Preparation of C6 carboxylic cellulose and adsorption for Cu2+
-
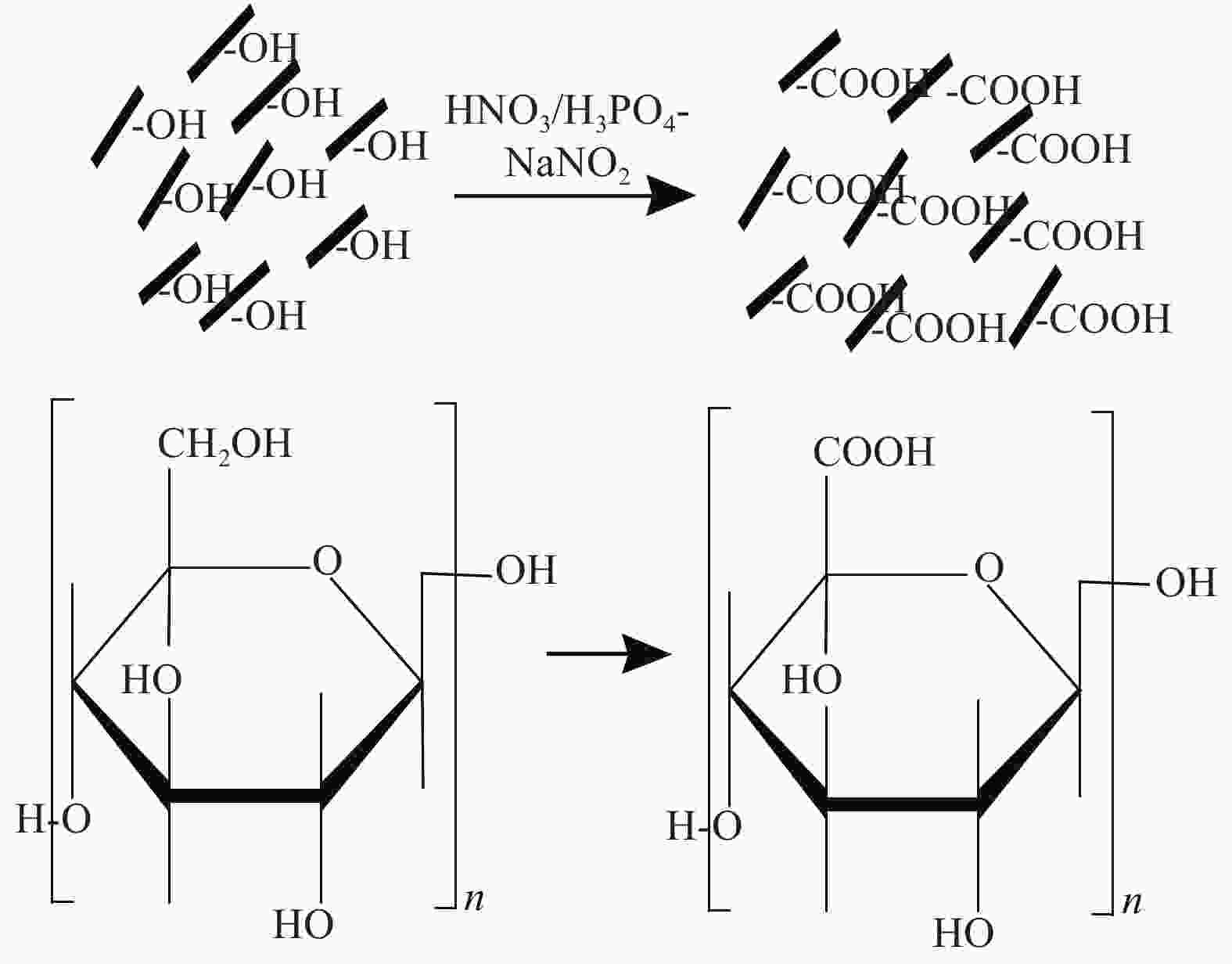
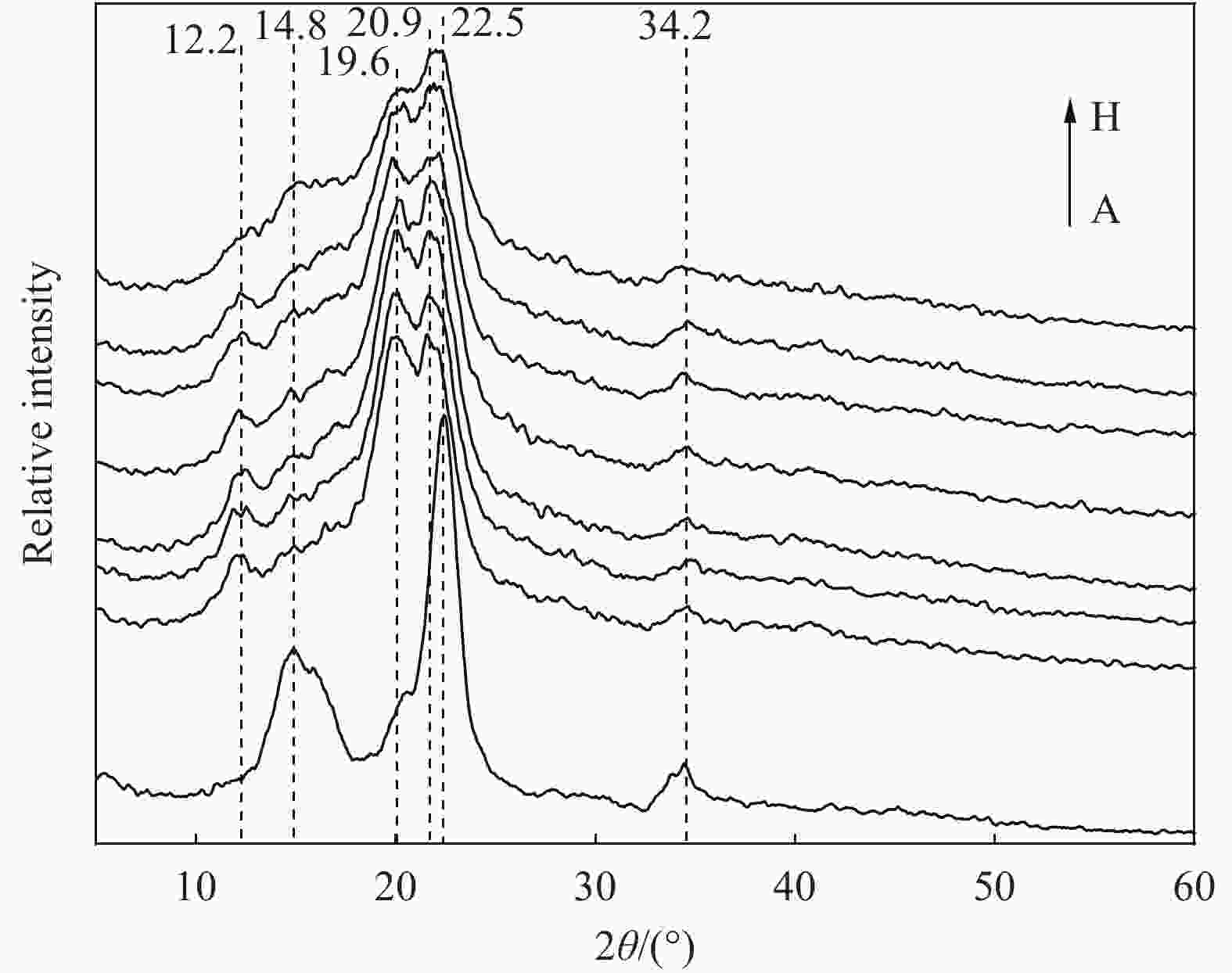
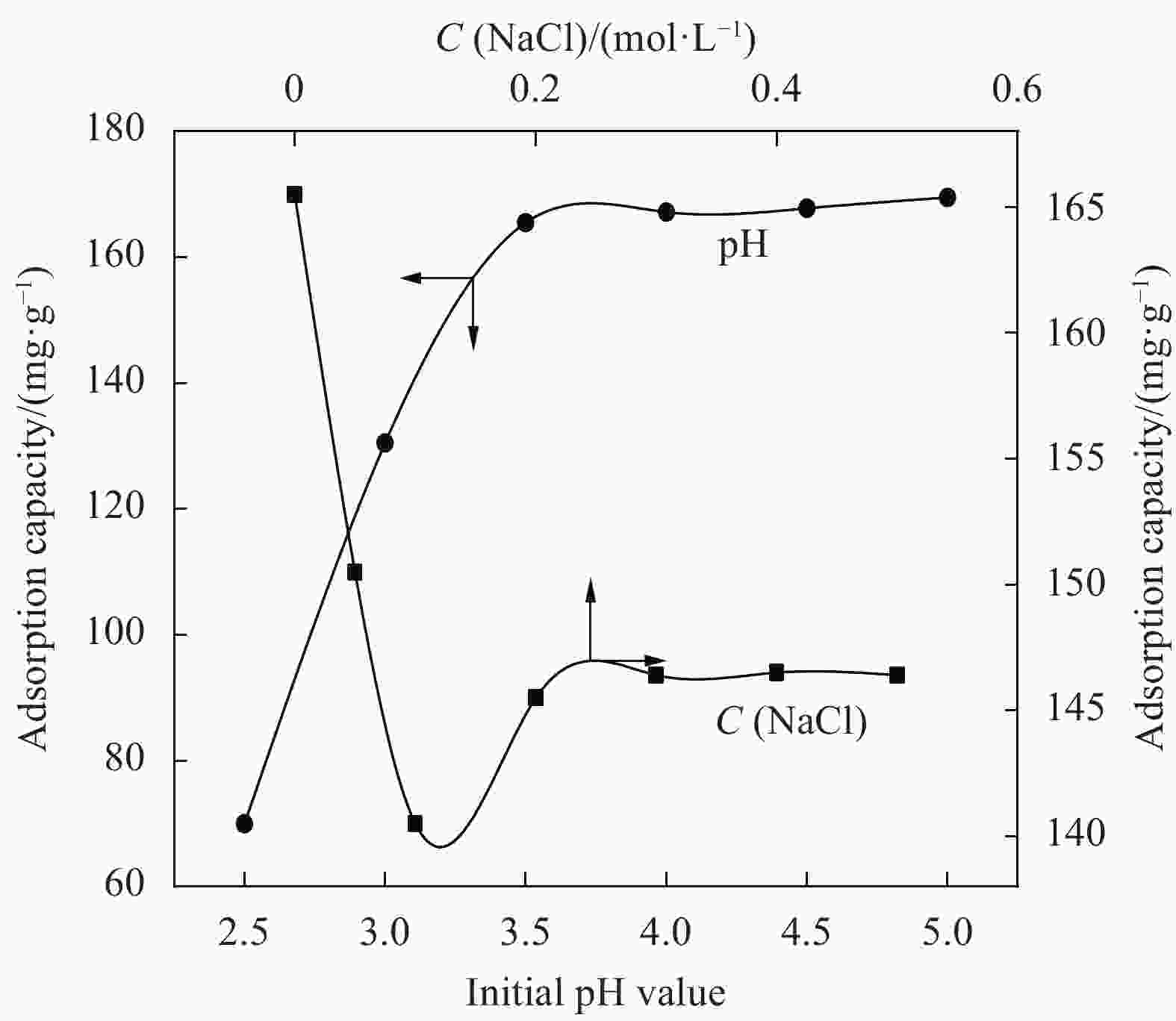
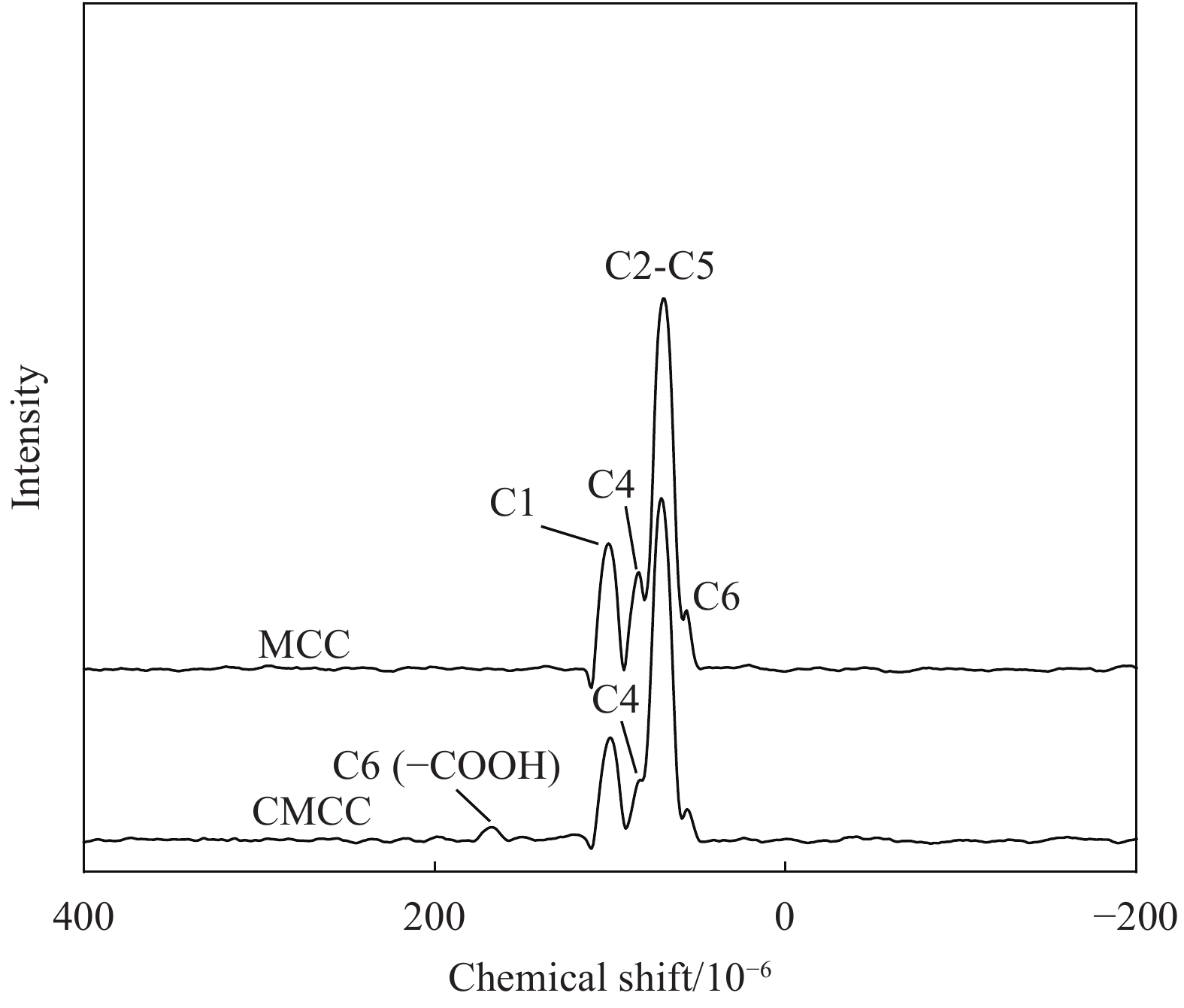
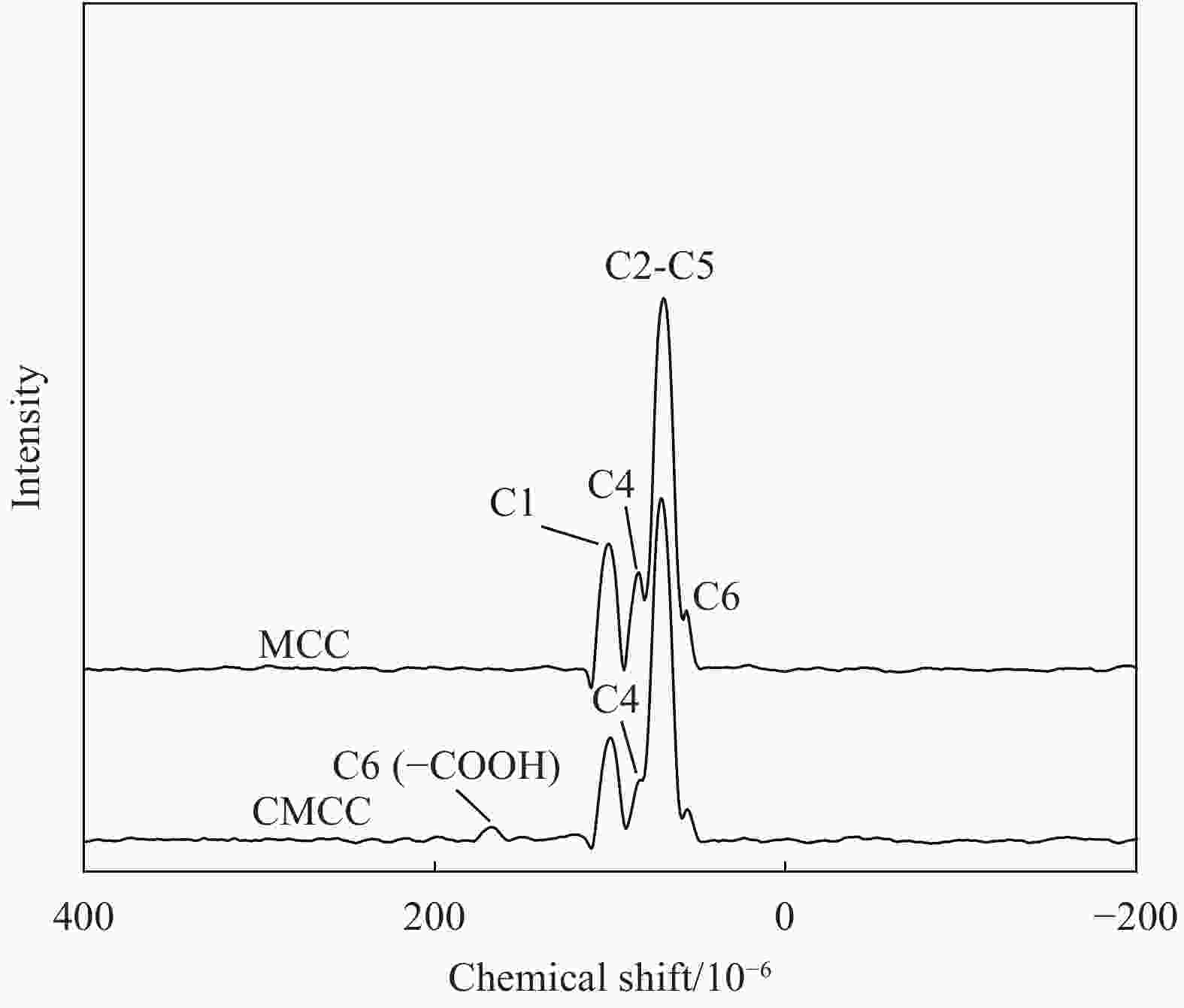
摘要: 为提高纤维素对金属离子污染物的吸附能力,本实验通过选择性氧化体系制备C6位羧基微晶纤维素(CMCC),利用现代测试表征技术分析了CMCC的氧化过程和氧化机制,并研究了CMCC对Cu2+吸附性能。结果表明,NMR和FTIR测试说明HNO3/H3PO4-NaNO2氧化体系可将微晶纤维素(MCC)大分子中吡喃葡萄糖环上C6位伯羟基选择性氧化成羧基。氧化反应对MCC表面产生一定程度的刻蚀,制备的CMCC吸湿性高、结晶度低,同时氧化会造成MCC热稳定性降低。Cu2+吸附实验显示CMCC吸附行为遵循准二级动力学模型和Langmuir等温线,对Cu2+的饱和吸附容量高达165.5 mg/g。吸附热力学分析发现,CMCC对Cu2+的吸附方式主要是通过羧基和金属离子化学反应的结合。实验表明该含有活性羧基的功能性纤维素可作为一种高效的吸附剂,广泛应用在金属离子污染物处理领域。Abstract: In order to improve the adsorption capacity of cellulose to metal ion pollutants, this study prepared C6 carboxylic microcrystalline cellulose (CMCC) by selective oxidation system. The oxidation process and oxidation mechanism of CMCC were analyzed by using modern test and characterization techniques, and the adsorption capacity of CMCC for Cu2+ was studied. The results show that the HNO3/H3PO4-NaNO2 system selectively oxidized the C6 primary hydroxyl group on the pyranose ring in microcrystalline cellulose (MCC) macromolecule to carboxyl group by NMR and FTIR testing. The oxidation reaction etches the MCC surface to a certain extent, which improves the hygroscopicity of the CMCC, decreases the crystallinity, and reduces the thermal stability of the CMCC. Cu2+ adsorption experiments show that the adsorption behavior of CMCC follows the quasi-second-order kinetic model and Langmuir isotherm, and the saturated adsorption capacity of Cu2+ is as high as 165.5 mg/g. The adsorption thermodynamic analysis show that the adsorption of Cu2+ by CMCC is mainly through the chemical reaction between the carboxyl group and metal ions. The results indicate that the functional cellulose containing carboxyl active group can be used as a highly efficient adsorbent and will be widely used in the field of metal ion pollutant treatment.
-
Key words:
- carboxylic microcrystalline cellulose /
- selective oxidation /
- crystallinity /
- copper ions /
- adsorption /
- pollutant
-
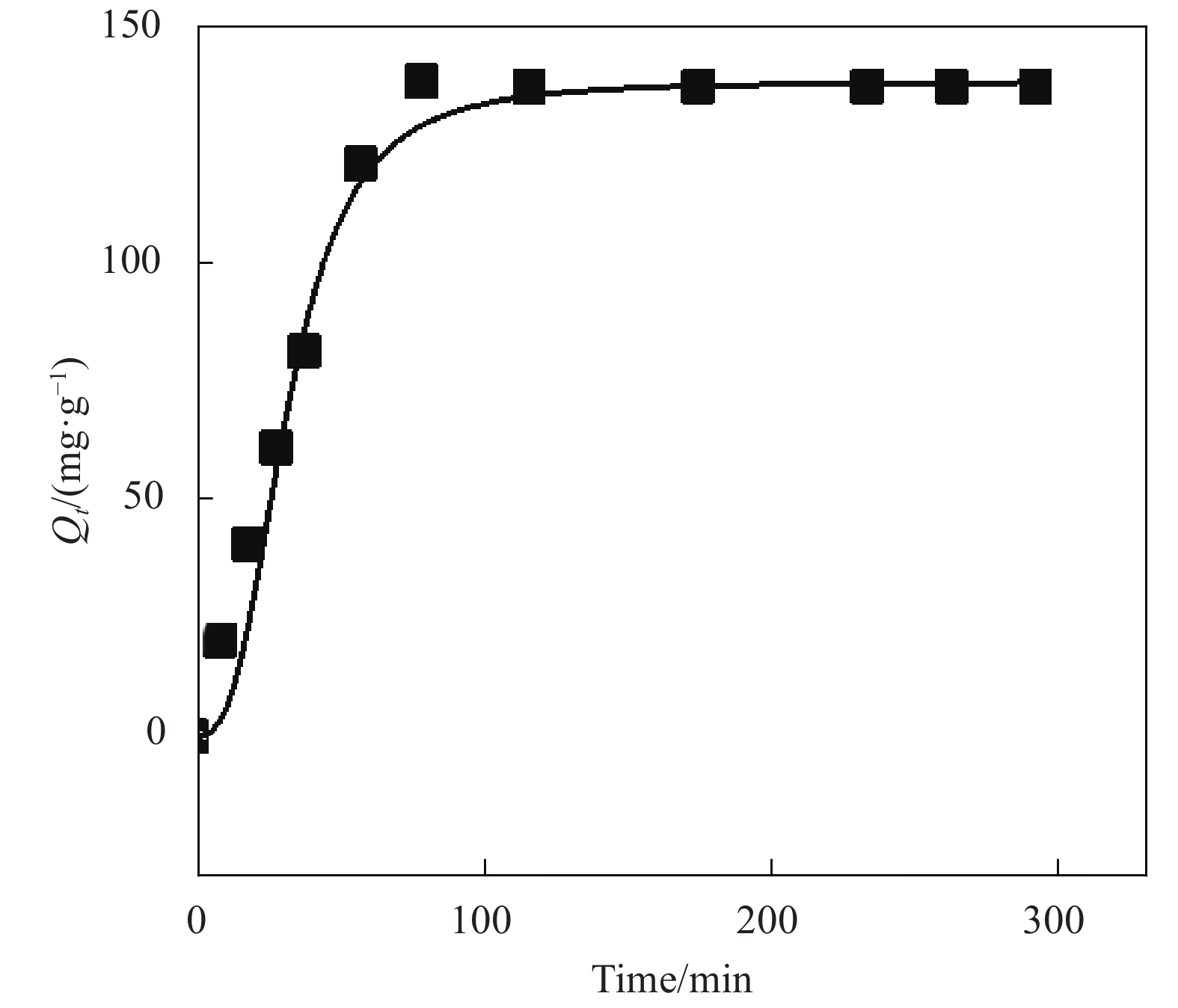
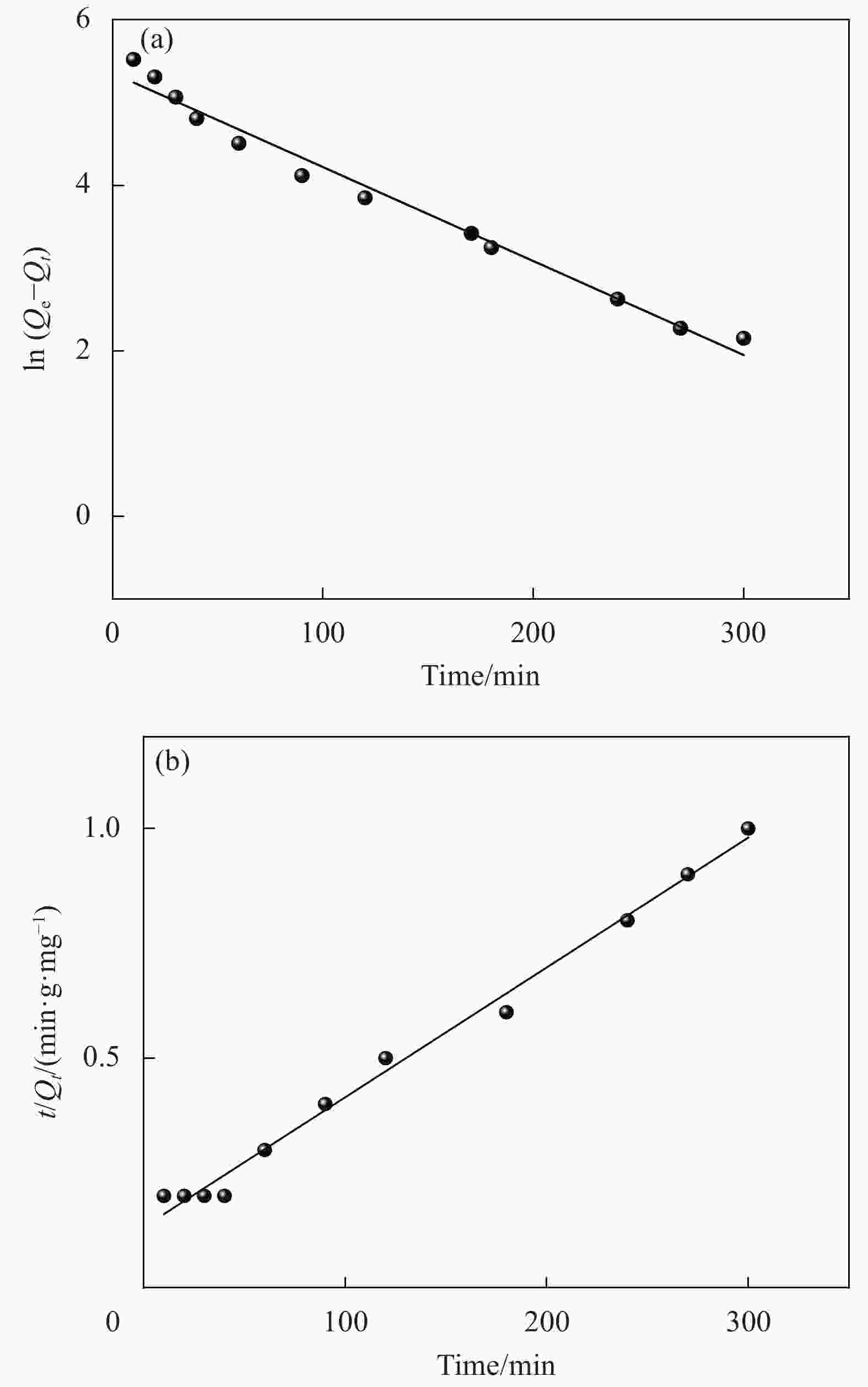
表 1 氧化3 h的CMCC吸附Cu2+的动力学模型拟合参数
Table 1. Parameters of kinetic adsorption models for Cu2+ onto CMCC oxidized for 3 h
Adsorbates Qexp/(mg·g−1) Pseudo-first-order model Pseudo-second-order model Q1e,cal/(mg·g−1) k1/min−1 R2 Q2e,cal/(mg·g−1) k2/(g·mg−1·min−1) R2 Cu2+ 162.5 91.2 1.06×10−2 0.9755 155.6 9.1×10−5 0.9967 Notes: k1, k2—Pseudo-first-order kinetic and Pseudo-second-order kinetic constants, respectively; Qe(cal)—Calculation amount of Cu2+ removed per unit mass of adsorbent; Qe(exp)—Experimental amount of Cu2+ removed per unit mass of adsorbent; R2—Correlation coefficient. 表 2 氧化3 h的CMCC对Cu2+的吸附等温拟合结果
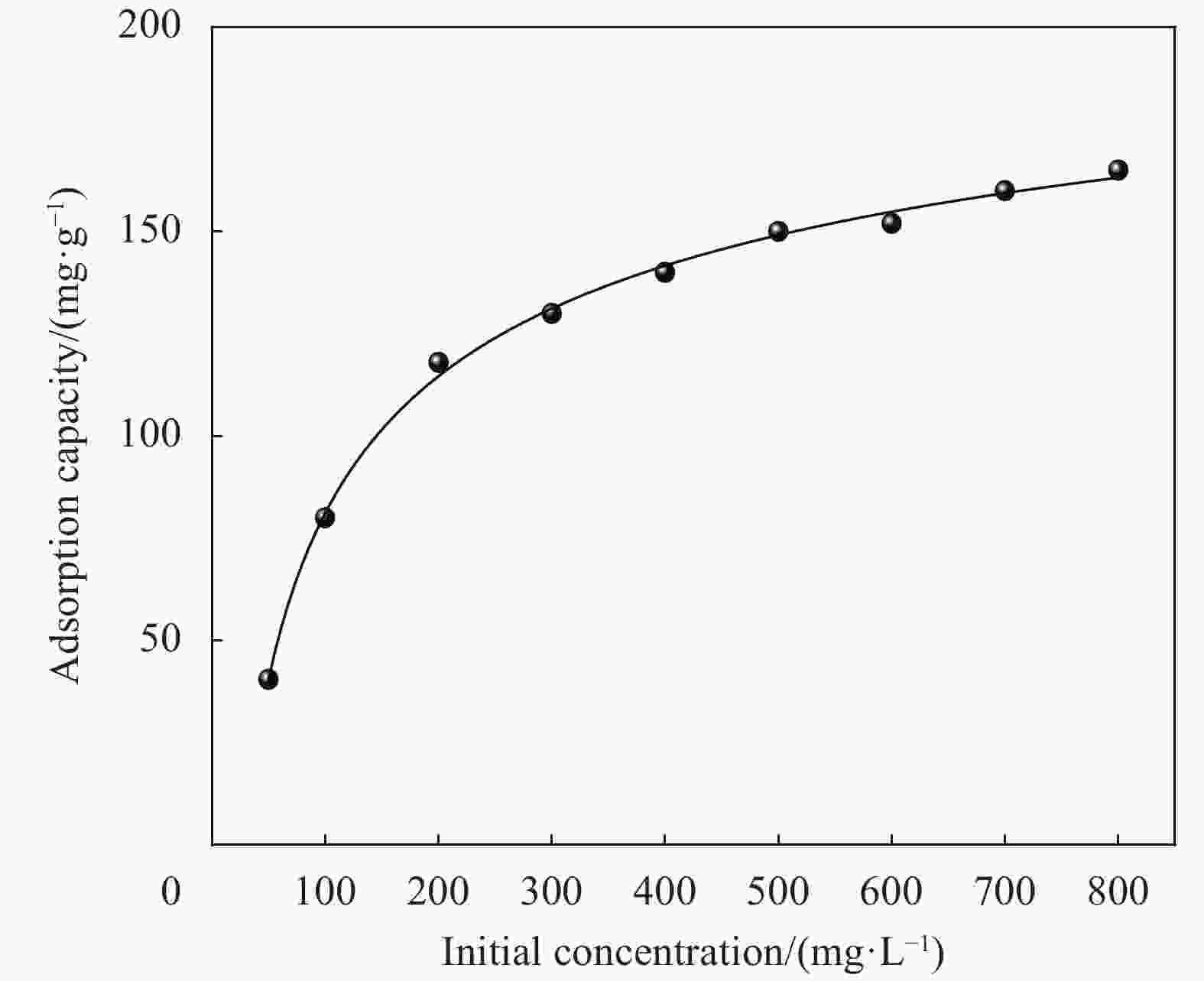
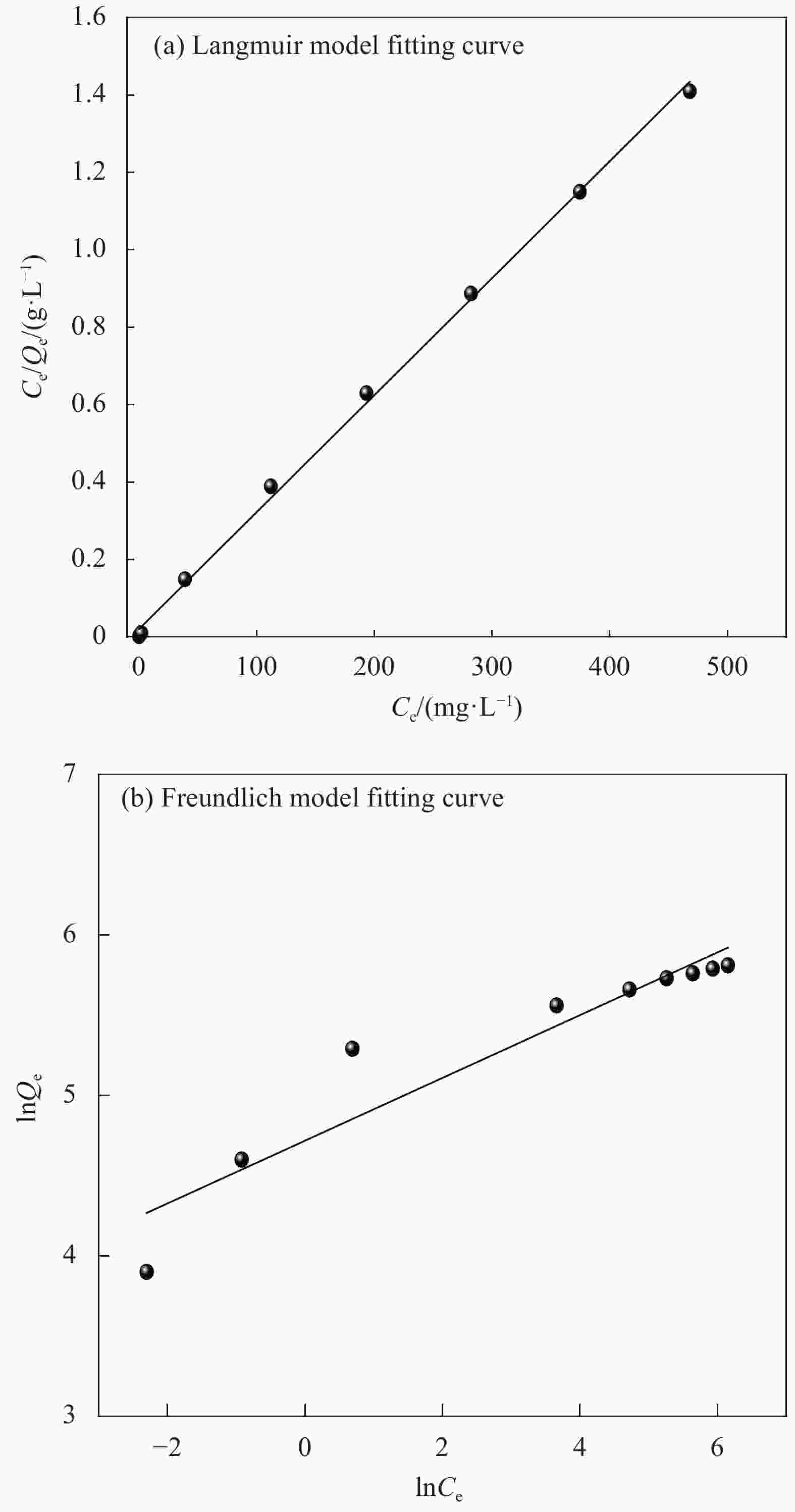
Table 2. Isothermal parameters for the adsorption of Cu2+ onto CMCC-3
Adsorbates Langmuir Freundlich Qm/(mg·g−1) kL/(L·mg−1) R2 kF/(L·mg−1) n R2 Cu2+ 165.5 0.0074 0.9985 7.8 2.2 0.7903 Notes: Qm—Langmuir adsorption maximum; kL—Langmuir coefficient of distribution of the adsorption;kF—Freundlich coefficient of distribution of the adsorption; n—Heterogeneous coefficient. 表 3 同类纤维素对Cu2+的吸附容量对比
Table 3. Adsorption capacity comparison of similar cellulose adsorbents to Cu2+
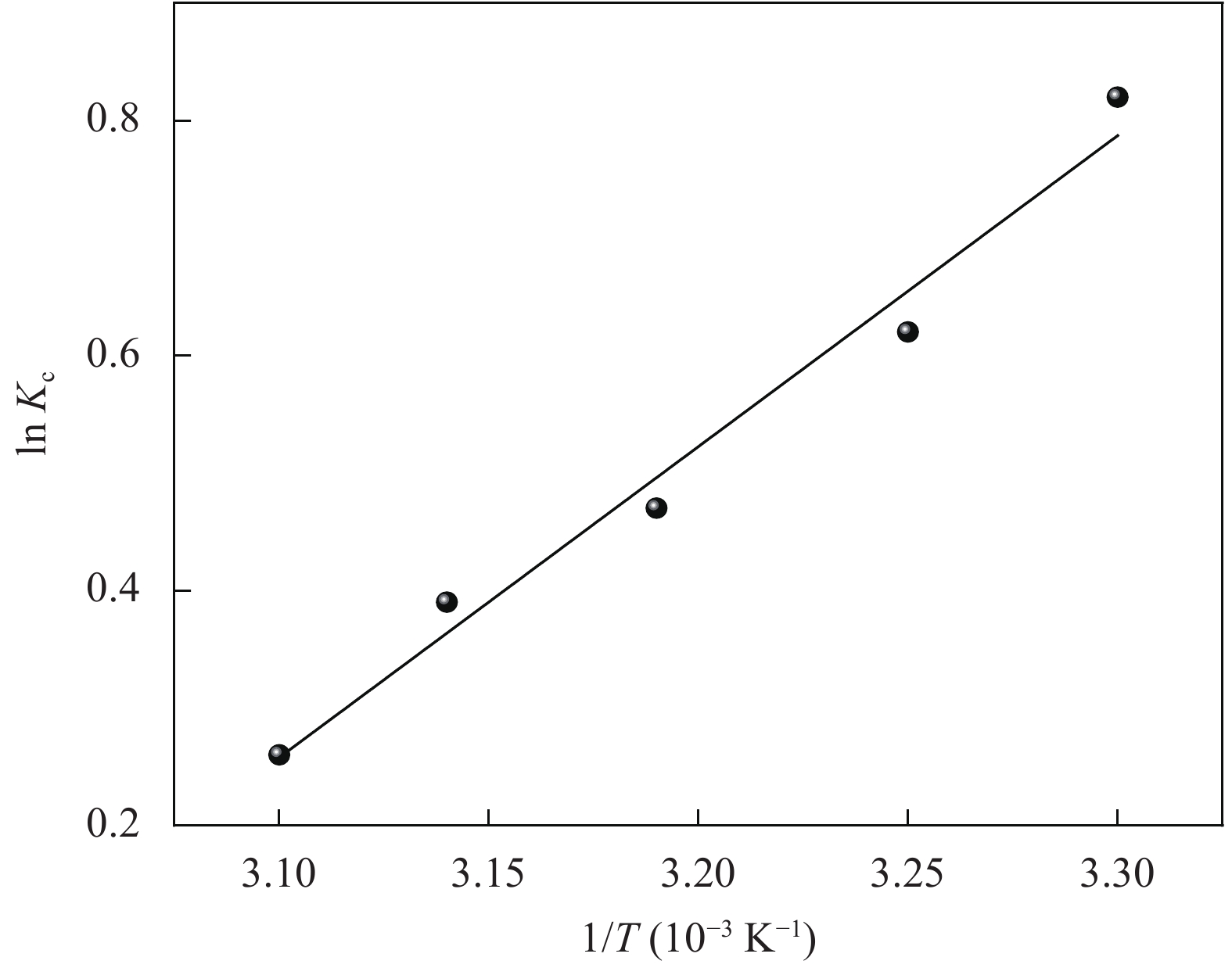
表 4 氧化3 h的CMCC吸附Cu2+的热力学参数
Table 4. Values of thermodynamic parameters for the adsorption of Cu2+ onto CMCC oxidized for 3 h
T/K ΔGo/(kJ·mol−1) ΔHo/(kJ·mol−1) ΔSo/(J·mol−1·K−1) 303 −2.5 −23 −65.4 308 −1.7 313 −1.4 318 −0.9 323 −0.7 Notes: ΔGo—Change in Gibbs free energy for the adsorption process; ΔHo—Change in enthalpy of adsorption; ΔSo—Entropy change of adsorption process. -
[1] DU Z L, CHEN H A, GUO X Y, et al. Mechanism and industrial application feasibility analysis on microwave-assisted rapid synthesis of amino-carboxyl functionalized cellulose for enhanced heavy metal removal[J]. Chemosphere,2021,268:128833. doi: 10.1016/j.chemosphere.2020.128833 [2] PEI L J, LIU J J, GU X M, et al. Adsorption kinetic and mechanism of reactive dye on cotton yarns with different wettability in siloxane non-aqueous medium[J]. Journal of the Textile Institute,2019,111(7):925-933. [3] PEI X P, GAN L, TONG Z H, et al. Robust cellulose-based composite adsorption membrane for heavy metal removal[J]. Journal of Hazardous Materials,2021,406:115-125. [4] 王瑞佳, 聂敬恒, 樊源源, 等. 微晶纤维素辅助制备C-TiO2复合材料及其对Cd2+的吸附性能[J]. 复合材料学报, 2020, 37(2):415-421.WANG Ruijia, NIE Jingheng, FAN Yuanyan, et al. Preparation of C-TiO2 composite with assistance of microcrystalline cellulose and its adsorption properties for Cd2+[J]. Acta Materiae Compositae Sinica,2020,37(2):415-421(in Chinese). [5] GUO X, XU D, YUAN H M, et al. A novel fluorescent nanocellulosic hydrogel based on carbon dots for efficient adsorption and sensitive sensing in heavy metals[J]. Journal of Materials Chemistry A,2019,7(47):27081-27088. doi: 10.1039/C9TA11502A [6] 张晓涛, 王喜明. 木质纤维素/纳米蒙脱土复合材料对废水中Cu(II)的吸附及解吸[J]. 复合材料学报, 2015, 32(2):385-394.ZHANG Xiaotao, WANG Ximing. Adsorption and desorption of Cu (II) in wastewater by lignocellulose/ nano-montmorillonite composites[J]. Acta Materiae Compositae Sinica,2015,32(2):385-394(in Chinese). [7] SARKAYA K, BAKHSHPOUR M, BENIZLI A. Ag+ ions imprinted cryogels for selective removal of silver ions from aqueous solutions[J]. Separation Science and Technology,2019,54(18):2993-3004. doi: 10.1080/01496395.2018.1556300 [8] SELLAOUI L, SOETAREDJO F, ISMADJI S, et al. Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems[J]. Chemical Engineering Journal,2018,354:569-576. doi: 10.1016/j.cej.2018.08.073 [9] TAO J, XIONG J, JIAO C, et al. Hybrid mesoporous silica based on hyperbranch-substrate nanonetwork as highly efcient adsorbent for water treatment[J]. ACS Sustainable Chemistry & Engineering,2016,4(1):60-68. [10] SELAMBAKKANNU S, OTHMAN N, BAKAR K, et al. Modification of radiation grafted banana trunk fibers for adsorption of anionic dyes[J]. Fibers and Polymers,2019,20(2):2556-2569. [11] VALENCIA L, MONTI S, KUMAR S, et al. Nanocellulose/graphene oxide layered membranes: Elucidating their behaviour during filtration of water and metal ions in real time[J]. Nanoscale,2019,11(46):22413-22422. doi: 10.1039/C9NR07116D [12] 谢水波, 罗景阳, 刘青, 等. 羟乙基纤维素/海藻酸钠复合膜对六价铀的吸附性能及吸附机制[J]. 复合材料学报, 2015, 32(1):268-275.XIE S B, LUO J Y, LIU Q, et al. Adsorption characteristics and mechanism of hydroxyethyl cellulose/sodium alginate blend films for uranium(Ⅵ)[J]. Acta Materiae Compo-sitae Sinica,2015,32(1):268-275(in Chinese). [13] HOU X B, LI Y C, PAN Y F, et al. Controlled release of agrochemicals and heavy metal ion capture dual-functional redox-responsive hydrogel for soil remediation[J]. Chemical Communications,2018,54(97):13714-13717. doi: 10.1039/C8CC07872F [14] QIN F M, FANG Z Q, ZHOU J, et al. Efficient removal of Cu2+ in water by carboxymethylated cellulose nanofibrils: Performance and mechanism[J]. Biomacromolecules,2019,20(12):4466-4475. doi: 10.1021/acs.biomac.9b01198 [15] HUANG Z J, HUANG Z Y, FENG L J, et al. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities[J]. Carbohydrate Polymers,2018,202:470-478. doi: 10.1016/j.carbpol.2018.08.136 [16] LI Y, XIAO H N, PAN Y F, et al. Study on cellulose microfilaments based composite spheres: Microwave-assisted synthesis, characterization, and application in pollutant removal[J]. Journal of Environmental Management,2018,228:85-92. [17] XU Y H, CHEN D D, Du Z F, et al. Structure and properties of silk fibroin grafted carboxylic cotton fabric via amide covalent modification[J]. Carbohydrate Polymers,2017,161:99-108. doi: 10.1016/j.carbpol.2016.12.071 [18] PISITSAK P, PHAMONPON W, SOONTORNCHATCHAVET P, et al. The use of water hyacinth fibers to develop chitosan-based biocomposites with improved Cu2+ removal efficiency[J]. Composites Communications,2019,16:1-4. doi: 10.1016/j.coco.2019.08.003 [19] ZHANG L L, LU H L, YU J, et al. Synthesis of lignocellulose-based composite hydrogel as a novel biosorbent for Cu2+ removal[J]. Cellulose,2018,25(12):7315-7328. doi: 10.1007/s10570-018-2077-8 [20] ALLOUSS D, ESSAMLALI Y, CHAKIR A, et al. Effective removal of Cu(II) from aqueous solution over graphene oxide encapsulated carboxymethy lcellulose- alginate hydrogel microspheres: Towards real wastewater treatment plants[J]. Cellulose, 2020, 27(7): 7476-7492. [21] TIAN C H, SHE J R, WU Y Q, et al. Reusable and cross-linked cellulose nanofibrils aerogel for the removal of heavy metal ions[J]. Polymer Composites,2018,39(12):4442-4451. doi: 10.1002/pc.24536 [22] MILHOMENS G C, DE ALMEIDA C G, ZANETTE R D S, et al. Biocompatibility and adsorption properties of hydrogels obtained by graft polymerization of acrylic acid on cellulose from rice hulls[J]. Iranian Polymer Journal,2018,27(12):1023-1032. doi: 10.1007/s13726-018-0672-z [23] MEHREZ E E N, EMAD K R, SHAIMAA T E, et al. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal[J]. International Journal of Biological Macromolecules, 2018, 113: 248-258. [24] SRIVASTAVA S, KARDAM, RAJ K R. Nanotech reinforcement onto cellulosic fibers: Green remediation of toxic metals[J]. International Journal of Green Nanotechnology,2012,4(1):46-53. doi: 10.1080/19430892.2012.654744 [25] LIU P, BORRALL P F, BOZIC M, et al. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+and Fe3+ from industrial effluents[J]. Journal of Hazardous Materials,2015,294:177-185. doi: 10.1016/j.jhazmat.2015.04.001 [26] SEHAQUI H, DE LARRAYA U P, LIU P, et al. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment[J]. Cellulose,2014,21:2831-2844. doi: 10.1007/s10570-014-0310-7 [27] LIU F L, HUA S, ZHOU L, et al. Development and characterization of chitosan functionalized dialdehyde viscose fiber for adsorption of Au(III) and Pd(II)[J]. International Journal of Biological Macromolecules,2021,173:457-466. doi: 10.1016/j.ijbiomac.2021.01.145 [28] DU K F, LI S K, ZHAO L S, et al. One-step growth of porous cellulose beads directly on bamboo fibers via oxidation-derived method in aqueous phase and their potential for heavy metal ions adsorption[J]. ACS Sustainable Chemistry & Engineering,2018,6(12):17068-17075. [29] LAI W J, LIN S C. Hydroxyethyl cellulose-grafted loofa sponge-based metal affinity adsorbents for protein purification and enzyme immobilization[J]. Process Biochemistry,2018,74:141-147. doi: 10.1016/j.procbio.2018.08.024 [30] MYERS A L. Thermodynamics of adsorption in porous materials[J]. Aiche Journal, 2002, 48(1): 145-160. -





 下载:
下载: