Research advances of MOF-based catalyst for photohydrolysis for hydrogen production
-
摘要: 随着能源枯竭和环境污染问题日益严重,人们不得不将目光转向更加清洁环保的氢能源。光解水制氢技术是一种获取氢能源经济且清洁的理想方式,通过光催化手段将太阳能转化为化学能也是一种很有前景的技术手段。然而如何选取高效、经济的光催化剂是制氢最关键的环节。金属-有机框架(Metal-organic frameworks, MOFs)由于比表面积大、孔尺寸可调节、结构易于修饰及活性位点丰富等特点,使其成为光解水制氢理想的光催化剂候选材料。国内外学者就MOFs光解水制氢开展了大量的研究,并且取得了丰硕的成果。本论文综述了MOF基材料作为催化剂在光解水制氢领域的研究进展,总结了MOFs作为催化剂的优点和局限性,并对MOFs及其相关材料在光催化水解制氢领域的发展前景提出展望,以期对未来研究提供参考。Abstract: The increasingly serious energy exhaustion and environmental pollution accelerates the development of clean hydrogen energy. Water splitting via photocatalysis technology provides an economical and clean way for the hydrogen production, converting solar energy into chemical energy through photocatalytic means is also a promising technical means. The rational selection of photocatalyst is the critical step for obtaining hydrogen energy in an efficient and economical way. Featuring the traits of large specific surface area, adjustable pore size, easy structure modification and abundant active sites, metal-organic frameworks (MOFs) are ideal candidates for photocatalytic hydrogen production scientists from domestic and foreign have carried out numerous researches on the water splitting with MOF-based photocatalysts. Currently, fruitful progresses have been achieved. In this paper, the state-of-the-art advances for the MOF-based materials as catalysts in the field of hydrogen production from splitting water was reviewed, and the advantages and limitations of MOFs as catalysts were summarized. The development prospect of MOFs and related materials in the field of photocatalytic hydrogen production was proposed, providing a valuable guideline for developing future photocatalysts.
-
Keywords:
- metal-organic frameworks /
- photocatalysis /
- catalyst /
- water splitting /
- hydrogen production
-
木材因其优良的力学性能、低廉的成本和独特的美学特性,在建筑和装饰材料领域被广泛应用[1-3]。然而,木材固有的可燃性限制了其在消防安全要求高的领域的实际应用,尤其是在人口密集的公共区域。因此,对木材进行阻燃处理,是有效提高木材防火安全性和避免潜在的火灾危害的措施之一[4-5]。
水凝胶具备富水的性质和保水的能力,其中所含有的水分具有较高的摩尔热容量和蒸发焓[6]。因此,水凝胶作为一道新屏障,在受热时通过水的蒸发可吸收大量的热,降低基体表面温度,稀释可燃气体,延缓材料的热降解,从而抑制火灾[7-8]。此外,水凝胶在分解过程中不释放有毒挥发物,对人体健康和环境均无危害,是一种极具发展前景的绿色木材阻燃涂料。Zhang等[9]使用明胶、壳聚糖(CS)和甘油制备出了木材阻燃涂层;结果表明,涂覆涂层的木材的点燃时间(TTI)增加了6倍,峰值热释放速率(pHRR)降低了24.0%,总热释放量(THR)降低了17.2%,与未涂覆涂层的木材相比,具有更长的耐火时间和快速的自熄性。Zhao等[10]制备出一种聚乙烯醇(PVA)/植酸(PA)水凝胶涂层,并通过冻融循环法来提高木材的阻燃性能;结果表明,与纯PVA水凝胶涂层相比,所获PVA/PA水凝胶涂层具有更好的粘附性能和防火性能。
对于阻燃涂层而言,有效的防火时间和基体材料的保护程度是衡量其阻燃性能的重要指标。而将水凝胶作为复合涂层应用于木材上,能延缓木材的点燃时间,从而延长火灾中的逃生时间;但单独使用而不添加其他阻燃剂时,其抑烟性能较差。此外,大多数水凝胶均由有机聚合物聚合而成,在受热水分蒸发后易被烧毁,且高温下只能保持约30 s的完好[11]。增加水凝胶涂层厚度是一种有效的基体材料保护手段,但其重量会随之大幅度增加,降低其实际应用效果;因此,延长有效的防火时间和增加水凝胶网络的保护程度是优化水凝胶涂层阻燃性能的发展方向[12]。
添加阻燃剂是一种提高水凝胶阻燃性能的有效手段。次磷酸铝(AHP)是公认的高效无卤阻燃剂之一[13],因其优良的阻燃性被广泛应用于聚合物材料的阻燃。Yuan等[14]以PVA和AHP为原料,采用溶剂共混法制备复合材料,当AHP负载量为15wt%时,复合材料的极限氧指数(LOI)达到28%,阻燃等级达到UL-94中的V-0级。但AHP的高负载量会对涂层的物理性能造成较大的负面影响,通常采用添加协同剂、表面改性和微胶囊等方法解决此问题[15-17];其中,添加协同剂是一种简单高效的手段。Zhou等[18]将AHP与热塑性聚氨酯(PT)共混制备阻燃材料,锥形量热测试结果表明,当AHP为22.5wt%,PT为2.5wt%时,pHRR、THR和总烟释放量(TSR)分别降低了91.2%、70%和63%。Yang等[19]探究了碳纳米管(m-CNT)和AHP对聚乳酸(PLA)/玄武岩(BF)的阻燃效果的影响;结果表明,含有19wt% AHP和1wt% m-CNT的PLA/BF在UL-94测试中达到了V-0级,LOI为31%,且与纯PLA/BF相比,pHRR和THR分别降低了约64%和27%,说明AHP和m-CNT显著提高了PLA/BF的阻燃性能。
玄武岩岩石在生产过程中会产生纤维和粉末等玄武岩填料[20],这是一种天然的、以矿物为基础的无机质硬填料,主要化学成分为43%~48%的SiO2、11%~12%的Al2O3、5%的Fe2O3、0%~5%的MgO等[21];根据以往的研究,SiO2和金属氧化物可作为增效剂,来提高聚合物材料的阻燃和抑烟性能。此外,我国玄武岩储量丰富,质量好,作为阻燃填料具有很好的前景[22]。然而,由于玄武岩表面活性低,分散性能差,作为阻燃填料直接使用时无法实现其高值化利用;故需对玄武岩进行改性处理,以提高其阻燃性能。
本文采用PVA和CS作为水凝胶基质,PVA可增强凝胶网络结构,以提高阻燃涂层与木材的结合强度,CS复配乙酸可形成膨胀型阻燃剂,AHP加入其中以进一步提高其阻燃性,此外,改性玄武岩和植酸金属盐分别作为阻燃填料和促炭剂,起到凝聚相阻燃作用,由此形成了气相/凝聚相阻燃体系,并对该复合阻燃体系对木材阻燃性能的影响进行了研究。通过SEM观察表面改性和沉淀法改性后玄武岩的形貌变化,采用FTIR分析其化学结构变化。利用UL-94阻燃等级标准和锥形量热(CONE)测试水凝胶复合阻燃涂层的阻燃性能;通过TGA测试对涂层的热稳定性进行研究;并测试了具有涂层木材的物理力学性能。
1. 实验部分
1.1 原材料
壳聚糖:粉料,脱乙酰度90%,上海源叶生物科技有限公司;聚乙烯醇:1788型,粉料,醇解度88%,聚合度
1700 ,西陇科学股份有限公司;玄武岩:粉料,粒径6000 目,天津硅酸盐研究所;植酸钠:分析纯,国药集团化学试剂有限公司;乙酸:分析纯,天津市科密欧化学试剂有限公司;乙酸镍(C4H6O4Ni·4H2O):分析纯,西陇科学股份有限公司;氢氧化钠(NaOH):分析纯,西陇科学股份有限公司;乙酸锌(C4H6ZnO4·2H2O):分析纯,天津市风船化学试剂科技有限公司;乙酸铝(Al(OH)2·(CH3COO)):分析纯,上海麦克林生化科技有限公司;次磷酸铝(Al(H2PO2)3):分析纯,上海麦克林生化科技有限公司;硅烷偶联剂(KH550):山东优索化工科技有限公司。1.2 实验过程
1.2.1 改性玄武岩的制备
(1) NaOH蚀刻处理:将玄武岩鳞片(30 g)放在
1000 mL的锥形瓶中,并加入900 mL的氢氧化钠溶液(4 mol·L−1),在100℃的水浴锅中磁力搅拌2 h,然后将玄武岩鳞片进行过滤、清洗(直至溶液pH=7.0)和干燥(105℃,24 h)。(2) KH550接枝处理:将上一步处理后的10 g玄武岩、1 g KH550和800 mL去离子水置于
1000 mL锥形瓶中,在80℃水浴锅中磁力搅拌3 h后,将玄武岩鳞片进行过滤、清洗(直至溶液pH=7.0)和干燥(105℃,24 h)。(3)共沉淀法改性阻燃剂:将上一步改性处理的5 g玄武岩鳞片、9.24 g植酸钠(0.01 mol)和800 mL去离子水置于
1000 mL锥形瓶中,并在80℃水浴锅中磁力搅拌1 h,最后加入0.03 mol的乙酸镍、乙酸铝或乙酸锌,继续反应2 h,然后将改性后的玄武岩鳞片进行过滤、清洗(直至溶液pH=7.0)和干燥(105℃,24 h),得到不同物质改性的阻燃玄武岩鳞片(BS-PA-Ni、BS-PA-Al、BS-PA-Zn)。图1为改性玄武岩的制备工艺流程。1.2.2 复合水凝胶阻燃涂层的制备
将4 g CS和4 g PVA溶于92 g醋酸水溶液(3%,v/v)中,在98℃下以200 r/min搅拌直至CS和PVA完全溶解。待CS/PVA溶液冷却至室温(25℃)后,分别加入3wt%的AHP和7wt%的BS (在该比例下,BS与AHP不容易发生团聚,分散效果最佳),以
1500 r/min的转速分散5 min,得到其水凝胶前驱体复合溶液。然后将不同组分的前驱体溶液涂覆于木材表面,制成厚度约为300 μm的水凝胶复合阻燃涂层。最后将所制备的样品在−20℃的冰箱中冷冻12 h。所有样品的组成列于表1中,Control为未添加AHP和BS的复合水凝胶涂层。表 1 水凝胶阻燃涂料配方Table 1. Hydrogel flame retardant coating formulaSample Hydrogel/wt% AHP/wt% Modified BS/wt% Control 100 0 0 BS-PA-Al 90 3 7 BS-PA-Ni 90 3 7 BS-PA-Zn 90 3 7 Notes: Hydrogel—The amount of hydrogel in the composite coating; AHP—The amount of aluminum hypophosphite in the composite coating; Modified BS—The amount of modified basalt in the composite coating. 1.3 测试与表征
采用配备了能量色散X射线分析装置(EDX)的Prisma型扫描电子显微镜(SEM,美国FEI公司)观察样品的微观形貌变化和元素的分布,测试时,对所有样品进行喷铂处理增加导电性,测量电压为20 kV。
采用ALPHA03040404型傅里叶红外光谱仪(FTIR,美国BRUKER公司)测试样品的化学结构变化,样品用溴化钾(KBr)压片法制得,扫描范围为400~
4000 cm−1。在N2条件下,使用PYris603190148型热重分析仪(TGA,美国PE公司),在室温到800℃的温度下以10℃/min的线性加热速率对复合材料的热降解行为进行测试,所有样品的质量都保持在5~10 mg以内。
锥形量热(CONE)测试在FTT007型锥形量热计(英国FTT公司)上进行,热通量为50 kW/m2,样品尺寸为100 mm×100 mm×5 mm。
根据GB/T 2408—2008测试标准[23],在FTT 0082型仪器(英国FTT公司)上进行UL-94测试来确定复合材料的可燃性试验,试验用试样尺寸为125 mm×13 mm×3 mm。
力学性能粘附试验的样品尺寸根据文献[24]确定,接触面积为13 mm×20 mm,并对尺寸为125 mm×13 mm×3 mm的涂层样品进行了应力-应变曲线分析。
2. 结果与讨论
2.1 改性玄武岩/水凝胶复合材料微观形貌分析
图2为所制备样品的SEM图像和EDX图像。由于玄武岩鳞片约由50%的SiO2组成,因此在图2(a)中可以观察到,样品BS的表面较为光滑,这不利于填料与材料之间的结合。在图2(b)中,经NaOH蚀刻后的玄武岩较未改性的具有更粗糙的表面,说明在NaOH蚀刻过程中,溶液中的OH−通过微小孔隙进入到玄武岩中,破坏了Si—O—Si键和Si—O—Al键,使得硅铝酸盐网格开始溶解,导致孔隙扩大[25]。而图2(c)、图2(e)和图2(g)则分别展示了BS-PA-Al、BS-PA-Ni和BS-PA-Zn的微观形貌,可以观察到,被蚀刻后的玄武岩鳞片经KH550接枝和植酸金属盐沉积后,表面变得更加粗糙,且从图2(d)、图2(f)和图2(h)右上角的EDX能谱图中可以看出,Al、Ni和Zn元素均匀分布在玄武岩表面,说明植酸铝、植酸镍和植酸锌的成功沉积,有利于阻燃效果的进一步改善。
![]() 图 2 样品的SEM图像:(a) BS;(b) NaOH蚀刻后的BS;(c) BS-PA-Al;(d) BS-PA-Al的EDX能谱图;(e) BS-PA-Ni;(f) BS-PA-Ni的EDX能谱图;(g) BS-PA-Zn;(h) BS-PA-Zn的EDX能谱图Figure 2. SEM images of samples: (a) Unmodified basalt; (b) NaOH etched basalt; (c) BS-PA-Al; (d) Element distribution of BS-PA-Al; (e) BS-PA-Ni; (f) Element distribution of BS-PA-Ni; (g) BS-PA-Zn; (h) Element distribution of BS-PA-Zn
图 2 样品的SEM图像:(a) BS;(b) NaOH蚀刻后的BS;(c) BS-PA-Al;(d) BS-PA-Al的EDX能谱图;(e) BS-PA-Ni;(f) BS-PA-Ni的EDX能谱图;(g) BS-PA-Zn;(h) BS-PA-Zn的EDX能谱图Figure 2. SEM images of samples: (a) Unmodified basalt; (b) NaOH etched basalt; (c) BS-PA-Al; (d) Element distribution of BS-PA-Al; (e) BS-PA-Ni; (f) Element distribution of BS-PA-Ni; (g) BS-PA-Zn; (h) Element distribution of BS-PA-Zn2.2 改性玄武岩/水凝胶复合材料化学结构分析
图3为BS、BS-KH550、BS-PA-Al、BS-PA-Ni和BS-PA-Zn的红外吸收光谱图。从图中可知,在850~
1284 cm−1出现较强的吸收峰,归属于Si—O—Si的对称与不对称拉伸振动。相比于BS,BS-KH550、BS-PA-Al、BS-PA-Ni和BS-PA-Zn在3554 cm−1处的—OH吸收峰均有所增强,说明经NaOH蚀刻后的玄武岩暴露出更多的—OH,增加了化学反应位点,使得表面更易发生化学修饰;而在1646 cm−1出现的—NH的吸收峰则证明KH550成功接枝到玄武岩表面[26]。此外,对于BS-PA-Al、BS-PA-Ni和BS-PA-Zn,在1063 cm−1出现了P—O的振动吸收峰,进一步证明了植酸铝、植酸镍、植酸锌成功沉积在玄武岩表面[27]。此外,在1075 ~1125 cm−1处出现了较弱的吸收峰,可归属于O=P的伸缩振动峰,但由于该峰与Si—O—Si的吸收峰部分重叠,导致该峰在图谱中不易被观察到。2.3 改性玄武岩/水凝胶复合材料热稳定性分析
利用热重分析(TGA)和热重微分(DTG)曲线来讨论复合涂层的降解过程,相关数据如表2所示。如图4(a)所示,未添加AHP和BS的复合水凝胶涂层(Control)的分解可分为3个阶段:第一阶段(40~170℃),由于Control组水凝胶复合涂层富含较多的水,因此主要是PVA和CS的脱水过程(释放吸附水);第二阶段(200~390℃),由于PVA是多羟基聚合物,且在分子链中保留了大量乙酸基团,在此阶段,残余的乙酸基团分解,CS大分子发生主链断裂和开环反应;第三阶段(400~750℃),水凝胶阻燃涂层完全降解后无碳残渣的PVA链裂解发生环化反应、CS的主链断裂和开环反应[28]。对于添加了AHP和改性玄武岩的水凝胶涂层,第一阶段的分解过程与Control组相似,第二阶段的分解主要是PVA、CS和AHP的分解,而在第三阶段中,由于第二阶段AHP的分解催化作用[14],使得涂层的热稳定性提高,此阶段最大热失重速率的温度较Control组后移约45~150℃。在分解过程中,玄武岩表面沉积的植酸金属离子可同时作为树脂交联和脱氢反应的催化剂,既能增加膨胀阻燃剂在高温下的交联程度,也能促进炭的形成,提高复合材料的热稳定性,达到更好的阻燃效果[29]。此外,结合图4(b)和表2的TGA测试数据可得,BS-PA-Al、BS-PA-Ni和BS-PA-Zn涂层的残炭率分别为22.4%、27.3%和20.8%,相较于Control组涂层的3.12%均有所增加,其中BS-PA-Ni组涂层的残炭率达到了27.3%,说明改性玄武岩和添加AHP后能有效防止涂层降解,提高其热稳定性。
表 2 复合涂料的主要TGA数据Table 2. Main TGA data of composite coatingsSample T10%/℃ T50%/℃ Residue/% Control 164.36 327.91 3.12 BS-PA-Al 129.07 339.87 22.4 BS-PA-Ni 123.64 382.43 27.3 BS-PA-Zn 116.88 351.55 20.8 Notes: T10%—Temperature at 10% mass loss; T50%—Temperature at 50% mass loss; Residue—Residual carbon content. 2.4 复合涂层/木材的阻燃性能
(1)锥形量热测试
为了更好地了解涂层的阻燃效果,将水凝胶涂层涂覆于木材表面,对样品进行阻燃效果测试,CONE的主要燃烧参数如表3所示,未涂覆涂层的木材(Pure wood)的点燃时间(TTI)为12 s,而水凝胶涂层木材(Control、BS-PA-Al、BS-PA-Ni、BS-PA-Zn)较Pure wood的TTI分别增加了292%、83%、125%和75%,这表明水凝胶阻燃复合涂层能有效提高木材的消防安全,TTI的延长对火灾救援具有重要意义。此外,从表3中可以观察到,BS-PA-Ni(dry)水凝胶涂层木材的TTI仍比未涂覆涂层的木材的长,表明失水后的水凝胶涂层仍具有阻燃性能。图5(a)和图5(b)为样品的HRR和THR变化曲线,水凝胶阻燃涂层木材的HRR和THR较未涂覆涂层木材明显降低;HRR曲线出现两个峰值:第一个峰值是由于水凝胶阻燃涂层降解释放可燃气体;第二个峰值是由于木材的涂层烧焦后,表面炭层裂解,可燃气体从裂缝中挥发,使得木材燃烧加剧,而涂覆水凝胶涂层的木材则延缓了第二峰值的出现。此外,从表3可得,Control、BS-PA-Al、BS-PA-Ni和BS-PA-Zn水凝胶涂层木材的pHRR较未涂覆涂层的木材分别降低48.6%、63.6%、64.2%和58.0%,THR分别降低18.9%、24.1%、32.7%和19.8%,说明改性玄武岩/水凝胶复合涂层对木材阻燃的改善效果较为显著。结合图5(c)、图5(d)和表3可知,BS-PA-Al、BS-PA-Ni和BS-PA-Zn水凝胶涂层木材的TSR较未涂覆涂层木材分别降低55.3%、51.3%和31.2%,而Control水凝胶涂层在燃烧时的TSR较未涂覆涂层的木材有所增加,这是由于该水凝胶阻燃涂层在物理和化学方面共同起到阻燃作用:首先水凝胶的高含水量能够吸收部分热量和稀释可燃气体,随着温度的升高,AHP分解产生的PO•和HPO•与燃烧过程中产生的自由基进行反应、终端燃烧链支化反应,达到阻燃效果;除此之外,玄武岩表面沉积的植酸金属离子亦能同时作为树脂交联和脱氢反应的催化剂,提高膨胀阻燃剂在高温下的交联程度以及促进炭的形成,而BS的存在也增加了炭层的致密性,使得热稳定性有了明显的提升,从而达到了更好的阻燃效果。其中BS-PA-Ni阻燃涂层的效果最佳,这可能是由于Ni+的催化性能最佳,在催化成炭,提高炭层的质量方面优于Al3+和Zn2+[30]。
表 3 水凝胶阻燃涂料锥形量热主要数据Table 3. Hydrogel flame retardant CONE main dataSample TTI/s pHRR/
(kW·m−2)THR/
(MJ·m−2)TSR/
m2Pure wood 12 572.90 44.17 1.99 Control 47 294.50 35.80 2.91 BS-PA-Al 22 210.51 33.51 0.89 BS-PA-Ni 27 205.38 29.74 0.97 BS-PA-Zn 21 240.68 35.44 1.37 BS-PA-Ni(dry) 17 397.90 39.14 3.16 Notes: TTI—Time to ignition of wood; pHRR—Peak heat release rate; THR—Total heat release; TSR—Total smoke release. (2)垂直燃烧测试
采用UL-94进一步验证水凝胶涂层木材的阻燃性能,相关数据列于表4。由表可知,未涂覆涂层的木材在被点燃后完全燃烧,而BS-PA-Al和BS-PA-Ni水凝胶涂层木材的UL-94等级达到V-0级,Control和BS-PA-Zn水凝胶涂层木材达到V-1级。其中BS-PA-Al和BS-PA-Ni水凝胶涂层木材在第一个和第二个10 s均未被点燃,且Control和BS-PA-Zn水凝胶涂层木材在点燃后也会在较短时间内熄灭,说明水凝胶阻燃涂层可赋予木材优异自熄性能和阻燃性能,其中BS-PA-Al和BS-PA-Ni水凝胶涂层木材的性能最佳。
表 4 水凝胶阻燃涂料的垂直燃烧数据Table 4. Vertical combustion data of hydrogel flame retardant coatingSample T1/s T2/s UL-94 Dripping or not Pure wood — — NR Yes Control 2 21 V-1 No BS-PA-Al 0 0 V-0 No BS-PA-Ni 0 0 V-0 No BS-PA-Zn 3 13 V-1 No Notes: T1—The first fire of wood; T2—The second fire of wood; UL-94—UL-94 combustion rating of wood; Dripping or not—Whether the coating drips during wood burning. 2.5 复合涂层/木材的力学性能
为了研究水凝胶阻燃涂层对木材力学性能的影响,对涂覆水凝胶阻燃涂层和未涂覆的木材进行拉伸实验对比,由图6(a)可知,未涂覆涂层的木材、Control、BS-PA-Al、BS-PA-Ni和BS-PA-Zn水凝胶阻燃涂层木材的抗拉强度分别为64.7、56.0、53.2、80.4和62.2 MPa,说明经过涂覆水凝胶阻燃涂层的木材与未涂覆涂层的木材的抗拉强度变化不大,对木材的力学性能影响较小。此外,涂层与基体材料的附着力是衡量涂层阻燃效果有否优异的一个重要指标,按照图6(b)所示的方案进行拉伸附着力测试,结果表明,Control、BS-PA-Al、BS-PA-Ni和BS-PA-Zn水凝胶阻燃涂层与木材之间的粘附力分别为5.0、1.4、3.4和2.0 MPa,说明AHP和改性BS的添加降低了水凝胶涂层与木材间的附着力,这是由于木材表面具有管孔结构,水凝胶涂层可渗透到木材表面,固化后在木材内部形成了凝胶网络互锁结构;此外,由于水凝胶涂层的成膜物质PVA和CS富含羟基,因此也能通过氢键与木材表面的羟基结合,来增加木材与水凝胶阻燃涂层间的附着力。
2.6 改性玄武岩/水凝胶复合涂层的阻燃机制
图7解释了水凝胶阻燃涂层的阻燃机制,该涂层由气相和凝聚相阻燃共同作用。当涂覆该涂层的木材受热时,由于CS中含有—NH2,可在高温下热分解生成NH3等不可燃气体,降低燃烧气体浓度。CS与乙酸进行复配后,构成的膨胀型阻燃体系能够初步起到催化成炭的作用,在木材表面形成炭层,保护木材内部不被燃烧。此外,由于水凝胶丰富的含水量,涂层内部的水首先被加热蒸发,吸收大部分热量,降低了木材表面温度,同时蒸发的水蒸气稀释样品周围的空气。当温度达到约290℃时,其含有的AHP开始分解,在该过程中会产生化学稳定性高的HPO和PO•自由基,以及化学性质活泼的H•和HO•自由基,PO•可以捕获H•和HO•自由基,减缓或终止自由基氧化反应的发生,达到良好的阻燃效果。此外,AHP会分解生成不可燃的磷酸盐固体物质如AlPO4、Al(H2PO4)3等,这些物质可以隔绝可燃气体和助燃气体的进入,起到保护木材的作用,且生成的H3PO4也能够起到催化成炭作用,使得聚乙烯醇和壳聚糖的脱氢交联反应,进一步生成更加紧密的炭层。化学反应过程如方程式(1)~(3)所示。
Al(H2PO2)3→AlPO4+2PH3↑+O2↑ (1) Al(H2PO2)3→Al(H2PO4)3+6PH3↑+3H2O↑ (2) PH3↑+2O2↑→H3PO4 (3) 除此之外,BS表面沉积的植酸金属络合物作为树脂交联和脱氢反应的催化剂,有助于提高复合材料的热稳定性、成膜物质在高温下的交联程度和热稳定性,以及促进炭的形成,并且其含有的磷酸基团可催化PVA的前期降解,改善PVA的阻燃性。而BS由于具有较好的力学性能,能够使得可连接残炭以保持凝聚相完整,增加炭层的致密性,而紧密的炭层在阻燃中可以起到物理屏障作用,减缓热量的传递,延缓基体材料的燃烧。因此该炭层可更好地保护火焰下的基体材料,从而减慢热和氧气的传递,两者协同作用共同改善了抑烟性能,同时提高了水凝胶的膨胀型阻燃体系的阻燃效率。
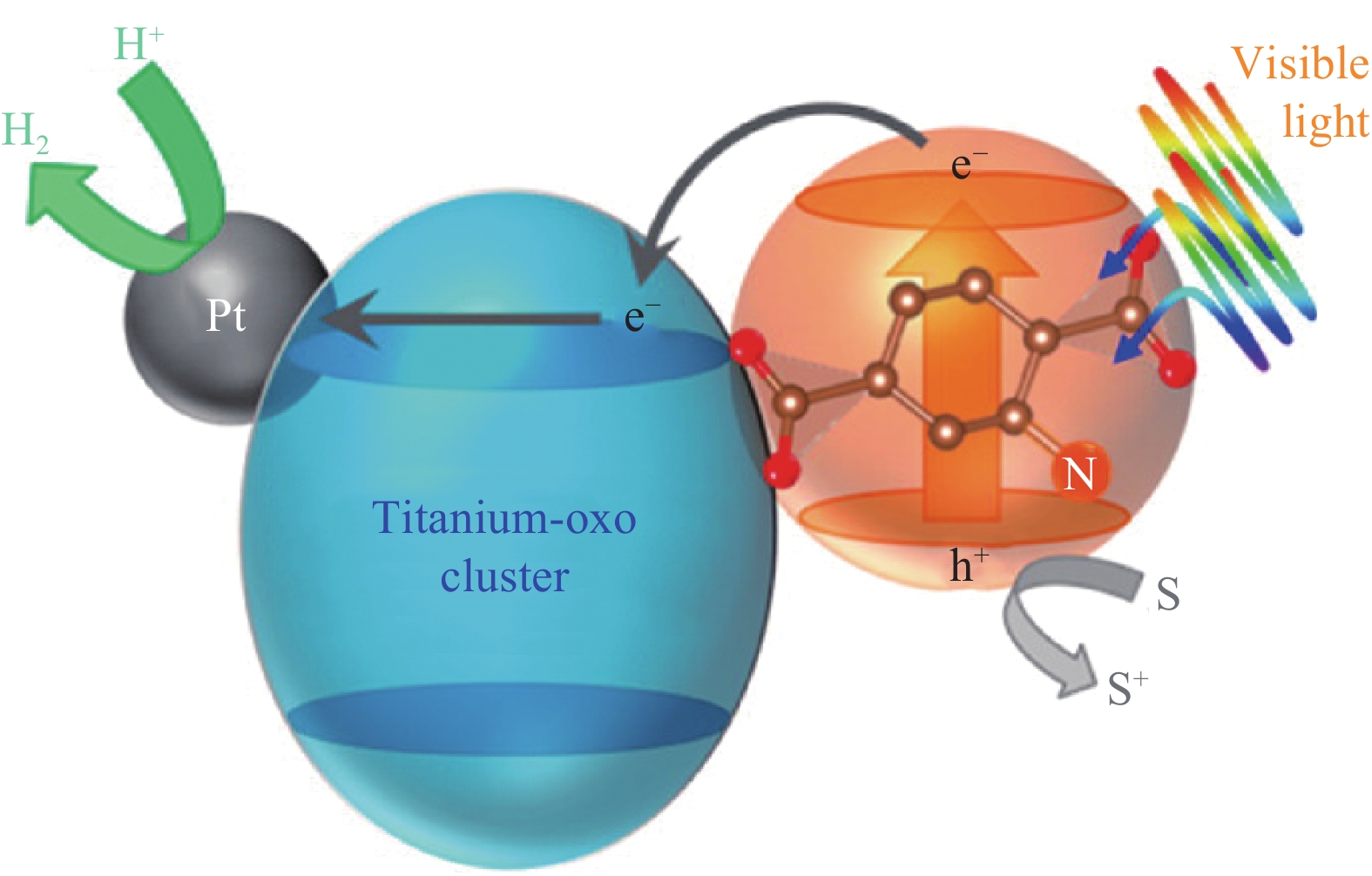
3. 结 论
以添加次磷酸铝阻燃剂的聚乙烯醇和壳聚糖作为水凝胶基质,经氢氧化钠预处理、硅烷偶联剂KH550接枝和植酸金属化合物改性后的玄武岩作为阻燃填料,两者复合后形成水凝胶阻燃涂层。
(1) SEM和FTIR结果表明,植酸铝、植酸镍和植酸锌在改性后的玄武岩表面成功沉积;
(2) TGA、CONE和垂直燃烧表明,经植酸镍改性的玄武岩与水凝胶基质复合得到的阻燃涂层具有最佳的阻燃性能,其UL-94测试达到V-0级,相较于未处理的木材,其残炭率增至27.3%,pHRR、THR和TSR降低64.2%、32.7%和51.3%。当涂层的厚度为300 µm时,抗拉强度较小,且其附着强度能达到3.4 MPa;
(3)改性玄武岩和次磷酸铝的添加能够提高水凝胶涂层的热稳定性,并提高涂层的阻燃抑烟性能,使木材具有良好的防火阻燃性能。
-
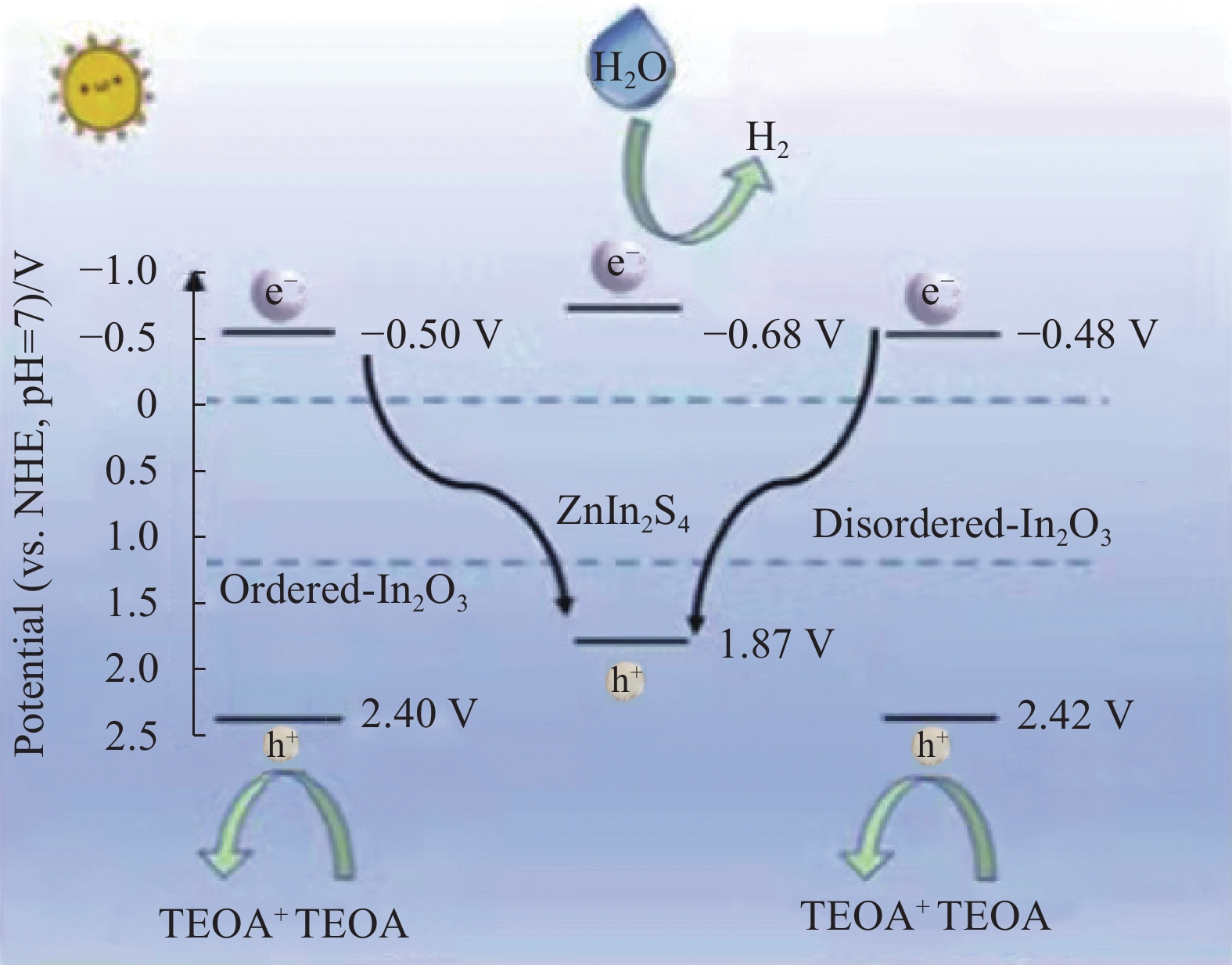
图 3 Al3(OH)3(HTCS)2 (AlTCS-1)在水和三乙醇胺(TEOA)混合溶剂中光催化裂解水的机制[24]
Figure 3. Mechanism of the photocatalytic water splitting process of Al3(OH)3(HTCS)2 (AlTCS-1) in mixed solvents of H2O and triethanolamine (TEOA)[24]
NHE—Normal hydrogen electrode; TCS—Tetrakis (4-oxycarbonylphenyl) silane; CB—Conduction band; VB—Valence band; Eg—Energy gap
图 13 Cu3P@CoP的曙红Y (EY) 敏化剂p-n异质结在可见光照射下水分解制氢过程机制图[78]
Figure 13. Mechanism diagram of the hydrogen production process by water decomposition of Eosin Y (EY) sensitizer p-n heterojunction of Cu3P@CoP under visible light irradiation[78]
SCE—Saturated calomel electrode; Ef—Fermi level; ISC—Inter system crossing
表 1 有关MOF/纳米颗粒复合材料作为可见光照射下的光催化析氢反应的催化剂
Table 1 MOF/nanoparticle composites as catalysts for photocatalytic hydrogen evolution under visible light irradiation
Photocatalyst Bandgap Eg/eV Sacrificial agents Co-catalysts H2 Production rate/(mmol·h−1 ·g−1) Recycled
timesSolution
stability/hRef. TiO2@ZIF-8 3.28 Methanol — 0.25 4 24 [56] MOF-199/Ni — Triethanolamine Pt 24.40 3 9 [57] Ru-Pt-UiO-67 — N, N-Dimethylacetamide — 34.00 3 30 [58] Pt@MIL-125/Au — Triethanolamine — 1.74 3 6 [59] Calix-3/Pt@UiO-66-NH2 — Methanol — 1.53 3 9 [60] Ni-MOF-74-CdS/Co3O4 1.97 Lactic acid — 0.58 4 20 [61] ZIF-9/Zn0.8Cd0.2S — Triethanolamine — 6.42 — — [62] 表 2 部分用于可见光催化析氢反应的MOFs衍生物
Table 2 Some MOFs derivatives used in visible light catalytic hydrogen evolution reaction
Photocatalyst MOF precursors Bandgap Eg/eV Sacrificial
agentsCo-catalysts H2 Production rate/(mmol·h−1·g−1) Recycled times Solution stability/h Cu0.9Co2.1S4@MoS2 ZIF-67(Cu/Co) — Triethanolamine — 40.16 3 30 FeO3.3C0.2H1.0 MIL-101(Fe) 1.61 — — 125.00 4 24 Pt-Zn3P2-CoP ZnCoZIF-8 — Methanol — 9.15 4 24 Co-Zn0.5Cd0.5S ZnCoZIF-8 — SO2−3/S2− — 17.36 6 30 Hollow Cu-TiO2/C SiO2@HKUST-1 — Methanol Pt 14.05 3 18 Hollow CdS nanoboxes Cd-MOF-74 2.30 Lactic acid Pt 21.65 4 16 Co/NGC@ZnIn2S4 ZIF-8@ZIF-67 2.10 Triethanolamine — 11.27 5 20 Notes: NGC—N-Doped graphitic carbon; HKUST-1—Hong Kong University of Science and Technology-1. -
[1] SOHAIL M, KIM H, KIM T W. Enhanced photocatalytic performance of a Ti-based metal-organic framework for hydrogen production: Hybridization with ZnCr-LDH nanosheets[J]. Scientific Reports,2019,9(1):1-11.
[2] PARIDA B, INIYAN S, GOIC R. A review of solar photovoltaic technologies[J]. Renewable and Sustainable Energy Reviews,2011,15(3):1625-1636. DOI: 10.1016/j.rser.2010.11.032
[3] TACHIBANA Y, VAYSSIERES L, DURRANT J R. Artificial photosynthesis for solar water-splitting[J]. Nature Photonics,2012,6(8):511-518. DOI: 10.1038/nphoton.2012.175
[4] KUDO A, MISEKI Y. Heterogeneous photocatalyst materials for water splitting[J]. Chemical Society Reviews,2009,38(1):253-278. DOI: 10.1039/B800489G
[5] LI Y, XU H, OUYANG S, et al. Metal-organic frameworks for photocatalysis[J]. Physical Chemistry Chemical Physics,2016,18(11):7563-7572. DOI: 10.1039/C5CP05885F
[6] FUJISHIMA A, HONDA K J N. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature,1972,238(5358):37-38. DOI: 10.1038/238037a0
[7] WEI J W, WEI S M, CHANG N, et al. Construction of Z-scheme Ag/In2S3/ZnO nanorods composite photocatalysts for degradation of 4-nitrophenol[J]. Nanotechnology,2021,32(10):105706. DOI: 10.1088/1361-6528/abcd63
[8] LOPEZ-VASQUEZ A, DELGADO-NINO P, SALAS-SIADO D. Photocatalytic hydrogen production by strontium titanate-based perovskite doped europium (Sr0.97Eu0.02-Zr0.1Ti0.9O3)[J]. Environmental Science and Pollution Research International,2019,26(5):4202-4214. DOI: 10.1007/s11356-018-3116-6
[9] NAWAZ A, SARAVANAN P. C-Dot TiO2 nanorod composite for enhanced quantum efficiency under direct sunlight[J]. RSC Advances,2020,10(33):19490-19500. DOI: 10.1039/D0RA03157G
[10] HUA X L, LI H G, TAN B E. COFs-based porous materials for photocatalytic applications[J]. Chinese Journal of Polymer Science,2020,38(7):673-684. DOI: 10.1007/s10118-020-2394-x
[11] LI S S, HU C, PENG Y N, et al. One-step scalable synthesis of honeycomb-like g-C3N4 with broad sub-band gap absorption for superior visible-light-driven photocatalytic hydrogen evolution[J]. RSC Advances,2019,9(56):32674-32682. DOI: 10.1039/C9RA07068K
[12] LIU X P, QIN H, FAN W L. Enhanced visible-light photocatalytic activity of a g-C3N4/m-LaVO4 heterojunction: Band offset determination[J]. Science Bulletin,2016,61(8):645-655. DOI: 10.1007/s11434-016-1053-7
[13] CONTRERAS D, MELIN V, PÉREZ-GONZÁLEZ G, et al. Advances and challenges in BiOX (X: Cl, Br, I)-based materials for harvesting sunlight[J]. Green Photocatalysts,2020,34:235-282.
[14] ZHONG Y, MA S, CHEN K, et al. Controlled growth of plasmonic heterostructures and their applications[J]. Science China Materials,2020,63(8):1398-1417. DOI: 10.1007/s40843-019-1262-6
[15] SONG Y, CHO D, VENKATESWARLU S, et al. Systematic study on preparation of copper nanoparticle embedded porous carbon by carbonization of metal-organic framework for enzymatic glucose sensor[J]. RSC Advances,2017,7(17):10592-10600. DOI: 10.1039/C7RA00115K
[16] LIU S J, ZHANG C, SUN Y D, et al. Design of metal-organic framework-based photocatalysts for hydrogen generation[J]. Coordination Chemistry Reviews,2020,413:213266. DOI: 10.1016/j.ccr.2020.213266
[17] HALL J N, BOLLINI P. Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks[J]. Reaction Chemistry & Engineering,2019,4(2):207-222.
[18] DUAN C X, YU Y, XIAO J, et al. Water-based routes for synthesis of metal-organic frameworks: A review[J]. Science China Materials,2020,63(5):667-685. DOI: 10.1007/s40843-019-1264-x
[19] BAVYKINA A, KOLOBOV N, KHAN I S, et al. Metal-organic frameworks in heterogeneous catalysis: Recent progress, new trends, and future perspectives[J]. Chemical Reviews,2020,120(16):8468-8535. DOI: 10.1021/acs.chemrev.9b00685
[20] ZHU S S, WANG D W. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities[J]. Advanced Energy Materials,2017,7(23):1700841.
[21] YU D Y, LI L B, WU M H, et al. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework[J]. Applied Catalysis B: Environmental,2019,251:66-75. DOI: 10.1016/j.apcatb.2019.03.050
[22] ALVARO M, CARBONELL E, FERRER B, et al. Semiconductor behavior of a metal-organic framework (MOF)[J]. Chemistry,2007,13(18):5106-5112. DOI: 10.1002/chem.200601003
[23] GOMES S C, LUZ I, LLABRES I X X, et al. Water stable Zr-benzene dicarboxylate metal-organic frameworks as photocatalysts for hydrogen generation[J]. Chemistry,2010,16(36):11133-11138. DOI: 10.1002/chem.200903526
[24] LAN Y Q, GUO Y Y, ZHANG J, et al. Syntheses of exceptionally stable Al(III) metal-organic frameworks: How to grow high quality large single crystals?[J]. Chemistry,2017,61(23):15518-15528.
[25] FENG Y N, CHEN C, LIU Z G, et al. Application of a Ni mercaptopyrimidine MOF as highly efficient catalyst for sunlight-driven hydrogen generation[J]. Journal of Materials Chemistry A,2015,3(13):7163-7169. DOI: 10.1039/C5TA00136F
[26] HU X L, SUN C Y, QIN C, et al. Iodine-templated assembly of unprecedented 3d-4f metal-organic frameworks as photocatalysts for hydrogen generation[J]. Chemical Communications,2013,49(34):3564-3566. DOI: 10.1039/c3cc39173f
[27] SANTACLARA J G, OLIVOS-SUAREZ A, FOSSÉ I, et al. Harvesting the photoexcited holes on a photocatalytic proton reduction metal-organic framework[J]. Faraday Discussions,2017,201:71-86. DOI: 10.1039/C7FD00029D
[28] GUESH K, CAIUBY C A D, MAYORAL Á, et al. Sustainable preparation of MIL-100(Fe) and its photocatalytic behavior in the degradation of methyl orange in water[J]. Crystal Growth & Design,2017,17(4):1806-1813.
[29] LIU M, QIAO L Z, DONG B B, et al. Photocatalytic coproduction of H2 and industrial chemical over MOF-derived direct Z-scheme heterostructure[J]. Applied Catalysis B: Environmental,2020,273:119066. DOI: 10.1016/j.apcatb.2020.119066
[30] CHEN Y J, JI S F, WANG Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2017,56(24):6937-6941. DOI: 10.1002/anie.201702473
[31] FANG X Z, SHANG Q C, WANG Y, et al. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis[J]. Advanced Materials,2018,30(7):1705112. DOI: 10.1002/adma.201705112
[32] LI J, HUANG H L, LIU P, et al. Metal-organic framework encapsulated single-atom Pt catalysts for efficient photocatalytic hydrogen evolution[J]. Journal of Catalysis,2019,375:351-360. DOI: 10.1016/j.jcat.2019.06.024
[33] ZUO Q, LIU T T, CHEN C S, et al. Ultrathin metal-organic framework nanosheets with ultrahigh loading of single Pt atoms for efficient visible-light-driven photocatalytic H2 evolution[J]. Angewandte Chemie International Edition,2019,58(30):10198-101203. DOI: 10.1002/anie.201904058
[34] HE T. Zirconium-porphyrin-based metal-organic framework hollow nanotubes for immobilization of noble-metal single atoms[J]. Angewandte Chemie International Edition,2018,57(13):3493-3498. DOI: 10.1002/anie.201800817
[35] JIAO L, JIANG H L. Metal-organic-framework-based single-atom catalysts for energy applications[J]. Chem,2019,5(4):786-804. DOI: 10.1016/j.chempr.2018.12.011
[36] YANG S J, CHOI J Y, CHAE H K. Preparation and enhanced hydrostability and hydrogen storage capacity of CNT@MOF-5 hybrid composite[J]. Chemistry of Materials,2009,21(9):1893-1897. DOI: 10.1021/cm803502y
[37] ZHU Q L, XU Q. Metal-organic framework composites[J]. Chemical Society Reviews,2014,43(16):5468-5512. DOI: 10.1039/C3CS60472A
[38] BUSO D, JASIENIAK J, LAY M D, et al. Highly luminescent metal-organic frameworks through quantum dot doping[J]. Small,2012,8(1):80-88. DOI: 10.1002/smll.201100710
[39] TIAN L, YANG X F, LIU Q Q, et al. Anchoring metal-organic framework nanoparticles on graphitic carbon nitrides for solar-driven photocatalytic hydrogen evolution[J]. Applied Surface Science,2018,455:403-409. DOI: 10.1016/j.apsusc.2018.06.014
[40] PAN Y T, LI D D, JIANG H L. Sodium-doped C3N4/MOF heterojunction composites with tunable band structures for photocatalysis: Interplay between light harvesting and electron transfer[J]. Chemistry,2018,24(69):18403-18407. DOI: 10.1002/chem.201803555
[41] SHI X F, ZHANG J H, CUI G W, et al. Photocatalytic H2 evo-lution improvement for H free-radical stabilization by electrostatic interaction of a Cu-BTC MOF with ZnO/GO[J]. Nano Research, 2017, 11(2):979-987.
[42] SU Y, ZHANG Z, LIU H, et al. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction[J]. Applied Catalysis B Environmental,2017,200:448-457. DOI: 10.1016/j.apcatb.2016.07.032
[43] ZHANG H, JIN M S, XIA Y N. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd[J]. Chemical Society Reviews,2012,41(24):8035-8049. DOI: 10.1039/c2cs35173k
[44] MEILIKHOV M, YUSENKO K, ESKEN D, et al. Metals@MOFs-loading MOFs with metal nanoparticles for hybrid functions[J]. European Journal of Inorganic Chemistry,2010(24):3701-3714. DOI: 10.1002/ejic.201000473
[45] CLAUDIA Z, RENATO C, FERMIN C, et al. Pd Nanoparticles embedded into a metal-organic framework: Synthesis, structural characteristics, and hydrogen sorption properties[J]. Journal of the American Chemical Society,2010,132(9):2991-2997. DOI: 10.1021/ja9084995
[46] SABO M, HENSCHEL A, FRÖDE H, et al. Solution infiltration of palladium into MOF-5: Synthesis, physisorption and catalytic properties[J]. Journal of Materials Chemistry,2007,17(36):3827. DOI: 10.1039/b706432b
[47] SCHROEDER F, ESKEN D, COKOJA M, et al. Ruthenium nanoparticles inside porous [Zn4O(bdc)3] by hydrogenolysis of adsorbed [Ru(cod)(cot)]: A solid-state reference system for surfactant-stabilized ruthenium colloids[J]. Journal of the American Chemical Society,2008,130(19):6119-6130. DOI: 10.1021/ja078231u
[48] GREATHOUSE J A, ALLENDORF M D. The interaction of water with MOF-5 simulated by molecular dynamics[J]. Journal of the American Chemical Society,2006,128(33):10678-10679. DOI: 10.1021/ja063506b
[49] CHAE H K, SIBERIO-PEREZ D Y, KIM J, et al. A route to high surface area, porosity and inclusion of large molecules in crystals[J]. Nature,2004,427(6974):523-527. DOI: 10.1038/nature02311
[50] PROCH S, HERRMANNSDÖRFER J, KEMPE R, et al. Pt@MOF-177: Synthesis, room-temperature hydrogen storage and oxidation catalysis[J]. Chemistry A European Journal,2008,27(14):8204-8212.
[51] ESKEN D, TURNER S, LEBEDEV O I, et al. Au@ZIFs: Stabilization and encapsulation of cavity-size matching gold clusters inside functionalized zeolite imidazolate frameworks ZIFs[J]. Chemistry of Materials, 2010, 22(23): 6393–6401.
[52] JIANG H L, AKITA T, ISHIDA T, et al. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework[J]. Journal of the American Chemical Society,2011,133(5):1304-1306. DOI: 10.1021/ja1099006
[53] JIANG H L, LIU B, AKITA T, et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework[J]. Journal of the American Chemical Society,2009,131(32):11302-11303. DOI: 10.1021/ja9047653
[54] LI P Z, ARANISHI K, XU Q. ZIF-8 immobilized nickel nanoparticles: Highly effective catalysts for hydrogen generation from hydrolysis of ammonia borane[J]. Chemical Communications,2012,26(48):3173-3175.
[55] HORIUCHI Y, TOYAO T, SAITO M, et al. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal-organic framework[J]. Journal of Physical Chemistry C,2012,116(39):20848-20853. DOI: 10.1021/jp3046005
[56] ZHANG M, SHANG Q G, WAN Y Q, et al. Self-template synthesis of double-shell TiO2@ ZIF-8 hollow nanospheres via sonocrystallization with enhanced photocatalytic activities in hydrogen generation[J]. Applied Catalysis B: Environmental,2019,241:149-158. DOI: 10.1016/j.apcatb.2018.09.036
[57] ZHAO J S, WANG Y, ZHOU J W, et al. A copper(II)-based MOF film for highly efficient visible-light-driven hydrogen production[J]. Journal of Materials Chemistry A,2016,4(19):7174-7177. DOI: 10.1039/C6TA00431H
[58] YANG S Z, FAN D H, HU W H, et al. Elucidating charge separation dynamics in a hybrid metal-organic framework photocatalyst for light-driven H2 evolution[J]. Journal of Physical Chemistry C,2018,122(6):3305-3311. DOI: 10.1021/acs.jpcc.8b00471
[59] XIAO J D, HAN L L, LOU J, et al. Integration of plasmonic effects and schottky junctions into metal-organic framework composites: Steering charge flow for enhanced visible-light photocatalysis[J]. Angewandte Chemie International Edition,2018,130(4):1115-1119. DOI: 10.1002/ange.201711725
[60] CHEN Y F, TAN L L, LIU J M, et al. Calix[4]arene based dye-sensitized Pt@UiO-66-NH2 metal-organic framework for efficient visible-light photocatalytic hydrogen production[J]. Applied Catalysis B Environmental,2017,206(8):426-433.
[61] ZHANG Y K, WANG G R, MA W, et al. CdS p-n heterojunction co-boosting with Co3O4 and Ni-MOF-74 for photocatalytic hydrogen evolution[J]. Dalton Transactions,2018,47(32):11176-11189. DOI: 10.1039/C8DT02294A
[62] RAN J R, QU J T, ZHANG H P, et al. Atomically dispersed single Co sites in zeolitic imidazole frameworks promoting high-efficiency visible-light-driven hydrogen production[J]. Chemistry-A European Journal,2019,25(41):9670-9677. DOI: 10.1002/chem.201901250
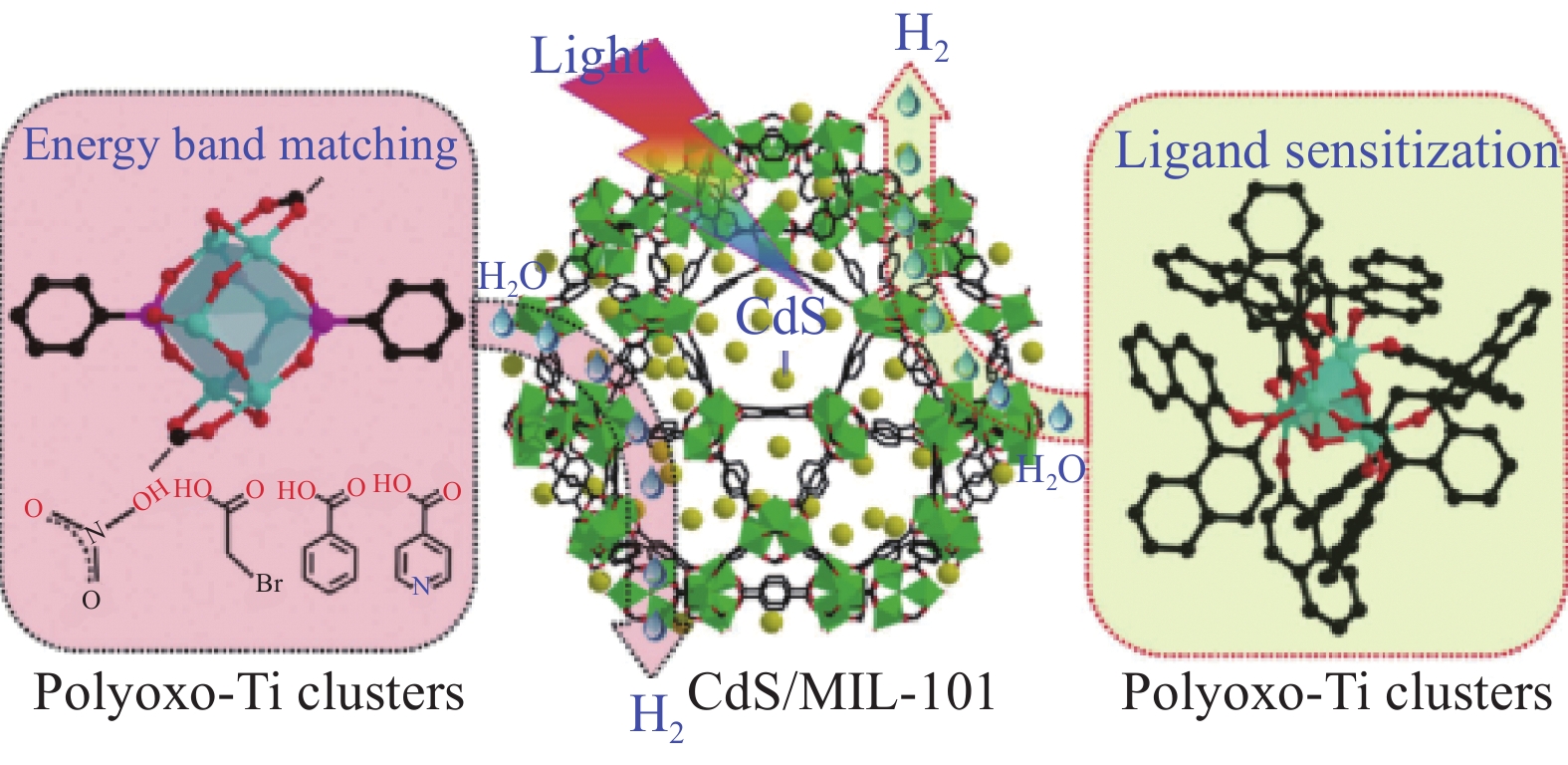
[63] ZHANG J, ZHANG J X, JIANG Z Q, et al. Assembling polyoxo-titanium clusters and CdS nanoparticles to a porous matrix for efficient and tunable H2 evolution activities with visible light[J]. Advanced Materials,2017,29(5):1603369.
[64] ZHANG Z M, ZHANG T, WANG C, et al. Photosensitizing metal-organic framework enabling visible-light-driven proton reduction by a Wells-Dawson-type polyoxometalate[J]. Journal of the American Chemical Society,2015,137(9):3197-3200. DOI: 10.1021/jacs.5b00075
[65] LI Z, XIAO J D, JIANG H L. Encapsulating a Co(II) molecular photocatalyst in metal-organic framework for visible-light-driven H2 production: Boosting catalytic efficiency via spatial charge separation[J]. ACS Catalysis,2016,6(8):5359-5365. DOI: 10.1021/acscatal.6b01293
[66] ZHANG F M, SHENG J L, YANG Z D, et al. Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors[J]. Angewandte Chemie International Edition,2018,57(37):12106-12110. DOI: 10.1002/anie.201806862
[67] LI F, WANG D K, XING Q J, et al. Design and syntheses of MOF/COF hybrid materials via postsynthetic covalent modification: An efficient strategy to boost the visible-light-driven photocatalytic performance[J]. Applied Catalysis B: Environmental,2019,243:621-628. DOI: 10.1016/j.apcatb.2018.10.043
[68] DEKRAFFT K E, WANG C, LIN W B. Metal-organic framework templated synthesis of Fe2O3/TiO2 nanocomposite for hydrogen production[J]. Advanced Materials,2012,24(15):2014-2018. DOI: 10.1002/adma.201200330
[69] YAN B L, SU X T, ZHANG L J, et al. Palladium-decorated hierarchical titania constructed from the metal-organic frameworks NH2-MIL-125(Ti) as a robust photocatalyst for hydrogen evolution[J]. Applied Catalysis B: Environmental,2017,218:743-750.
[70] VALERO-ROMERO M J . Photocatalytic properties of TiO2 and Fe-doped TiO2 prepared by metal organic framework-mediated synthesis[J]. Chemical Engineering Journal,2019,360:75-88. DOI: 10.1016/j.cej.2018.11.132
[71] ZHAI L Z, QIAN Y H, WANG Y X, et al. In situ formation of micropore-rich titanium dioxide from metal-organic framework templates[J]. ACS Applied Msterials & Interfaces,2018,10(43):36933-36940.
[72] ZHAO X X, FENG J R, LIU J, et al. An efficient, visible-light-driven, hydrogen evolution catalyst NiS/ZnxCd1-xS nanocrystal derived from a metal-organic framework[J]. Angewandte Chemie International Edition,2018,130(31):9938-9942. DOI: 10.1002/ange.201805425
[73] WANG S B, GUAN B Y, WANG X, et al. Formation of hierarchical Co9S8@ZnIn2S4 heterostructured cages as an efficient photocatalyst for hydrogen evolution[J]. Journal of the American Chemical Society,2018,140(45):15145-15148. DOI: 10.1021/jacs.8b07721
[74] ZHUANG G X, FANG Q H, WEI J X, et al. Branched In2O3 mesocrystal of ordered architecture derived from the oriented alignment of a metal-organic framework for accelerated hydrogen evolution over In2O3-ZnIn2S4[J]. ACS Applied Materials & Interfaces,2021,13(8):9804-9813.
[75] WEI S, ZHAO X X, FENG J R, et al. An efficient, visible-light-driven, hydrogen evolution catalyst NiS/ZnxCd1-xS nanocrystal derived from a metal-organic framework[J]. Angewandte Chemie International Edition, 2018, 130(31): 9938-9942.
[76] XU J X, QI Y H, WANG C, et al. NH2-MIL-101(Fe)/Ni(OH)2-derived C, N-codoped Fe2P/Ni2P cocatalyst modified g-C3N4 for enhanced photocatalytic hydrogen evolution from water splitting[J]. Applied Catalysis B Environmental,2019,241:178-186. DOI: 10.1016/j.apcatb.2018.09.035
[77] HU C Y, ZHOU J, SUN C Y, et al. HKUST-1 derived hollow C-Cu2xS nanotube/g-C3N4 composites for visible-light CO2 photoreduction with H2O vapor[J]. Chemistry A European Journal,2019,25(1):379-385. DOI: 10.1002/chem.201804925
[78] ZHANG L J, WANG G R, HAO X Q, et al. MOFs-derived Cu3P@CoP p-n heterojunction for enhanced photocataly-tic hydrogen evolution[J]. Chemical Engineering Journal,2020,395(4):125113.
[79] MA B, CHEN T T, LI Q Y, et al. Bimetal-organic-framework-derived nanohybrids Cu0.9Co2.1S4@MoS2 for high-perfor-mance visible-light-catalytic hydrogen evolution reaction[J]. ACS Applied Energy Materials,2019,2(2):1134-1148. DOI: 10.1021/acsaem.8b01691
[80] XU J Y, ZHAI X P, GAO L F, et al. In situ preparation of a MOF-derived magnetic carbonaceous catalyst for visible-light-driven hydrogen evolution[J]. RSC Advances,2016,6(3):2011-2018. DOI: 10.1039/C5RA23838B
[81] TANG X, ZHAO J H, LI Y H, et al. Co-Doped Zn1-xCdxS nanocrystals from metal-organic framework precur-sors: Porous microstructure and efficient photocatalytic hydrogen evolution[J]. Dalton Transactions,2017,46(32):10553-10557. DOI: 10.1039/C7DT01970J
[82] LI Y L, JIN T, MA G, et al. Metal-organic framework assisted and in situ synthesis of hollow CdS nanostructures with highly efficient photocatalytic hydrogen evolution[J]. Dalton Transactions, 2019, 48(17): 5649-5655.
[83] LAN M, GUO R M, DOU Y B, et al. Fabrication of porous Pt-doping heterojunctions by using bimetallic MOF template for photocatalytic hydrogen generation[J]. Nano Energy,2017,33:238-246. DOI: 10.1016/j.nanoen.2017.01.046
[84] CHEN H, GU Z G, MIRZA S, et al. Hollow Cu-TiO2/C nanospheres derived from a Ti precursor encapsulated MOF coating for efficient photocatalytic hydrogen evolution[J]. Journal of Materials Chemistry A,2018,6(16):7175-7181. DOI: 10.1039/C8TA01034J
[85] SU B, HUANG L J, XIONG Z, et al. Branch-like ZnS-DETA/CdS hierarchical heterostructures as an efficient photocatalyst for visible light CO2 reduction[J]. Journal of Materials Chemistry A,2019,7(47):26877-26883. DOI: 10.1039/C9TA10470D
-





 下载:
下载: