Action mechanism of expanded graphite in the composite of expanded graphite/MnO2 supercapacitor electrode materials
-
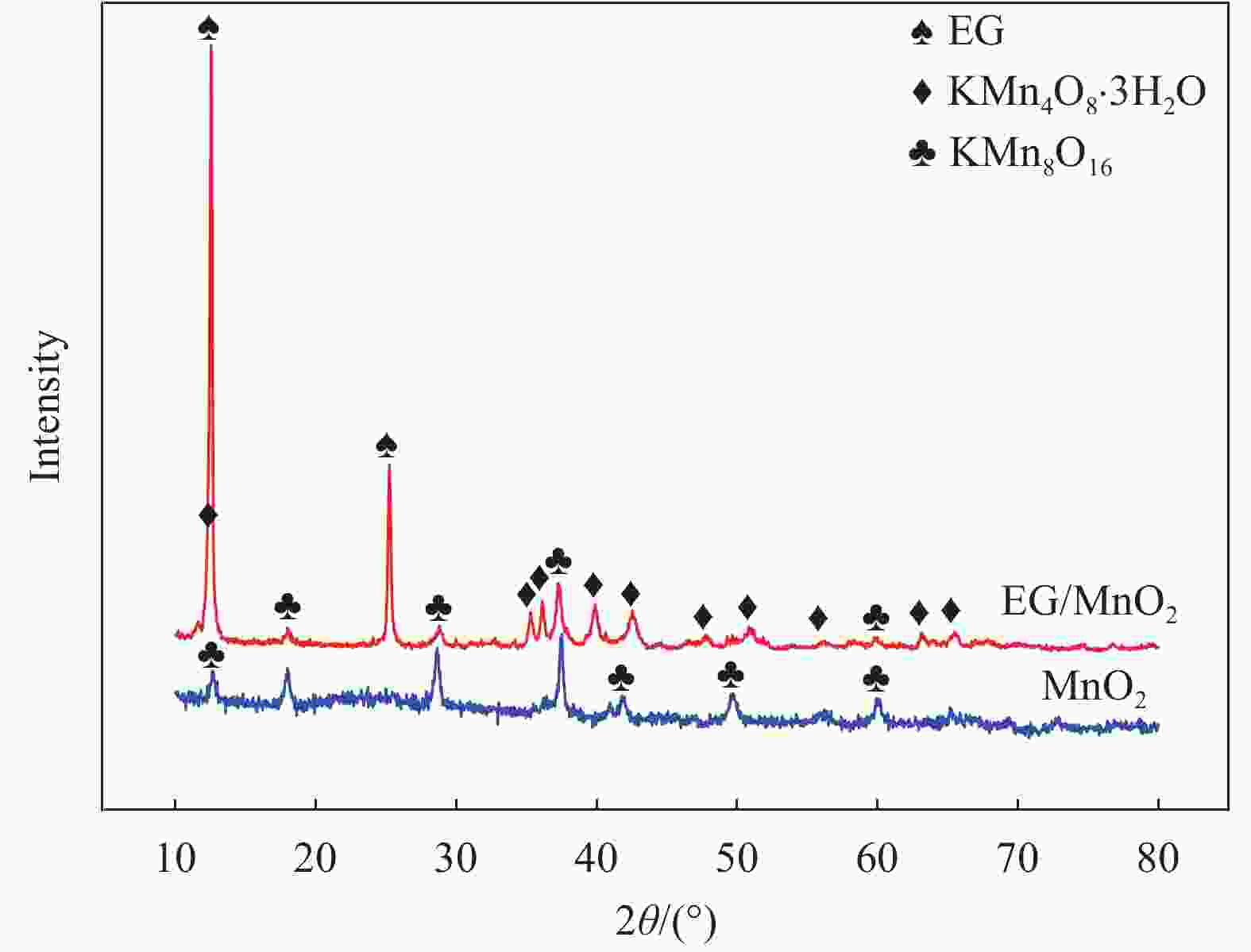
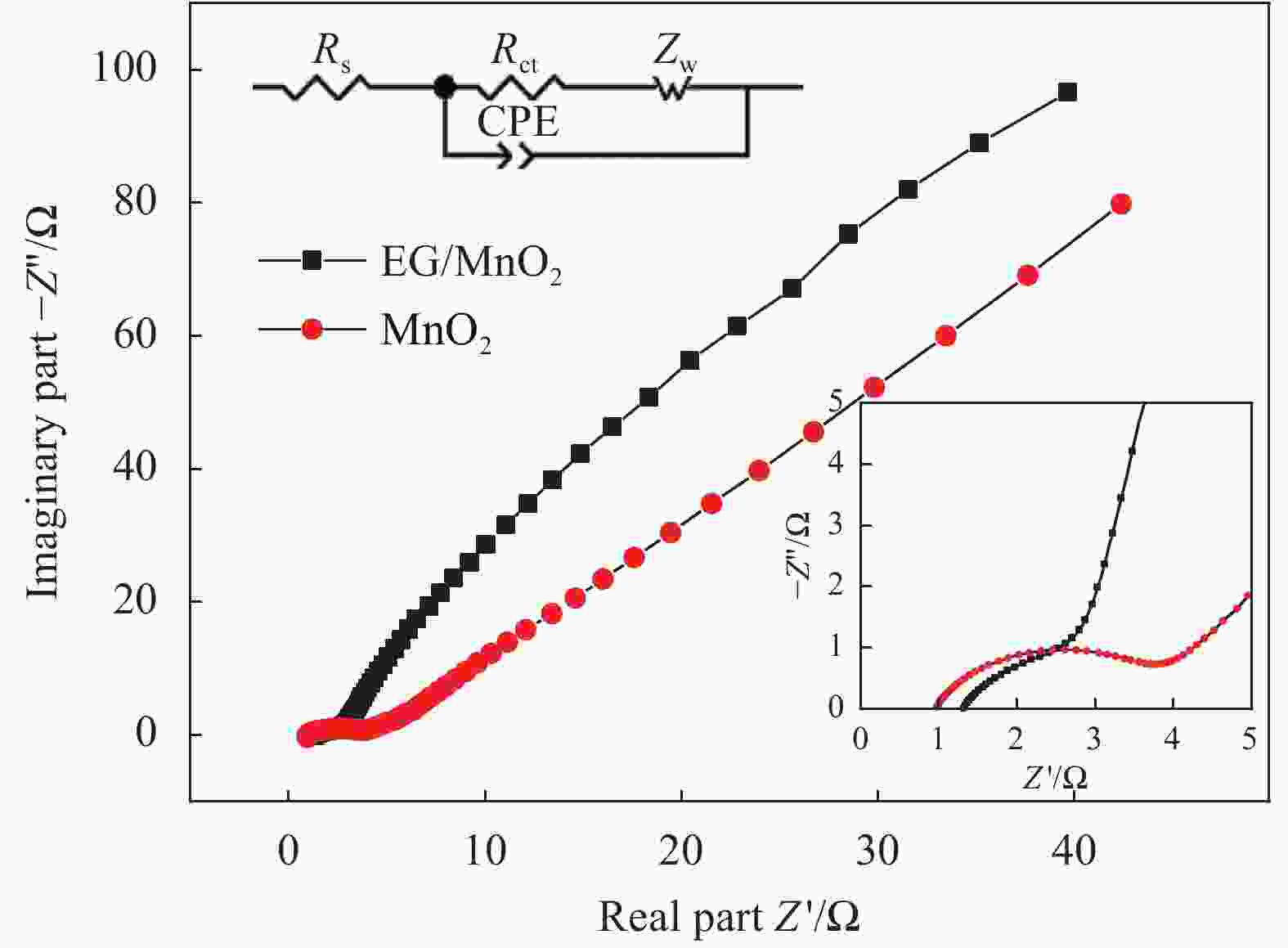
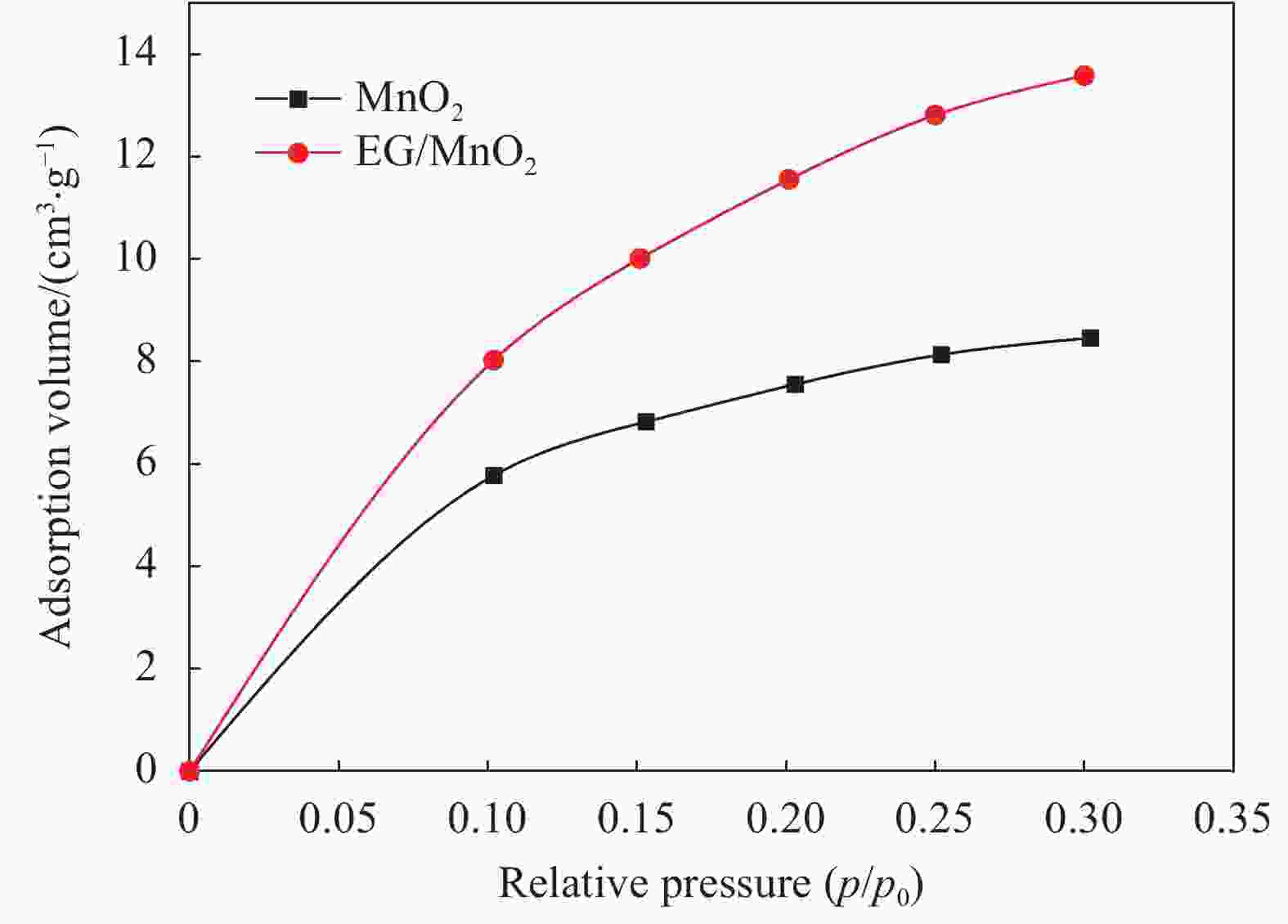
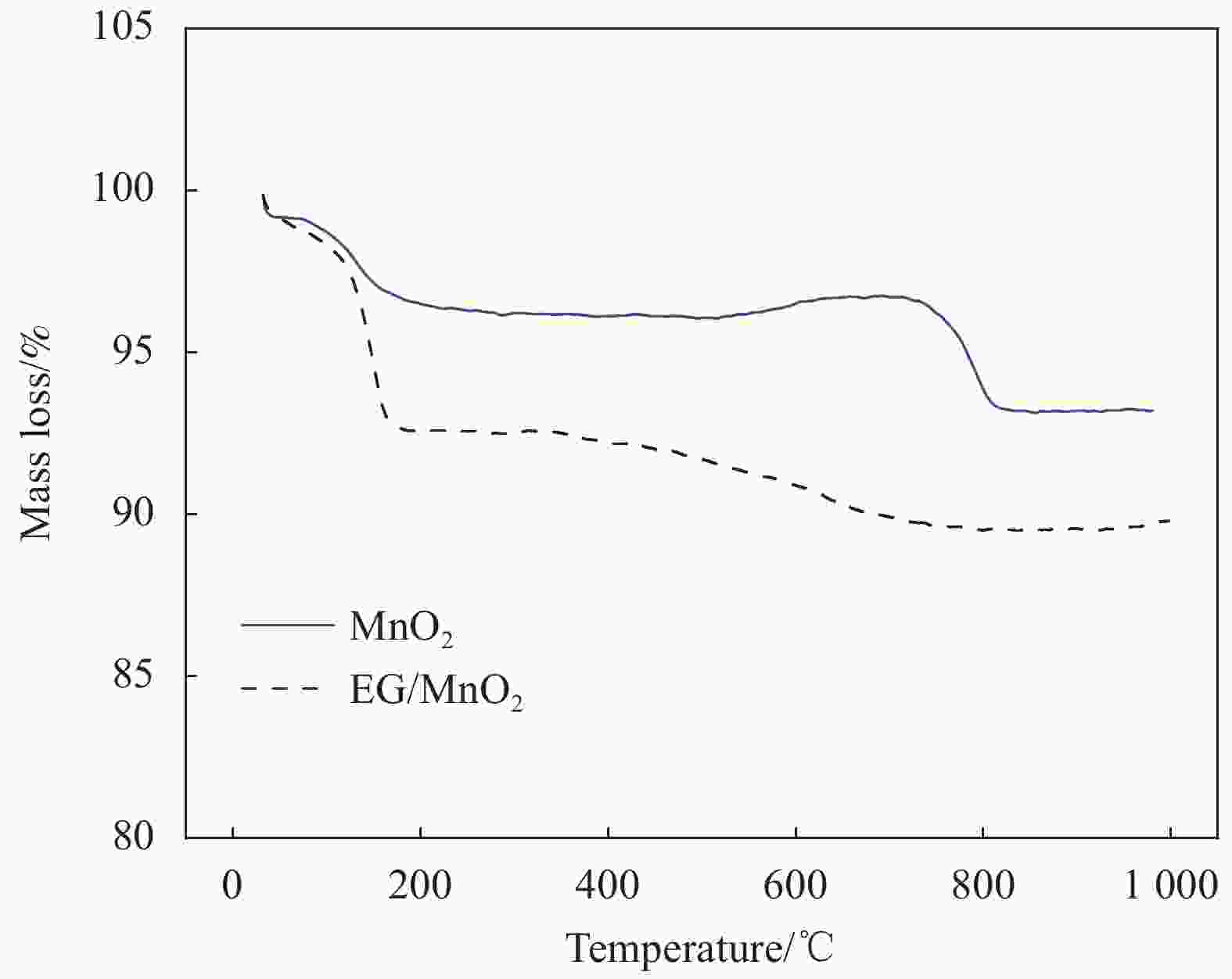
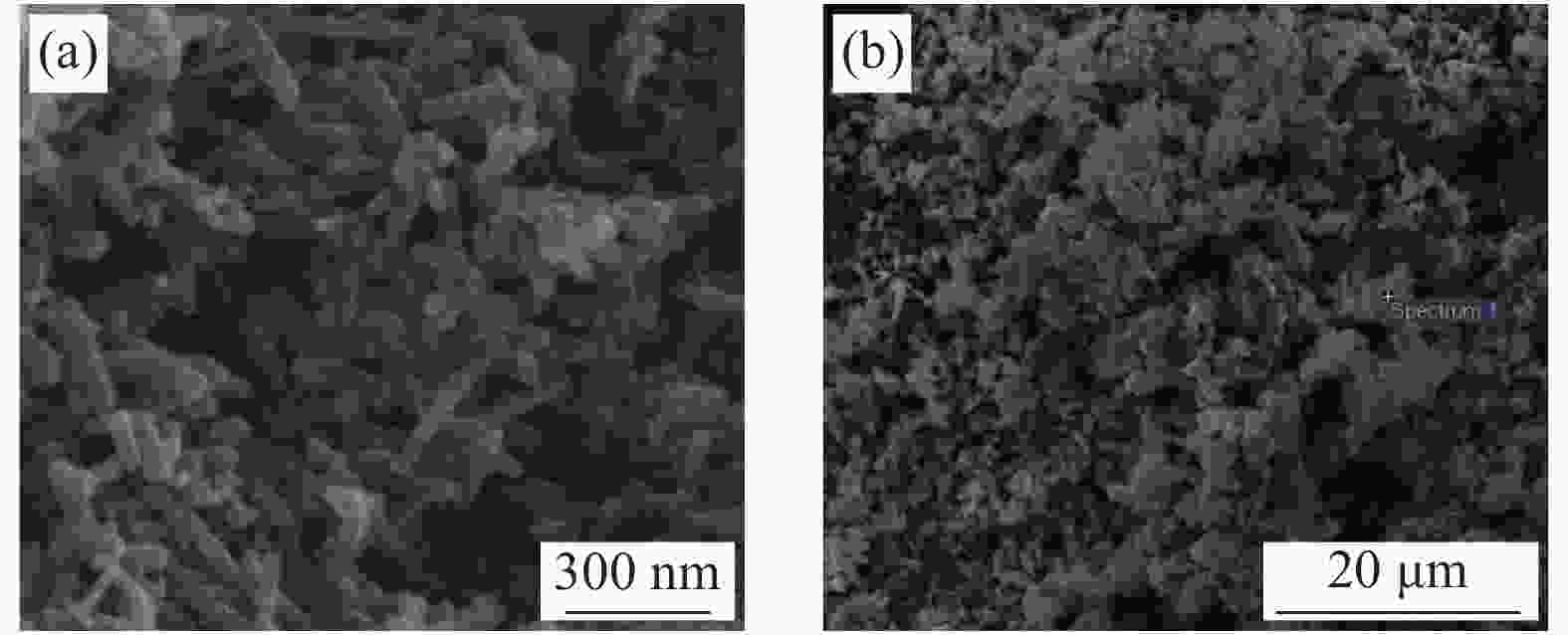
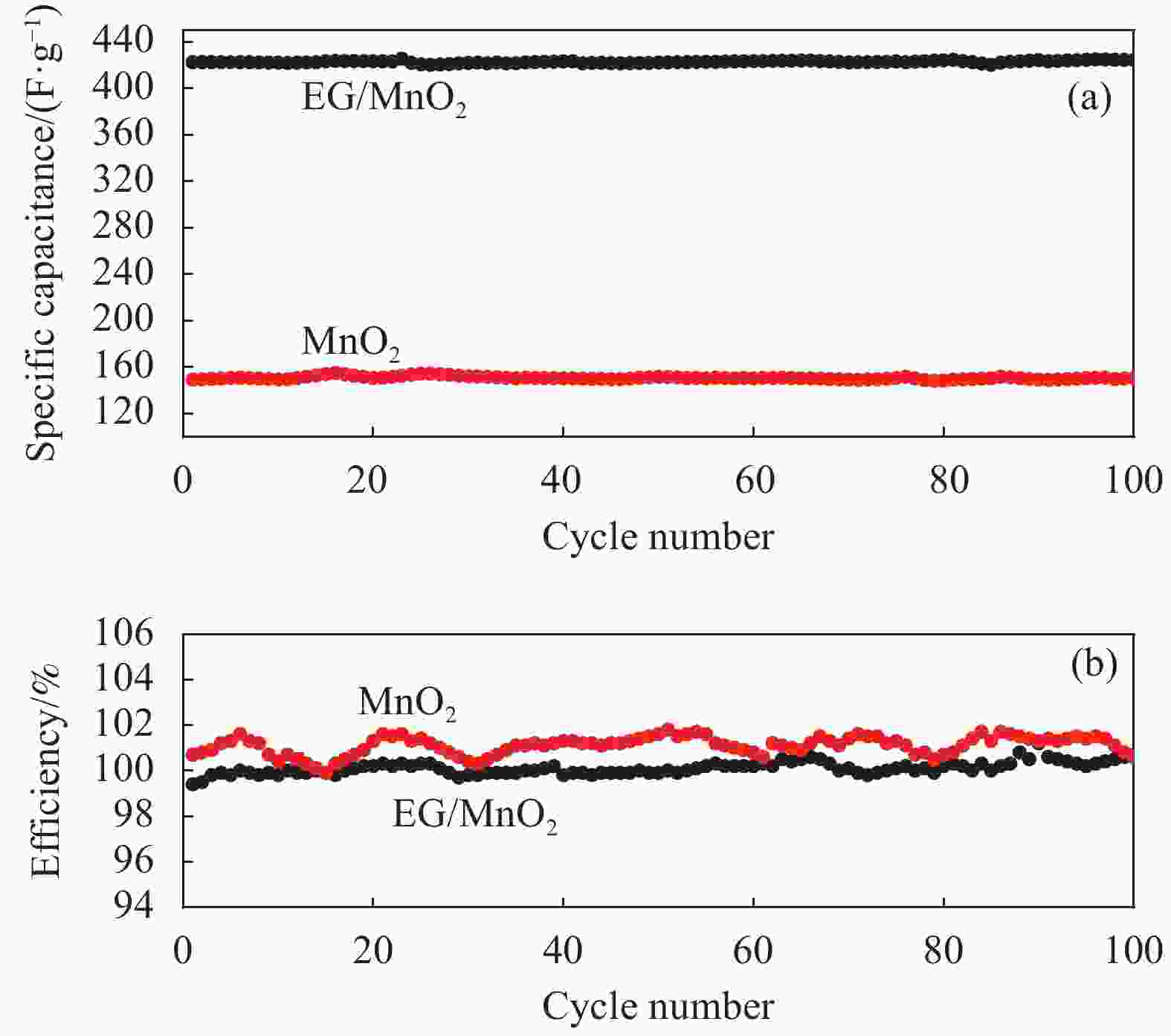
摘要: 采用一种简便的方法制备出了单相α-MnO2和膨胀石墨(EG)/MnO2复合物。实验结果表明EG/MnO2复合物有着比单相α-MnO2更高的比电容和更加优异的倍率性能。随后采用XRD、TG、EIS、BET等手段对复合材料中EG的作用机制进行了研究,结果发现在EG/MnO2复合物中出现了δ相MnO2 (δ-MnO2为层状结构,相较于α-MnO2更加有利于离子的扩散和传输),δ-MnO2大约占复合物总质量的30wt%,EG占3.4wt%。EG/MnO2复合物的电荷转移电阻显著低于单相α-MnO2。EG/MnO2的比表面积为38.7 m2·g−1,而单相α-MnO2的比表面积只有21.6 m2·g−1,更大的比表面积使材料与电解液接触更为充分,从而降低了电荷转移电阻。综上,复合材料中EG的作用机制是通过自身的层状结构诱导了MnO2在其上沉积形成了层状的δ-MnO2,同时抑制了MnO2颗粒的生长,增加了颗粒与电解液的接触面积,降低了电荷转移电阻,从而增大了比电容,也提高了其倍率性能。Abstract: The pure MnO2 and the composite of expanded graphite (EG)/MnO2 were prepared by a very facile method. The experimental results show that the composite presents higher specific capacitance and more stable rate capacity. The action mechanism of EG in the composite was investigated by the measurements of XRD, TG, EIS, BET, etc. It is found that the δ-MnO2 appears in the composite, whose mass percentage is approximately 30% compared with 3.4% of EG in the composite. The δ-MnO2 has a special layered structure compared with the channel structure of α-MnO2, which makes the ions diffuse and transport easier. The charge exchange resistance of the EG/MnO2 composite is significantly lower than that of pure α-MnO2 which may be caused by more tiny particles size of composite because the specific surface area of the composite being 38.7 m2·g−1, with the pure α-MnO2 of21.6 m2·g−1. The larger specific surface area makes the contact between the material particles and the electrolyte solution more sufficient along with lower charge exchange resistance. In all, the action mechanism of EG in the composite can be summed as follow. The δ-MnO2 with the layered structure is induced to grow on the surface of the EG which has the similar layered structure. At the same time, the grows-up of the MnO2 particles is restrained by EG which makes the contact of particles with electrolyte solution more sufficient with the result of the reduce of charge exchange resistance. And, therefore, the specific capacitance is elevated accompanied with more stable rate capacity.
-
Key words:
- supercapacitor /
- manganese dioxide /
- graphite /
- composite /
- mechanisms
-
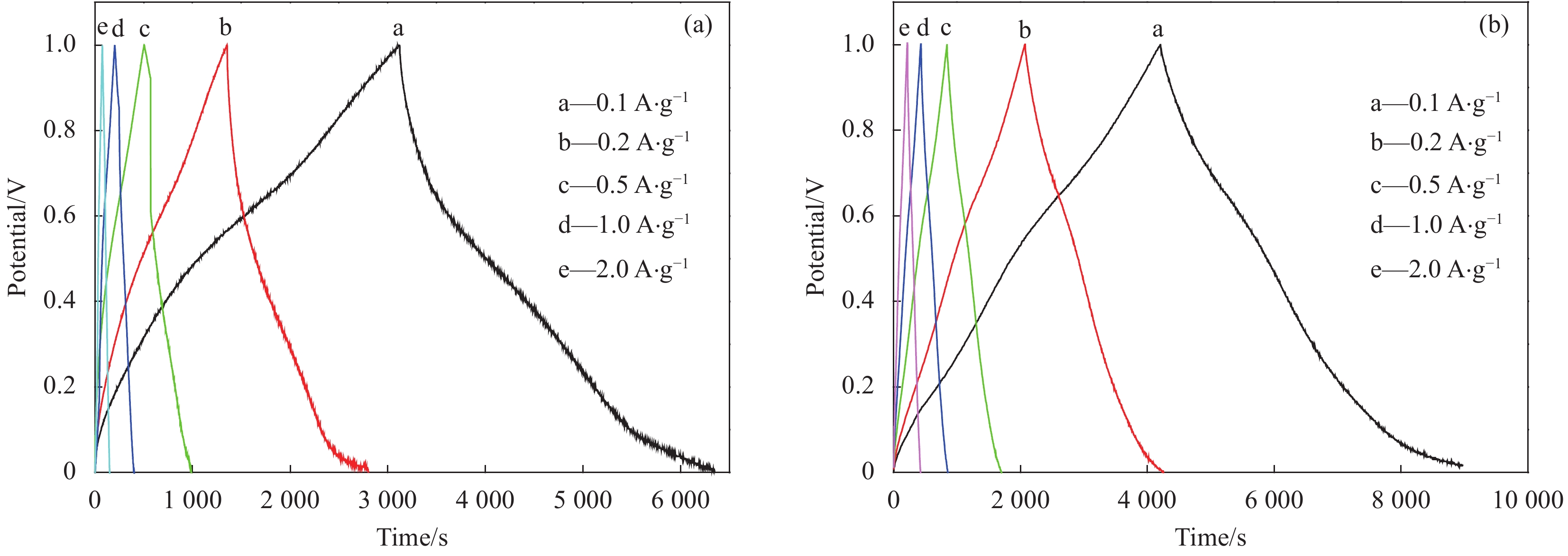
表 1 MnO2和EG/MnO2在不同电流密度下的比电容Cm (F·g−1)
Table 1. Specific capacitance Cm of single MnO2 and EG/MnO2 composite under different current density (F·g−1)
Current density/(A·g−1) 0.1 0.2 0.5 1.0 2.0 MnO2 327 271 258 150 106 EG/MnO2 476 435 428 422 410 表 2 通过EIS拟合得到的MnO2和EG/MnO2的RS和Rct
Table 2. RS and Rct of MnO2 and EG/MnO2 fitted by EIS data
Rs/(Ω·cm2) Rct/(Ω·cm2) MnO2 1.050 3.535 EG/MnO2 1.247 2.519 表 3 本研究中EG/MnO2复合材料的储能性能与文献中类似研究结果的对比
Table 3. Comparison of energy-storing capability of EG/MnO2 in this work with other work
Compounds C m /
(F·g−1)Measure
rateElectrolyte/
(Na2SO4 mol·L−1)Rate
capabilityCycle
stabilityLiterature UN-MnO2 810 1.0 A·g−1 1 58.5% (1.0-20 A·g−1) 88.45% (5000cyc@5.0 A·g−1) [2] δ-MnO2/Ni foam 325 1.0 A·g−1 1 67.6% (1-5 A·g−1) 86% (1000cyc@30 mV·s−1) [11] MnO2/CNFs 324.5 0.5 A·g−1 1 55.4% (0.5-2 A·g−1) 97.0 (1000cyc@3.5 A·g−1) [15] δ-MnO2/Corn-C 520 5 mV·s−1 1 53.5% (5-100 mV·s−1) 80.9% (5000cyc@5 A·g−1) [16] Ni/δ-MnO2 883 10 mV·s−1 1 46.2% (0.01-0.2 V·s−1) 73.8% (2000cyc@1 mA·cm−2) [17] δ-MnO2 HMS 394 1.0 A·g−1 1 71.3% (1-10 A·g−1) 95.2% (5000cyc@5 A·g−1) [20] C@δ-MnO2 345 0.5 A·g−1 1 55.9% (0.5-5 A·g−1) 92.8% (5000cyc@ 5 A·g−1) [22] α-MnO2/CNTs 276 3.0 A·g−1 0.5 65.4% (3–9.5 A·g−1) 91.6% (5000cyc@3.0 A·g−1) [23] EG/MnO2 220 2 mV·s−1 1 95% (0.1-0.5 A·g−1) 100% (400cyc@5 mV·s−1) [24] EG/MnO2 161 0.1 A·g−1 1 68.3% (0.1-5 A·g−1) 100% (1000cyc@1 A·g−1) [25] EG/MnO2 428 0.5 A·g−1 1 86% (0.1-2 A·g−1) 100% (100cyc@0.5 A·g−1) This work Notes: UN-MnO2—Ultrasonic and NH4+ assisted MnO2 deposited on Ni foam substrate; CNFs—Carbon nanofibers; HMS—Hollow microsphere; CNTs—Carbon nanotubes; Cm—Specific capacitance. -
[1] XIA A, YU W R, YI J, et al. Synthesis of porous δ-MnO2 nanosheets and their supercapacitor performance[J]. Journal of Electroanalytical Chemistry,2019,839:25-31. doi: 10.1016/j.jelechem.2019.02.059 [2] ZHANG M, YANG D Y, LI J T. Ultrasonic and NH4+ assisted Ni foam substrate oxidation to achieve high performance MnO2 supercapacitor[J]. Applied Surface Science,2021,541:148546. doi: 10.1016/j.apsusc.2020.148546 [3] SU X H, YU L, CHENG G, et al. Controllable hydrothermal synthesis of Cu-doped δ-MnO2 films with different morphologies for energy storage and conversion using supercapacitors[J]. Applied Energy,2014,134:439-445. doi: 10.1016/j.apenergy.2014.08.050 [4] ZHAO S Q, LIU T M, MUHAMMAD S J, et al. Rational synthesis of Cu-doped porous δ-MnO2 microsphere for high performance supercapacitor applications[J]. Electrochimica Acta,2016,191:716-723. doi: 10.1016/j.electacta.2016.01.106 [5] GAO Q, WANG J X, KE B, et al. Fe doped δ-MnO2 nanoneedles as advanced supercapacitor electrodes[J]. Ceramics International,2018,44(15):18770-18775. doi: 10.1016/j.ceramint.2018.07.108 [6] LI J J, HU B, NIE P F, et al. Fe-regulated δ-MnO2 nanosheet assembly on carbon nanofiber under acidic condition for high performance supercapacitor and capacitive deionization[J]. Applied Surface Science,2021,542:148715. doi: 10.1016/j.apsusc.2020.148715 [7] ZHAO G Y, ZHANG D, ZHANG L, et al. Ti@δ-MnO2 core-shell nanowire arrays as self-supported electrodes of supercapacitors and Li ion batteries[J]. Electrochimica Acta,2016,202:8-13. doi: 10.1016/j.electacta.2016.03.203 [8] RADHAMANI A V, SURENDRA M, RAO M S. Zn doped δ-MnO2 nano flakes: An efficient electrode material for aqueous and solid state asymmetric supercapacitors[J]. Applied Surface Science,2018,450:209-218. doi: 10.1016/j.apsusc.2018.04.081 [9] DENG X C, BAI X J, CAI Z H, et al. Renewable carbon foam/δ-MnO2 composites with well-defined hierarchical microstructure as supercapacitor electrodes[J]. Journal of Materials Research and Technology,2020,9(4):8544-8555. doi: 10.1016/j.jmrt.2020.05.130 [10] WANG X S, CHEN L, ZHANG S Q, et al. Compounding δ-MnO2 with modified graphene nanosheets for highly stable asymmetric supercapacitors[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2019,573:57-66. [11] PANG M J, LONG G H, JIANG S, et al. One pot low-temperature growth of hierarchical δ-MnO2 nanosheets on nickel foam for supercapacitor applications[J]. Electrochimica Acta,2015,161:297-304. doi: 10.1016/j.electacta.2015.02.089 [12] SHEN H J, KONG X D, ZHANG Pu, et al. In-situ hydrothermal synthesis of δ-MnO2/soybean pod carbon and its high performance application on supercapacitor[J]. Journal of Alloys and Compounds,2021,853:157357. doi: 10.1016/j.jallcom.2020.157357 [13] FAN Z J, XIE M M, JIN X, et al. Characteristics and electrochemical performances of supercapacitors using double-walled carbon nanotube/δ-MnO2 hybrid material electrodes[J]. Journal of Electroanalytical Chemistry,2011,659(2):191-195. doi: 10.1016/j.jelechem.2011.05.025 [14] LIU J Q, ZHAO W, WEN G L, et al. Hydrothermal synthesis of well-standing δ-MnO2 nanoplatelets on nitrogen-doped reduced graphene oxide for high-performance supercapacitor[J]. Journal of Alloys and Compounds,2019,787:309-317. doi: 10.1016/j.jallcom.2019.02.090 [15] LIU C S, HUANG C L, FANG H C, et al. MnO2-based carbon nanofiber cable for supercapacitor applications[J]. Journal of Energy Storage,2021,33:102130. doi: 10.1016/j.est.2020.102130 [16] Synthesis of δ-MnO2/C assisted with carbon sheets by directly carbonizing from corn stalk for high-performance supercapacitor[J]. Materials Letters, 2021, 285: 129116. [17] XIE W L, SUN M Y, LI Y Q, et al. Three-dimensional Ni/MnO2 nanocylinder array with high capacitance for supercapacitors[J]. Results in Physics,2019,12:1411-1416. doi: 10.1016/j.rinp.2019.01.041 [18] XIE A J, JIANG C, SUN W L, et al. A coralliform-structured γ-MnO2/polyaniline nanocomposite for high-performance supercapacitors[J]. Journal of Electroanalytical Chemistry,2017,789:29-37. doi: 10.1016/j.jelechem.2017.02.032 [19] BAI X L, TONG X L, GAO Y L, et al. Hierarchical multidimensional MnO2 via hydrothermal synthesis for high performance supercapacitors[J]. Electrochimica Acta,2018,281:525-533. doi: 10.1016/j.electacta.2018.06.003 [20] CHAI C J, LIU A F, WANG Y, et al. A MoS2-templated oxidation-etching strategy to synthesize hollow δ-MnO2 nanospheres as a high-performance electrode for supercapacitor[J]. Ceramics International,2018,44(14):16923-16930. doi: 10.1016/j.ceramint.2018.06.132 [21] LONG X, TIAN L, WANG J, et al. Interconnected δ-MnO2 nanosheets anchored on activated carbon cloth as flexible electrode for high-performance aqueous asymmetric supercapacitors[J]. Journal of Electroanalytical Chemistry,2020,877:114656. doi: 10.1016/j.jelechem.2020.114656 [22] WANG X, CHEN S, LI D, et al. Direct interfacial growth of MnO2 nanostructure on hierarchically porous carbon for high-performance asymmetric supercapacitors[J]. ACS Sustainable Chemistry & Engineering,2018,6:633-641. [23] RAMESH S, KIM H S, HALDORAI Y, et al. Fabrication of nanostructured MnO2/carbon nanotube composite from 3D precursor complex for high performance supercapacitor[J]. Materials Letters,2017,196:132-136. doi: 10.1016/j.matlet.2017.03.044 [24] 万传云, AZUMI K, KONNO H. 不同工作介质下二氧化锰/膨胀石墨复合材料的电化学电容器行为[J]. 化学学报, 2007, 65(17):1911-1916.WAN C Y, AZUMI K, KONNO H. Behavior of manganese dioxide/exfoliated graphite Composite as materials for electrochemical capacitor in different Electrolytes[J]. Acta chimica sinica,2007,65(17):1911-1916(in Chinese). [25] 徐德芳, 宋燕, 田晓冬, 等. 纳米MnO2/膨胀石墨复合材料的制备及其电化学性能[J]. 新型炭材料, 2016, 31(6):615-620.XU D F, SONG Y, TIAN X D, et al. Preparation and electrochemical properties of nanostructured MnO2/ exfoliated graphite composites[J]. New carbon materials,2016,31(6):615-620(in Chinese). [26] 向震, 范宝安, 史东辉, 等. 表面改性对膨胀石墨电容性能的影响[J]. 电源技术, 2019, 43(4):81-84.XIANG Z, FAN B A, SHI D H, et al. Research on capacitance prope of surface-modified expanded graphite[J]. Chinese Journal of Power Sources,2019,43(4):81-84(in Chinese). -





 下载:
下载: