,2021,869:158918-158939. doi: 10.1016/j.jallcom.2021.158918 [75] MENEZES P W, INDRA A, GONZALEZ-FLORES D, et al. High-performance oxygen redox catalysis with multifunctional cobalt oxide nanochains: Morphology-dependent activity[J]. ACS Catalysis,2015,5(4):2017-2027. doi: 10.1021/cs501724v [76] WANG S Y, CHEN S M, MA L T, et al. Recent progress in cobalt-based carbon materials as oxygen electrocatalysts for zinc-air battery applications[J]. Materials Today Energy,2021,20:100659-100690. doi: 10.1016/j.mtener.2021.100659 [77] WANG X, LIAO Z Q, FU Y B, et al, Confined growth of porous nitrogen-doped cobalt oxide nanoarrays as bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries[J]. Energy Storage Materials, 2020, 26: 157-164. [78] ZOU X X, ZHANG Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chemical Society Reviews,2015,44:5148-5180. doi: 10.1039/C4CS00448E [79] JIN Z, LI P, XIAO D. Metallic Co2P ultrathin nanowires distinguished from CoP as robust electrocatalysts for overall water-splitting[J]. Green Chemistry,2015,18(6):1459-1464. [80] LI N, LIU X, LI G D, et al. Vertically grown CoS nanosheets on carbon cloth as efficient hydrogen evolution electrocatalysts[J]. International Journal of Hydrogen Energy,2017,42(15):9914-9921. doi: 10.1016/j.ijhydene.2017.01.191 [81] MUTHURASU A, MARUTHAPANDIAN V, KIM H Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction[J]. Applied Catalysis B: Environmental,2019,248:202-210. doi: 10.1016/j.apcatb.2019.02.014 [82] ELAKKIY R, RAMKUMAR R, MADURAIVEERAN G. Flower-like nickel-cobalt oxide nanomaterials as bi-functional catalyst for electrochemical water splitting[J]. Materials Research Bulletin,2019,116:98-105. doi: 10.1016/j.materresbull.2019.04.016 -





 下载:
下载: