Preparation method of a new self-supporting electrode based on carbon fiber
-
摘要:
借鉴自支撑电极的制备原理,利用电化学沉积结合(NH4)2S2O8和NaOH沉积液进行表面处理等手段制备了基于碳纤维表面Cu(OH)2纳米结构的自支撑电极,分析测试了碳纤维表面的微观形貌、表面元素组成及其分布和表面物质的晶型以及利用水热反应在其表面附着电化学物质MnO2后的电化学性能。结果发现,当(NH4)2S2O8的浓度为0.43 g/L、NaOH浓度为30.48 g/L、处理时间为12 min时,由SEM观察发现碳纤维表面的Cu(OH)2纳米纤维的直径、长度、数量都较适宜;XPS、XRD和EDS的测试结果,沉积液处理后碳纤维表面部分单质铜转化为Cu(OH)2,此结构非常有利于电化学物质的负载而由此构成开放、具有核壳结构的高性能电极材料;恒电流充放电(GCD)测试结果表明此电极材料具有极快的充放电速度。因此本文首次成功地在碳纤维表面的铜层表面原位生长出Cu(OH)2纳米纤维,为未来以超级电容器为代表的能源设备的性能提升和商业化应用开拓了一种新的电极制备方法。
-
关键词:
- 自支撑电极 /
- 电化学沉积 /
- 沉积液 /
- Cu(OH)2纳米纤维 /
- 超级电容器
Abstract:Based on the preparation principle of self-supporting electrodes, a self-supporting electrode based on carbon fiber surface Cu(OH)2 nanostructures was prepared by electrochemical deposition combined with (NH4)2S2O8 and NaOH deposition solution for surface treatment. The electrochemical performance of the electrochemical substance MnO2 attached to its surface was analyzed by the hydrothermal reaction. The microscopic morphology of the carbon fiber surface, the composition and distribution of surface elements and the crystalline form of the surface substance were tested. It was found that when the concentration of (NH4)2S2O8 is 0.43 g/L, the concentration of NaOH is 30.48 g/L and the treatment time is 12 min, the diameter, length and quantity of Cu(OH)2 nano-fibers on the surface of the carbon fiber are more suitable for SEM observation; The test results of XPS, XRD and EDS show that the elemental copper on the surface of the carbon fiber is converted to Cu(OH)2 after being treated by the deposition solution; Galvanostatic charge-discharge (GCD) test results show that the electrode material has extremely fast charge and discharge speed. The structure is very conducive to the loading of electrochemical substances, and results in the open, high-performance electrode material with a core-shell structure. The Cu(OH)2 nanofibers are successfully grown on the surface of copper layer on the surface of carbon fiber for the first time, which opens up a new electrode preparation method for the performance improvement and commercial application of energy equipment represented by supercapacitors in the future.
-
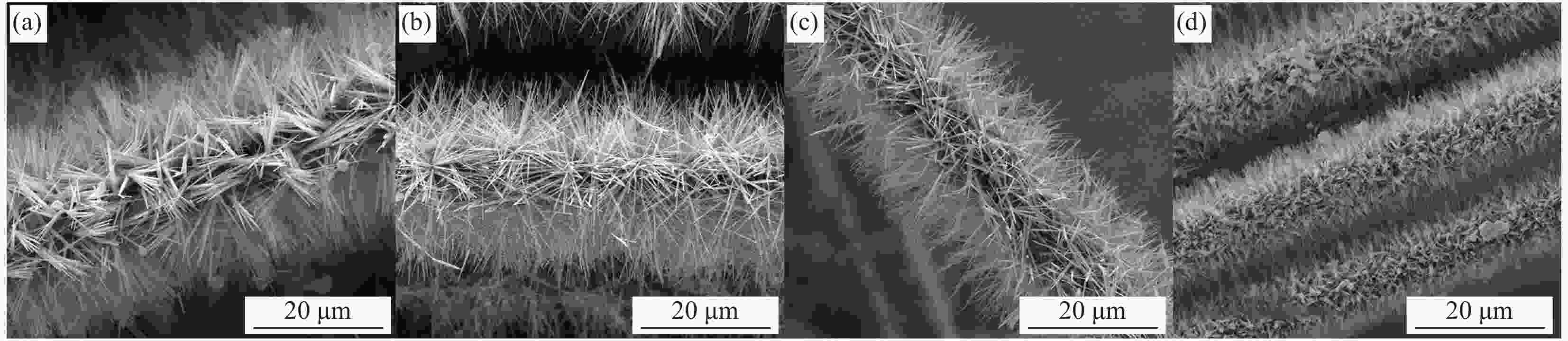
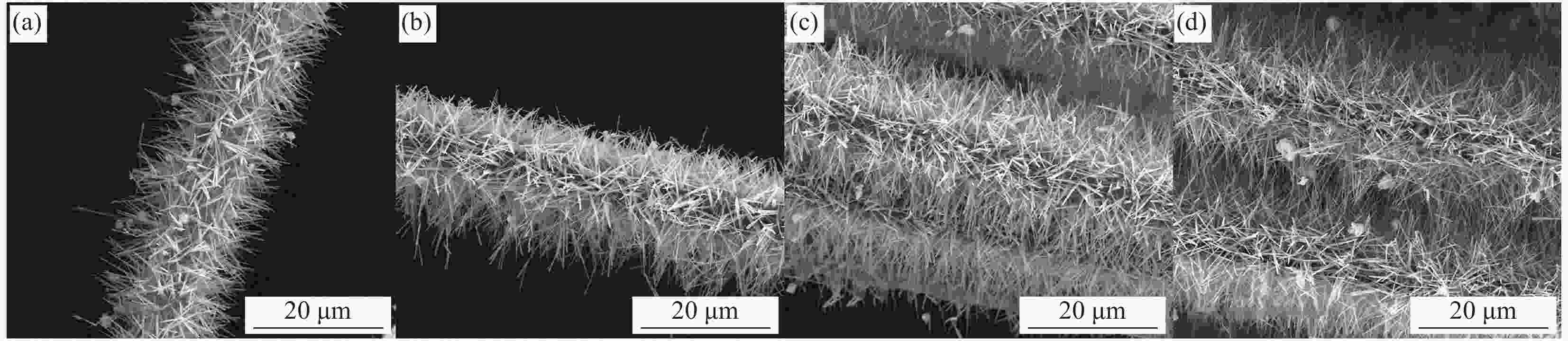
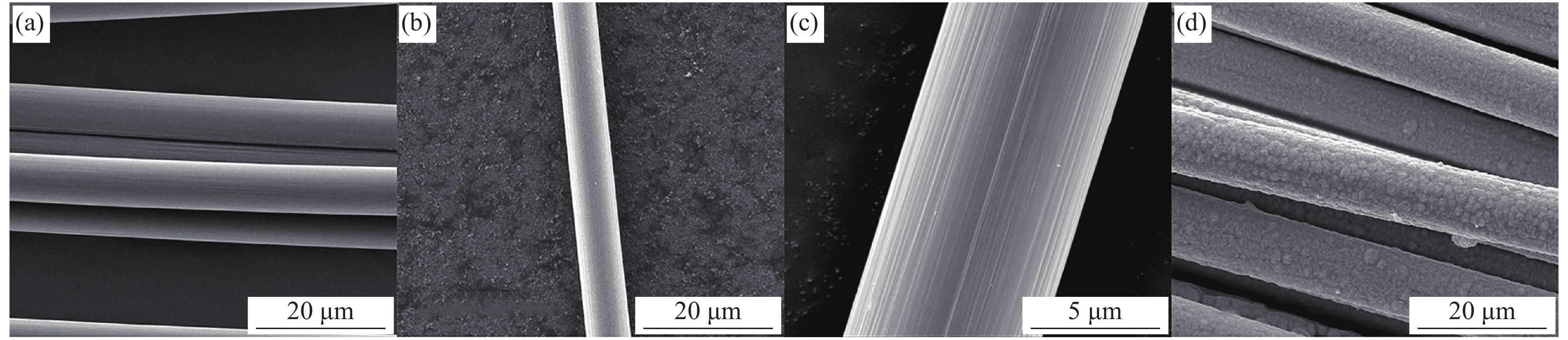
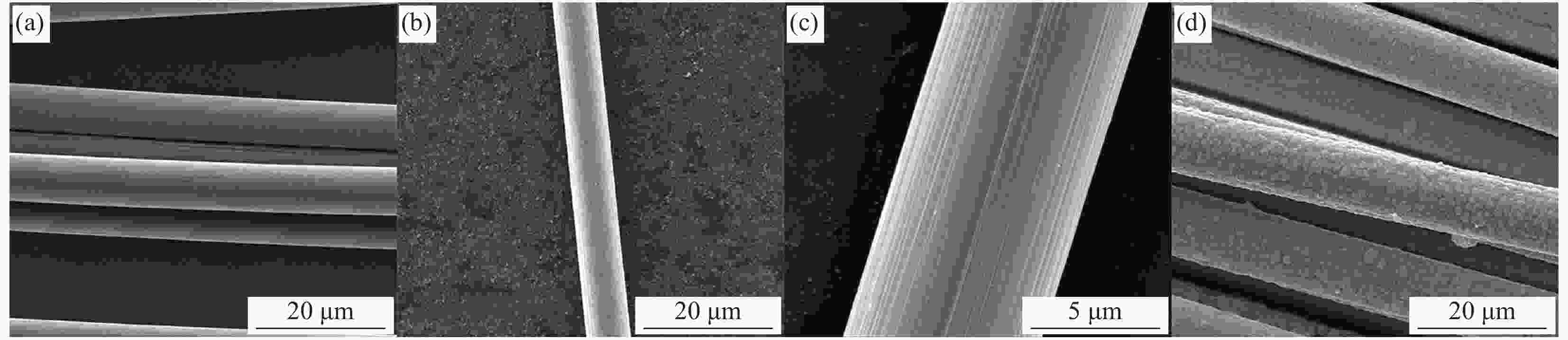
图 3 采用相同物质比例但浓度不同的沉积液处理后碳纤维的表面形貌
Figure 3. Surface morphologies of carbon fiber obtained by deposition solutions of the same molar ratio and different concentrations for different time((a) CF-Cu(OH)2-50-2; (b) CF-Cu(OH)2-50-5; (c) CF-Cu(OH)2-50-10; (d) CF-Cu(OH)2-50-20; (e) CF-Cu(OH)2-75-2; (f) CF-Cu(OH)2-75-5; (g) CF-Cu(OH)2-75-10; (h) CF-Cu(OH)2-75-20; (i) CF-Cu(OH)2-100-2; (j) CF-Cu(OH)2-100-5; (k) CF-Cu(OH)2-100-10; (l) CF-Cu(OH)2-100-20)
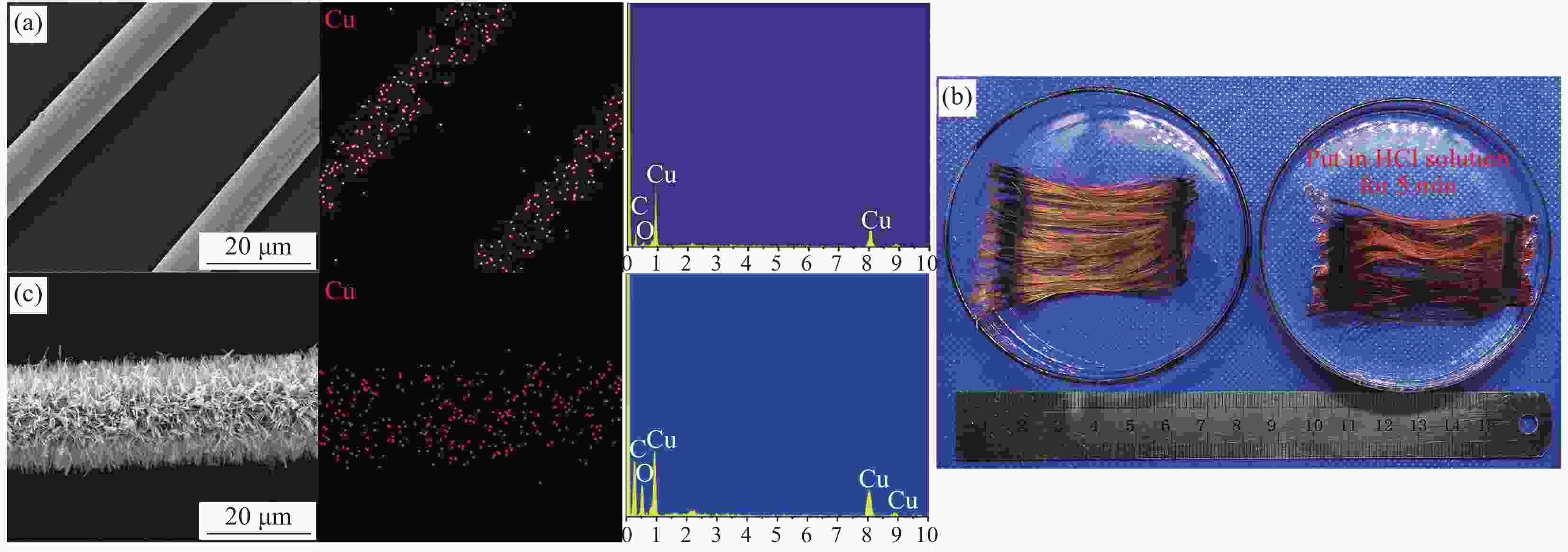
图 6 电化学沉积后碳纤维表面的EDS元素分布图(a);浸入HCl溶液前后碳纤维的实物照片(b);沉积液处理后碳纤维表面的EDS元素分布图(c)
Figure 6. EDS mapping results of the sample after electrochemical deposition (a); Photo of the sample before and after put in HCl solution (b); EDS mapping results of the sample after the treatment of the deposition solution (c)
表 1 电化学沉积过程的工艺参数
Table 1. Optimal electrochemical deposition process
Project Detail CuSO4·5H2O/(g·L−1) 40.00 C4H4O6KNa·4H2O/(g·L−1) 20.00 C6H5Na3O7·2H2O/(g·L−1) 180.00 KNO3/(g·L−1) 24.00 PEG/(g·L−1)(Mw=6000) 0.20 Current density/(mA·cm−2) 0.95 Plating time/min 60 Temperature/℃ 25 Notes: PEG—Polyethylene glycol; Mw—Weight average molecular weight. 表 2 不同的沉积液组成及处理时间
Table 2. Composition of the deposition solution and the treatment time
Sample name (NH4)2S2O8 concentration/% NaOH concentration/% Time/min CF-Cu(OH)2-100-2 100 100 2 CF-Cu(OH)2-100-5 100 100 5 CF-Cu(OH)2-100-10 100 100 10 CF-Cu(OH)2-100-20 100 100 20 CF-Cu(OH)2-75-2 75 75 2 CF-Cu(OH)2-75-5 75 75 5 CF-Cu(OH)2-75-10 75 75 10 CF-Cu(OH)2-75-20 75 75 20 CF-Cu(OH)2-50-2 50 50 2 CF-Cu(OH)2-50-5 50 50 5 CF-Cu(OH)2-50-10 50 50 10 CF-Cu(OH)2-50-20 50 50 20 Note: Concentration of 100% is 17.40 g/L for (NH4)2S2O8 and 30.48 g/L for NaOH. 表 3 不同沉积液的组成
Table 3. Different compositions of the deposition solutions
Sample name (NH4)2S2O8/% NaOH/% Time/min CF-Cu(OH)2-50/100-20 50.0 100 20 CF-Cu(OH)2-37.5/100-20 37.5 100 20 CF-Cu(OH)2-25/100-20 25.0 100 20 CF-Cu(OH)2-22.5/100-20 22.5 100 20 表 4 相同沉积液的不同处理时间
Table 4. Different treat time of the same deposition solution
Sample name (NH4)2S2O8/% NaOH/% Time/min CF-Cu(OH)2-25/100-10 25 100 10 CF-Cu(OH)2-25/100-12 25 100 12 CF-Cu(OH)2-25/100-14 25 100 14 CF-Cu(OH)2-25/100-16 25 100 16 CF-Cu(OH)2-25/100-18 25 100 18 表 5 水热反应实验参数
Table 5. Experimental parameters of hydrothermal reaction
KMnO4/(g·L-1) NaOH/(g·L-1) Time/h Temperature/℃ 1.19 0.030 12 155 表 6 不同碳纤维样品表面的元素比例
Table 6. Ratio of elements on the fiber surface of different carbon fiber samples
Sample Cu/% O/% Before treatment 23.14 76.86 After treatment 9.33 90.67 -
[1] POONAM, SHARMA K, ARORA A, et al. Review of supercapacitors: Materials and devices[J]. Journal of Energy Storage,2019,21:801-825. doi: 10.1016/j.est.2019.01.010 [2] 何水剑, 陈卫. 碳基三维自支撑超级电容器电极材料研究进展[J]. 电化学, 2015, 21(6):518-533.HE Shuijian, CHEN Wei. Research progress of carbon-based three-dimensional self-supporting supercapacitor electrode materials[J]. Electrochemistry,2015,21(6):518-533(in Chinese). [3] HO Kuochuan, LIN Luyin. A review of electrode materials based on core-shell nanostructures for electrochemical supercapacitors[J]. Journal of Materials Chemistry A,2019,7:3516-3530. doi: 10.1039/C8TA11599K [4] MICHIO Inagaki, HIDETAKA Konno, OSAMU Tanaike. Carbon materials for electrochemical capacitors[J]. Journal of Power Sources,2010,195:7880-7903. doi: 10.1016/j.jpowsour.2010.06.036 [5] 岳红伟, 陈淑君, 卢帆, 等. 高性能自支撑不锈钢网@MoS2锂离子电池负极材料[J]. 复合材料学报, 2020, 37(6):1476-1482.YUE Hongwei, CHEN Shujun, LU Fan, et al. High perfor-mance freestanding stainless steel net@MoS2 lithium-ion battery anode material[J]. Acta Materiae Compositae Sinica,2020,37(6):1476-1482(in Chinese). [6] 李寒, 孙志鹏, 贾殿赠. 柔性钛箔上生长的自支撑TiO2@NiCo2S4阵列复合材料用作高性能非对称超级电容器电极[J]. 材料导报, 2020, 34(1):1187-1195.LI Han, SUN Zhipeng, JIA Dianzeng. Self-supporting TiO2@NiCo2S4 array composites grown on flexible titanium foils are used as high-performance asymmetric supercapacitor electrodes[J]. Materials Reports,2020,34(1):1187-1195(in Chinese). [7] KANG Jiahui, SHENG Jiali, XIE Jinqi, et al. Tubular Cu(OH)2 arrays decorated with nanothorny Co-Ni bimetallic carbonate hydroxide supported on Cu foam: A 3D hierarchical core-shell efficient electrocatalyst for the oxygen evolution reaction[J]. Journal of Materials Chemistry A,2018,6:10064-10073. doi: 10.1039/C8TA02492H [8] ZHANG Dongbin, SHAO Yuan, KONG Xianggui, et al. Facile fabrication of large-area hybrid Ni-Co hydroxide/Cu(OH)2/copper foam composites[J]. Electrochimica Acta,2016,218:294-302. doi: 10.1016/j.electacta.2016.09.137 [9] ZHANG Tengyuan, LI Xia, EATON Asher, at al. Paper with power: Engraving 2D materials on 3D structures for printed, high-performance, binder-free, and all-solid-state supercapacitors[J]. Advanced Functional Materials,2018,28:1803600. doi: 10.1002/adfm.201803600 [10] WANG Jiexi, ZHANG Qiaobao, LI Xinhai, et al. Smart construction of three-dimensional hierarchical tubular transition metal oxide core/shell heterostructures with high-capacity and long-cycle-life lithium storage[J]. Nano Energy,2015,12:437-446. doi: 10.1016/j.nanoen.2015.01.003 [11] LIU Pengfei, ZHOU Jiaojiao, LI Guochang, et al. A hierarchical NiO/NiMn-layered double hydroxide nanosheet array on Ni foam for high performance supercapacitors[J]. Dalton Transaction,2017,46:7388-7391. doi: 10.1039/C7DT00932A [12] ZHOU Enmin, TIAN Liangliang, CHENG Zhengfu, et al. Design of NiO flakes@CoMoO4 nanosheets core-shell architecture on Ni foam for high-performance super-capacitors[J]. Nanoscale Research Letters,2019,14:221. doi: 10.1186/s11671-019-3054-3 [13] YAN Hailong, ZHANG Deyang, XU Jinyou, et al. Solution growth of NiO nanosheets supported on Ni foam as high-performance electrodes for supercapacitors[J]. Nanoscale Research Letters,2014,9:424. doi: 10.1186/1556-276X-9-424 [14] WANG Huanwen, YI Huan, CHEN Xiao, et al. Facile synthesis of a nano-structured nickel oxide electrode with outstanding pseudocapacitive properties[J]. Electrochimica Acta,2013,105:353-361. doi: 10.1016/j.electacta.2013.05.031 [15] XIN Guoxiang, WANG Yanhui, ZHANG Jinhui, et al. A self-supporting graphene/MnO2 composite for high-perfor-mance supercapacitors[J]. International Journal of Hydrogen Energy,2015,40:10176-10184. doi: 10.1016/j.ijhydene.2015.06.060 [16] YANG Jie, LI Pengfa, WANG Liujie, et al. In-situ synthesis of Ni-MOF@CNT on graphene/Ni foam substrate as a novel self-supporting hybrid structure for all-solid-state supercapacitors with a high energy density[J]. Journal of Electroanalytical Chemistry,2019,848:113301. doi: 10.1016/j.jelechem.2019.113301 [17] 窦元运, 罗民, 梁森, 等. 电泳沉积耦合电化学还原法制备柔性石墨烯自支撑薄膜电极超级电容器[J]. 中国有色金属学报: 英文版, 2014, 24(5):1425-1433. doi: 10.1016/S1003-6326(14)63208-8DOU Yuanyun, LUO Min, LIANG Sen, et al. Preparation of flexible graphene self-supporting film electrode super-capacitors by electrophoretic deposition coupled with electrochemical reduction[J]. The Chinese Journal of Nonferrous Metals: English Edition,2014,24(5):1425-1433(in Chinese). doi: 10.1016/S1003-6326(14)63208-8 [18] LAURENT Schlur, KARINE Bonnot, DENIS Spitzer. Synthesis of Cu(OH)2 and CuO nanotubes arrays on a silicon wafer[J]. RSC Advances,2015,5:6061-6070. doi: 10.1039/C4RA10155C [19] WU Yuanzhan, LIU Suqin, ZHAO Kuangmin, et al. Chemical deposition of MnO2 nanosheets on graphene-carbon nanofiber paper as free-standing and flexible electrode for supercapacitors[J]. Ionics,2016,22(7):1185-1195. doi: 10.1007/s11581-015-1625-6 [20] 辛国祥, 王蒙蒙, 翟耀, 等. 一步法合成具有优异循环性能的聚苯胺纳米线/自支撑石墨烯复合材料[J]. 复合材料学报, 2021, 38(4):1-11.XIN Guoxiang, WANG Mengmeng, ZHAI Yao, et al. One-step synthesis of polyaniline nanowire/self-supported graphene composite with excellent cycling stability[J]. Acta Materiae Compositae Sinica,2021,38(4):1-11(in Chinese). [21] 张鹏, 刘洋, 陈明华, 等. 高性能自支撑CuS/SnS2锂电池负极材料[J]. 复合材料学报, 2021, 38(3):871-878.ZHANG Peng, LIU Yang, CHEN Minghua, et al. High-performance self-supporting CuS/SnS2 lithium battery anode material[J]. Acta Materiae Compositae Sinica,2021,38(3):871-878(in Chinese). [22] 施萍萍, 王金杰, 任芝龙, 等. 自支撑的活性碳布/MnO2/碳纳米管/聚苯胺复合电极用于高性能超级电容器的研究[J]. 化工新型材料, 2020, 48(2):121-124.SHI Pingping, WANG Jinjie, REN Zhilong, et al. Research on self-supporting activated carbon cloth/MnO2/carbon nanotube/polyaniline composite electrode for high-performance supercapacitors[J]. New Chemical Materials,2020,48(2):121-124(in Chinese). [23] LI Dan, LAN Wei, LIU Zhongqing, et al. Powder sintered Ni–P/CNTs composites as three-dimensional self-supported efficient electrocatalysts for hydrogen evolution reaction[J]. Journal of Alloys and Compounds,2020,825:153920. doi: 10.1016/j.jallcom.2020.153920 [24] STEEVE Rousselot, PHILIPPE Antitomaso, LAURENCE Savignac, et al. PEDOT assisted CNT self-supported electrodes for high energy and power density[J]. Electrochimica Acta,2020,349:136418. doi: 10.1016/j.electacta.2020.136418 [25] ZHU Fangfang, LIU Weijing, LIU Yu, et al. Construction of porous interface on CNTs@NiCo-LDH core-shell nano-tube arrays for supercapacitor applications[J]. Chemical Engineering Journal,2020,383:123150. doi: 10.1016/j.cej.2019.123150 -





 下载:
下载: