Preparation of calcite/biochar composite by co-pyrolysis and its adsorption properties and mechanism for Pb(II)
-
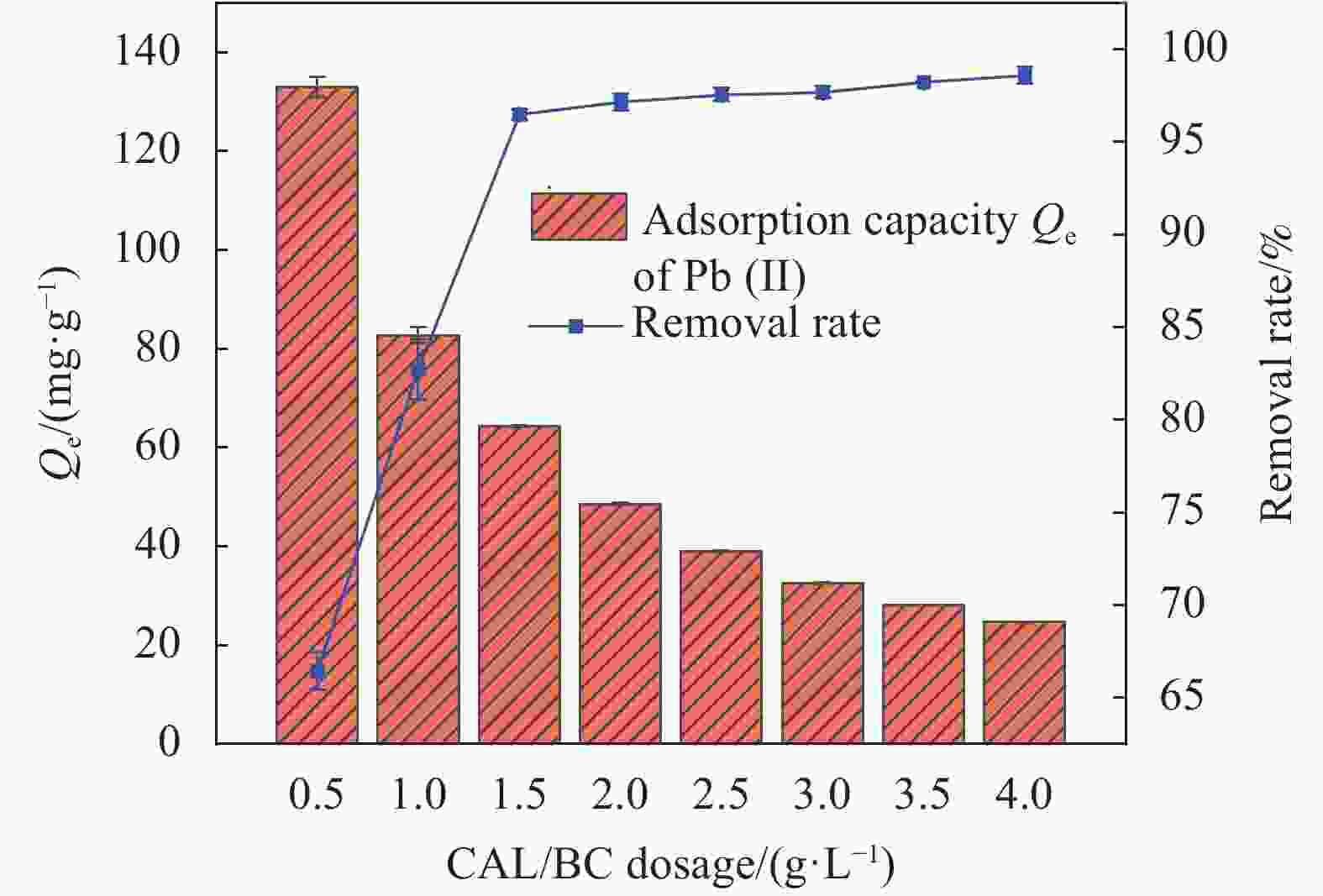
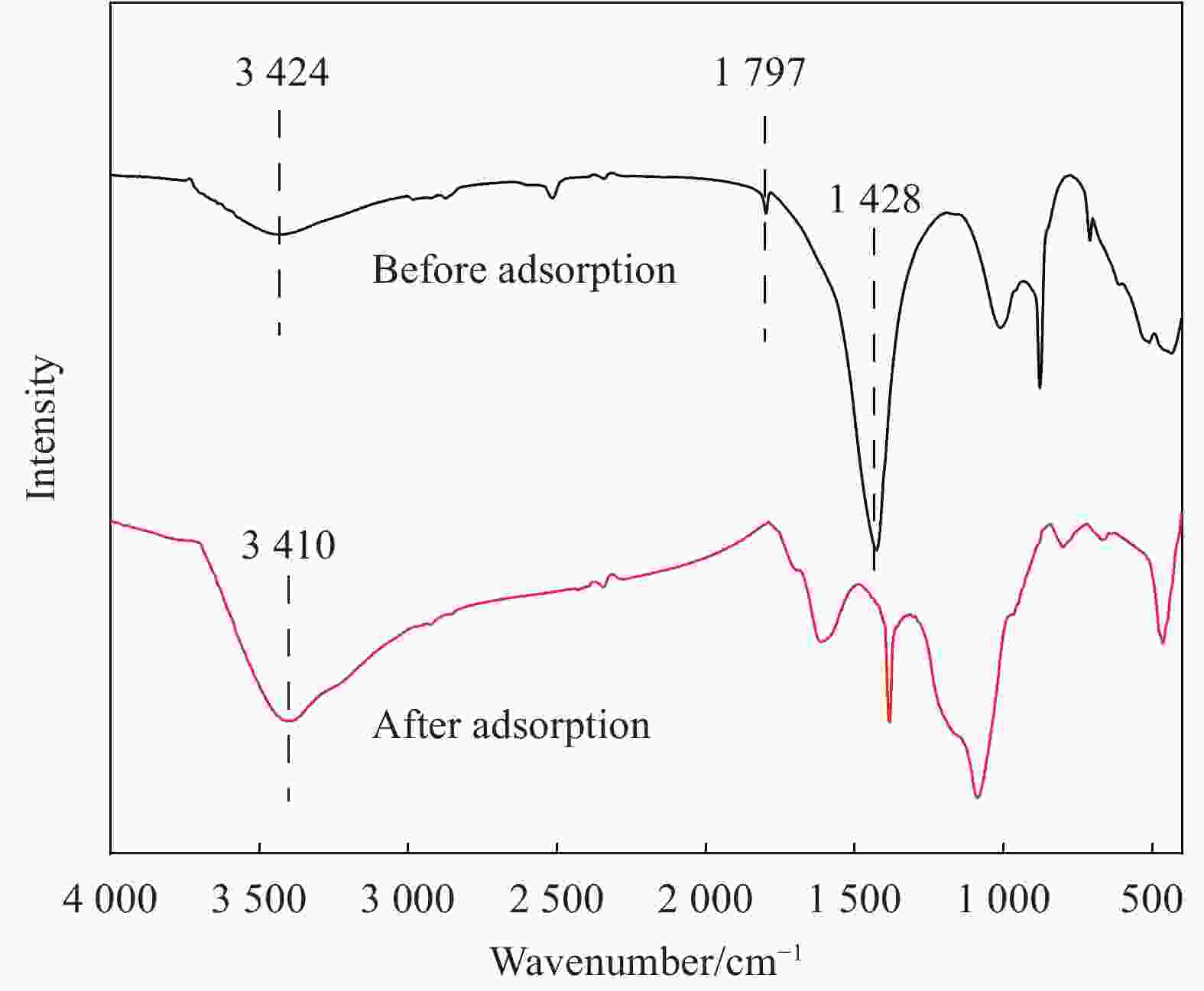
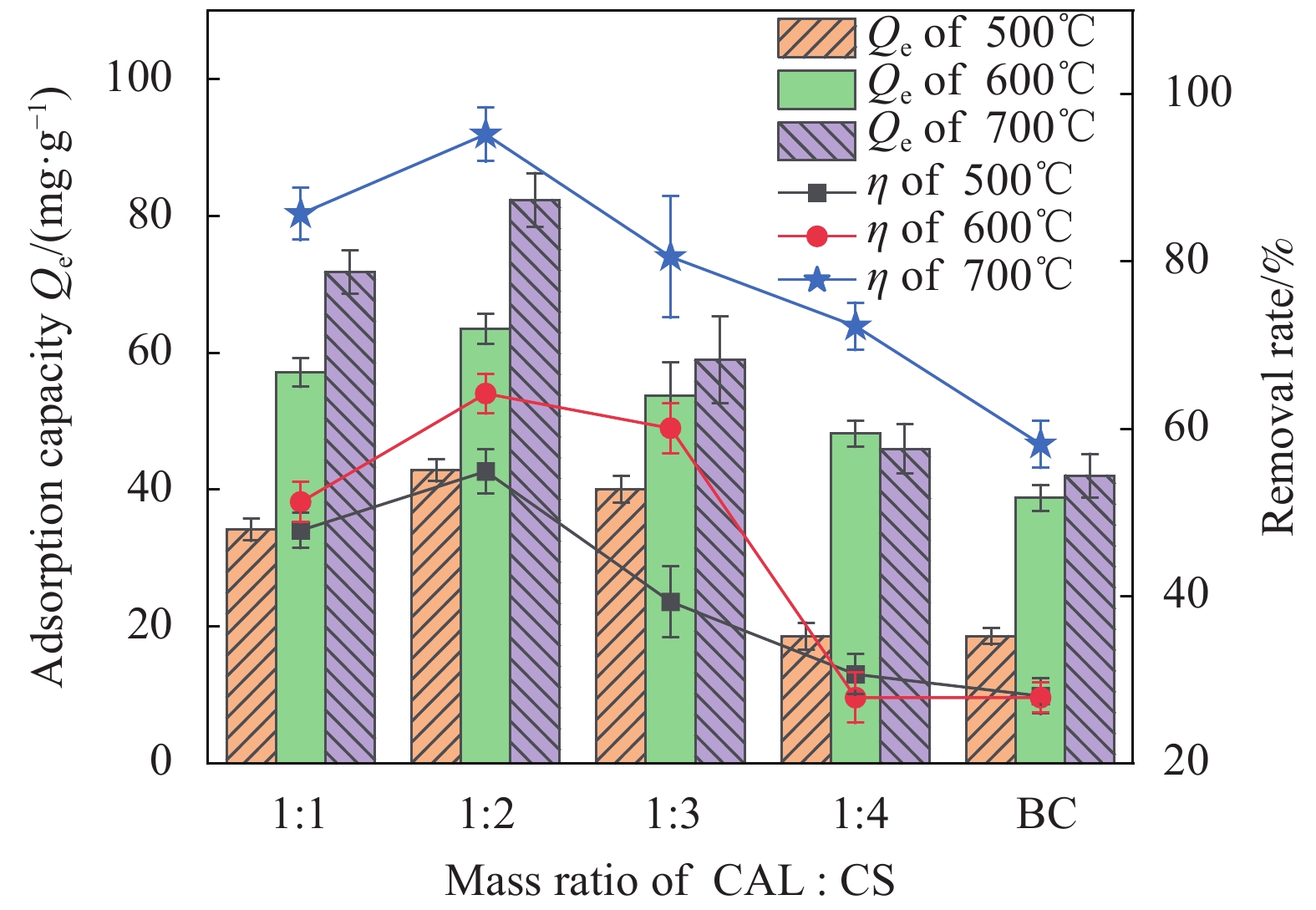
摘要: 为了制备一种高效吸附含Pb(II)废水的生物炭材料,以椰壳(CS)和方解石(CAL)为原料,采用共热解法分别在500℃、600℃、700℃制备了方解石/生物炭(CAL/BC)复合材料。通过SEM、ICP-MS、BET、XRD、FTIR等方法对CAL/BC复合材料的表面微观形态和结构进行了表征。结果发现,三种热解温度条件下,CAL均能够与CS紧密结合,而且CAL/BC具有较大的比表面积,表面含有丰富的官能团。批量吸附实验结果表明,CAL和CS质量比为1∶2,pH值为5.5,吸附剂添加量为1.5 g·L−1,此时CAL/BC复合材料对Pb(II)的吸附量分别为95.24 mg·g−1(500℃)、99.01 mg·g−1(600℃)、185.19 mg·g−1(700℃),可见热解温度为700℃时,吸附效果最佳。吸附过程符合二级动力学模型和Langmuir等温线模型。CAL/BC复合材料吸附Pb(II)的主要机制是沉淀、离子交换、阳离子-π作用、孔隙填充和静电引力。此外,CAL/BC复合材料在4次吸附-解吸循环后仍能保持较高的Pb(II)去除率。因此,共热解法制备的CAL/BC复合材料在处理废水中的Pb(II)方面具有广阔的应用前景。Abstract: In order to obtain a biochar material which adsorbs PB (II) in wastewater efficiently, calcite/biochar (CAL/BC) composite was prepared by co-pyrolysis at 500℃, 600℃ and 700℃, using coconut shell (CS) and calcite (CAL) as raw materials. The surface morphology and structure of CAL/BC composites were characterized by SEM, ICP-MS, BET, XRD and FTIR. The results show that CAL and CS combine tightly under the three pyrolysis tempera-tures, and CAL/BC has a large specific surface area and a large number of functional groups. The maximum adsorption capacities of PB (II) on CAL/BC composite (CAL∶ CS=1∶ 2, mass ratio) prepared at 500℃, 600℃, and 700℃ are 95.24 mg·g−1, 99.01 mg·g−1, and 185.19 mg·g−1. The optimum adsorption condition is pH=5.5 and the amount of adsorbent is 1.5 g·L−1. The adsorption process conforms to the second-order kinetic model and Langmuir isotherm model. The mechanisms of adsorption of Pb(II) on CAL/BC composites are precipitation, ion exchange, cation-π action, pore filling and electrostatic gravitation. In addition, the removal rate of Pb(II) by CAL/BC composite remains high level after 4 adsorption-desorption cycles. Therefore, the CAL/BC composite prepared by co-pyrolysis has a excellent application prospect in the treatment of Pb(II) in wastewater.
-
Key words:
- calcite /
- coconut shell /
- biochar /
- composites /
- Pb(II) /
- adsorption
-
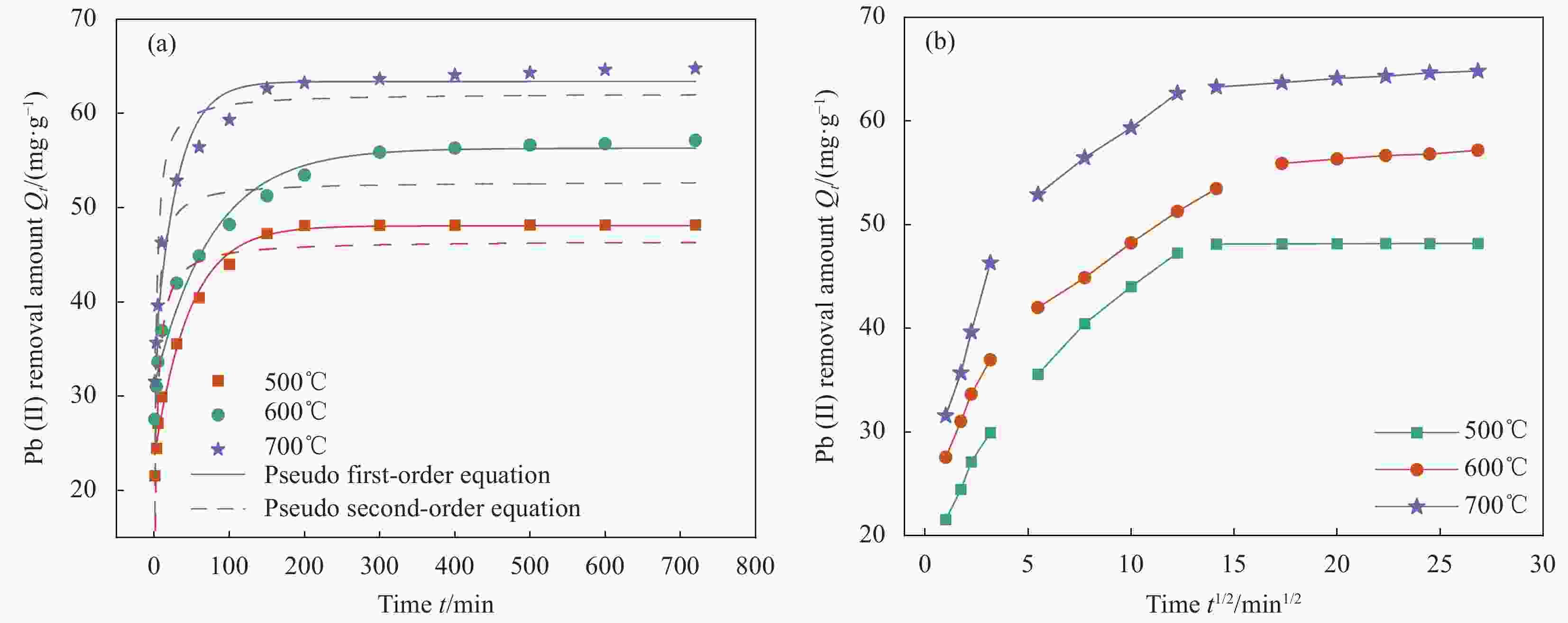
图 4 吸附时间对不同温度条件下制备的CAL/BC复合材料对Pb(II)吸附量的影响的准一级和准二级动力学模型拟合 (a) 和颗粒内扩散模型 (b)
Figure 4. Effect of adsorption time on Pb(II) adsorption capacity by CAL/BC composite prepared at different temperatures and fits of pseudo first-order and pseudo second-order kinetic models (a), intra-particle diffusion model (b)
表 1 不同温度下制备的CAL/BC复合材料吸附Pb(II)的动力学参数
Table 1. Kinetic parameters of Pb(II) adsorption by the CAL/BC composite prepared at different temperatures
Temprature/℃ Qe,exp/(mg·g−1) Pseudo-first-order Pseudo-second-order k1/min−1 Qe,calu/(mg·g−1) R2 k2/(g·mg−1·min−1) Qe,calu/(mg·g−1) R2 500 48.1844 3.1670 23.7362 0.9847 0.0035 48.7805 0.9998 600 57.1612 3.1963 24.4419 0.9752 0.0017 57.8035 0.9986 700 64.8022 3.0806 21.7715 0.9151 0.0029 64.9351 0.9999 Note: Qe,exp—Equilibrium sorption capacity obtained from experiment; k1—First-order apparent sorption rate constant; Qe,calu—Equilibrium sorption capacity calculated by pseudo-first order kinetics or pseudo-second order kinetics; k2—Second-order apparent sorption rate constant; R2—Correlation coefficient. 表 2 不同温度下制备的CAL/BC复合材料吸附Pb(II)的颗粒内扩散参数
Table 2. Intra-particle diffusion parameters of Pb(II) adsorption by the CAL/BC composite prepared at different temperatures
Temprature/
℃Film diffusion Intra-particle diffusion Equilibrium stage Kp1/
(mg·g−1·min−0.5)C1/
(mg·g−1)R2 Kp2/
(mg·g−1·min−0.5)C2/
(mg·g−1)R2 Kp3/
(mg·g−1·min−0.5)C3/
(mg·g−1)R2 500 3.9312 17.7720 0.9894 1.7142 26.6200 0.9910 0.0043 48.0720 0.9938 600 4.3769 23.3990 0.9932 1.3454 34.6150 0.9987 0.1284 53.7210 0.9845 700 6.8956 24.2800 0.9960 1.4274 45.1810 0.9987 0.1239 61.5500 0.9912 Note:Kp1, Kp2, Kp3—Rate constants at different stages of internal diffusion; C1, C2, C3—Intercept of corresponding concentration. 表 3 不同温度下制备的CAL/BC复合材料吸附Pb(II)的Langmuir和Freundlich吸附等温线参数
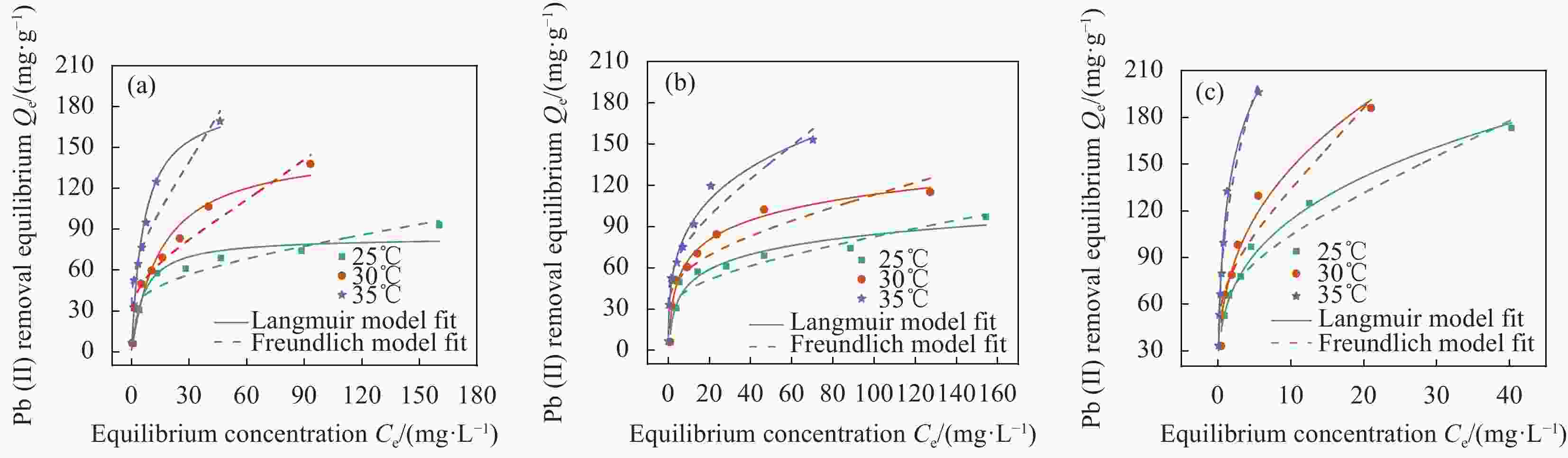
Table 3. Langmuir and Freundlich adsorption isotherm parameters of Pb(II) adsorption by the CAL/BC composite prepared at different temperatures
Adsorption temprature/℃ Temprature/℃ Langmuir Freundlich Qmax/(mg·g−1) KL/(L·mg−1) R2 1/n KF/(mg·g−1) R2 25 500 95.24 0.0798 0.9831 0.4455 2.7511 0.7557 600 99.01 0.0728 0.9742 0.4512 2.6437 0.7659 700 185.19 0.2935 0.9903 0.3461 219.7260 0.9717 30 500 151.52 0.0746 0.9718 0.5204 6.7442 0.9408 600 123.46 0.1070 0.9913 0.2761 67.4520 0.9672 700 204.08 0.4153 0.9925 0.4022 328.3842 0.9372 35 500 185.19 0.1949 0.9811 0.4841 70.2934 0.9691 600 158.73 0.2079 0.9816 0.3882 82.7148 0.9548 700 222.22 1.3235 0.9941 0.4343 1 150.8854 0.9763 Note: Qmax—Maximum sorption capacity; KL—Adsorptive constant of Langmuir model; 1/n—Empirical parameter varied with the degree of heterogeneity of adsorbing sites; KF—Adsorptive constant of Freundlich model. 表 4 复合前后材料元素含量及BET比表面积
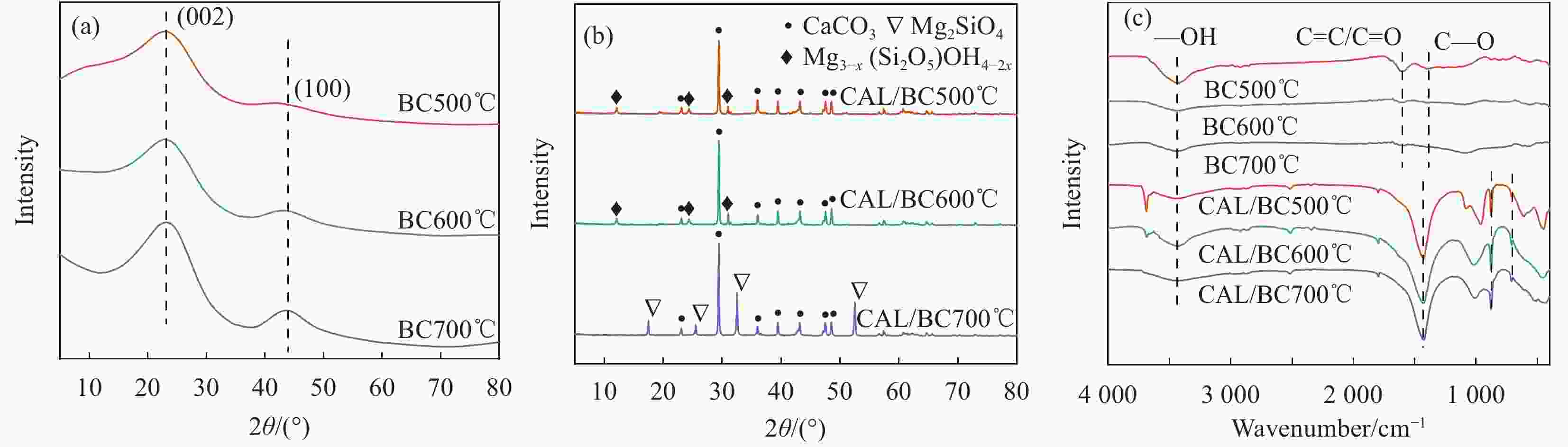
Table 4. Element mass and BET specific surface area of materials before and after composite
Material Element mass fraction/wt% SBET/(m2·g−1) C O Ca Mg BC500℃ 67.44 0 0.26 0.15 5.69 BC600℃ 77.45 0 0.10 0.06 103.39 BC700℃ 85.48 3.81 0.10 0.04 347.43 CAL 4.54 47.80 22.00 14.58 9.30 CAL/BC500℃ 15.81 16.59 18.09 13.82 50.86 CAL/BC600℃ 21.12 14.89 17.66 10.43 93.20 CAL/BC700℃ 0 15.07 18.03 10.65 285.89 Note: SBET—BET surface area. -
[1] BOGUSZ A, OLESZCZUK P, DOBROWOLSKI R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water[J]. Bioresource Technology,2015,196:540-549. doi: 10.1016/j.biortech.2015.08.006 [2] LU X W, NING X A, LEE P H, et al. Transformation of hazardous lead into lead ferrite ceramics: Crystal structures and their role in lead leaching[J]. Journal of Hazardous Materials,2017,336(15):139-145. [3] CUONG D V, LIU N L, NGUYEN V A, et al. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal[J]. Science of the Total Environment,2019,692:844-853. doi: 10.1016/j.scitotenv.2019.07.125 [4] GUPTA V K, AGARWAL S, SALEH T A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal[J]. Journal of Hazardous Materials,2011,185(1):17-23. doi: 10.1016/j.jhazmat.2010.08.053 [5] BAI J, CHAO Y Q, CHEN Y M, et al. The effect of interaction between Bacillus subtilis DBM and soil minerals on Cu(II) and Pb(II) adsorption.[J]. Journal of Environmental Sciences (China),2019,78:328-337. doi: 10.1016/j.jes.2018.11.012 [6] RWIZA M J, OH S, KIM K, et al. Comparative sorption isotherms and removal studies for Pb(II) by physical and thermochemical modification of low-cost agro-wastes from Tanzania[J]. Chemosphere,2018,195:135-145. doi: 10.1016/j.chemosphere.2017.12.043 [7] AWUAL M R, ISLAM A, HASAN M M, et al. Introducing an alternate conjugated material for enhanced lead(II) capturing from wastewater[J]. Journal of Cleaner Production,2019,224:920-929. doi: 10.1016/j.jclepro.2019.03.241 [8] BOUABIDI Z B, EL-NAAS M H, CORTES D, et al. Steel-Making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution[J]. Chemical Engineering Journal,2018,334:837-844. doi: 10.1016/j.cej.2017.10.073 [9] KAZEMI M, JAHANSHAHI M, PEYRAVI M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate[J]. Journal of Hazardous Materials,2018,344:12-22. doi: 10.1016/j.jhazmat.2017.09.059 [10] ROQUE F, DIAZ K, ANCCO M, et al. Biodepuration of domestic sewage, textile effluents and acid mine drainage using starch-based xerogel from recycled potato peels[J]. Water Science and Technology,2018,77(5):1250-1261. doi: 10.2166/wst.2018.008 [11] SONG M, WEI Y X, CAI S P, et al. Study on adsorption properties and mechanism of Pb2+ with different carbon based adsorbents[J]. Science of The Total Environment,2018,618:1416-1422. doi: 10.1016/j.scitotenv.2017.09.268 [12] HU B W, GUO X J, ZHENG C, et al. Plasma-enhanced amidoxime/magnetic graphene oxide for efficient enrichment of U(VI) investigated by EXAFS and modeling techniques[J]. Chemical Engineering Journal,2019,357:66-74. doi: 10.1016/j.cej.2018.09.140 [13] QIU M Q, WANG M, ZHAO Q Z, et al. XANES and EXAFS investigation of uranium incorporation on nZVI in the presence of phosphate[J]. Chemosphere,2018,201:764-771. doi: 10.1016/j.chemosphere.2018.03.057 [14] GAO L Y, DENG J H, HUANG G F, et al. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge[J]. Bioresource Technology,2019,272:114-122. doi: 10.1016/j.biortech.2018.09.138 [15] JIANG Y H, LI A Y, DENG H, et al. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks[J]. Bioresource Technology,2019,276:183-189. doi: 10.1016/j.biortech.2018.12.079 [16] WANG J L, WANG S Z. Preparation, modification and environmental application of biochar: A review[J]. Journal of Cleaner Production,2019,227:1002-1022. doi: 10.1016/j.jclepro.2019.04.282 [17] ABDELHAFEZ A A, LI J H. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel[J]. Journal of the Taiwan Institute of Chemical Engineers,2016,61:367-375. doi: 10.1016/j.jtice.2016.01.005 [18] WANG H Y, GAO B, WANG S S, et al. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood[J]. Bioresource Technology,2015,197:356-362. doi: 10.1016/j.biortech.2015.08.132 [19] ZHANG J J, SHAO J G, JIN Q Z, et al. Effect of deashing on activation process and lead adsorption capacities of sludge-based biochar[J]. Science of the Total Environment,2020,716:137016. doi: 10.1016/j.scitotenv.2020.137016 [20] 卢琨, 侯媛媛. 海南省椰子产业分析与发展路径研究[J]. 广东农业科学, 2020, 47(6):145-151.LU K, HOU Y Y. Analysis and development path of coconut industry in Hainan province[J]. Guangdong Agricultural Sciences,2020,47(6):145-151(in Chinese). [21] ADENIYI A G, ONIFADE D V, IGHALO J O, et al. A review of coir fiber reinforced polymer composites[J]. Composites Part B: Engineering,2019,176:107305. doi: 10.1016/j.compositesb.2019.107305 [22] PRAJAPATI A K, MONDAL M K. Comprehensive kinetic and mass transfer modeling for methylene blue dye adsorption onto CuO nanoparticles loaded on nanoporous activated carbon prepared from waste coconut shell[J]. Chemosphere,2020,307:112949. [23] UMAR I A, ABDULRAHEEM G, BALA S, et al. Kinetics, equilibrium and thermodynamics studies of C. I. Reactive Blue 19 dye adsorption on coconut shell based activated carbon[J]. International Biodeterioration & Biodegradation,2015,102:265-273. [24] ZHAO X Y, ZENG X L, QIN Y, et al. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon[J]. Chemosphere,2018,206:285-292. doi: 10.1016/j.chemosphere.2018.04.126 [25] DASSHARMA D, SAMANTA S, KUMAR S D N, et al. A mechanistic insight into enrofloxacin sorptive affinity of chemically activated carbon engineered from green coconut shell[J]. Journal of Environmental Chemical Engineering,2020,8(5):104140. doi: 10.1016/j.jece.2020.104140 [26] JANG H M, KAN E. Engineered biochar from agricultural waste for removal of tetracycline in water[J]. Bioresource Technology,2019,284:437-447. doi: 10.1016/j.biortech.2019.03.131 [27] LYU H H, TANG J C, HUANG Y, et al. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite[J]. Chemical Engineering Journal,2017,322:516-524. doi: 10.1016/j.cej.2017.04.058 [28] MA H, LI J B, LIU W W, et al. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal[J]. Bioresource Technology,2015,190:13-20. doi: 10.1016/j.biortech.2015.04.048 [29] LI X P, WANG C B, ZHANG J G, et al. Preparation and application of magnetic biochar in water treatment: A critical review[J]. Science of The Total Environment,2020,711:134847. doi: 10.1016/j.scitotenv.2019.134847 [30] ZHU R H, ZHU R L, GE F, et al. Effect of heating temperature on the sequestration of Cr3+ cations on montmorillonite[J]. Applied Clay Science,2016,121-122:111-118. doi: 10.1016/j.clay.2015.11.027 [31] YAO Y, GAO B, FANG J, et al. Characterization and environmental applications of clay-biochar composites[J]. Chemical Engineering Journal,2014,242:136-143. doi: 10.1016/j.cej.2013.12.062 [32] HEBERLING F, TRAINOR T P, LÜTZENKIRCHEN J, et al. Structure and reactivity of the calcite-water interface[J]. Journal of Colloid and Interface Science,2011,354(2):843-857. doi: 10.1016/j.jcis.2010.10.047 [33] SDIRI A., HIGASHI T, JAMOUSSI F, et al. Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems[J]. Journal of Environmental Management,2012,93(1):245-253. doi: 10.1016/j.jenvman.2011.08.002 [34] MERRIKHPOUR H, JALALI M. Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions[J]. Clean Technologies and Environmental Policy,2012,14(5):845-855. doi: 10.1007/s10098-012-0450-0 [35] SADJADI S, AKBARI M, LÉGER B, et al. Eggplant-derived biochar-halloysite nanocomposite as supports of pd nanoparticles for the catalytic hydrogenation of nitroarenes in the presence of cyclodextrin[J]. ACS Sustainable Chemistry & Engineering,2019,7:6720-6731. [36] KIM S A, KAMALA-KANNAN S, LEE K, et al. Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zerovalent iron composite[J]. Chemical Engineering Journal,2013,217:54-60. doi: 10.1016/j.cej.2012.11.097 [37] ZHAO Y L, ZHANG R Y, LIU H B, et al. Green preparation of magnetic biochar for the effective accumulation of Pb(II): Performance and mechanism[J]. Chemical Engineering Journal,2019,375:122011. doi: 10.1016/j.cej.2019.122011 [38] ZHOU X H, ZHOU J J, LIU Y C, et al. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water[J]. Fuel,2018,233:469-479. doi: 10.1016/j.fuel.2018.06.075 [39] BUDIMIROVIĆ D, VELIČKOVIĆ Z S, DJOKIĆ V R, et al. Efficient As(V) removal by α-FeOOH and α-FeOOH/α-MnO2 embedded PEG-6-arm functionalized multiwall carbon nanotubes[J]. Chemical Engineering Research and Design,2017,119:75-86. doi: 10.1016/j.cherd.2017.01.010 [40] ZAHEER Z, BAWAZIR W A, AL-BUKHARI S M, et al. Adsorption, equilibrium isotherm, and thermodynamic studies to the removal of acid orange 7[J]. Materials Chemistry and Physics,2019,232:109-120. doi: 10.1016/j.matchemphys.2019.04.064 [41] 陈泽文, 周子晗, 吴美仪, 等. 埃洛石纳米管/聚间苯二胺复合材料去除Cr(Ⅵ)的性能[J]. 复合材料学报, 2020, 37(3):493-503.CHEN Z W, ZHOU Z H, WU M Y, et al. Adsorption properties of halloysite nanotubes/poly (m-phenylenediamine) composites for Cr(Ⅵ)[J]. Acta Materiae Compositae Sinica,2020,37(3):493-503(in Chinese). [42] ZHENG L C, YANG Y B, MENG P P, et al. Absorption of cadmium (II) via sulfur-chelating based cellulose: Characterization, isotherm models and their error analysis[J]. Carbohydrate Polymers,2019,209:38-50. doi: 10.1016/j.carbpol.2019.01.012 [43] SONG Z G, LIAN F, YU Z H, et al. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution[J]. Chemical Engineering Journal,2014,242(242):36-42. [44] CAO Y Y, SHEN G H, ZHANG Y, et al. Impacts of carbonization temperature on the Pb(II) adsorption by wheat straw-derived biochar and related mechanism[J]. Science of the Total Environment,2019,692:479-489. doi: 10.1016/j.scitotenv.2019.07.102 [45] FAN Y H, WANG H, DENG L Y, et al. Enhanced adsorption of Pb(II) by nitrogen and phosphorus Co-doped biochar derived from Camellia oleifera shells[J]. Environmental Research,2020,191:110030. doi: 10.1016/j.envres.2020.110030 [46] 贾陆营, 连勇, 张津, 等. 羟基硅酸镁粉体表面改性及作为润滑油添加剂的摩擦学性能研究[J]. 表面技术, 2020, 49(4):213-221.JIA L Y, LIAN Y, ZHANG J, et al. Surface modification and tribological properties of magnesium silicate hydroxide powder as lubricant additive[J]. Surface Technology,2020,49(4):213-221(in Chinese). [47] LIU J, YANG X Y, LIU H H, et al. Modification of calcium-rich biochar by loading Si/Mn binary oxide after NaOH activation and its adsorption mechanisms for removal of Cu(II) from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2020,601:124960. doi: 10.1016/j.colsurfa.2020.124960 [48] XU X B, HU X, DING Z H, et al. Waste-art-paper biochar as an effective sorbent for recovery of aqueous Pb(II) into value-added PbO nanoparticles[J]. Chemical Engineering Journal,2017,308:863-871. doi: 10.1016/j.cej.2016.09.122 [49] CAI D Q, WANG L H, ZHANG G L, et al. Controlling pesticide loss by natural porous micro/nano composites: Straw ash-based biochar and biosilica[J]. ACS Applied Materials & Interfaces,2013,5(18):9212-9216. [50] 张晓涛, 王喜明. 木质纤维素/纳米蒙脱土复合材料对废水中Cu(II)的吸附及解吸[J]. 复合材料学报, 2015, 32(2):385-394.ZHANG X T, WANG X M. Adsorption and desorption of Cu(II) in wastewater by lignocellulose/nano-montmorillonite composites[J]. Acta Materiae Compositae Sinica,2015,32(2):385-394(in Chinese). [51] WU X P, GAO P, ZHANG X L, et al. Synthesis of clay/carbon adsorbent through hydrothermal carbonization of cellulose on palygorskite[J]. Applied Clay Science,2014,95:60-66. doi: 10.1016/j.clay.2014.03.010 [52] YAMADA S. Cation–π interactions in organic crystals[J]. Coordination Chemistry Reviews,2020,415:213301. doi: 10.1016/j.ccr.2020.213301 [53] MAZIARZ P, MATUSIK J, RADZISZEWSKA A. Halloysite-zero-valent iron nanocomposites for removal of Pb(II)/Cd(II) and As(V)/Cr(VI): Competitive effects, regeneration possibilities and mechanisms[J]. Journal of Environmental Chemical Engineering,2019,7(6):103507. doi: 10.1016/j.jece.2019.103507 -






 下载:
下载: