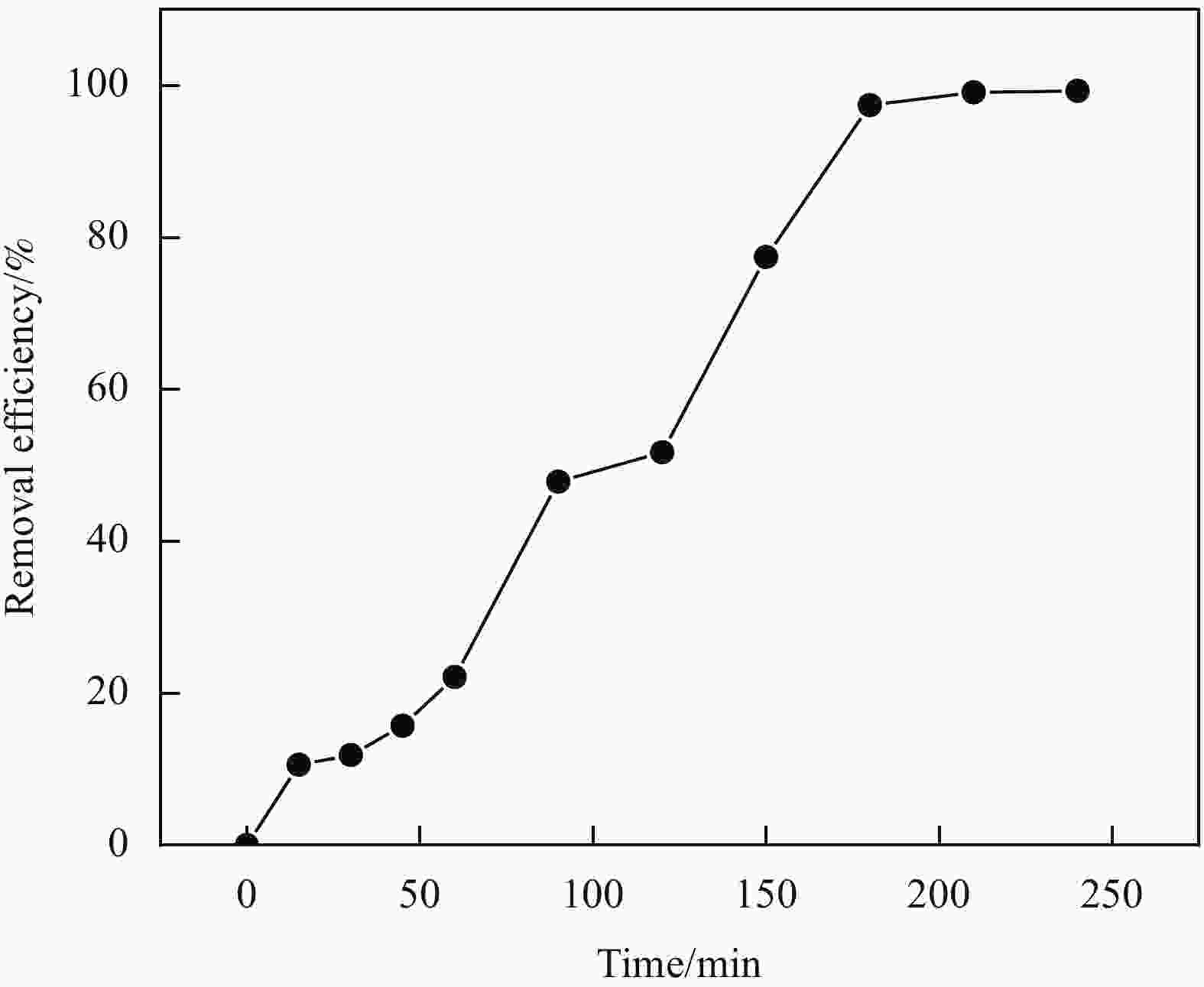
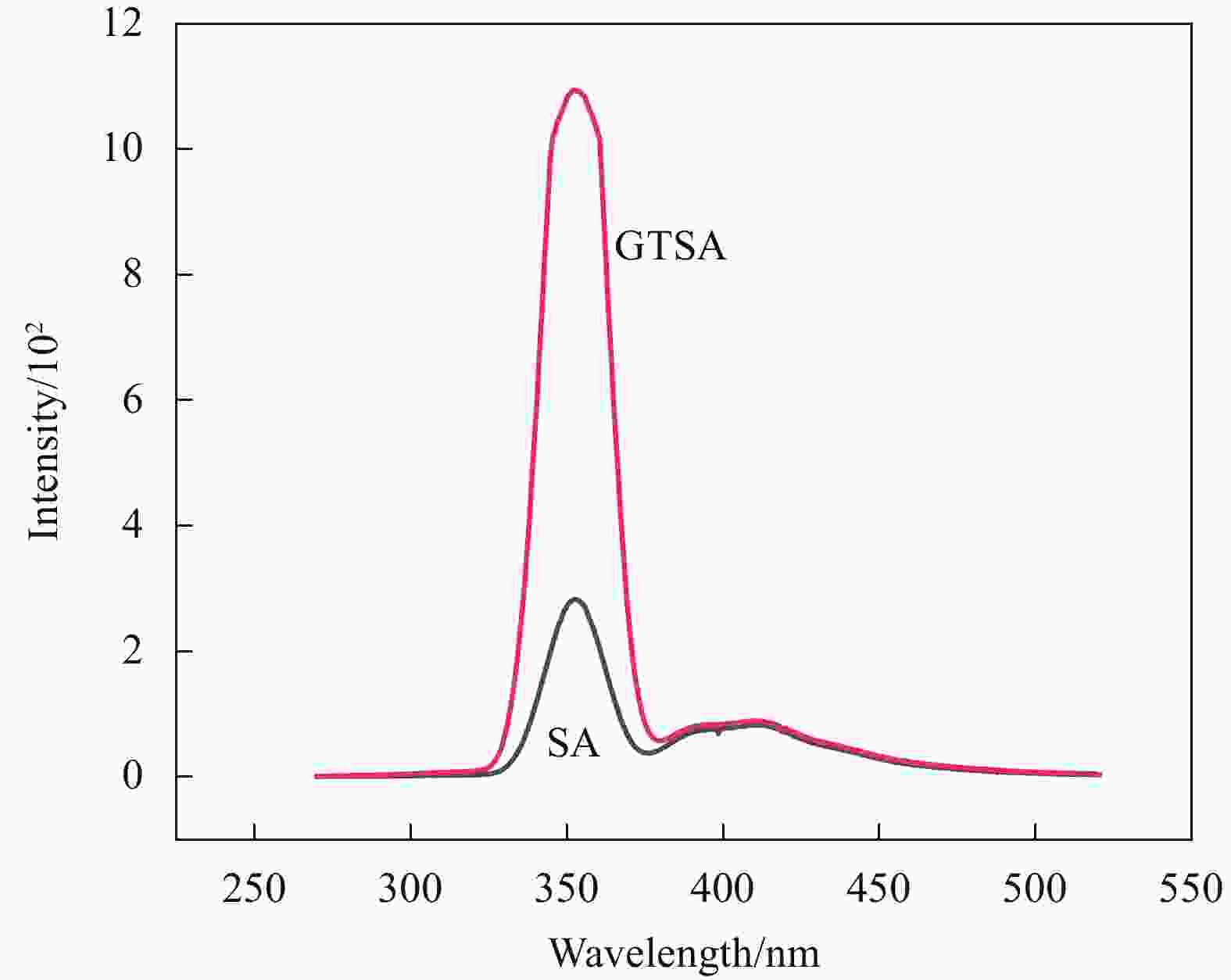
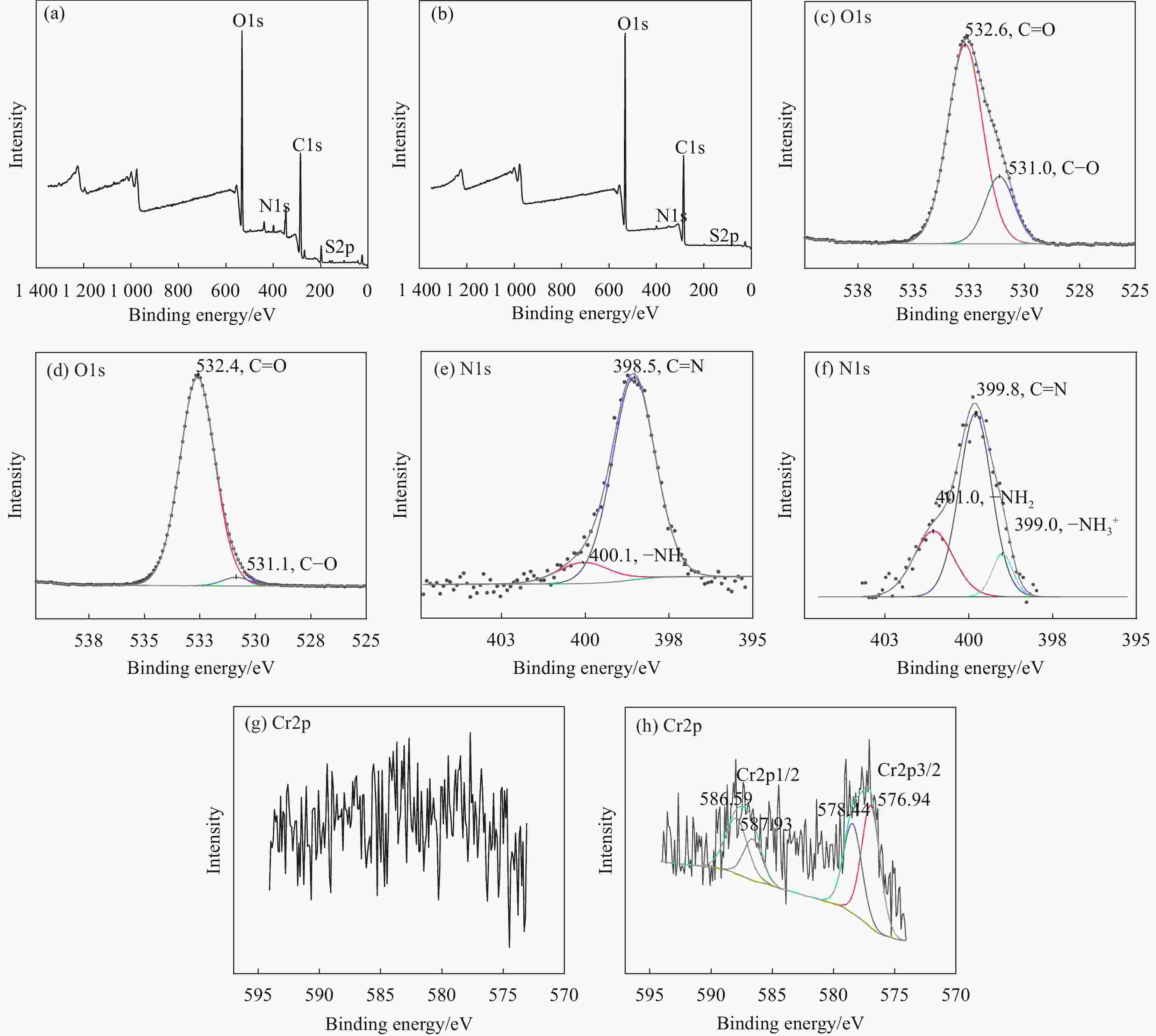
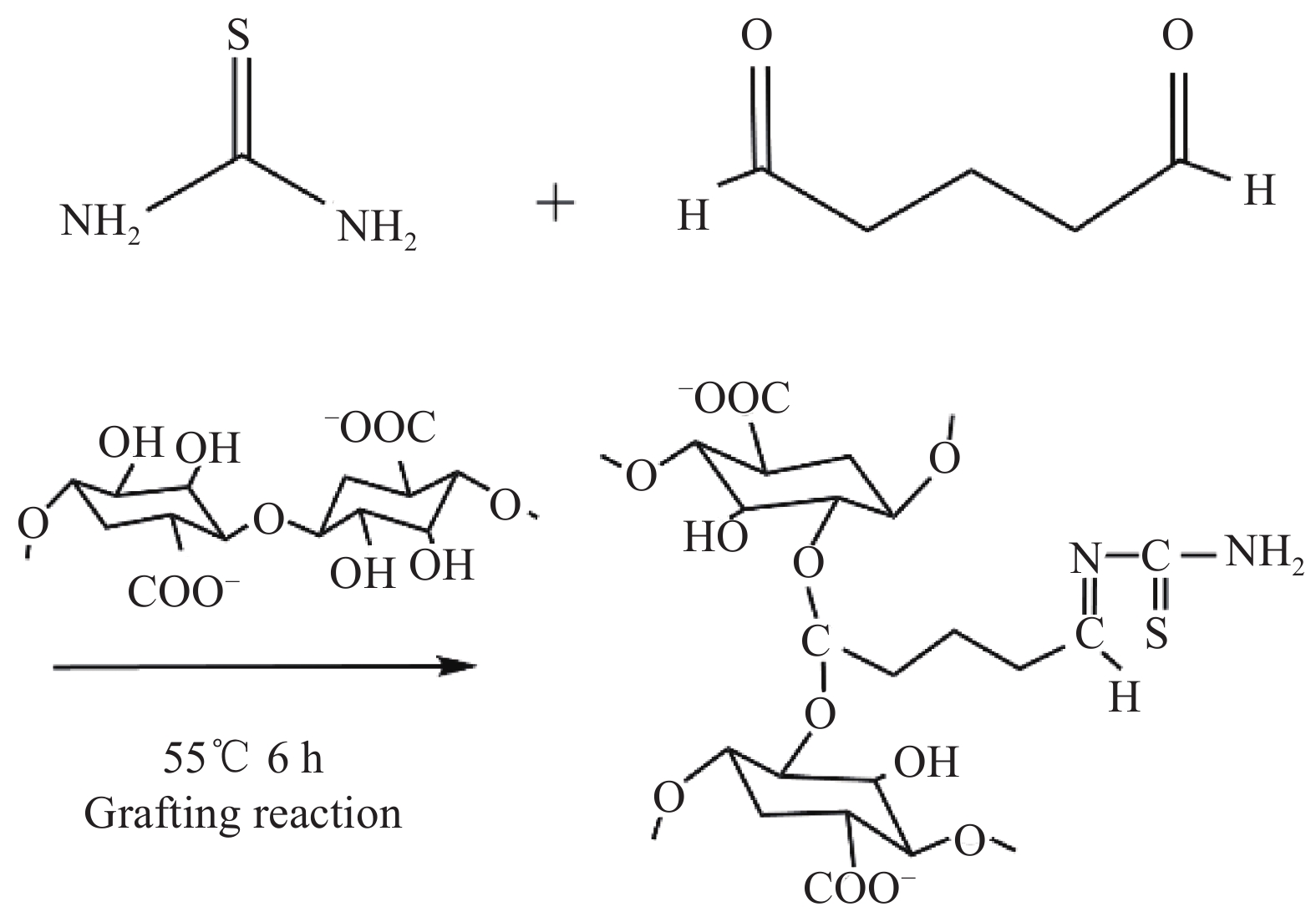
| [1] |
EDELSTEIN M, BEN-HUR M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops[J]. Scientia Horticulturae,2018,234:431-444. doi: 10.1016/j.scienta.2017.12.039
|
| [2] |
GODWIN P M, PAN Y, XIAO H, et al. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater[J]. Journal of Bioresources and Bioproducts,2019,4(1):31-42. doi: 10.21967/jbb.v4i1.180
|
| [3] |
SALCEDO, ANGEL, FRANCES. Removal of nickel by homogeneous granulation in a fluidized-bed reactor[J]. Chemosphere Environmental Toxicology & Risk Assessment,2016,164:59-67.
|
| [4] |
UZAŞÇI S, FILIZ T, BEDIA E F. Removal of hexavalent chromium from aqueous solution by barium ion cross-linked alginate beads[J]. International Journal of Environment,2014,18:1861-1868.
|
| [5] |
BARAKAT M A. New trends in removing heavy metals from industrial wastewater[J]. Arabian Journal of Chemistry,2011,4(4):361-377. doi: 10.1016/j.arabjc.2010.07.019
|
| [6] |
FU F, WANG Q. Removal of heavy metal ions from wastewater: A review[J]. Journal of Environmental Management,2011,92(3):407-418. doi: 10.1016/j.jenvman.2010.11.011
|
| [7] |
BORBA C E, GUIRARDELLO R, SILVA E A, et al. Removal of nickel(II) ions from aqueous solution by biosorption in a fixed bed column: Experimental and theoretical breakthrough curves[J]. Biochemical Engineering Journal,2006,30(2):184-191. doi: 10.1016/j.bej.2006.04.001
|
| [8] |
FARZANA M H, MEENAKSHI S. Photocatalytic aptitude of titanium dioxide impregnated chitosan beads for the reduction of Cr(VI)[J]. International Journal Macromolecules,2014,72:1265-1271.
|
| [9] |
孙俊芝, 王静霞, 倪茂君, 等. 改性海藻酸钠微球对 Pb(II)吸附性能的研究[J]. 环境科学与技术, 2019, 42(7):100-104, 110.SUN J Z, WANG J X, NI M J, et al. Adsorption of Pb(II) on modified sodium alginate microspheres[J]. Environmental Science and Technology,2019,42(7):100-104, 110(in Chinese).
|
| [10] |
周利民, 王一平, 黄群武, 等. 改性磁性壳聚糖对 Cu(II), Cd(II)和 Ni(II)的吸附性能[J]. 物理化学学报, 2007, 12:1979-1984. doi: 10.3866/PKU.WHXB20071228ZHOU L M, WANG Y P, HUANG Q W, et al. Adsorption of Cu(II), Cd(II) and Ni(II) by modified magnetic chitosan[J]. Journal of Physical Chemistry,2007,12:1979-1984(in Chinese). doi: 10.3866/PKU.WHXB20071228
|
| [11] |
蔡华敏, 韩巍, 蒋鑫, 等. 水中铬(Ⅵ)离子的去除研究进展[J]. 山东化工, 2020, 49(3):53-54, 56. doi: 10.3969/j.issn.1008-021X.2020.03.020CAI H M, HAN W, JIANG X, et al. Research progress in the removal of chromium (Ⅵ) ions from water[J]. Shandong Chemical Industry,2020,49(3):53-54, 56(in Chinese). doi: 10.3969/j.issn.1008-021X.2020.03.020
|
| [12] |
郭成, 高翔鹏, 李明阳, 等. 海藻酸钠基吸附材料去除水中重金属离子的研究进展[J]. 过程工程学报, 2021, 21(1):3-17. doi: 10.12034/j.issn.1009-606X.220059GUO C, GAO X P, LI M Y, et al. Research progress in the removal of heavy metal ions from water by sodium alginate based adsorbent[J]. Chinese Journal of Process Engineering,2021,21(1):3-17(in Chinese). doi: 10.12034/j.issn.1009-606X.220059
|
| [13] |
SADIQ A, CHOUBEY A, BAJPAI A K, et al. Biosorption of chromium ions by calcium alginate nanoparticles[J]. Journal of the Chilean Chemical Society,2018,63(3):4077-4081. doi: 10.4067/s0717-97072018000304077
|
| [14] |
张雪彦, 金贵锋, 刘贵峰, 等. 重金属离子吸附材料的研究进展[J]. 生物质化学工程, 2017, 51(1):51-58. doi: 10.3969/j.issn.1673-5854.2017.01.009ZHANG X Y, JIN G F, LIU G F, et al. Research progress of heavy metal ion adsorption materials[J]. Biomass Chemical Engineering,2017,51(1):51-58(in Chinese). doi: 10.3969/j.issn.1673-5854.2017.01.009
|
| [15] |
ZHOU C, NI J, ZHANG D, et al. Cellulosic adsorbent functionalized with macrocyclic pyridone pentamer for selectively removing metal cations from aqueous solutions[J]. Carbohydrate Polymers,2019,217:1-5. doi: 10.1016/j.carbpol.2019.04.048
|
| [16] |
VAKILI M, DENG S, CAGNETTA G, et al. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review[J]. Separation and Purification Technology,2019,224:373-387. doi: 10.1016/j.seppur.2019.05.040
|
| [17] |
ZHANG X, LIN X Y, HE Y, et al. Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment[J]. International Journal of Biological Macromolecules,2019,124:418-428. doi: 10.1016/j.ijbiomac.2018.11.218
|
| [18] |
WANG Y, LI Y, LIU S L, et al. Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent[J]. Carbohydrate Polymers,2015,120:53-59. doi: 10.1016/j.carbpol.2014.12.005
|
| [19] |
GAO X P, GUO C, HAO J J, et al. Selective adsorption of Pd (II) by ion-imprinted porous alginate beads: Experimental and density functional theory study[J]. International Journal of Biological Macromolecules,2020,157:401-413. doi: 10.1016/j.ijbiomac.2020.04.153
|
| [20] |
SANDIP S, ANJALI P, SUBRATA K, et al. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction[J]. Journal of Surfaces and Colloids,2010,26(4):2885-2893.
|
| [21] |
YU C L, WANG S L. Chromium(VI) reactions of polysaccharide biopolymers[J]. Chemical Engineering Journal,2012,181:479-485.
|
| [22] |
王磊, 白成玲, 朱振亚. 氧化石墨烯/海藻酸钠复合膜对Pb(Ⅱ)的吸附性能和机制[J]. 复合材料学报, 2020, 37(3):681-689.WANG L, BAI C L, ZHU Z Y. Adsorption of Pb(Ⅱ) by graphene oxide/sodium alginate composite membrane[J]. Acta Materialia Compositae Sinica,2020,37(3):681-689(in Chinese).
|
| [23] |
包炳钦, 张军, 宋卫锋, 等.磁性复合凝胶球对Pb(Ⅱ)的吸附特性与机制[J].复合材料学报, 2021, 38(6): 1929-1938.BAO B Q, ZHANG J, SONG W F, et al. Magnetic composite gel ball on the adsorption characteristics and mechanism of Pb (Ⅱ)[J]. Acta Materiae Compositae Sinica, 2021, 38(6): 1929-1938(in Chinese).
|
| [24] |
黄攀丽, 沈晓骏, 陈京环, 等. 海藻酸钠的提取与功能化改性研究进展[J]. 林产化学与工业, 2017, 37(4):13-22. doi: 10.3969/j.issn.0253-2417.2017.04.002HUANG P L, SHEN X J, CHEN J H, et al. Progress in extraction and functionalization of sodium alginate[J]. Che-mistry and Industry of Forest Products,2017,37(4):13-22(in Chinese). doi: 10.3969/j.issn.0253-2417.2017.04.002
|
| [25] |
于长江, 董心雨, 王苗, 等. 海藻酸钙/生物炭复合材料的制备及其对 Pb(Ⅱ)的吸附性能和机制[J]. 环境科学, 2018, 39(8):3719-3728.YU C J, DONG X Y, WANG M, et al. Preparation of calcium alginate/biochar composite and its adsorption perfor-mance and mechanism for Pb(Ⅱ)[J]. Environmental Science,2018,39(8):3719-3728(in Chinese).
|
| [26] |
姚温浩, 于飞, 马杰. 海藻酸盐复合凝胶吸附材料的合成及其在水处理中的应用[J]. 化学进展, 2018, 30(11):1722-1733.YAO W H, YU F, MA J. Synthesis of alginate composite gel adsorbent and its application in water treatment[J]. Progress in Chemistry,2018,30(11):1722-1733(in Chinese).
|
| [27] |
SHEN W, AN Q, XIAO Z Y, et al. Alginate modified graphitic carbon nitride composite hydrogels for efficient removal of Pb(II), Ni(II) and Cu(II) from water[J]. International Journal of Biological Macromolecules,2020,148:1298-1306. doi: 10.1016/j.ijbiomac.2019.10.105
|
| [28] |
郭成, 郝军杰, 李明阳, 等. 海藻酸钠/聚乙烯亚胺凝胶球的合成及对Cr (VI)的吸附性能和机制[J]. 复合材料学报, 2021, 38(7):2140-2151.GUO C, HAO J J, LI M Y, et al. Adsorption of Cr(VI) on polyethyleneimine grafted porous sodium alginate beads and its mechanistic study[J]. Acta Materiae Compositae Sinica,2021,38(7):2140-2151(in Chinese).
|
| [29] |
WANG Z Q, WU S, ZHANG Y, et al. Preparation of modified sodium alginate aerogel and its application in removing lead and cadmium ions in wastewater[J]. International Journal of Biological Macromolecules,2020,157:687-694. doi: 10.1016/j.ijbiomac.2019.11.228
|
| [30] |
SOLOMONS T G. Organic chemistry[M]. New York: Wiley Press Inc, 1980: 703-714.
|
| [31] |
杨庆, 梁伯润, 窦丰栋, 等. 以乙二醛为交联剂的壳聚糖纤维交联机理探索[J]. 纤维素科学与技术, 2005, 30(3):13-20.YANG Q, LIANG B R, DOU F D, et al. Study on crosslinking mechanism of chitosan fiber with glyoxal as crosslinking agent[J]. Cellulose Science and Technology,2005,30(3):13-20(in Chinese).
|
| [32] |
国家环境保护局. 水质六价铬的测定二苯碳酰二肼分光光度法: GB 7467—1987[S]. 北京: 中国标准出版社, 1987.State Department of Environmental Conservation. Water quality: Determination of chromium(Ⅵ): 1, 5-diphenylcar-bohydrazide spectrophotometric method: GB 7467—1987[S]. Beijing: China Standards Press, 1987(in Chinese).
|
| [33] |
PRIYABRAT M, SANTOSH K S, KULAMANI P. Photocatalytic reduction of hexavalent chromium in aqueous solution over sulphate modified titania[J]. Photochemistry and Photobiology A,2005,170:189-194. doi: 10.1016/j.jphotochem.2004.08.012
|
| [34] |
YANG J K, LEE S M, FARROKHI M. Photocatalytic removal of Cr(VI) with illuminated TiO2[J]. Desalination and Water Treatment,2013,46:375-380.
|
| [35] |
THAKUR S, PANDEY S, AROTIBA O A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue[J]. Carbohydrate Polymers, 2016, 153: 34–46.
|
| [36] |
GAO X, ZHANG Y, ZHAO Y, et al. Zinc oxide templating of porous alginate beads for the recovery of gold ions[J]. Polymers,2018,200:297-304.
|
| [37] |
OOMENS J, STEILL J D. Free carboxylate stretching modes[J]. Physical Chemistry,2008,112:3281-3283.
|
| [38] |
GAO X, LIU J, LI M, et al. Mechanistic study of selective adsorption and reduction of Au (III) to gold nanoparticles by ion-imprinted porous alginate microspheres[J]. Chemical Engineering Journal,2020,385:123897.
|
| [39] |
GAO X, ZHANG Y, ZHAO Y. Biosorption and reduction of Au (III) to gold nanoparticles by thiourea modified alginate[J]. Carbohydrate Polymers,2017,159:108-115. doi: 10.1016/j.carbpol.2016.11.095
|
| [40] |
RAO C N R, VENKATARAGHAVAN R. The C=S stretching frequency and the “—N—C= S bands” in the infrared[J]. Spectrochimica Acta Part A: Molecular Spectroscopy,1962,18:541-547. doi: 10.1016/S0371-1951(62)80164-7
|
| [41] |
GAVILAN K, PESTOV A V, GARCIA H M, et al. Mercury sorption on a thiocarbamoyl derivative of chitosan[J]. Journal of Hazardous Materials,2009,165:415-426. doi: 10.1016/j.jhazmat.2008.10.005
|
| [42] |
ZHU D D, ZHOU Q X. Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic activities under visible light[J]. Applied Catalysis: Environmental,2021,281:119474.
|
| [43] |
YANG X L, QIAN F F, ZOU G J, et al. Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation[J]. Applied Catalysis: Environmental,2016,193:22-35. doi: 10.1016/j.apcatb.2016.03.060
|
| [44] |
曾雄丰, 王梦幻, 王建省, 等.TiO2/石墨烯夹层结构复合材料的制备及光催化性能[J].复合材料学报, 2022, 39(2): 656-663.ZENG X F, WANG M H, WANG J S, et al. Preparation and photocatalytic performance of TiO2/graphene laminated composite[J]. Acta Materialia Compositae Sinica, 2022, 39(2): 656-663(in Chinese).
|
| [45] |
OCINSKI D, JACUKOWICZACV S I, KOCLOEK B E. Alginate beads containing water treatment residuals for arsenic removal from water-formation and adsorption studies[J]. Environmental Science and Pollution Research,2016,23(24):24527-24539. doi: 10.1007/s11356-016-6768-0
|
| [46] |
TIAN X, WANG W, WANG Y, et al. Polyethylenimine functionalized halloysite nanotubes for efficient removal and fixation of Cr(VI)[J]. Microporous and Mesoporous Materials,2015,207:46-52. doi: 10.1016/j.micromeso.2014.12.031
|
| [47] |
KOUSALYA G N, RAJIV M, MEENAKSHI S. Removal of toxic Cr(VI) ions from aqueous solution using nanohydroxyapatite-based chitin and chitosan hybrid compo-sites[J]. Adsorption Science and Technology,2010,28:49-64. doi: 10.1260/0263-6174.28.1.49
|
| [48] |
李小燕, 何登武, 李冠超, 等. Bi2O3-Bi2WO6直接Z-Scheme异质结的制备、表征及光催化还原U(VI)的性能[J].复合材料学报, 2021, 38(8): 2646-2654.LI X Y, HE D W, LI G C, et al. Bi2O3-Bi2WO6 directly Z-scheme heterojunction and photocatalytic reduction of U(VI) under visible light irradiation[J]. Acta Materiae Compositae Sinica, 2021, 38(8): 2646-2654(in Chinese).
|
| [49] |
CHAKRABARTI S, CHAUDHURI B, BHATTACHARJEE S, et al. Photo-reduction of hexavalent chromium in aqueous solution in the presence of zinc oxide as semiconductor catalyst[J]. Chemical Engineering Journal,2009,153:86-93. doi: 10.1016/j.cej.2009.06.021
|





 下载:
下载: