Basic scientific problems of Ni rich cathode materials for Li-ion battery: Surface residual Li and its removal
-
摘要: 层状富Ni正极材料具有高可逆容量、低自放电性能和低成本优势,被认为是最有潜力的Li+电池正极材料。然而,材料结构不稳定、容量衰退和安全性差等问题,阻碍了富Ni正极材料的实际应用。当Ni含量大于80%时,富镍正极易与空气中的水分和CO2反应,在材料表面生成Li2CO3、LiHCO3、LiOH等残Li化合物。残Li存在不仅导致材料不稳定和电化学性能衰退,还造成电池安全问题。本文首先综述了残Li化合物的形成机制及其危害,再探讨了水洗过程中的水洗温度、时间、干燥温度等因素对材料性能的影响,并阐述了水洗造成结构衰退和容量衰减的机制。此外,还论述了其他去除残Li化合物的方法,特别是无水洗表面包覆的方法在去除残Li化合物影响方面呈现出巨大应用潜力。Abstract: The layered Ni-rich cathode materials are considered as the most promising cathode materials for Li-ion batteries due to their high reversible capacity, low self-discharge performance and low cost. However, they have some disadvantages, such as the unstable material structure, capacity decay and poor safety, hindered their practical application. The Ni-rich cathode materials with nickel content over 80% are easy to react with moisture and CO2 in the air, generating residual Li compounds such as Li2CO3, LiHCO3 and LiOH on the surface of materials. The presence of residual Li not only leads to structure instability and electrochemical performance degradation, but also causes battery safety problems. In this paper, the mechanism of the formation of residual Li and its hazards are reviewed. Then, the effect of factors (e.g. washing temperature, time, drying temperature, etc.) during water washing on the material property are discussed, and the mechanisms of structural deterioration and capacity degradation induced by water washing are elaborated. Lastly, other methods for removing residual Li compounds are introduced, especially the non-washing surface coating method, which shows great application potential in removing the influence of residual Li compounds.
-
Key words:
- nanomaterials /
- preparation /
- surface /
- Ni-rich cathode /
- residual lithium /
- washing /
- Li-ion battery
-
图 2 未处理和包覆Li2CO3、LiF涂层LiNi0.8Co0.1Mn0.1O2的首周放电容量 (a) 和200周循环性能 (b)[49]、碳酸锂对富镍正极首周充放电过程相转变的影响 (c)[50]、富镍正极材料产生气体机制 (d)[34]
Figure 2. Fresh, Li2CO3-coated and LiF-coated LiNi0.8Co0.1Mn0.1O2 first cycle discharge capacity (a) , the cycle performance during 200 cycles (b)[49], effects of Li2CO3 on the phase transition of Ni-rich cathode during first cycle (c)[50]and gas generation mechanism of nickel-rich cathode materials (d)[34]
图 3 水洗时间为0 min (a)、5 min (b)、15 min (c) 及30 min (d) 的LiNi0.83Co0.13Mn0.04O2的SEM图像[60];不同水洗时间的LiNi0.83Co0.13Mn0.04O2的XRD图谱 (e) 和循环50次的容量保持率 (f)[60]
Figure 3. SEM images of LiNi0.83Co0.13Mn0.04O2 cathode after different washing time: 0 min (a), 5 min (b), 15 min (c) and 30 min (d)[60]; Powder XRD patterns (e) and capacity retention after 50 cycles for LiNi0.83Co0.13Mn0.04O2 after different washing time (f)[60]
图 4 未水洗 (a) 和材料与水比值为1 ∶ 0.7 (b)、1 ∶ 1 (c)、1 ∶ 2 (d) 1 ∶ 2及1 ∶ 5 (e) 的水洗涤后LiNi0.80Co0.15Mn0.05O2的SEM图像[23];未水洗和水洗后材料的循环性能 (f)[23];残锂量和容量保持率与洗涤用水量的关系 (g)[23];未水洗和经不同洗涤次数的LiNi0.83Co0.15Al0.02O2的充放电容量曲线 (h)[61]
Figure 4. SEM images of fresh (a) and washed LiNi0.80Co0.15Mn0.05O2 with ratio of material to water fixed about 1 ∶ 0.7 (b), 1 ∶ 1 (c), 1 ∶ 2 (d) and 1 ∶ 5 (e) [23]; Cycling performance of fresh and washed materials (f)[23]; Relationship between the residual lithium amount and capacity retention rate and the amount of water used for washing (g)[23]; Charge-discharge capacity curve of fresh and washed LiNi0.83Co0.15Al0.02O2 with different washing times (h)[61]
图 5 未处理 (a) 和水洗后不同干燥温度80 ℃ (b)、120 ℃ (c) 和190 ℃ (d) 材料的SEM图像[23];TGA-MS分析未处理和水洗两次LiNi0.85Co0.1Mn0.05O2随温度升高的质量损失和气体释放 (e)[62]
Figure 5. SEM images of fresh material (a) and washed and dried at 80 ℃ (b), 120 ℃ (c) and 190 ℃ (d) [23]; TGA-MS analysis of the mass loss and gas release of fresh and two times washed LiNi0.85Co0.1Mn0.05O2 (e)[62]
图 6 未处理 ((a), (b)) 和水洗 ((c), (d)) 后富镍正极材料在空气中储存30天后SEM和TEM图像[63];在空气中储存7天 ((e), (f)) 和30天 ((g), (h)) 未处理和水洗材料在不同倍率下充放电曲线[63]
Figure 6. SEM and TEM images of fresh ((a), (b)) and washed ((c), (d)) nickel-rich cathode materials after storage in air for 30 days[63]; Charge and discharge curves of fresh and washed materials stored in air for 7 days ((e), (f)) and 30 days ((g), (h)) at different rates[63]
FFT—Fast Fourier transform
图 7 未处理 (a) 和水洗后二次煅烧热处理 (b) LiNi0.88Co0.11Al0.01O2第300周电化学阻抗谱和等效电路[14];未处理和水洗后二次煅烧热处理LiNi0.88Co0.11Al0.01O2的循环性能 (c)[14];水洗和热处理结构变化的示意简图 ((d)~(f))[14]
Figure 7. Electrochemical impedance spectra and the equivalent circuits of fresh (a) and secondary calcination treated (b) LiNi0.88Co0.11Al0.01O2 at 300th cycle[14]; Cycling performance of fresh and secondary calcination treated LiNi0.88Co0.11Al0.01O2 (c)[14]; Schematic illustration of structural changes during washing and heat treatment ((d)-(f))[14]
Re, Rs and Rct—Resistance of liquid electrolyte, resistance of solid electrolyte interphase film and charge transfer resistance, respectively; L1—Warburg impedance connected with the lithium ions diffusion through the solid particles
图 8 (a)水洗富Ni正极滤液pH随水洗时间变化(NCA:LiNi0.83Co0.15Al0.02O2,NCM523PC:多晶LiNi0.5Co0.2Mn0.3O2,NCM523SC:单晶LiNi0.5Co0.2Mn0.3O2)[64];(b)两次独立测量未处理和水洗后不同温度干燥的富Ni正极的平均阻抗,误差线表示两次测量的最小值/最大值[62];(c)循环前后放电过程中Li嵌入的示意图[14]
Figure 8. (a) Changes of pH for the filtrate obtained at different washing time (NCA: LiNi0.83Co0.15Al0.02O2, NCM523 PC: Polycrystalline LiNi0.5Co0.2Mn0.3O2, NCM523 SC: single-crystalline LiNi0.5Co0.2Mn0.3O2)[64]; (b) Average impedance from two independent measurements for fresh and washed and dried Ni-rich cathode, the error bars indicate the minimum/maximum of two measurements[62]; (c) Schematic illustration of the Li intercalation during the discharge process before/after cycle[14]
表 1 不同水洗温度下材料的Li、Ni、Co和Al含量变化[58]
Table 1. Changes in the content of Li, Ni, Co and Al under different washing temperature[58]
Washing temperature/℃ Li/wt% Ni/wt% Co/wt% Al/wt% Fresh 7.28 48.83 9.16 1.38 5 7.23 48.87 9.19 1.40 15 7.21 48.88 9.18 1.39 25 7.16 48.92 9.20 1.41 35 7.08 48.99 9.22 1.41 45 6.92 49.14 9.31 1.42 表 2 不同水洗时间样品的晶胞结构参数及I(003)/I(104)值[60]
Table 2. Unit cell structure parameters and I(003)/I(104) values of samples with different washing time[60]
Washing time/min a/nm c/nm c/a I(003)/I(104) Fresh 0.2874 1.4206 4.943 1.14 5 0.2872 1.4203 4.945 1.21 15 0.2872 1.4204 4.946 1.17 30 0.2873 1.4208 4.945 1.15 Notes: a, c—Lattice parameters of the crystal; c/a—Ratio of c to a; I(003)/I(104)—Intensity ratio of (003) to (104) peaks in XRD. 表 3 未处理以及水洗干燥LiNi0.80Co0.15Mn0.05O2中的总残Li量[23]
Table 3. Total amount of residual Li of fresh and washed and dried LiNi0.80Co0.15Mn0.05O2[23]
Drying
temperature/℃Total amount of
residual lithium/10−6Fresh 2319 80 1203 120 1130 190 916 表 4 未处理和经水洗干燥的LiNi0.85Co0.1Mn0.05O2在石墨全电池的电化学性能[62]
Table 4. Electrochemical performance of fresh and washed and dried LiNi0.85Co0.1Mn0.05O2 in full battery with graphite anode[62]
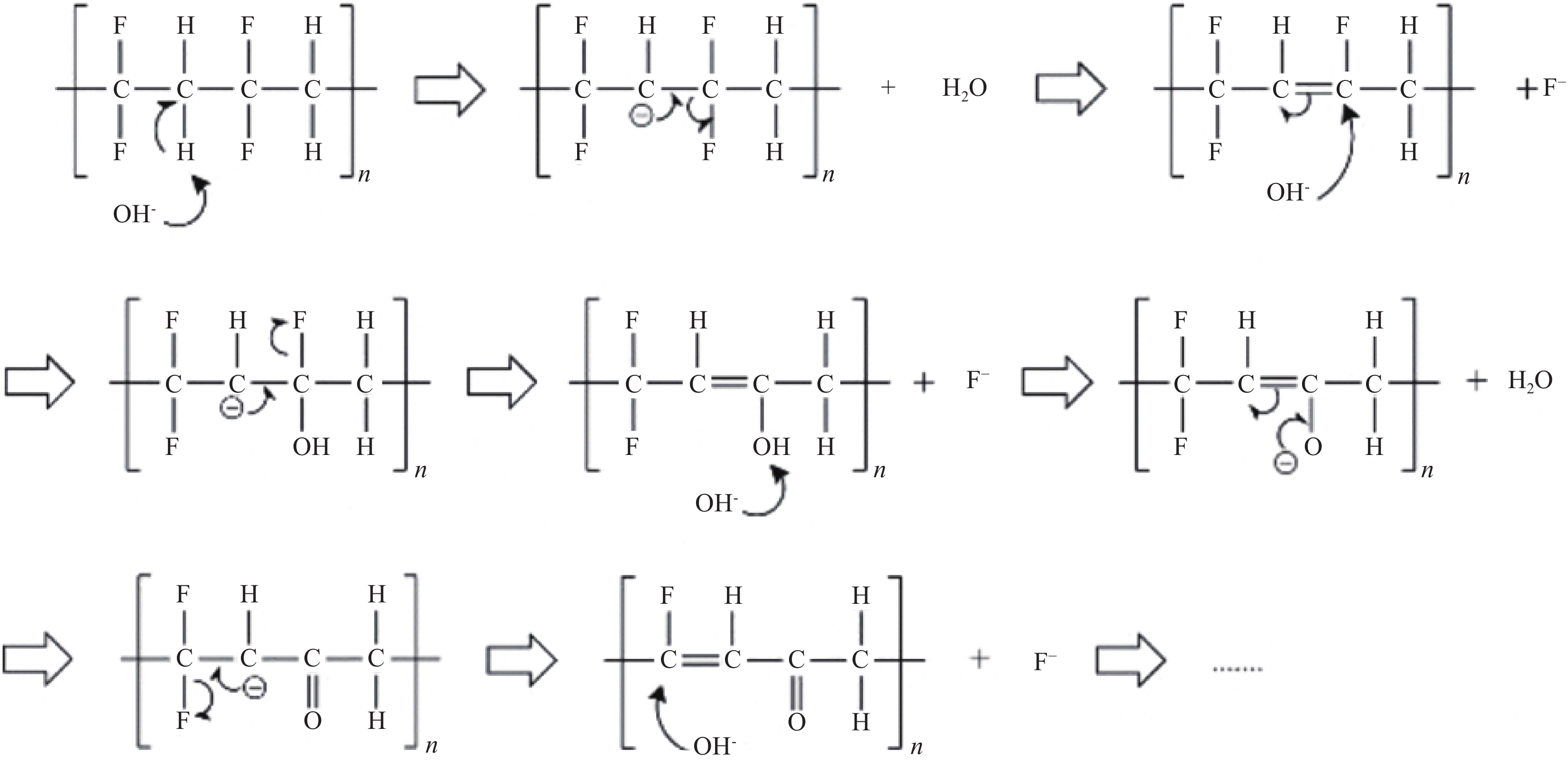
Drying temperature/℃ Discharge capacity of 0.1C
cycle 2 times/(mA·h·g−1)Discharge capacity
loss of 0.1 C to 1 C/%Discharge capacity
loss after 198 cycles of 1 C/%Fresh 188 5 12 80 178 11 20 180 175 16 25 300 130 31 92 -
[1] KIM U H, KIM J H, HWANG J Y, et al. Compositionally and structurally redesigned high-energy Ni-rich layered cathode for next-generation lithium batteries[J]. Materials Today,2018,23:26-36. [2] ZHENG Jianming, YAN Pengfei, ESTEVEZ Luis, et al. Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries[J]. Nano Energy,2018,49:538-548. doi: 10.1016/j.nanoen.2018.04.077 [3] HOU Peiyu, YIN Jiangmei, DING Meng, et al. Surface/Interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: advances and perspectives[J]. Small,2017,13(45):1-29. [4] SARI H M K, LI X F. Controllable cathode-electrolyte interface of LiNi0.8Co0.1Mn0.1O2 for lithium ion batteries: A review[J]. Advanced Energy Materials,2019,9(39):1-31. [5] ZHAO Wengao, ZHENG Jianming, ZOU Lianfeng, et al. High voltage operation of Ni-rich NMC cathodes enabled by stable electrode/electrolyte interphases[J]. Advanced Energy Materials,2018,8(19):1-9. [6] VILLEVIEILLE Claire, LANZ Patrick, BUNZLI Christa, et al. Bulk and surface analyses of ageing of a 5V-NCM positive electrode material for lithium-ion batteries[J]. Journal of Materials Chemistry A,2014,2:6488-6493. doi: 10.1039/c3ta13112b [7] RYU H H, PARK K J, YOON D R, et al. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability[J]. Advanced Energy Materials,2019,9(44):1-7. [8] HASHEM A M A, ABDEL-GHANY A E, EID A E, et al. Study of the surface modification of LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion battery[J]. Journal of Power Sources,2011,196(20):8632-8637. doi: 10.1016/j.jpowsour.2011.06.039 [9] ZHANG Shu, MA Jun, HU Zhenglin, et al. Identifying and Addressing Critical Challenges of High-Voltage Layered Ternary Oxide Cathode Materials[J]. Chemistry of Materials,2019,31(16):6033-6065. doi: 10.1021/acs.chemmater.9b01557 [10] SUN Y K, KIM D H, YOON C S, et al. A novel cathode material with a concentration-gradient for high-energy and safe lithium-ion batteries[J]. Advanced Functional Materials,2010,20(3):485-491. doi: 10.1002/adfm.200901730 [11] DU Rui, BI Yujing, YANG Wenchao, et al. Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5 V[J]. Ceramics International, 2015, 41(5): 7133-7139. [12] LU Chao, WU Hao, CHEN Baojun, et al. Improving the electrochemical properties of Li1.2Mn0.52Co0.08Ni0.2O2 cathode material by uniform surface nanocoating with samarium fluoride through depositional-hydrothermal route[J]. Journal of Alloys and Compounds,2015,634:75-82. doi: 10.1016/j.jallcom.2015.02.056 [13] PAN C C, BANKS C E, SONG W X, et al. Recent development of LiNixCoyMnzO2: Impact of micro/nano structures for imparting improvements in lithium batteries[J]. Transactions of Nonferrous Metals Society of China,2013,23(1):108-119. doi: 10.1016/S1003-6326(13)62436-X [14] LEE Wontae, LEE Donghyun, KIM Yunok, et al. Enhancing the structural durability of Ni-rich layered materials by post-process: washing and heat-treatment[J]. Journal of Materials Chemistry A,2020,8(20):10206-10216. doi: 10.1039/D0TA01083A [15] LEE M J, NOH MJ, PARK M H, et al. The role of nanoscale-range vanadium treatment in LiNi0.8Co0.15Al0.05O2 cathode materials for Li-ion batteries at elevated temperatures[J]. Journal of Materials Chemistry A,2015,3(25):13453-13460. doi: 10.1039/C5TA01571E [16] KIM Junhyeok, LEE Hyomyung, CHA Hyungyeon, et al. Prospect and reality of Ni-rich cathode for commercialization[J]. Advanced Energy Materials,2017,8(6):1-25. [17] XIONG Xunhui, DING Dong, BU Yunfei, et al. Enhanced electrochemical properties of a LiNiO2-based cathode material by removing lithium residues with (NH4)2HPO4[J]. Journal of Materials Chemistry A,2014,2(30):11691-11696. doi: 10.1039/C4TA01282H [18] DING Yu, LIU Zhi, HUANG Mengke, et al. Depth-aware saliency detection using convolutional neural networks[J]. Journal of Visual Communication and Image Representation,2019,61:1-9. doi: 10.1016/j.jvcir.2019.03.019 [19] DAI Hongliu, XI Kai, LIU Xin, et al. Cationic surfactant-based electrolyte additives for uniform lithium deposition via lithiophobic repulsion mechanisms[J]. Journal of the American Chemical society, 2018, 140(50): 17515-17521. [20] CHEN Shi, HE Tao, SU Yuefeng, et al. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode for lithium-ion batteries[J]. ACS Applied Materials & Interfaces,2017,9(35):29732-29743. [21] QIAN Kun, HUANG Binhua, LIU Yuxiu, et al. Increase and discretization of the energy barrier for individual LiNixCoyMnyO2 (x+2y=1) particles with the growth of a Li2CO3 surface film[J]. Journal of Materials Chemistry A,2019,7(20):12723-12731. doi: 10.1039/C9TA01443H [22] ZHENG Xiaobo, LI Xinhai, WANG Zhixing, et al. Investigation and improvement on the electrochemical performance and storage characteristics of LiNiO2-based materials for lithium ion battery[J]. Electrochimica Acta,2016,191:832-840. doi: 10.1016/j.electacta.2016.01.142 [23] PARK J H, CHOI B J, KANG Y S, et al. Effect of residual lithium rearrangement on Ni-rich layered oxide cathodes for lithium-ion batteries[J]. Energy Technology,2018,6(7):1361-1369. doi: 10.1002/ente.201700950 [24] CHO D H, JO C H, CHO W S, et al. Effect of residual lithium compounds on layer Ni-rich LiNi0.7Mn0.3O2[J]. Journal of the Electrochemical Society,2014,161(6):A920-A926. doi: 10.1149/2.042406jes [25] ZHANG Mingjian, HU Xiaobing, LI Maofan, et al. Cooling induced surface reconstruction during synthesis of high-Ni layered oxides[J]. Advanced Energy Materials,2019,9(43):1-10. [26] MARTINEZ A C, GRUGEON S, CAILLEU D, et al. High reactivity of the nickel-rich LiNi1-x-yMnxCoyO2 layered materials surface towards H2O/CO2 atmosphere and LiPF6-based electrolyte[J]. Journal of Power Sources,2020:468. [27] BICHON Marie, SOTTA Dane, DUPRE Nicolas, et al. Study of immersion of LiNi0.5Mn0.3Co0.2O2 material in water for aqueous processing of positive electrode for Li-ion batteries[J]. ACS Applied Materials & Interfaces,2019,11(20):18331-18341. [28] LIU H S, ZHANG Z R, GONG Z L, et al. Origin of deterioration for LiNiO2 cathode material during storage in air[J]. Electrochemical and Solid-State Letters,2004,7(7):A190-A193. doi: 10.1149/1.1738471 [29] LIU Hansan, YANG Yong, ZHANG Jiujun. Investigation and improvement on the storage property of LiNi0.8Co0.2O2 as a cathode material for lithium-ion batteries[J]. Journal of Power Sources,2006,162(1):644-650. doi: 10.1016/j.jpowsour.2006.07.028 [30] LIU Hansan, YANG Yong, ZHANG Jiujun. Reaction mechanism and kinetics of lithium ion battery cathode material LiNiO2 with CO2[J]. Journal of Power Sources,2007,173(1):556-561. doi: 10.1016/j.jpowsour.2007.04.083 [31] MIJUNG Noh, LEE Youngil, CHO Jaephil. Water adsorption and storage characteristics of optimized LiCoO2 and LiNi1/3Co1/3Mn1/3O2 composite cathode material for Li-Ion cells[J]. Journal of The Electrochemical Society,2006,153(5):A935-A940. doi: 10.1149/1.2186041 [32] EOM Junho, KIM Mingyu, CHO Jaephil. Storage characteristics of LiNi0.8Co0.1+xMn0.1−xO2 (x=0, 0.03, and 0.06) cathode materials for lithium batteries[J]. Journal of the Electrochemical Society, 2008, 155(3): A239-A245. [33] FAENZA N V, BRUCE L, LEBENS-HIGGINS Z W, et al. Growth of ambient induced surface impurity species on layered positive electrode materials and impact on electrochemical performance[J]. Journal of the Electrochemical Society,2017,164(14):A3727-A3741. doi: 10.1149/2.0921714jes [34] HATSUKADE Toru, SCHIELE Alexander, HARTMANN Pascal, et al. The origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes[J]. ACS Applied Materials & Interfaces,2018,10(45):38892-38899. [35] SHKROB I A, GILBERT J A, PHILLIPS P J, et al. Chemical weathering of layered Ni-rich oxide electrode materials: Evidence for cation exchange[J]. Journal of the Electrochemical Society,2017,164(7):A1489-A1498. doi: 10.1149/2.0861707jes [36] TOMA Takahiro, MAEZONO Ryo, HONGO Kenta. Electrochemical properties and crystal structure of Li+/H+ cation-exchanged LiNiO2[J]. ACS Applied Energy Materials,2020,3(4):4078-4087. doi: 10.1021/acsaem.0c00602 [37] SU Yuefeng, CHEN Gang, CHEN Lai, et al. Clean the Ni-rich cathode material surface with boric acid to improve its storage performance[J]. Frontiers in Chemistry,2020,8:1-11. doi: 10.3389/fchem.2020.00001 [38] TASAKI Ken, GOLDBERG Alex, LIAN Jianjie, et al. Solubility of lithium salts formed on the lithium-ion battery negative electrode surface in organic solvents[J]. Journal of the Electrochemical Society,2009,156(12):A1019-A1027. doi: 10.1149/1.3239850 [39] ROSS G J, WATTS J F, HILL M P, et al. Surface modification of poly (vinylidene fluoride) by alkaline treatment Part 2. Process modification by the use of phase transfer catalysts[J]. Polymer,2001,42(2):403-413. doi: 10.1016/S0032-3861(00)00328-1 [40] MARCHANDBRYNAERT J, JONGEN N, DEWEZ J L. Surface hydroxylation of poly (vinylidene fluoride) (PVDF) film[J]. Polymer Chemistry,1997,35(7):1227-1235. doi: 10.1002/(SICI)1099-0518(199705)35:7<1227::AID-POLA8>3.0.CO;2-Z [41] LOGINOVA N N, MADORSKAYA L Y, PODLESSKAYA N K. Relations between the thermal stability of partially fluorinated polymers and their structure[J]. Polymer Science USSR,1983,25(12):2995-3000. doi: 10.1016/0032-3950(83)90052-7 [42] ROSS G J, WATTS J F, HILL M P, et al. Surface modification of poly(vinylidene fluoride) by alkaline treatment 1. The degradation mechanism[J]. Polymer,2000,41(5):1685-1696. doi: 10.1016/S0032-3861(99)00343-2 [43] SEONG Wonmo, KIM Youngjin, MANTHIRAM Arumugam. Impact of residual lithium on the adoption of high-nickel layered oxide cathodes for lithium-ion batteries[J]. Chemistry of Materials,2020,32(22):9479-9489. doi: 10.1021/acs.chemmater.0c02808 [44] KIM Youngjin, PARK Hyoju, WARNER J H, et al. Unraveling the intricacies of residual lithium in high-Ni Cathodes for lithium-ion batteries[J]. ACS Energy Letters,2021,6(3):941-948. doi: 10.1021/acsenergylett.1c00086 [45] HE Tao, LU Yun, SU Yuefeng, et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of nickel-rich cathode materials for lithium-ion batteries[J]. Chemsuschem,2018,11(10):1639-1648. doi: 10.1002/cssc.201702451 [46] SEONG W M, CHO K H, PARK J W, et al. Controlling residual lithium in high-nickel (>90%) lithium layered oxides for cathodes in lithium-ion batteries[J]. Angewandte Chemie-International Edition, 2020, 59(42): 18662-18669. [47] KIM T H, ONO L K., FLECK N, et al. Transition metal speciation as a degradation mechanism with the formation of a solid-electrolyte interphase (SEI) in Ni-rich transition metal oxide cathodes[J]. Journal of Materials Chemistry A,2018,6(29):14449-14463. doi: 10.1039/C8TA02622J [48] CHEN Anqi, WANG Kun, LI Jiaojiao, et al. The formation, detriment and solution of residual lithium compounds on Ni-rich layered oxides in lithium-ion batteries[J]. Frontiers in Energy Research,2020,8:1-16. doi: 10.3389/fenrg.2020.00001 [49] BI Yujing, WANG Tao, LIU Meng, et al. Stability of Li2CO3 in cathode of lithium ion battery and its influence on electrochemical performance[J]. RSC Advances,2016,6(23):19233-19237. doi: 10.1039/C6RA00648E [50] GRENIER A, LIU H, WIADEREK K M., et al. Reaction heterogeneity in LiNi0.8Co0.15Al0.05O2 induced by surface layer[J]. Chemistry of Materials,2017,29(17):7345-7352. doi: 10.1021/acs.chemmater.7b02236 [51] RENFREW S E, MCCLOSKEY B D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides[J]. Journal of the American Chemical Society,2017,139(49):17853-17860. doi: 10.1021/jacs.7b08461 [52] SHARIFI-ASL Soroosh, LU Jun, AMINE Khalil, et al. Oxygen release degradation in Li-ion battery cathode materials: Mechanisms and mitigating approaches[J]. Advanced Energy Materials,2019,9(22):1900551. doi: 10.1002/aenm.201900551 [53] KIM Yongseon. Mechanism of gas evolution from the cathode of lithium-ion batteries at the initial stage of high-temperature storage[J]. Journal of Materials Science,2013,48(24):8547-8551. doi: 10.1007/s10853-013-7673-2 [54] KIM Yongseon. Investigation of the gas evolution in lithium ion batteries: Effect of free lithium compounds in cathode materials[J]. Journal of Solid State Electrochemistry, 2013, 17(7): 1961-1965. [55] LUO Kun, ROBERTS Matthew R., HAO Rong, et al. Charge-compensation in 3D-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen[J]. Nature Chemistry,2016,8(7):684-691. doi: 10.1038/nchem.2471 [56] JUNG Roland, METZGER Michael, MAGLIA Filippo, et al. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries[J]. Journal of the Electrochemical Society,2017,164(7):A1361-A1377. doi: 10.1149/2.0021707jes [57] JUNG Roland, MORASCH Robert, KARAYAYLALI Pinar, et al. Effect of ambient storage on the degradation of Ni-rich positive electrode materials (NMC811) for Li-ion batteries[J]. Journal of the Electrochemical Society,2018,165(2):A132-A141. doi: 10.1149/2.0401802jes [58] 刘万民. 锂离子电池LiNi0.8Co0.15Al0.05O2正极材料的合成、改性及储存性能研究[D]. 长沙: 中南大学, 2012.LIU Wanmin. Synthesis, modification and storage research of LiNi0.8Co0.15Al0.05O2 cathode materials for lithium ion batteries[D]. Changsha: Central South University, 2012(in Chinese). [59] XIAO Lifen, YANG Yanyan, ZHAO Yanqiang, et al. Synthesis and electrochemical properties of submicron LiNi0.8Co0.2O2 by a polymer-pyrolysis method[J]. Electrochimica Acta,2008,53(6):3007-3012. doi: 10.1016/j.electacta.2007.11.013 [60] XU Shiguo, WANG Xingning, ZHANG Wenyan, et al. The effects of washing on LiNi0.83Co0.13Mn0.04O2 cathode materials[J]. Solid State Ionics,2019,334:105-110. doi: 10.1016/j.ssi.2019.01.037 [61] KIM Jisuk, HONG Youngsik, RYU Kwang Sun, et al. Washing effect of a LiNi0.83Co0.15Al0.02O2 cathode in water[J]. Electrochemical and Solid-State Letters,2006,9(1):A19-A23. doi: 10.1149/1.2135427 [62] PRITZL D, TEUFL T, FREIBERG A T S, et al. Washing of nickel-rich cathode materials for lithium-ion batteries: Towards a mechanistic understanding[J]. Journal of the Electrochemical Society,2019,166(16):A4056-A4066. doi: 10.1149/2.1351915jes [63] XIONG Xunhui, WANG Zhixing, YUE Peng, et al. Washing effects on electrochemical performance and storage characteristics of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium-ion batteries[J]. Journal of Power Sources,2013,222:318-325. doi: 10.1016/j.jpowsour.2012.08.029 [64] HAMAM Ines, ZHANG Ning, LIU Aaron, et al. Study of the reactions between Ni-rich positive electrode materials and aqueous solutions and their relation to the failure of Li-ion cells[J]. Journal of the Electrochemical Society,2020,167(13):130521. [65] PAN Junqing, SUN Yanzhi, WAN Pingyu, et al. Synthesis, characterization and electrochemical performance of battery grade NiOOH[J]. Electrochemistry Communications,2005,7(8):857-862. doi: 10.1016/j.elecom.2005.05.004 [66] LI J, CHEN B R, ZHOU H M. Effects of washing and heat-treatment on structure and electrochemical charge/discharge property of LiNi0.8Co0.15Al0.05O2 Powder[J]. Journal of Inorganic Materials,2016,31(7):773-778. doi: 10.15541/jim20150644 [67] HUANG X, DUAN J, HE J, et al. Ions transfer behavior during water washing for LiNi0.815Co0.15Al0.035O2: Role of excess lithium[J]. Materials Today Energy,2020,17:100440. [68] PARK K J, HWANG J Y, RYU H H, et al. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical[J]. ACS Energy Letters,2019,4(6):1394-1400. doi: 10.1021/acsenergylett.9b00733 [69] LIU Wanmin, QIN Mulan, XU Lü, et al. Washing effect on properties of LiNi0.8Co0.15Al0.05O2 cathode material by ethanol solvent[J]. Transactions of Nonferrous Metals Society of China,2018,28(8):1626-1631. doi: 10.1016/S1003-6326(18)64805-8 [70] XU Sheng, DU Chunyu, XU Xing, et al. A mild surface washing method using protonated polyaniline for Ni-rich LiNi0.8Co0.1Mn0.1O2 material of lithium ion batteries[J]. Electrochimica Acta, 2017, 248: 534-540. [71] KIM Yoojung, CHO Jaephil. Lithium-reactive Co3(PO4)2 nanoparticle coating on high-capacity LiNi0.8Co0.16Al0.04O2 cathode material for lithium rechargeable batteries[J]. Journal of the Electrochemical Society,2007,154(6):A495-A499. doi: 10.1149/1.2716556 [72] EOM Junho, RYU Kwangsun, CHO Jaephil. Dependence of electrochemical behavior on concentration and annealing temperature of LixCoPO4 phase-grown LiNi0.8Co0.16Al0.04O2 cathode materials[J]. Journal of the Electrochemical Society,2008,155(3):A228-A233. doi: 10.1149/1.2829887 [73] PARK Kwangjin, PARK Junho, HONG Sukgi, et al. Enhancement in the electrochemical performance of zirconium/phosphate bi-functional coatings on LiNi0.8Co0.15Mn0.05O2 by the removal of Li residuals[J]. Physical Chemistry Chemical Physics,2016,18(42):29076-29085. doi: 10.1039/C6CP05286J [74] DING Yan, DENG Bangwei, WANG Hao, et al. Improved electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode material by reducing lithium residues with the coating of Prussian blue[J]. Journal of Alloys and Compounds,2019,774:451-460. doi: 10.1016/j.jallcom.2018.09.286 -






 下载:
下载: