Forming mechanism of surface nitriding of high strength and high modulus carbon fiber by electrochemical modification
-
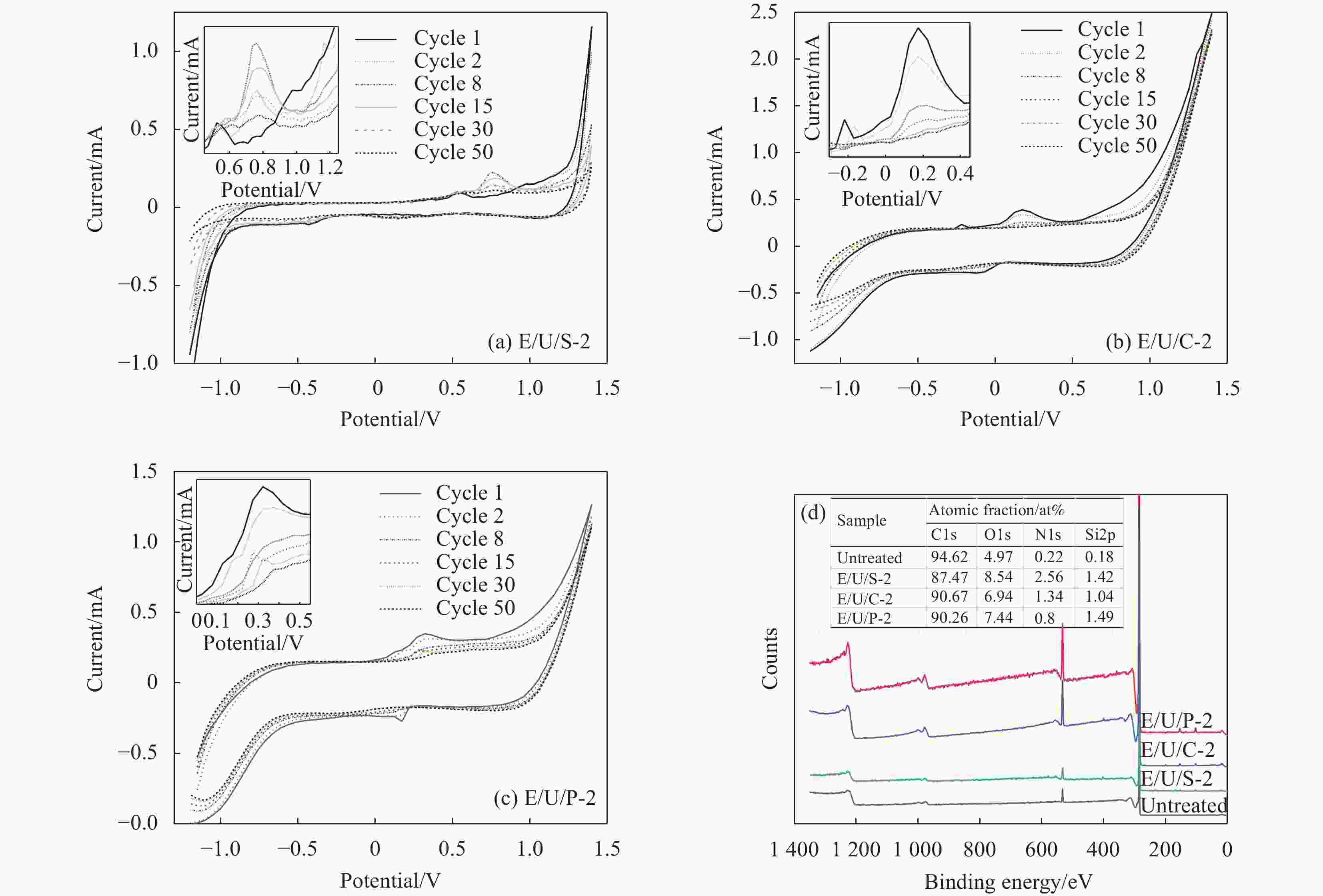
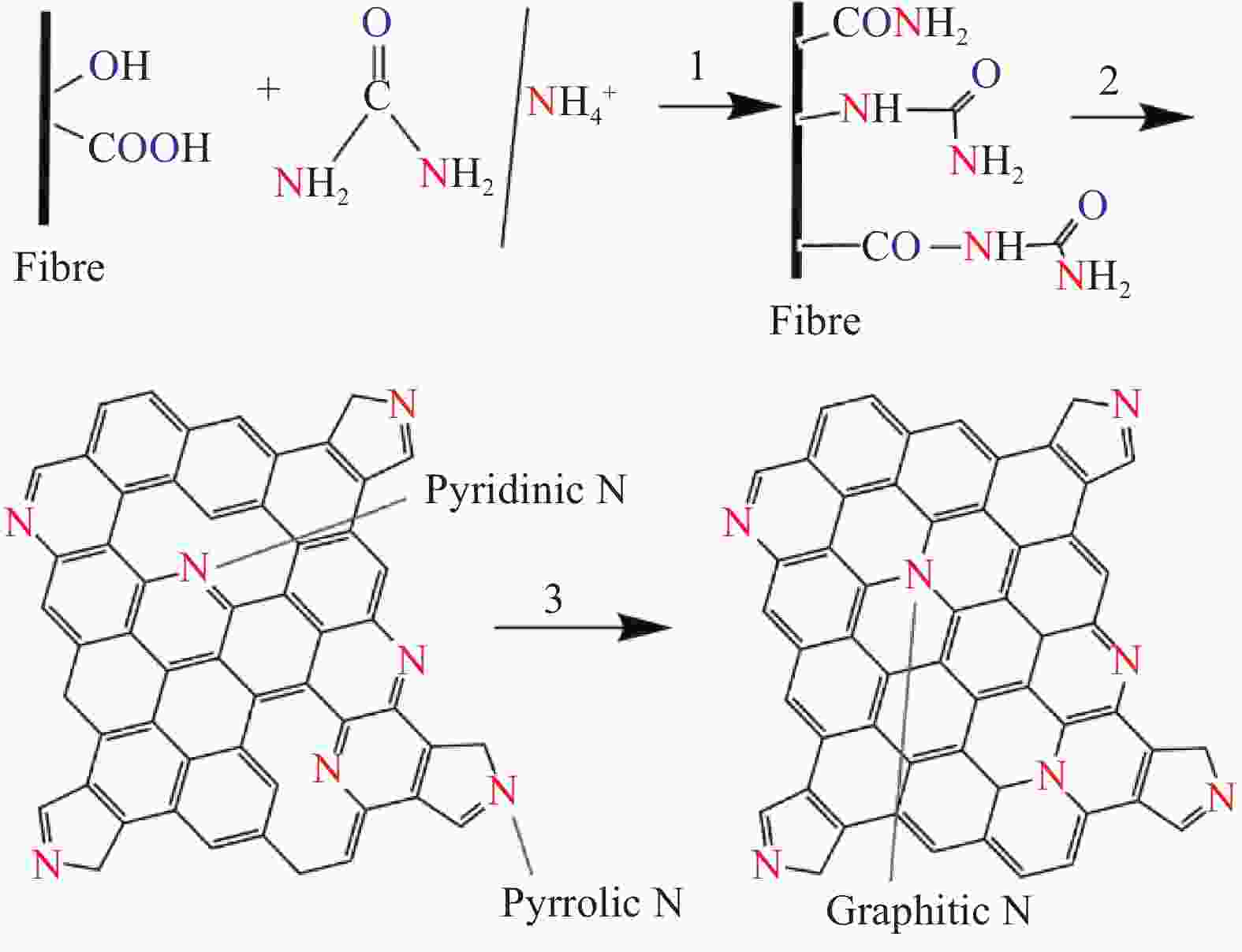
摘要: 高温石墨化使高强高模碳纤维(CF)表面光滑,反应活性低,导致其复合材料界面粘接性能差。杂原子改性是改善CF表面反应活性的有效手段之一。采用循环伏安(CV)方法在有机复合电解液中对高强高模CF进行了表面氧化和氮化改性,采用CV优选的复合电解液进行恒流电化学氧化处理,研究了CV扫描次数和电解液中含氮有机物对CF表面化学组成的影响。电化学处理前后CF表面化学元素组成和微观形态变化通过XPS、SEM及拉曼光谱表征。基于实验数据探讨了CF表面含氮官能团的生成及转变机制。研究结果显示,有机溶剂、有机氮源和含硫铵盐的协同作用使CF表面N含量从0.28at%增至4.77at%。适量的水存在,可以使CF表面O含量显著提高。CF表面的含氧官能团可以与CO(NH2)2中的—NH2及电解液中的NH4+反应形成酰胺基团,随着反应时间延长,CF表面的酰胺N会先转变成氧化氮,随后转变成吡啶和吡咯N,并最终转换成石墨N。恒流电化学处理后CF/环氧树脂复合材料的层间剪切强度(ILSS)较未处理的提高了132%,同时CF拉伸强度略有提高,表明有机复合电解液是一种温和、有效的CF表面电化学处理液。Abstract: CF has smooth surface and low reactivity due to high temperature graphitization, resulting in poor interfacial adhesion of CF composites. Heteroatom modification is one of the effective methods to improve the surface reactivity of CF. In the organic composite electrolyte solution, the surface oxidation and nitridation treatment of the high-strength and high-modulus carbon fiber (CF) were carried out by cyclic voltammetry (CV). The surface element composition and microscopic morphology of CF were characterized by XPS, SEM and Raman spectroscopy. A comparative analysis based on the obtained data reveals the nitriding mechanism of the CF surface and the source of the substance introducing the nitrogen-containing functional group. The results show that under the synergistic action of organic solvent, organic nitrogen source and S-containing ammonium salt, the surface nitrogen content of CF increases from 0.28at% to 4.77at%. When there is an appropriate amount of water in the solution, the number of oxygen-containing functional groups is significantly increased. The reaction between the acidic oxygen-containing functional groups formed during oxidation, the amino group of urea, and the ammonium ions in the solution is the key to forming C—N bonds on the surface of CFs. As the reaction time prolongs, the nitrogen-containing functional groups on the surface of the CF gradually transform from amide nitrogen to nitrogen oxides, then to pyridine and pyrrole, and finally to the graphitized nitrogen. The interlayer shear strength (ILSS) of CF/epoxy composites after constant current electrochemical treatment is 132% higher than that of untreated, and the tensile strength of CF increased slightly. The results show that the organic composite electrolyte is a mild and effective solution for electrochemical treatment of CF surface.
-
表 1 循环伏安(CV)实验参数
Table 1. Cyclic voltammetry (CV) experimental conditions
Electrolyte Sample Scanning
speed/(mV·s−1)EG/NH4HSO4 E/S 150 EG/urea/NH4HSO4 E/U/S 150 EG/urea/NH4HSO4/2wt%H2O E/U/S-2 150 EG/urea/NH4HCO3/2wt%H2O E/U/C-2 150 EG/urea/NH4H2PO3/2wt%H2O E/U/P-2 150 EG/urea/NH4HSO4 E/U/S 20 EG/urea/NH4HSO4/1wt%H2O E/U/S-1 20 EG/urea/NH4HSO4/2wt%H2O E/U/S-2 20 EG/urea/NH4HSO4/5wt%H2O E/U/S-5 20 EG/urea/NH4HSO4/20wt%H2O E/U/S-20 20 EG/urea/NH4HSO4/50wt%H2O E/U/S-50 20 H2O/urea/NH4HSO4 H2O/U/S 20 Note: EG—Ethylene glycol. 表 2 不同CV循环次数下CF表面元素含量
Table 2. Element contents on CF surface after treatment with different CV cycles of E/U/S electrolyte
Cycles 0 1 8 15 20 50 70 O/at% 4.97 6.59 8.52 9.33 13.00 7.80 10.30 N/at% 0.22 1.90 2.59 3.22 4.77 2.38 3.78 表 3 不同CV循环次数下CF表面的含氧官能团含量
Table 3. Contents of oxygen-containing functional groups on CF surface with different CV cycles
Cycle Atomic fraction of functional groups/at% C—C C—OH, C—N,
C—O—RC=O COOH
COOR0 88.4 8.81 0.36 2.43 1 76.3 12.64 0.23 10.83 8 66.1 21.10 — 12.71 15 68.6 19.33 — 12.07 20 82.8 11.79 1.23 4.18 50 90.3 6.87 1.72 1.02 70 76.0 12.05 0.49 11.46 表 4 不同水含量下CV处理的CF表面元素组成
Table 4. Element contents on CF surface after CV treatment with different water content electrolytes
Sample C/at% O/at% N/at% Si/at% E/U/S 88.14 7.80 2.38 1.68 E/U/S-1 86.93 9.85 3.05 0.17 E/U/S-2 78.55 16.59 4.74 0.12 E/U/S-5 82.82 13.52 3.66 0.00 E/U/S-20 79.98 15.59 4.31 0.13 E/U/S-50 88.79 8.72 2.06 0.44 H2O/U/S 95.06 4.26 0.49 0.19 表 5 电解液E/U/S-50中处理后CF力学性能变化
Table 5. Changes of mechanical properties of CF before and after treatment with E/U/S-50 electrolyte
Sample σ/MPa E/GPa ε/% ILSS/MPa Untreated CF1 4052 541 0.7 22 Treated-CF1 4478 567 0.8 51 Untreated CF2 4082 507 0.8 40 Treated-CF2 4280 499 0.8 60 Notes: σ—Tensile strength; E—Tensile modulus; ε—Elongation at break; ILSS—Interlaminar shear strength. -
[1] WEN Z, XU C, QIAN X, et al. A two-step carbon fiber surface treatment and its effect on the interfacial properties of CF/EP composites: The electrochemical oxidation followed by grafting of silane coupling agent[J]. Applied Surface Science,2019,486:546-554. doi: 10.1016/j.apsusc.2019.04.248 [2] DANIEL J E, KARYN J, ANDERS J B, et al. Improving the effects of plasma polymerization on carbon fiber using a surface modification pretreatment[J]. Composites Part A: Applied Science and Manufacturing,2021,143:106319. doi: 10.1016/j.compositesa.2021.106319 [3] XIONG S, ZHAO Y, WANG Y, et al. Enhanced interfacial properties of carbon fiber/epoxy composites by coating carbon nanotubes onto carbon fiber surface by one-step dipping method[J]. Applied Surface Science,2021,546:149135. doi: 10.1016/j.apsusc.2021.149135 [4] PAIVA M C, BERNARDO C A, NARDIN M. Mechanical, surface and interfacial characterization of pitch and PAN-based carbon fibers[J]. Carbon,2000,38(9):1323-1337. doi: 10.1016/S0008-6223(99)00266-3 [5] YU J, MENG L, FAN D, et al. The oxidation of carbon fibers through K2S2O8/ AgNO3 system that preserves fiber tensile strength[J]. Composites Part B: Engineering,2014,60:261-267. doi: 10.1016/j.compositesb.2013.12.037 [6] ZHANG L, KEIKO W. The influence of carboxyl group on nitrogen doping for defective carbon nanotubes toward oxygen reduction reaction[J]. Carbon,2022,189:369-376. doi: 10.1016/j.carbon.2021.12.087 [7] LOPEZ R O, SANCHEZ G R, SCHULZ J M E, et al. On site formation of N-doped carbon nanofibers, an efficient electrocatalyst for fuel cell applications[J]. International Journal of Hydrogen Energy,2017,42(5):30339-30348. [8] FAN Y, ZHAO Z, ZHOU Q, et al. Nitrogen-doped carbon microfibers with porous textures[J]. Carbon,2013,58:128-133. doi: 10.1016/j.carbon.2013.02.040 [9] RODRIGUEZ-CORVERA C L, FAJARDO-DIAZ J L, CORTES-LOPEZ A J, et al. Nitrogen-doped carbon fiber sponges by using different nitrogen precursors: Synthesis, characterization, and electrochemical activity[J]. Materials Today Chemistry,2019,14:100200. doi: 10.1016/j.mtchem.2019.100200 [10] PARK S J, KIM M H, LEE J R, et al. Effect of fiber-polymer interactions on fracture toughness behavior of carbon fiber-reinforced epoxy matrix composites[J]. Journal of Colloid Interface Science,2000,228(2):287-291. doi: 10.1006/jcis.2000.6953 [11] PEERA S G, SAHU A K, ARUNCHANDER A, et al. Nitrogen and fluorine co-doped graphite nanofibers as high durable oxygen reduction catalyst in acidic media for polymer electrolyte fuel cells[J]. Carbon,2015,93:130-142. doi: 10.1016/j.carbon.2015.05.002 [12] MORITA T, TAKAMI N. Characterization of oxidized boron-doped carbon fiber anodes for Li-ion batteries by analysis of heat of immersion[J]. Electrochim Acta,2004,49(16):2591-2599. doi: 10.1016/j.electacta.2004.02.010 [13] LU T, LI Q, SHAO J, et al. Nitrogen and sulfur co-doped porous carbons from polyacrylonitrile fibers for CO2 adsorption[J]. Journal of the Taiwan Institute of Chemical Engineers,2021,128:148-155. doi: 10.1016/j.jtice.2021.08.043 [14] MA T Y, RAN J, DAI S, et al. Phosphorus-doped graphitic carbon nitrides grown in situ on carbon-fiber paper: Flexible and reversible oxygen electrodes[J]. Angewandte Chemie-International Edition,2014,54(15):1-6. [15] 刘福杰, 王浩静, 范立东, 等. MJ系列碳纤维微观结构的剖析[J]. 化工新型材料, 2009, 37(1):41-43.LIU Fujie, WANG Haojing, FAN Lidong, et al. Analysis on the microstructure of MJ series carbon fiber[J]. New Chemical Materials,2009,37(1):41-43(in Chinese). [16] 张莎, 田艳红, 张学军. 电化学氧化对高强高模碳纤维表面结构及力学性能的影响[J]. 复合材料学报, 2012, 29(3):1-8.ZHANG Sha, TIAN Yanhong, ZHANG Xuejun, et al. Effect of electrochemical oxidation on the surface structure and mechanical performance of high strength and high modulus carbon fibres[J]. Acta Materiae Compositae Sinica,2012,29(3):1-8(in Chinese). [17] 乔伟静, 田艳红, 张学军. 国产聚丙烯腈基高强高模碳纤维电化学氧化表面处理工艺[J]. 复合材料学报, 2018, 35(9):2449-2457.QIAO Weijing, TIAN Yanhong, ZHANG Xuejun. Electrochemical oxidation surface treatment of domestic polyacrylonitrile-based high strength and high modulus carbon fiber[J]. Acta Materiae Compositae Sinica,2018,35(9):2449-2457(in Chinese). [18] KAINOURGIOS P, KARTSONAKIS I A, DRAGATOGIANNIS D A, et al. Electrochemical surface functionalization of carbon fibers for chemical affinity improvement with epoxy resins[J]. Applied Surface Science,2017,416:593-604. doi: 10.1016/j.apsusc.2017.04.214 [19] TIWARI S, BIJWE J. Surface treatment of carbon fibers-A review[J]. Procedia Technology,2014,14:505-512. doi: 10.1016/j.protcy.2014.08.064 [20] DONNET J B, GUILPAIN G. Surface treatments and properties of carbon fibers[J]. Carbon,1989,27(5):749-757. doi: 10.1016/0008-6223(89)90209-1 [21] BASOVA Y V, HATORI H, YAMADA Y, et al. Effect of oxidation–reduction surface treatment on the electrochemical behavior of PAN-based carbon fibers[J]. Electrochemistry Communications,1999,1(11):540-544. doi: 10.1016/S1388-2481(99)00112-5 [22] BISMARCK A, KUMRU M E, SPRINGER J, et al. Surface properties of PAN-based carbon fibers tuned by anodic oxidation in different alkaline electrolyte systems[J]. Applied Surface Science,1999,143(1-4):45-55. doi: 10.1016/S0169-4332(98)00929-5 [23] GEORGIOU P, WALTON J, SIMITZIS J. Surface modification of pyrolyzed carbon fibers by cyclic voltammetry and their characterization with XPS and dye adsorption[J]. Electrochimica Acta,2010,55(3):1207-1216. doi: 10.1016/j.electacta.2009.09.068 [24] RAHMANI H, ASHORI A, VARNASERI N. Surface modification of carbon fiber for improving the interfacial adhesion between carbon fiber and polymer matrix[J]. Polymers for Advanced Technologies,2016,27(6):805-811. doi: 10.1002/pat.3720 [25] LIU J, TIAN Y, CHEN Y, et al. Interfacial and mechanical properties of carbon fibers modified by electrochemical oxidation in (NH4HCO3)/(NH4)2C2O4·H2O aqueous compound solution[J]. Applied Surface Science,2010,256(21):6199-6204. doi: 10.1016/j.apsusc.2010.03.141 [26] WANG Y Q, ZHANG F Q, SHERWOOD P M A. X-ray photoelectron spectroscopic studies of carbon fiber surfaces. 25. Interfacial interactions between PEKK polymer and carbon fibers electrochemically oxidized in nitric acid and degradation in a saline solution[J]. Chemistry of Materials,2001,13(3):832-841. doi: 10.1021/cm000555t [27] 徐显亮, 张月义, 马全胜, 等. 国产PAN基高强中模型碳纤维的电化学表面改性研究[J]. 玻璃钢/复合材料, 2016(12):81-85.XU Xianliang, ZHANG Yueyi, MA Quansheng, et al. Study on electrochemical surface modification of PAN-based carbon fiber of high strength and middle modulus[J]. Fibre Reinforced Plastics/Composites,2016(12):81-85(in Chinese). [28] QIAN X. Effect of ammonium-salt solutions on the surface properties of carbon fibers in electrochemical anodic oxidation[J]. Applied Surface Science, 2012, 259: 238-244. [29] 吴波, 郑帼, 孙玉, 等. 有机电解液电化学改性PAN基碳纤维的表面性能[J]. 材料工程, 2016, 44(9):52-57.WU Bo, ZHENG Guo, SUN Yu, et al. Surface properties of PAN-based carbon fibers modified by electrochemical oxidization in organic electrolyte systems[J]. Journal of Materials Engineering,2016,44(9):52-57(in Chinese). [30] HAN F, DUAN D, JING W, et al. Synthesis and plasma treatment of nitrogen-doped graphene fibers for high-perfor-mance supercapacitors[J]. Ceramics International,2022,48(2):2058-2067. doi: 10.1016/j.ceramint.2021.09.291 [31] HOU X, HU Q, ZHANG P, et al. Oxygen reduction reaction on nitrogen-doped graphene nanoribbons: A density functional theory study[J]. Chemical Physics Letters, 2016, 663: 123-127. [32] ZENG K, WEI M H, LI C, et al. PPy-derived N, P co-doped hollow carbon fiber decorated with island-like Ni2P nanoparticles as bifunctional oxygen electrocatalysts[J]. Jour-nal of Electroanalytical Chemistry,2021,882:115013. doi: 10.1016/j.jelechem.2021.115013 [33] KIM Y J, LEE H J, LEE S W, et al. Effects of sulfuric acid treatment on the microstructure and electrochemical performance of a polyacrylonitrile (PAN)-based carbon anode[J]. Carbon,2005,43(1):163-169. doi: 10.1016/j.carbon.2004.09.001 -





 下载:
下载: