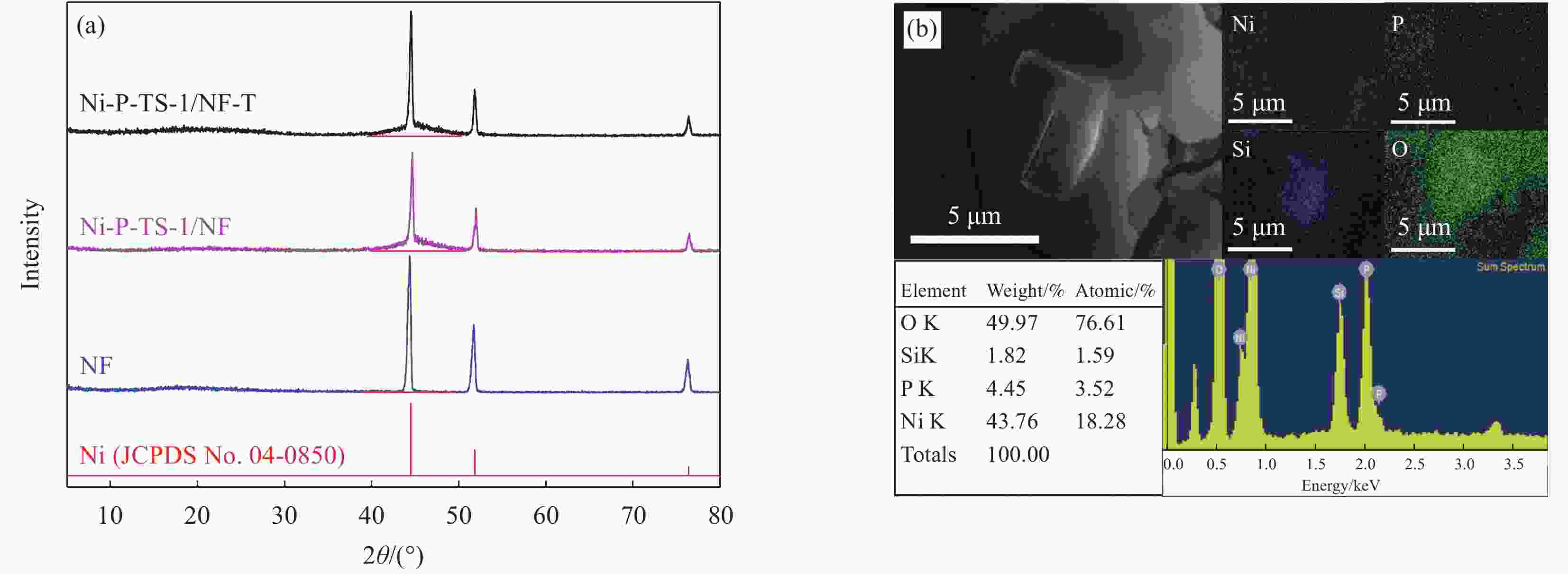
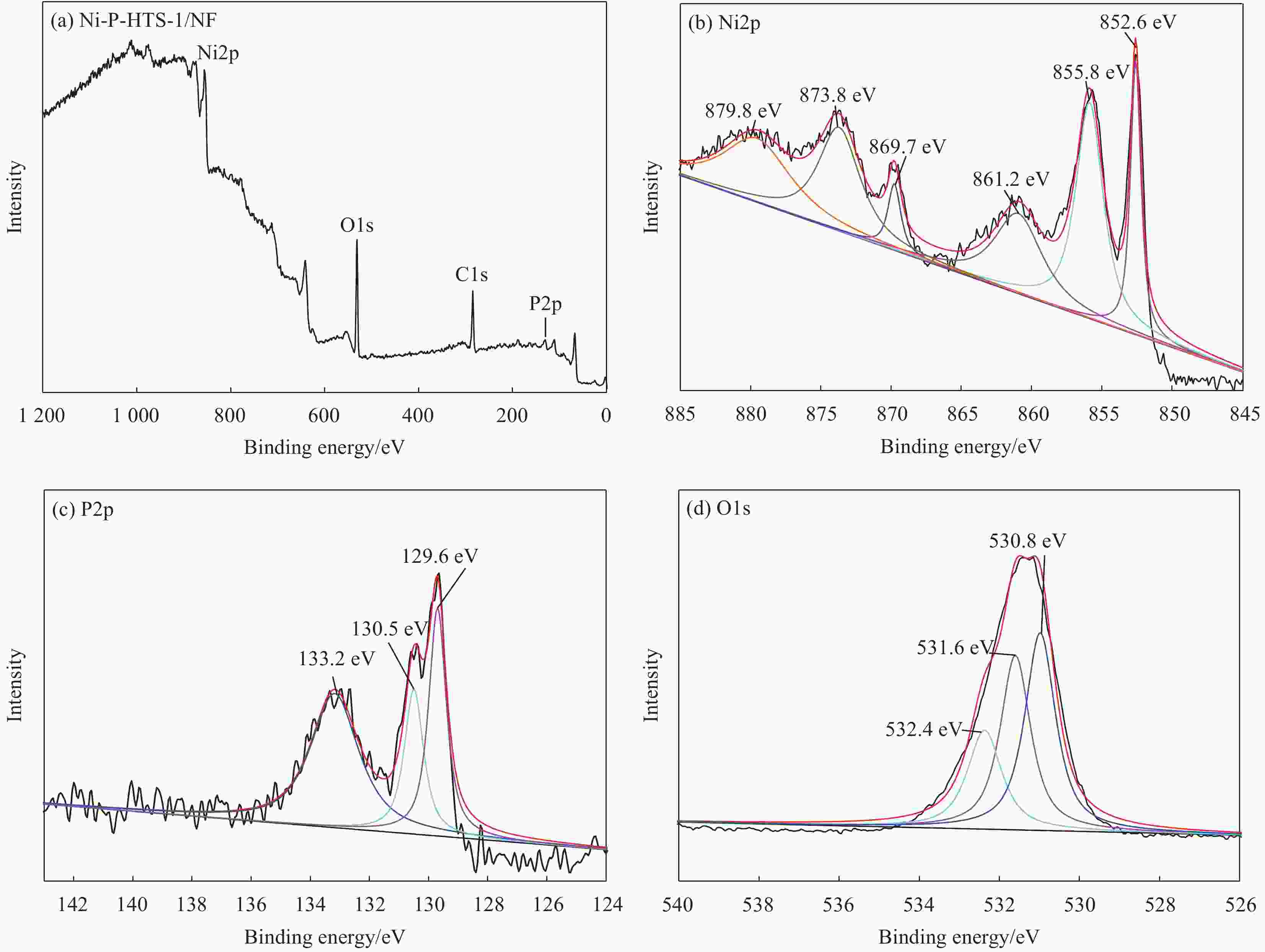
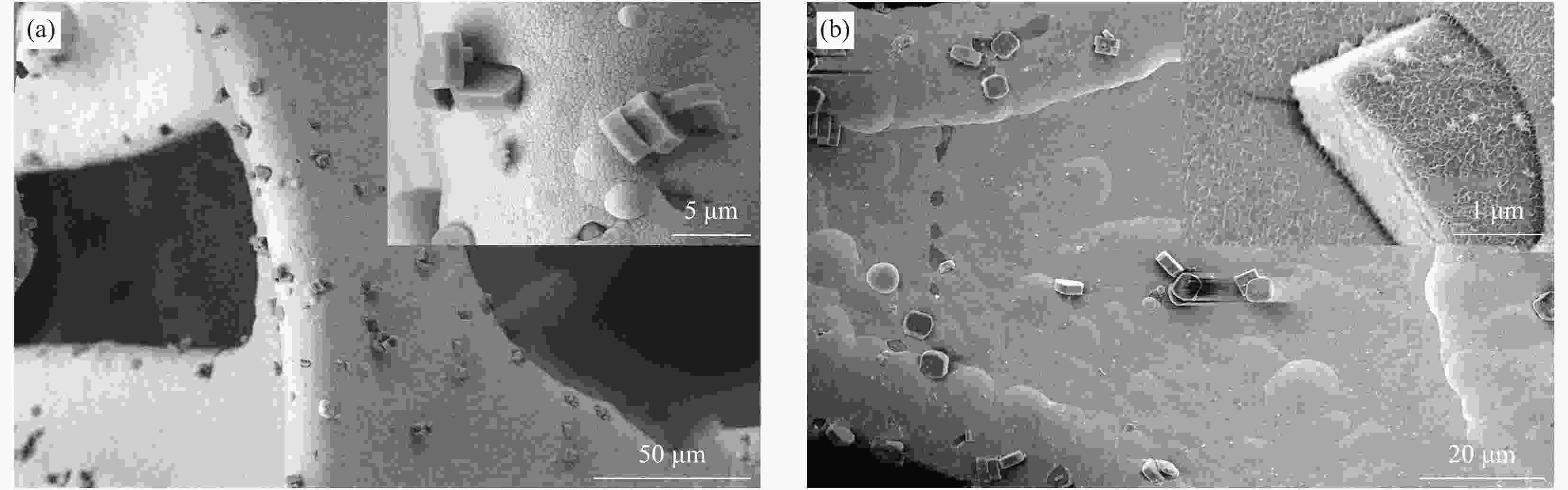
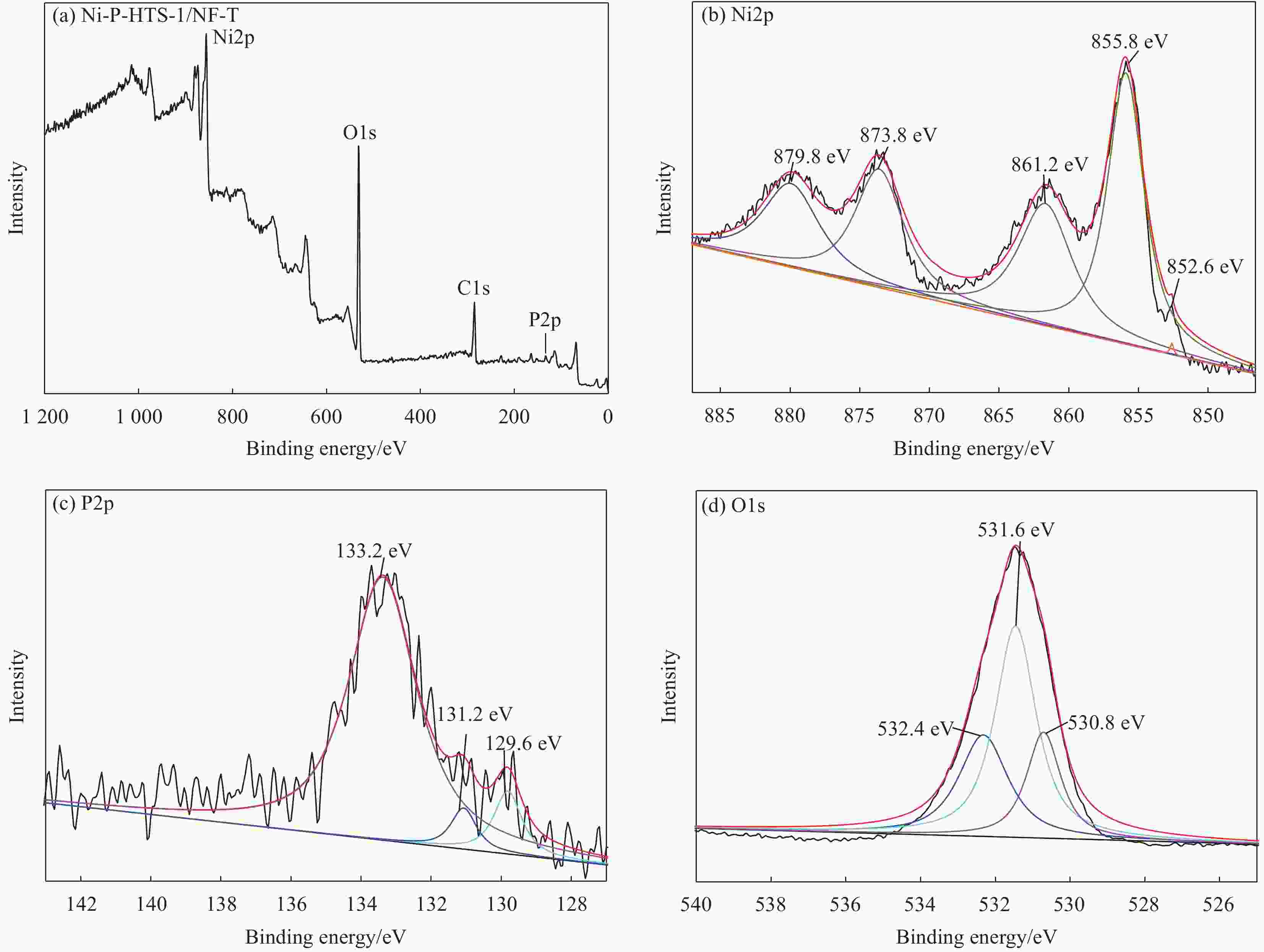
Preparation and catalytic performance of nickel-phosphorus-titanium silicalite zeolite composite
-
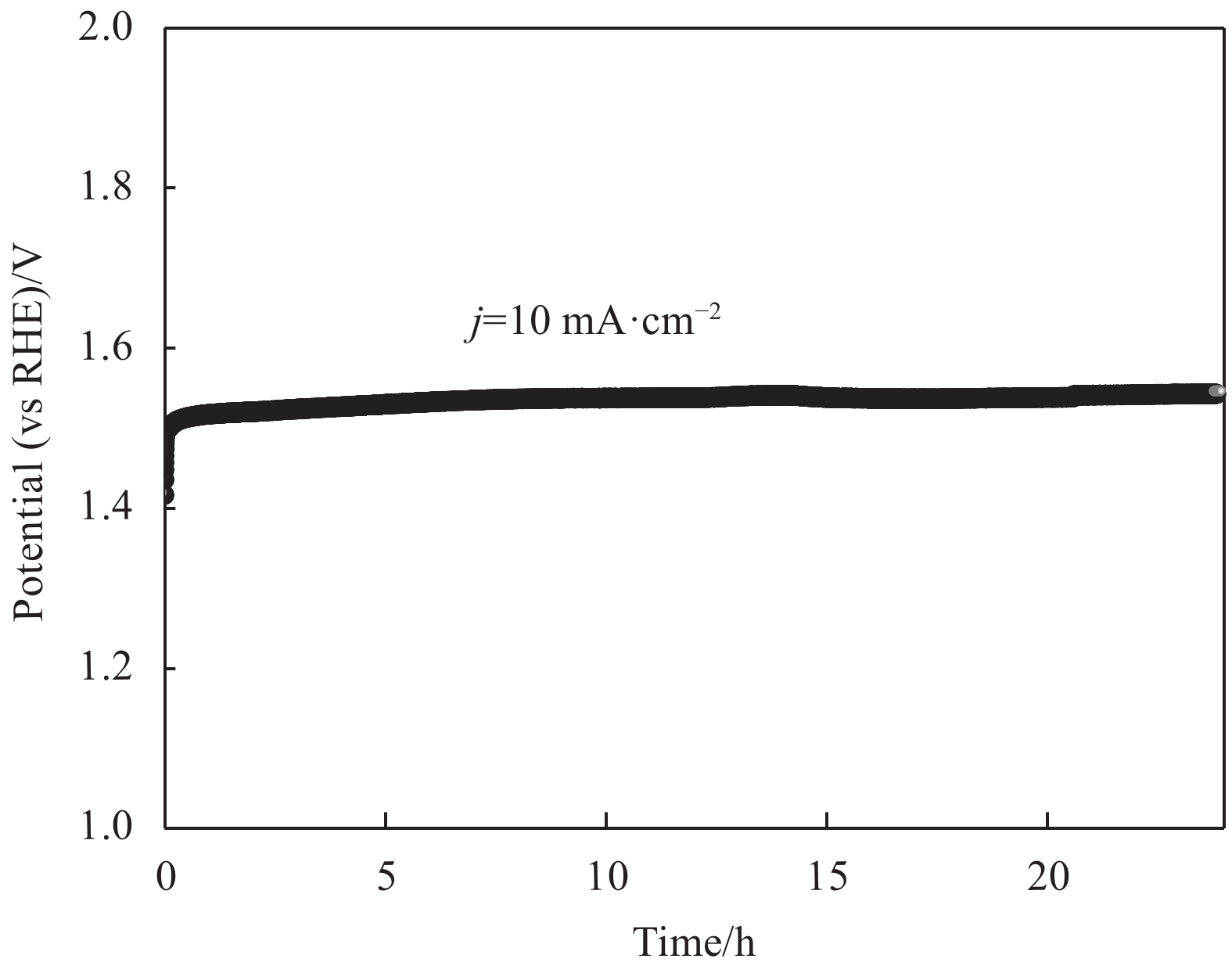
摘要: 以多级孔钛硅分子筛(HTS-1)为复合相、泡沫镍(NF)为基体,采用化学复合镀和原位处理工艺制备了镍-磷-钛硅分子筛(Ni-P-HTS-1/NF-T)复合材料,并对其电催化析氧反应(OER)性能进行研究。结果表明,Ni-P-HTS-1/NF-T复合材料在1 mol/L的KOH电解液中具有较快的OER动力学及电子转移速率,优异的OER性能及长期运行稳定性。HTS-1分子筛的掺杂及原位处理工艺降低了Ni-P-HTS-1/NF-T复合材料的电子转移电阻,增大其电催化活性表面积,此外还改变了复合材料的化学组成,生成了能为电催化析氧反应提供催化活性中心的镍的磷化物和氢氧化物,从而有效提升了Ni-P-HTS-1/NF-T复合材料的OER性能。
-
关键词:
- 钛硅分子筛 /
- 化学复合镀 /
- 镍-磷-钛硅分子筛复合材料 /
- 非晶态 /
- 电催化析氧反应(OER)
Abstract: Nickel-phosphorus-titanium silicalite zeolite (Ni-P-HTS-1/NF-T) composite was prepared by electroless composite plating and in-situ treatment process with hierarchical titanium silicalite (HTS-1) zeolite as composite phase and nickel foam (NF) as matrix materials, and its electrocatalytic oxygen evolution reaction (OER) performance was studied. The results show that Ni-P-HTS-1/NF-T composite has faster OER kinetics and electron transfer rate, excellent OER performance and long-term stability in 1.0 mol/L KOH electrolyte. The doping of HTS-1 zeolite and in-situ treatment process reduce the electron transfer resistance of Ni-P-HTS-1/NF-T composite, increase its electrocatalytic active surface area, and change the chemical composition of the composite to form nickel phosphide and hydroxide which can provide catalytic active centers for electrocatalytic oxygen evolution reaction, thus effectively improving the OER performance of Ni-P-HTS-1/NF-T composite. -
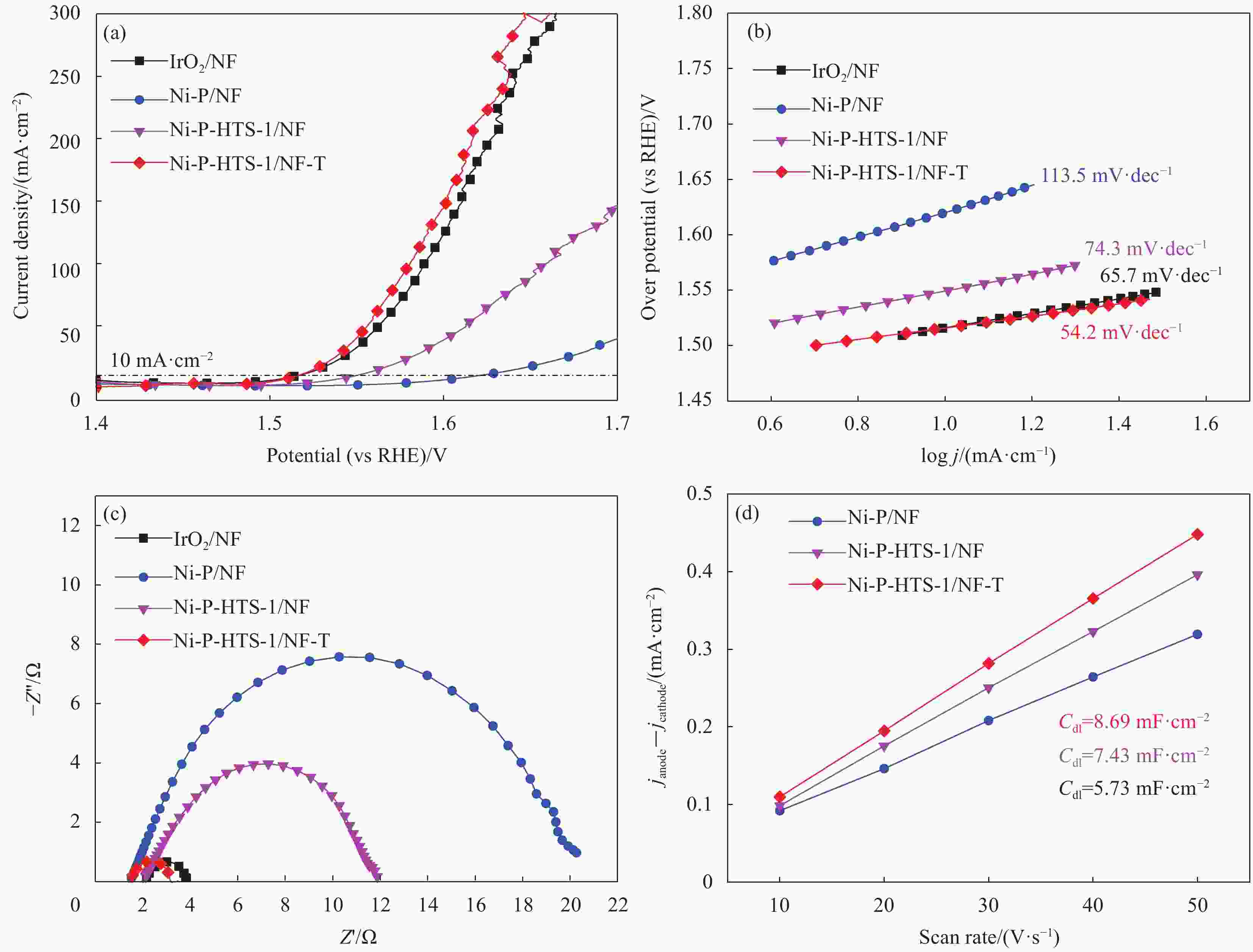
图 6 Ni-P-HTS-1/NF-T复合材料的极化曲线(a)、Tafel斜率(b)、奈奎斯特曲线(c)和双层电容曲线(d)
Figure 6. Polarization curves (a), Tafel plots (b), Nyquist plots (c) and Electrochemical double-layer capacitance (d) of Ni-P-HTS-1/NF-T composite
j —Current density; Z′—Real impedance; Z′′—Imaginary impedance; Cdl—Electrochemical double-layer capacitance; janodic, jcathodic—Difference of current density between anode and cathode corresponding to fixed potential in cyclic voltammetry curve at a certain scanning speed
表 1 化学复合镀的配方及工艺参数
Table 1. Bath components and operating conditions of electroless composite plating
Bath component and operating condition Effect of component Amount Nickel sulfate(NiSO4·6H2O) Mental salts 25 g·L−1 Sodium hypophosphite(NaH2PO2·H2O) Reducing agent 30 g·L−1 Lactic acid(C3H6O3) Complexing agent 20 mL·L−1 Sodium citrate(Na3C6H5O7·2H2O) Complexing agent 12.5 g·L−1 Sodium acetate(CH3COONa·3H2O) Buffer 25 g·L−1 Titanium silicalite zeolite(TS-1) Composite phase 0.2 g·L−1 Sodium dodecyl sulfate(SDS) Surfactant 0.1 g·L−1 Potassium iodate(KIO3) Stabilizer 0.002 g·L−1 pH value − 5 Temperature − 80℃ 表 2 钛硅分子筛的结构性质
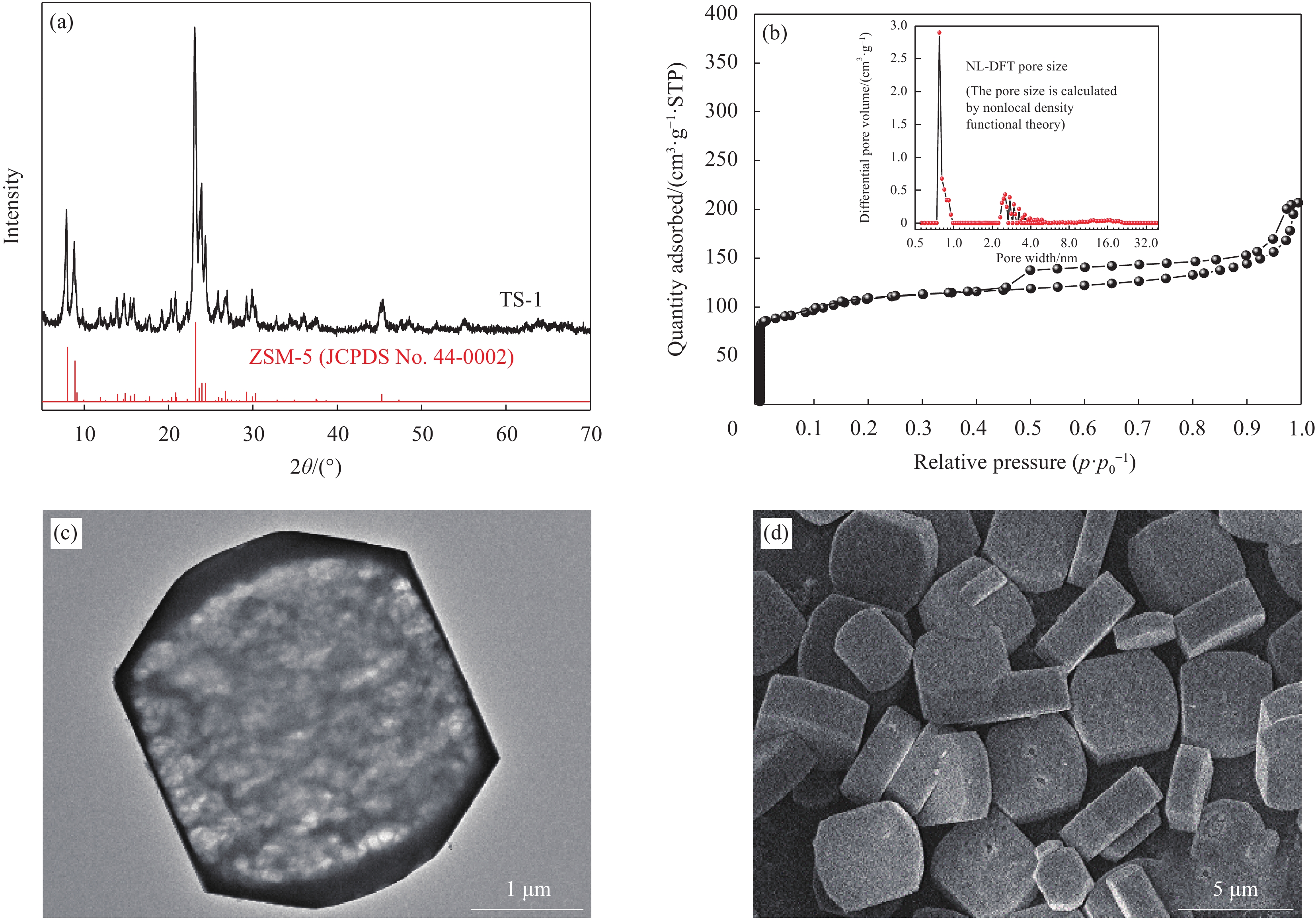
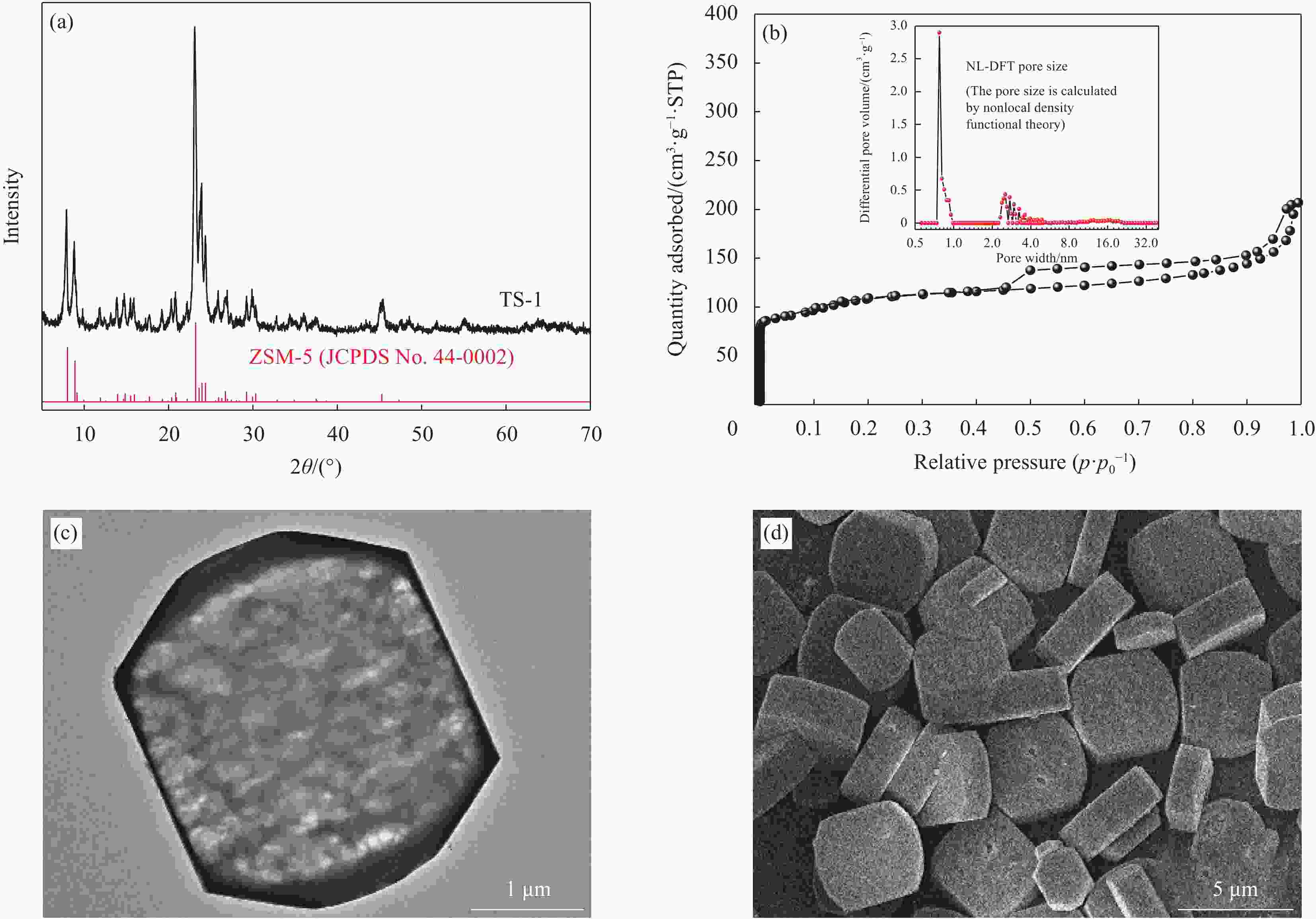
Table 2. Texture properties of titanium silicalite zeolites
Materials ST/(m2·g−1) SM/(m2·g−1) SE/(m2·g−1) VT/(cm−3·g−1) VM/(cm−3·g−1) TS-1 (cal) 363.30 113.49 249.81 0.45 0.064 HTS-1 (cal) 462.28 177.63 284.65 0.57 0.096 Notes: ST—Surface area of micropores; SM—Surface area of mesopores by t−plot method; SE—Surface area by Brunnauer-Emmet-Teller method; VM—Micropore volume by t−plot method; VT—Total pore volume. -
[1] HUSSAIN A, ARIF S M, ASLAM M. Emerging renewable and sustainable energy technologies: State of the art[J]. Renewable and Sustainable Energy Reviews,2017,71:12-28. doi: 10.1016/j.rser.2016.12.033 [2] 孟培媛, 郭明媛, 乔勋. WS2/g-C3N4异质结光催化分解水制氢性能及机理[J]. 复合材料学报, 2021, 38(2):591-600.MENG Peiyuan, GUO Mingyuan, QIAO Xun. Establish WS2/g-C3N4 heterojunction to improve the H2 production performance of photocatalyst and mechanism study[J]. Acta Materiae Compositae Sinica,2021,38(2):591-600(in Chinese). [3] MINUTILLO M, PERNA A, SORCE A. Green hydrogen production plants via biogas steam and autothermal reforming processes: Energy and exergy analyses[J]. Applied Energy,2020,277:115452. doi: 10.1016/j.apenergy.2020.115452 [4] LI L, LIN J, WU N Y, et al. Review and outlook on the international renewable energy development[J]. Energy and Built Environment,2020,17:12-19. [5] LIU W G, ZUO H B, WANG J S, et al. The production and application of hydrogen in steel industry[J]. International Journal of Hydrogen Energy,2020,12(17):10548-10569. [6] AMORES E, SANCHE M, ROJAS N, et al. Sustainable fuel technologies handbook [M]. New York: Academic Press, 2021: 271-313. [7] WANG H, YUAN Y, GU J L, et al. Facile one-pot synthesis of layered double hydroxides nanosheets with oxygen vacancies grown on carbon nanotubes for efficient oxygen evolution reaction[J]. Journal of Power Sources,2020,467:228354. doi: 10.1016/j.jpowsour.2020.228354 [8] LV Z H, ZHANG Y, WANG K L. High performance of Co-P/NF electrocatalyst for oxygen evolution reaction[J]. Materials Chemistry and Physics,2019,235:121772. doi: 10.1016/j.matchemphys.2019.121772 [9] CHEN D W, QIAO M, LIU Y R, et al. Preferential cation vacancies inperovskite hydroxide for the oxygen evolution reaction[J]. Angewandte Chemie International Edition,2018,57(28):8691-8696. doi: 10.1002/anie.201805520 [10] LI R Q, WAN X Y, CHEN B L, et al. Hierarchical Ni3N/Ni0.2Mo0.8N hetero- structure nanorods arrays as efficient electrocatalysts for overall water and urea electrolysis[J]. Chemical Engineering Journal,2021,409:12840. [11] CHEN D, LU R H, PU Z H, et al. Ru-doped 3D flower-like bimetallic phosphide with a climbing effect on overall water splitting[J]. Applied Catalysis B,2020,279:119396. doi: 10.1016/j.apcatb.2020.119396 [12] BADAM R, HARA M, HUANG H H, et al. Synthesis and electrochemical analysis of novel IrO2 nanoparticle catalysts supported on carbon nanotube for oxygen evolution reaction[J]. International Journal of Hydrogen Energy,2018,43(39):18095-18104. doi: 10.1016/j.ijhydene.2018.08.034 [13] RANI B J, PRADEEPA S S, HASAN Z M, et al. Supercapacitor and OER activity of transition metal (Mo, Co, Cu) sulphides[J]. Journal of Physics and Chemistry of Solids,2020,138:109240. doi: 10.1016/j.jpcs.2019.109240 [14] WANG K X, WANG X Y, LI Z J, et al. Designing 3D dual transition metal electro-catalysts for oxygen evolution reaction in alkaline electrolyte: Beyond oxides[J]. Nano Energy,2020,77:105162. doi: 10.1016/j.nanoen.2020.105162 [15] VIJ V, SULTAN S, HARZANDI A M, et al. Nickel-based electrocatalysts for energy-related applications: oxygen reduction, oxygen evolution, and hydrogen evolution reactions[J]. ACS Catalysis,2017,7(10):7196-7225. doi: 10.1021/acscatal.7b01800 [16] PAN C C, LIU Z C, HUANG M H. 2D iron-doped nickel MOF nanosheets grown on nickel foam for highly efficient oxygen evolution reaction[J]. Applied Surface Science,2020,529:147201. doi: 10.1016/j.apsusc.2020.147201 [17] WAN K, LUO J S, ZHANG X, et al. In-situ formation of Ni (oxy)hydroxide on Ni foam as an efficient electrocatalyst for oxygen evolution reaction[J]. International Journal of Hydrogen Energy,2020,45(15):8490-8496. doi: 10.1016/j.ijhydene.2020.01.043 [18] 涂言言, 赵子涵, 孙一强. FeOOH-Ni(OH)2复合材料的制备及其电催化析氧性能[J]. 复合材料学报, 2020, 37(8):1944-1950.TU Yanyan, ZHAO Zihan, SUN Yiqiang. Synthesis and electrocatalytic oxygen evolution performances of FeOOH- Ni(OH)2 composites[J]. Acta Materiae Compositae Sinica,2020,37(8):1944-1950(in Chinese). [19] WANG K H, SI Y Y, LV Z H, et al. Efficient and stable Ni-Co-Fe-P nanosheet arrays on Ni foam for alkaline and neutral hydrogen evolution[J]. International Journal of Hydrogen Energy,2020,45(4):2504-2512. doi: 10.1016/j.ijhydene.2019.11.154 [20] LI M, PAN X C, JIANG M Q, et al. Interface engineering of oxygen-vacancy-rich CoP/CeO2 heterostructure boosts oxygen evolution reaction[J]. Chemical Engineering Journal,2020,395:125160. doi: 10.1016/j.cej.2020.125160 [21] TIAN Y H, XU L, QIU J X, et al. Rational design of sustainable transition metal-based bifunctional electrocatalysts for oxygen reduction and evolution reactions[J]. Sustainable Materials and Technologies,2020,25:e00204. doi: 10.1016/j.susmat.2020.e00204 [22] LIU X G, GE P P, ZHANG Y M, et al. Highly oriented thin membrane fabrication with hierarchically porous zeolite seed[J]. Crystal Growth & Design,2018,18(8):4544-4554. [23] SHAO Y C, WANG H J, LIU X F, et al. Single-crystalline hierarchically-porous TS-1 zeolite catalysts via a solid-phase transformation mechanism[J]. Micro-porous and Mesoporous Materials,2021,313:110828. doi: 10.1016/j.micromeso.2020.110828 [24] LIU X G, SONG H J, SUN W J, et al. Strong nano size effect of titanium silicalite (TS-1) zeolites for electrorheological fluid[J]. Chemical Engineering Journal,2020,384(15):123267. [25] ZHANG P P, LV Z H, LIU X G, et al. Electroless nickel plating on alumina ceramic activated by metallic nickel as electrocatalyst for oxygen evolution reaction[J]. Catalysis Communications,2021,149:106238. doi: 10.1016/j.catcom.2020.106238 [26] GUO X M, QIAN Y, ZHANG W, et al. Amorphous Ni-P with hollow dendritic architecture as bifunctional electrocatalyst for overall water splitting[J]. Journal of Alloys and Compounds,2018,765:835-840. doi: 10.1016/j.jallcom.2018.06.321 [27] YANG Q P, LV C C, HUANG Z P, et al. Amorphous film of ternary Ni-Co-P alloy on Ni foam for efficient hydrogen evolution by electroless deposition.[J]. International Journal of Hydrogen Energy,2018,43(16):7872-7880. doi: 10.1016/j.ijhydene.2018.03.003 [28] DING Y D, LI H Y, HOU Y. Phosphorus-doped nickel sulfides/nickel foam as electrode materials for electrocatalytic water splitting[J]. International Journal of Hydrogen Energy,2018,43(41):19002-19009. doi: 10.1016/j.ijhydene.2018.08.083 -





 下载:
下载: