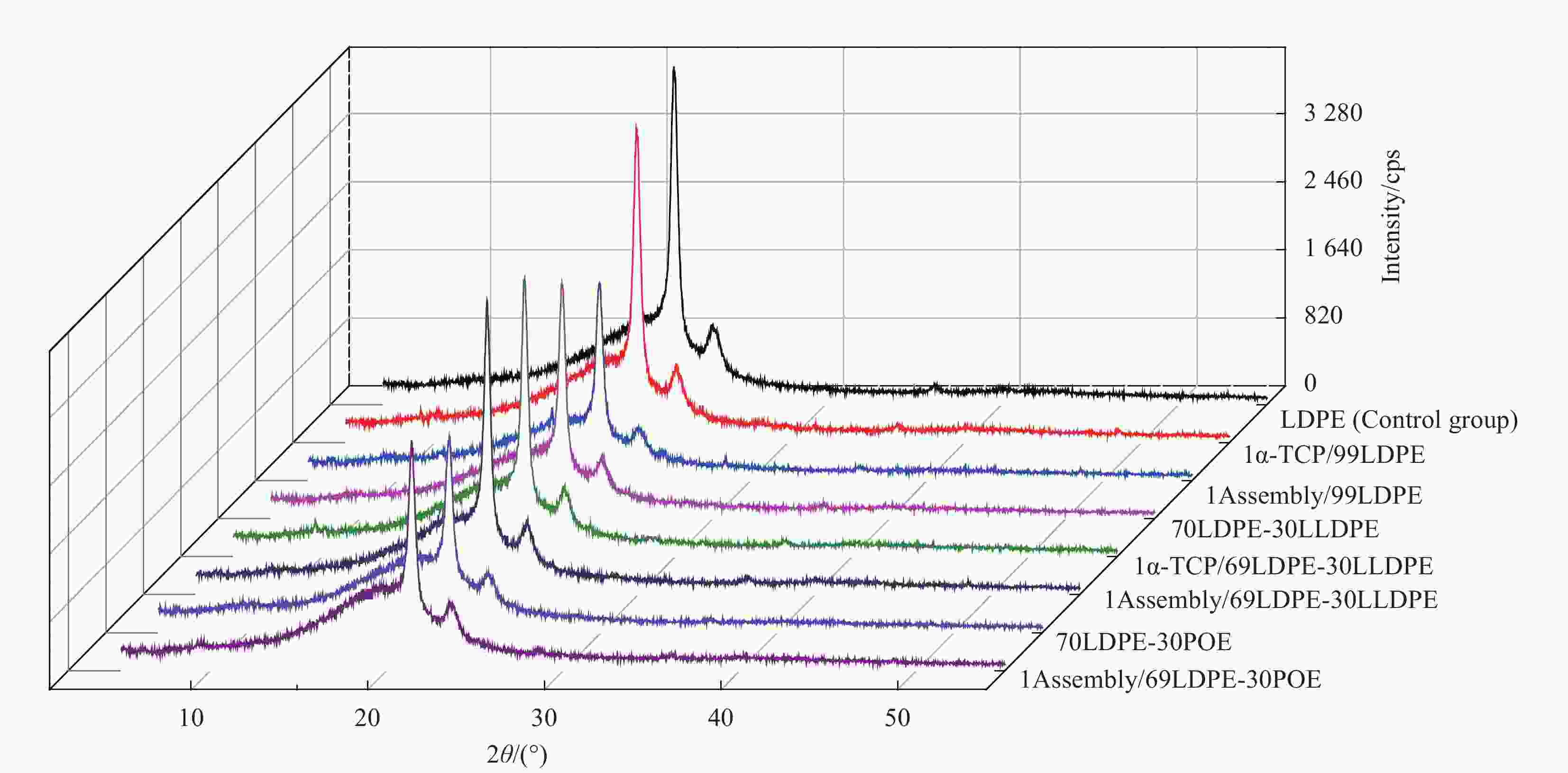
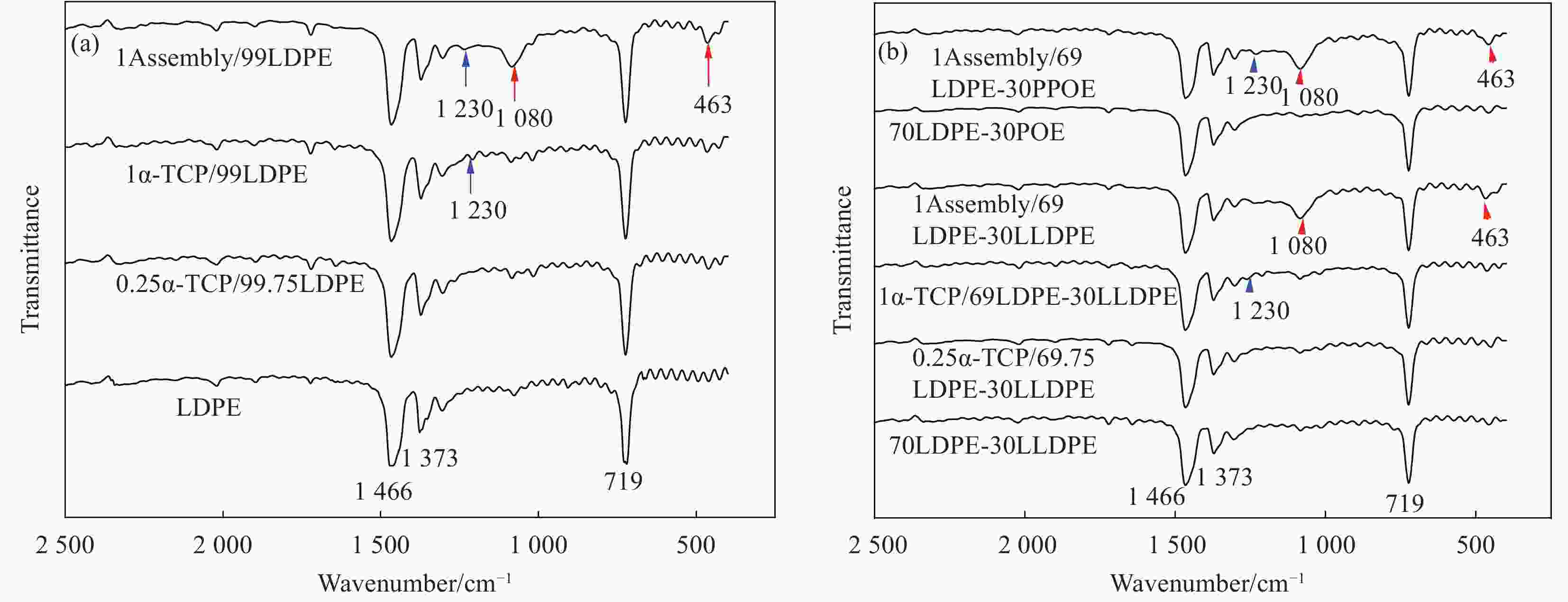
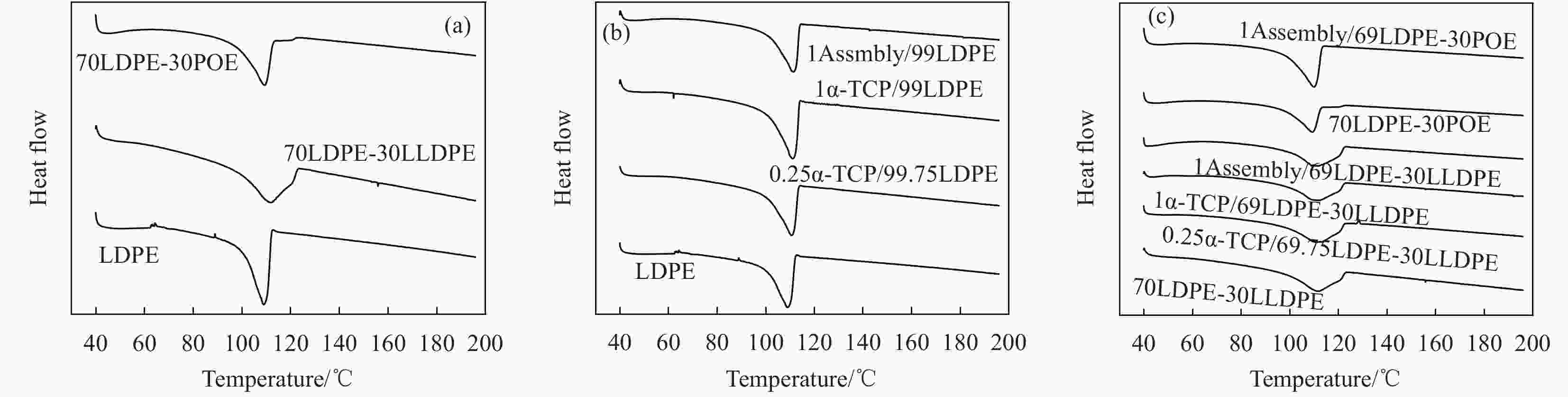
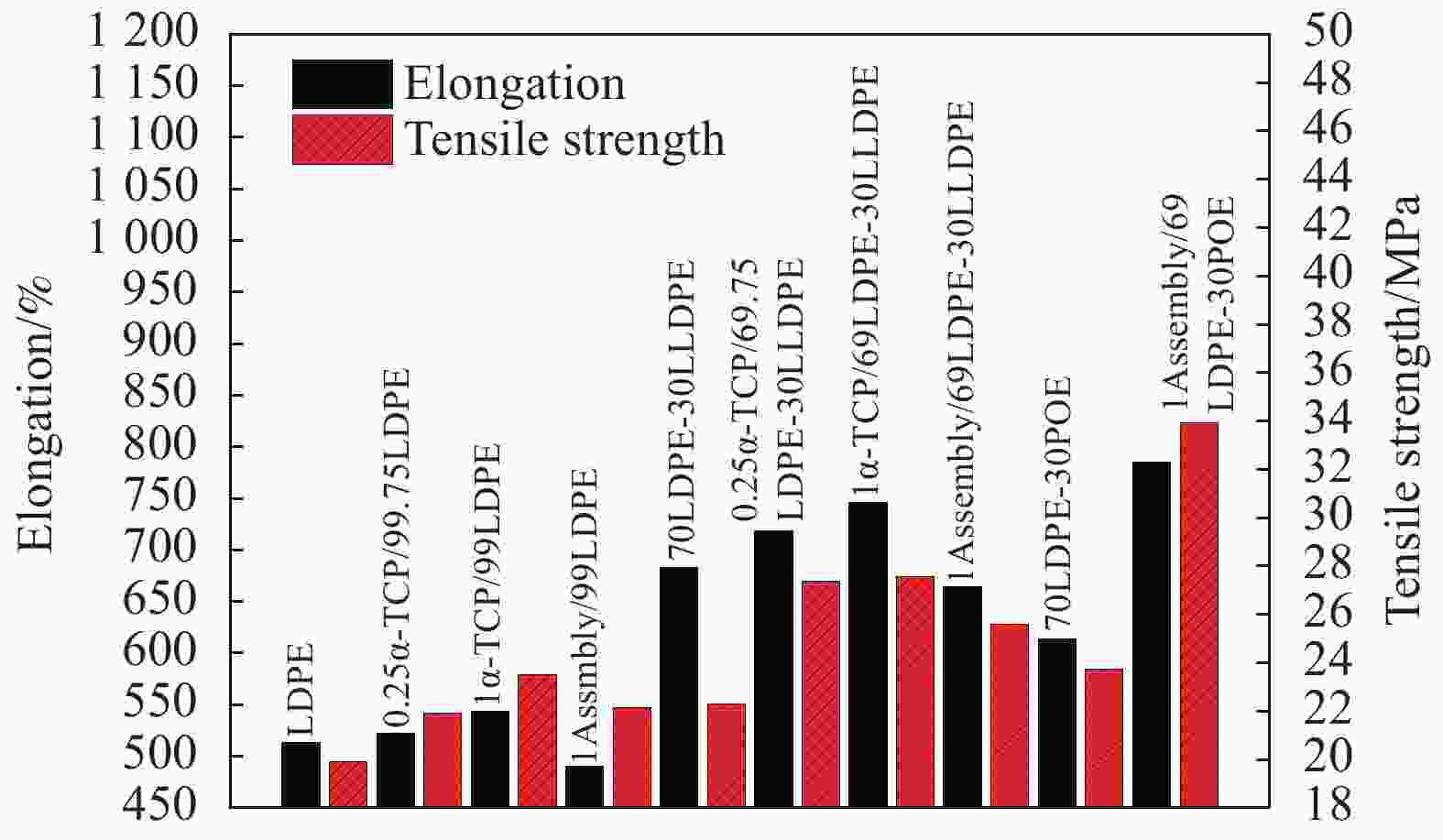
Preparation and properties of polyethylene-based blended films base on mesoporous nano-SiO2 antioxidant active assembly
-
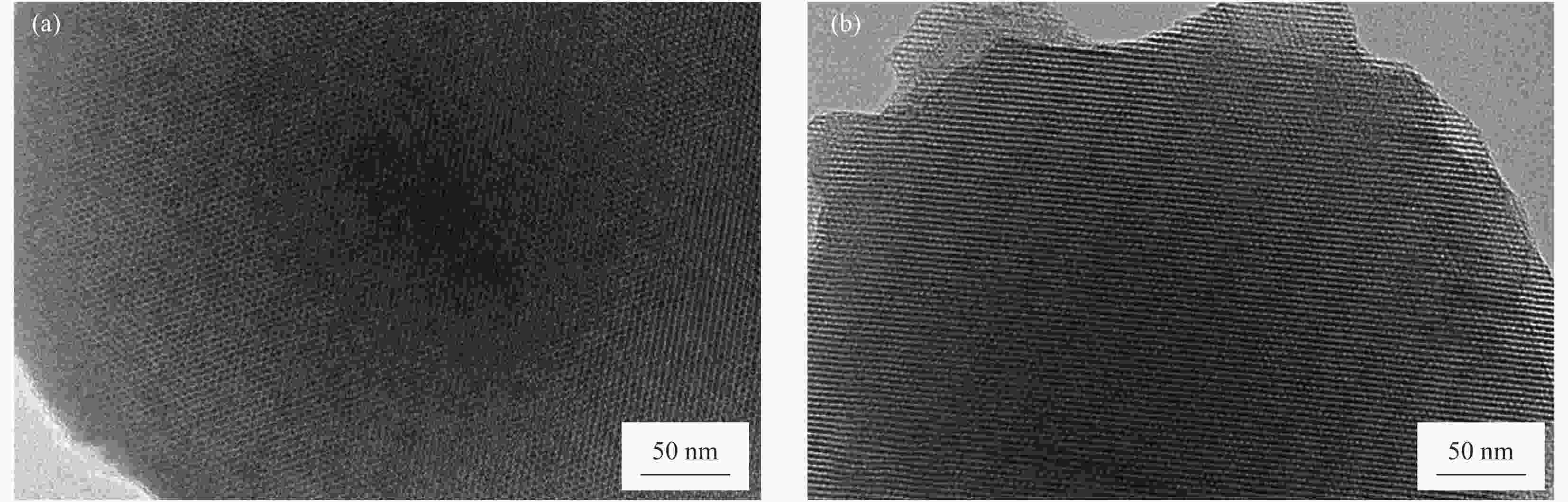
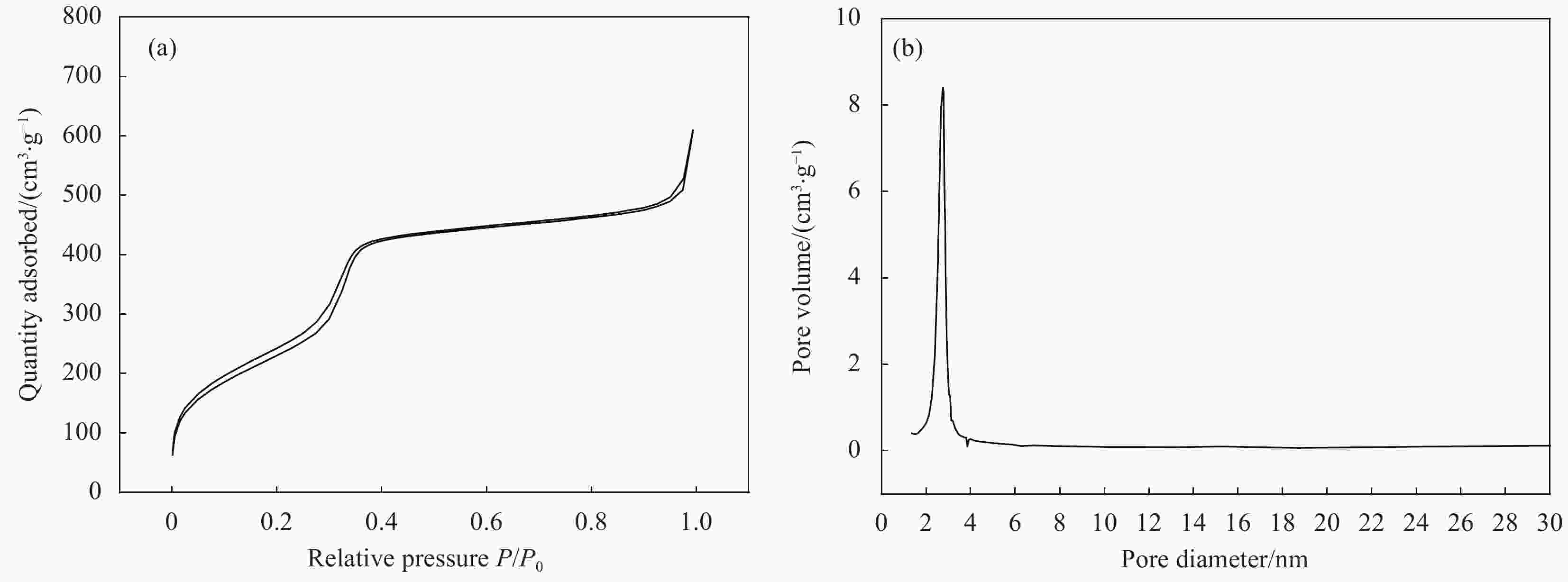
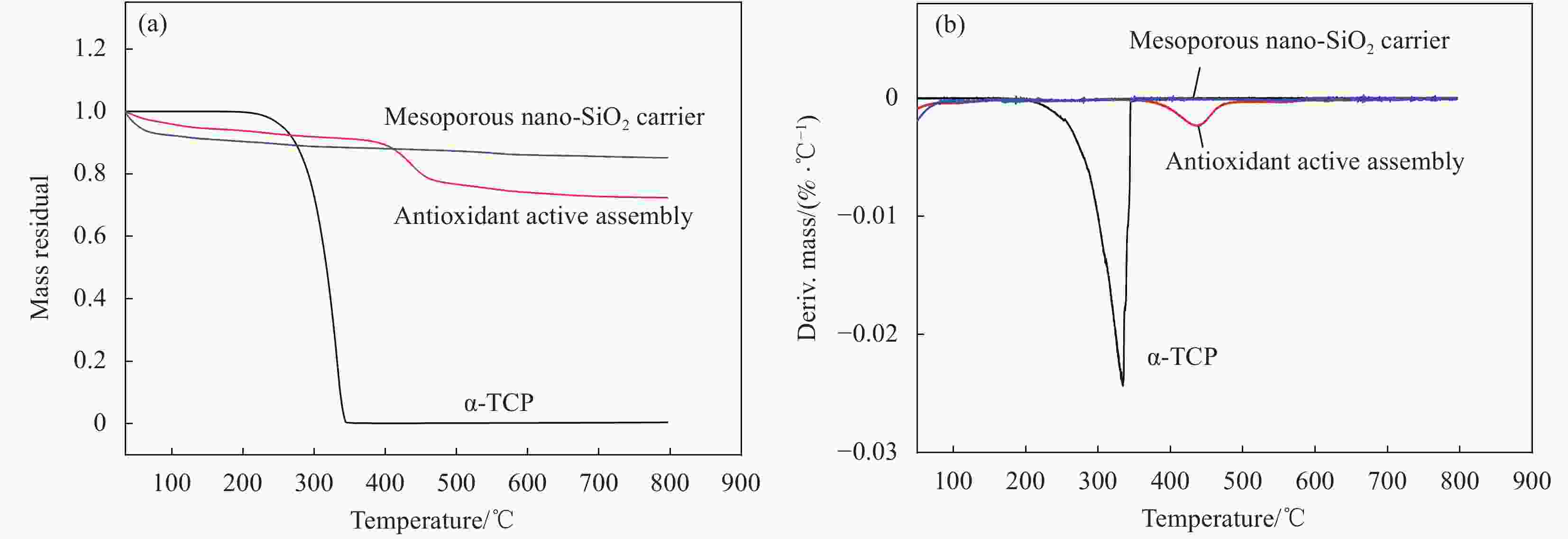
摘要: 以十六烷基三甲溴化铵(CTAB)为模板剂,制备介孔纳米SiO2,以其为载体搭载α-生育酚形成抗氧活性组装体(简称组装体)。以组装体为抗氧活性因子,以低密度聚乙烯(LDPE)为基材,引入线性低密度聚乙烯(LLDPE)、聚烯烃类热塑性弹性体(POE)进行共混改性,并以挤出流延法制备系列控释型抗氧活性膜。通过TEM、XRD、氮气吸附脱附、TGA、力学性能测试和释放性能测试等,研究了LLDPE、POE、α-生育酚、组装体四种添加物对活性膜力学、控释等性能的影响,并研究添加物间协同作用。结果表明,引入添加物令活性膜热加工、力学、控释性能显著提升:α-生育酚搭载于组装体后热分解温度提升70%,利于活性膜热加工;各添加物皆不同程度提升薄膜力学性能,其中,POE及组装体的组合添加具有协同增强作用,薄膜拉伸强度提升率为其他样品2.5倍以上;活性膜控释性能显著,组装体令α-生育酚扩散系数下降45%~47%,薄膜结晶度与α-生育酚释放速率呈负相关,利于活性膜释放调控。所制备活性膜可用于软塑活性包装释放调控,且力学、热加工等性能优于改性前,在功能性活性包装材料领域具有广阔的应用前景。Abstract: The mesoporous nano-SiO2 carrier was prepared with cetyltrimethylammonium bromide (CTAB) that served as template. Then α-tocopherol (α-TCP) was integrated into the carrier to form antioxidant active assembly. Low-density polyethylene (LDPE) active films were prepared by flat extrusion. Linear low-density polyethylene (LLDPE) and polyolefin thermoplastic elastomer (POE) were used as modified resin to regulate the films for better performance. The assembly and α-TCP were introduced into the films, serving as antioxidant active factor. The structure of the nano-SiO2 carrier was studied by TEM, XRD and nitrogen adsorption/desorption apparatus. The thermal stability of the assembly was investigated by thermal gravimetric analysis (TGA). The physical and controlled release properties of the films were determined by mechanical performance test, release performance test, etc. Meanwhile, the additives’ synergistic effects on the films were studied. The results show that the active films’ thermal processing, mechanical and controlled release properties are significantly improved with introduction of the additives. The assembly’s insulating effect leads to thermal resistant temperature of α-TCP, which is integrated into assembly, increase by 70% when compared with the bared α-TCP. As compared with pure LDPE film, the mechanical properties of the active films are significantly improved by the additives. The tensile strength increase rate of the film modified by POE and assembly is more than 2.5 times as other samples’. As compared with the films modified with the bared α-TCP, the assembly reduces the diffusion coefficient of α-TCP by 45%-47%, which shows the remarkable controlled release property of the active films. The films’ crystallinity is negatively correlated with release rate of the antioxidant substance. As a result, the prepared materials can be used to regulate active substances’ release rate of antioxidant active package. And it shows a good potential for strengthing and toughing package films.
-
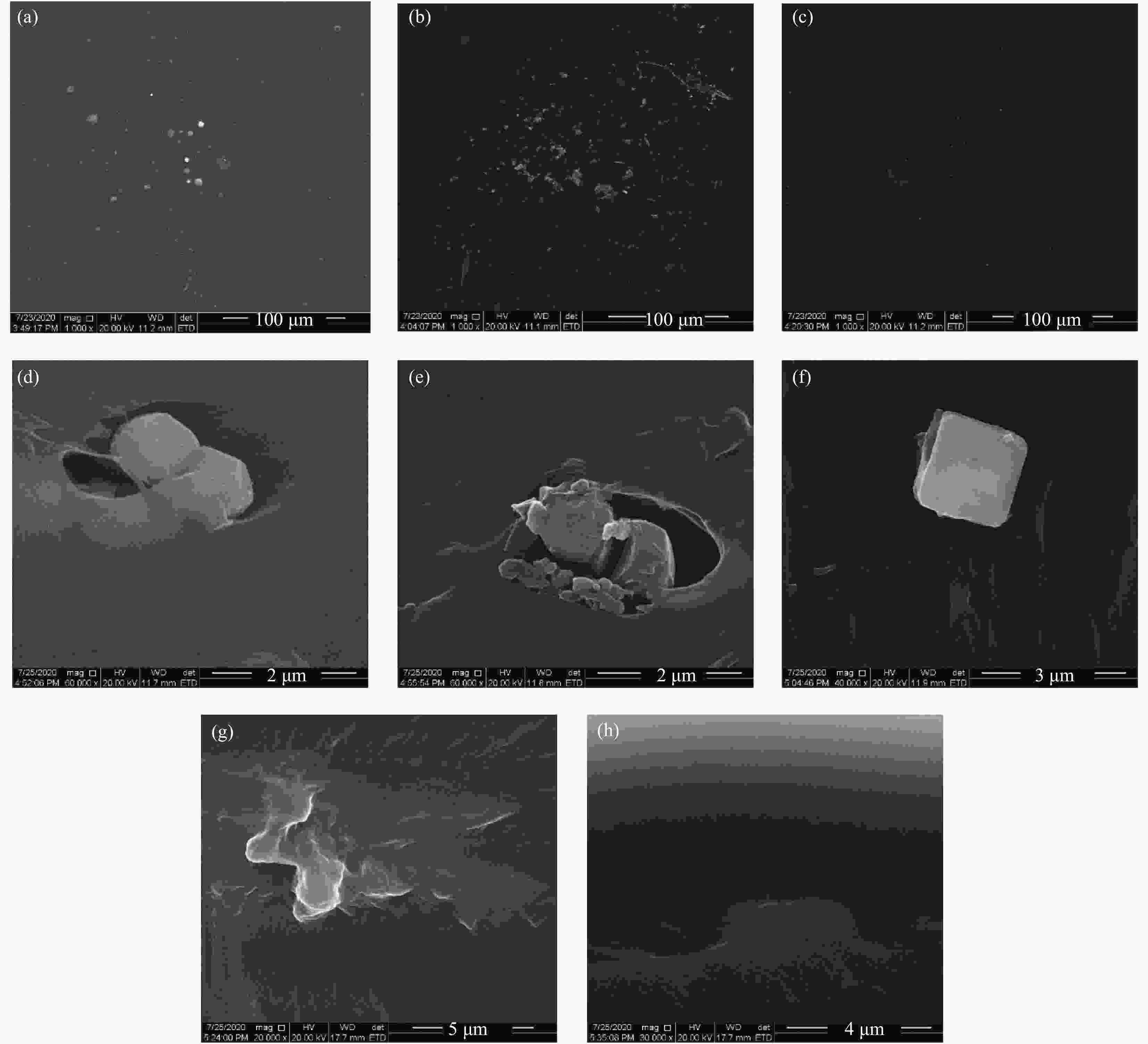
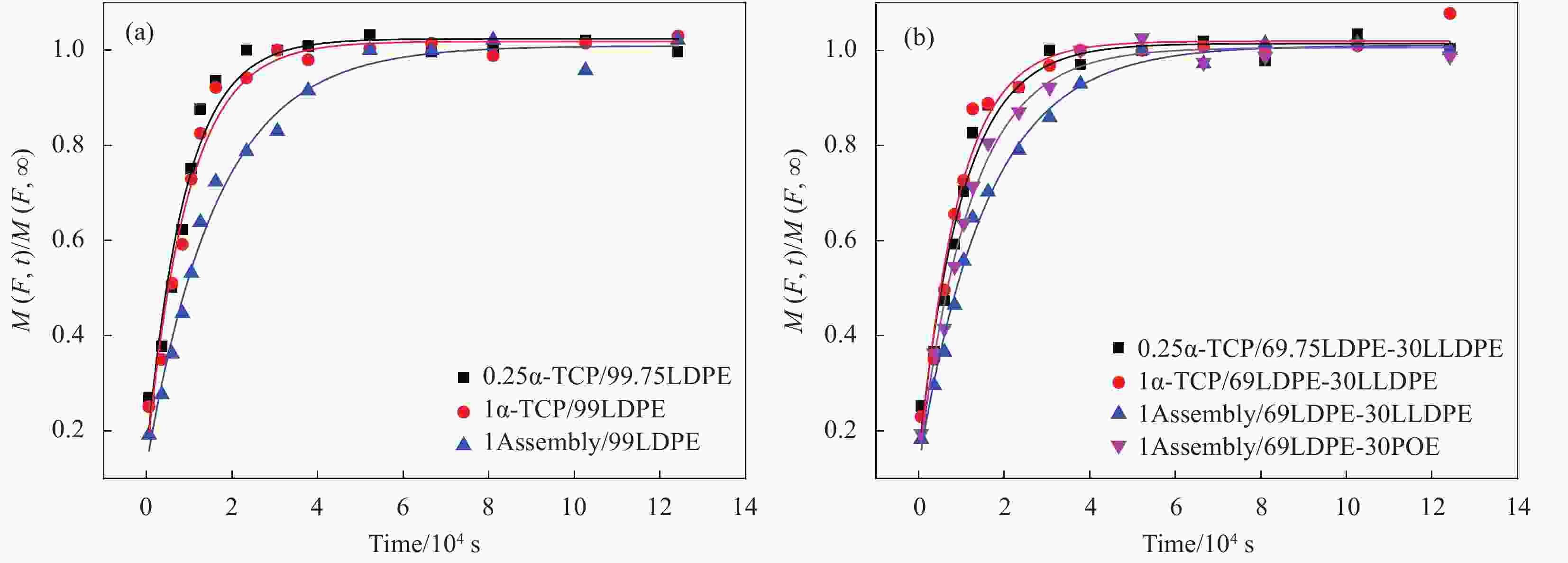
图 5 薄膜表面和截面SEM图像:(a) 1Assembly/99LDPE表面;(b) 1Assembly/69LDPE-30LLDPE表面;(c) 1Assembly/69LDPE-30POE 表面;(d) 1Assembly/99LDPE截面;(e) 1Assembly/69LDPE-30LLDPE截面;(f) 1Assembly/69LDPE-30POE 截面;(g) 1α-TCP/99LDPE 截面; (h) 1α-TCP/69LDPE-30LLDPE截面
Figure 5. SEM images of surface and cross section of films: (a) 1Assembly/99LDPE surface; (b) 1Assembly/69LDPE-30LLDPE surface; (c) 1Assembly/69LDPE-30POE surface; (d) 1Assembly/99LDPE section; (e) 1Assembly/ 69LDPE-30LLDPE section; (f) 1Assembly/69LDPE-30POE Section; (g) 1α-TCP/99LDPE section; (h) 1α-TCP/69LDPE-30LLDPE
表 1 薄膜配方
Table 1. Formula of films
Sample α-TCP/g Assembly/g LDPE/g LLDPE/g POE/g LDPE 0 0 200 0 0 0.25α-TCP/99.75LDPE 0.5 0 199.5 0 0 1α-TCP/99LDPE 2 0 198 0 0 1Assembly/99LDPE 0 2 198 0 0 70LDPE-30LLDPE 0 0 140 60 0 0.25α-TCP/69.75LDPE-30LLDPE 0.5 0 139.5 60 0 1α-TCP/69LDPE-30LLDPE 2 0 138 60 0 1Assembly/69LDPE-30LLDPE 0 2 138 60 0 70LDPE-30POE 0 0 140 0 60 1Assembly/69LDPE-30POE 0 2 138 0 60 Notes: α-TCP—α-tocopherol; Antioxidant active assembly (referred as Assembly)—Mesoporous nano-SiO2 that carried α-TCP; LDPE—Low-density polyethylene; LLDPE—Linear low-density polyethylene; POE—Polyolefin thermoplastic elastomer; All 10 formulations above were used to prepare extruded films in this study. 表 2 不同活性膜DSC结果对比
Table 2. Comparison of DSC results of different films
Sample Tm/℃ $\Delta {H_{\rm{m}}}$/(J·g−1) ${\chi _{\rm{c}}}$/% Transmittance/% Haze/10−4 WP/(10−15cm3· cm·cm−2·s·Pa) OP/(10−13cm3·
cm·cm−2·s·Pa)LDPE(Control group) 109.22 54.81 19.80 90.87 5.28 9.64±0.93 1.88±0.07 0.25α-TCP/99.75LDPE 111.07 56.35 20.35 90.27 5.61 5.06±1.25 1.98±0.07 1α-TCP/99LDPE 111.14 53.33 19.26 90.82 5.44 7.88±0.45 2.01±0.01 1Assembly/99LDPE 111.48 58.22 21.03 90.42 5.47 7.65±0.74 2.06±0.02 70LDPE-30LLDPE 111.90 54.00 20.24 91.45 6.44 7.31±0.47 1.94±0.06 0.25α-TCP/69.75LDPE-30LLDPE 113.21 56.33 21.77 91.30 5.18 6.78±0.94 2.12±0.08 1α-TCP/69LDPE-30LLDPE 112.02 52.16 21.47 91.48 5.77 6.69±0.75 2.04±0.05 1Assembly/69LDPE-30LLDPE 112.81 57.07 22.06 91.55 7.34 7.05±0.74 2.16±0.03 70LDPE-30POE 109.54 41.30 14.91 92.48 3.98 8.87±0.42 4.30±0.13 1Assembly/70LDPE-30POE 109.91 47.82 17.27 91.13 9.96 10.36±0.51 4.76±0.04 Notes: Tm—Melting temperature of films; $\Delta {H_{\rm{m}}}$—Melting enthalpy of films; ${\chi _{\rm{c}}}$—Crystallinity of films; WP—Water vapor permeability of films; OP—Oxygen permeability of films. 表 3 相对LDPE组的不同薄膜拉伸强度和断裂伸长率变化率对比
Table 3. Change rates of tensile strength and elongation at break for different films in contrast to group LDPE
Sample Tensile strength change rate/% Elongation change rate/% LDPE(Control group) 0 0 70LDPE-30LLDPE 13.6 33.1 70LDPE-30POE 20.7 19.6 1Assembly/99LDPE 12.1 −4.5 1Assembly/69LDPE-30LLDPE 30.9 29.5 1Assembly/69LDPE-30POE 76.2 53.0 Note: Tensile strength and elongation of group LDPE are 18.97 MPa and 513.14%, respectively 表 4 活性膜厚度Lp及α-生育酚扩散系数D对比
Table 4. Comparison of active film thickness Lp and α-TCP diffusion coefficient D
Sample 0.25α-TCP/
99.75LDPE1α-TCP/
99LDPE1Assembly/
99LDPE0.25α-TCP/
69.75LDPE-
30LLDPE1α-TCP/
69LDPE-
30LLDPE1Assembly/
69LDPE-
30LLDPE1Assembly/
69LDPE-
30POELp/μm 82.1±1.4 86.3±1.5 82.9±1.1 86.1±1.1 84.0±1.0 84.1±1.7 81.4±0.6 D/(10−9 cm2s−1) 3.1861 3.2998 1.6901 2.9468 3.0019 1.6048 2.0134 -
[1] TIAN F, DECKER E A, GODDARD J M. Controlling lipid oxidation of food by active packaging technologies[J]. Food & Function,2013:4. [2] WILSON R, FERNIE C E, SCRIMGEOUR C M, et al. Dietary epoxy fatty acids are absorbed in healthy women[J]. European Journal of Clinical Investigation,2015,32(2):79-83. [3] ZIA J, PAUL U C, JOSÉ A G, et al. Low-density polyethylene/curcumin melt extruded composites with enhanced vapor barrier and antioxidant properties for active food packaging[J]. Polymer,2019,175:137-145. doi: 10.1016/j.polymer.2019.05.012 [4] JOAQUÍN G E, CAROL L D, PILAR H M, et al. Advances in antioxidant active food packaging[J]. Trends in Food ence & Technology,2014,35(1):42-51. [5] SAEED G H, MORTEZA E, HOSSEIN A K, et al. Controlled-release of ferulic acid from active packaging based on LDPE/EVA blend: Experimental and modeling[J]. Food Packaging and Shelf Life,2019,22:100392. doi: 10.1016/j.fpsl.2019.100392 [6] ALMASI H, OSKOUIE M J, SALEH A. A review on techniques utilized for design of controlled release food active packaging[J]. Critical Reviews in Food Science and Nutrition,2021,61(15):2601-2621. [7] 孙莉楠. 基于MCM-41介孔分子筛抗氧化活性包装膜的制备及控释行为研究[D]. 无锡: 江南大学, 2020.SUN L N. Antioxidant active packaging films development based on MCM-41 mesoporous silica materials and controlled release profiles investigation[D]. Wuxi: Jiangnan University, 2020 (in Chinese). [8] WEI F, HOU Q, YANG J Y, et al. Multifunctional NO-delivery vessel derived from aminopropyl-modified mesoporous zeolites[J]. Journal of Colloid & Interface ence,2011,356(2):526-535. [9] YUAN L, TANG Q, YANG D, et al. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery[J]. Journal of Physical Chemistry C, 2011, 115(20): 9926–9932. [10] KYAW B M, CHAMPAKALAKSHMI R, SAKHARKAR M K, et al. Biodegradation of low density polythene (LDPE) by pseudomonas species[J]. Indian Journal of Microbiology,2012,52(3):411-419. doi: 10.1007/s12088-012-0250-6 [11] GARGIULO N, ATTIANESE I, BUONOCORE G G, et al. Alpha-Tocopherol release from active polymer films loaded with; functionalized SBA-15 mesoporous silica[J]. Microporous & Mesoporous Materials,2013,167(Complete):10-15. [12] HEIRLINGS L, SIRO I, DEVLIEGHERE F, et al. Influence of polymer matrix and adsorption onto silica materials on the migration of α-tocopherol into 95% ethanol from active packaging[J]. Food Additives & Contaminants,2004,21(11):1125-1136. [13] SUN L N, LU L X, QIU X L, et al. Development of low-density polyethylene antioxidant active films containing α-tocopherol loaded with MCM-41(Mobil Composition of Matter No. 41) mesoporous silica[J]. Food Control,2017,71:193-199. doi: 10.1016/j.foodcont.2016.06.025 [14] LI C, QIU X L, LU L X, et al. Preparation of low-density polyethylene film with quercetin and α-tocopherol loaded with mesoporous silica for synergetic-release antioxidant active packaging[J]. Journal of Food Process Engineering,2019,42(5):e13088.1-e13088.9. [15] SUN L N, LU L X, WANG L Q, et al. Influence of α-tocopherol/MCM-41 assembly on physical and antioxidant release properties of low-density polyethylene antioxidant active films[J]. Polymer Engineering & Science,2018,58(10):1710-1716. [16] LU L J, LU L X. Preparation and properties of quercetin-incorporated high density polyethylene/low density polyethylene antioxidant multilayer film[J]. Packaging Technology and Science,2018,31(6):433-439. doi: 10.1002/pts.2371 [17] KHONAKDAR H A, JAFARI S H, SINGH S P. Rheology, morphology and estimation of interfacial tension of LDPE/EVA and HDPE/EVA blends[J]. Polymer Bulletin, 2005, 54(2): 75-84. [18] BRANCIFORTI M C, GUERRINI L M, MACHADO R, et al. Correlations between processing parameters, morphology, and properties of blown films of LLDPE/LDPE blends: Part 2—Crystalline and amorphous biaxial orientation by WAXD pole figures[J]. Journal of Applied Polymer Science,2010,102(3):2760-2767. [19] LI S C, LI B, QIN X M. Properties of LLDPE film modified by LDPE[J]. Polymer Plastics Technology & Engineering,2012,51(3):307-310. [20] PATEL R M, KARJALA T P, SAVARGAONKAR N R, et al. Fundamentals of structure-property relationships in blown films of linear low density polyethylene/low density polyethylene blends[J]. Journal of Plastic Film and Sheeting,2019,35(4):401-421. [21] BAGHAEI B, JAFARI S H, KHONAKDAR H A, et al. Thermal properties of novel clay containing nanocomposites based on low density polyethylene/ ethylene-octene copolymer blends[J]. E-Polymers, 2009:082. [22] VELÁZQUEZ O D, HATZIKIRIAKOS S G, SENTMANAT M L. Thermorheological properties of LLDPE/LDPE blends: Effects of production technology of LLDPE[J]. Journal of Polymerence Part B—Polymer Physics,2008,46(16):1669-1683. doi: 10.1002/polb.21504 [23] LIU S, WANG K, ZHANG Z, et al. Effects of ethylene, ctene copolymer (POE) on the brittle to ductile transition of high-density polyethylene/POE blends[J]. Polymer Engineering and Science,2020,60(10):2640-2652. doi: 10.1002/pen.25532 [24] MAL N K, FUJIWARA M, TANAKA Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica[J]. Nature,2003,421(6921):350-353. doi: 10.1038/nature01362 [25] 中国国家标准化管理委员会. 塑料拉伸性能的测定第3部分: 薄膜和薄片的验条件: GB/T 1040.3—2006[S]. 北京: 中国标准出版社, 2006.Standardization Administration of the People’s Republic of China. Determination of tensile properties of plastics Part 3: Test conditions for films and sheets: GB/T 1040.3—2006[S]. Beijing: China Standard Press, 2006 (in Chinese). [26] 中国国家标准化管理委员会. 塑料薄膜和薄片气体透过性试验方法-压差法: GB/T 1038—2000[S]. 北京: 中国标准出社, 2000.Standardization Administration of the People’s Republic of China. Test method for gas permeability of plastic films and sheets-Differential pressure method: GB/T 1038—2000[S]. Beijing: China Standard Press, 2000 (in Chinese). [27] 中国国家标准化管理委员会. 塑料薄膜和片材透水蒸气性试验方法杯式: GB 1037—1988[S]. 北京: 中国标准出版社, 1988.Standardization Administration of the People’s Republic of China. Plastic film and sheet water vapor permeability test method cup method: GB 1037—1988[S]. Beijing: China Standard Press, 1988 (in Chinese). [28] 中国国家标准化管理委员会. 透明塑料透光率和雾度的测定: GB/T 2410—2008[S]. 北京: 中国标准出版社, 2008.Standardization Administration of the People’s Republic of China. Determination of light transmittance and haze of transparent plastics: GB/T 2410—2008[S]. Beijing: China Standard Press, 2008 (in Chinese). [29] 中国国家标准化管理委员会. 食品接触材料塑料中受限物质塑料中物质向食品及食品模拟物特定迁移: GB/T 23296.1—2009[S]. 北京: 中国标准出版社, 2009.Standardization Administration of the People’s Republic of China. Restricted substances in food contact materials plastics specific migration of substances in plastics to food and food simulants: GB/T 23296.1—2009[S]. Beijing: China Standards Press, 2009 (in Chinese). [30] 赵东元. 有序介孔分子筛材料[M]. 北京: 高等教育出版社, 2013.ZHAO D Y. Ordered mesoporous molecular sieve materials[M]. Beijing: Higher Education Press, 2013 (in Chinese). [31] 王家博. 纳米多孔材料隔热性能的研究[D]. 鞍山: 辽宁科技大学, 2015.WANG J B. Study on thermal insulation properties of nanoporous materials[D]. Anshan: University of Science and Technology Liaoning, 2015 (in Chinese). [32] 窦绿叶. 二氧化硅纳米纤维基弹性气凝胶的制备及其隔热性能研究[D]. 上海: 东华大学, 2020.DOU L Y. Fabrication and thermal insulation properties of silica nanofiber based aerogels with super elasticity[D]. Shanghai: Donghua University, 2020 (in Chinese). [33] 马传国. 纳米碳酸钙/聚丙烯复合材料中界面与微相结构的设计与研究[D]. 广州: 中山大学, 2005.MA C G. Design of the interface and micro-phase structure in nano-CaCO3/polypropylene composites[D]. Guangzhou: Sun Yat-sen University, 2005 (in Chinese). [34] WANG L, DONG Y, MEN H, et al. Preparation and characterization of active films based on chitosan incorporated tea polyphenols[J]. Food Hydrocolloids,2013,32(1):35-41. doi: 10.1016/j.foodhyd.2012.11.034 [35] 张师军, 乔金樑. 聚乙烯树脂及其应用[M]. 北京: 化学工业出版社, 2011.ZHANG S J, QIAO J L. Polyethylene resin and its application[M]. Beijing: Chemical Industry Press, 2011 (in Chinese). [36] 李瑞. LLDPE/LDPE共混物的结构与性能[J]. 合成树脂及塑料, 2016, 33(3):52-55.LI R. Structure and properties of linear low density polyethylene/low density polyethylene blend[J]. China Synthetic Resin And Plastics,2016,33(3):52-55(in Chinese). [37] 王新鹏, 张军. LDPE/POE共混物的结晶行为和力学性能[J]. 合成树脂及塑料, 2009, 26(1):10-14. doi: 10.3969/j.issn.1002-1396.2009.01.003WANG X P, ZHANG J. The crystallization behavior and mechanical properties of LDPE/POE blends[J]. China Synthetic Resin and Plastics,2009,26(1):10-14(in Chinese). doi: 10.3969/j.issn.1002-1396.2009.01.003 [38] SCARFATO P, AVALLONE E, GALDI M R, et al. Preparation, characterization, and oxygen scavenging capacity of biodegradable α-tocopherol/PLA microparticles for active food packaging applications[J]. Polymer Composites,2017,38(5):981-986. doi: 10.1002/pc.23661 [39] PARK I, PENG H G, GIDLEY D W, et al. Epoxy-Silica mesocomposites with enhanced tensile properties and oxygen permeability[J]. Chemistry of Materials,2006,18(3):650-656. doi: 10.1021/cm051768r -





 下载:
下载: