Basic scientific problems of nickel-rich cathode for lithium-ion battery: Morphology and accumulation regulation of primary particles
-
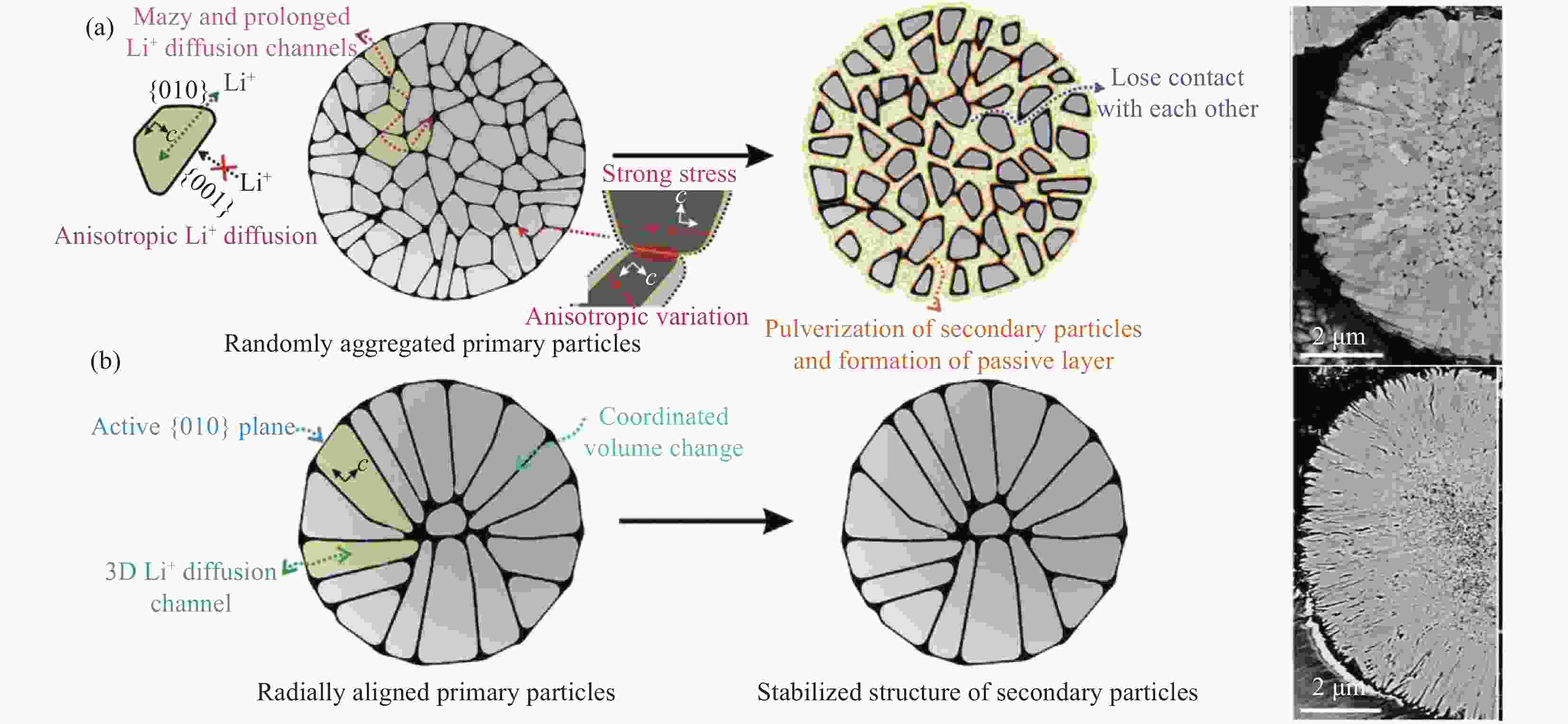
摘要: 晶粒辐射状组装的富镍正极具有较常规无序多晶更优的断裂韧性和Li+扩散速率,是快充长寿命锂离子电池理想的正极材料。近年来,部分研究者报道了系列富镍正极晶粒形态及组装方式调控研究进展,所开发的辐射状富镍正极性能优异,代表着全球顶尖水平,且相关技术应用于韩国电池巨头LG化学。然而,高度辐射状富镍正极材料的合成在国内尚处于起步阶段,且尚无合成辐射状富镍正极所需晶粒形态及组装方式调控的系统阐述。本文首先介绍富镍正极晶粒形态及组装方式调控的必要性;再综述了晶粒辐射状富镍正极所需前驱体沉淀结晶与其受控锂化的研究进展,并对沉淀及高温煅烧结晶调控晶粒形态及组装方式所涉及机制进行了分析,以期为国内相关专业人员开发高端富镍正极材料提供参考。Abstract: Compared with randomly oriented cathodes, nickel-rich cathodes assembled with radially oriented primary particle, which are ideal materials for fast charging and long-life lithium ion battery, have better fracture toughness and Li+ diffusion rate. In recent years, Some researchers have reported a series of studies on morphology and accumulation regulation of primary particles for nickel-rich cathode. The performance of the cathodes developed by his group is excellent, representing the top level in the world, and the related technology has recently been transferred to the South Korean battery giant LG Chem. However, the synthesis of cathodes assembled with radially oriented primary particles is still in its infancy in China, and there is no systematic elaboration for the morphology and accumulation regulation of primary particles required to synthesize the nickel-rich cathode. In this paper, the necessity for the morphology and accumulation regulation of primary particles is first introduced. Then, the research progresses about the precipitation and high-temperature lithiation crystallization of precursor for the cathodes are summarized, and the mechanism involved in regulation of precipitation and high temperature calcination crystallization is analyzed. This pare is hoped to provide reference for relevant domestic specialist staff when developing high-end nickel-rich cathode materials.
-
Key words:
- nickel rich cathode material /
- precursor /
- precipitation crystallization /
- doping /
- radial
-
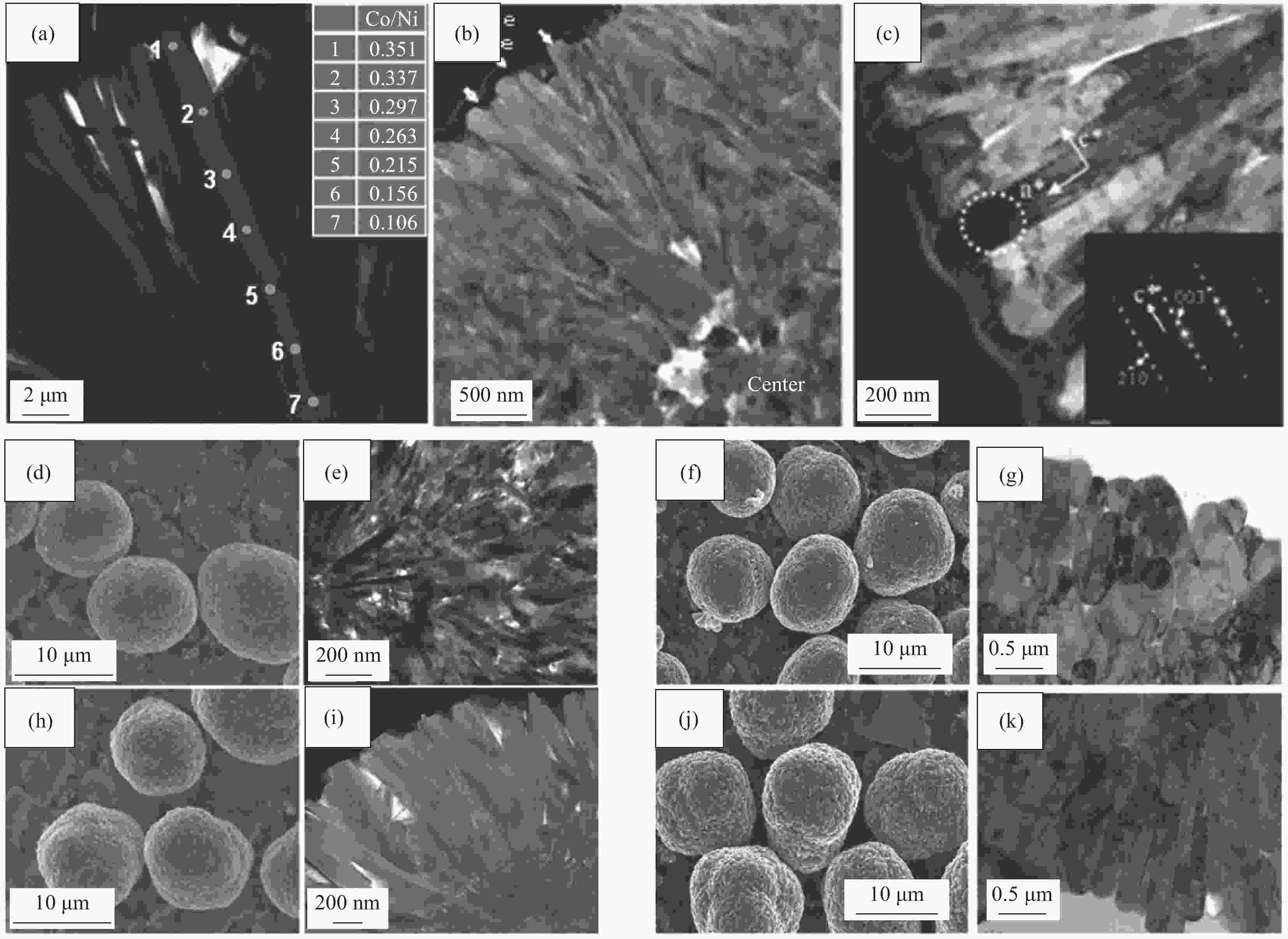
图 3 pH值与氨浓度分别为10.5与6 mol·L−1 ((a), (d))、11与4 mol·L−1 ((b), (e))、11.5与2 mol·L−1 ((c), (f)) 合成Ni0.6Co0.2Mn0.2(OH)2前驱体及其正极的截面SEM图像[5]
Figure 3. Cross-sectional SEM images of precursor Ni0.6Co0.2Mn0.2(OH)2 and corresponding cathodes synthesized at 10.5 and 6 mol·L−1 ((a), (d)), 11 and 4 mol·L−1 ((b), (e)), 11.5 and 2 mol·L−1 ((c), (f))[5]
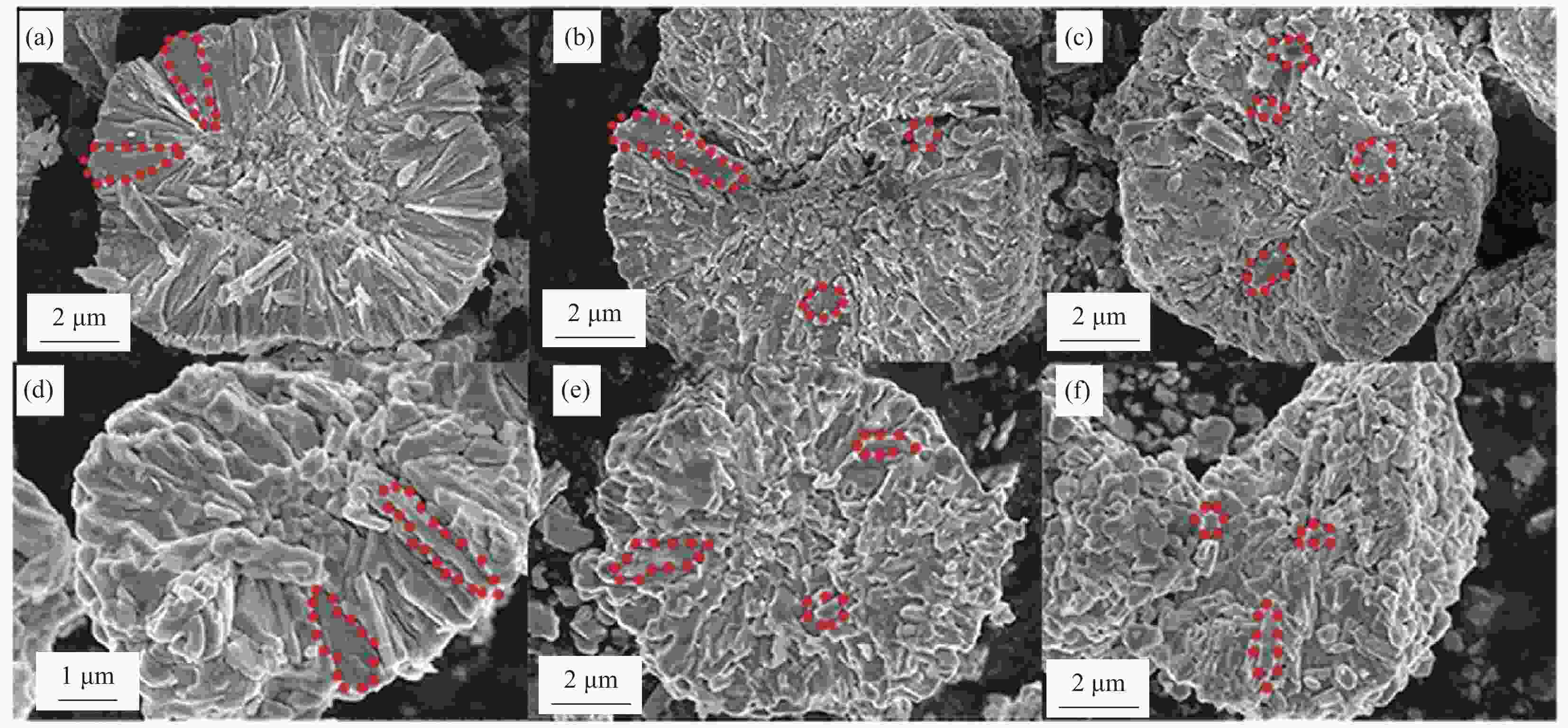
图 4 全浓度梯度前驱体 (a) 及其正极 ((b), (c)) 截面TEM图像及SEM图像[7];((d)~(g)) 无序梯度壳前驱体及其正极SEM图像及截面TEM图像;((h)~(k)) 辐射状梯度壳前驱体及其正极SEM图像及截面TEM图像[20]
Figure 4. Cross-sectional TEM and SEM images of the full concentration gradient precursor (a) and corresponding cathodes ((b), (c))[7]; ((d)-(g)) SEM and TEM images of concentration gradient core-shell precursor and cathode with a nanoparticle shell; ((h)-(k)) SEM and TEM images of concentration gradient core-shell precursor and cathode with a nanorod shell[20]
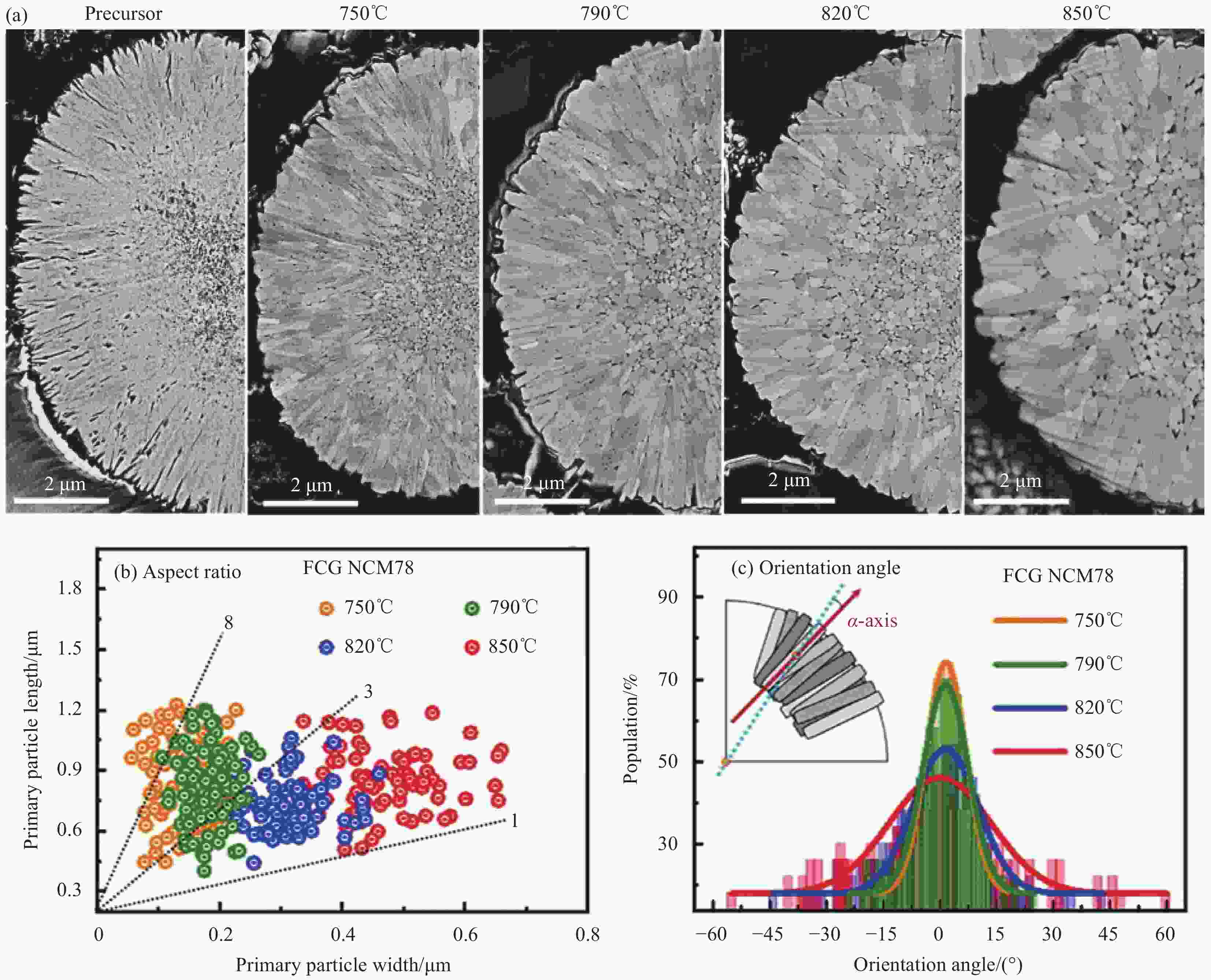
图 6 (a) 前驱体和不同温度煅烧LiNi0.78Co0.1Mn0.12O2 (FCG NCM78)的截面SEM图像;不同温度煅烧FCG NCM78的一次晶粒测量的宽度与长度比 (b) 及晶粒取向 (c)[11]
Figure 6. (a) Cross-sectional SEM images of the precursor and LiNi0.78Co0.1Mn0.1O2 (FCG NCM78) calcined at different temperature; Measured length and width ratio (b) and orientation (c) of primary FCG NCM78 particles calcined at various temperatures[11]
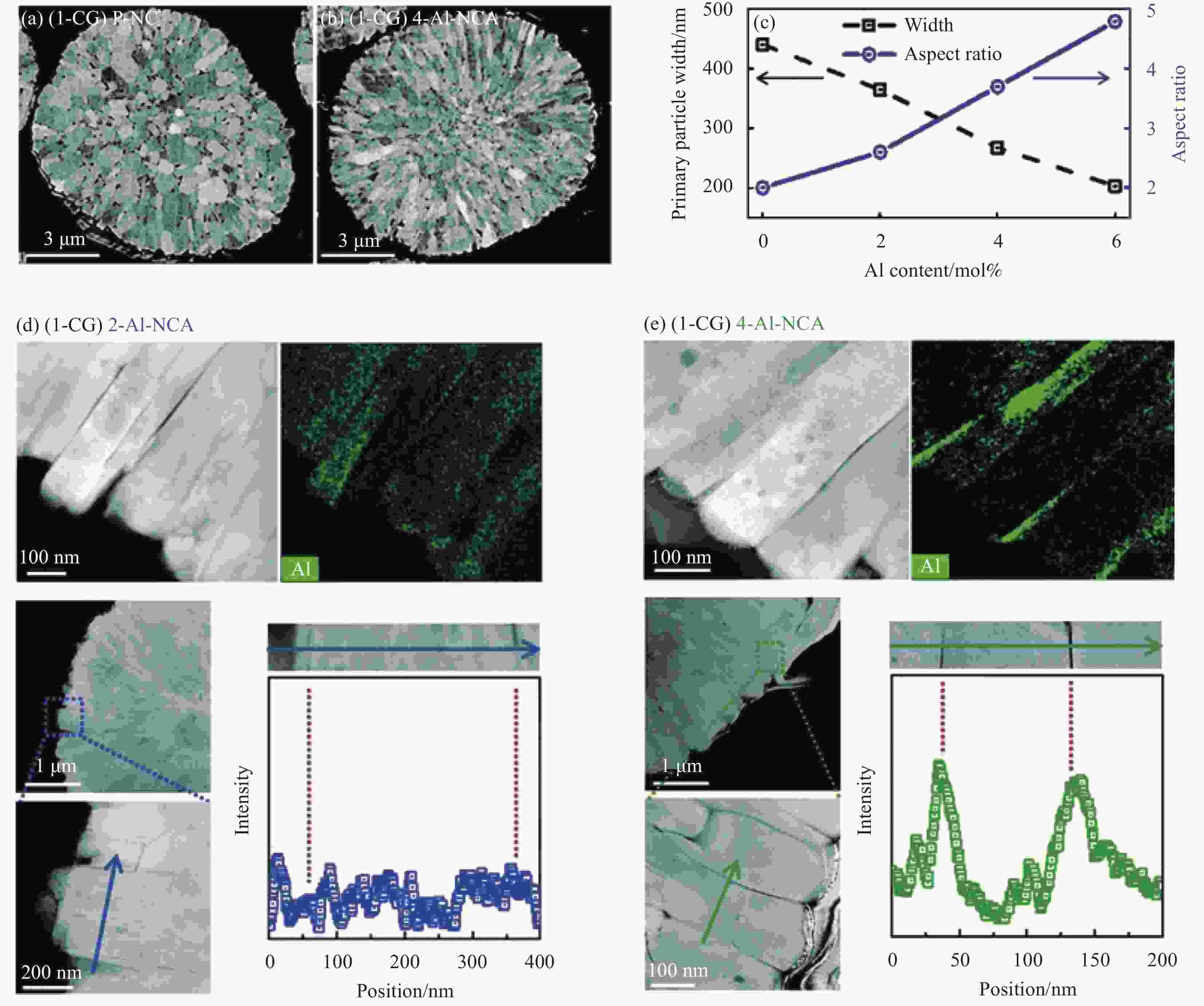
图 7 在720°C煅烧的LiNi0.9Co0.1O2(P-NC) (a) 和LiNi0.86Co0.1Al0.04O2(4-Al-NCA) (b) 正极的截面SEM图像; (c) 在720℃煅烧的不同Al含量正极的一次晶粒的宽度及长宽比;在720℃煅烧的LiNi0.88Co0.1Al0.02O2(2-Al-NCA) (d) 和4-Al-NCA (e) 的TEM-EDX元素面分布和线扫结果[16]
1-CG—Al doped Ni0.9Co0.1(OH)2
Figure 7. Cross sectional SEM images of LiNi0.9Co0.1O2 (P-NC) (a) and LiNi0.86Co0.1Al0.04O2 (4-Al-NCA) (b) cathodes lithiated at 720℃; (c) Primary particle width and aspect ratio (length/width) of cathodes with different Al content lithiated at 720°C; TEM-EDX elemental mapping and line scan results of LiNi0.88Co0.1Al0.02O2 (2-Al-NCA) (d) and 4-Al-NCA (e) cathodes lithiated at 720°C[16]
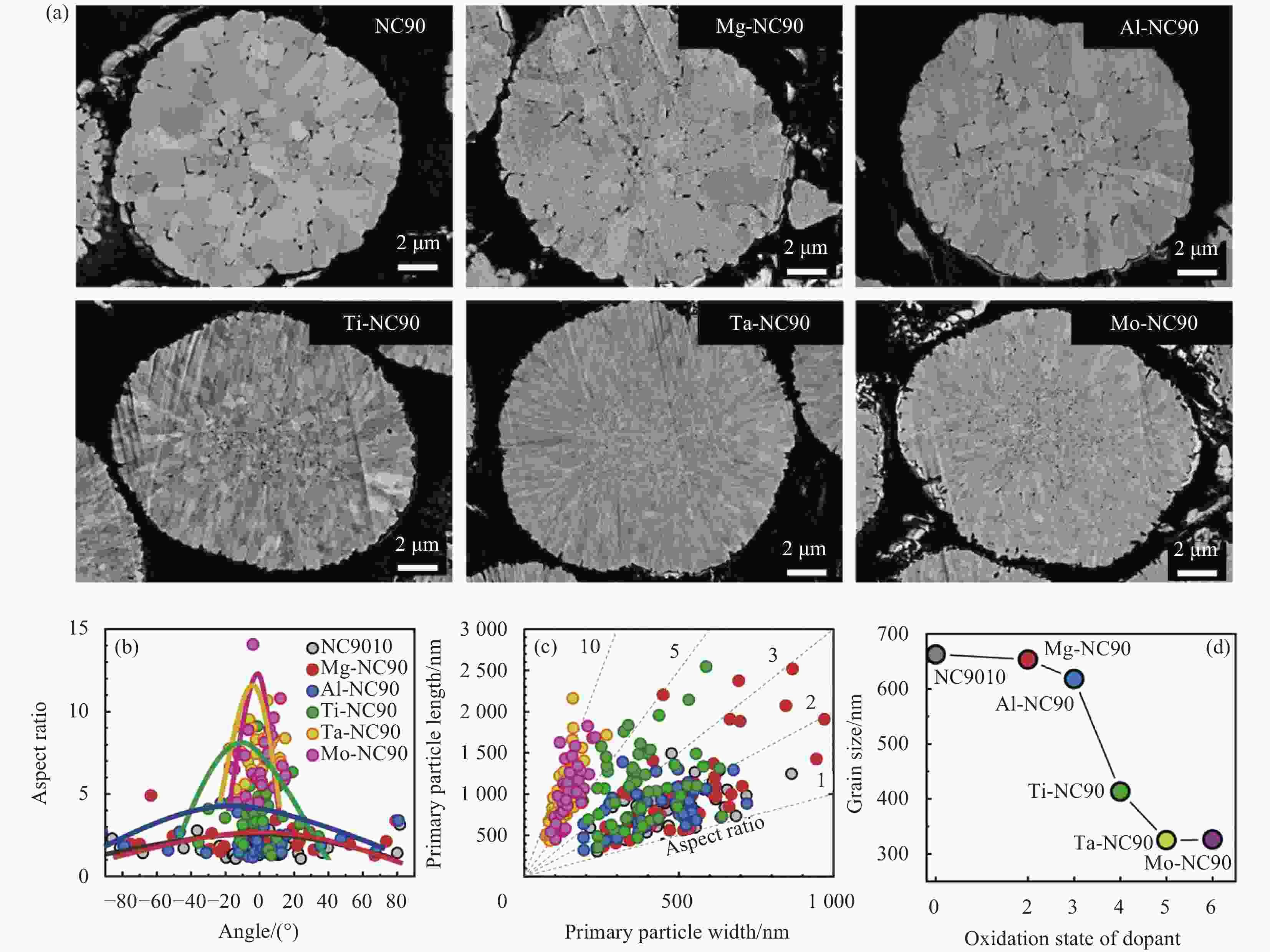
图 8 (a)未掺杂LiNi0.9Co0.1O2 (NC-90)及掺杂Mg、Al、Ti、Ta和Mo的LiNi0.9Co0.1O2(Mg-NC90、Al-NC90、Ti-NC90、Ta-NC90和Mo-NC90)正极截面SEM图像[21];长径比与相对晶粒取向 (b)、长径比(长/宽) (c)和晶粒尺寸 (d) 作为氧化态函数的关系
Figure 8. (a) Cross-sectional SEM images of pristine LiNi0.9Co0.1O2 (NC-90) and doped LiNi0.9Co0.1O2 cathodes with Mg, Al, Ti, Ta and Mo (Mg-NC90, Al-NC90, Ti-NC90, Ta-NC90 and Ta-NC90)[21]; Relationship between aspect ratio and relative grain orientation (b), aspect ratio (length/width) (c) and grain size (d) as a function of oxidation state
-
[1] SU Y, CHEN G, CHEN L, et al. Advances and prospects of surface modification on nickel-rich materials for lithium-ion batteries[J]. Chinese Journal of Chemistry,2020,38(12):1817-1831. doi: 10.1002/cjoc.202000385 [2] SU Y, LI L, CHEN G, et al. Strategies of removing residual lithium compounds on the surface of Ni-rich cathode materials[J]. Chinese Journal of Chemistry,2021,39(1):189-198. doi: 10.1002/cjoc.202000386 [3] AMINE K, LIU J, BELHAROUAK I, et al. Advanced cathode materials for high-power applications[J]. Journal of Power Sources,2005,146(1-2):111-115. doi: 10.1016/j.jpowsour.2005.03.227 [4] XU X, HUO H, JIAN J Y, et al. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Advanced Energy Materials,2019,9(15):1803963. doi: 10.1002/aenm.201803963 [5] YANG C K, QI L Y, ZUO Z, et al. Insights into the inner structure of high-nickel agglomerate as high-performance lithium-ion cathodes[J]. Journal of Power Sources,2016,331:487-494. doi: 10.1016/j.jpowsour.2016.09.068 [6] ERICKSON E M, BOUZAGLO H, SCLAR H, et al. Synthesis and electrochemical performance of nickel-rich layered-structure LiNi0.65Co0.08Mn0.27O2 cathode materials comprising particles with Ni and Mn full concentration gradients[J]. Journal of the Electrochemical Society,2016,163(7):A1348-A1358. doi: 10.1149/2.0951607jes [7] NOH H J, CHEN Z, YOON C S, et al. Cathode material with nanorod structure-An application for advanced high-energy and safe lithium batteries[J]. Chemistry of Materials,2013,25(10):2109-2115. doi: 10.1021/cm4006772 [8] NOH H J, JU J W, SUN Y K. Comparison of nanorod-structured LiNi0.54Co0.16Mn0.30O2 with conventional cathode materials for Li-ion batteries[J]. Chemsuschem,2014,7(1):245-252. doi: 10.1002/cssc.201300379 [9] KIM U H, LEE E J, YOON C S, et al. Compositionally graded cathode material with long-term cycling stability for electric vehicles application[J]. Advanced Energy Materials,2016,6(22):1601417. doi: 10.1002/aenm.201601417 [10] YOON C S, KIM S J, KIM U H, et al. Microstructure evolution of concentration gradient LiNi0.75Co0.10Mn0.15O2 cathode for lithium-ion batteries[J]. Advanced Functional Materials,2018,28 (28):1802090. doi: 10.1002/adfm.201802090 [11] PARK G T, RYU H H, NOH T C, et al. Microstructure-optimized concentration-gradient NCM cathode for long-life Li-ion batteries[J]. Materials Today, 2022, 52: 9-18. [12] LIM B B, MYUNG S T, YOON C S, et al. Comparative study of Ni-rich layered cathodes for rechargeable lithium batteries: LiNi0.85Co0.11Al0.04O2 and LiNi0.84Co0.06Mn0.09Al0.01O2 with two-step full concentration gradients[J]. ACS Energy Letters,2016,1(1):283-289. doi: 10.1021/acsenergylett.6b00150 [13] LIM B B, YOON S J, PARK K J, et al. Advanced concentration gradient cathode material with two-slope for high-energy and safe lithium batteries[J]. Advanced Functional Materials,2015,25(29):4673-4680. doi: 10.1002/adfm.201501430 [14] YOON C S, PARK K J, KIM U H, et al. High-energy Ni-rich Li[NixCoyMn1–x–y]O2 cathodes via compositional partitioning for next-generation electric vehicles[J]. Chemistry of Materials,2017,29(24):10436-10445. doi: 10.1021/acs.chemmater.7b04047 [15] PARK K J, CHOI M J, MAGLIA F, et al. High-capacity concentration gradient Li[Ni0.865Co0.120Al0.015]O2 cathode for lithium-ion batteries[J]. Advanced Energy Materials,2018,8(19):1703612. doi: 10.1002/aenm.201703612 [16] PARK G T, PARK N Y, NOH T C, et al. High-performance Ni-rich LiNi0.9-xCo0.1AlxO2 cathodes via multi-stage microstructural tailoring from hydroxide precursor to the lithiated oxide[J]. Energy & Environmental Science,2021,14(9):5084-5095. [17] PARK N Y, RYU H H, KUO L Y, et al. High-energy cathodes via precision microstructure tailoring for next-generation electric vehicles[J]. ACS Energy Letters,2021,6(12):4195-4202. [18] RYU H H, PARK N Y, NOH T C, et al. Microstrain alleviation in high-energy Ni-rich NCMA cathode for long battery life[J]. ACS Energy Letters,2021,6(1):216-223. doi: 10.1021/acsenergylett.0c02281 [19] LEE E J, NOH H J, YOON C S, et al. Effect of outer layer thickness on full concentration gradient layered cathode material for lithium-ion batteries[J]. Journal of Power Sources,2015,273:663-669. doi: 10.1016/j.jpowsour.2014.09.161 [20] YOON S J, MYUNG S T, NOH H J, et al. Nanorod and nanoparticle shells in concentration gradient core-shell lithium oxides for rechargeable lithium batteries[J]. Chemsuschem,2014,7(12):3295-3303. doi: 10.1002/cssc.201402389 [21] SUN H H, KIM U H, PARK J H, et al. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries[J]. Nature Communications,2021,12(1):6552. doi: 10.1038/s41467-021-26815-6 [22] KIM U H, PARK G T, SON B K, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nature Energy,2020,5(11):860-869. [23] RYU H H, PARK N Y, YOON D R, et al. New class of Ni-rich cathode materials LiNixCoyB1-x-yO2 for next lithium batteries[J]. Advanced Energy Materials, 2020, 10(25): 2000495. [24] RYU H H, PARK N Y, SEO J H, et al. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries[J]. Materials Today,2020,36:73-82. doi: 10.1016/j.mattod.2020.01.019 [25] PARK K J, JUNG H G, KUO L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries[J]. Advanced Energy Materials,2018,8(25):1801202. doi: 10.1002/aenm.201801202 [26] CHOI C M, PARK J H, SUN Y K, et al. Ultra-stable cycling of multi-doped (Zr, B) LiNi0.885Co0.1Al0.015O2 cathode[J]. Journal of Power Sources,2021,513:230548. [27] SEO J H, KIM U H, SUN Y K, et al. Multi-doped (Ga, B) LiNi0.885Co0.1Al0.015O2 cathode[J]. Journal of the Electrochemical Society,2020,167(10):100557. [28] KIM U H, PARK N Y, PARK G T, et al. High-energy W-doped LiNi0.95Co0.04Al0.01O2 cathodes for next-generation electric vehicles[J]. Energy Storage Materials,2020,33:399-407. doi: 10.1016/j.ensm.2020.08.013 [29] RYU H H, PARK K J, YOON D R, et al. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability[J]. Advanced Energy Materials,2019,9(44):1-7. [30] KIM U H, PARK J H, AISHOVA A, et al. Microstructure engineered Ni-rich layered cathode for electric vehicle batteries[J]. Advanced Energy Materials,2021,11(25):2100884. [31] NGUYEN T T, KIM U H, YOON C S, et al. Enhanced cycling stability of Sn-doped LiNi0.9Co0.05Mn0.05O2 via optimization of particle shape and orientation[J]. Chemical Engi-neering Journal,2021,405(1):126887. -





 下载:
下载: