Adsorption of platinum by thiosemicarbazide/quaternary ammonium lignin
-
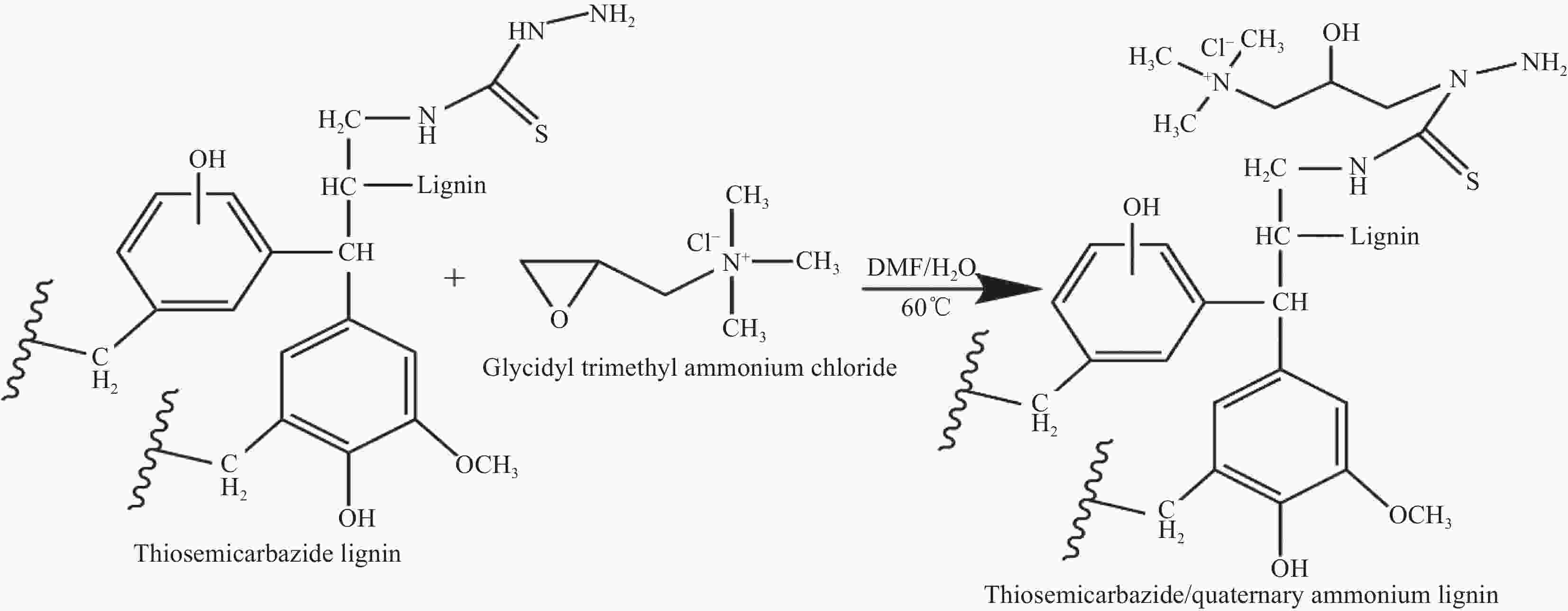
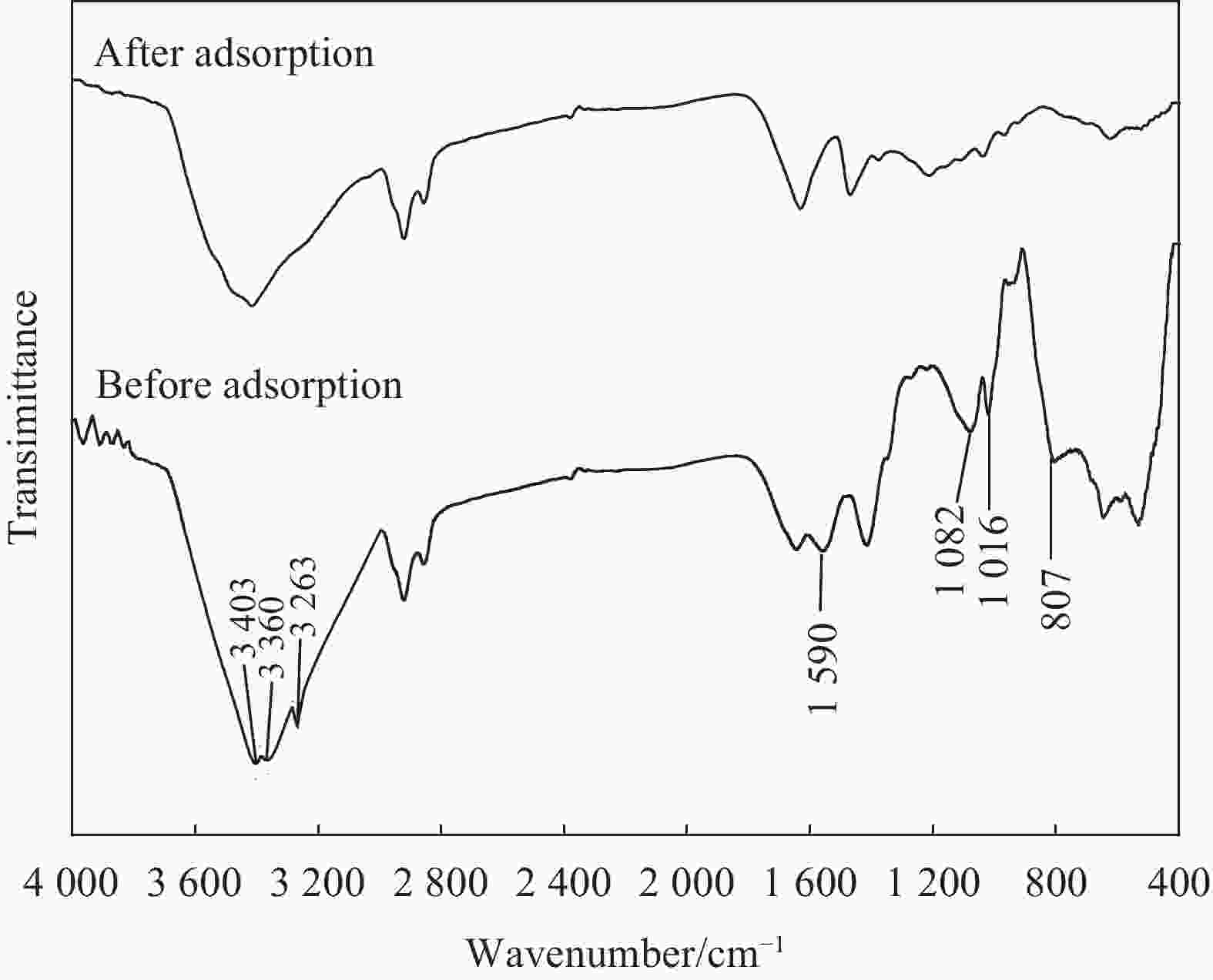
摘要: 木质素及其衍生物因具有经济高效和无污染等特点,在有价金属提取和有毒金属离子去除等领域具有广阔的应用前景。本研究采用氨基硫脲/季铵木质素吸附铂,通过FTIR分析揭示了吸附过程的机制,考察了盐酸浓度、Pt(IV)初始浓度、吸附时间和吸附剂用量对吸附效果的影响。结果表明,改性木质素中含有大量的酚羟基、胺基和季铵官能团,PtCl62−被氨基硫脲/季铵木质素中的酚羟基还原成PtCl42−后与胺基发生配合反应和氯离子发生离子交换反应,在盐酸浓度为0.5 mol·L−1、Pt(IV)初始浓度为1 170 mg·L−1和吸附时间为120 min的条件下,1 g·L−1氨基硫脲/季铵木质素对Pt(IV)的饱和吸附容量为267.80 mg·g−1,7 g·L−1时的最大吸附率为88.50%。吸附过程遵循Freundlich模型和准二级动力学模型,表明吸附过程为单分子层非均质的化学吸附。Abstract: Lignin and its derivatives have broad application prospects in the fields of extraction of valuable metals and removal of toxic metal ions because of the characteristics of economical, efficient and friendly. Platinum was adsorbed by thiosemicarbazide/quaternary ammonium lignin in this study. The mechanism of adsorption was revealed by FTIR and the influence factors such as initial concentration of Pt(IV), hydrochloric acid concentration, adsorption time and adsorbent dosage on adsorption were optimized. The results show that the modified lignin contains a large number of phenolic hydroxyl, amino and quaternary ammonium functional groups. PtCl62− is reduced to PtCl42− by phenolic hydroxyl and then reacts with amino group by coordination reaction and chloride by ion exchange reaction. Using the optimum conditions, which include hydrochloric acid of 0.5 mol·L−1, Pt(IV) of 1 170 mg·L−1, adsorption time of 120 min and thiosemicarbazide/quaternary ammonium lignin addition of 1 g·L−1, the maximum adsorption capacity is 267.80 mg·g−1. Under the same conditions besides 7 g·L−1 thiosemicarbazide/quaternary ammonium lignin, the maximum adsorption ratio is 88.50%. The adsorption process can be simulated by Freundlich model and quasi-second-order kinetic model, which indicates that adsorption is chemisorptions of monolayer heterogeneous.
-
Key words:
- thiosemicarbazide /
- quaternary ammonium lignin /
- platinum /
- adsorption mechanism /
- adsorption behavior
-
表 1 不同种类吸附剂对Pt(IV)的吸附容量
Table 1. Adsorption capacities of different kinds of adsorbents for Pt(IV)
Adsorbents Adsorption
capacities/(mg·g−1)Ref. Magnetic cellulose functionalized with thiol and amine 40.48 [28] Macrocyclic polyphenol resin 44.85 [20] Silicon dioxide and copolymer of 4-vinylpyridine and 2-hydroxyethylmethacrylate 188.00 [22] Ion recognition rice straw lignin 218.75 [29] Thiosemicarbazide/quaternary ammonium lignin 267.80 This work 表 2 氨基硫脲/季铵木质素对PtCl62−的等温吸附模型参数
Table 2. Parameters of adsorption isotherm models for the adsorption of PtCl62− by thiosemicarbazide/quaternary ammonium lignin
Adsorption isotherm models Parameters Langmuir qm/(mg·g−1) KL/(L·mg−1) R2 270.40 0.00859 0.894 Freundlich 1/n KF/(L·mg−1) R2 0.45 12.034 0.978 Temkin RTb−1 KT R2 76.86 0.0307 0.949 Notes: qm—Maximum adsorption capacity; KL—Langmuir equilibrium constant of adsorption; R2—Linear regression coefficient; 1/n—Inverse of the concentration index; KF—Freundlich adsorption equilibrium constant; R—Thermodynamic gas constant(8.314 J·mol−1·K−1); T—Temperature; b—Related constant; KT—Temkin adsorption equilibrium constant. 表 3 氨基硫脲/季铵木质素吸附PtCl62−的动力学模型参数
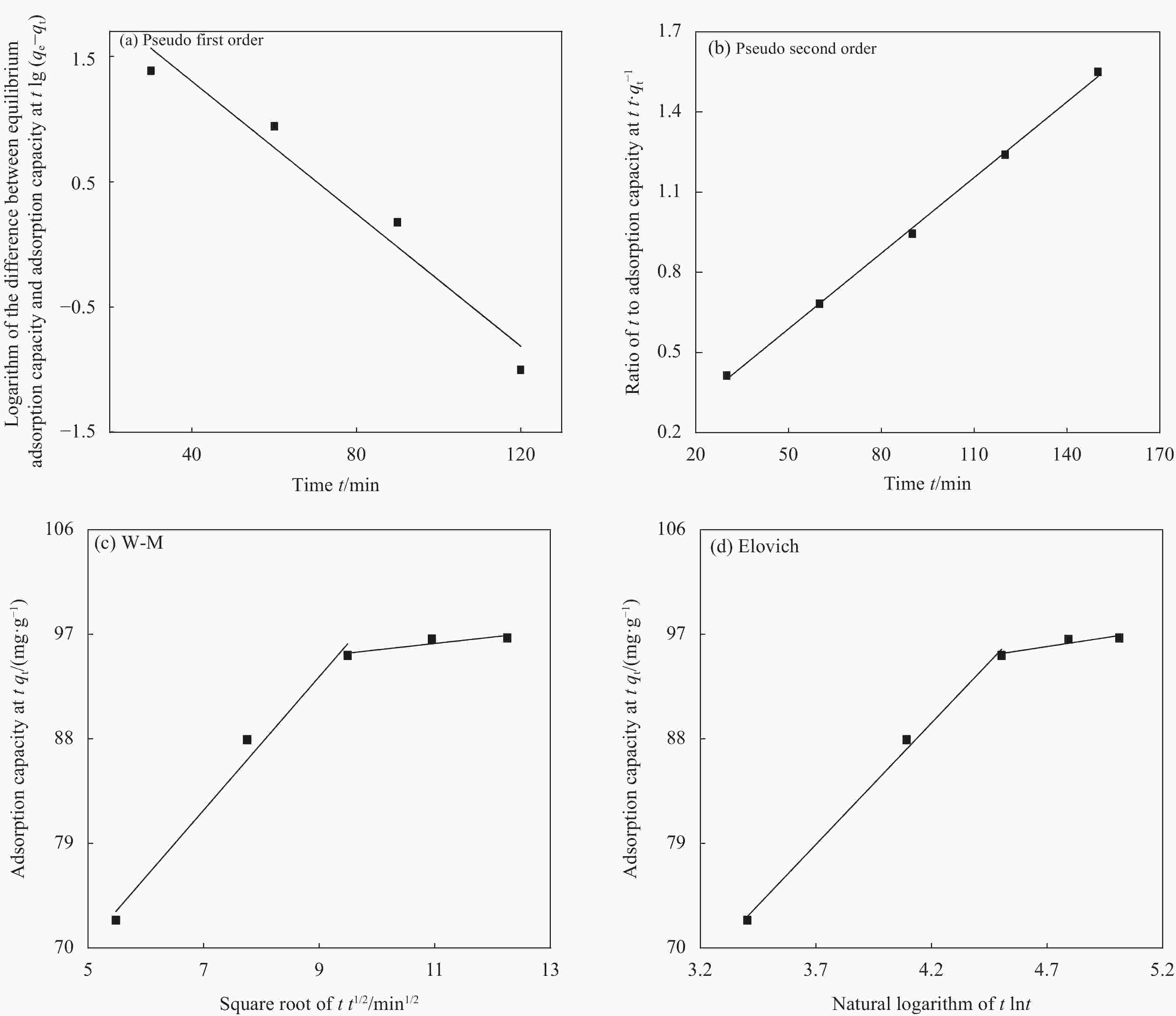
Table 3. Kinetic model parameters of PtCl62− adsorption by thiosemicarbazide/quaternary ammonium lignin
Kinetic models Parameters Pseudo first order K1/(min−1) qe,cal/(mg·g−1) R2 0.0608 227.77 0.938 Pseudo second order K2/(g·mg−1·min−1) qe,cal/(mg·g−1) R2 0.000759 106.00 0.998 W-M K31/(mg·g−1·min−1/2) A1 R12 5.74 41.68 0.966 K32/(mg·g−1·min−1/2) A2 R22 0.55 90.15 0.652 Elovich K41/(mg·g−1·min−1/2) B1 R12 20.91 1.59 0.993 K42/(mg·g−1·min−1/2) B2 R22 3.04 81.68 0.718 Notes: K1—Pseudo-first-order rate constant; qe,cal—Adsorption capacity at equilibrium; R2—Linear regression coefficient; K2—Pseudo-second-order rate constant; K31—Diffusion rate constants in the first stage; K32—Diffusion rate constant in the second stage; A1—First stage boundary layer thickness constant; A2—Second stage boundary layer thickness constant; R12—First stage linear regression coefficient; R22—Second stage linear regression coefficient; K41—First stage of the surface distribution of heterogeneous rate constant; K42—Second stage of the surface distribution of heterogeneous rate constant; B1—First stage Elovich constant; B2—Second stage Elovich constant. -
[1] GUAN H L, CHEN Y, RUAN C Y, et al. Versatile application of wet-oxidation for ambient CO abatement over Fe(OH)x supported subnanometer platinum group metal catalysts[J]. Chinese Journal of Catalysis,2020,41(4):613-621. doi: 10.1016/S1872-2067(19)63489-3 [2] OSMIERI L, PARK J, CULLEN D A, et al. Status and challenges for the application of platinum group metal-free catalysts in proton-exchange membrane fuel cells[J]. Current Opinion in Electrochemistry,2021,25:23-26. doi: 10.1016/j.coelec.2020.08.009 [3] MCCORMICK W, MCCRUDDEN D. Development of a highly nanoporous platinum screen-printed electrode and its application in glucose sensing[J]. Journal of Electroanalytical Chemistry,2020,860:11-18. doi: 10.1016/j.jelechem.2020.113912 [4] YANG X M, LEZA D S, PORCEL E, et al. A facile one-pot synthesis of versatile pegylated platinum nanoflowers and their application in radiation therapy[J]. International Journal of Molecular Sciences,2020,21(5):1619. doi: 10.3390/ijms21051619 [5] ZHANG L, LI S B, XIN J J, et al. A non-enzymatic voltammetric xanthine sensor based on the use of platinum nanoparticles loaded with a metal-organic framework of type MIL-101(Cr). Appliction to simultaneous detection of dopamine, uric acid, xanthine and hypoxanthine[J]. Microchimica Acta,2019,186(1):8-9. doi: 10.1007/s00604-018-3136-4 [6] KRAEMER D, JUNGE M, BAU M. Oxidized ores as future resource for platinum group metals: Current state of research[J]. Chemie Ingenieur Technik,2017,89(1-2):53-63. doi: 10.1002/cite.201600092 [7] 郭利果, 刘玉平, 苏文超, 等. 四川大岩子铂族元素低温成矿特征: BSE微区分析证据[J]. 矿物学报, 2006, 26(2):203-209. doi: 10.3321/j.issn:1000-4734.2006.02.014GUO Liguo, LIU Yuping, SU Wenchao, et al. Low temperature metallogenic characteristics of platinum group elements in Dayanzi, Sichuan: Evidence from BSE microanalysis[J]. Journal of Mineralogy,2006,26(2):203-209(in Chinese). doi: 10.3321/j.issn:1000-4734.2006.02.014 [8] DING Y J, ZHENG H D, ZHANG S G, et al. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection[J]. Resources, Conservation and Recycling,2020,155:104644. doi: 10.1016/j.resconrec.2019.104644 [9] ARSENOV P V, VLASOV I S, EFIMOV A A, et al. Aerosol jet printing of platinum microheaters for the application in gas sensors[J]. IOP Conference Series: Materials Science and Engineering,2019,473(8):012042. [10] WANG J H, ZHONG Y Q, TONG Y, et al. Removal of aluminum nitride from secondary aluminum ash by fire process[J]. Journal of Central South University,2021,28(2):386-397. doi: 10.1007/s11771-021-4610-4 [11] BOUDESOCQUE S, MOHAMADOU A, CONREUX A, et al. The recovery and selective extraction of gold and platinum by novel ionic liquids[J]. Separation and Purification Technology,2019,210(15):824-834. doi: 10.1016/j.seppur.2018.09.002 [12] KWAK I S, BAE M A, WON S W. Sequential process of sorption and incineration for recovery of gold from cyanide solutions: Comparison of ion exchange resin, activated carbon and biosorbent[J]. Chemical Engineering Journal,2010,165(2):440-446. doi: 10.1016/j.cej.2010.09.027 [13] HUANG C D, SHI X F, WANG C, et al. Boosted selectivity and enhanced capacity of As(V) removal from polluted water by triethylenetetramine activated lignin-based adsorbents[J]. International Journal of Biological Macromolecules,2019,140:1167-1174. doi: 10.1016/j.ijbiomac.2019.08.230 [14] WU F F, CHEN L, HU P, et al. Industrial alkali lignin-derived biochar as highly efficient and low-cost adsorption material for Pb(II) from aquatic environment[J]. Bioresource Technology,2021,322:124539. doi: 10.1016/j.biortech.2020.124539 [15] CHEN X, SUN S N, WANG X L, et al. One-pot preparation and characterization of lignin-based cation exchange resin and its utilization in Pb(II) removal[J]. Bioresource Technology,2020,295:1-5. doi: 10.1016/j.biortech.2019.122297 [16] SHI X F, WANG C, DONG B B, et al. Cu/N doped lignin for highly selective efficient removal of As(V) from polluted water[J]. International Journal of Biological Macromolecules,2020,161:147-154. doi: 10.1016/j.ijbiomac.2020.06.016 [17] 白成玲, 王磊, 朱振亚, 等. 氧化石墨烯/海藻酸钙水凝胶复合膜对水中Cd(II)的吸附[J]. 复合材料学报, 2020, 37(6):1458-1465. doi: 10.13801/j.cnki.fhclxb.20191016.001BAI Chengling, WANG Lei, ZHU Zhenya, et al. Adsorption of Cd(II) in water by graphene oxide/calcium alginate hydrogel composite membrane[J]. Acta Materiae Compo-sitae Sinica,2020,37(6):1458-1465(in Chinese). doi: 10.13801/j.cnki.fhclxb.20191016.001 [18] 张保平, 马钟琛, 刘运, 等. 木质素的化学改性及其对AuCl4-的吸附[J]. 化工学报, 2017, 68(5):1946-1953.ZHANG Baoping, MA Zhongchen, LIU Yun, et al. Chemical modification of lignin and adsorption of AuCl4-[J]. Journal of Chemical Industry,2017,68(5):1946-1953(in Chinese). [19] PARAJULI D, KHUNATHAI K, ADHIKARI C R, et al. Total recovery of gold, palladium, and platinum using lignophenol derivative[J]. Minerals Engineering,2009,22(13):1173-1178. doi: 10.1016/j.mineng.2009.06.003 [20] PRIASTOMO Y, MORISADA S, KAWAKITA H, et al. Synthesis of macrocyclic polyphenol resin by methylene crosslinked calix[4]arene (MC-[4]H) for the adsorption of palladium and platinum ions[J]. New Journal of Chemistry,2019,43(21):8015-8023. doi: 10.1039/C9NJ00435A [21] LEBEDEVA O V, SIPKINA E I, POZHIDAEV Y N. Adsorption of platinum(IV) by a composite based on silicon dioxide and copolymer of 4-vinylpyridine and 2-hydroxyethylmethacrylate[J]. Protection of Metals and Physical Chemistry of Surfaces,2017,53(1):80-84. doi: 10.1134/S2070205117010130 [22] LOSEV V N, ELSUFIEV E V, BUYKO O V, et al. Extraction of precious metals from industrial solutions by the pine (Pinus sylvestris) sawdust-based biosorbent modified with thiourea groups[J]. Hydrometallurgy,2018,176:118-128. doi: 10.1016/j.hydromet.2018.01.016 [23] 张保平, 杨芳, 马钟琛, 等. 稻草木质素的硫酸法提取与表征[J]. 河北大学学报: 自然科学版, 2016, 36(5): 474-479, 486. ZHANG Baoping, YANG Fang, MA Zhongchen, et al. Extraction and characterization of rice straw lignin by sulfuric acid method[J]. Journal of Hebei University: Natural Science Edition, 2016, 36(5): 474-479, 486(in Chinese). [24] DUVAL A, LANGE H, LAWOKO M, et al. Reversible crosslinking of lignin via the furan-maleimide Diels-Alder reaction[J]. Green Chemistry,2015,17(11):4991-5000. doi: 10.1039/C5GC01319D [25] ZHANG B P, MA Z C, YANG F, et al. Adsorption properties of ion recognition rice straw lignin on PdCl42−: Equilibrium, kinetics and mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2017,514:260-268. doi: 10.1016/j.colsurfa.2016.11.069 [26] 高秀清, 刘俊峰, 樊慧菊, 等. 五乙烯六胺改性活性炭对Cd离子的吸附效能[J]. 复合材料学报, 2019, 36(7):1769-1775. doi: 10.13801/j.cnki.fhclxb.20181023.002GAO Xiuqing, LIU Junfeng, FAN Huiju, et al. Adsorption efficiency of Cd ion on activated carbon modified by pentethylene hexamine[J]. Acta Materiae Compositae Sinica,2019,36(7):1769-1775(in Chinese). doi: 10.13801/j.cnki.fhclxb.20181023.002 [27] BIROL I, VOLKAN U. Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies[J]. International Journal of Biological Macromolecules,2021,167(3):1156-1167. doi: 10.1016/j.ijbiomac.2020.11.070 [28] ANBIA M, RAHIMI F. Adsorption of platinum(IV) from an aqueous solution with magnetic cellulose functionalized with thiol and amine as a nano-active adsorbent[J]. Jour-nal of Applied Polymer Science,2017,134(39):1-8. doi: 10.1002/app.45361 [29] ZHANG B P, SHEN B W, GUO M C, et al. Adsorption of PtCl62- from hydrochloric acid solution by chemically modified lignin based on rice straw[J]. Australian Journal of Chemistry,2018,71(12):931-938. doi: 10.1071/CH18282 [30] LI H, WANG F H, LI J N, et al. Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: Kinetics, isotherms, thermodynamics, and molecular dynamics simulation[J]. Chemosphere,2021,264(P2):128556. doi: 10.1016/j.chemosphere.2020.128556 [31] POPOVIC A L, RUSMIROVIC L D, VELICKOVIC L, et al. Kinetics and column adsorption study of diclofenac and heavy-metal ions removal by amino-functionalized lignin microspheres[J]. Journal of Industrial and Engineering Chemistry,2021,93:302-314. doi: 10.1016/j.jiec.2020.10.006 [32] HASSAN K, UDDIN A S M I, CHUNG G S. Fast-response hydrogen sensors based on discrete Pt/Pd bimetallicultra-thin films[J]. Sensors and Actuators B: Chemical,2016,234:435-445. doi: 10.1016/j.snb.2016.05.013 -






 下载:
下载: