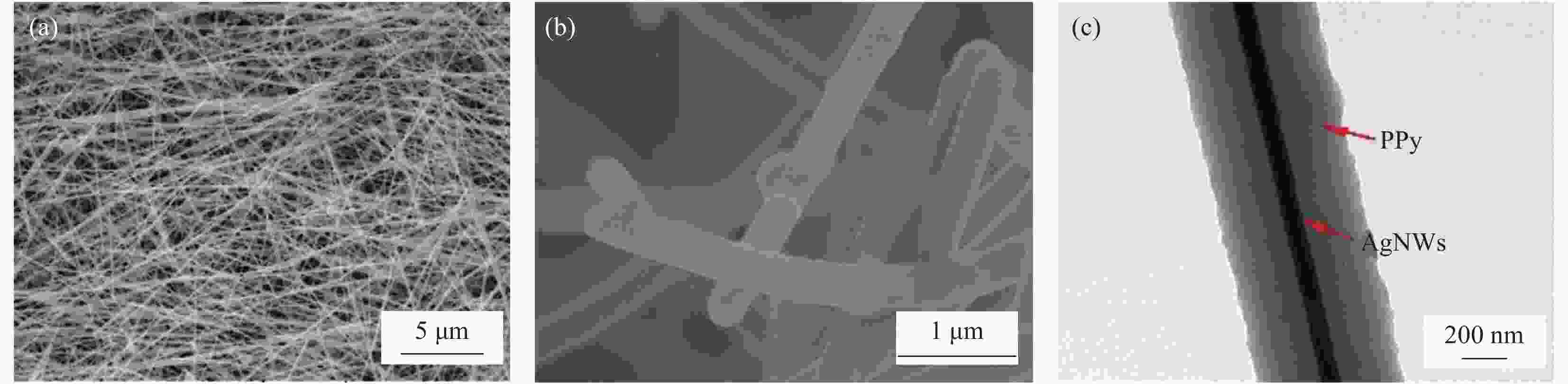
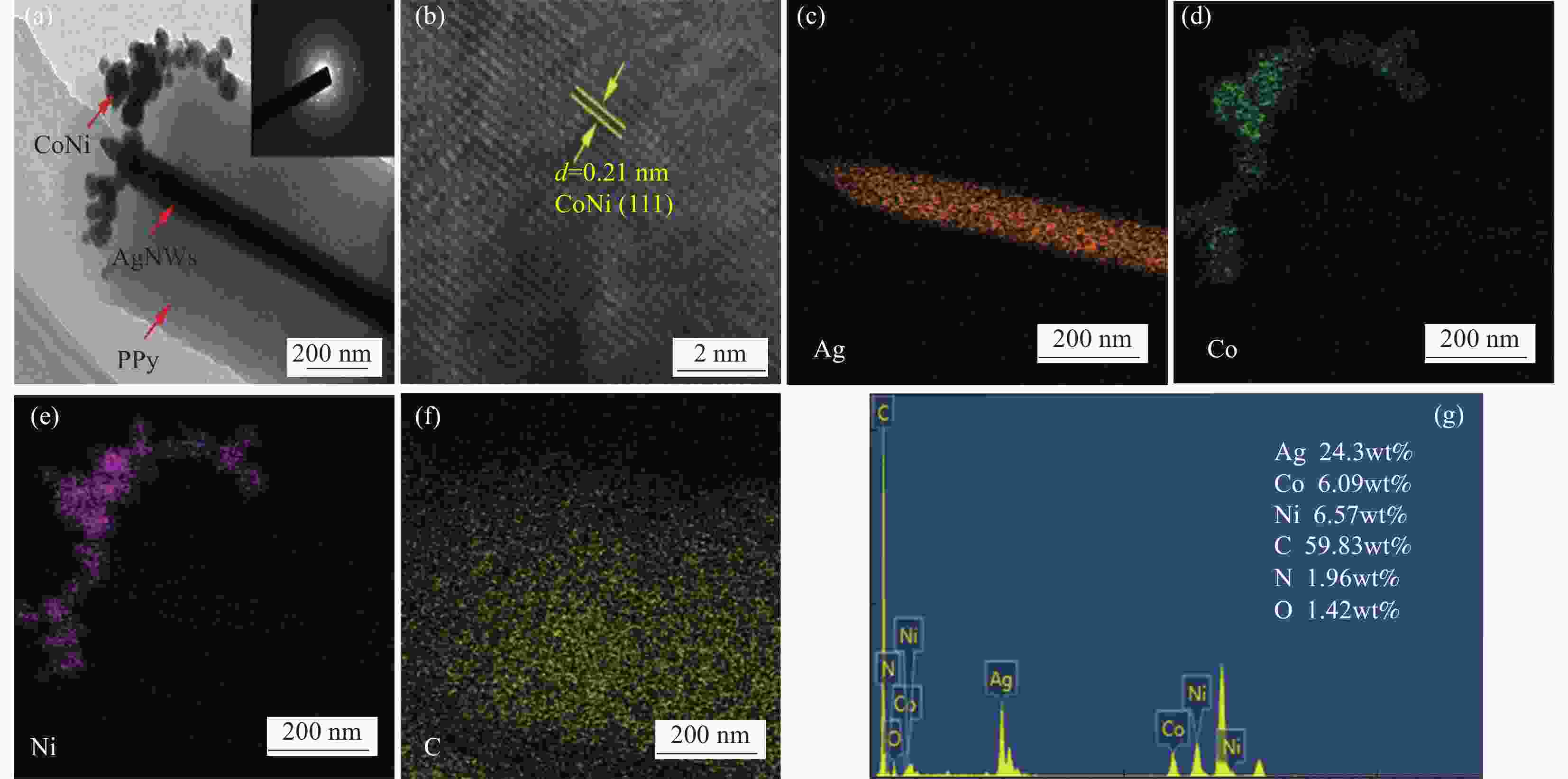
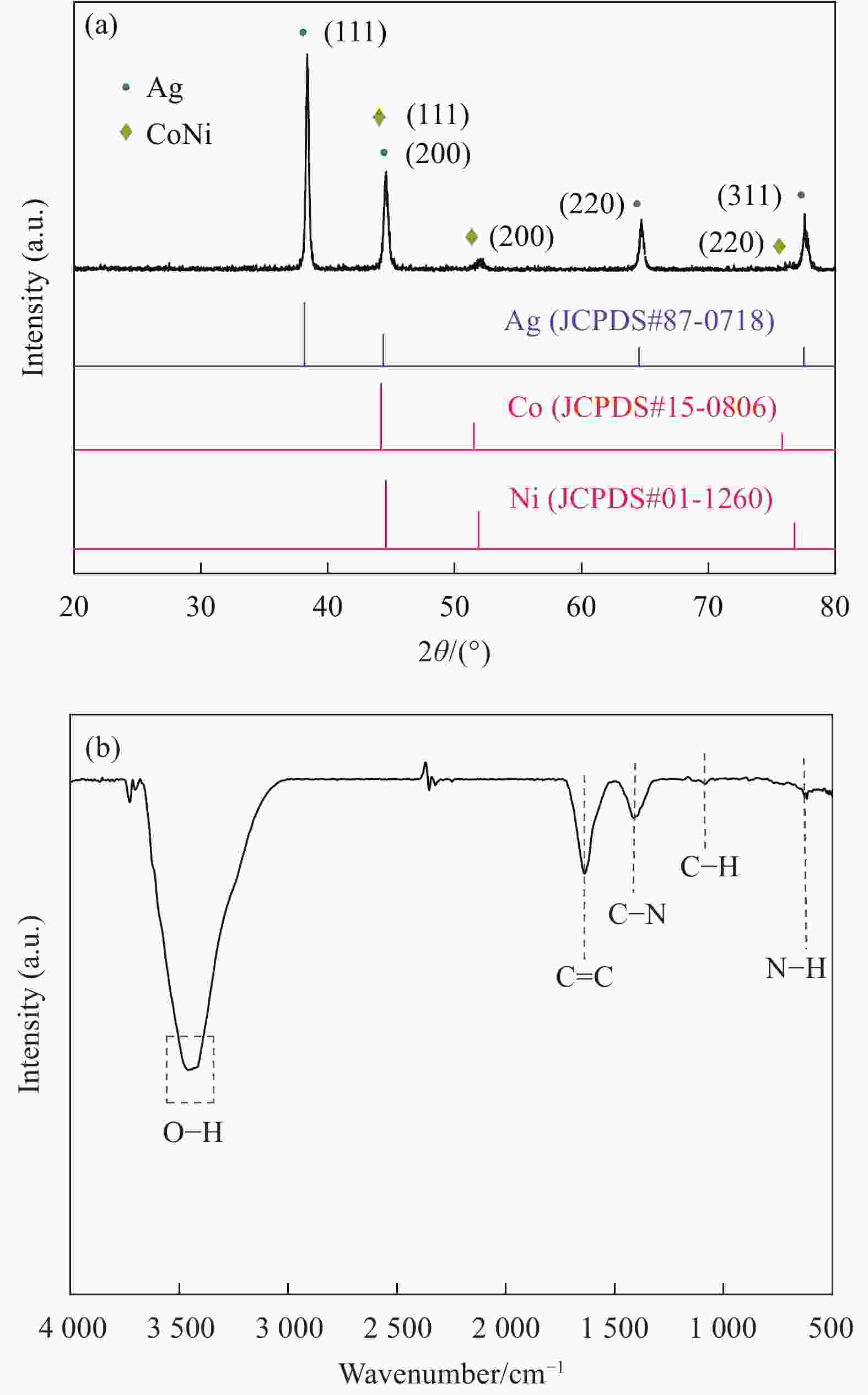
Synthesis and electrocatalytic oxygen evolution performances of high conductivity silver nanowire@polypyrrole@CoNi alloy aerogels
-
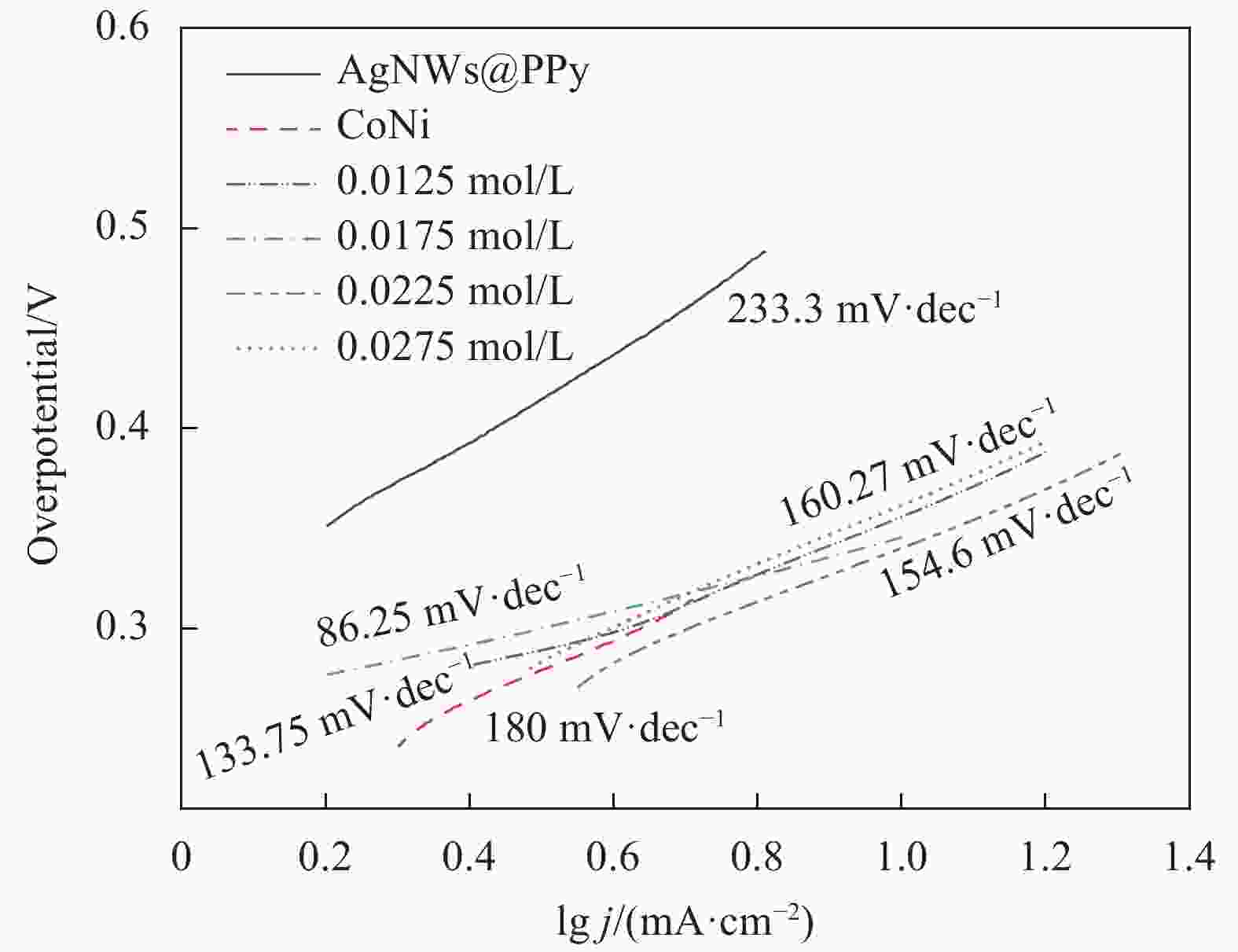
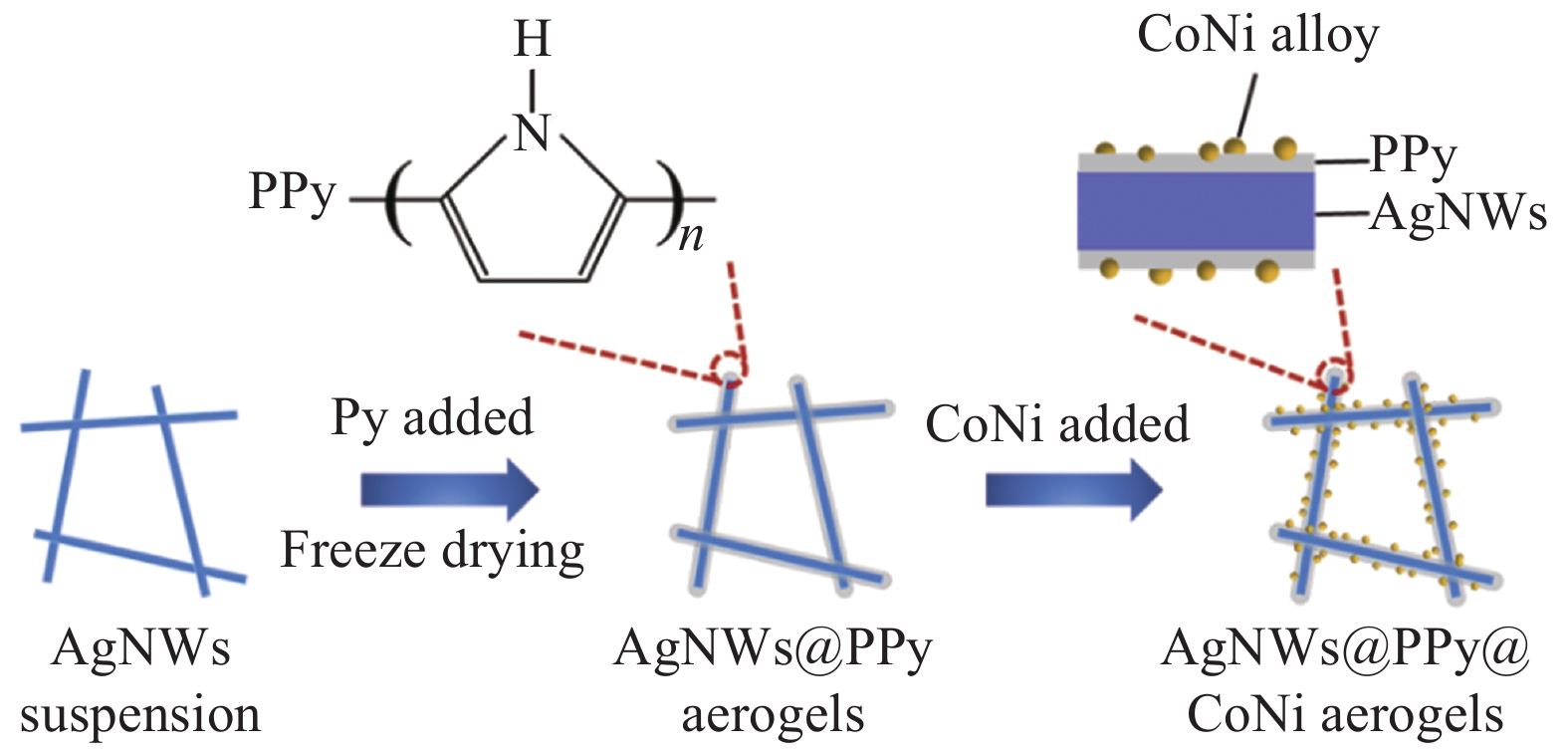
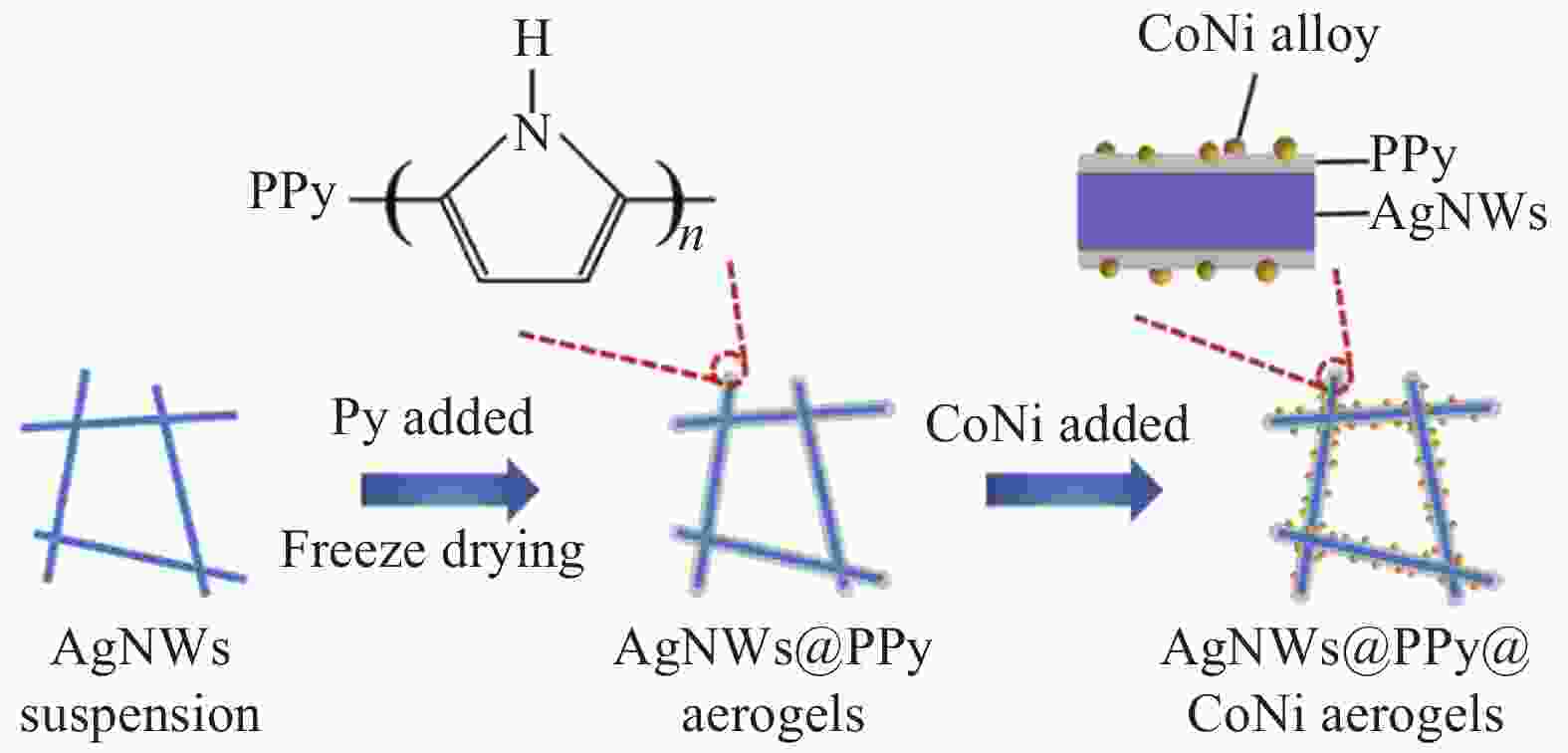
摘要: 针对传统贵金属析氧反应(OER)电催化剂的电导率较低、催化活性较差、成本高等问题,开发高效耐久、低成本且具有高暴露活性表面和优良导电性的电催化剂已迫在眉睫。通过冷冻干燥法合成银纳米线@聚吡咯(AgNWs@PPy)气凝胶,然后使用溶剂热法在AgNWs@PPy气凝胶骨架表面生长纳米CoNi合金,获得了OER电催化性能良好的AgNWs@PPy@CoNi气凝胶。结果表明,随着Co2+、Ni2+浓度的增加,AgNWs@PPy@CoNi气凝胶的催化性能先增强后减弱,当Co2+、Ni2+浓度为0.0175 mol/L制备的AgNWs@PPy@CoNi气凝胶在电流密度10 mA·cm−2时,过电位为346 mV,Tafel斜率为86.25 mV·dec−1,在恒定电压下经过10 h的稳定性测试,电流保持率达93.9%,具有良好的稳定性。三维独立型AgNWs@PPy气凝胶提供优良的导电性,CoNi合金提供丰富的活性位点,二者的共同作用表现出优异的OER催化性能,将有望替代贵金属催化剂而成为新型的OER催化材料。
-
关键词:
- 溶剂热 /
- CoNi合金 /
- AgNWs@PPy气凝胶 /
- 电催化 /
- 析氧反应
Abstract: Given the low conductivity, poor catalytic activity, and high cost of traditional noble metal-oxygen evolution reaction (OER) electrocatalysts, it is urgent to develop electrocatalysts with high efficiency, durability, low cost, high exposed active surface, and excellent electrical conductivity. The Silver nanowire@Polypyrrole (AgNWs@PPy) aerogels were synthesized by freeze-drying method, and then nano-CoNi alloy was grown on the surface of AgNWs@PPy aerogels framework by solvothermal method, and AgNWs@PPy@CoNi aerogels with good OER electrocatalytic performance was obtained. The results show that the catalytic performance of AgNWs@PPy@CoNi aerogels first increase and then weaken with the increase of Co2+ and Ni2+ concentrations. When the Co2+ and Ni2+ concentrations are 0.0175 mol/L, the AgNWs@PPy@CoNi aerogels exhibite high current density. At 10 mA·cm−2, the overpotential is 346 mV, and the Tafel slope is 86.25 mV·dec−1. After a 10-hour stability test under a constant voltage, the current retention rate reaches 93.9%, which has good stability. The three-dimensional independent AgNWs@PPy aerogels provide excellent electrical conductivity, and the CoNi alloy provides abundant active sites. The combined effect of them shows excellent OER catalytic performance, which is expected to replace precious metal catalysts and become a new type of OER catalytic material.-
Key words:
- solvothermal method /
- CoNi alloy /
- AgNWs@PPy aerogels /
- electrocatalysis /
- OER
-
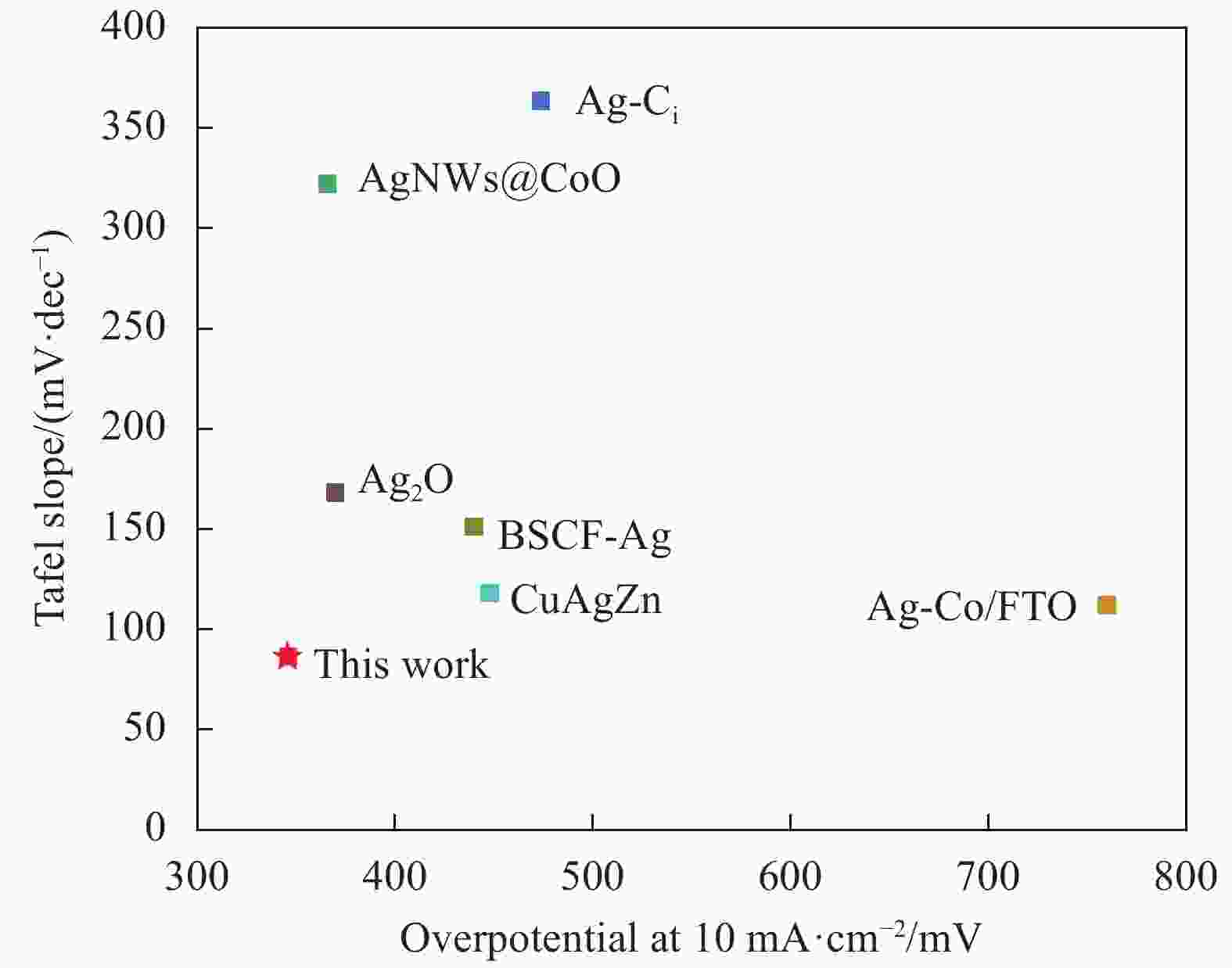
图 12 AgNWs@PPy@CoNi气凝胶与其他Ag基电催化剂在10 mA·cm−2下的过电位和塔菲尔斜率的对比图
Figure 12. Comparison of overpotential and Tafel slope between AgNWs@PPy@CoNi aerogels and other Ag-based electrocatalysts at 10 mA·cm−2
BSCF-Ag—Silver doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ; Ag-Co/FTO—Ag-doped Co3O4 nanowire arrays supported on FTO fluorine-doped tin oxide; Ag-Ci—Catalyst containing silver and carbon
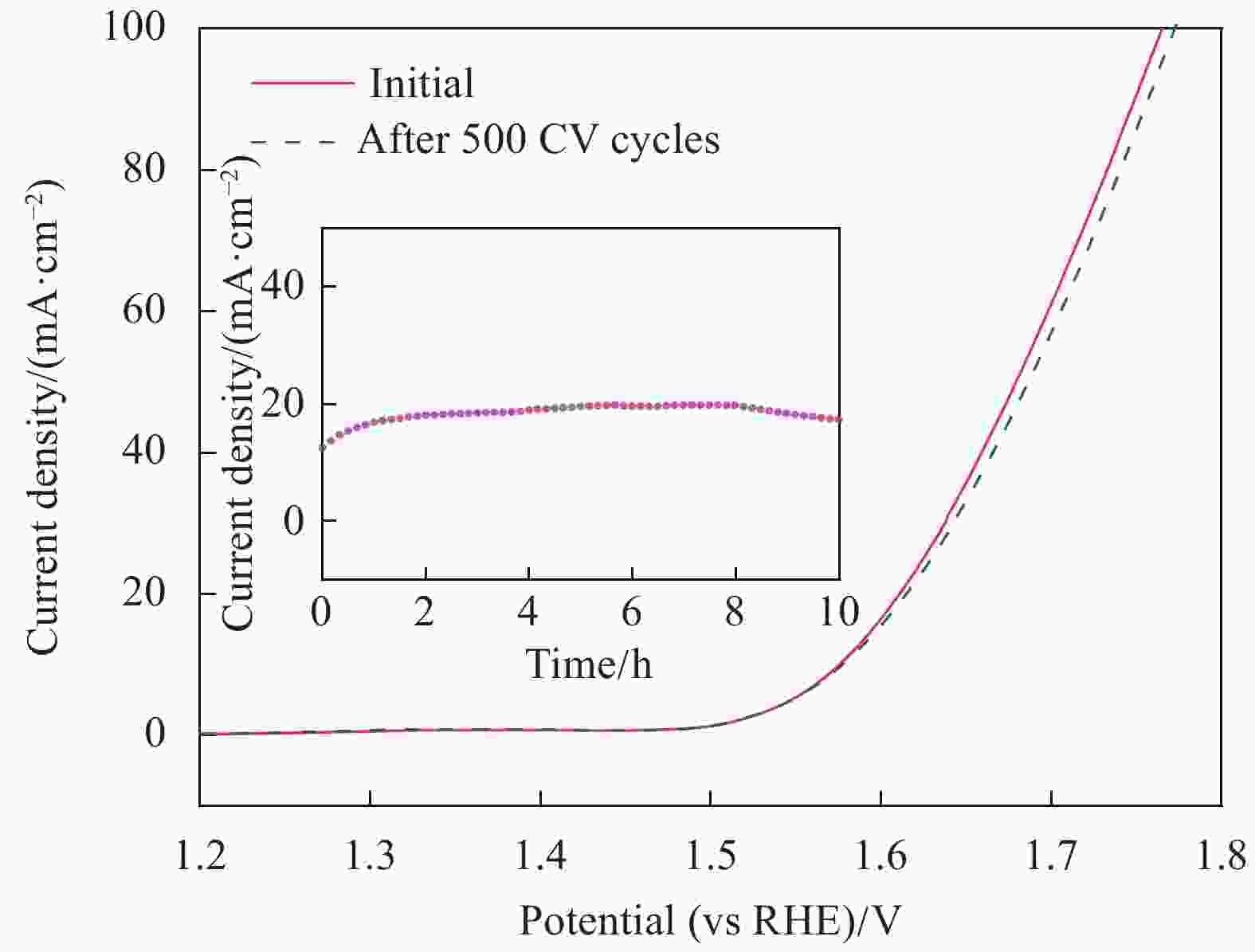
图 13 浓度为0.0175 mol/L的AgNWs@PPy@CoNi气凝胶在500圈CV前后的LSV曲线;插图为浓度0.0175 mol/L的样品在过电位为346 mV时进行10 h的稳定性实验
Figure 13. LSV curve of the 0.0175 mol/L AgNWs@PPy@CoNi aerogels before and after 500 cycles of CV; Inset shows that a sample with a concentration of 0.0175 mol/L was subjected to a stability experiment for 10 h at an overpotential of 346 mV
-
[1] ISLAM D A, BARMAN K, ACHARYA H, et al. Ag-nanoparticle-anchored rGO-coated MIL-88B(Fe) hybrids as robust electrocatalysts for the highly efficient oxygen evolution reaction at neutral pH[J]. ChemElectroChem,2017,4(12):3110-3118. doi: 10.1002/celc.201700883 [2] 张淑娟, 杨婕妤, 张翊青, 等. Pd-Sn-Co纳米粒子修饰还原氧化石墨烯/CuBi2O4复合材料的制备及电催化性能[J]. 复合材料学报, 2020, 37(6):222-229.ZHANG Shujuan, YANG Jieshu, ZHANG Linqing, et al. Preparation and electrocatalytic properties of Pd-Sn-Co nanoparticles modified reduced graphene oxide/CuBi2O4 composites[J]. Acta Materiae Compositae Sinica,2020,37(6):222-229(in Chinese). [3] YU M, MOON G H, CASTILLO R G, et al. Dual role of silver moieties coupled with ordered mesoporous cobalt oxide towards electrocatalytic oxygen evolution reaction[J]. Angewandte Chemie International Edition,2020,59(38):16544-16552. doi: 10.1002/anie.202003801 [4] KIM H, KIM Y, NOH Y, et al. Ultrathin amorphous α-Co(OH)2 nanosheets grown on Ag nanowire surfaces as a highly active and durable electrocatalyst for oxygen evolution reaction[J]. Dalton Transactions,2016:13686-13690. [5] TANG C, WANG H F, CHEN X, et al. Topological defects in metal-free nanocarbon for oxygen electrocatalysis[J]. Advanced Materials,2016,28(32):7030-7030. doi: 10.1002/adma.201670225 [6] ZHANG Y, FAN X, JIAN J, et al. A general polymer-assisted strategy enables unexpected efficient metal-free oxygen-evolution catalysis on pure carbon nanotubes[J]. Energy and Environmental Science,2017,9(11):1-268. [7] ISABELA C M, HAI Y S, FEDERICO C V, et al. Cover picture: universality in oxygen evolution electrocatalysis on oxide surfaces[J]. ChemCatChem,2011,3(7):1085-1085. doi: 10.1002/cctc.201190027 [8] CASALONGUE H G S, NG M L, KAYA S, et al. Insitu observation of surface species on iridium oxide nanoparticles during the oxygen evolution reaction[J]. Angewandte Chemie International Edition,2014,126(28):7297-7300. [9] KIM J, SHIH P C, TSAO K C, et al. High-performance pyrochlore-type yttrium ruthenate electrocatalyst for oxygen evolution reaction in acidic media[J]. Journal of the American Chemical Society,2017,139(34):12076-12083. doi: 10.1021/jacs.7b06808 [10] ROGER I, SHIPMAN M A, SYMES M D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting[J]. Nature Reviews Chemistry,2017,1(1):0003. doi: 10.1038/s41570-016-0003 [11] ZHANG J, ZHAO Z, XIA Z, et al. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions[J]. Nature Nanotechnology,2015,10(5):444-452. doi: 10.1038/nnano.2015.48 [12] WANG B, TANG C, WANG H F, et al. A nanosized CoNi hydroxide@hydroxysulfide core-shell heterostructure for enhanced oxygen evolution[J]. Advanced Materials,2019,31(4):1805658.1-1805658. [13] ZHANG J, LIU J, XI L, et al. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction[J]. Journal of the American Chemical Society,2018,140(11):3876-3879. doi: 10.1021/jacs.8b00752 [14] CHENG C, ZHENG F, ZHANG C, et al. High-efficiency bifunctional electrocatalyst based on 3d freestanding Cu foam in situ armored CoNi alloy nanosheet arrays for overall water splitting[J]. Journal of Power Sources,2019,427(JUL. 1):184-193. [15] SUBBARAMAN R, TRIPKOVIC D, CHANG K, et al. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr(oxy)oxide catalysts[J]. Nature Materials,2012,11(6):550-557. doi: 10.1038/nmat3313 [16] ATKINSON A, BARNETT S, GORTE R J, et al. Advanced anodes for high-temperature fuel cells[J]. Nature Materials,2004,3(1):17-27. doi: 10.1038/nmat1040 [17] ZHANG L, BAIN J A, ZHU J G, et al. Dynamic domain motion of thermal-magnetically formed marks on CoNi/Pt multilayers[J]. Journal of Applied Physics,2006,100(5):2767. [18] NING H G, CHEN Y, ZHANG K K, et al. Porous N-doped carbon-encapsulated CoNi alloy nanoparticles derived from MOFs as efficient bifunctional oxygen electrocatalysts[J]. ACS Applied Materials & Interfaces,2019,11(2):1957-1968. doi: 10.1021/acsami.8b13290 [19] YAN D, LI Y, HUO J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Advanced Materials,2017,29(48):1606459. [20] CHEN D, DONG C L, ZOU Y, et al. In situ evolution of highly dispersed amorphous CoOx clusters for oxygen evolution reaction[J]. Nanoscale,2017,9(33):11969-11975. doi: 10.1039/C7NR04381C [21] DE S, HIGGINS T M, LYONS P E, et al. Silver nanowire networks as flexible, transparent, conducting films: extremely high DC to optical conductivity ratios[J]. ACS Nano,2009,3(7):1767-1774. doi: 10.1021/nn900348c [22] ZHAO X, ZHANG H, YAN Y, et al. Engineering the electri-cal conductivity of lamellar silver-doped cobalt(Ⅱ) selenide nanobelts for enhanced oxygen evolution[J]. Angewandte Chemie International Edition,2017,9(33):11969-11975. [23] 胡金娟, 马春雨, 王佳琳, 等. Ag-Ag2O/TiO2-g-C3N4纳米复合材料的制备及可见光催化性能[J]. 复合材料学报, 2020, 37(6):181-190.HU Jinjuan, MA Chunyu, WANG Jialin, et al. Preparation and photocatalytic properties of Ag-Ag2O/TiO2-g-C3N4 nanocomposites[J]. Acta Materiae Compositae Sinica,2020,37(6):181-190(in Chinese). [24] HOU Y, LIU Y, GAO R, et al. Ag@CoxP core@shell heterogeneous nanoparticles as efficient oxygen evolution reaction catalysts[J]. ACS Catalysis,2017,7(10):7038-7042. doi: 10.1021/acscatal.7b02341 [25] GL E Y, AYTEKIN A, KARABUDAK E, et al. Investigation of oxygen evolution reaction performance of Ag doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite structure[J]. Journal of Applied Electrochemistry,2020(7411):1037-1043. [26] MAKHLOUFI L, HAMMACHE H, SAIDANI B. Electrocataly-tic reduction of proton on polypyrrole coatings onto aluminium modified by the electrochemical cementation process[J]. Electrochemistry Communications,2000,2(8):552-556. doi: 10.1016/S1388-2481(00)00081-3 [27] AGHA H, FLEURY J B, GALERNE Y. Polypyrrole coating films for nanoparticles[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects,2014,462:217-224. [28] HE W, LI G, ZHANG S, et al. Polypyrrole/silver coaxial nanowire aero-sponges for temperature-independent stress sensing and stress-triggered joule heating[J]. ACS Nano,2015,9(4):4244-4251. doi: 10.1021/acsnano.5b00626 [29] YUKSEL R, ALPUGAN E, UNALAN H E. Coaxial silver nanowire/polypyrrole nanocom-posite supercapacitors[J]. Organic Electronics,2018,52:272-282. doi: 10.1016/j.orgel.2017.10.012 [30] QIAO M T, WEI D, HE X, et al. Novel yolk-shell Fe3O4@void@SiO2@PPy nanochains toward microwave absorption application[J]. Journal of Materials Science,2021,56(2):1-16. [31] JITKA S, ŠKODOVá K, DUŠAN M, et al. Polypyrrole-silver composites prepared by the reduction of silver ions with polypyrrole nanotubes[J]. Polymer Chemistry,2013,4(12). [32] FENG X, YE Q, HOU W, et al. Ag/polypyr-role core-shell nanostructures: Interface poly-merization, characterization, and modification by gold nanoparticles[J]. The Journal of Physical Chemistry C,2007,111(24):1493-1504. [33] MAO H, GUO X, FU Y, et al. Efficiently improving oxygen evolution activity using hierarchical α-Co(OH)2/polypyrrole/graphene oxide nanosheets[J]. Applied Surface Science,2019,485(15):554-563. [34] CHENG M, WEN M, ZHOU S, et al. Solvothermal synthesis of NiCo alloy icosahedral nanocrystals[J]. Inorganic Chemistry,2012,51(3):1495-1500. doi: 10.1021/ic201763j [35] QIAO M, LEI X, MA Y, et al. Application of yolk-shell Fe3O4@N-doped carbon nanochains as highly effective microwave-absorption material[J]. Nano Research,2018,11:1500-1519. doi: 10.1007/s12274-017-1767-0 [36] RAFIQUE M Y, PAN L, KHAN W S, et al. Controlled synthesis, phase formation, growth mechanism, and magnetic properties of 3-D CoNi alloy microstructures composed of nanorods[J]. CrystEngComm,2013,15(26):5314-5325. doi: 10.1039/c3ce40385h [37] NING H H, LI G Q, CHEN Y, et al. Porous N-doped carbon encapsulated CoNi alloy nanoparticles derived from MOFs as efficient bifunctional oxygen electrocatalysts[J]. ACS Applied Materials and Interfaces,2018,11(2):1957-1968. [38] YAN K L, CHI J Q, LIU Z Z, et al. Coupling Ag-doping and rich oxygen vacancies in mesoporous NiCoO nanorods supported on nickel foam for highly efficient oxygen evolution[J]. Inorganic Chemistry Frontiers,2017,4(11):1783-1790. [39] XIAO D J, MA J, CHEN C L, et al. Oxygen-doped carbonaceous polypyrrole nanotubes-supported Ag nanoparticle as electrocatalyst for oxygen reduction reaction in alkaline solution[J]. Materials Research Bulletin,2018,105:184-191. doi: 10.1016/j.materresbull.2018.04.030 [40] SINGU B S, YOON K R. Highly exfoliated GO-PPy-Ag ternary nanocomposite for electr-ochemical supercapacitor[J]. Electrochimica Acta,2018,268(1):304-315. [41] DIONIGI F, ZENG Z, SINEV I, et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution[J]. Nature Communications,2020,11(1):2522-2531. doi: 10.1038/s41467-020-16237-1 [42] YAN K L, CHI J Q, XIE J Y, et al. Mesoporous Ag-doped Co3O4 nanowire arrays supported on FTO as efficient electrocatalysts for oxygen evolution reaction in acidic media[J]. Renewable Energy,2018,119:54-61. doi: 10.1016/j.renene.2017.12.003 [43] LIU X W, WANG R C, GUO R, et al. Construction of alternating layered quasi-three-dimensional electrode AgNWs/CoO for water splitting: A discussion of catalytic mechanism[J]. Electrochimica Acta,2019,317(10):468-477. [44] ZHAO Q, YU Z, YUAN W, et al. Metal-Ci oxygen-evolving catalysts generated in situ in a mild H2O/CO2 environment[J]. International Journal of Hydrogen Energy,2013,38(13):5251-5258. doi: 10.1016/j.ijhydene.2013.02.034 [45] HUANG X, XIE M, CHEN Y, et al. Copper-silver oxide nanowires grown on an alloy electrode as an efficient electrocatalyst for water oxidation[J]. RSC Advances,2015,5(33):26150-26156. doi: 10.1039/C5RA00820D -





 下载:
下载: