Study on paraffin modifying inorganic composite phase change heat storage system
-
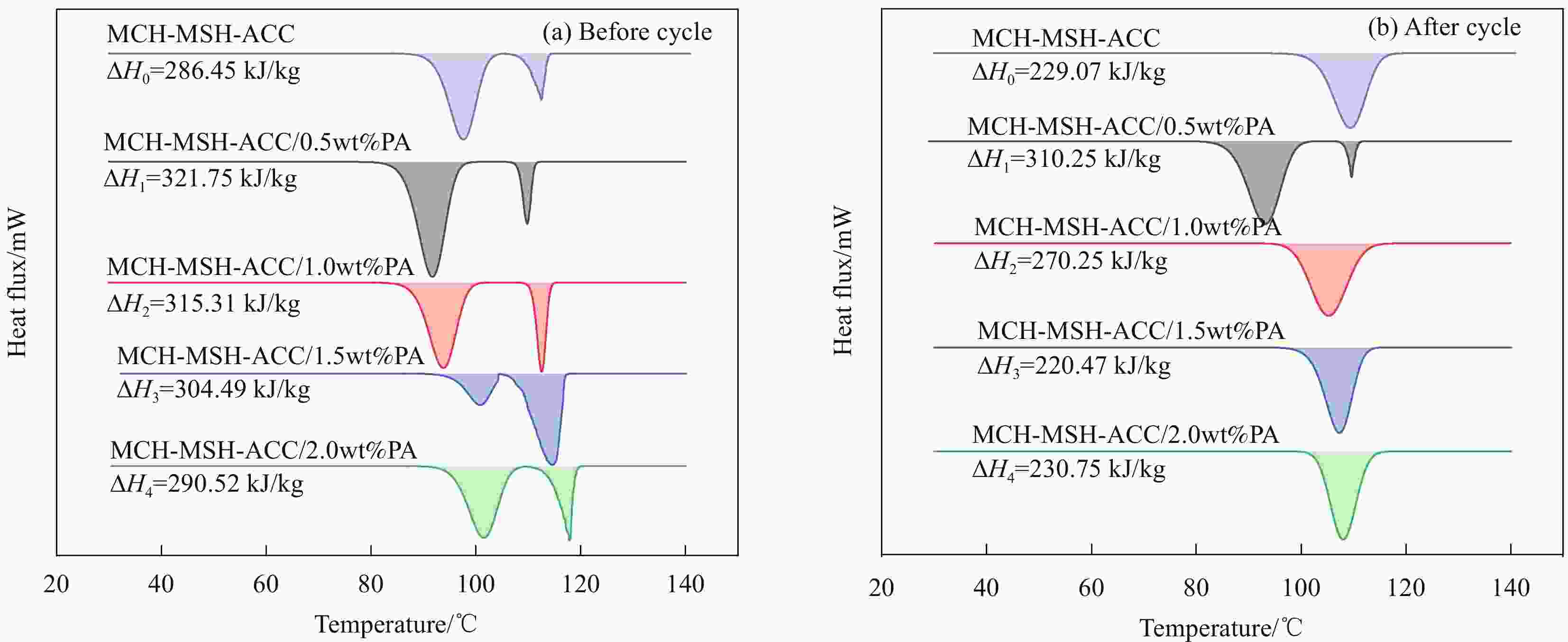
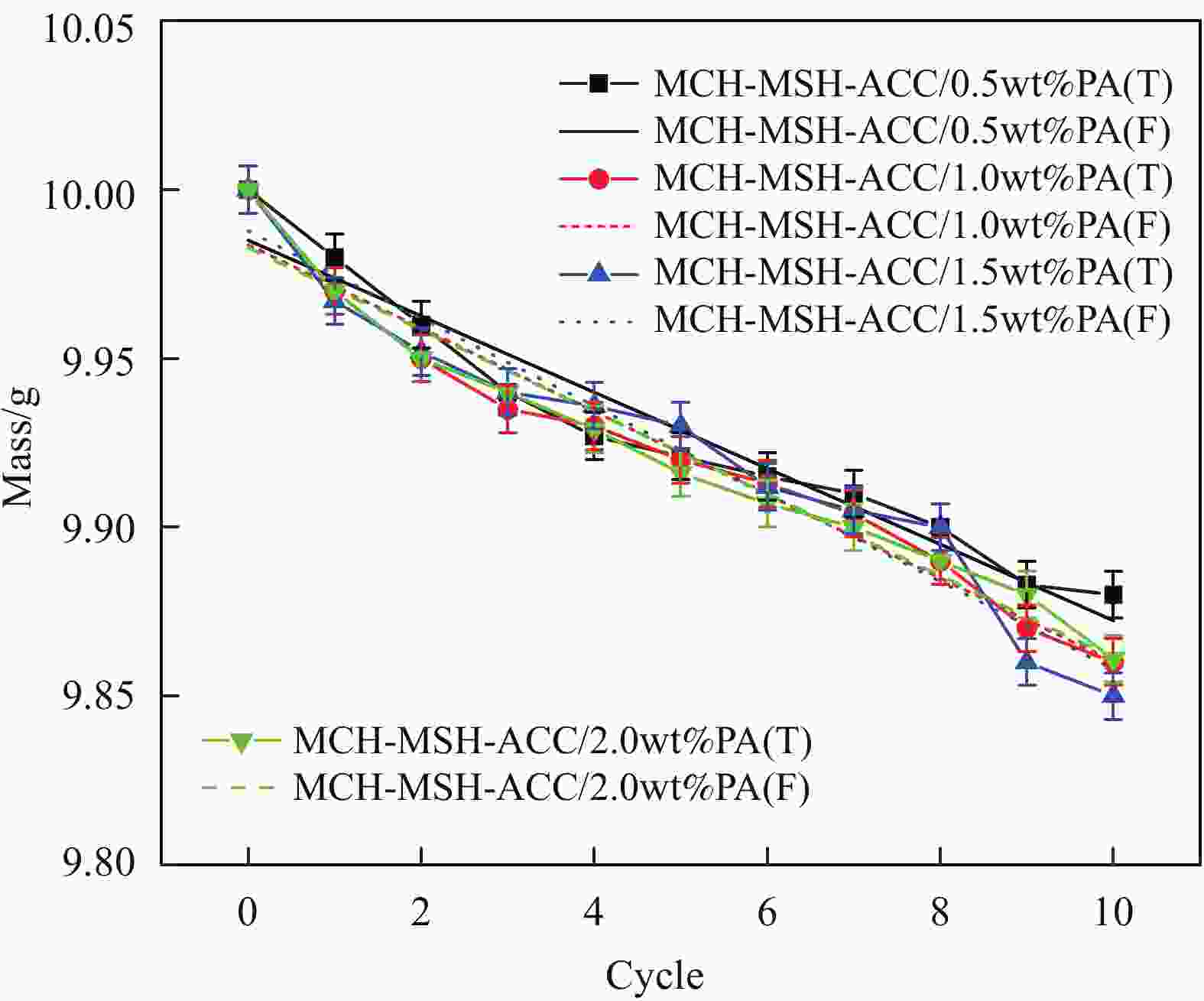
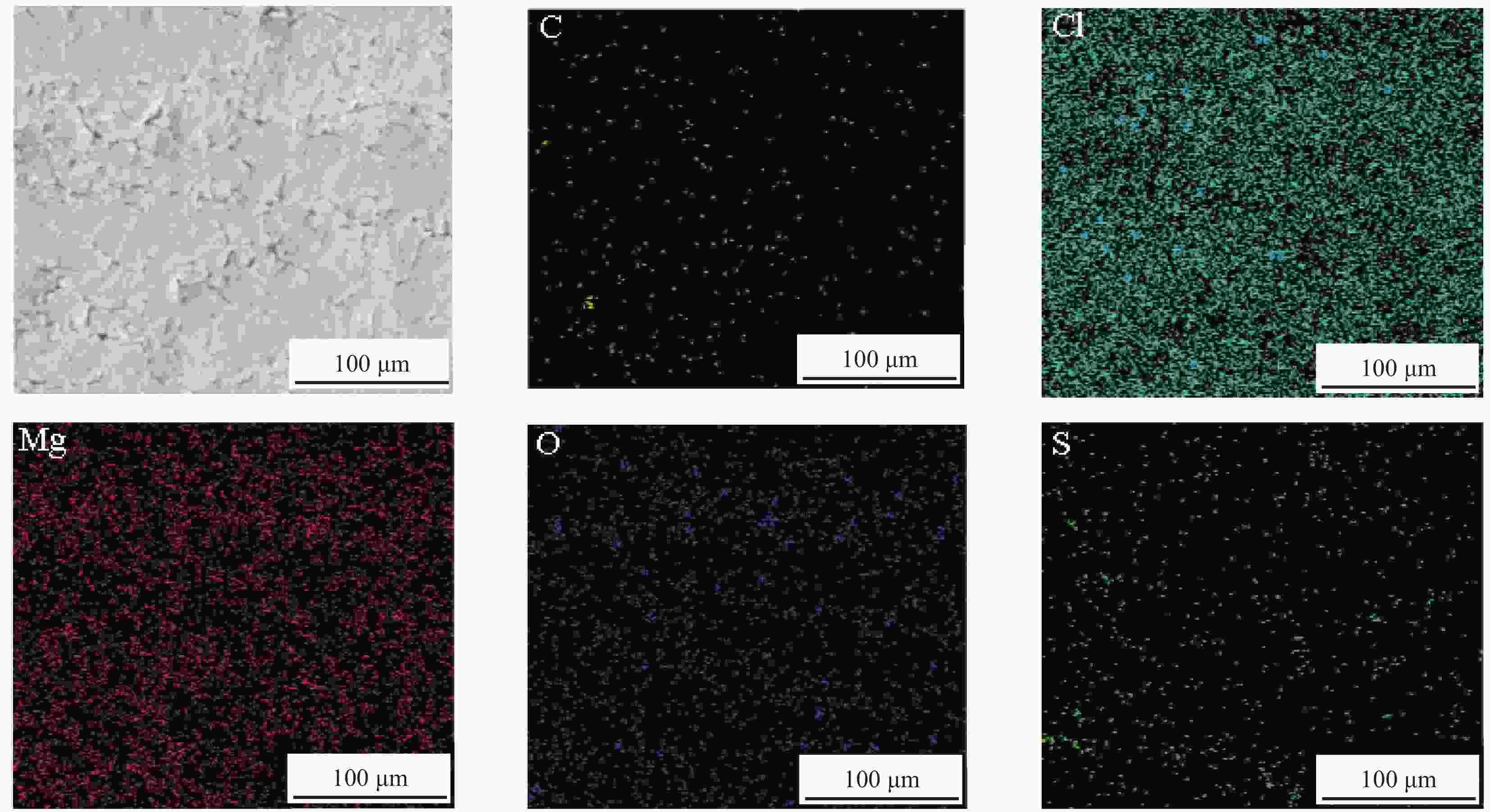
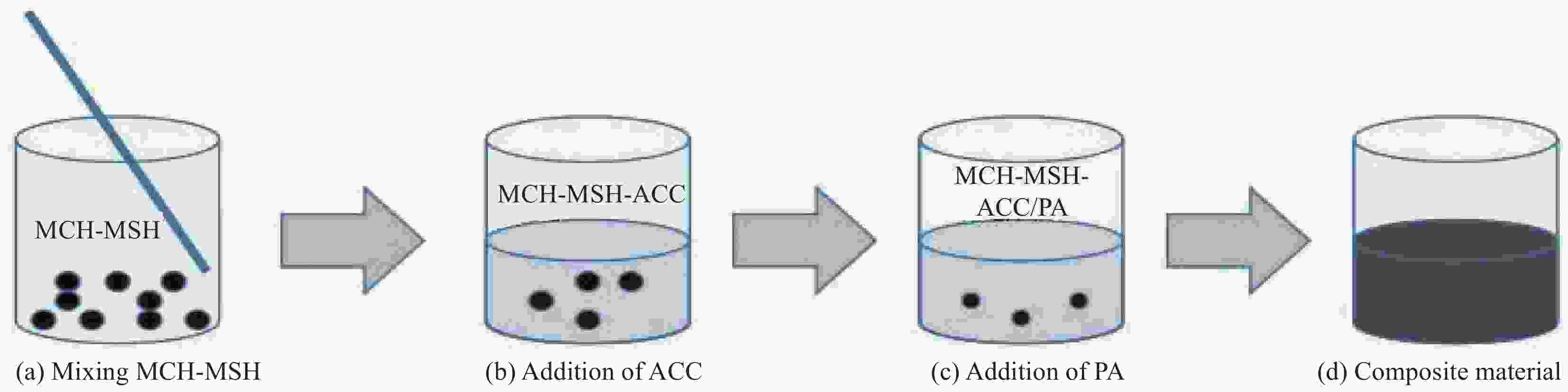
摘要: 水合盐相变储热材料普遍存在的过冷和相分离现象是影响其热稳定性和热性能的关键问题。以中低温水合盐相变储热材料MgCl2∙6H2O(MCH)和MgSO4∙7H2O(MSH)为研究对象,以活性炭(ACC)为添加剂,采用熔融共混法制备了MCH-MSH-ACC混合体系,并以石蜡(PA)为调节剂,制备了MCH-MSH-ACC/PA复合材料相变体系。研究了PA对复合相变体系的相变焓、相变温度、过冷度及相分离的影响。结果表明:微量PA的添加有助于提升MSH-MCH-ACC/PA体系的相变储热性能,和其他PA含量体系相比较,PA含量为0.5wt%的体系在储热阶段所需时间最短,而放热阶段持续时间最长,其初始相变焓值可达到321.75 kJ/kg,循环试验后相变焓稳定在310.25 kJ/kg。所制备的新型共混MSH-MCH-3wt%ACC/0.5wt%PA复合相变体系具有良好的储热性能和循环稳定性能。Abstract: The supercooling and phase separation of hydrated salt phase change heat storage materials are two key issues which affect its thermal stability and thermal performance. MgCl2∙6H2O (MCH) and MgSO4∙7H2O (MSH) are used as the research materials, which are the phase change heat storage materials at moderate and low tempera-tures, and activated carbon (ACC) is used as the additive, The MCH-MSH-ACC composite phase change system was prepared by the melt blending method, then, the MCH-MSH-ACC/PA composite phase change system is prepared with paraffin (PA) as the modifier. The effects of PA on the phase change enthalpy, phase change temperature, subcooling degree and phase separation phenomenon of the composite phase change materials were studied. The re-sults show that the addition of trace PA is beneficial to improve the phase change heat storage performance of the MSH-MCH-ACC/PA system. Compared with other PA content systems, the system with 0.5wt% PA perform the shortest time in the heat storage stage and the maximum heat release time in the exothermic phase, whose initial phase change enthalpy value reach 321.75 kJ/kg, and the stable enthalpy is 310.25 kJ/kg after the cycle tests. The prepared new blend MSH-MCH-3wt%ACC/0.5wt%PA composite material has good heat storage property and good thermal cycle stability.
-
Key words:
- composite material /
- heat storage property /
- subcooling degree /
- thermal cycle stability /
- paraffin
-
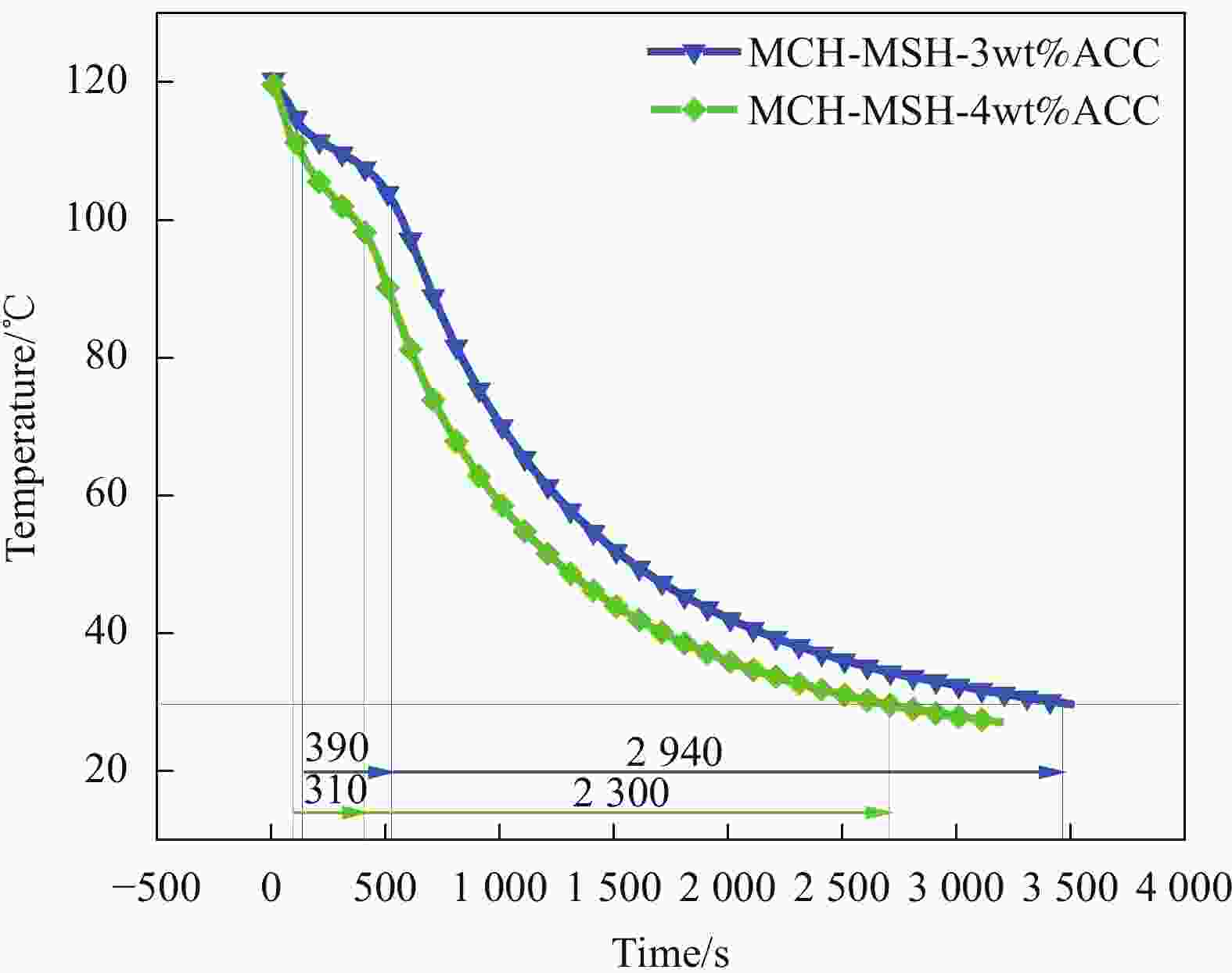
表 1 不同ACC浓度下MCH-MSH-ACC系统的过冷度数值
Table 1. Subcooling value of the MCH-MSH-ACC system under different ACC concentrations
Sample Undercooling/℃ MCH-MSH 6.63 MCH-MSH-1wt%ACC 4.74 MCH-MSH-2wt%ACC 1.12 MCH-MSH-3wt%ACC 0 MCH-MSH-4wt%ACC 0 表 2 不同PA含量的MCH-MSH-ACC/PA复合材料的相变特性
Table 2. Phase change properties of MCH-MSH-ACC/PA composite PCMs with different PA contents
Condition Sample Onset temperature
Tm/℃Peak temperature
Tp/℃End temperature
Te/℃Before cycle MCH-MSH-ACC 88.87 97.82 115.98 MCH-MSH-ACC/0.5wt%PA 82.16 91.78 111.96 MCH-MSH-ACC/1.0wt%PA 85.30 93.29 115.31 MCH-MSH-ACC/1.5wt%PA 93.11 100.69 117.33 MCH-MSH-ACC/2.0wt%PA 92.07 101.03 119.82 After cycle MCH-MSH-ACC 98.46 109.77 117.14 MCH-MSH-ACC/0.5wt%PA 83.62 93.15 111.70 MCH-MSH-ACC/1.0wt%PA 96.12 105.17 115.21 MCH-MSH-ACC/1.5wt%PA 98.83 107.17 114.30 MCH-MSH-ACC/2.0wt%PA 101.36 108.01 115.11 -
[1] DINCER I, DOST S, LI X G. Thermal energy storage applications from an energy saving perspective[J]. International Journal of Global Energy Issues,1997,9(4-6):351-364. [2] PUTRA N, RAWI S, MMAD M A, et al. Preparation of beeswax/multi-walled carbon nanotubes as novel shape- stable nanocomposite phase-change material for thermal energy storage[J]. Journal of Energy Storage,2019,21(2):32-39. [3] SWA B, TYA B, ZKA B, et al. Corrigendum to: “Thermal conductivity enhancement on phase change materials for thermal energy storage: A review”[J]. Energy Storage Materials,2020,33(6):88-97. [4] XU S Z, LEMINGTON, WANG R Z, et al. A zeolite 13X/magnesium sulfate-water sorption thermal energy storage device for domestic heating[J]. Energy Conversion and Management,2018,17:98-109. [5] MOHAN G, MAHESH B V, VIDAL J G, et al. Assessment of a novel temary eutectic chloride salt for next generation high-temperature sensible heat storage[J]. Energy Conversion and Management,2018,167:156-164. doi: 10.1016/j.enconman.2018.04.100 [6] WANG Yan, YU Kaixiang, HAO Peng, et al. Preparation and thermal properties of sodium acetate trihydrate as a novel phase change material for energy storage[J]. Energy,2019,167(10):269-274. [7] XIE Baoshan, LI Chuanchang, ZHANG Bo, et al. Evaluation of stearic acid/coconut shell charcoal composite phase change thermal energy storage materials for tankless solar water heater[J]. Energy and Built Environment,2020,1(2):187-198. doi: 10.1016/j.enbenv.2019.08.003 [8] FERCHAUD C J, ZONDAG H A, VELDHUI J B, et al. Study of the reversible water vapour sorption process of MgSO4·7H2O and MgCl2·6H2O under the conditions of seasonal solar heat storage[J]. Journal of Physics Conference,2012,395(1):91-95. [9] POSERN K, KAPS C. Calorimetric studies of thermochemical heat storage material based on mixtures of MgSO4 and MgCl2 [J]. Thermochimica Acta,2010,502(1-2):73-76. doi: 10.1016/j.tca.2010.02.009 [10] JAB BA RI-HICHRI A, BENNICI S, UROUX A A. Water sorption heats on silica-alumina-based composites for interseasonal heat storage[J]. Journal of Thermal Analysis and Calorimetry,2014,118(2):1111-1118. doi: 10.1007/s10973-014-3886-0 [11] SUGIMOTO K, DINNEBIER R E, HANSON J C. Structures of three dehydration products of bischofite from in situ synchrotron powder diffraction data (MgCl2·nH2O; n=1, 2, 4)[J]. Acta Crystallographica Section B Structural Science,2010,63(2):235-242. [12] SHKATUOV A, RYU J, KATO Y, et al. Composite material “Mg(OH)2/vermiculite”: A promising new candidate for storage of middle temperature heat[J]. Energy,2012,44(1):1028-1034. doi: 10.1016/j.energy.2012.04.045 [13] CAO Yufeng, FAN Dongli, LIN Shaohui, et al. Phase change materials based on comb-like polynorbornenes and octadecylamine-functionalized graphene oxide nanosheets for thermal energy storage[J]. Chemical Engineering Journal,2020,389:124318. doi: 10.1016/j.cej.2020.124318 [14] RAN Xiaofeng, WANG Haoran, ZHONG Yajuan, et al. Thermal properties of eutectic salts/ceramics/expanded graphite composite phase change materials for high-temperature thermal energy storage[J]. Solar Energy Materials and Solar Cells,2021,225:111047. doi: 10.1016/j.solmat.2021.111047 [15] 杨希贤, 黄宇宏, 王智辉, 等. 碳纳米/氢氧化锂复合材料的低温化学蓄热性能研究[J]. 工程热物理学报, 2016, 37(12):2512-2516.YANG Xixian, HUANG Hongyu, WANG Zhihui, et al. Study on low temperature chemical heat storage properties of carbon nanometer/lithium hydroxide composites[J]. Journal of Engineering Thermophysics,2016,37(12):2512-2516(in Chinese). [16] YU N, WANG R Z, LU Z S, et al. Study on consolidated composite sorbents impregnated with LiCl for thermal energy storage[J]. International Journal of Heat and Mass Transfer,2015,84:660-670. doi: 10.1016/j.ijheatmasstransfer.2015.01.065 [17] 马晓春, 刘延君, 刘函, 等. 石蜡@TiO2/CNTs复合相变材料制备及其热物性[J]. 浙江工业大学学报, 2020, 48(1):85-89. doi: 10.3969/j.issn.1006-4303.2020.01.014MA Xiaochun, LIU Yanjun, LIU Han, et al. Preparation and thermal properties of paraffin@TiO2/CNTs composite phase change materials[J]. Journal of Zhejiang University of Technology,2020,48(1):85-89(in Chinese). doi: 10.3969/j.issn.1006-4303.2020.01.014 [18] HOLGER U, RAMMELBERG, THOMAS O, et al. Thermochemical heat storage materials-performance of mixed salt hydrates[J]. Solar Energy,2016,136(10):571-589. [19] 满亚辉. 相变潜热机理及其应用技术研究[D]. 长沙: 国防科学技术大学, 2010.MAN Yahui. Study on latent heat mechanism of phase change and its application technology[D]. Changsha: University of Defence Science and Technology, 2010(in Chinese). [20] 李玉婷, 周永全, 葛飞, 等. 无机水合盐相变储能材料的过冷及相分离研究进展[J]. 盐湖研究所, 2018, 26(1):81-86.LI Yuting, ZHOU Yongquan, GE Fei, et al. Research progress on supercooling and phase separation of inorganic hydrate phase change energy storage materials[J]. Salt Lake Institute,2018,26(1):81-86(in Chinese). [21] 赵长颖, 潘智豪, 王倩, 等. 多孔介质的相变和热化学储热性能[J]. 科学通报, 2016, 61(17):1897-1911.ZHAO Changying, PAN Zhihao, WANG Qin, et al. Phase transition and thermochemical thermal storage properties of porous media[J]. Scientific Bulletin,2016,61(17):1897-1911(in Chinese). [22] 卢竼漪, 侯峰, 徐贵钰. 碳纳米管-无机盐复合成核剂对MgCl2·6H2O-CaCl2·6H2O体系储热性能的影响[J]. 稀有金属材料与工程, 2018, 47(S1):283-287.LU Fanyi, HOU Feng, XU Guiyu. The effect of carbon nano-tube-inorganic salt composite nucleating agent on the heat storage performance of MgCl2·6H2O-CaCl2·6H2O system[J]. Rare Metal Materials and Engineering,2018,47(S1):283-287(in Chinese). [23] WU Shaofei, YAN Ting, KUAI Zihan, et al. Experimental and numerical study of modified expanded graphite/hydrated salt phase change material for solar energy storage[J]. Solar Energy,2020,205:474-486. doi: 10.1016/j.solener.2020.05.052 [24] 吴东灵, 李廷贤, 何峰, 等. 三水醋酸钠相变储能复合材料改性制备及储/放热特性[J]. 化工学报, 2018, 69(7):2860-2868.WU Dongling, LI Tingxian, HE Feng, et al. Preparation of modified storage/exothermic properties of sodium acetate phase change energy storage composites[J]. Journal of Chemical Engineering,2018,69(7):2860-2868(in Chinese). [25] 李敬会, 姜贵文, 黄菊花. 铝蜂窝增强膨胀石墨/石蜡复合材料的制备和性能研究[J]. 化工新型材料, 2018, 46: 551(8): 95-98.LI Jinghui, JIANG Guiwen, HUANG Juhua. Preparation and properties of aluminum honeycomb reinforced expanded graphite/paraffin composites[J]. New Chemical Materials, 2018, 46: 51(8): 95-98(in Chinese). [26] 赵康. 复合式相变蓄热建筑围护结构的构建及节能效果研究[D] 锦州: 辽宁工业大学, 2018.ZHAO Kang. Construction and energy saving effect of complex phase change thermal storage building enclosure[D]. Jinzhou: Liaoning University of Technology, 2018(in Chinese). [27] BO Yu, LAN Xiang. Composition and morphology of the thermal decomposition products of 3Mg(OH)2·MgCl2·8H2O nanowires[J]. Particuology, 2017, 30(1): 129-134. [28] LELE A F, KUZNIK F, OPEL O, et al. Performance analysis of a thermochemical based heat storage as an addition to cogeneration systems[J]. Energy Conversion and Management,2015,106:1327-1344. doi: 10.1016/j.enconman.2015.10.068 -





 下载:
下载: