Expanded graphite/sulfur-fluorinated vapor-deposited carbon fiber bilayer cathode
-
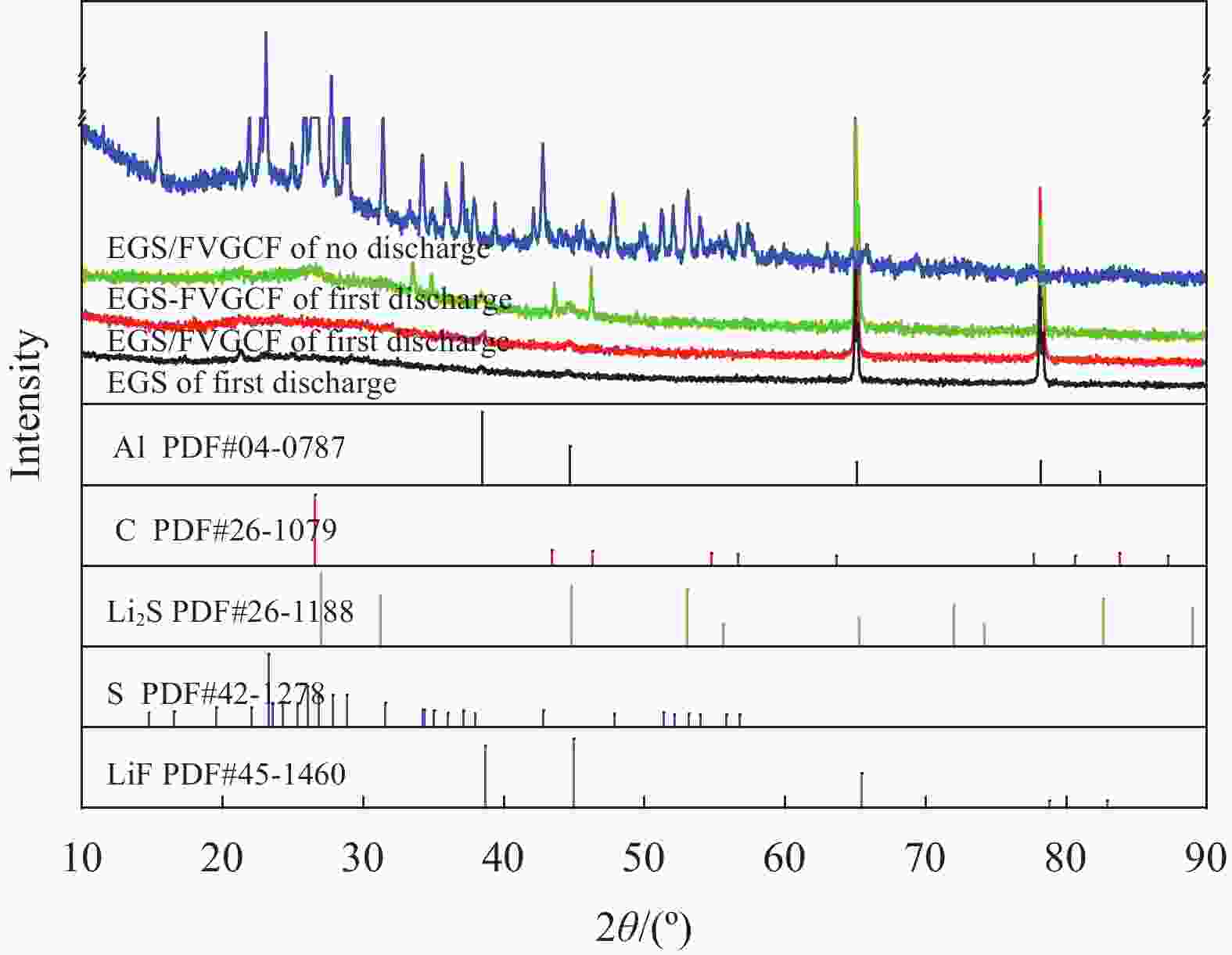
摘要: 对于高性能储能设备的迫切需求,使得理论能量密度达到2600 W·h/kg的锂硫电池(LSBs)变得极具吸引力。然而,低的容量可逆性和硫自身绝缘性的天然缺陷制约了其商业化进程。为了有效改善硫的导电性能,同时抑制多硫化物的穿梭效应,达到提高LSBs电化学性能的目的。本文采用逐层涂覆法在膨胀石墨(EG)/硫(S)复合正极极片表面涂覆氟化气相沉积碳纤维(FVGCF),通过首次放电至2.5 V实现FVGCF嵌锂,在EG/S正极极片表面形成LiF和FVGCF复合层。电化学性能测试和形貌表征结果表明:采用FVGCF新型正极材料具有最佳的循环寿命,EGS-FVGCF在1 C电流密度下的初始放电比容量为691.8 mA·h/g,100次循环之后剩余比容量为549.5 mA·h/g。相对于EGS涂覆的单层结构,在EGS上面涂覆FVGCF的双层电池性能具备极大应用优势,放电过程中生成的LiF能够抑制多硫化物从正极到负极的穿梭。同时,放充电后的电极形貌表征发现FVGCF层的加入减少了极片表面的裂纹,表明FVGCF层在一定程度上缓冲了硫正极的体积膨胀。这种简单易操作的复合结构为开发高性能LSBs提供了一定参考。Abstract: The urgent need for high-performance energy storage devices makes lithium-sulfur batteries (LSBs) with theoretical energy densities up to 2600 W·h/kg very attractive. However, the low capacity reversibility and the natural defect of sulfur's self-insulating property restrict its commercialization. In order to effectively improve the electrical conductivity of sulfur while suppressing the shuttle effect of polysulfides, the purpose of improving the electrochemical performance of LSBs is achieved. In this paper, a layer-by-layer coating method was used to coat the surface of the expanded graphite (EG)/sulfur (S) composite cathode with fluorinated vapor-deposited carbon fiber (FVGCF). A composite layer of LiF and FVGCF is formed on the surface of the pole piece. The electrochemical performance test and morphological characterization results show that the new cathode material using FVGCF has the best cycle life. The initial discharge specific capacity of EGS-FVGCF at 1 C current density is 691.8 mA h/g, and the remaining specific capacity after 100 cycles is 549.5 mA h/g. Compared with the EGS-coated single-layer structure, the double-layer battery coated with FVGCF on EGS has great application advantages, and the LiF generated during the discharge process can inhibit the shuttle of polysulfides from the positive electrode to the negative electrode. At the same time, the electrode morphology characterization after discharge and charge found that the addition of the FVGCF layer reduced the cracks on the surface of the pole piece, indicating that the FVGCF layer buffered the volume expansion of the sulfur cathode to a certain extent. This simple and easy-to-operate composite structure provides a certain reference for the development of high-performance LSBs.
-
图 4 EGS-FVGCF、EGS、EGS/FVGCF正极的电池电化学性能特性;(a)首次放充电曲线;(b) EGS-FVGCF在0.2 C电流密度的第1、10、50、100次循环放/充电曲线;(c) 0.1 mV·s−1扫描速率下的循环伏安曲线;(d)阻抗曲线;(e) 0.2 C电流密度下100次循环性能;(f) 1 C电流密度下循环100圈性能及相应库伦效率
Figure 4. Battery electrochemical performance characteristics of EGS-FVGCF, EGS, EGS/FVGCF cathodes: (a) First discharge and charge curves; (b) EGS-FVGCF at the 1st, 10th, 50th and 100th cycle discharge/charge curves at 0.2 C current density; (c) Cyclic voltammetry curve at 0.1 mV·s−1 scan rate; (d) Impedance curve; (e) 100 cycle performance at 0.2 C current density; (f) 100 cycle performance and corresponding coulombic efficiency at 1 C current density
表 1 本文与文献报道的部分上集流体材料性能对比
Table 1. Properties of fluid materials in this study compared with those reported in the literature
Upper current collector material Sulfur Sulfur load/(mg·cm–1) Current density/(mA·g–1) Specific capacity/(mA·h·g–1) Ref. VGCF S 2 50 1100 (First cycle) [33] Fe3O4/RGO CSC/S 1.0 500 434 (400 cycles) [39] Carbon paper S 1.7 335 631 (200 cycles) [40] Fe-N-C KB/S 1.0 335 428 (500 cycles) [41] M-S-LTO S 1.3 335 960 (200 cycles) [42] Waste cotton cloth S 3.05 335 423 (200 cycles) [43] FVGCF EGS 1.1 1675 549.5 (100 cycles) This work Notes: VGCF—Vapor grown carbon fiber; RGO—Reduced graphene oxide; Fe-N-C—Fe-N-doped carbon; M—Multi-walled carbon nanotubes; LTO—Li4Ti5O12; CSC—Coconut shell charcoal; KB—Ketjen black. -
[1] TARASCON M A A J M. Building better batteries[J]. Nature,2008,451:652-657. doi: 10.1038/451652a [2] WANG G X, HE P G, FAN L Z. Asymmetric polymer electrolyte constructed by metal-organic framework for solid-state, dendrite-free lithium metal battery[J]. Advanced Functional Materials,2021,31(3):2007198. doi: 10.1002/adfm.202007198 [3] LIU Y, HARIDAS A A K, LEE Y, et al. Freestanding porous sulfurized polyacrylonitrile fiber as a cathode material for advanced lithium sulfur batteries[J]. Applied Surface Science, 2019, 472(1): 135-142. [4] 陈宗宗, 张瑞丰. 基于Polymer-S-C/SiO2多层结构大孔电极锂硫离子电池的制备与性能[J]. 复合材料学报, 2014, 31(2): 525-531.CHEN Zongzong, ZHANG Ruifeng. Preparation and performance of lithium-sulfur batteries based on multilayer structure in polymer-S-C/SiO2 macroporous electrodes[J]. Acta Materiae Compositae Sinica, 2014, 31(2): 525-531(in Chinese). [5] 黄雅盼, 孙晓刚, 王杰, 等. 羟基化多壁碳纳米管掺杂抑制锂硫电池的穿梭效应[J]. 复合材料学报, 2019, 36(5): 1335-1341.HUANG Yapan, SUN Xiaogang, WANG Jie, et al. Inhibiting shuttle effect of lithium sulfur batteries by introducing hydroxylated multi-walled carbon nanotube[J]. Acta Materiae Compositae Sinica, 2019, 36(5): 1335-1341(in Chinese). [6] 施再发, 杨少彬, 刘景东, 等. 化学沉淀法制备S-FeS/介孔碳复合材料及其电化学性能[J]. 复合材料学报, 2015, 32(2): 341-346.SHI Zaifa, YANG Shaobin, LIU Jingdong, et al. Preparation of S-FeS/mesoporous carbon composites by chemical precipitation and its electrochemical properties[J]. Acta Materiae Compositae Sinica, 2015, 32(2): 341-346(in Chinese). [7] 闫崇, 李向南, 曹朝霞, 等. 高能球磨法制备PTFE/科琴黑-C柔性复合材料及其电化学应用[J]. 复合材料学报, 2016, 33(10): 2390-2396.YAN Chong, LI Xiangnan, CAO Zhaoxia, et al. Preparation of PTFE/Ketjen Black-C flexible composites by high energy ball milling method and its electrochemical application[J]. Acta Materiae Compositae Sinica, 2016, 33(10): 2390-2396(in Chinese). [8] HERBERT J U D. Electric dry cells and storage batteries: US Patent, US3043896[P]. 1962-07-10. [9] CHUNG S H, MANTHIRAM A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator[J]. Journal of Physical Chemistry Letters,2014,5(11):1978-1983. doi: 10.1021/jz5006913 [10] SINGHAL R, CHUNG S H, MANTHIRAM A, et al. A free-standing carbon nanofiber interlayer for high-performance lithium-sulfur batteries[J]. Journal of Materials Chemistry A,2015,3(8):4530-4538. doi: 10.1039/C4TA06511E [11] CUISINIER M, CABELGUEN P E, ADAMS B D, et al. Unique behaviour of nonsolvents for polysulphides in lithium-sulphur batteries[J]. Energy & Environmental Science,2014,7(8):2697-2705. [12] ZHANG S S. Binder based on polyelectrolyte for high capacity density lithium/sulfur battery[J]. Journal of the Electrochemical Society,2012,159(8):A1226-A1229. doi: 10.1149/2.039208jes [13] LI G R, CAI W L, LIU B H, et al. A multi functional binder with lithium ion conductive polymer and polysulfide absorbents to improve cycleability of lithium-sulfur batteries[J]. Journal of Power Sources,2015,294:187-192. doi: 10.1016/j.jpowsour.2015.06.083 [14] CHEN W, LEI T, QIAN T, et al. A new hydrophilic binder enabling strongly anchoring polysulfides for high-performance sulfur electrodes in lithium-sulfur battery[J]. Advanced Energy Materials, 2018, 8(12): 1702889. [15] CHENG X B, HUANG J Q, ZHANG Q. Review-Li metal anode in working lithium-sulfur batteries[J]. Journal of the Electrochemical Society,2018,165(1):A6058-A6072. doi: 10.1149/2.0111801jes [16] LI Z, JIANG Y, YUAN L X, et al. A highly ordered meso@microporous carbon-supported sulfur@smaller sulfur core-shell structured cathode for Li-S batteries[J]. ACS Nano,2014,8(9):9295-9303. doi: 10.1021/nn503220h [17] PONRAJ R, KANNAN A G, AHN J H, et al. Improvement of cycling performance of lithium-sulfur batteries by using magnesium oxide as a functional additive for trapping lithium polysulfide[J]. ACS Applied Materials & Interfaces,2016,8(6):4000-4006. [18] LIU H, LIU X, LI W, et al. Porous carbon composites for next generation rechargeable lithium batteries[J]. Advanced Energy Materials, 2017, 7(24): 1700283. [19] QI M L, LIANG X Q, WANG F, et al. Sulfur-impregnated disordered SnO2/carbon aerogel core-shell microspheres cathode for lithium-sulfur batteries[J]. Journal of Alloys and Compounds,2019,799:345-350. doi: 10.1016/j.jallcom.2019.05.366 [20] GONG Y, FU C, DONG A, et al. Polar ultrathin self-doping carbon nitride nanosheets with intrinsic polysulfide adsorption for high performance lithium-sulfur batteries[J]. Batteries & Supercaps,2018,1(5):192-201. [21] HE D, XIANG J, ZHA C, et al. The efficient redox electron transfer and powered polysulfide confinement of carbon doped tungsten nitride with multi-active sites towards high-performance lithium-polysulfide batteries[J]. Applied Surface Science,2020:525(30): 146625-146632. [22] LIU M, ZHOU D, JIANG H R, et al. A highly-safe lithium-ion sulfur polymer battery with SnO2 anode and acrylate-based gel polymer electrolyte[J]. Nano Energy,2016,28:97-105. doi: 10.1016/j.nanoen.2016.08.033 [23] SUN W, OU X, YUE X, et al. A simply effective double-coating cathode with MnO2 nanosheets/graphene as functionalized interlayer for high performance lithium-sulfur batteries[J]. Electrochimica Acta,2016,207:198-206. doi: 10.1016/j.electacta.2016.04.135 [24] ZEGEYE T A, KUO C F J, WOTANGO A S, et al. Hybrid nanostructured microporous carbon-mesoporous carbon doped titanium dioxide/sulfur composite positive electrode materials for rechargeable lithium-sulfur batteries[J]. Journal of Power Sources,2016,324:239-252. doi: 10.1016/j.jpowsour.2016.05.080 [25] LI C, ZHANG P, DAI J, et al. Rational method for improving the performance of lithium-sulfur batteries: Coating the separator with lithium fluoride[J]. ChemElectroChem,2017,4(6):1535-1543. doi: 10.1002/celc.201700154 [26] WU F, QIAN J, CHEN R, et al. An effective approach to protect lithium anode and improve cycle performance for Li-S batteries[J]. ACS Applied Materials & Interfaces,2014,6(17):15542-15549. [27] LIU Y, QIN X, ZHANG S, et al. Fe3O4-decorated porous graphene interlayer for high-performance lithium-sulfur batteries[J]. ACS Applied Materials & Interfaces,2018,10(31):26264-26273. doi: 10.1021/acsami.8b07316 [28] RAULO A, BANDYOPADHYAY S, AHAMAD S, et al. Bio-inspired poly(3, 4-ethylenedioxythiophene) : poly(styrene sulfonate)-sulfur@polyacrylonitrile electrospun nanofibers for lithium-sulfur batteries[J]. Journal of Power Sources,2019,431:250-258. doi: 10.1016/j.jpowsour.2019.05.055 [29] LIANG C D, DUDNEY N J, HOWE J Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery[J]. Chemistry of Materials,2009,21(19):4724-4730. doi: 10.1021/cm902050j [30] ZHANG S, UENO K, DOKKO K, et al. Recent advances in electrolytes for lithium-sulfur batteries[J]. Advanced Energy Materials,2015,5(16):1500117. [31] WANG Q, WEN Z, YANG J, et al. Electronic and ionic co-conductive coating on the separator towards high-performance lithiume sulfur batteries[J]. Journal of Power Sources,2016,306:347-353. doi: 10.1016/j.jpowsour.2015.11.109 [32] YAO H, YAN K, LI W, et al. Improved lithium-sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode-separator interface[J]. Energy & Environmental Science,2014,7(10):3381-3390. doi: 10.1039/C4EE01377H [33] ZHANG Y Y, LI K, LI H, et al. High sulfur loading lithium-sulfur batteries based on a upper current collector electrode with lithium-ion conductive polymers[J]. Journal of Materials Chemistry A,2017,5(1):97-101. doi: 10.1039/C6TA08264E [34] QIE L, MANTHIRAM A. High-energy-density lithium-sulfur batteries based on blade-cast pure sulfur electrodes[J]. ACS Energy Letters,2016,1(1):46-51. doi: 10.1021/acsenergylett.6b00033 [35] SUN L, LI M Y, JIANG Y, et al. Sulfur nanocrystals confined in carbon nanotube network as a binder-free electrode for high-performance lithium sulfur batteries[J]. Nano Letters,2014,14(7):4044-4049. doi: 10.1021/nl501486n [36] ZHU X, WEN Z, GU Z, et al. Electrochemical characterization and performance improvement of lithium/sulfur polymer batteries[J]. Journal of Power Sources,2005,139(1-2):269-273. doi: 10.1016/j.jpowsour.2004.07.002 [37] SAYAHPOUR B, HIRSH H, BAI S, et al. Revisiting discharge mechanism of CFx as a high energy density cathode material for lithium primary battery[J]. Advanced Energy Materials, 2022, 12(5): 2103196. [38] LEI T, XIE Y, WANG X, et al. TiO2 feather duster as effective polysulfides restrictor for enhanced electrochemical kinetics in lithium-sulfur batteries[J]. Small, 2017, 13(37): 1701013. [39] CHENG P, GUO P Q, LIU D Q, et al. Fe3O4/RGO modified separators to suppress the shuttle effect for advanced lithium-sulfur batteries[J]. Journal of Alloys and Compounds,2019,784:149-156. doi: 10.1016/j.jallcom.2019.01.041 [40] LI Y, MENG L, JIN L, et al. A wet-laid carbon paper with 3D conductive structure as an interlayer for lithium-sulfur batteries[J]. Materials Research Express,2019,6(12):125547-125555. [41] SONG X, WANG S Q, CHEN G P, et al. Fe-N-doped carbon nanofiber and graphene modified separator for lithium-sulfur batteries[J]. Chemical Engineering Journal,2018,333:564-571. doi: 10.1016/j.cej.2017.09.186 [42] YUAN W, QIU Z Q, WANG C, et al. Design and interface optimization of a sandwich-structured cathode for lithium-sulfur batteries[J]. Chemical Engineering Journal,2020,381:122648-122660. [43] JOSHI A, RAULO A, BANDYOPADHYAY S, et al. Waste cotton cloth derived flexible current collector with optimized electrical properties for high performance lithium-sulfur batteries[J]. Carbon,2022,192:429-437. doi: 10.1016/j.carbon.2022.03.018 -






 下载:
下载: