Preparation and electrochemical sodium storage performance of polypyrrole coated FeCl3-intercalated graphite intercalation compound
-
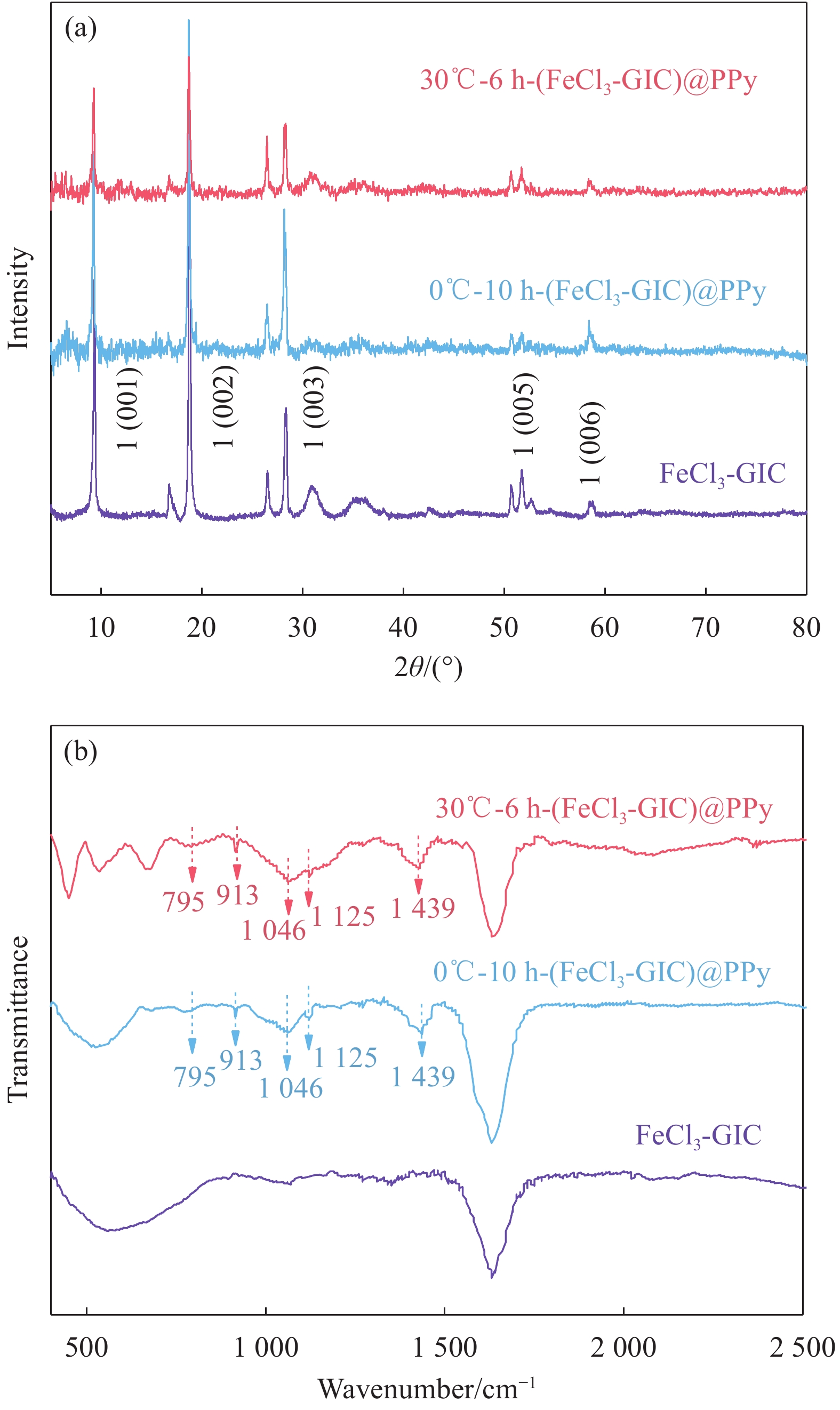
摘要: 以FeCl3和天然鳞片石墨为原料,通过融盐法制得1阶FeCl3插层的石墨层间化合物(FeCl3-GIC)。用原位聚合法对FeCl3-GIC进行聚吡咯(PPy)包覆改性,形成具有核壳结构的(FeCl3-GIC)@PPy复合材料。通过多种表征方法研究聚吡咯包覆前后FeCl3-GIC的表面形貌和微观结构变化。结果表明:聚吡咯均匀致密地包覆在十微米级的FeCl3-GIC颗粒外部,包覆层厚度为35 nm,经过聚吡咯包覆后(FeCl3-GIC)@PPy的导电性能显著提高((FeCl3-GIC)@PPy粉末电阻率2.3×10−3 Ω·cm,FeCl3-GIC粉末电阻率3.1×10−3 Ω·cm)。采用多种电化学测试探究产物的钠离子存储特性,聚吡咯外壳能够显著提高FeCl3-GIC作为钠离子电池负极材料的充放电容量、倍率性能和循环性能。在0.1 A·g−1电流密度下循环100次后,FeCl3-GIC的比容量逐渐衰减到157 mA·h·g−1,而(FeCl3-GIC)@PPy材料的比容量达到281 mA·h·g−1左右且容量基本保持不变;在电流密度1 A·g−1的条件下循环500次后,(FeCl3-GIC)@PPy的比容量仍有181 mA·h·g−1,容量保持率约为89%。Abstract: The 1-stage FeCl3-intercalated graphite intercalation compound (FeCl3-GIC) were prepared by a molten salt method using FeCl3 and natural flake graphite as raw materials. Subsequently, a conductive layer of polypyrrole (PPy) were uniformly coated on the surface of the FeCl3-GIC particles by in-situ polymerization to form a core-shell structured (FeCl3-GIC)@PPy composite material. Various characterization methods were employed to study the surface morphology and microstructure evolution of FeCl3-GIC before and after polypyrrole coating. The results show that a uniform and dense polypyrrole layer with a thickness of 35 nm is tightly coated on the surface of the micro-sized FeCl3-GIC particles. After coating, the conductivity of the (FeCl3-GIC)@PPy composite is significantly improved for the powder resistivity is reduced from 3.1×10−3 Ω·cm of FeCl3-GIC to 2.3×10−3 Ω·cm of (FeCl3-GIC)@PPy. As an anode material for sodium ion storage, it is found that the (FeCl3-GIC)@PPy anode exhibits the improved reversible capacitiy, rate capability and cycling stability compared with the naked FeCl3-GIC anode. Specially, the specific capacity of (FeCl3-GIC)@PPy remains steady with a high sodium storage value of 281 mA·h·g−1 after 100 cycles at the current density of 0.1 A·g−1, while the FeCl3-GIC anode shows a continuous capacity decay with a low value of 157 mA·h·g−1 after 100 cycles. Additionally, even at a high current density of 1.0 A·g−1, the (FeCl3-GIC)@PPy anode delivers a remained sodium storage capacity of 181 mA·h·g−1 after 500 cycles, accompanying with a fascinating capacity retention ratio of 89%.
-
Key words:
- polypyrrole /
- graphite intercalation compound /
- ferric chloride /
- sodium ion storage /
- secondary battery
-
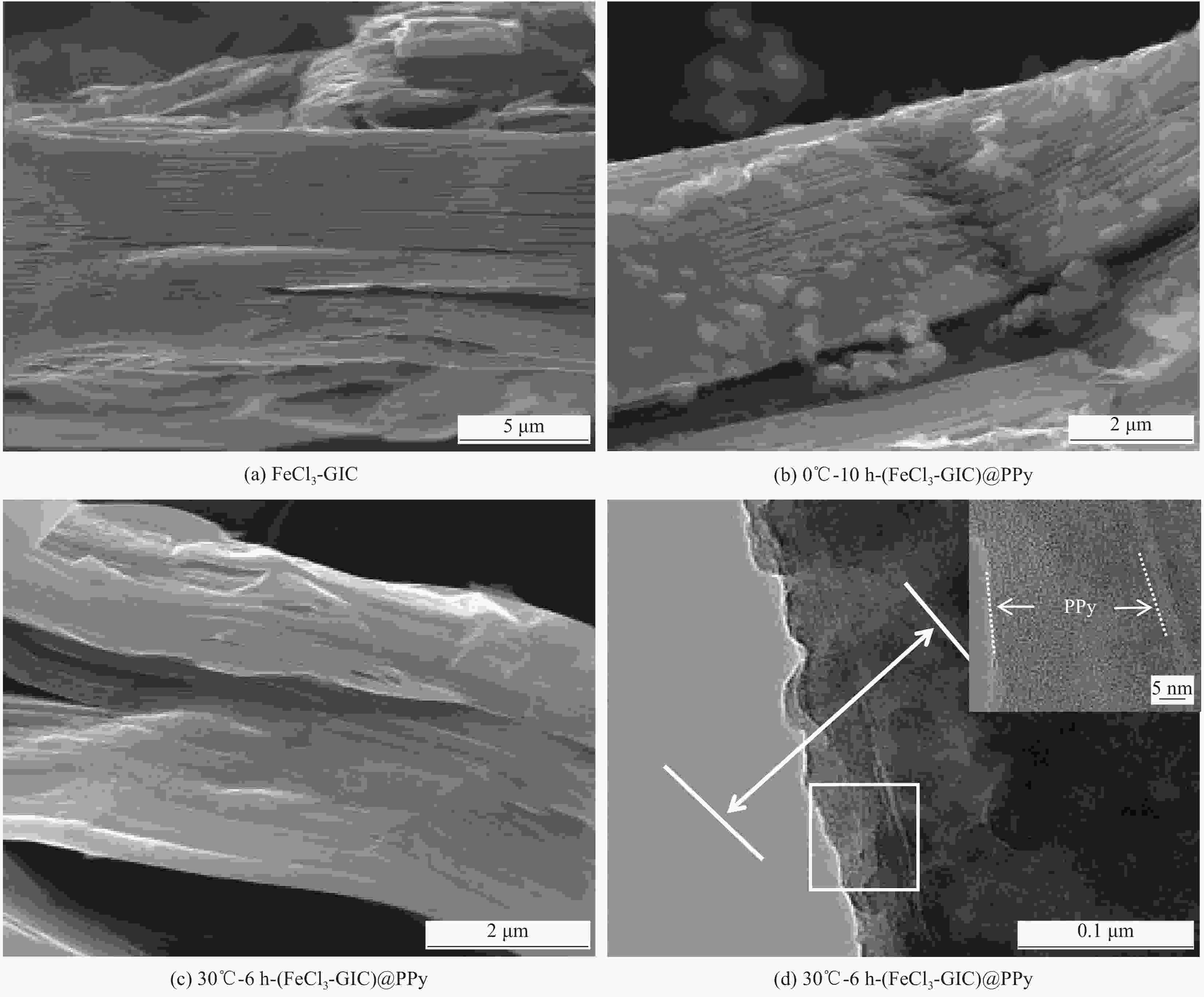
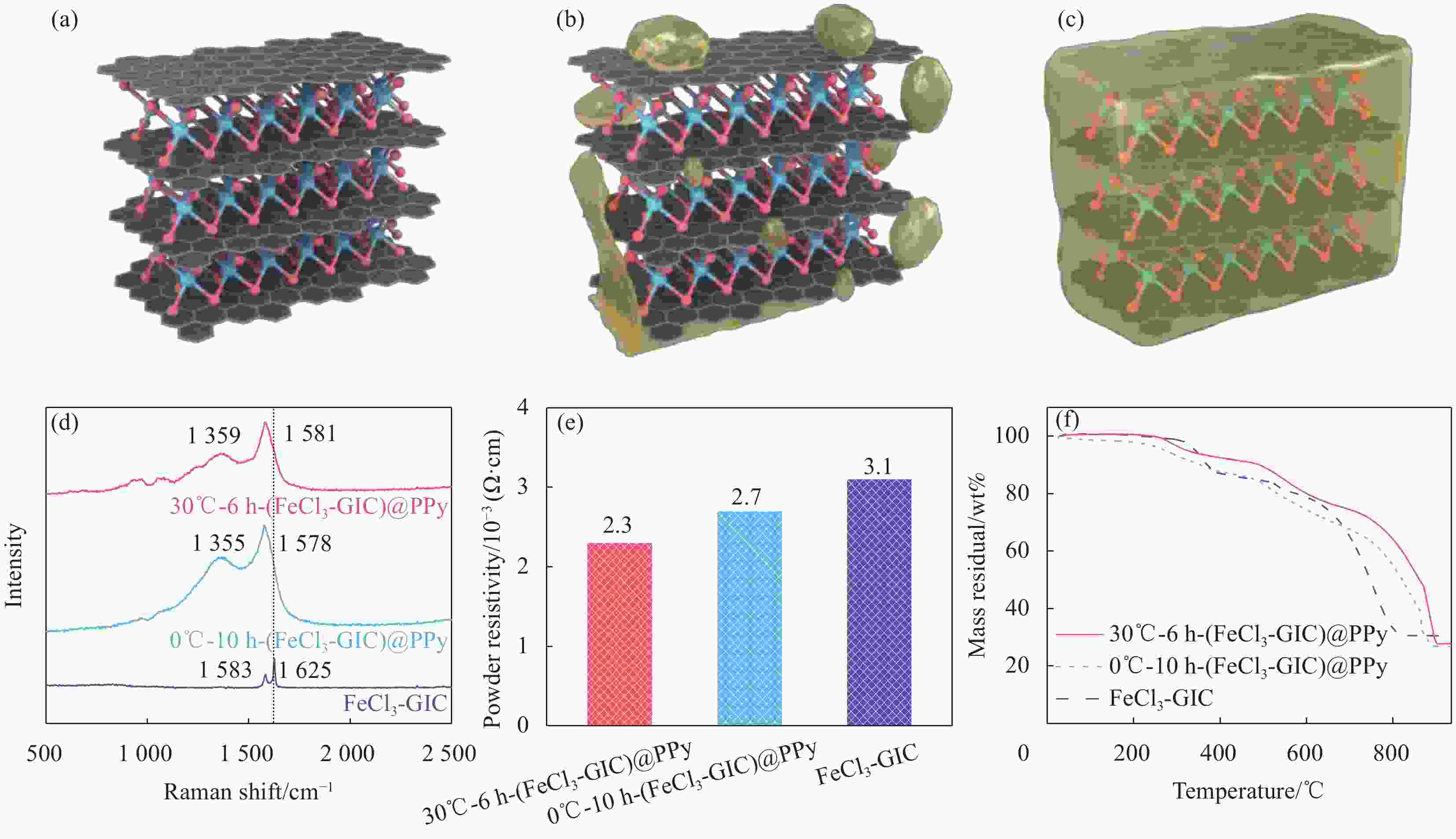
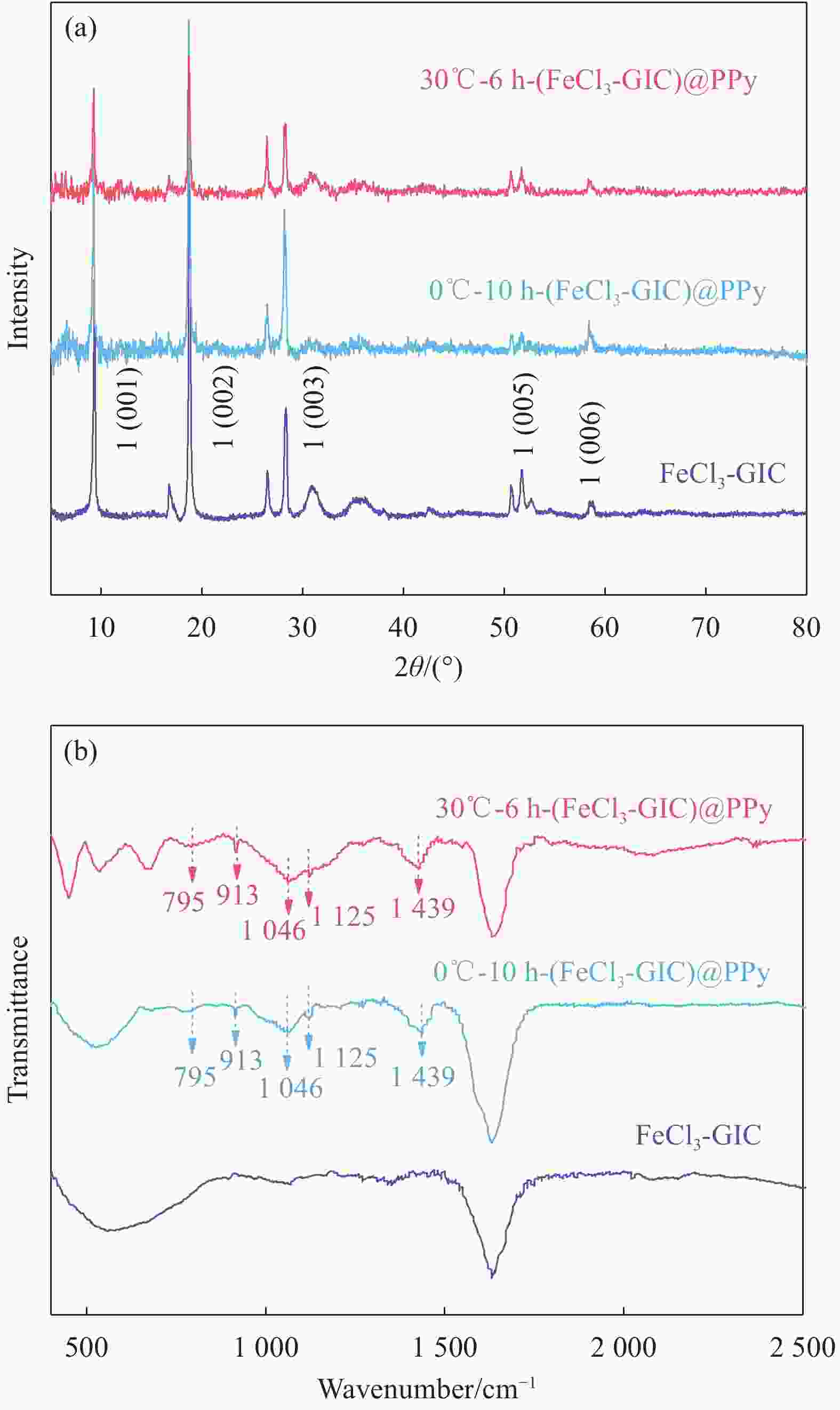
图 3 FeCl3-GIC、0℃-10 h-(FeCl3-GIC)@PPy和30℃-6 h-(FeCl3-GIC)@PPy的微观结构示意图 ((a)~(c))、拉曼光谱 (d)、粉末电阻率 (e)、热重曲线 (f)
Figure 3. Schematic diagram of the microstructure ((a)-(c)), Raman spectra (d), powder electronic resistivity (e) and thermogravimetry curves (f) of FeCl3-GIC, 30℃-6 h-(FeCl3-GIC)@PPy and 0℃-10 h-(FeCl3-GIC)@PPy
图 4 (a) 30℃-6 h-(FeCl3−GIC)@PPy电极的循环伏安曲线;(b)恒流充放电曲线;(c) FeCl3−GIC、30℃-6 h-(FeCl3−GIC)@PPy、0℃-10 h-(FeCl3−GIC)@PPy和PPy电极在100 mA·g−1电流密度下的循环性能图;(d) FeCl3−GIC电极循环后FeCl3溶解逃逸示意图;(e) 30℃-6 h-(FeCl3−GIC)@PPy电极循环100次之后的SEM和(f) TEM图片
Figure 4. Cyclic voltagmmograms (a) and galvanostatic discharge/charge profiles (b) of the 30℃-6 h-(FeCl3−GIC)@PPy anode; (c) Comparison of cycle performance of FeCl3−GIC, 0℃-10 h-(FeCl3−GIC)@PPy, 30℃-6 h-(FeCl3−GIC)@PPy and PPy at 100 mA·g−1 for SIBs; (d) FeCl3 dissolution and escape diagram of the FeCl3−GIC anode after cycling several times; SEM (e) and TEM (f) of 30℃-6 h-(FeCl3−GIC)@PPy anode after cycling 100 times
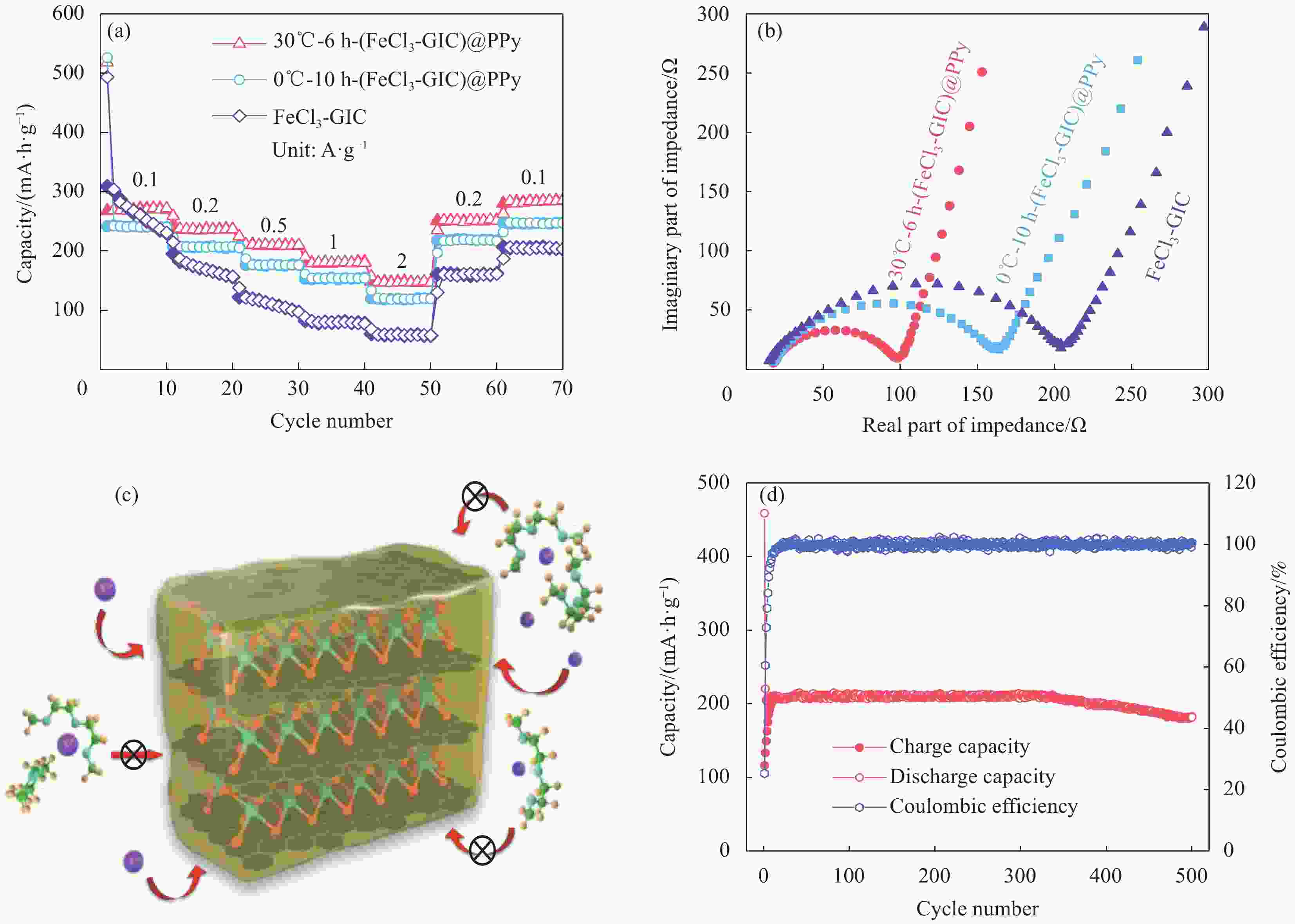
图 5 (a) FeCl3-GIC、0℃-10 h-(FeCl3-GIC)@PPy和30℃-6 h-(FeCl3-GIC)@PPy电极的倍率性能;(b) FeCl3-GIC、30℃-6 h-(FeCl3-GIC)@PPy和0℃-10 h-(FeCl3-GIC)@PPy电极的奈奎斯特曲线;(c) 30℃-6 h-(FeCl3-GIC)@PPy外层PPy阻碍溶剂化Na+插层示意图;(d) 30℃-6 h-(FeCl3-GIC)@PPy电极的长循环性能
Figure 5. (a) Rate permance of FeCl3-GIC, 0℃-10 h-(FeCl3-GIC)@PPy and 30℃-6 h-(FeCl3-GIC)@PPy anode; (b) Nyquist plots of FeCl3-GIC, 30℃-6 h-(FeCl3-GIC)@PPy and 0℃-10 h-(FeCl3-GIC)@PPy anode; (c) Schematic diagram of out layer PPy for 30℃-6 h-(FeCl3-GIC)@PPy blocking solvated Na+ intercalating; (d) Long-cycle performance of the 30℃-6 h-(FeCl3-GIC)@PPy anode
-
[1] MASSÉ R C, LIU C, LI Y, et al. Energy storage through intercalation reactions: electrodes for rechargeable batteries[J]. National Science Review,2017,4(1):26-53. doi: 10.1093/nsr/nww093 [2] WHITTINGHAM M S. Ultimate limits to intercalation reactions for lithium batteries[J]. Chemical Reviews,2014,114(23):11414-11443. doi: 10.1021/cr5003003 [3] KINOSHITA H, JEON I, MARUYAMA M, et al. Highly conductive and transparent large-area bilayer graphene realized by MoCl5 intercalation[J]. Advanced Materials,2017,29(41):1702141. doi: 10.1002/adma.201702141 [4] XU J, DOU Y, WEI Z, et al. Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries[J]. Advanced Science,2017,4(10):1700146. doi: 10.1002/advs.201700146 [5] PENG F, MENG F, GUO Y, et al. Intercalating hybrids of sandwich-like Fe3O4-graphite: synthesis and their synergistic enhancement of microwave absorption[J]. ACS Sustainable Chemistry & Engineering,2018,6(12):16744-16753. [6] BOINTON T H, JONES G F, DE SANCTIS A, et al. Large-area functionalized CVD graphene for work function matched transparent electrodes[J]. Scientific Reports,2015,5(1):1-6. doi: 10.9734/JSRR/2015/14076 [7] JIANG J, YAN P, ZHOU Y, et al. Interplanar growth of 2D non-Van der Waals Co2N-based heterostructures for efficient overall water splitting[J]. Advanced Energy Materials,2020,10(44):2002214. doi: 10.1002/aenm.202002214 [8] 杨绍斌, 夏英凯, 刘凤霞, 等. 活化剂对焦煤基多孔碳制备的影响及其在锂硫电池中的应用[J]. 复合材料学报, 2020, 37(3):716-723.YANG Shaobin, XIA Yingkai, LIU Fengxia, et al. Effect of activator on perparation of coal-based porous carbon and its application in lithium-sulfur batteries[J]. Acta Materiae Compositae Sinica,2020,37(3):716-723(in Chinese). [9] ZHANG L, WANG H. Intercalation of multiply solvated hexafluorophospate anion into graphite electrode from mixtures of methyl acetate, ethyl methyl and ethylene carbonates[J]. Journal of Energy Chemistary,2021,58:233-236. doi: 10.1016/j.jechem.2020.10.012 [10] DIVYA M L, NATARAJAN S, LEE Y S, et al. Highly reversible Na-intercalation into graphite recovered from spent Li-ion batteries for high-energy Na-ion capacitor[J]. Chemsuschem,2020,13(21):5654-5663. doi: 10.1002/cssc.202001355 [11] ZHANG C, MA J, HAN F, et al. Strong anchoring effect of ferric chloride-graphite intercalation compounds(FeCl3-GICs) with tailored epoxy groups for high-capacity and stable lithium storage[J]. Journal of Materials Chemistry A,2018,6(37):17982-17993. doi: 10.1039/C8TA06670A [12] KIM K, GUO Q, TANG L, et al. Reversible insertion of Mg-Cl superhalides in graphite as a cathode for aqueous dual-ion batteries[J]. Angewandte Chemie,2020,132(45):20096-20100. doi: 10.1002/ange.202009172 [13] LEI Y, CHEN Y, WANG H, et al. A graphite intercalation composite as the anode for the potassium-ion oxygen battery in a concentrated ether-based electrolyte[J]. ACS Applied Materials & Interfaces,2020,12(33):37027-37033. [14] ZHOU W, SIT P H L. First-principles understanding of the staging properties of the graphite intercalation compounds towards dual-ion battery applications[J]. ACS Omega,2020,5(29):18289-18300. doi: 10.1021/acsomega.0c01950 [15] WANG F, YI J, WANG Y, et al. Graphite intercalation compounds(GICs): A new type of promising anode material for lithium-ion batteries[J]. Advanced Energy Materials,2014,4(2):1300600. doi: 10.1002/aenm.201300600 [16] LI Z, ZHANG C, HAN F, et al. Improving the cycle stability of FeCl3-graphite intercalation compounds by polar Fe2O3 trapping in lithium-ion batteries[J]. Nano Research,2019,12(8):1836-1844. doi: 10.1007/s12274-019-2444-2 [17] 魏文丽. 聚吡咯基电极材料的设计及其在锂电池中的应用[D]. 兰州: 兰州大学, 2019.WEI Wenli. Design of polypyrrole-based electrode materials and its application in lithium battery[D]. Lanzhou: Lanzhou University, 2019(in Chinese). [18] XU J, WANG D X, YUAN Y, et al. Polypyrrole/reduced graphene oxide coated fabric electrodes for supercapacitor application[J]. Organic Eletronics,2015,24:153-159. [19] ZHONG X B, WANG H Y, YANG Z Z, et al. Facile synthesis of mesoporous ZnCo2O4 coated with polypyrrole as an anode material for lithiun-ion batteries[J]. Journal of Power Sources,2015,296:298-304. doi: 10.1016/j.jpowsour.2015.07.047 [20] CHEN G F, LI XX, ZHANG L Y, et al. A porous perchlorate-doped polypyrrole nanocoating on nickel nanotube arrays for stable wide-potential-window supercapacitors[J]. Advanced Materials,2016,28(35):7680-7687. doi: 10.1002/adma.201601781 [21] 汪杰. 聚吡咯及其复合材料的制备及性能研究[D]. 合肥: 中国科学技术大学, 2017.WANG Jie. Fabrication and application of polypyrrole and its composites[D]. Hefei: University of Science and Technology of China, 2017(in Chinese). [22] 沈兰波. 导电聚合物合成新策略探索[D]. 济南: 山东大学, 2020.SHEN Lanbo. New strategies for the synthesis of conducting polymers[D]. Jinan: Shandong University, 2020(in Chinese). [23] LI Z, ZHANG C, HAN F, et al. Towards high-volumetric performance of Na/Li-ion batteries: a better anode material with molybdenum pentachloride-graphite intercalation compounds(MoCl5-GICs)[J]. Journal of Materials Chemistry A,2020,8(5):2430-2438. doi: 10.1039/C9TA12651A [24] LI D, ZHU M, CHEN L, et al. Sandwich-like FeCl3@C as high-performance anode materials for potassium-ion batteries[J]. Advanced Materials Interfaces,2018,5(15):1800606. doi: 10.1002/admi.201800606 [25] SUN Y, HAN F, ZHANG C, et al. Intercalated microcrystalline graphite enables high volumetric capacity and good cycle stability for lithium-ion batteries[J]. Energy Technology,2019,7(4):1801091. doi: 10.1002/ente.201801091 [26] HAN F, LI D, LI W, et al. Nanoengineered polypyrrole-coated Fe2O3@C multifunctional composites with an improved cycle stability as lithium-ion anodes[J]. Advanced Functional Materials,2013,23(13):1692-1700. doi: 10.1002/adfm.201202254 [27] HAN F, ZHANG C, SUN B, et al. Dual-carbon phase-protective cobalt sulfide nanoparticles with cable-type and mesoporous nanostructure for enhanced cycling stability in sodium and lithium ion batteries[J]. Carbon,2017,118:731-742. doi: 10.1016/j.carbon.2017.03.038 [28] CHUNG G C, KIM H J, YU S I, et al. Origin of graphite exfoliation an investigation of the important role of solvent cointercalation[J]. Journal of The Electrochemical Society,2000,147(12):4391-4398. doi: 10.1149/1.1394076 [29] JACHE B, ADELHELM P. Use of graphite as a highly reversibie electrode with superior cycle life for sodium-ion batteries by making use of co-intercalation phenomena[J]. Angewandte Chemie International Edition,2014,53(38):10169-10173. doi: 10.1002/anie.201403734 -





 下载:
下载: