Preparation and electrochemical properties of Ag/MnO2 composite electrode materials
-
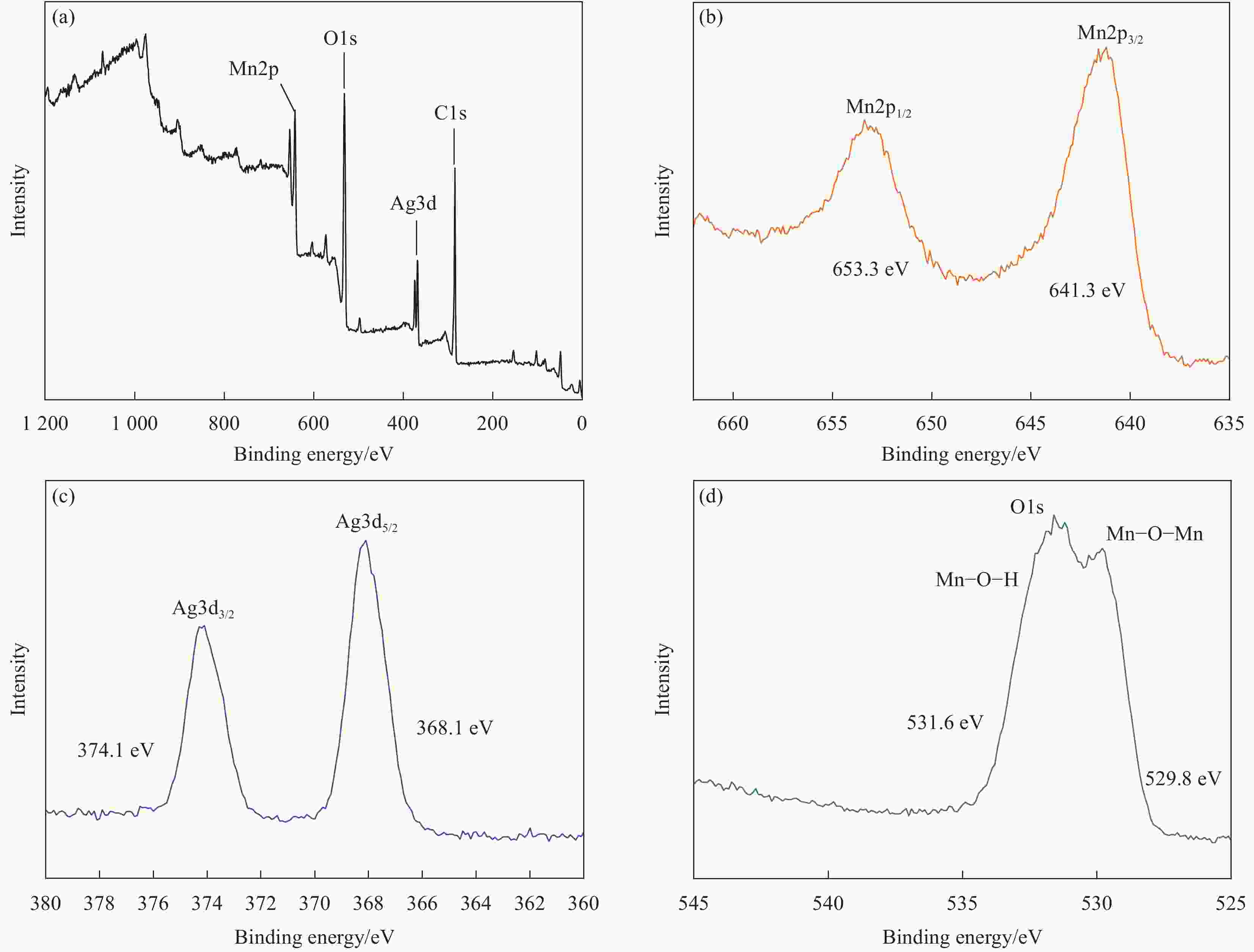
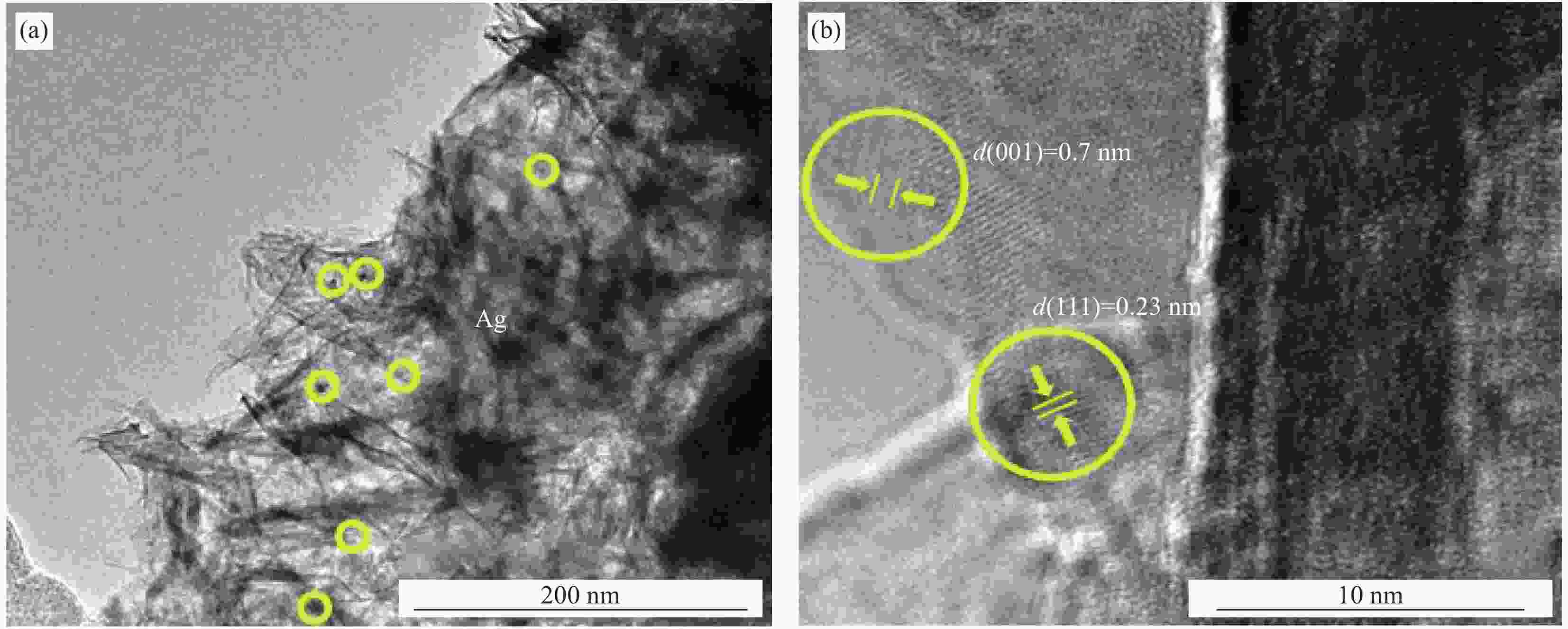
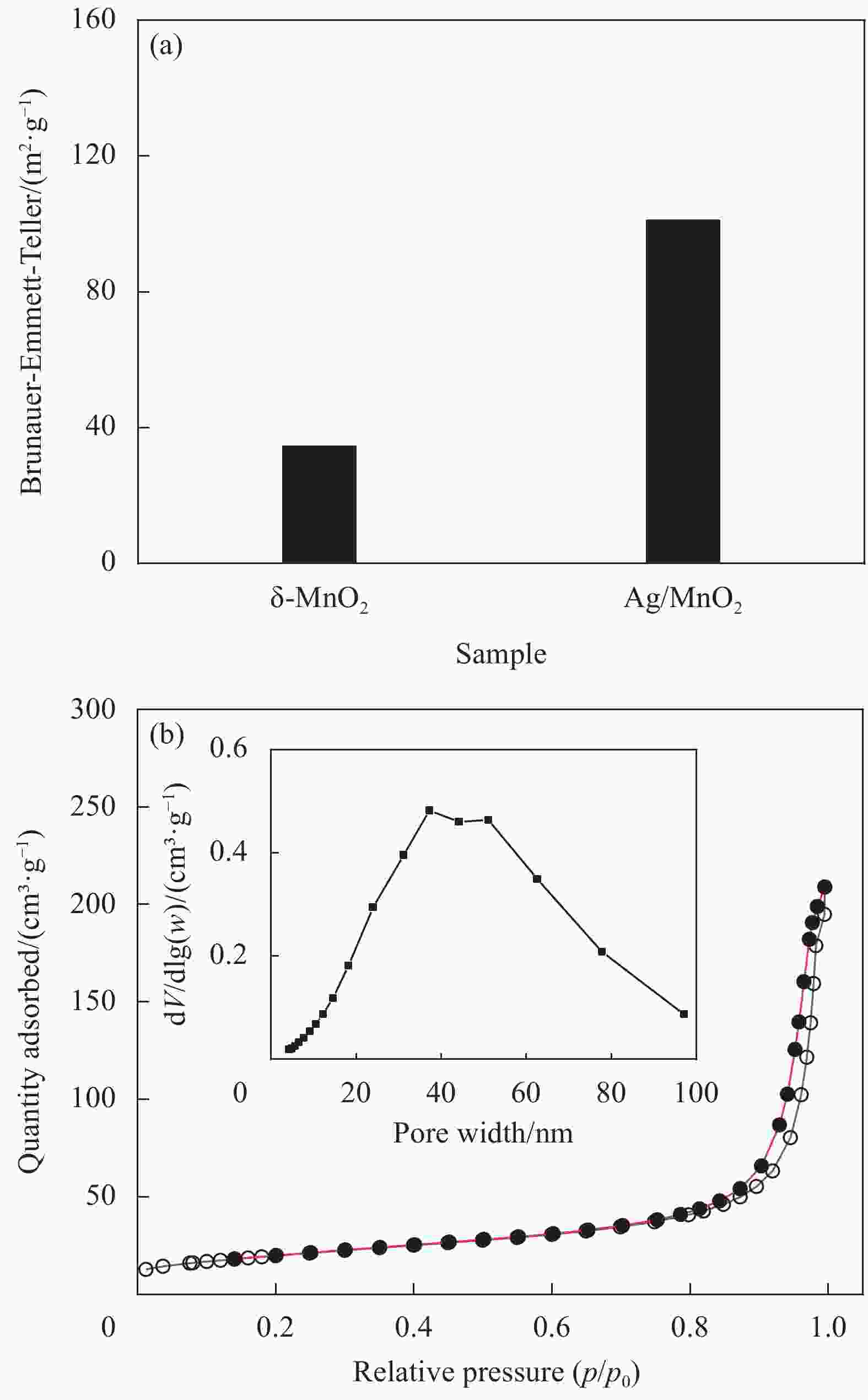
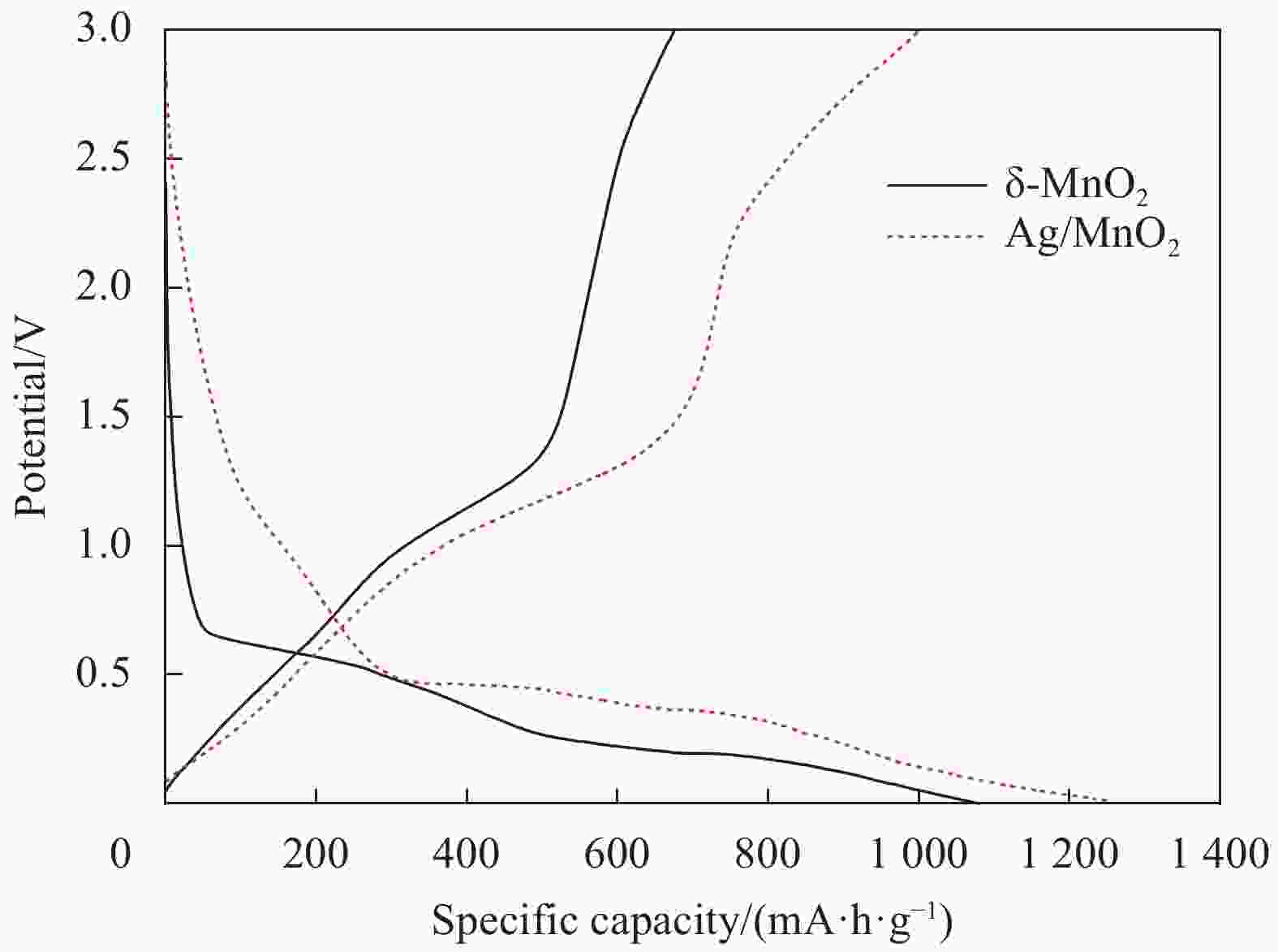
摘要: 过渡金属氧化物MnO2因其制备工艺简单、储量丰富、环保且具有较高的理论比容量,在电池储能方面有较大潜力。本论文借助溶胀法对水热合成的δ-MnO2进行剥离制得MnO2纳米片。再利用紫外光照以及NaBH4的还原作用在MnO2纳米片表面负载Ag纳米颗粒,从而得到Ag/MnO2复合材料。对Ag/MnO2复合材料进行了结构和形貌表征以及电化学性能测试。结果表明,作为锂离子电池负极材料,Ag/MnO2的电化学性能明显优于纯相δ-MnO2。Ag/MnO2在100 mA/g电流密度下的首次可逆比容量达到1001.1 mA·h/g,库伦效率为79.9%;在0.1、0.2、0.5、1.0、2.0 A/g电流密度下的平均可逆比容量分别为936.3、607.5、429.5、351.1和278 mA·h/g,当电流密度重新回到0.1 A/g时,其平均可逆比容量仍可达到658.7 mA·h/g。MnO2电化学性能的改善归因于均匀负载的导电Ag颗粒,使得电极材料的导电性显著提升,利于带电粒子的传输。此外,Ag/MnO2复合材料的纳米结构使得锂离子在固相中的传输路径缩短,进而提高了锂离子扩散速率。Abstract: Transition metal oxide MnO2 has great potential in battery storage because of its simple preparation process, abundant reserves, environmental protection and high theoretical specific capacity. In this paper, MnO2 nanosheets were prepared by exfoliating the hydrothermally synthesized δ-MnO2 by the swelling method. The Ag/MnO2 composites were obtained by loading Ag nanoparticles on the surface of MnO2 nanosheets under UV irradiation and NaBH4 reduction. The structure and morphology of Ag/MnO2 composites were characterized and their electrochemical properties were tested. The results show that the electrochemical performance of Ag/MnO2 as anode material for lithium ion batteries is obviously better than that of the pure phase δ-MnO2. The first reversible specific capacity of Ag/MnO2 at the current density of 100 mA/g reaches 1001.1 mA·h/g, and the coulombic efficiency is 79.9%. At the current density of 0.1, 0.2, 0.5, 1.0 and 2.0 A/g, the average reversible specific capacity is 936.3, 607.5, 429.5, 351.1 and 278 mA·h/g, respectively. When the current density returns to 0.1 A/g, the average reversible specific capacity can still reach 658.7 mA·h/g. The improvement of the electrochemical performance of Ag/MnO2 is attributed to the fact that the uniformly loaded conductive Ag particles significantly enhance the electrical conductivity of the electrode material, which is conducive to the transport of charged particles. In addition, the nano-structure of Ag/MnO2 composites shortens the transport path of lithium ions in the solid phase, thus increasing the diffusion rate of lithium ions.
-
Key words:
- MnO2 /
- Ag /
- anode material /
- lithium ion battery /
- electrochemical properties
-
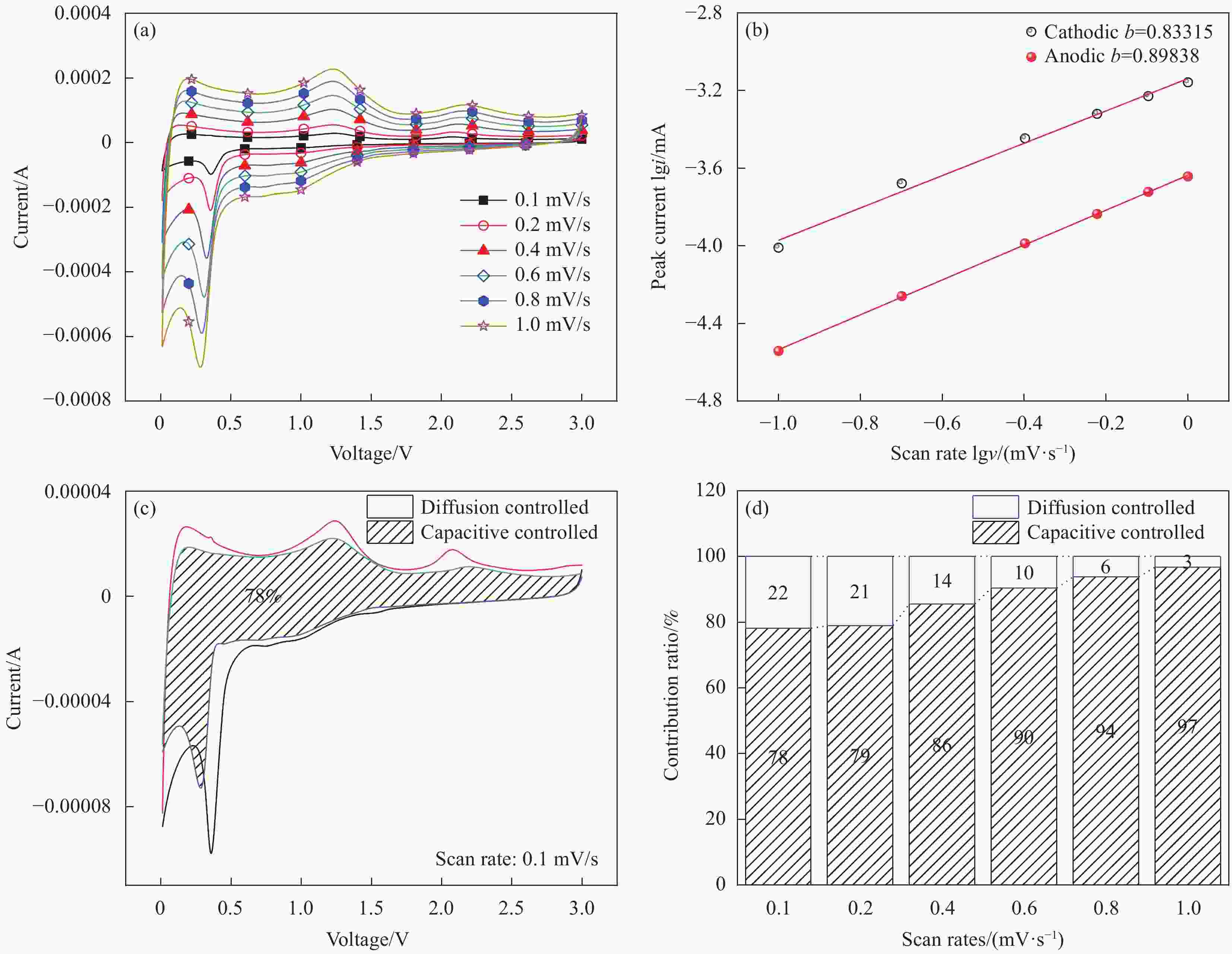
图 12 (a) Ag/MnO2电极在不同扫速下的CV曲线;(b) Ag/MnO2电极b值计算;(c) Ag/MnO2电极在0.1 mV/s扫速下赝电容贡献面积比例;(d) Ag/MnO2电极在不同扫速下赝电容比例
Figure 12. (a) Cyclic voltammetry curves of Ag/MnO2 electrode at various scan rates; (b) Calculation of b value of Ag/MnO2 electrode; (c) Contribution area ratio of pseudocapacitance at 0.1 mV/s scanning speed of Ag/MnO2 electrode; (d) Normalized contribution ratios of capacitive and diffusion-controlled capacities at different scan rates
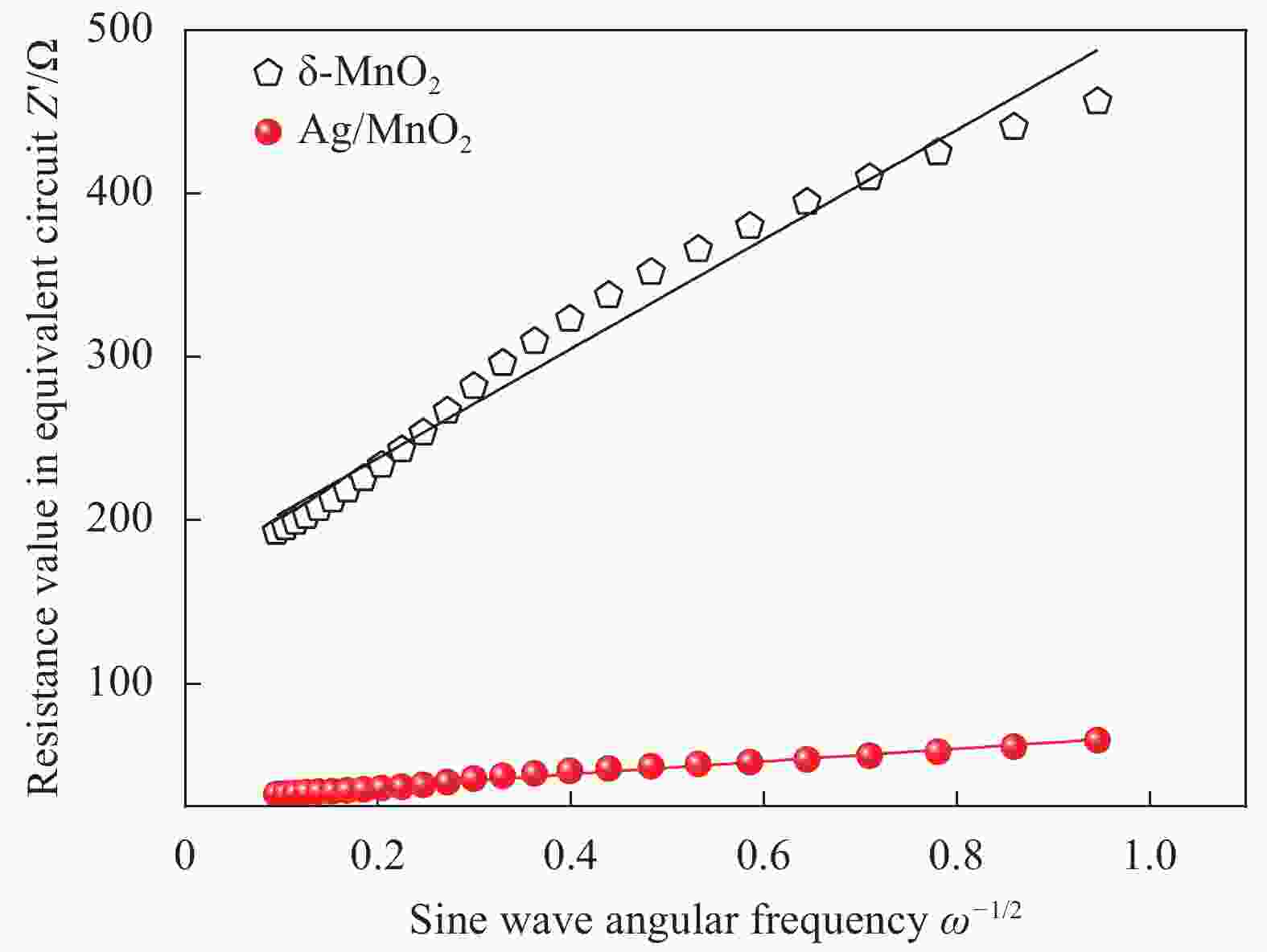
图 13 δ-MnO2 (a) 和Ag/MnO2 (b) 的电化学阻抗图谱 (内插图为模拟等效电路图)
Figure 13. Electrochemical impedance spectra of δ-MnO2 (a) and Ag/MnO2 (b) (Fitting circuit diagram are in inset)
Rs—Ohmic resistance; Rct—Charge transfer resistance; CPE—Equivalent circuit defines the double-layer capacitance; W—Warburg resistance
表 1 δ-MnO2和Ag/MnO2合成样品的电化学阻抗谱(EIS)模型参数
Table 1. Electrochemical impedance spectroscopy (EIS) model parameters for the synthesized of δ-MnO2 and Ag/MnO2 samples
Sample Rs/Ω Rct/Ω CPE
/FW/Ω δ-MnO2 77.33 283.40 3.45×10−3 8.95×10−5 Ag/MnO2 5.83 34.50 4.68×10−3 7.89×10−4 Notes: Rs—Ohmic resistance; Rct—Charge transfer resistance; CPE—Equivalent circuit defines the double-layer capacitance; W—Warburg resistance. 表 2 δ-MnO2和Ag/MnO2复合材料的阻抗因子(σ)和扩散系数(DLi+)
Table 2. Impedance factor (σ) and diffusion rate (DLi+) of δ-MnO2 and Ag/MnO2 composites
Sample σ/(Ω·cm2·S−1/2) DLi+/(cm2·S−1) δ-MnO2 334.55 2.95×10−22 Ag/MnO2 39.00 2.17×10−20 -
[1] 罗述东, 李祖德, 赵慕岳, 等. 锰在粉末冶金材料中的应用[J]. 粉末冶金材料科学与工程, 2007, 12(6): 321-329.LUO Shudong, LI Zude, ZHAO Muyue, et al. Application of manganese in powder metallurgical materials[J]. Materials Science and Engineering of Powder Metallurgy, 2007, 12(6): 321-329(in Chinese). [2] 顾鑫, 徐化云, 杨剑, 等. 二氧化锰纳米材料在锂离子电池负极材料中的应用[J]. 科学通报, 2013, 58(31):3108-3114. doi: 10.1360/972013-712GU Xin, XU Huayun, YANG Jian, et al. Application of MnO2 nanomaterials as an anode for lithiumion batteries[J]. Chinese Science Bulletin,2013,58(31):3108-3114(in Chinese). doi: 10.1360/972013-712 [3] WU M S, CHIANG P C J, LEE J T, et al. Synthesis of manganese oxide electrodes with interconnected nanowire structure as an anode material for rechargeable lithium ion batteries[J]. Journal of Physical Chemistry B,2005,109(49):23279-23284. doi: 10.1021/jp054740b [4] WU M S, CHIANG P C J. Electrochemically deposited nanowires of manganese oxide as an anode material for lithium-ion batteries[J]. Electrochemistry Communications,2006,8(3):383-388. doi: 10.1016/j.elecom.2005.12.014 [5] 孔祥荣, 胡如男, 吴延红, 等. MnO2纳米材料的制备及应用[J]. 应用化工, 2016, 45(8):1549-1552.KONG Xiangrong, HU Runan, WU Yanhong, et al. Prearation and application of MnO2 nanomaterials[J]. Applied Chemical Industry,2016,45(8):1549-1552(in Chinese). [6] 夏熙. 二氧化锰的物理、化学性质与其电化学活性的相关(5)[J]. 电池, 2006, 36(4):276-279. doi: 10.3969/j.issn.1001-1579.2006.04.012XIA Xi. The relation between chemical, physical properties and electrochemical activity for manganese dioxides(Ⅴ)[J]. Battery Bimonthly,2006,36(4):276-279(in Chinese). doi: 10.3969/j.issn.1001-1579.2006.04.012 [7] 郑龙. 类钙钛矿结构中氧八面体调制及物性研究[D]. 南京: 南京大学, 2013.ZHENG Long. Study of octahedron structure and properties on perovskite like oxides[D]. Nanjing: Nanjing University, 2013(in Chinese). [8] VOSKANYAN A A, HO C K, CHAN K Y. 3D δ-MnO2 nanostructure with ultralarge mesopores as high-performance lithium-ion battery anode fabricated via colloidal solution combustion synthesis[J]. Journal of Power Sources, 2019, 421: 162-168. [9] LI X, CHEN Y, YAO H, et al. Core/shell TiO2-MnO2/MnO2 heterostructure anodes for high-performance lithium-ion batteries[J]. RSC Advances,2014,4:39906. doi: 10.1039/C4RA06981A [10] XING L L, HE B, NIE Y X, et al. SnO2-MnO2-SnO2 sandwich-structured nanotubes as high-performance anodes of lithium ion battery[J]. Materials Letters,2013,105(15):169-172. [11] GUO Z, GUAN Y, DAI C, et al. Ag/MnO2 nanorod as electrode material for high-performance electrochemical supercapacitors[J]. Journal of Nanoscience and Nanotechnology,2019,18(7):4904-4909. [12] LU S Q, YAN D L, CHEN L, et al. One-pot fabrication of hierarchical Ag/MnO2 nanoflowers for electrochemical capacitor electrodes[J]. Materials Letters,2016,168(1):40-43. [13] HWAN K J, CHANGSOON C, MYEONG L J, et al. Ag/MnO2 composite sheath-core structured yarn super-capacitors[J]. Scientific Reports,2018,8(1):13309. doi: 10.1038/s41598-018-31611-2 [14] YANG X F, WANG G C, WANG R Y, et al. A novel layered manganese oxide/poly(aniline-co-O-anisidine) nanocomposite and its application for electrochemical supercapaci-tor[J]. Electrochimica Acta,2010,55(19):5414-5419. doi: 10.1016/j.electacta.2010.04.067 [15] YU L, XU Z Y, WANG D W, et al. Snowflake-like core-shell α-MnO2@δ-MnO2 for high performance asymmetric supercapacitor[J]. Electrochimica Acta,2017,251:344-354. doi: 10.1016/j.electacta.2017.08.146 [16] DI W H, ZHANG X, QIN W. Single-layer MnO2 nanosheets for sensitive and selective detection of glutathione by a colorimetric method[J]. Applied Surface Science,2017,400:200-205. doi: 10.1016/j.apsusc.2016.12.204 [17] KOO W T, JANG H Y, KIM C, et al. MOF derived ZnCo2O4 porous hollow spheres functionalized with Ag nanoparticles for a long-cycle and high-capacity lithium ion battery anode[J]. Journal of Materials Chemistry A,2017,5(43):22717-22725. doi: 10.1039/C7TA07573A [18] KARMAKAR N, FERNANDES R, JAIN S P, et al. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite[J]. Sensors and Actuators B: Chemical,2017,242:118-126. doi: 10.1016/j.snb.2016.11.039 [19] AN J, SUN G H, XIA H A. Aerobic oxidation of 5 hydroxymethylfurfural to high-yield 5 hydroxymethyl-2-furancarboxylic acid by poly(vinylpyrrolidone)-capped Ag nanoparticle catalysts[J]. ACS Sustainable Chemistry & Engineering,2019,7(7):6696-6706. [20] LI Y, ZHOU X Z, BAI Y, et al. Building an electronic bridge via Ag-decoration to enhance kinetics of iron fluoride cathode in lithium ion batteries[J]. Applied Materials & Interfaces,2017,9(23):19852-19860. [21] 张伟, 谈发堂, 乔学亮, 等. 光化学还原法制备纳米银溶胶[J]. 材料导报, 2012, 26(12):32-35. doi: 10.3969/j.issn.1005-023X.2012.12.010ZHANG Wei, TAN Fatang, QIAO Xueliang, et al. Preparation of nano-silver sol by photochemical reduction method[J]. Materials Reports,2012,26(12):32-35(in Chinese). doi: 10.3969/j.issn.1005-023X.2012.12.010 [22] 姚素薇, 曹艳蕊, 张卫国. 光还原法制备不同形貌银纳米粒子及其形成机理[J]. 应用化学, 2006, 23(4):438-440. doi: 10.3969/j.issn.1000-0518.2006.04.022YAO Suwei, CAO Yanru, ZHANG Weiguo. Preparation of silver nanoparticles of different shapes via photoreduction method[J]. Chinese Jouranal of Applied Chemistry,2006,23(4):438-440(in Chinese). doi: 10.3969/j.issn.1000-0518.2006.04.022 [23] ZANG J, YE J, QIAN H, et al. Hollow carbon sphere with open pore encapsulated MnO2, nanosheets as high-performance anode materials for lithium ion batteries[J]. Electrochimica Acta,2017,260:783-788. [24] CAO Zhiguang, CHEN Xiaoqiao, XING Lidang, et al. Nano-MnO2@TiO2 microspheres: A novel structure and excellent performance as anode of lithium-ion batteries[J]. Journal of Power Sources, 2018, 379: 174-181. [25] ZHANG Y X, LIAN F, LU J H, et al. Identification of rever-sible insertion-type lithium storage reaction of manganese oxide with long cycle lifespan[J]. Journal of Energy Che-mistry, 2020(46): 144-151. [26] LIU H D, HU Z L, TIAN L L, et al. Reduced graphene oxide anchored with δ-MnO2 nanoscrolls as anode materials for enhanced Li-ion storage[J]. Ceramics International, 2016, 42(12): 13519-13524. [27] 郑国涛. 锰基氧化物电极材料制备及电化学性能研究[D]. 辽宁: 大连海事大学, 2019.ZHENG Guotao. Preparation and electrochemical properties of manganese based oxide electrode materials[D]. Liaoning: Dalian Maritime University, 2019(in Chinese). -





 下载:
下载: