Epoxy resin anticorrosive coating modified by the co-doping of polyhedral silsesquioxane/hexagonal boron nitride/aniline trimer
-
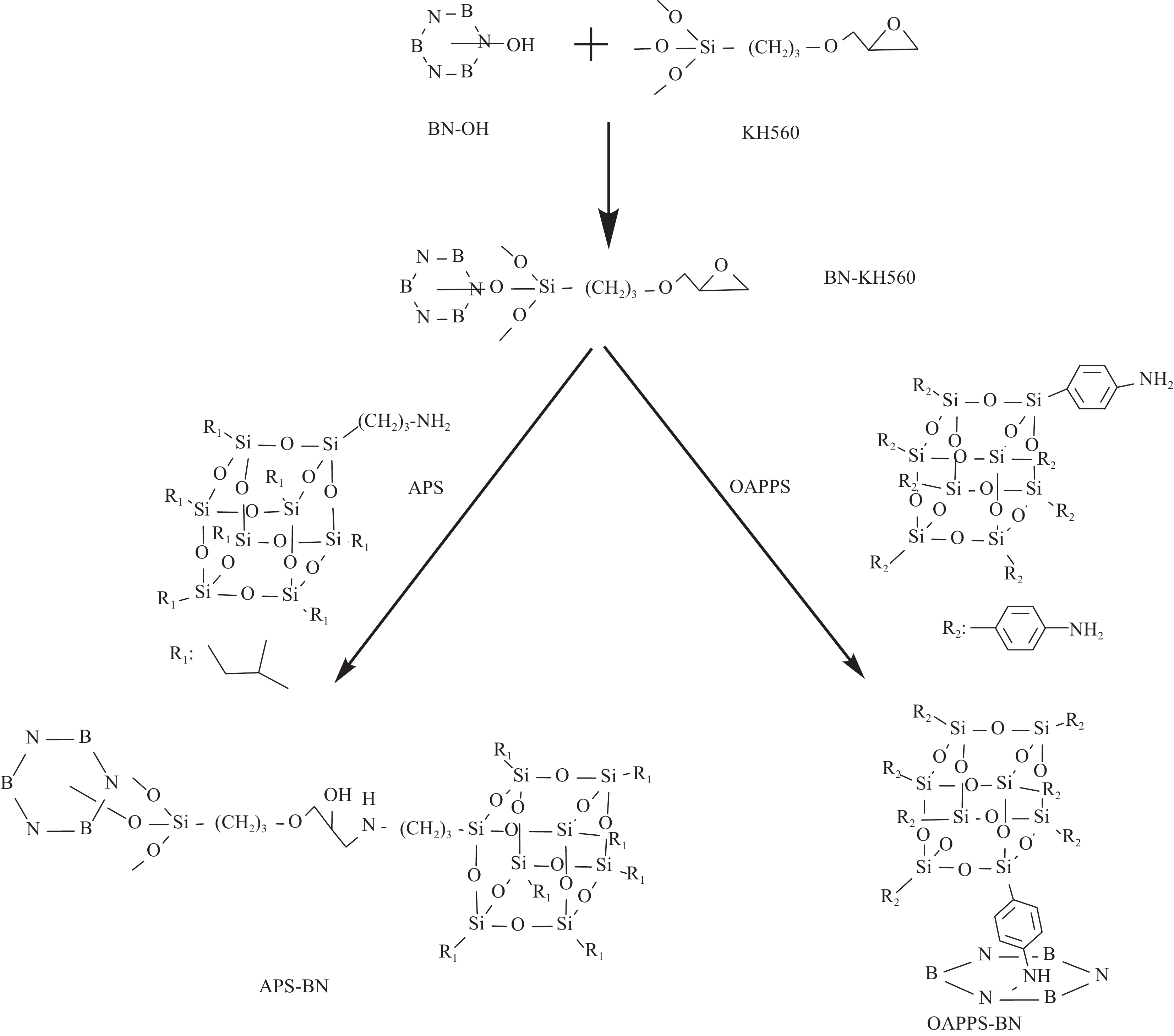
摘要: 为了改善环氧树脂(EP)涂层防腐性能,采用笼形聚倍半硅氧烷(POSS)修饰的六方氮化硼(h-BN)和苯胺三聚体(AT)作为填料加入环氧固化体系中进行共掺杂,研究两种POSS分子、两种添加方式以及不同添加量对复合涂层的性能影响。首先通过高温剥离六方氮化硼得到羟基化氮化硼(OH-BN),而后采用硅烷偶联剂KH-560对其表面进行乙氧基功能化修饰,再分别将氨丙基七异丁基POSS(APS)和八氨苯基POSS(OAPPS)与之接枝,经反应合成出两种新型的POSS杂化氮化硼功能助剂APS-BN和OAPPS-BN;进一步将它们和AT通过π-π相互作用共混,以不同的添加比例分散到环氧树脂中制备有机无机杂化的环氧复合防腐涂层材料,最后表征涂层的交流阻抗谱、塔菲尔曲线、盐雾试验、接触角、热性能和力学性能等。结果表明,与纯环氧涂层相比,掺杂0.5wt% OAPPS-BN-AT的环氧复合涂层性能提升幅度最大,阻抗值为1.27×1011 Ω·cm2;腐蚀电位提高了0.35 V,达到−0.052 V;耐盐雾性能也有明显提高,30天未出现点蚀和起泡。此外,基于POSS杂化氮化硼功能助剂中POSS表面迁移作用和h-BN屏障作用,复合涂层铅笔硬度提高到3H级别;表面疏水性有所提升,接触角从纯环氧涂层的67.1°增大到93.2°,并且还显示出优良的附着力、耐冲击性、柔韧性和耐热性能,说明0.5wt%OAPPS-BN-AT/EP防腐涂层在金属腐蚀与防护领域将具有一定的潜在应用前景。Abstract: In order to improve the corrosion resistance of epoxy resin (EP) coatings, both polyhedral oligomeric silsesquioxane (POSS) modified hexagonal boron nitride (h-BN) and aniline trimer (AT) were co-doped into the epoxy curing system using as functional fillers. The hydroxylated boron nitride OH-BN was obtained by peeling off the hexagonal boron nitride at high temperature. The surface of OH-BN was modified with the silane coupling agent KH-560, and then the aminopropyl heptaisobutyl POSS (APS) and octaaminophenyl POSS (OAPPS) were grafted to synthesize APS-BN and OAPPS-BN. The two modified h-BN and aniline trimer were dispersed into EP to prepare organic-inorganic hybrid coatings through π-π interactions. The coating's AC impedance spectroscopy, Tafel curve, salt spray test, and contact angle, thermal and mechanical properties were characterized by some techniques. The results show that, compared with the pure epoxy coating, the composited coating doped with 0.5wt% OAPPS-BN-AT has the largest improvement in performance with an impedance value of 1.27×1011 Ω·cm2. The corrosion potential is −0.052 V with an increasing of 0.35 V. The salt spray resistance test exhibites that no pitting or blistering occurred for 30 days. Based on the POSS surface migration effect and h-BN barrier effect, the pencil hardness of the composited coating is increased to 3H level. The surface contact angle is changed from 67.1° to 93.2°. In a word, the composited 0.5wt%OAPPS-BN-AT/EP coating has excellent adhesion, impact resistance, flexibility and heat resistance, which showed a promising potential in the field of anti-corrosion.
-
图 5 环氧树脂(EP)、0.5wt%OH-BN-苯胺三聚体(AT)/EP、0.5wt%APS-BN-AT/EP、0.5wt%BN-OPPS-AT/EP、0.25wt%~0.75wt%OAPPS-BN-AT/EP浸泡1天的Bode-Phase图 (a)、1天的Nyquist图 (b)、15天的Bode-Phase图 (c)、15天的Nyquist图 (d)、30天的Bode-Phase图 (e) 和30天的Nyquist图 (f)
Figure 5. Bode-Phase diagram soaked for 1 day (a), 15 days (c), 30 days (e) and Nyquist diagram soaked for 1 day (b), 15 days (d), 30 days (f) of epoxy resin (EP), 0.5wt%OH-BN-aniline trimer (AT)/EP, 0.5wt%APS-BN-AT/EP, 0.5wt%BN-OPPS-AT/EP, 0.25wt%-0.75wt%OAPPS-BN-AT/EP
Z—Polarization impedance; Z'—Real impedance; −Z''—Imaginary impedance
图 7 EP复合防腐涂层盐雾箱放置30天后的光学照片:(a) EP;(b) 0.5wt%OH-BN-AT/EP;(c) 0.5wt%APS-BN-AT/EP;(d) 0.5wt%BN-OPPS-AT/EP;((e)~(g)) 0.25wt%~0.75wt%OAPPS-BN-AT/EP
Figure 7. Optical photographs of EP coatings put in salt spray tank after 30 days: (a) EP; (b) 0.5wt%OH-BN-AT/EP; (c) 0.5wt%APS-BN-AT/EP; (d) 0.5wt%BN-OPPS-AT/EP; ((e)-(g)) 0.25wt%-0.75wt%OAPPS-BN-AT/EP
图 8 EP ((a)、(f))、0.5wt%OH-BN-AT/EP ((b)、(g))、0.5wt%APS-BN-AT/EP ((c)、(h))、0.5wt%BN-OPPS-AT/EP ((d)、(i))、0.5wt%OAPPS-BN-AT/EP ((e)、(j))涂层及其分别放置30天后的表面接触角测试图
Figure 8. Contact angle of EP ((a), (f)), 0.5wt%OH-BN-AT/EP ((b), (g)), 0.5wt%APS-BN-AT/EP ((c),(h)), 0.5wt%BN-OPPS-AT/EP ((d), (i)) and 0.5wt%OAPPS-BN-AT/EP ((e), (j)) coatings at initial state and being placed for 30 days
图 9 EP涂层断面SEM图像:(a) EP;(b) 0.5wt%OH-BN-AT-EP;(c) 0.5wt%APS-BN-AT-EP; (d) 0.5wt%BN-OPPS-AT-EP;(e) 0.25wt%OAPPS-BN-AT/EP;(f) 0.5wt%OAPPS-BN-AT/EP;(g) 0.75wt%OAPPS-BN-AT/EP
Figure 9. SEM images of EP coatings: (a) EP; (b) 0.5wt%OH-BN-AT-EP; (c) 0.5wt%APS-BN-AT-EP; (d) 0.5wt%BN-OPPS-AT-EP; (e) 0.25wt%OAPPS-BN-AT/EP; (f) 0.5wt%OAPPS-BN-AT/EP; (g) 0.75wt%OAPPS-BN-AT/EP
图 10 EP涂层高低温交变实验后的光学照片:(a) EP;(b) 0.5wt%OH-BN-AT/EP;(c) 0.5wt%APS-BN-AT/EP;(d) 0.5wt%BN-OPPS-AT/EP;(e) 0.5wt%OAPPS-BN-AT/EP
Figure 10. Optical photographs of EP coatings after high and low temperature alternation experiment: (a) EP; (b) 0.5wt%OH-BN-AT/EP; (c) 0.5wt%APS-BN-AT/EP; (d) 0.5wt%BN-OPPS-AT/EP; (e) 0.5wt%OAPPS-BN-AT/EP
表 1 EP、0.5wt%OH-BN-AT/EP、0.5wt%APS-BN-AT/EP、0.5wt%BN-OPPS-AT/EP、 0.25tw%~0.75wt%OAPPS-BN-AT/EP涂层的塔菲尔曲线拟合值
Table 1. Tafel curves fitting value of EP, 0.5wt%OH-BN-AT/EP, 0.5wt%APS-BN-AT/EP, 0.5wt%BN-OPPS-AT/EP, 0.25wt%-0.75wt%OAPPS-BN-AT/EP coatings
Sample Ecorr /V Icorr /(A·cm−2) EP −0.40958 2.971×10−11 0.5wt%OH-BN-AT/EP −0.14316 8.511×10−11 0.5wt%APS-BN-AT/EP −0.10602 6.109×10−14 0.5wt%BN-OPPS-AT/EP −0.20480 3.548×10−13 0.25wt%OAPPS-BN-AT/EP −0.10013 8.551×10−13 0.5wt%OAPPS-BN-AT/EP −0.05206 4.677×10−14 0.75wt%OAPPS-BN-AT/EP −0.19233 9.898×10−11 Notes: Ecorr—Corrosion potential; Icorr—Corrosion current density. 表 2 环氧复合防腐涂层的物理性能表
Table 2. Physical properties of epoxy composite anticorrosive coating
Sample Thickness/μm Adhesion Impact resistance Flexibility Pencil hardness EP 35 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.25wt%OH-BN-AT/EP 31 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.5wt%OH-BN-
AT/EP30 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.75wt%OH-BN-AT/EP 31 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.25wt%APS-BN-AT/EP 33 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.5wt%APS-BN-
AT/EP37 Level 0 50 cm,
no crack2 mm,
no peeling2H 0.75wt%APS-BN-AT/EP 37 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.25wt%BN-OPPS-AT/EP 31 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.5wt%BN-OPPS-AT/EP 39 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.75wt%BN-OPPS-AT/EP 35 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.25wt%OAPPS-BN-AT/EP 39 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.5wt%OAPPS-BN-AT/EP 33 Level 0 50 cm,
no crack2 mm,
no peeling3H 0.75wt%OAPPS-BN-AT/EP 35 Level 0 50 cm,
no crack2 mm,
no peeling3H -
[1] WANG H Y, DI D Y, ZHAO Y M, et al. A multifunctional polymer composite coating assisted with pore-forming agent: Preparation, superhydrophobicity and corrosion resistance[J]. Progress in Organic Coatings,2019,132:370-378. doi: 10.1016/j.porgcoat.2019.04.027 [2] LU H, ZHANG S T, ZHAO Z H, et al. Preparation and corrosion protection of VB 2 modified trimer aniline-reduced graphene oxide(VTA-rGO) coatings[J]. Progress in Organic Coatings,2019,132:95-99. doi: 10.1016/j.porgcoat.2019.03.030 [3] YE Y W, LIU W, LIU Z Y, et al. Anti-corrosion performance of aniline trimer-containing sol-gel hybrid coatings for mild steel substrate[J]. Journal of Sol-Gel Science and Technology,2018,87:464-477. doi: 10.1007/s10971-018-4716-9 [4] SHARMA V, KAGDADA H L, JHA P K, et al. Thermal transport properties of boron nitride based materials: A review[J]. Renewable and Sustainable Energy Reviews,2020,120:109622. doi: 10.1016/j.rser.2019.109622 [5] CHILKOOR G, KARANAM S P, STAR S, et al. Hexagonal boron nitride: The thinnest insulating barrier to microbial corrosion[J]. ACS nano,2018,12(3):2242-2252. doi: 10.1021/acsnano.7b06211 [6] WANG Y, MAO J, MENG X, et al. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications[J]. Chemical Reviews,2018,119(3):1806-1854. [7] LI J, GAN L, LIU Y, et al. Boron nitride nanosheets reinforced waterborne polyurethane coatings for improving corrosion resistance and antifriction properties[J]. European Polymer Journal,2018,104:57-63. doi: 10.1016/j.eurpolymj.2018.04.042 [8] CUI M, REN S, QIN S, et al. Non-covalent functionalized hexagonal boron nitride nanoplatelets to improve corrosion and wear resistance of epoxy coatings[J]. RSC Advances,2017,7(70):44043-44053. doi: 10.1039/C7RA06835B [9] ZHENG Z, COX M C, LI B. Surface modification of hexagonal boron nitride nanomaterials: A review[J]. Journal of Materials Science,2018,53(1):66-99. doi: 10.1007/s10853-017-1472-0 [10] LIU X L, CHEN S G, ZHANG Y J, et al. Preparation of graphene oxide-boron nitride hybrid to reinforce the corrosion protection coating[J]. Corrosion Reviews, 2021, 39(2): 123-136. [11] YE Y, ZHANG D, LI J, et al. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields[J]. Corrosion Science,2019,147:9-21. [12] ZHANG D, YUAN T, WEI G, et al. Preparation of self-healing hydrophobic coating on AA6061 alloy surface and its anti-corrosion property[J]. Journal of Alloys and Compounds,2018,774:495-501. [13] WU Y, FANG L, HUYAN J, et al. Low dielectric and high thermal conductivity epoxy nanocomposites filled with NH2-POSS/n-BN hybrid fillers[J]. Journal of Applied Polymer Science, 2015, 132(19): 41951. [14] GU L, LIU S, ZHAO H C, et al. Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings[J]. ACS Applied Materials & Interfaces,2015,7(32):17641-17648. [15] KOECH J K, SHAO Q, MUTUA F N, et al. Application of hydrazine hydrate in the synthesis of octa (aminophenyl) silsesquioxane (OAPS) POSS [J]. Advances in Chemical Engineering and Science, 2013, 3(1): 93-97. [16] GUO Y Q, LYU Z Y, YANG X T, et al. Enhanced thermal conductivities and decreased thermal resistances of functionalized boron nitride/polyimide composites[J]. Composites Part B: Engineering,2019,164:732-739. doi: 10.1016/j.compositesb.2019.01.099 [17] ZHAO Y, ZHOU M, CHEN G, et al. Hybridization of polyhedral oligomeric silsesquioxane and boron nitride for epoxy composites with improved dielectric, thermal and tensile properties[J]. Journal of Materials Science: Materials in Electronics,2019,30(11):10360-10368. doi: 10.1007/s10854-019-01375-0 [18] 中国国家标准化管理委员会. 色漆和清漆 弯曲试验(圆柱轴): GB/T 6742—2007[S]. 北京: 中国标准出版社, 2007.Standardization Administration of China. Paints and varnishes: Bending test (cylindrical shaft): GB/T 6742—2007[S]. Beijing: China Standards Press, 2007(in Chinese). [19] 中国国家标准化管理委员会. 色漆和清漆 铅笔法测定漆膜硬度: GB/T 6739—2006[S]. 北京: 中国标准出版社, 2006.Standardization Administration of China. Paints and varnishes: Determination of film hardness by pencil test: GB/T 6739—2006[S]. Beijing: China Standards Press, 2006(in Chinese). [20] 中国国家标准化管理委员会. 漆膜耐冲击性测定法: GB/T 1732—1993[S]. 北京: 中国标准出版社, 1993.Standardization Administration of China. Paint film—Determination of impact resistance: GB/T 1732—1993[S]. Beijing: China Standards Press, 1993(in Chinese). [21] 中国国家标准化管理委员会. 色漆和清漆 漆膜的划格试验: GB/T 9286—1998[S]. 北京: 中国标准出版社, 1998.Standardization Administration of China. Paints and varnishes: Marking test of paint film: GB/T 9286—1998[S]. Beijing: China Standards Press, 1998(in Chinese). [22] LI J, XIAO X, XU X, et al. Activated boron nitride as an effective adsorbent for metal ions and organic pollutants[J]. Scientific Reports,2013,3:3208. [23] HU L, JIANG P, BIAN G, et al. Effect of octa(aminopropyl) polyhedral oligomeric silsesquioxane (OAPPOSS) functionalized graphene oxide on the mechanical, thermal, and hydrophobic properties of waterborne polyurethane composites[J]. Journal of Applied Polymer Science, 2016, 134(6): 44440. [24] SINGLA P, GOEL N, KUMAR V, et al. Boron nitride nanomaterials with different morphologies: Synthesis, characterization and efficient application in dye adsorption[J]. Ceramics International,2015,41(9):10565-10577. doi: 10.1016/j.ceramint.2015.04.151 [25] LIANG H, ZHU P, GANG L, et al. Spherical and flake-like BN filled epoxy composites: Morphological effect on the thermal conductivity, thermo-mechanical and dielectric properties[J]. Journal of Materials Science Materials in Electronics,2015,26(6):3564-3572. doi: 10.1007/s10854-015-2870-1 [26] LIU Y, SHI Z X, XU H J, et al. Preparation, characterization, and properties of novel polyhedral oligomeric silsesquio-xane-polybenzimidazole nanocomposites by Friedel-Crafts reaction[J]. Macromolecules,2010,43:6731-6738. doi: 10.1021/ma1011792 [27] YONG N, ZHENG S, NIE K. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes[J]. Polymer,2004,45(16):5557-5568. doi: 10.1016/j.polymer.2004.06.008 [28] HUANG H, HUANG X, XIE Y, et al. Fabrication of h-BN-rGO@PDA nanohybrids for composite coatings with enhanced anticorrosion performance[J]. Progress in Organic Coatings,2019,130:124-131. doi: 10.1016/j.porgcoat.2019.01.059 [29] ZHONG J, ZHOU G, HE P, et al. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide[J]. Carbon,2017,117:421-426. doi: 10.1016/j.carbon.2017.02.102 -






 下载:
下载: