Preparation of MnO2-rGO/bamboo cellulose based carbon aerogel and its application in supercapacitors
-
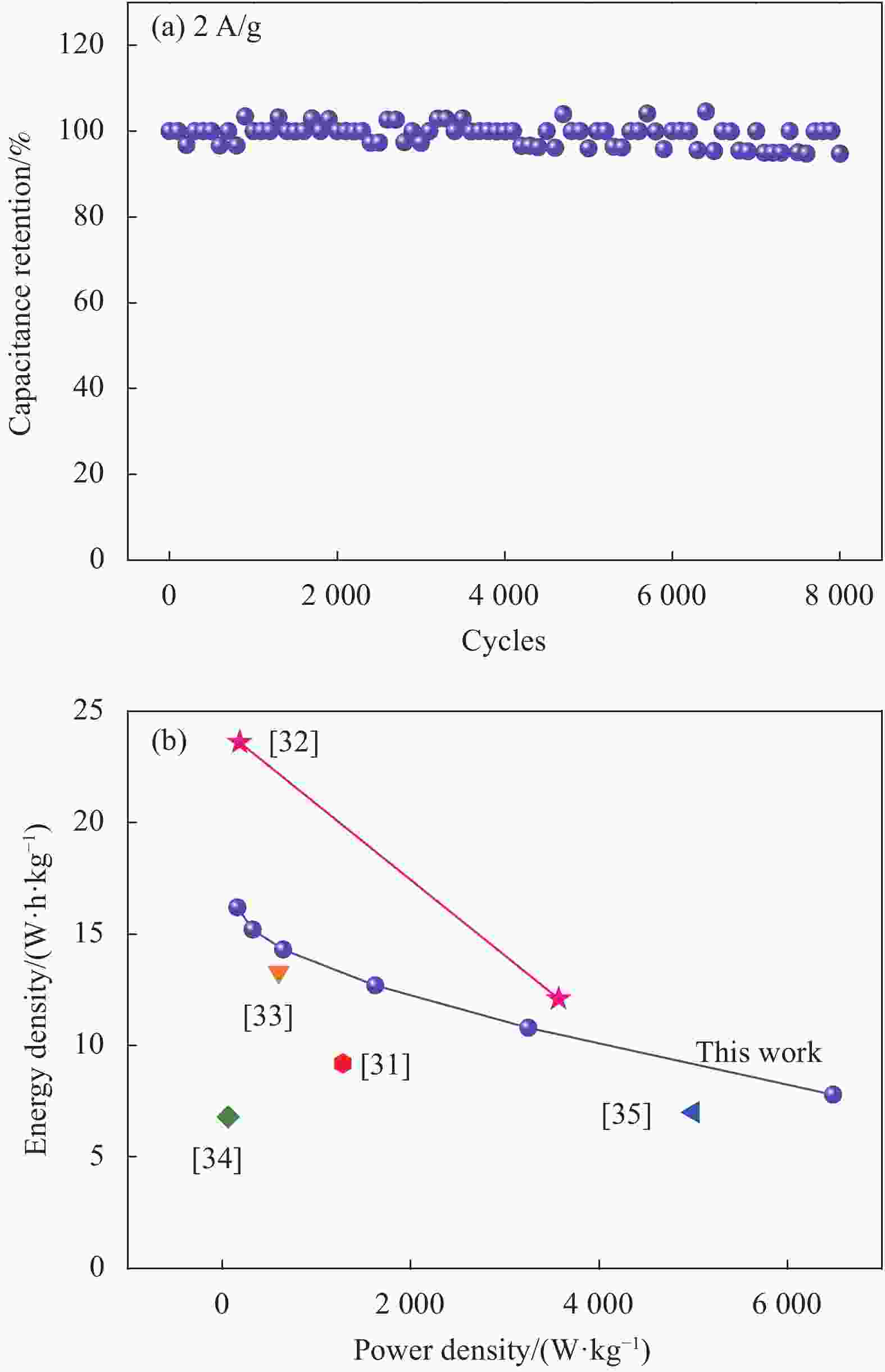
摘要: 研究以竹浆纤维为原料,在氢氧化钠和尿素溶解体系中加入氧化石墨烯,经过凝胶、热解制备还原氧化石墨烯(rGO)/竹纤维素基炭气凝胶。以此作为导电基质,采用水热法负载δ-MnO2纳米片,研究了MnO2负载量对复合材料的电化学性能影响,探讨了复合材料的储能机制。结果显示,随着MnO2负载量的增加,复合材料的电化学性能呈先提高后降低的趋势。当初始参与反应的KMnO4含量为0.005 mol时,复合材料比电容可达330 F/g。将其作为正极材料组装的非对称超级电容器在0.5 A/g的电流密度下,比电容高达68.8 F/g;在功率密度为163 W/kg时,能量密度高达16.2 W•h/kg;在2 A/g电流密度下循环8000次后仍能保持94%的初始容量。Abstract: Reduced graphene oxide (rGO)/bamboo cellulose based carbon aerogel was prepared after gel and pyrolysis by adding GO in sodium hydroxide and urea solution system. Then, the δ-MnO2 nanosheets were deposited on this conductive substrate by hydrothermal method. The effect of MnO2 deposition content on the electrochemical properties of the composites and the energy storage mechanism of the composites were studied. The results show that the electrochemical properties of the composites are firstly improved and then decreased with increasing MnO2 deposition amount. When 0.005 mol KMnO4 is added, the specific capacitance of the composite material is 330 F/g. In addition, the asymmetric supercapacitor exhibits a high specific capacitance of 68.8 F/g at 0.5 A/g, and reveals a high energy density of 16.2 W•h/kg at a power density of 163 W/kg. It retains 94% of the initial specific capacitance after 8000 cycles at 2 A/g.
-
Key words:
- bamboo pulp fiber /
- MnO2 /
- graphene /
- carbon aerogel /
- supercapacitor /
- electrochemical properties /
- energy storage mechanism
-
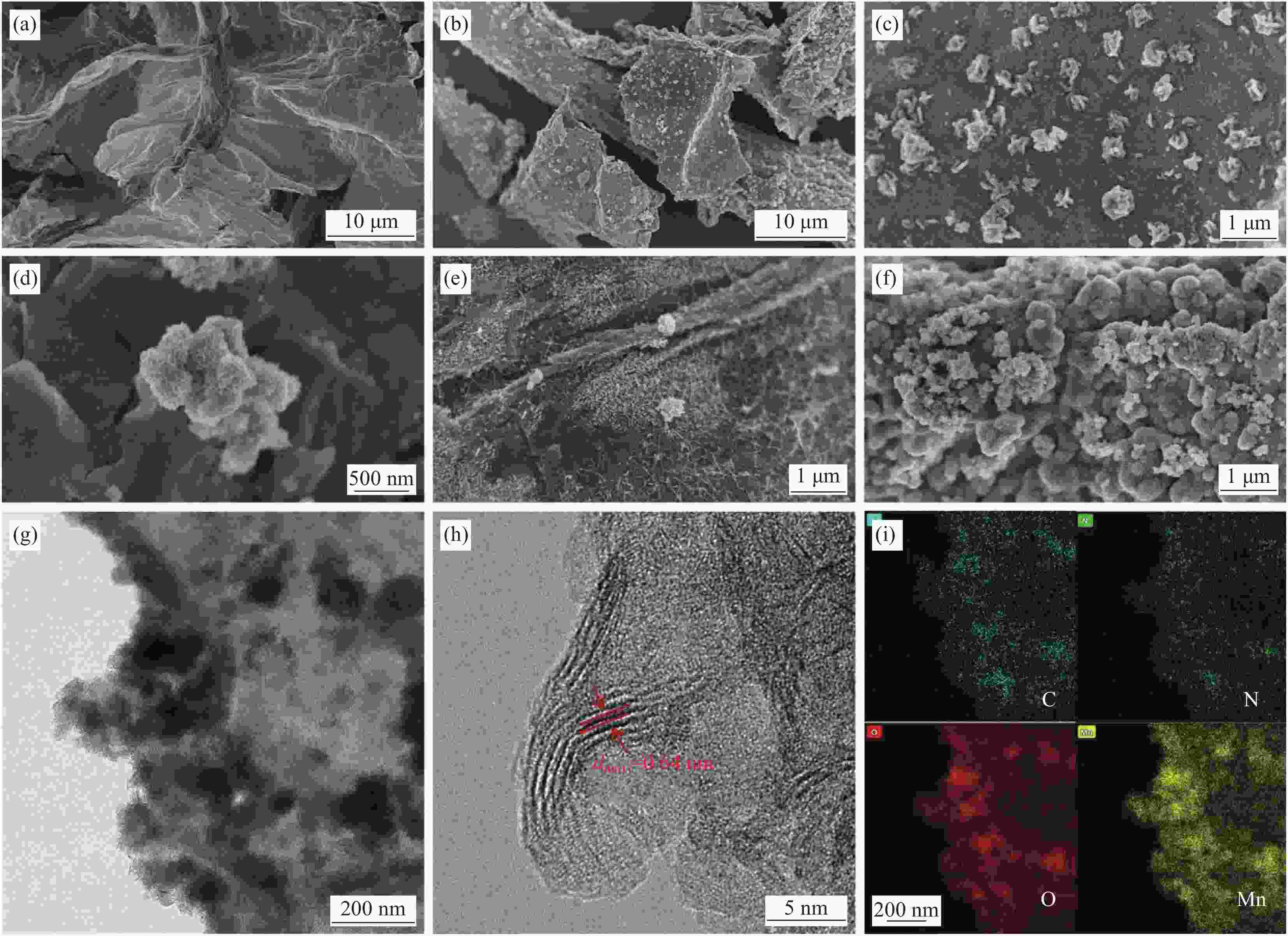
图 3 不同MnO2负载量下炭气凝胶的扫描电镜图:rGO/BCCA (a)、MnO2-rGO/BCCA5 ((b)~(d))、MnO2-rGO/BCCA1 (e)、MnO2-rGO/BCCA10 (f)、KMnO4含量为0.005 mol时的低倍和高倍透射电镜 ((g), (h)) 及EDS能谱图 (i)
Figure 3. SEM images of the carbon aerogel under different MnO2 contents: rGO/BCCA (a), MnO2-rGO/BCCA5 ((b)-(d)), MnO2-rGO/BCCA1 (e), MnO2-rGO/BCCA10 (f); TEM and HRTEM images ((g), (h)) and EDS spectrum diagrams (i) of the carbon aerogel synthetized with 0.005 mol KMnO4
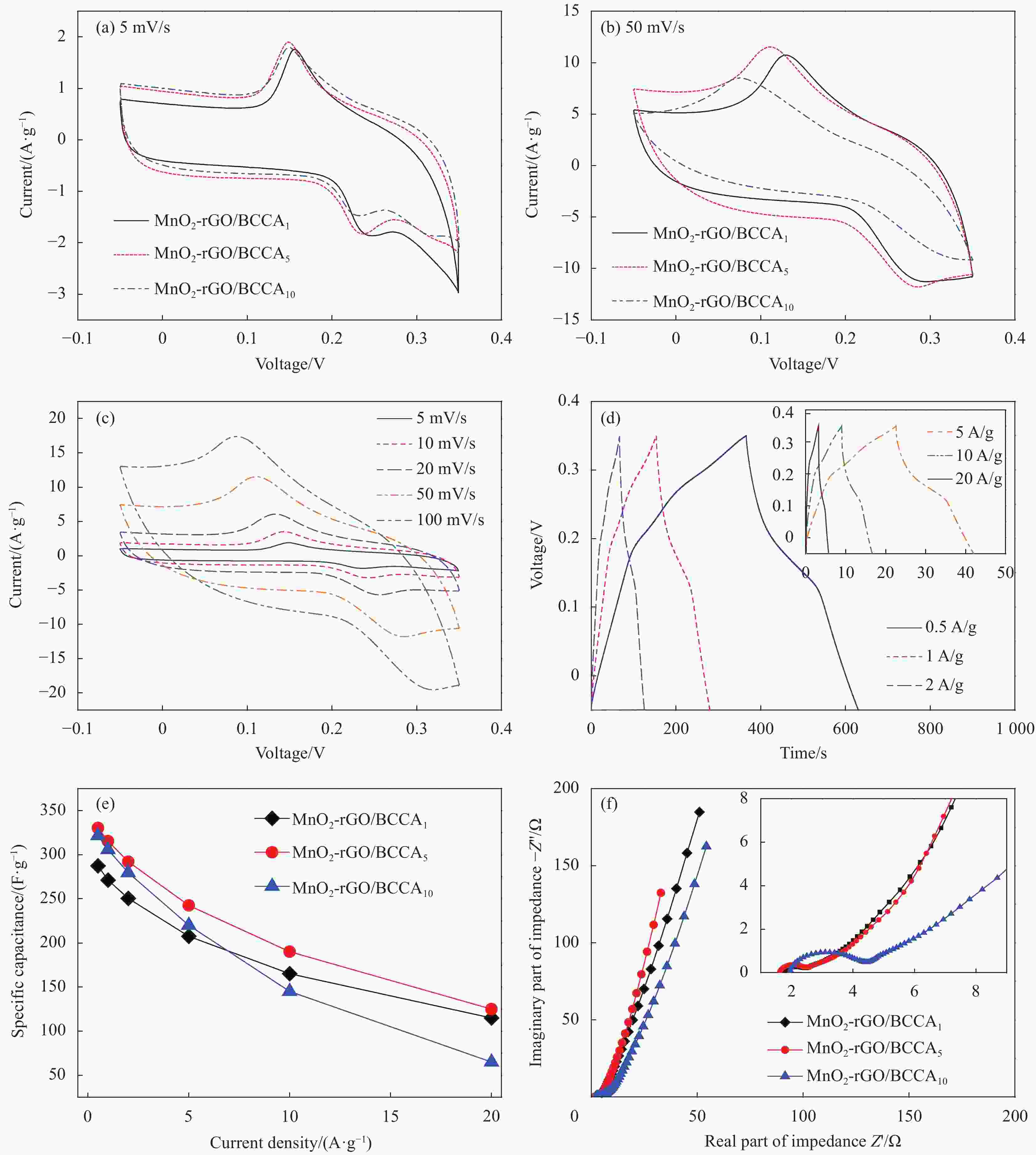
图 5 不同MnO2负载量下炭气凝胶的电化学性能:三个样品在5 mV/s (a)和50 mV/s (b)扫描速率下的循环伏安曲线、MnO2-rGO/BCCA5样品的循环伏安曲线(c)和恒流充放电曲线(d)、三个样品的质量比电容(e)和尼奎斯特图(f)
Figure 5. Electrochemical performance of the carbon aerogel under different MnO2 content: the CV curves at scan rate of 5 mV/s (a) and 50 mV/s (b) of three samples, the CV curves (c) and GCD curves (d) of MnO2-rGO/BCCA5, the capacitance retentions (e) and Nyquist plots (f) of three samples
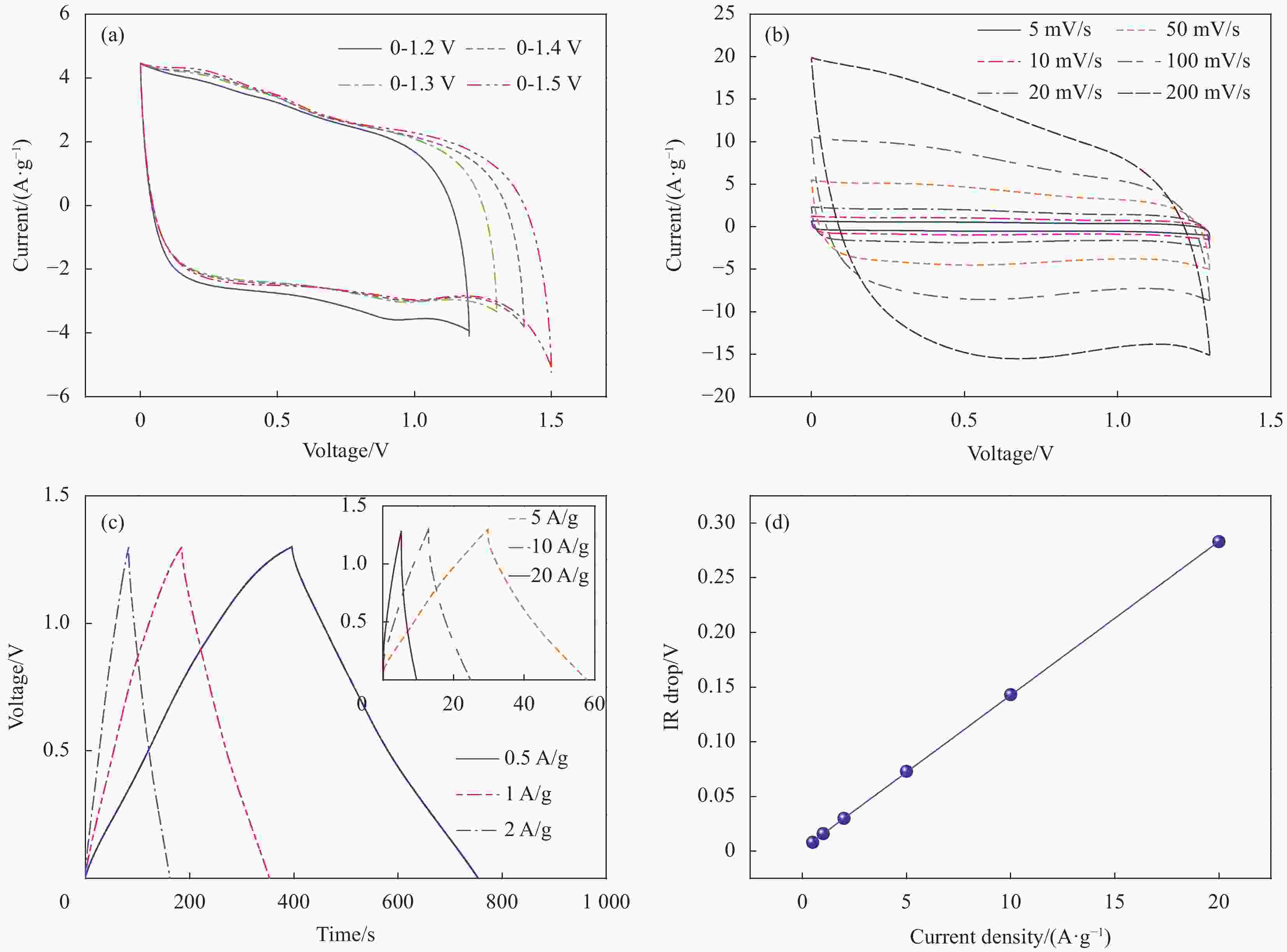
图 6 MnO2-rGO/BCCA5//N/BCCA非对称超级电容器的电化学性能:在不同电压窗口(a)和不同扫描速率(b)下的循环伏安曲线、不同电流密度下的恒流充放电曲线(c)和电压降与电流密度关系图(d)
Figure 6. Electrochemical performance of MnO2-rGO/BCCA5//N/BCCA asymmetric supercapacitor: the CV curves at different voltage windows (a) and different scan rates (b), the GCD surves at different current densities (c) and dependence of the IR drop on current density (d)
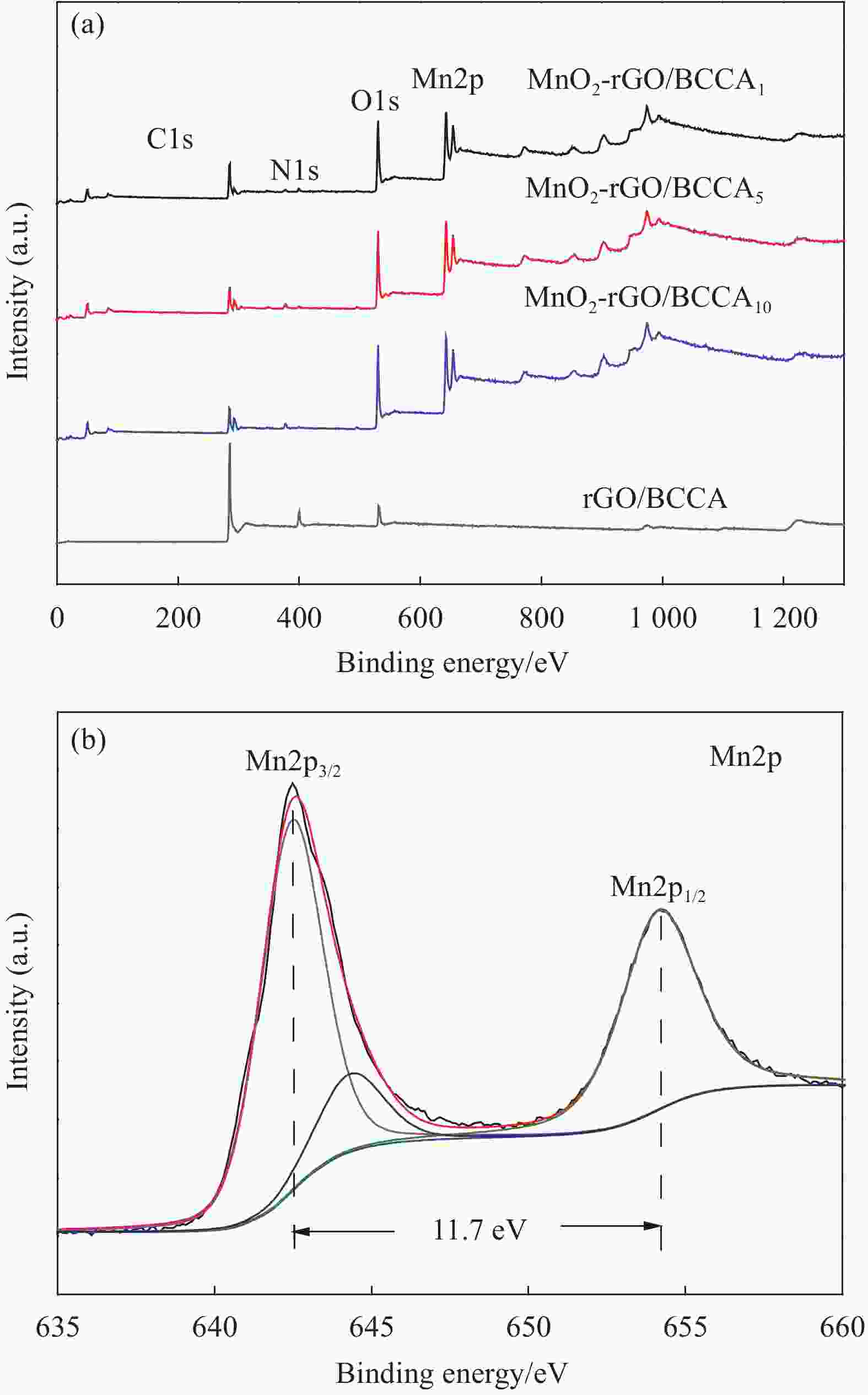
表 1 不同MnO2负载量下炭气凝胶的元素含量
Table 1. Element contents of the carbon aerogel under different MnO2 contents
Sample Element contents/wt% C1s N1s O1s Mn2p rGO/BCCA 81.62 9.49 8.90 — MnO2-rGO/BCCA1 43.95 3.04 37.26 15.74 MnO2-rGO/BCCA5 37.19 2.08 42.38 18.35 MnO2-rGO/BCCA10 35.59 1.62 43.96 18.83 Notes: MnO2-rGO/BCCAx(x=1, 5, 10)—MnO2-reduced graphene oxide/bamboo cellulose carbon aerogel composites with the initial KMnO4 contents involved in the reaction are 0.001 mol, 0.005 mol, 0.01 mol, respectively. -
[1] MAYER S T, PEKALA R W, KASCHMITTER J L. The aero-capacitor: An electrochemical double layer energy storage device[J]. Journal of the Electrochemical Society,1993,140(2):446. doi: 10.1149/1.2221066 [2] PEKALA R W, ALVISO C T, KONG F M, et al. Aerogels derived from multifunctional organic monomers[J]. Journal of Non-Crystalline Solids,1992,145:90-98. doi: 10.1016/S0022-3093(05)80436-3 [3] BIESMANS G, MERTENS A, DUFFOURS L, et al. Polyurethane based organic aerogels and their transformation into carbon aerogels[J]. Journal of Non-Crystalline Solids,1998,225:64-68. doi: 10.1016/S0022-3093(98)00010-6 [4] TAN C B, FUNG B M, NEWMAN J K, et al. Organic aerogels with very high impact strength[J]. Advanced Materials,2001,13(9):644-646. doi: 10.1002/1521-4095(200105)13:9<644::AID-ADMA644>3.0.CO;2-# [5] HAO P, ZHAO Z, LENG Y, et al. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors[J]. Nano Energy,2015,15:9-23. doi: 10.1016/j.nanoen.2015.02.035 [6] BAKIERSKA M, MOLENDA M, MAJDA D, et al. Functional starch based carbon aerogels for energy applications[J]. Procedia Engineering,2014,98:14-19. doi: 10.1016/j.proeng.2014.12.481 [7] YANG X, FEI B H, MA J F, et al. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes[J]. Carbohydrate Polymers,2018,180:385-392. doi: 10.1016/j.carbpol.2017.10.013 [8] LEE Y J, JUNG J C, YI J, et al. Preparation of carbon aerogel in ambient conditions for electrical double-layer capacitor[J]. Current Applied Physics, 2010, 10(2): 682-686. [9] SUBRAMANIAN V, ZHU H W, VAJTAI R, et al. Hydro-thermal synthesis and pseudocapacitance properties of MnO2 nanostructures[J]. Journal of Physical Chemistry B,2005,109(43):20207-20214. doi: 10.1021/jp0543330 [10] EL-KADY M F, IHNS M, LI M, et al. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage[J]. Proceedings of the National Academy of Sciences,2015,112(14):4233-4238. doi: 10.1073/pnas.1420398112 [11] TOUPIN M, BROUSS T, BELANGER D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor[J]. Chemistry of Materials,2004,16(16):3184-3190. doi: 10.1021/cm049649j [12] LANG X, HIRATA A, FUJITA T, et al. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors[J]. Nature Nanotechnology,2011,6(4):232-236. doi: 10.1038/nnano.2011.13 [13] LI D, LIN J, LU Y, et al. MnO2 nanosheets grown on N-doped agaric-derived three-dimensional porous carbon for asymmetric supercapacitors[J]. Journal of Alloys and Compounds,2020,815:152344. doi: 10.1016/j.jallcom.2019.152344 [14] BAI X L, GAO Y L, GAO Z Y, et al. Supercapacitor perfor-mance of 3D-graphene/MnO2 foam synthesized via the combination of chemical vapor deposition with hydrothermal method[J]. Applied Physics Letters,2020,117(18):183901. doi: 10.1063/5.0018708 [15] WEISS N O, ZHOU H, LIAO L, et al. Graphene: An emerg-ing electronic material[J]. Advanced Materials,2012,24(43):5782-5825. doi: 10.1002/adma.201201482 [16] MO M, CHEN C, GAO H, et al. Wet-spinning assembly of cellulose nanofibers reinforced graphene/polypyrrole microfibers for high performance fiber-shaped supercapacitors[J]. Electrochimica Acta,2018,269:11-20. doi: 10.1016/j.electacta.2018.02.118 [17] SEVILLA M, FERRERO G A, DIEZ N, et al. One-step synthesis of ultra-high surface area nanoporous carbons and their application for electrochemical energy storage[J]. Carbon,2018,131:193-200. doi: 10.1016/j.carbon.2018.02.021 [18] RAKHI R B, CHEN W, CHA D, et al. Nanostructured ternary electrodes for energy-storage applications[J]. Advanced Energy Materials,2012,2(3):381-389. doi: 10.1002/aenm.201100609 [19] JIN X, ZHOU W, ZHANG S, et al. Nanoscale microelectrochemical cells on carbon nanotubes[J]. Small,2007,3(9):1513-1517. doi: 10.1002/smll.200700139 [20] JANES A, KURIG H, LUST E. Characterisation of activated nanoporous carbon for supercapacitor electrode mater-ials[J]. Carbon,2007,45(6):1226-1233. doi: 10.1016/j.carbon.2007.01.024 [21] SUN M, LAN B, YU L, et al. Manganese oxides with diffe-rent crystalline structures: Facile hydrothermal synthesis and catalytic activities[J]. Materials Letters,2012,86:18-20. doi: 10.1016/j.matlet.2012.07.011 [22] LIANG S, TENG F, BULGAN G, et al. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation[J]. Journal of Physical Chemistry C,2008,112(14):5307-5315. doi: 10.1021/jp0774995 [23] MA S B, AHN K Y, LEE E S, et al. Synthesis and characteri-zation of manganese dioxide spontaneously coated on carbon nanotubes[J]. Carbon,2007,45(2):375-382. doi: 10.1016/j.carbon.2006.09.006 [24] YU N, YIN H, ZHANG W, et al. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics[J]. Advanced Energy Materials,2016,6(2):1501458. doi: 10.1002/aenm.201501458 [25] ZHUANG R, DONG Y, LI D, et al. Polyaniline-mediated coupling of Mn3O4 nanoparticles on activated carbon for high-performance asymmetric supercapacitors[J]. Journal of Alloys and Compounds,2021,851:156871. doi: 10.1016/j.jallcom.2020.156871 [26] DENG L, HAO Z, WANG J, et al. Preparation and capaci-tance of grapheme/multiwall carbon nanotubes/MnO2 hybrid material for high-performance asymmetrical electrochemical capacitor[J]. Electrochimica Acta,2013,89:191-198. doi: 10.1016/j.electacta.2012.10.106 [27] LI Z, MI Y, LIU X, et al. Flexible graphene/MnO2 composite papers for supercapacitor electrodes[J]. Journal of Mater-ials Chemistry,2011,21(38):14706-14711. doi: 10.1039/c1jm11941a [28] MESSAOUDI B, JOIRET S, KEDDAM M, et al. Anodic behaviour of manganese in alkaline medium[J]. Electrochimica Acta,2001,46(16):2487-2498. doi: 10.1016/S0013-4686(01)00449-2 [29] JIANG J, KUCERNAK A. Electrochemical supercapacitor material based on manganese oxide: Preparation and characterization[J]. Electrochimica Acta,2002,47(15):2381-2386. doi: 10.1016/S0013-4686(02)00031-2 [30] CONWAY B E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage[J]. Journal of the Electrochemical Society,1991,138(6):1539-1548. doi: 10.1149/1.2085829 [31] LI M, YU J, WANG X, et al. 3D porous MnO2@carbon nanosheet synthesized from rambutan peel for high-performing supercapacitor electrodes materials[J]. Applied Surface Science,2020,530:147230. doi: 10.1016/j.apsusc.2020.147230 [32] LI Y, YU N, YAN P, et al. Fabrication of manganese dioxide nanoplates anchoring on biomass-derived cross-linked carbon nanosheets for high-performance asymmetric supercapacitors[J]. Journal of Power Sources,2015,300:309-317. doi: 10.1016/j.jpowsour.2015.09.077 [33] LI L, HU Z A, AN N, et al. Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability[J]. Journal of Physical Chemistry C,2014,118(40):22865-22872. doi: 10.1021/jp505744p [34] HE Y, CHEN W, LI X, et al. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes[J]. ACS Nano,2013,7(1):174-182. doi: 10.1021/nn304833s [35] WU Z S, REN W, WANG D W, et al. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors[J]. ACS Nano,2010,4(10):5835-5842. doi: 10.1021/nn101754k -





 下载:
下载: