Recent advances in uranium adsorption by biomass based composite
-
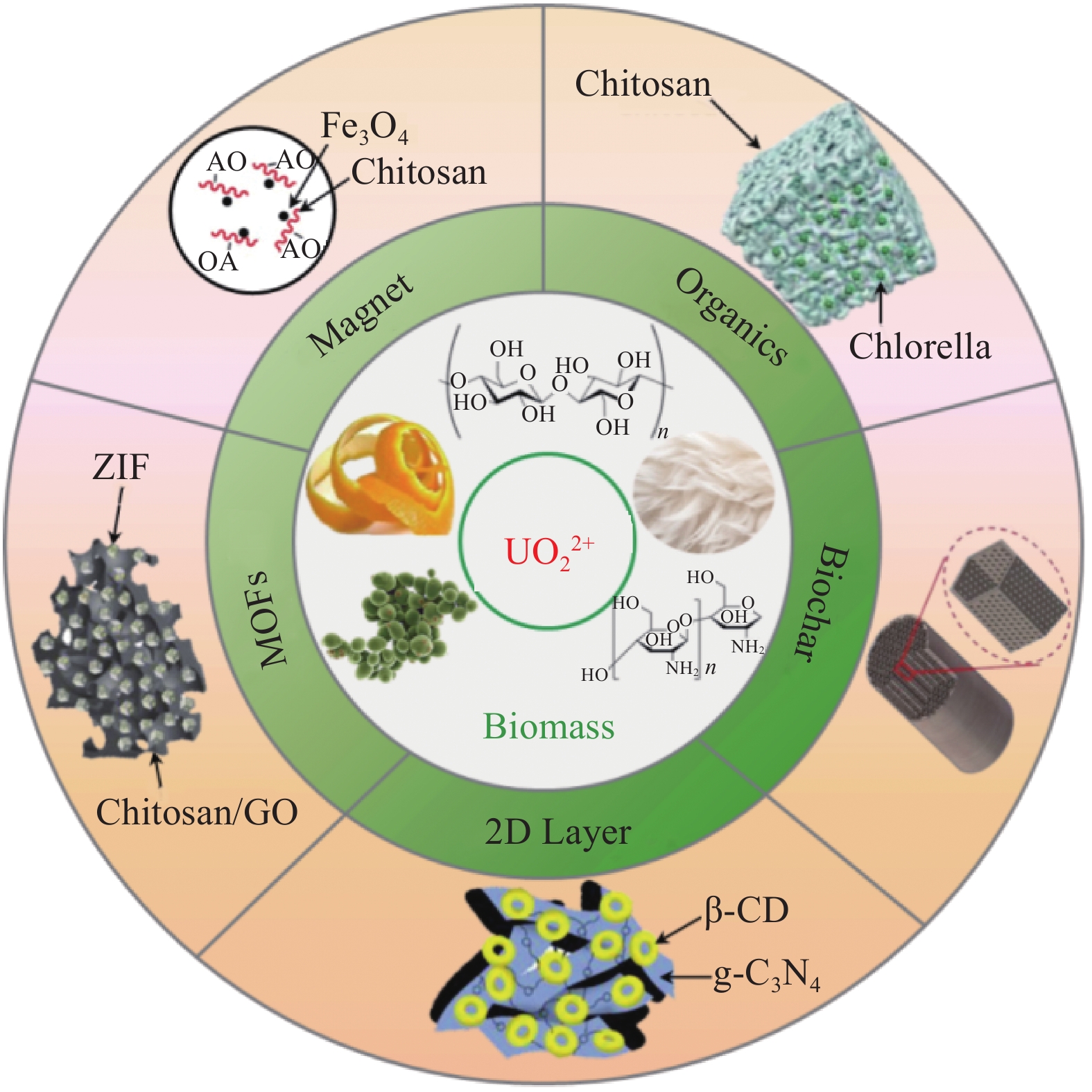
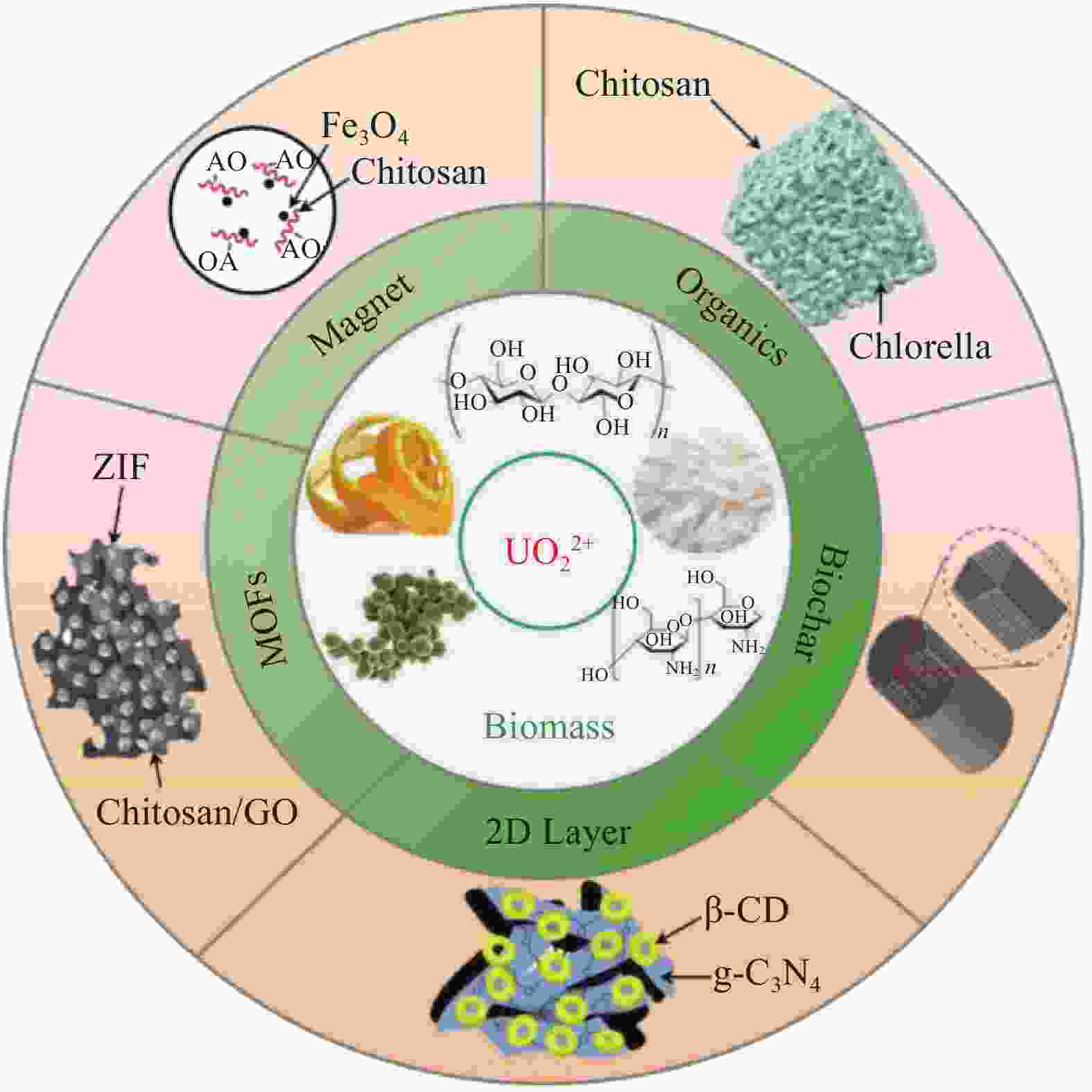
摘要: 在核能的开发利用中会产生大量放射性含铀废水,给生态安全和人类健康带来巨大威胁。与此同时,随着陆地铀的逐渐消耗,为保证长期充足的核燃料供应,开采海水铀势在必行。近年来,吸附法在废水铀治理及海水铀利用中备受关注。以环境友好、储量丰富、成本低廉的生物质材料加工制备性能优异、附加值高的吸附材料,是铀吸附的绿色、经济、可持续的发展策略。本文系统综述了生物质基复合材料在铀吸附应用领域的最新研究成果,详细介绍了铀吸附性能和铀吸附机制,最后对其应用前景和发展趋势进行了展望。Abstract: With the development of nuclear energy, a large amount of uranium-containing radioactive wastewater has been produced, posing a great threat to ecological safety and human health. At the same time, with the gradual depletion of terrestrial uranium, it is imperative to exploit uranium from seawater to ensure long-term sufficient supply of nuclear fuel. In recent years, the adsorption method has attracted increasing attention in the treatment of uranium-contaminated wastewater and the extraction of uranium from seawater. And it is a green, economical and sustainable development strategy for uranium adsorption to prepare high value-added biomass-based materials with excellent performance based on its eco-friendliness, abundant reserves and low cost. This review focuses on the main progress of biomass-based uranium adsorption composite materials, covering its adsorption performance and adsorption mechanism. Finally, the future prospects and research direction are proposed for better biomass-based adsorbents.
-
Key words:
- biomass /
- composite materials /
- uranium /
- adsorption /
- mechanism /
- nuclear energy
-
图 2 (a) 聚丙烯酸/聚乙烯亚胺(PAA/PEI)复合丝瓜络吸附剂的合成示意图[34];(b) 磷酸化壳聚糖/纤维素复合材料(CSP-CMCP)的合成示意图[37]
Figure 2. (a) Synthesis of polyacrylic acid/polyethyleneimine (PAA/PEI) modified Luffa cylindrical[34]; (b) Synthetic procedures of phosphorylated chitosan-phosphatedecorated carboxymethyl cellulose composite (CSP-CMCP)[37]
CS—Chitosan; MSP—Monosodium phosphate; STMP—Trisodium trimetaphosphate; CMC—Carboxymethyl cellulose
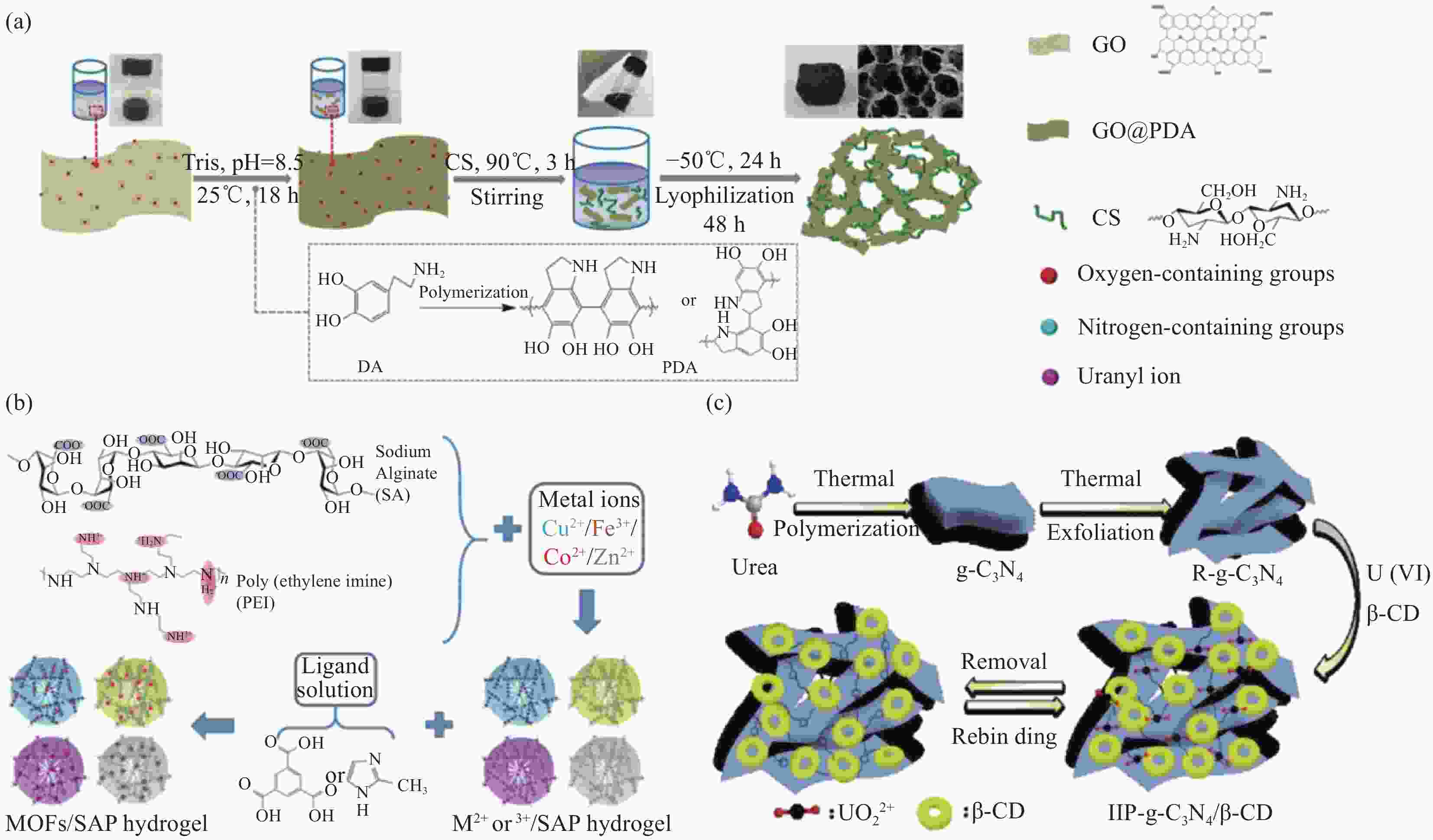
图 4 (a) 氧化石墨烯@聚多巴胺/壳聚糖(GO@PDA/CS)铀吸附气凝胶的制备[62];(b) ZIF-67/SAP复合凝胶的制备[66];(c)表面铀印迹石墨氮化碳/β-环糊精(IIP-g-C3N4/β-CD)复合吸附剂的制备[67]
Figure 4. (a) Pathway to fabricate the GO@Polydopamine/Chitosan (GO@PDA/CS) aerogel[62]; (b) Fabrication process of the ZIF-67/SAP composite hydrogel[66]; (c) Synthesis of ion-imprinted β-cyclodextrin modified graphitic carbon nitride polymer (IIP-g-C3N4/β-CD)[67]
表 1 单一生物质铀吸附材料及性能对比
Table 1. Uranium adsorption abilities of pure biomass materials
Category Material Condition Maximum adsorption
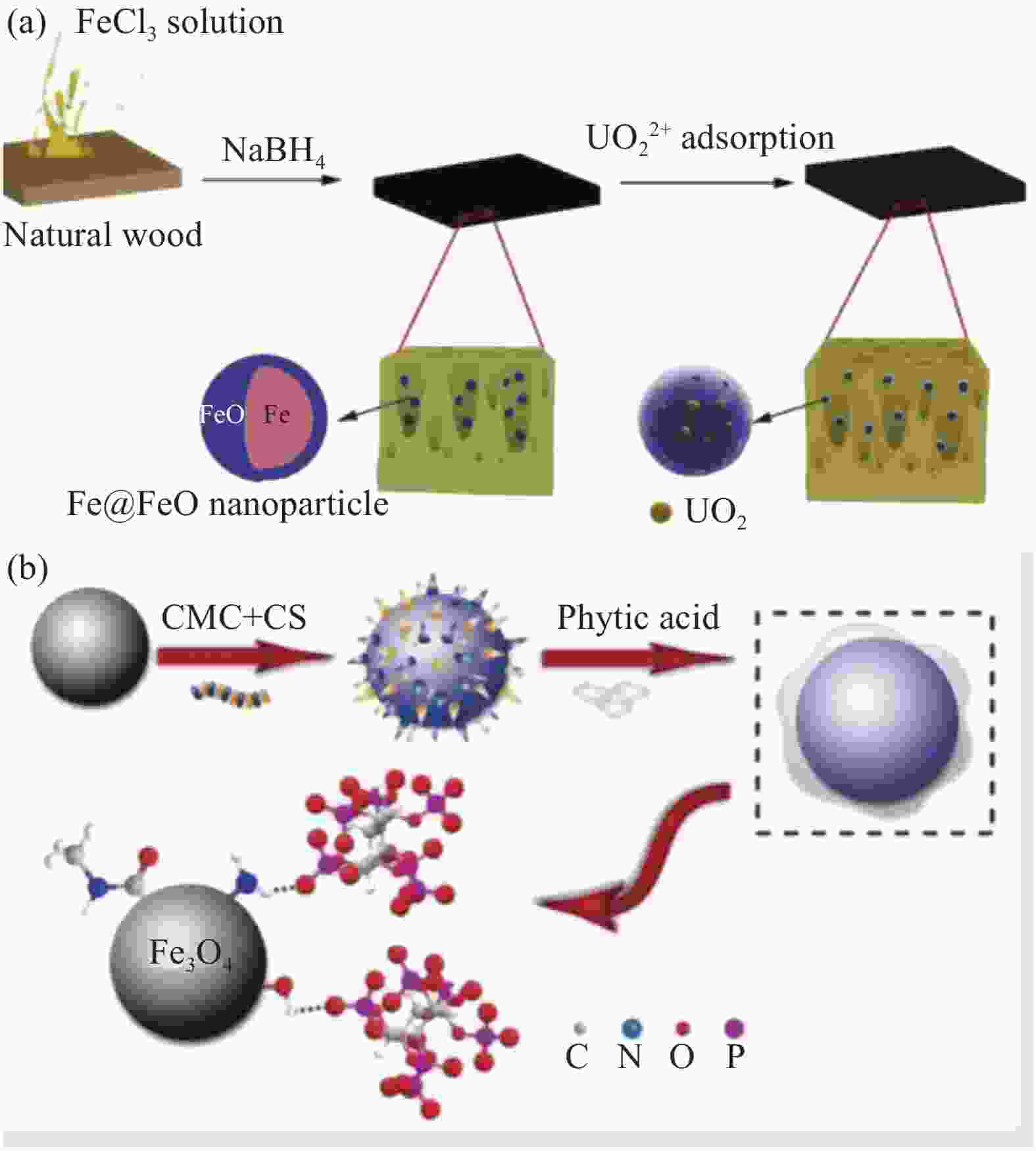
capacity/(mg·g−1)Ref. C0/(mg·L−1) pH (best) s/l/(g·L−1) Microbial Formaldehyde-treated Fusarium (fungus) 20-400 4.0 0.6 318.04 [11] Amidoximated Aspergillus niger (fungus) 0.2-0.8 5.0 0.05-0.35 621 [12] Yeast (fungus) 10-350 5.0 0.06-1.20 341.2 [13] Bacillus subtilis (bacteria) 10-350 5.0 0.06-1.20 512.5 [13] Chlorella (algae) 10-350 5.0 0.06-1.20 356.5 [13] Nature polymer Ultrafine cellulose nanofibers 100 6.5 0.3 167 [15] Amine-impregnated cellulose 25-350 0.1-3 2.5 56.5 [16] Chitosan beads 100-2 000 5.0 1.0 236.9 [17] Chitosan films 10~100 5.0 0.5 197.74 [18] Agroforestry waste Amidoximated wool fibers 80-1530 5.0 - 78.19 [25] Coir pith 200-800 4.5 0.025-0.2 218.3 [26] Garlic dregs 20-100 6.0 5.0 38.0 [27] Sunflower straw 10-800 5.0 2.0 251.52 [28] Grapefruit peel 50-500 5.0 2.0 140.79 [29] Notes: C0—Uranium concentration;s/l—Solid/liquid. 表 2 不同体系生物质基复合材料的铀吸附性能
Table 2. Uranium adsorption properties of biomass based composites in different systems
Category Material Condition Maximum adsorption
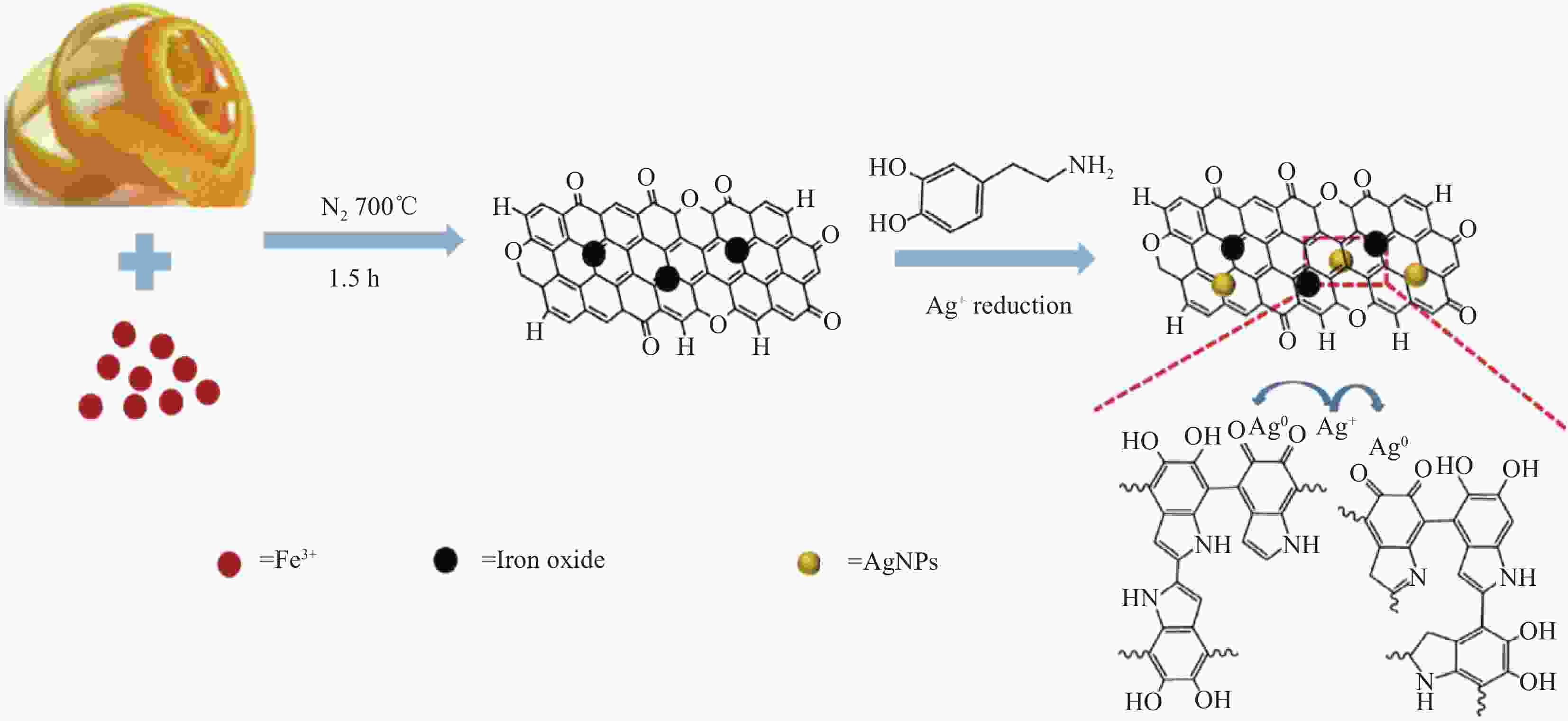
capacity/(mg·g−1)Ref. C0/(mg·L−1) pH (best) s/l/(g·L−1) Organics composite Cellulose/camphor soot 50-250 6.0 0.2-1.0 410 [30] Polyvinylpyrroldone/chitosan 0.3-300 5.0-8.0 1.0 167 [31] Hydroxyethyl cellulose/sodium alginate 5-150 5.0 0.5 357.1 [32] Acrylic acid/polyethyleneimine modified Luffa cylindrical 50-350 6.0 0.4 444.4 [34] Chlorella pyrenoidosa/chitosan 10-800 4.0-5.0 0.5 571 [35] Polyethylenimine/guanidyl functionalized Hemp fibers 5-400 7.0 0.4 414.93 [36] Phosphorylated chitosan/carboxymethyl cellulose 0.08 5.5-8.5 0.05 977.54 [37] Poly(amidoxime)/chitosan 2-8 6.0 5.0 1013 [22] Poly(amidoxime)/cellulose 2-16 6.0 5.0 465 [38] Spidroin/super uranyl-binding protein 2-16 6.0 20 12.33 (Natural seawater) [39] Dual-superb-uranyl binding protein 2-16 5.0 20 17.45 (Natural seawater) [40] Magnetic composite Nano-Fe3O4/aspergillus niger 2-10 7.0 0.15 60.05 [41] Polyethyleneimine/magnetic yeast 5-40 6.0 0.2 173.99 [42] Nano-Fe3O4/pseudomonas aeruginosa 30 6.0 1.4 92.483 [43] Diethylenetriamine-magnetic chitosan 10-80 3.5 0.2 62.75 [44] Tripolyphosphate-linked magnetic chitosan 80 4.5 0.4 166.7 [45] Magnetic chitosan 100 5.0 0.4 178 [46] Ion-imprinted magnetic chitosan 80 5.0 0.2-1.0 187.26 [47] Magnetic chitosan 40-140 5.0 0.6 161.3 [48] Amidoxime-magnetic chitosan 10-600 6.0 1.0 117.65 [49] Amidoxime-magnetic chitosan 5-350 4.0 0.1-0.25 405 [50] Fe@FeO/wood 100-600 4.0 - 2.08 g UO22+/g Fe@FeO [51] Magnetic carboxymethylcellulose 25-550 8.0 0.5 625 [52] Fe0@Ni0/collagen 20-260 5.0-6.0 0.2-1.0 129.5 [56] Fe0/carboxymethyl-cellulose 20-100 5.0 3.0 322.58 [57] Novel-nanomaterials Carboxymethyl-cellulose/carbon nanotubes 54 5.0 0.1-1.0 127 [58] Chitosan/graphene oxide 2-100 5.0 0.05-0.40 227.3 [60] Konjac glucomannan/graphene oxide 90 5.0 0.25 513.4 [61] Polydopamine-grapheneoxide/chitosan 10-220 5.5-7.0 0.3 415.9 [62] Phosphorylated-graphene oxide/chitosan 13.5 5.5-6.5 0.05 779.44 [63] Chitosan/graphite felt electrode 1000 - - 1523 [64] Chitosan@graphene oxide/ZIF 50 8.0 0.2 361.01 [65] Polyethyleneimine@sodium alginate/ZIF-67 10-100 8.0 0.3 480.87 [66] β-Cyclodextrin/g-C3N4 50-800 7.0-8.0 0.5 855.96 [67] Fungal hyphae/graphene/MoS2 90 6.0 0.16 275 [68] Bacterial cellulose/ MoS2-x 8-100 5.0 0.5 342 [69] Biochar composite Salophe/luffa-biochar 3-2000 5.5 0.33 833 [76] Amidoxime/starch-biochar 5-130 5.0 0.1 724.6 [77] Fe/dopamine-biochar 20 6.0-8.0 - 232.54 [78] Magnetic activated carbon/poly-dopamine/Ag 50-500 8.0 0.5 657.89 [79] -
[1] LENZEN M. Life cycle energy and greenhouse gas emissions of nuclear energy: a review[J]. Energy Conversion and Management,2008,49(8):2178-2199. doi: 10.1016/j.enconman.2008.01.033 [2] WU X, LV C, YU S, et al. Uranium(VI) removal from aqueous solution using iron-carbon micro-electrolysis packing[J]. Separation and Purification Technology,2020,234:116104. doi: 10.1016/j.seppur.2019.116104 [3] World Health Organization. Guidelines for drinking-water quality: ISBN 978 92 4 154815 1[S]. Switzerlan: World Health Organization Press, 2011. [4] 熊洁, 文君, 胡胜, 等. 中国海水提铀研究进展[J]. 核化学与放射化学, 2015, 37(5):257-265. doi: 10.7538/hhx.2015.37.05.0257XIONG J, WEN J, HU S, et al. Progress in extracting uranium from seawater of China[J]. Journal of Nuclear and Radiochemistry,2015,37(5):257-265(in Chinese). doi: 10.7538/hhx.2015.37.05.0257 [5] 李昊, 文君, 汪小琳. 中国海水提铀研究进展[J]. 科学通报, 2018, 63(5-6):481-494. doi: 10.1360/N972017-01122LI H, WEN J, WANG X L. Research advances on extracting uranium from seawater in China[J]. Chinese Science Bulletin,2018,63(5-6):481-494(in Chinese). doi: 10.1360/N972017-01122 [6] ABNEY C W, MAYES R T, SAITO T, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews,2017,117(23):13935-14013. doi: 10.1021/acs.chemrev.7b00355 [7] GOMEZ-MALDONADO D, ERRAMUSPE I B V, PERESIN M S. Natural polymers as alternative adsorbents and treatment agents for water remediation[J]. Bioresources,2019,14(4):10093-10160. doi: 10.15376/biores.14.4.Gomez-Maldonado [8] MUDHOO A, GARG V K, WANG S. Removal of heavy metals by biosorption[J]. Environmental Chemistry Letters,2011,10(2):109-117. [9] MICHALAK I, CHOJNACKA K, WITEK-KROWIAK A. State of the art for the biosorption process—A review[J]. Applied Biochemistry and Biotechnology,2013,170(6):1389-416. doi: 10.1007/s12010-013-0269-0 [10] 陈保冬, 孙玉青, 张莘, 等. 菌根真菌重金属耐性机制研究进展[J]. 环境科学, 2015, 36(3):1124-1132.CHEN B D, SUN Y Q, ZHANG X, et al. Underlying mechanisms of the heavy metal tolerance of mycorrhizal fungi[J]. Environmental Science,2015,36(3):1124-1132(in Chinese). [11] CHEN F, TAN N, LONG W, et al. Enhancement of uranium(VI) biosorption by chemically modified marine-derived mangrove endophytic fungus Fusarium sp. #ZZF51[J]. Journal of Radioanalytical and Nuclear Chemistry,2014,299(1):193-201. doi: 10.1007/s10967-013-2758-6 [12] LI L, HU N, DING D, et al. Adsorption and recovery of U(VI) from low concentration uranium solution by amidoxime modified Aspergillus niger[J]. RSC Advances,2015,5(81):65827-65839. doi: 10.1039/C5RA13516H [13] 马佳林, 聂小琴, 董发勤, 等. 三种微生物对铀的吸附行为研究[J]. 中国环境科学, 2015, 35(3):825-832.MA J L, NIE X Q, DONG F Q, et al. The adsorption behavior on uranium by three kinds of microorganisms[J]. China Environmental Science,2015,35(3):825-832(in Chinese). [14] DASSANAYAKE R S, ACHARYA S, ABIDI N. Biopolymer-based materials from polysaccharides: properties, processing, characterization and sorption applications[M]. IntechOpen: 2018, 1-24. [15] MA H, HSIAO B S, CHU B. Ultrafine cellulose nanofibers as efficient adsorbents for removal of UO22+ in water[J]. ACS Macro Letters,2011,1(1):213-216. doi: 10.1021/mz200047q [16] ORABI A H, EL-SHEIKH E M, SALEH W H, et al. Potentiality of uranium adsorption from wet phosphoric acid using amine-impregnated cellulose[J]. Journal of Radiation Research and Applied Sciences,2019,9(2):193-206. doi: 10.1016/j.jrras.2015.12.003 [17] SURESHKUMAR M K, DAS D, MALLIA M B, et al. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads[J]. Journal of Hazardous Materials,2010,184(1-3):65-72. doi: 10.1016/j.jhazmat.2010.07.119 [18] WANG Y, LI Y, LI L, et al. Preparation of three-dimensional fiber-network chitosan films for the efficient treatment of uranium-contaminated effluents[J]. Water Science and Technology,2020,81(1):52-61. doi: 10.2166/wst.2020.075 [19] BETHKE K, PALANT KEN S, ANDREI V, et al. Functionalized cellulose for water purification, antimicrobial applications, and sensors[J]. Advanced Functional Materials,2018,28(23):1800409. doi: 10.1002/adfm.201800409 [20] GARBA Z N, LAWAN I, ZHOU W, et al. Microcrystalline cellulose (MCC) based materials as emerging adsorbents for the removal of dyes and heavy metals-a review[J]. The Science of the Total Environment,2020,717:135070. doi: 10.1016/j.scitotenv.2019.135070 [21] HAMED I, ÖZOGUL F, REGENSTEIN J M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review[J]. Trends in Food Science & Technology,2016,48:40-50. doi: 10.1016/j.jpgs.2015.11.007 [22] SHI S, LI B, QIAN Y, et al. A simple and universal strategy to construct robust and anti-biofouling amidoxime aerogels for enhanced uranium extraction from seawater[J]. Chemical Engineering Journal,2020,397:125337. doi: 10.1016/j.cej.2020.125337 [23] MOHAMMADZADEH PAKDEL P, PEIGHAMBARDOUST S J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application[J]. Carbohydrate Polymers,2018,201:264-279. doi: 10.1016/j.carbpol.2018.08.070 [24] RAO P, RATHOD V. Valorization of food and agricultural waste: a step towards greener future[J]. Chemical Record,2019,19(9):1858-1871. doi: 10.1002/tcr.201800094 [25] ZHANG F, CHEN M, HU S, et al. Chemical treatments on the cuticle layer enhancing the uranium(VI) uptake from aqueous solution by amidoximated wool fibers[J]. Journal of Radioanalytical and Nuclear Chemistry,2017,314(3):1927-1937. doi: 10.1007/s10967-017-5548-8 [26] PARAB H, JOSHI S, SHENOY N, et al. Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies[J]. Bioresource Technology,2005,96(11):1241-8. doi: 10.1016/j.biortech.2004.10.016 [27] 王扬, 牛洁, 徐乐昌. 大蒜渣生物质材料对铀的吸附性能研究[J]. 铀矿冶, 2020, 39(4):278-281.WANG Y, NIU J, XU L C. Study on the adsorption property for uranium by garlic dregs biomass materia[J]. Uranium Mining & Metallurgy,2020,39(4):278-281(in Chinese). [28] AI L, LUO X, LIN X, et al. Biosorption behaviors of uranium(VI) from aqueous solution by sunflower straw and insights of binding mechanism[J]. Journal of Radioanalytical and Nuclear Chemistry,2013,298(3):1823-1834. doi: 10.1007/s10967-013-2613-9 [29] ZOU W H, ZHAO L, ZHU L. Efficient uranium(VI) biosorption on grapefruit peel: kinetic study and thermodynamic parameters[J]. Journal of Radioanalytical and Nuclear Chemistry,2012,292(3):1303-1315. doi: 10.1007/s10967-011-1602-0 [30] SINGH N, BALASUBRAMANIAN K. An effective technique for removal and recovery of uranium(VI) from aqueous solution using cellulose–camphor soot nanofibers[J]. RSC Advances,2014,4(53):27691-27701. doi: 10.1039/C4RA01751J [31] CHRISTOU C, PHILIPPOU K, KRASIA-CHRISTOFOROU T, et al. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers[J]. Carbohydrate Polymers,2019,219:298-305. doi: 10.1016/j.carbpol.2019.05.041 [32] 谢水波, 罗景阳, 刘清, 等. 羟乙基纤维素/海藻酸钠复合膜对六价铀的吸附性能及吸附机制[J]. 复合材料学报, 2015, 32(1):268-275.XIE S B, LUO J Y, LIU Q, et al. Adsorption characteristics and mechanism of hydroxyethyl cellulose/sodium alginate blend films for uranium(VI)[J]. Acta Materiae Compositae Sinica,2015,32(1):268-275(in Chinese). [33] LUO W, XIAO G, TIAN F, et al. Engineering robust metal–phenolic network membranes for uranium extraction from seawater[J]. Energy & Environmental Science,2019,12(2):607-614. DOI: 10.1039/C8EE01438H. [34] SU S, CHEN R, LIU Q, et al. High efficiency extraction of U(VI) from seawater by incorporation of polyethyleneimine, polyacrylic acid hydrogel and Luffa cylindrical fibers[J]. Chemical Engineering Journal,2018,345:526-535. doi: 10.1016/j.cej.2018.03.164 [35] JIANG X, WANG H, HU E, et al. Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel[J]. Microporous and Mesoporous Materials,2020,305:110383. doi: 10.1016/j.micromeso.2020.110383 [36] BAI Z, LIU Q, ZHANG H, et al. A novel 3D reticular anti-fouling bio-adsorbent for uranium extraction from seawater: Polyethylenimine and guanidyl functionalized hemp fibers[J]. Chemical Engineering Journal,2020,382:122555. doi: 10.1016/j.cej.2019.122555 [37] CAI Y W, CHEN L, YANG S T, et al. Rational synthesis of novel phosphorylated chitosan-carboxymethyl cellulose composite for highly effective decontamination of U(VI)[J]. ACS Sustainable Chemistry & Engineering,2019,7(5):5393-5403. doi: 10.1021/acssuschemeng.8b06416 [38] GAO J, YUAN Y, YU Q, et al. Bio-inspired antibacterial cellulose paper-poly(amidoxime) composite hydrogel for high-efficient uranium(VI) capture from seawater[J]. Chemical Communications,2020,56(28):3935-3938. doi: 10.1039/C9CC09936K [39] YUAN Y, YU Q, WEN J, et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber[J]. Angewandte Chemie International Edition,2019,58(34):11785-11790. doi: 10.1002/anie.201906191 [40] YU Q, YUAN Y, FENG L, et al. Spidroin-inspired, high-strength, loofah-shaped protein fiber for capturing uranium from seawater[J]. Angewandte Chemie International Edition,2020,59(37):15997-16001. doi: 10.1002/anie.202007383 [41] 成彬, 李乐, 丁德馨, 等. 黑曲霉磁性生物吸附剂制备及其吸附低浓度铀性能研究[J]. 应用化工, 2018, 47(2):219-223. doi: 10.3969/j.issn.1671-3206.2018.02.003CHENG B, LI L, DING D X, et al. Preparation of nano-Fe3O4 modified Aspergillus niger and its properties for adsorption of low concentration uranium(VI)[J]. Applied Chemical Industry,2018,47(2):219-223(in Chinese). doi: 10.3969/j.issn.1671-3206.2018.02.003 [42] 伍随意, 李仕友, 胡俊毅, 等. 聚乙烯亚胺改性磁性酵母复合材料去除铀(VI)的性能[J]. 复合材料学报, 2020, 37(9):3073-3083.WU S Y, LI S Y, HU J Y, et al. Adsorption properties of polyethyleneimine modified magnetic yeast composites for uranium (VI)[J]. Acta Materiae Compositae Sinica,,2020,37(9):3073-3083(in Chinese). [43] 吴伟林, 谢水波, 谢磊, 等. 纳米Fe3O4负载铜绿假单胞菌吸附U(VI)的热力学与动力学研究[J]. 安全与环境学报, 2013, 13(6):26-30.WU W L, XIE S B, XIE L, et al. Dynamic and thermodynamic study on U(VI) adsorption onto nano-Fe3O4 immobilized Pseudomonas aeruginosa[J]. Journal of Safety and Environment,2013,13(6):26-30(in Chinese). [44] XU J, CHEN M, ZHANG C, et al. Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan[J]. Journal of Radioanalytical and Nuclear Chemistry,2013,298(2):1375-1383. doi: 10.1007/s10967-013-2571-2 [45] 陈小松, 周利民, 刘峙嵘. 三聚磷酸钠交联磁性壳聚糖树脂对铀酰离子的吸附特性[J]. 原子能科学技术, 2015, 49(6):972-978. doi: 10.7538/yzk.2015.49.06.0972CHEN X S, ZHOU L M, LIU Z R. Adsorption of UO22+ ion onto tripolyphosphate-crosslinked magnetic chitosan resin[J]. Atomic Energy Science and Technology,2015,49(6):972-978(in Chinese). doi: 10.7538/yzk.2015.49.06.0972 [46] SHENG L, ZHOU L, HUANG Z, et al. Facile synthesis of magnetic chitosan nano-particles functionalized with N/O-containing groups for efficient adsorption of U(VI) from aqueous solution[J]. Journal of Radioanalytical and Nuclear Chemistry,2016,310(3):1361-1371. doi: 10.1007/s10967-016-5014-z [47] ZHOU L, SHANG C, LIU Z, et al. Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins[J]. Journal of Colloid and Interface Science,2012,366(1):165-172. doi: 10.1016/j.jcis.2011.09.069 [48] 黄国林, 陈中胜, 梁喜珍, 等. 磁性交联壳聚糖对水溶液中铀(VI)离子的吸附行为[J]. 化工学报, 2012, 663(3):834-840. doi: 10.3969/j.issn.0438-1157.2012.03.023HUANG G L, CHEN Z S, LIANG X Z, et al. Adsorption behavior of U(VI) ions from aqueous solution on novel cross-linked magnetic chitosan beads[J]. CIESC Journal,2012,663(3):834-840(in Chinese). doi: 10.3969/j.issn.0438-1157.2012.03.023 [49] ZHUANG S, CHENG R, KANG M, et al. Kinetic and equilibrium of U(VI) adsorption onto magnetic amidoxime-functionalized chitosan beads[J]. Journal of Cleaner Production,2018,188:655-661. doi: 10.1016/j.jclepro.2018.04.047 [50] HAMZA M F, ROUX J-C, GUIBAL E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles[J]. Chemical Engineering Journal,2018,344:124-137. doi: 10.1016/j.cej.2018.03.029 [51] WANG Z, WANG J, ZHU L, et al. Scalable Fe@FeO core-shell nanoparticle-embedded porous wood for high-efficiency uranium(VI) adsorption[J]. Applied Surface Science,2020,508:144709. doi: 10.1016/j.apsusc.2019.144709 [52] GUO X, CHEN R, LIU Q, et al. Superhydrophilic phosphate and amide functionalized magnetic adsorbent: a new combination of anti-biofouling and uranium extraction from seawater[J]. Environmental Science: Nano,2018,5(10):2346-2356. doi: 10.1039/C8EN00545A [53] CANTRELL K J, KAPLAN D I, WIETSMA T W. Zero-valent iron for the in situ remediation of selected metals in groundwater[J]. Journal of Hazardous Materials,1995,42(2):201-212. doi: 10.1016/0304-3894(95)00016-N [54] HU B, MEI X, LI X, et al. Decontamination of U(VI) from nZVI/CNF composites investigated by batch, spectroscopic and modeling techniques[J]. Journal of Molecular Liquids,2017,237:1-9. doi: 10.1016/j.molliq.2017.04.084 [55] ZHANG H, RUAN Y, LIANG A, et al. Carbothermal reduction for preparing nZVI/BC to extract uranium: Insight into the iron species dependent uranium adsorption behavior[J]. Journal of Cleaner Production,2019,239:117873. doi: 10.1016/j.jclepro.2019.117873 [56] LIAO H, YU J, ZHU W, et al. Nano-zero-valent Fe/Ni particles loaded on collagen fibers immobilized by bayberry tannin as an effective reductant for uranyl in aqueous solutions[J]. Applied Surface Science,2020,507:145075. doi: 10.1016/j.apsusc.2019.145075 [57] POPESCU I-C, FILIP P, HUMELNICU D, et al. Removal of uranium(VI) from aqueous systems by nanoscale zero-valent iron particles suspended in carboxy-methyl cellulose[J]. Journal of Nuclear Materials,2013,443(1-3):250-255. doi: 10.1016/j.jnucmat.2013.07.018 [58] SHAO D, JIANG Z, WANG X, et al. Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution[J]. Journal of Physical Chemistry B,2009,113:860-864. doi: 10.1021/jp8091094 [59] ZOLFONOUN E, YOUSEFI S R. On-line extraction and determination of uranium in aqueous samples using multi-walled carbon nanotubes-coated cellulose acetate membrane[J]. International Journal of Environmental Analytical Chemistry,2016,96(3):203-211. doi: 10.1080/03067319.2015.1137909 [60] 李仕友, 史冬峰, 唐振平, 等. 壳聚糖/氧化石墨烯复合材料吸附U(VI)的特性与机理[J]. 环境科学学报, 2017, 37(4):1388-1395.LI S Y, SHI D F, TANG Z P, et al. Characteristics and mechanism of uranium(Ⅵ) adsorption by chitosan/grapheme oxide composite[J]. Acta Scientiae Circumstantiae,2017,37(4):1388-1395(in Chinese). [61] CHEN T, ZHANG J, LI M, et al. Biomass-derived composite aerogels with novel structure for removal/recovery of uranium from simulated radioactive wastewater[J]. Nanotechnology,2019,30(45):455602. doi: 10.1088/1361-6528/ab3991 [62] LIAO Y, WANG M, CHEN D. Preparation of polydopamine-modified graphene oxide/ chitosan aerogel for uranium (VI) adsorption[J]. Industrial & Engineering Chemistry Research,2018,57(25):8472-8483. doi: 10.1021/acs.iecr.8b01745 [63] CAI Y, WU C, LIU Z, et al. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(VI)[J]. Environmental Science: Nano,2017,4(9):1876-1886. doi: 10.1039/C7EN00412E [64] CHI F T, ZHANG S, WEN J, et al. Highly efficient recovery of uranium from seawater using an electrochemical approach[J]. Industrial & Engineering Chemistry Research,2018,57(23):8078-8084. doi: 10.1021/acs.iecr.8b01063 [65] GUO X, YANG H, LIU Q, et al. A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater[J]. Chemical Engineering Journal,2020,382:122850. doi: 10.1016/j.cej.2019.122850 [66] BAI Z, LIU Q, ZHANG H, et al. Anti-biofouling and water-stable balanced charged metal organic framework-based polyelectrolyte hydrogels for extracting uranium from seawater[J]. ACS Applied Materials & Interfaces,2020,12(15):18012-18022. doi: 10.1021/acsami.0c03007 [67] HAO X, CHEN R R, LIU Q, et al. A novel U(VI)-imprinted graphitic carbon nitride composite for the selective and efficient removal of U(VI) from simulated seawater[J]. Inorganic Chemistry Frontiers,2018,5(9):2218-2226. doi: 10.1039/C8QI00522B [68] LI Y, ZOU G, YANG S, et al. Integration of bio-inspired adsorption and photodegradation for the treatment of organics-containing radioactive wastewater[J]. Chemical Engineering Journal,2019,364:139-145. doi: 10.1016/j.cej.2019.01.169 [69] CHEN T, LIU B, LI M, et al. Efficient uranium reduction of bacterial cellulose-MoS2 heterojunction via the synergistically effect of Schottky junction and S-vacancies engineering[J]. Chemical Engineering Journal,2021,406:126791. doi: 10.1016/j.cej.2020.126791 [70] 李小燕, 张明, 刘义保, 等. 花生壳活性炭吸附溶液中的铀[J]. 化工环保, 2013, 33(3):202-205. doi: 10.3969/j.issn.1006-1878.2013.03.004LI X Y, ZHANG M, LIU Y B, et al. Adsorption of uranium from aqueous solution with peanut shell activated carbon[J]. Environmental Protection of Chemical Industry,2013,33(3):202-205(in Chinese). doi: 10.3969/j.issn.1006-1878.2013.03.004 [71] YAKOUT S M, METWALLY S S, EL-ZAKLA T. Uranium sorption onto activated carbon prepared from rice straw: Competition with humic acids[J]. Applied Surface Science,2013,280:745-750. doi: 10.1016/j.apsusc.2013.05.055 [72] PU D, KOU Y, ZHANG L, et al. Waste cigarette filters: Activated carbon as a novel sorbent for uranium removal[J]. Journal of Radioanalytical and Nuclear Chemistry,2019,320(3):725-731. doi: 10.1007/s10967-019-06502-z [73] LI B, MA L, TIAN Y, et al. A catechol-like phenolic ligand-functionalized hydrothermal carbon: one-pot synthesis, characterization and sorption behavior toward uranium[J]. Journal of Hazardous Materials,2014,271:41-49. doi: 10.1016/j.jhazmat.2014.01.060 [74] SUN Y B, WU Z Y, WANG X X, et al. Macroscopic and microscopic investigation of U(VI) and Eu(III) adsorption on carbonaceous nanofibers[J]. Environmental Science & Technology,2016,50(8):4459-4467. doi: 10.1021/acs.est.6b00058 [75] LIATSOU I, MICHAIL G, DEMETRIOU M, et al. Uranium binding by biochar fibres derived from Luffa cylindrica after controlled surface oxidation[J]. Journal of Radioanalytical and Nuclear Chemistry,2016,311(1):871-875. doi: 10.1007/s10967-016-5063-3 [76] LIATSOU I, PASHALIDIS I, NICOLAIDES A. Triggering selective uranium separation from aqueous solutions by using salophen-modified biochar fibers[J]. Journal of Radioanalytical and Nuclear Chemistry,2018,318(3):2199-2203. doi: 10.1007/s10967-018-6186-5 [77] ZHANG Z B, DONG Z M, DAI Y, et al. Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution[J]. RSC Advances,2016,6(104):102462-102471. doi: 10.1039/C6RA21986A [78] ZHU K, CHEN C, XU M, et al. In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction[J]. Chemical Engineering Journal,2018,331:395-405. doi: 10.1016/j.cej.2017.08.126 [79] ZHANG F, ZHANG H, CHEN R, et al. Mussel-inspired antifouling magnetic activated carbon for uranium recovery from simulated seawater[J]. Journal of Colloid and Interface Science,2019,534:172-182. doi: 10.1016/j.jcis.2018.09.023 -





 下载:
下载: