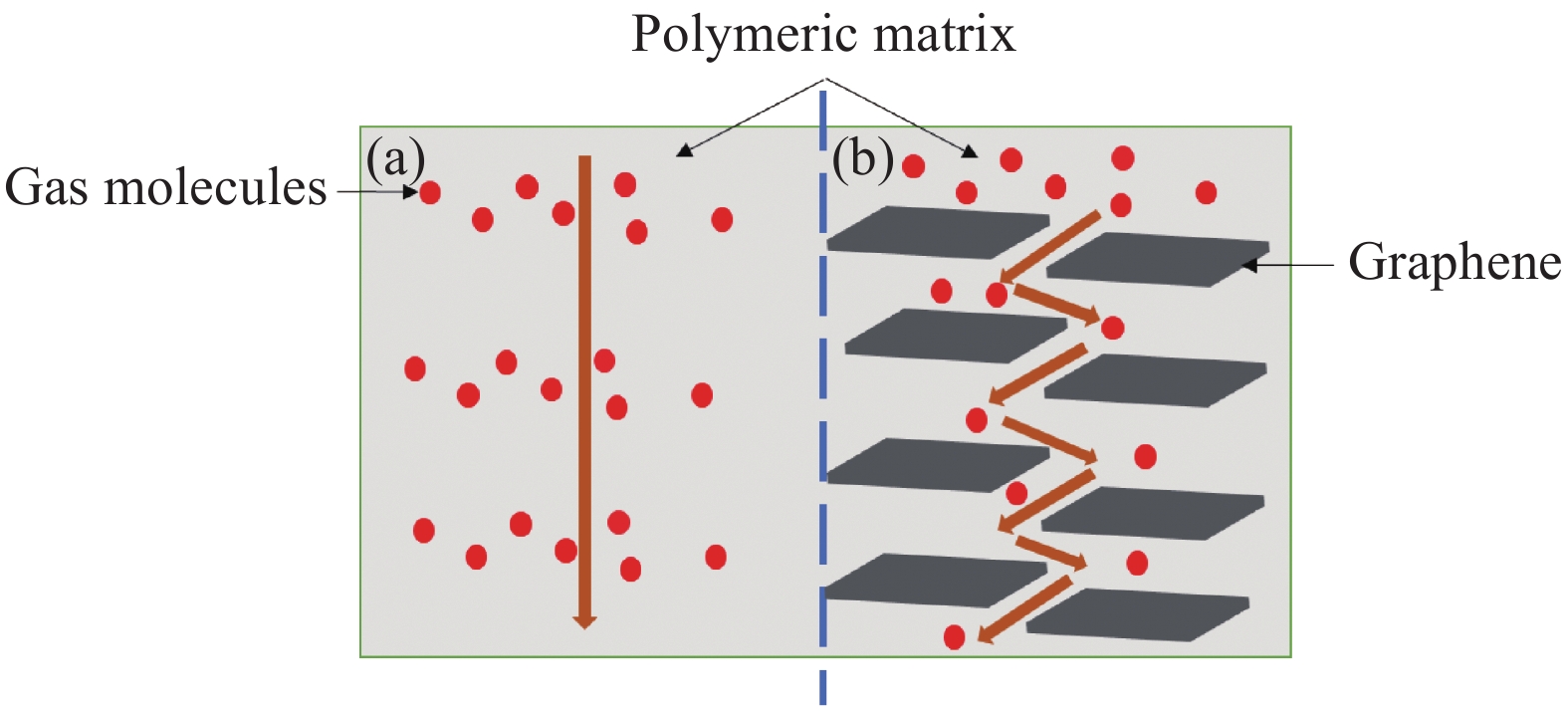
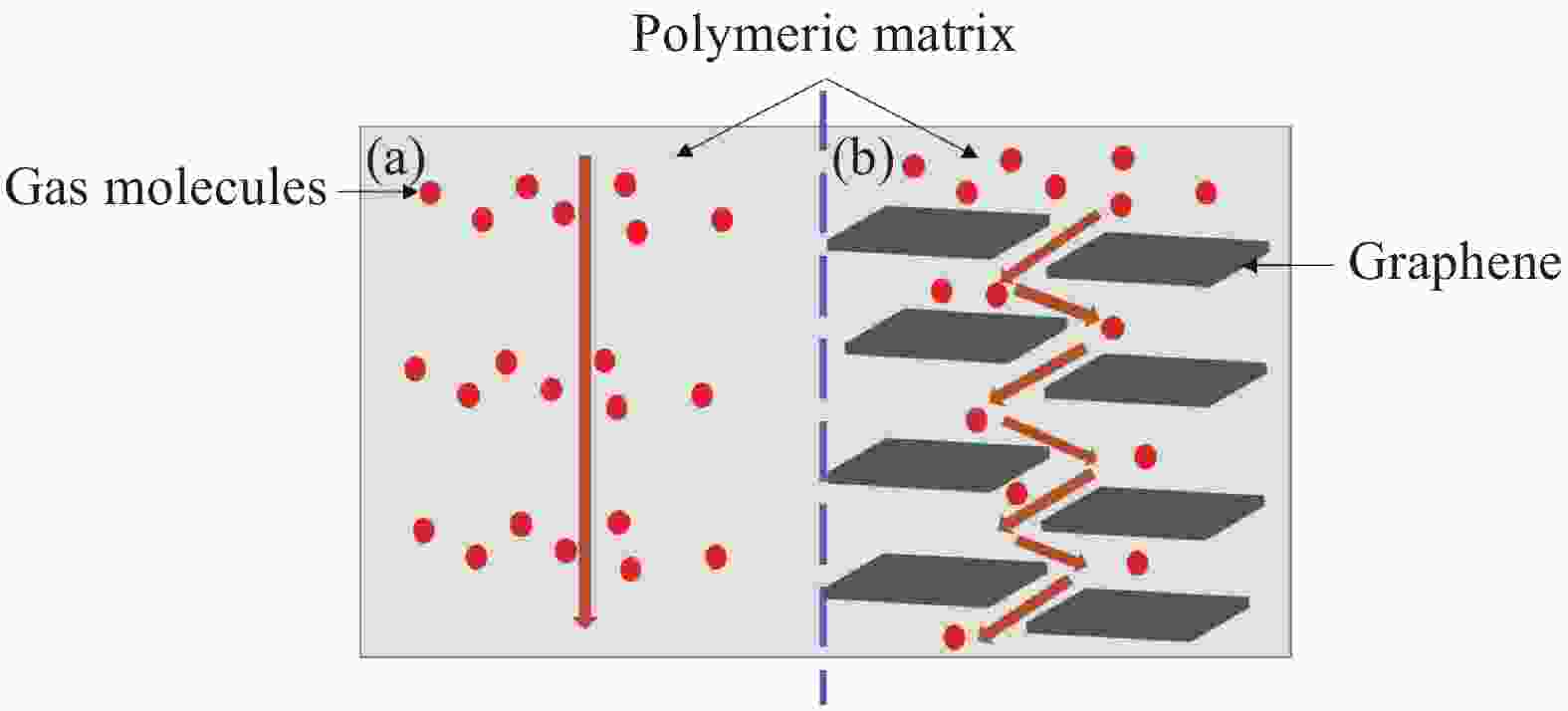
Research progress in the enhanced polymer airtightness of graphene
-
摘要: 石墨烯被认为是一种极具前景的气体阻隔填料,完整的单层石墨烯可以阻挡绝大多数气体小分子通过其表面,因此石墨烯被广泛用作提高聚合物气密性的填料。本论文综述了近几年石墨烯及其衍生物增强聚合物气密性的研究进展,介绍了石墨烯的特征以及其在聚合物基体中起到的阻隔机制,根据小分子渗透理论模型来讨论影响层状石墨烯/聚合物纳米复合材料阻隔性的主要因素;重点分类介绍了石墨烯及其衍生物增强不同聚合物气密性的方法,并分析了这些方法对聚合物气密性的提高效果。最后,对石墨烯及其衍生物增强聚合物气密性的研究进行了总结并展望了未来的发展方向。Abstract: Graphene is considered to be a promising gas barrier filler, complete monolayer graphene blocks the vast majority of small gaseous molecules through its surface, so graphene is widely used as a filler to improve the gas density of the polymer. The research progress of graphene and its derivatives enhancing polymer airtightness in recent years is reviewed. The characteristics of graphene and its barrier mechanism in polymer matrix are introduced, the main factors affecting the barrier of layered graphene/polymer nanocomposite are discussed. The methods of enhancement of graphene and the derivatives of different polymer gas density are analyzed. Finally, the research of graphene and its derivatives enhancing polymer airtightness is summarized and the future development direction is prospected.
-
Key words:
- graphene /
- polymer /
- airtightness /
- nanocomposites /
- research progress
-
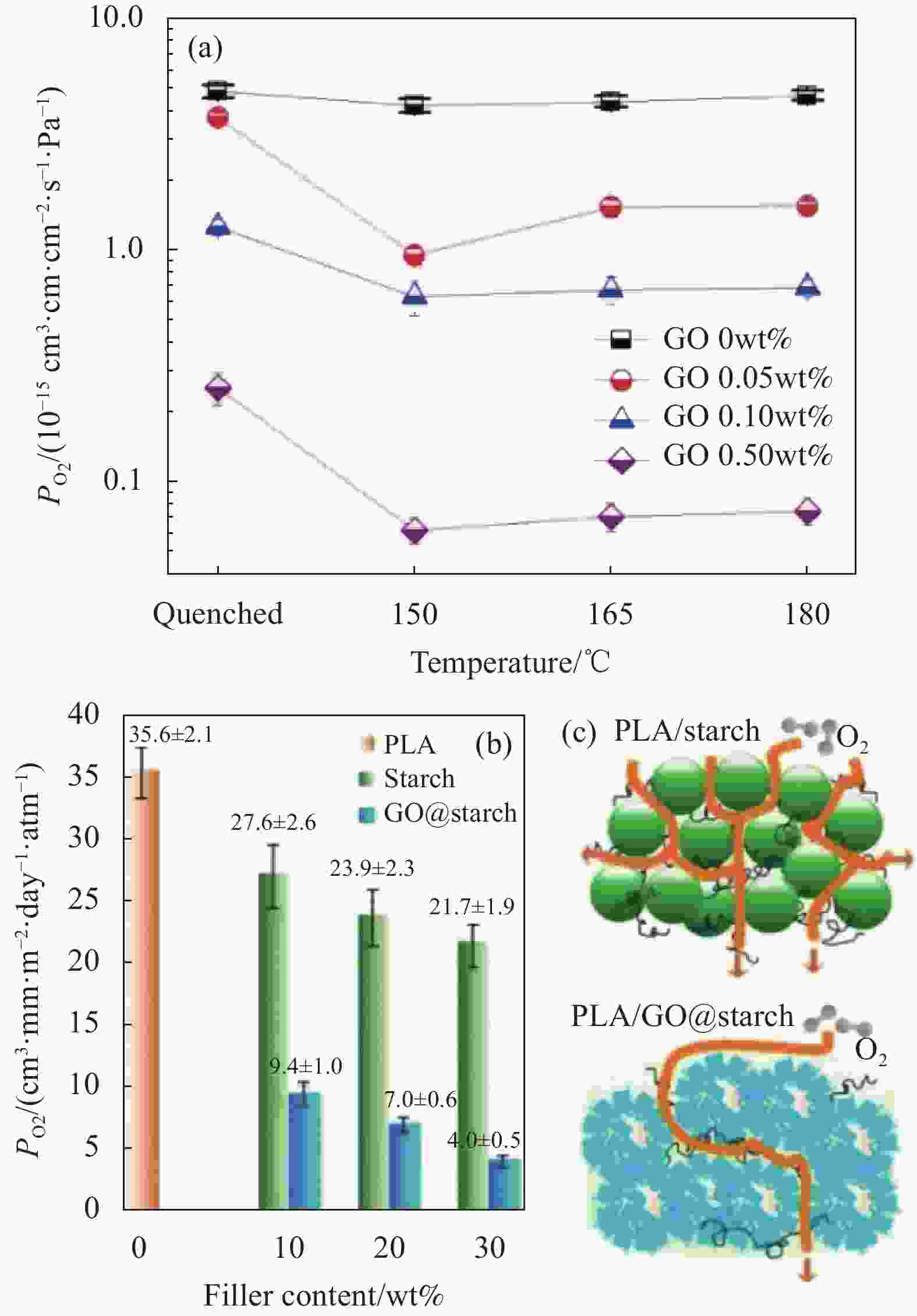
图 2 GO/聚乳酸(GO/PLA)复合材料的O2渗透性的变化图以及渗透路径图:(a)淬火和退火处理不同GO/PLA复合膜的O2渗透系数曲线[24];(b)在PLA中分别加入淀粉和淀粉包裹GO的O2渗透性随着其添加量的变化图[26];(c) O2通过PLA/淀粉的相对简单路径和通过PLA/GO@淀粉的曲折路径的示意图[26]
Figure 2. Change diagram of O2 permeability and penetration path diagram of GO/polylactic acid (GO/PLA) composite: (a) O2 permeability coefficient curve of different GO/PLA composite films treated by quenching and annealing[24]; (b) O2 permeability of starch and starch wrapped GO in PLA with its amount[26]; (c) Schematic diagram of O2 relative simple path through PLA/starch and a tortuous path through PLA/GO@starch[26]
PO2—O2 permeability coefficient of the material
图 3 十八胺改性氧化石墨烯/马来酸酐接枝聚丙烯(mGO-ODA/MAPP)复合材料的气体渗透性随着mGO-ODA含量变化图: (a) mGO-ODA/MAPP的H2GTR和渗透系数随着mGO-ODA含量变化图;(b) mGO-ODA/MAPP的O2GTR和渗透系数随着mGO-ODA含量变化图[34]
Figure 3. Gas permeability of octadectamine-modified GO/maleic anhydride-grafted polypropylene (mGO-ODA/MAPP) composite changes with mGO-ODA content: (a) H2GTR and permeability coefficient of mGO-ODA/MAPP change with mGO-ODA content; (b) O2GTR and permeability coefficient of mGO-ODA/MAPP change with mGO-ODA content[34]
PH2—H2 permeability coefficient of the material; GTR—Gas transmission rates
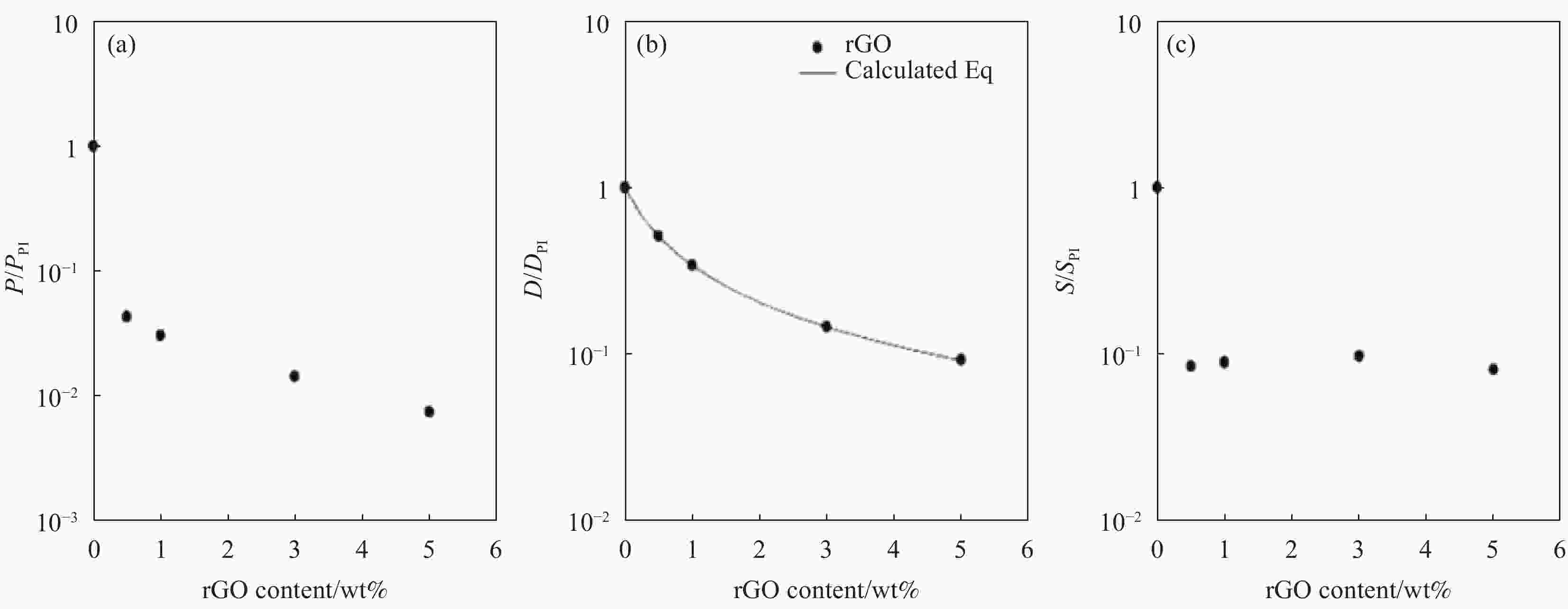
图 4 吡啶功能化还原氧化石墨烯/聚酰亚胺( Py-rGO/PI)纳米复合材料的O2渗透性随着rGO含量的变化图: (a) Py-rGO/PI纳米复合材料的O2渗透系数随着rGO含量的变化图;(b) Py-rGO/PI纳米复合材料的O2扩散系数随着rGO含量的变化图;(c) Py-rGO/PI纳米复合材料的O2溶解度系数随着rGO含量的变化图[46]
Figure 4. O2 permeability of pyridine-functionalized reduced graphene oxide/polyimide (Py-rGO/PI) nanocomposites changes with rGO content: (a) Changes of O2 perfiltration coefficient of Py-rGO/PI nanocomposites; (b) Changes of O2 diffusion coefficient of Py-rGO/PI nanocomposites; (c) Change of O2 solubility of Py-rGO/PI nanocomposites[46]
P/PPI—Permeability coefficient of the Py-rGO/PI composite; D/DPI—Diffusivity coefficient of the Py-rGO/PI composite; S/SPI—Solubility coefficient of the Py-rGO/PI composite
-
[1] CUI Y, KUNDALWAL S I, KUMAR S. Gas barrier perfor-mance of graphene/polymer nanocomposites[J]. Carbon,2016,98:313-333. doi: 10.1016/j.carbon.2015.11.018 [2] YOU J, WON S, JIN H J, et al. Nano-patching defects of reduced graphene oxide by cellulose nanocrystals in scalable polymer nanocomposites[J]. Carbon,2020,165:18-25. doi: 10.1016/j.carbon.2020.04.024 [3] REN P G, LIU X H, REN F,et al. Biodegradable graphene oxide nanosheets/poly-(butylene adipate-co-terephthalate) nanocomposite film with enhanced gas and water vapor barrier properties[J]. Polymer Testing,2017,58:173-180. doi: 10.1016/j.polymertesting.2016.12.022 [4] REN F, TAN W, DUAN Q, et al. Ultra-low gas permeable cellulose nanofiber nanocomposite films filled with highly oriented grapheneoxide nanosheets induced by shear field[J]. Carbohydrate Polymers,2019,209:310-319. doi: 10.1016/j.carbpol.2019.01.040 [5] YU Q, ZHU L, LIU T, et al. Preparation of nacre-like polyimide/montmorillonite composite films with excellent water vapor barrier properties by gravity-induced deposition[J]. Advanced Materials Interfaces,2020,8:2001786. doi: 10.1002/admi.202001786 [6] KONG J, LI Z, CAO Z, et al. The excellent gas barrier properties and unique mechanical properties of poly(propylene carbonate)/organo-montmorillonite nanocomposites[J]. Polymer Bulletin,2017,74(12):5065-5082. doi: 10.1007/s00289-017-2002-6 [7] LIU Y W, LIU J J, DING Q, et al. Enhanced gas barrier and thermal properties of polyimide/montmorillonite nanocomposites as a result of “dual-plane” structure effect[J]. Polymer Composites,2018,39(S3):1725-1732. doi: 10.1002/pc.24713 [8] XIA M, SHI K, ZHOU M, et al. Effects of chain extender and uniaxial stretching on the properties of PLA/PPC/mica composites[J]. Polymers for Advanced Technologies,2019,30(9):2436-2446. doi: 10.1002/pat.4691 [9] SANCHEZ-GARCIA M D, LAGARON J M. Novel clay-based nanobiocomposites of biopolyesters with synergistic barrier to UV light, gas, and vapour[J]. Journal of Applied Polymer Science,2010,118(1):188-199. doi: 10.1002/app.31986 [10] YOO B M, SHIN H J, YOON H W, et al. Graphene and graphene oxide and their uses in barrier polymers[J]. Journal of Applied Polymer Science,2014,131(1):39628. doi: 10.1002/app.39628 [11] KALAITZIDOU K, FUKUSHIMA H, DRZAL L T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets[J]. Carbon,2007,45(7):1446-1452. doi: 10.1016/j.carbon.2007.03.029 [12] LEE C, WEI X, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science,2008,321(5887):385-388. doi: 10.1126/science.1157996 [13] STOLLER M, PARK S, ZHU Y, et al. Graphene-based ultracapacitors[J]. Nano Letters,2008,8(10):3498-3502. doi: 10.1021/nl802558y [14] NAIR R R, BLAKE P, GRIGORENKO A N, et al. Fine structure constant defines visual transparency of graphene[J]. Science,2008,320(5881):1308. doi: 10.1126/science.1156965 [15] BOLOTIN K I, SIKES K J, JIANG Z, et al. Ultrahigh electron mobility in suspended graphene[J]. Solid State Communications,2008,146(9):351-355. doi: 10.1016/j.ssc.2008.02.024 [16] BALANDIN A A, GHOSH S, BAO W, et al. Superior thermal conductivity of single-Layer graphene[J]. Nano Letters,2008,8(3):902-907. doi: 10.1021/nl0731872 [17] DAS T K, PRUSTY S. Graphene-based polymer composites and their applications[J]. Polymer-Plastics Technology and Engineering,2013,52(4):319-331. doi: 10.1080/03602559.2012.751410 [18] GAO W, ALEMANY L B, CI L, et al. New insights into the structure and reduction of graphite oxide[J]. Nature Chemistry,2009,1(5):403-408. doi: 10.1038/nchem.281 [19] HILTNER A, LIU R Y F, HU Y S, et al. Oxygen transport as a solid-state structure probe for polymeric materials: A review[J]. Journal of Polymer Science Part B: Polymer Physics,2005,43(9):1047-1063. doi: 10.1002/polb.20349 [20] TAN B, THOMAS N L. A review of the water barrier properties of polymer/clay and polymerigraphene nanocomposites[J]. Journal of Membrane Science,2016,514:595-612. doi: 10.1016/j.memsci.2016.05.026 [21] ANDREUCCI D, CIRILLO E N M, COLANGELI M, et al. Fick and fokker–planck diffusion law in inhomogeneous media[J]. Journal of Statistical Physics,2019,174(2):469-493. doi: 10.1007/s10955-018-2187-6 [22] ROSENBERG R M, PETICOLAS W L. Henry's law: A retrospective[J]. Journal of Chemical Education,2004,81(11):1647-1652. doi: 10.1021/ed081p1647 [23] TERRONES M, MARTIN O, GONZÁLEZ M, et al. Inter-phases in graphene polymer-based nanocomposites: Achievements and challenges[J]. Advanced Materials,2011,23(44):5302-5310. doi: 10.1002/adma.201102036 [24] XU H, XIE L, FENG Z X. Graphene oxide-driven design of strong and flexible biopolymer barrier films: From smart crystallization control to affordable engineering[J]. ACS Sustainable Chemistry & Engineering,2016,4(1):334-349. doi: 10.1021/acssuschemeng.5b01273 [25] HUANG H D, ZHOU S Y, ZHOU D, et al. Highly efficient “composite barrier wall” consisting of concentrated graphene oxide nanosheets and impermeable crystalline structure for poly(lactic acid) nanocomposite films[J]. Industrial & Engineering Chemistry Research,2016,55(35):9544-9554. doi: 10.1021/acs.iecr.6b02168 [26] XU H, XIE L, WU D, et al. Immobilized graphene oxide nanosheets as thin but strong nanointerfaces in biocomposites[J]. ACS Sustainable Chemistry & Engineering,2016,4(4):2211-2222. doi: 10.1021/acssuschemeng.5b01703 [27] XU P P, ZHANG S M, HUANG H D, et al. Highly efficient three-dimensional gas barrier network for biodegradable nanocomposite films at extremely low loading levels of graphene oxide nanosheets[J]. Industrial & Engineering Chemistry Research,2020,59(13):5818-5827. doi: 10.1021/acs.iecr.9b06810 [28] LI F F, ZHANG C L, WENG Y N, et al. Enhancement of gas barrier properties of graphene oxide/poly (lactic acid) films using a solvent-free method[J]. Materials,2020,13:3024. doi: 10.3390/ma13133024 [29] BELOSHENKO V A, VOZNYAK A V, VOZNYAK Y, et al. Effect of simple shear induced orientation process on the morphology and properties of polyolefin/graphite nanoplates composites[J]. Composites Science and Technology,2017,139:47-56. doi: 10.1016/j.compscitech.2016.12.009 [30] HUI J, REN P G, SUN Z F, et al. Influences of interfacial adhesion on gas barrier property of functionalized graphene oxide/ultra-high-molecular-weight polyethylene composites with segregated structure[J]. Composite Interfaces,2017,24(8):729-741. doi: 10.1080/09276440.2017.1269517 [31] KAROLINA G, ROLAND K, ANDRZEJ R, et al. Gas barrier, thermal, mechanical and rheological properties of highly aligned graphene-LDPE nanocomposites[J]. Polymers,2017,9(7):294. doi: 10.3390/polym9070294 [32] HONAKER K, VAUTARD F, DRZAL L T. Influence of processing methods on the mechanical and barrier properties of HDPE-GNP nanocomposites[J]. Advanced Composites and Hybrid Materials,2021,4:492-504. doi: 10.1007/s42114-020-00181-1 [33] KIM S H, KIM K H, PARK O O. Poly(propylene)-grafted thermally reduced graphene oxide and its compatibilization effect on poly(propylene)-graphene nanocomposites[J]. RSC Advances,2016,6(91):87828-87835. doi: 10.1039/C6RA17934G [34] LI X Y, BANDYOPADHYAY P, NGUYEN T T, et al. Fabrication of functionalized graphene oxide/maleic anhydride grafted polypropylene composite film with excellent gas barrier and anticorrosion properties[J]. Journal of Membrane Science,2018,547:80-92. doi: 10.1016/j.memsci.2017.10.031 [35] CHOI B K, PARK S J, SEO M K. Effect of graphene oxide on thermal, optical, and gas permeability of graphene oxide/poly(vinyl alcohol) hybrid films using the boric acid[J]. Journal of Nanoscience & Nanotechnology,2017,17(10):7368-7372. doi: 10.1166/jnn.2017.14784 [36] DING J, ZHAO C, ZHAO L, et al. Synergistic effect of α-ZrP and graphene oxide nanofillers on the gas barrier properties of PVA films[J]. Journal of Applied Polymer Science,2018,135(27):46455. doi: 10.1002/app.46455 [37] REN P G, WANG H, YAN D X, et al. Ultrahigh gas barrier poly (vinyl alcohol) nanocomposite film filled with congregated and oriented Fe3O4@GO sheets induced by magneticfield[J]. Composites Part A: Applied Science and Manufacturing,2017,97:1-9. doi: 10.1016/j.compositesa.2017.02.026 [38] TSOU C H, ZHAO L, GAO C, et al. Characterization of network bonding created by intercalated functionalized graphene and polyvinyl alcohol in nanocomposite films for reinforced mechanical properties and barrier perfor-mance[J]. Nanotechnology,2020,31(38):385703. doi: 10.1088/1361-6528/ab9786 [39] SCHUEREN B, MAROUAZI H, MOHANTY A, et al. Polyvinyl alcohol-few layer graphene composite films prepared from aqueous colloids. Investigations of mechanical, conduc-tive and gas barrier properties[J]. Nanomaterials,2020,10(5):858. doi: 10.3390/nano10050858 [40] MADHAD H V, VASAVA D V. Review on recent progress in synthesis of graphene-polyamide nanocomposites[J]. Journal of Thermoplastic Composite Materials,2019,(3):1-29. doi: 10.1177/0892705719880942 [41] YOUSEF S, SARWAR Z, EREIKA J, et al. A new industrial technology for mass production of graphene/PEBA membranes for CO2/CH4 selectivity with high dispersion, thermal and mechanical performance[J]. Polymers,2020,12:831. doi: 10.3390/polym12040831 [42] RAINE T P, ISTRATE O M, KING B E, et al. Graphene/polyamide laminates for supercritical CO2 and H2S barrier app-lications: An approach toward permeation shutdown[J]. Advanced Materials Interfaces,2018,5(15):1800304. doi: 10.1002/admi.201800304 [43] LI X, BANDYOPADHYAY P, GUO M, et al. Enhanced gas barrier and anticorrosion performance of boric acid induced cross-linked poly(vinyl alcohol-co-ethylene)/graphene oxide film[J]. Carbon,2018,133:150-161. doi: 10.1016/j.carbon.2018.03.036 [44] YANG Z, GUO H, KANG C, et al. Enhanced gas barrier properties of polymer substrates for flexible OLEDs by adjusting the backbone rigidity and incorporating 2D nanosheets[J]. New Journal of Chemistry,2021,45(27):12945-12956. [45] NAM K H, KIM K, KIM S G, et al. Sustainable production of reduced graphene oxide using elemental sulfur for multifunctional composites[J]. Composites Part B Engineering,2019,176:107236. doi: 10.1016/j.compositesb.2019.107236 [46] LIM J, YEO H, KIM S G, et al. Pyridine-functionalized graphene/polyimide nanocomposites: Mechanical, gas barrier, and catalytic effects[J]. Composites Part B Engi-neering,2017,114:280-288. doi: 10.1016/j.compositesb.2016.12.057 [47] LIN Y, LIU S, PENG J, et al. Constructing a segregated graphene network in rubber composites towards improved electrically conductive and barrier properties[J]. Composites Science & Technology,2016,131:40-47. doi: 10.1016/j.compscitech.2016.05.012 [48] LUO Y, WU Y, LUO K, et al. Structures and properties of alkanethiol-modified graphene oxide/solution-polymerized styrene butadiene rubber composites: Click chemistry and molecular dynamics simulation[J]. Composites Science and Technology,2018,161:32-38. doi: 10.1016/j.compscitech.2018.03.036 [49] RAEF M, RAZZAGHI-KASHANI M. The role of interface in gas barrier properties of styrene butadiene rubber-reduced graphene oxide composites[J]. Polymer,2019,182:121816. doi: 10.1016/j.polymer.2019.121816 [50] ZHAO L, SUN H, KIM N, et al. Hydrogen gas barrier pro-perty of polyelectrolyte/GO layer-by-layer films[J]. Jour-nal of Applied Polymer Science,2015,132(20):41973. [51] PARK W B, BANDYOPADHYAY P, NGUYEN T T, et al. Effect of high molecular weight polyethyleneimine functionalized graphene oxide coated polyethylene terephthalate film on the hydrogen gas barrier properties[J]. Compo-sites Part B Engineering,2016,106:316-323. doi: 10.1016/j.compositesb.2016.09.048 [52] WU Y H, LIN Y, WEI Y, et al. Constructing interconnected graphene network in fluoroelastomer composites by F-H polar interaction for enhanced mechanical and barrier properties[J]. Composites Science and Technology,2017,148:35-42. doi: 10.1016/j.compscitech.2017.05.014 [53] LIU M F, CATAIDI P, YOUNG R J, et al. High-performance fluoroelastomer-graphene nanocomposites for advanced sealing applications[J]. Composites Science and Technology,2021,202:108592. doi: 10.1016/j.compscitech.2020.108592 [54] BANDYOPADHYAY P, NGUYEN T T, LI X, et al. Enhanced hydrogen gas barrier performance of diaminoalkane functionalized stitched graphene oxide/polyurethane compo-sites[J]. Composites Part B Engineering,2017, 117:101-110. [55] LI X, BANDYOPADHYAY P, KSHETRI T, et al. Novel hydroxylated boron nitride functionalized p-phenylenediamine-grafted graphene: An excellent filler for enhancing the barrier properties of polyurethane[J]. Journal of Materials Chemistry A,2018,6(43):21501-21515. doi: 10.1039/C8TA08351G [56] FEIJANI E, TAVASSOLI A, MAHDAVI H, et al. Effective gas separation through graphene oxide containing mixed matrix membranes[J]. Journal of Applied Polymer Science,2018,135:46271. doi: 10.1002/app.46271 [57] LI J, WANG S, LAI L, et al. Synergistic enhancement of gas barrier and aging resistance for biodegradable films with aligned graphene nanosheets[J]. Carbon,2021,172(9):31-40. [58] SUNG S J, PARK J, CHO Y S, et al. Enhanced gas barrier property of stacking-controlled reduced graphene oxide films for encapsulation of polymer solar cells[J]. Carbon,2019,150:275-283. doi: 10.1016/j.carbon.2019.04.120 [59] GHANEM A F, YASSIN M A, RABIE A M, et al. Investigation of water sorption, gas barrier and antimicrobial properties of polycaprolactone films contain modified graphene[J]. Journal of Materials Science,2021,56(1):497-512. doi: 10.1007/s10853-020-05329-4 [60] WANG C, GE X, JIANG Y. Synergistic effect of graphene oxide/montmorillonite-sodium carboxymethycellulose ter-nary mimic-nacre nanocomposites prepared via a facile evaporation and hot-pressing technique[J]. Carbohydrate Polymers,2019,222:115026. doi: 10.1016/j.carbpol.2019.115026 [61] MIANEHROW H, LO RE G, CAROSIO F, et al. Strong reinforcement effects in 2D cellulose nanofibril–graphene oxide (CNF–GO) nanocomposites due to GO-induced CNF ordering[J]. Journal of Materials Chemistry A,2020,8(34):17608-17620. doi: 10.1039/D0TA04406G [62] RAMEZANI H, BEHZAD T, BAGHERI R. Synergistic effect of graphene oxide nanoplatelets and cellulose nanofibers on mechanical, thermal, and barrier properties of thermoplastic starch[J]. Polymers for Advanced Technologies,2020,31(3):553-565. doi: 10.1002/pat.4796 -





 下载:
下载: