Design of BiMn-doped Pd modified GO/MOF-74-Co composites for electrocatalytic oxidation of ethylene glycol
-
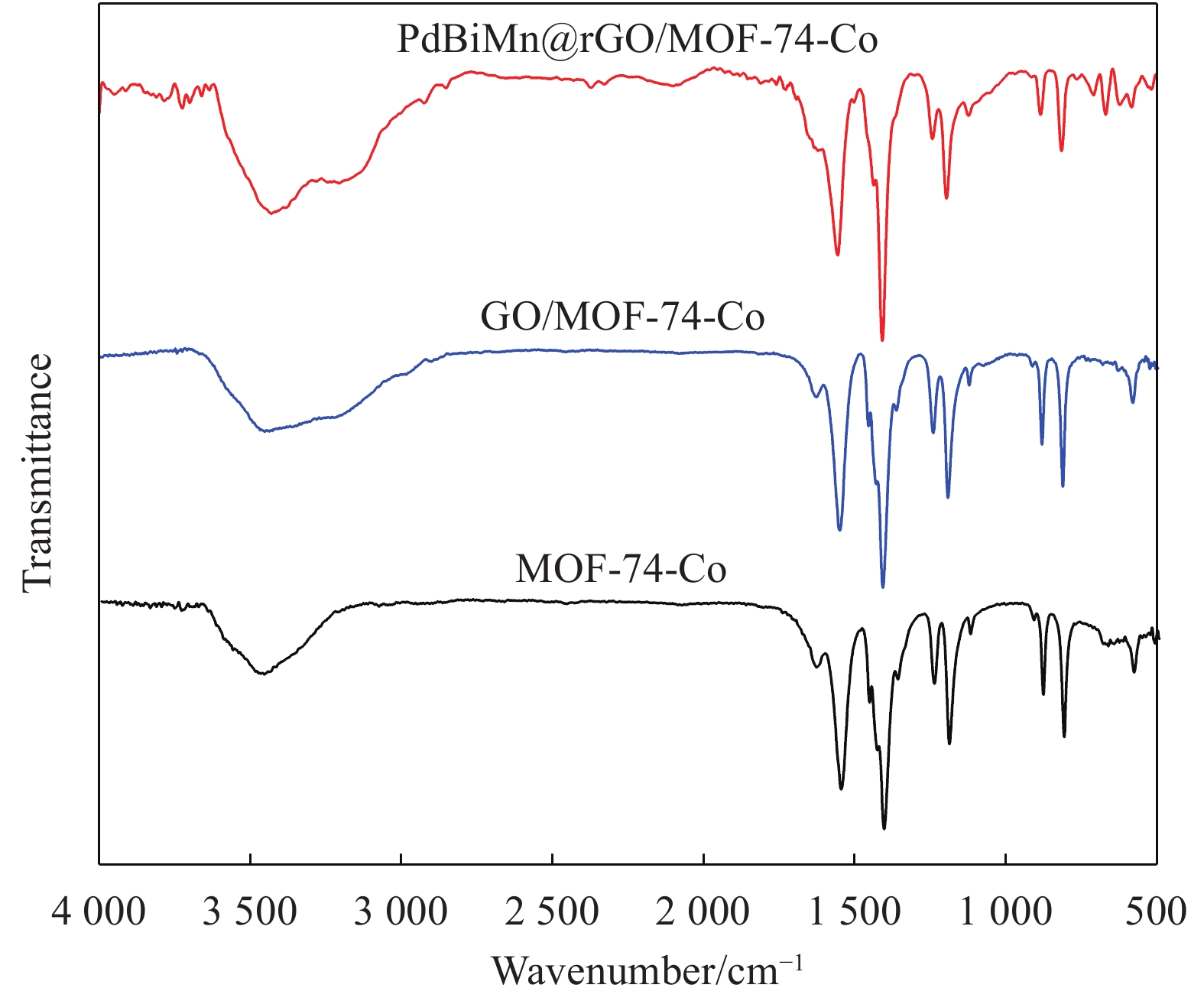
摘要: 将BiMn共掺Pd的合金纳米粒子成功地修饰到三维棱锥柱状的还原氧化石墨烯(rGO)/金属有机骨架(MOFs)-74-Co上,得到一种新型复合电催化剂PdBiMn@rGO/MOF-74-Co,其中Pd-Bi-Mn的平均粒径约7.8 nm,MOF-74-Co为多孔的面心六角单元结构。该PdBiMn@rGO/MOF-74-Co催化剂在碱性条件下电催化氧化乙二醇(EG)表现出最高的催化活性和耐久性。优异的电催化性能主要归结于Pd-Bi-Mn纳米合金的形成,其具有强的协同效应和较多的反应活性中心,有利于含氧物种的吸附,使其抗毒性和稳定性显著增强;同时载体rGO/MOF-74-Co独特的微结构使催化剂具有优异的电子传递性能和多孔特性。此外,对EG的电催化氧化机制给出了详细分析。这种新型复合材料的设计和杰出的催化性能为直接醇类燃料电池(DAFCs)的开发和应用提供了重要参考。
-
关键词:
- Pd-Bi-Mn纳米合金 /
- 氧化石墨烯 /
- MOF-74-Co /
- 电催化氧化 /
- 乙二醇
Abstract: BiMn co-doped Pd alloy nanoparticles were successfully modified on the 3D pyramidal columnar-like reduced graphene oxide (rGO)/metal-organic frameworks (MOFs)-74-Co to obtain a new composite electrocatalyst PdBiMn@rGO/MOF-74-Co in which the mean particle diameter of Pd-Bi-Mn particles was about 7.8 nm, and MOF-74-Co was a porous face-centered hexagonal unit cell. PdBiMn@rGO/MOF-74-Co catalyst exhibites the highest catalytic activity and durability towards ethylene glycol (EG) electrooxidation under alkaline condition. The excellent electrocatalytic performance is mainly attributed to the strong synergistic effects of Pd, Bi and Mn nanoalloys and abundant reaction active sites in the catalyst, which are beneficial to adsorb more oxygen-containing substances and significantly enhance the resistance to toxicity and stability of the ternary catalyst. Furthermore, the unique microstructure of rGO/MOF-74-Co can provide superior electron transfer properties and multi-porous for the PdBiMn@rGO/MOF-74-Co electrocatalyst. Besides, the mechanism of electrocatalytic oxidation of EG drew up a detailed analysis. The novel composites’ design and outstanding performance would provide an essential reference for the application and development of direct alcohol fuel cells (DAFCs).-
Key words:
- Pd-Bi-Mn nanoalloys /
- graphene oxide /
- MOF-74-Co /
- electrocatalytic oxidation /
- ethylene glycol
-
图 3 GO/MOF-74 (a)和PdBiMn@rGO/MOF-74-Co ((b)、(c))的FE-SEM图像及Co、C、O、Mn、Pd、Bi HAAD-SSEM元素映射(d)和EDS元素分布(e)图谱
Figure 3. FE-SEM images of GO/MOF-74-Co (a) and PdBiMn@rGO/MOF-74-Co ((b),(c)) and HAAD-SSEM elemental mapping of Co, C, O, Mn, Pd, Bi (d) and statistics of EDS element distribution of PdBiMn@rGO/MOF-74-Co (e)
图 8 PdBiMn@rGO/MOF-74-Co在1 mol/L KOH和0.5 mol/L EG溶液中不同扫描速率下的循环伏安图(a)和四种不同催化剂的峰值电流密度与扫描速率平方根(V1/2)曲线图(b)
Figure 8. Cyclic voltammograms of PdBiMn@rGO/MOF-74-Co in 1 mol/L KOH and 0.5 mol/L EG solution at various scan rates (a) and curves of peak current density of four different catalysts and square root of the scanning rate (V1/2) (b)
-
[1] HU T, WANG Y, XIAO H, et al. Shape-control of super-branched Pd-Cu alloys with enhanced electrocatalytic performance for ethylene glycol oxidation[J]. Chemical Communications,2018,54(95):13363-13366. doi: 10.1039/C8CC06901H [2] XIE Y X, CEN S Y, MA Y T, et al. Facile synthesis of platinum-rhodium alloy nanodendrites as an advanced electrocatalyst for ethylene glycol oxidation and hydrogen evolution reactions[J]. Journal of Colloid and Interface Science,2020,579:250-257. doi: 10.1016/j.jcis.2020.06.061 [3] ZHOU H C, LONG J R, YAGHI O M. Introduction to metal-organic frameworks[J]. Chemical Reviews,2012,112:673-674. doi: 10.1021/cr300014x [4] WU H B, LOU X W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: promises and challenges[J]. Science Advances,2017,3(12):928-935. [5] STRAUSS I, MUNDSTOCK A, HINRICHS D, et al. On the interaction of guest molecules with Co-MOF-74: A Vis/NIR and Raman approach[J]. Angewandte Chemie International Edition,2018,57(25):7434-7439. doi: 10.1002/anie.201801966 [6] JIANG H, WANG Q, WANG H. et al. MOF-74 as an efficient catalyst for the low-temperature selective catalytic reduction of NOx with NH3[J]. ACS Applied Materials & Interfaces,2016,8(40):26817-26826. [7] FERNANDEZ V, ABDELKADER C K, FERNANDES D M, et al. Noble metal-free MOF-74 derived nanocarbons: insights on metal composition and doping effects on the electrocatalytic activity towards oxygen reactions[J]. ACS Applied Energy Materials,2019,2(3):1854-1867. doi: 10.1021/acsaem.8b02010 [8] HE Q, JI J, ZHANG Q, et al. PdMn and PdFe nanoparticles over a reduced graphene oxide carrier for methanol electro-oxidation under alkaline conditions[J]. Ionics,2020,26:2421-2433. doi: 10.1007/s11581-019-03343-4 [9] GEORGAKILAS V, TIWARI J N, KEMP K C, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic and biomedical applications[J]. Chemical Reviews,2016,116(9):5464-5519. doi: 10.1021/acs.chemrev.5b00620 [10] CAI J, HUANG Y, GUO Y. Bi-modified Pd/C catalyst via irreversible adsorption and its catalytic activity for ethanol oxidation in alkaline medium[J]. Electrochimica Acta,2013,99(6):22-29. [11] WAQAS M, LAN J, ZHANG X, et al. Fabrication of non-enzymatic electrochemical glucose sensor based on Pd-Mn alloy nanoparticles supported on reduced graphene oxide[J]. Electroanalysis,2020,32:1-12. doi: 10.1002/elan.201900434 [12] XU H, YAN B, ZHANG K, et al. Synthesis and characterization of core-shell PdAu convex nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation[J]. Journal of Alloys & Compounds,2017,723:36-42. [13] MENG X, GENG D, LIU J, et al. Controllable synthesis of graphene-based titanium dioxide nanocomposites by atomic layer deposition[J]. Nanotechnology,2011,22(16):165602-165612. doi: 10.1088/0957-4484/22/16/165602 [14] ZHANG S J, LIU L, YANG J Y, et al. Pd-Ru-Bi nanoalloys modified three-dimensional reduced graphene oxide/MOF-199 composites as a highly efficient electrocatalyst for ethylene glycol electrooxidation[J]. Applied Surface Science,2019,492:617-625. doi: 10.1016/j.apsusc.2019.06.228 [15] ADHIKARI A K, LIN K S. Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74 (Ni, Co) by doping palladium-containing activated carbon[J]. Chemical Engineering Journal,2016,284:1348-1360. doi: 10.1016/j.cej.2015.09.086 [16] LIANG M, HUI H, HSU A, et al. PdRu/C catalysts for ethanol oxidation in anion-exchange membrane direct ethanol fuel cells[J]. Journal of Power Sources,2013,241:696-702. doi: 10.1016/j.jpowsour.2013.04.051 [17] SHEN S Y, ZHAO T S, XU J B, et al. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells[J]. Journal of Power Sources,2010,195(4):1001-1006. doi: 10.1016/j.jpowsour.2009.08.079 [18] NETO A, TUSI M, POLANCO N, et al. PdBi/C electrocatalysts for ethanol electro-oxidation in alkaline medium[J]. International Journal of Hydrogen Energy,2011,36(17):10522-10526. doi: 10.1016/j.ijhydene.2011.05.154 [19] YUAN X L, ZHANG Y, CAO M, et al. Bi(OH)3/PdBi composite nanochains as highly active and durable electrocatalysts for ethanol oxidation[J]. Nano Letter,2019,19(7):4752-4759. doi: 10.1021/acs.nanolett.9b01843 [20] LIAO H, ZHU J, HOU Y. Synthesis and electrocatalytic properties of PtBi nanoplatelets and PdBi nanowires[J]. Nanoscale,2013,6(2):1049-1055. [21] ZHANG M, GAO J P, HONG W, et al. Bimetallic Mn and Co encased within bamboo-like N-doped carbon nanotubes as efficient oxygen reduction reaction electrocatalysts[J]. Journal of Colloid and Interface Science,2019,537:238-246. doi: 10.1016/j.jcis.2018.11.022 [22] CHENG Y, GUO M S, YU Y, et al. Fabrication of coral-like Pd based porous MnO2 nanosheet arrays on nickel foam for methanol electrooxidation[J]. Industrial & Engineering Chemistry Research,2018,57(32):10893-10904. [23] GU Z L, XIONG Z P, REN F F, et al. Flower-like PdCu catalyst with high electrocatalytic properties for ethylene glycol oxidation[J]. Journal of the Taiwan Institute of Chemical Engineers,2018,83:32-39. doi: 10.1016/j.jtice.2017.12.010 [24] WAN Z R, BAI X, MO H, et al. Multi-porous NiAg-doped Pd alloy nanoparticles immobilized on reduced graphene oxide/CoMoO4 composites as a highly active electrocatalyst for direct alcohol fuel cell[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2021,614:126048. [25] LIU J G, ZHAO T S, CHEN R, et al. The effect of methanol concentration on the performance of a passive DMFC[J]. Electrochemistry Communications,2005,7(3):288-294. doi: 10.1016/j.elecom.2005.01.011 [26] XU H, SONG P P, FERNEZ C, et al. Sophisticated construction of binary PdPb alloy nanocubes as robust electrocatalysts toward ethylene glycol and glycerol oxidation[J]. ACS Applied Materials & Interfaces,2018,10(15):12659-12665. -






 下载:
下载: