Enhanced conductivity and regulated mechanism of PEDOT:PSS film with biomass-derived gallic acid
-
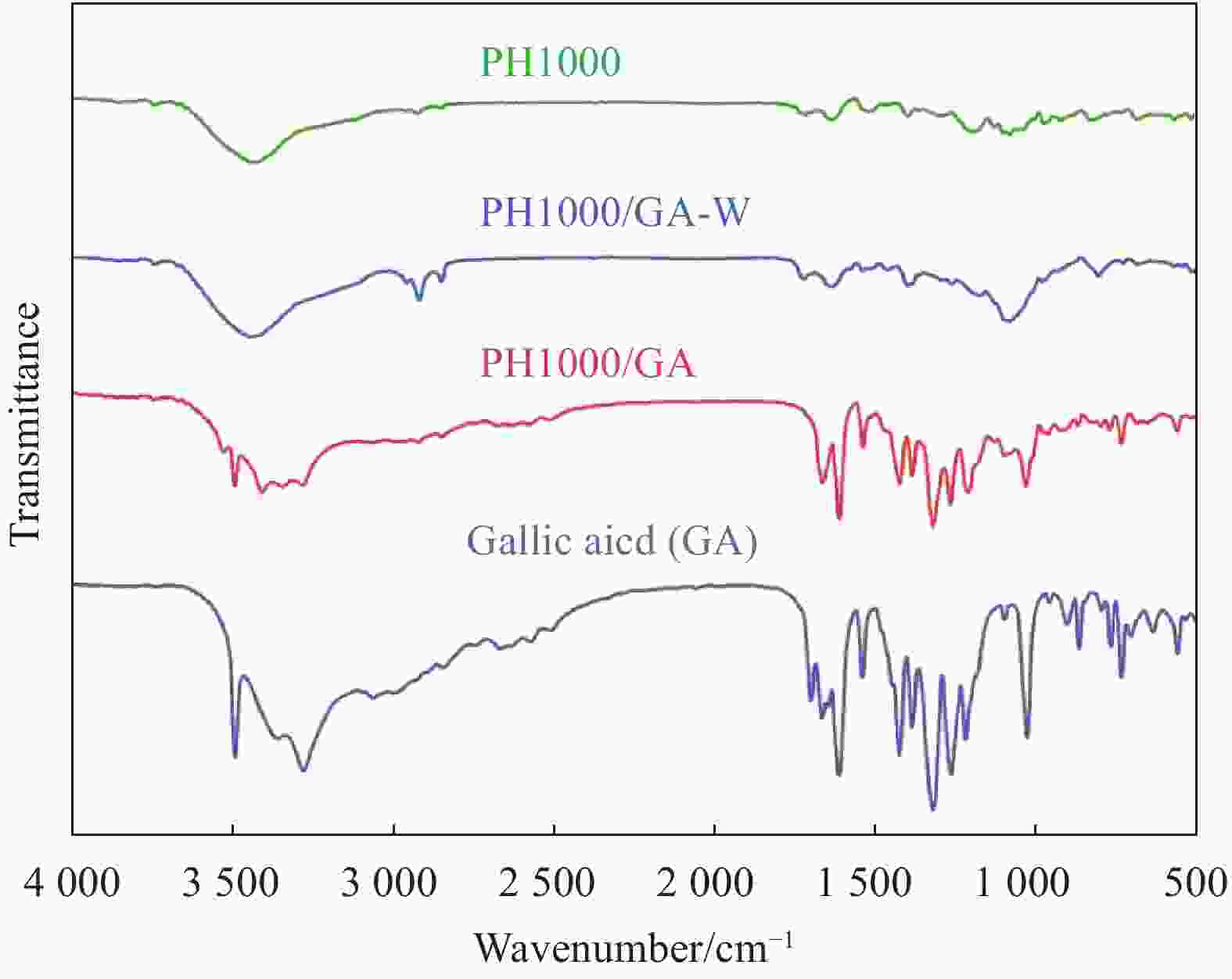
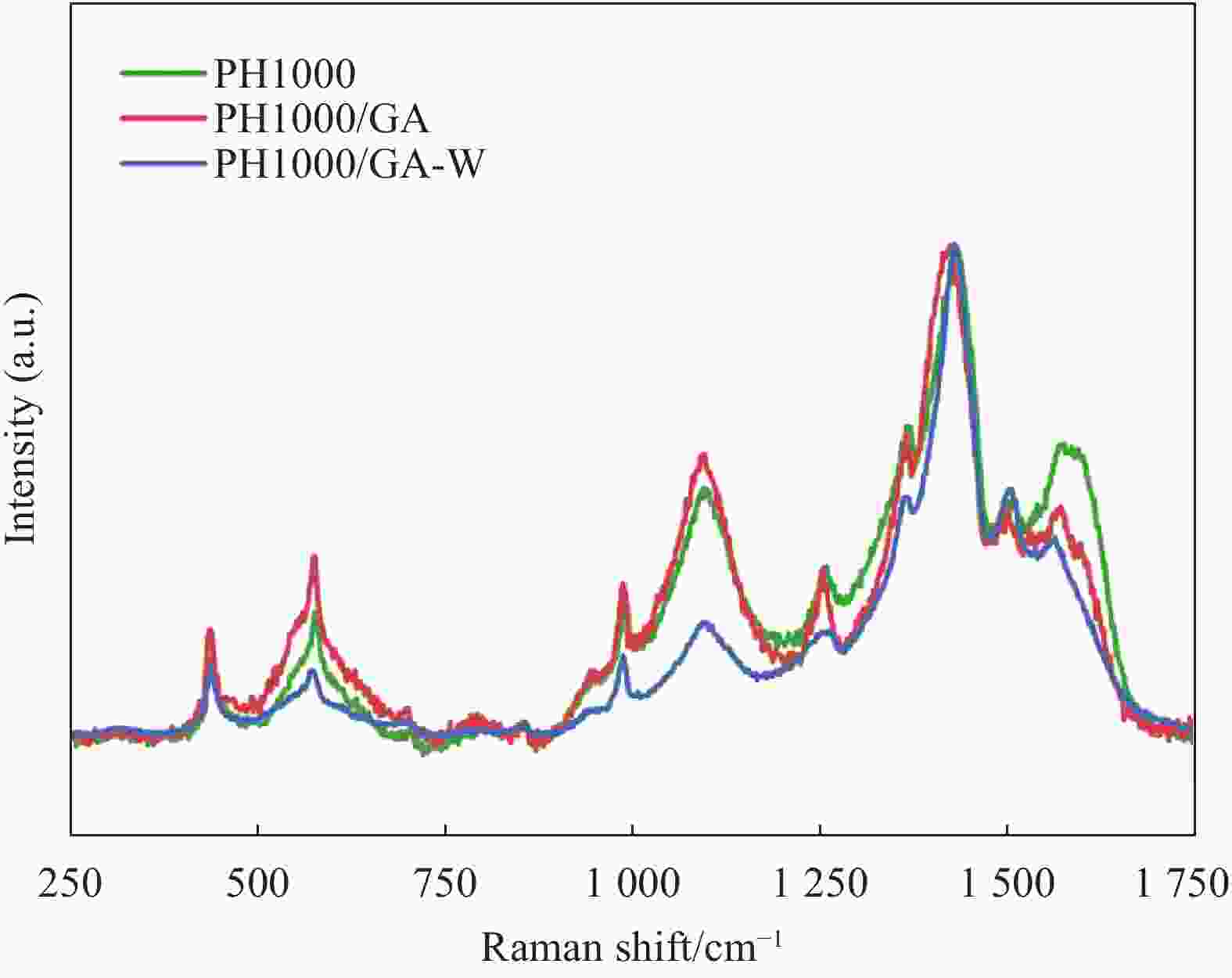
摘要: 聚3, 4-乙烯二氧噻吩(PEDOT)因其具有柔性可拉伸、生物相容性高、导电及功函数可调控等优势在柔性可穿戴电子器件中显示出广阔的应用前景。近年来,随着资源危机的日益凸显,针对PEDOT:聚苯乙烯磺酸(PSS)体系,研究开发高效绿色可持续的生物质基掺杂剂,已引起相关研发人员的高度关注。本研究首次报道了采用生物质芳香弱酸−没食子酸(GA,pKa为4.41)掺杂制备高性能PEDOT导电膜的新途径。GA独特的邻多酚羟基结构创造了稳定的GA-PSSH双氢键,使得GA-PSSH的分子结合能显著高于GA的石油基强酸异构体(2, 4, 6-三羟基苯甲酸,pKa=1.68)与PSSH的分子结合能。GA掺杂不仅可实现PEDOT-PSS的高效相分离,而且优化了PEDOT分子链的构象、聚集结构的形貌及其排列方向。这赋予GA具有很高的掺杂效率,当GA掺杂量为1.2%时,PEDOT导电膜的电导率可提升三个数量级,达到1050 S/cm,导电性能达到已报道的生物质基掺杂剂的最高水平,且掺杂效率明显优于其它生物质基掺杂剂及其石油基强酸异构体。
-
关键词:
- 没食子酸 /
- 导电膜 /
- 聚3, 4-乙烯二氧噻吩 /
- 电导率 /
- 掺杂剂
Abstract: Since poly(3, 4-ethylenedioxythiophene) (PEDOT) has advantages of flexible stretchable, high biocompatibility, and controllable conductivity and work function, it has emerged extensive application prospects in the flexible wearable electronic devices. In recent years, as the increasingly resources crisis, research and development of the high efficient, green, and sustainable bio-based dopant for PEDOT: PSS (polystyrene sulfonate) system has attracted serious concerns of the relevant researchers. For the first time, this work reported a new approach to prepare high-performance PEDOT conductive film using biomass-derived aromatic weak acid, i.e., gallic acid (GA, pKa of 4.41). Its special structure of adjacent multiple phenolic hydroxyl groups created a stable dual-hydrogen bonds combination with PSSH. The binding energy of GA-PEDOT is significantly higher than that of its petroleum-based strong acidic isomer (2, 4, 6-trihydroxybenzoic acid with pKa of 1.68, ) with PEDOT. GA doping not only realized the high efficient phase separation of PEDOT-PSS, but also optimized the conformational of PEDOT molecular chain, the optimal morphology and orientation of aggregation structure. This endowed GA with high doping efficiency, and the conductivity of PEDOT conductive film can be upgraded by three orders of magnitude to 1050 S/cm, only with 1.2% of doping amount of GA. That has reached the highest conductive feature in all reported bio-based dopants, and the doping efficiency of GA is significantly higher than that of bio-based dopant and its petroleum-based strong acidic isomer.-
Key words:
- gallic acid /
- conductive film /
- poly(3, 4-ethylenedioxythiophene) /
- conductivity /
- dopant
-
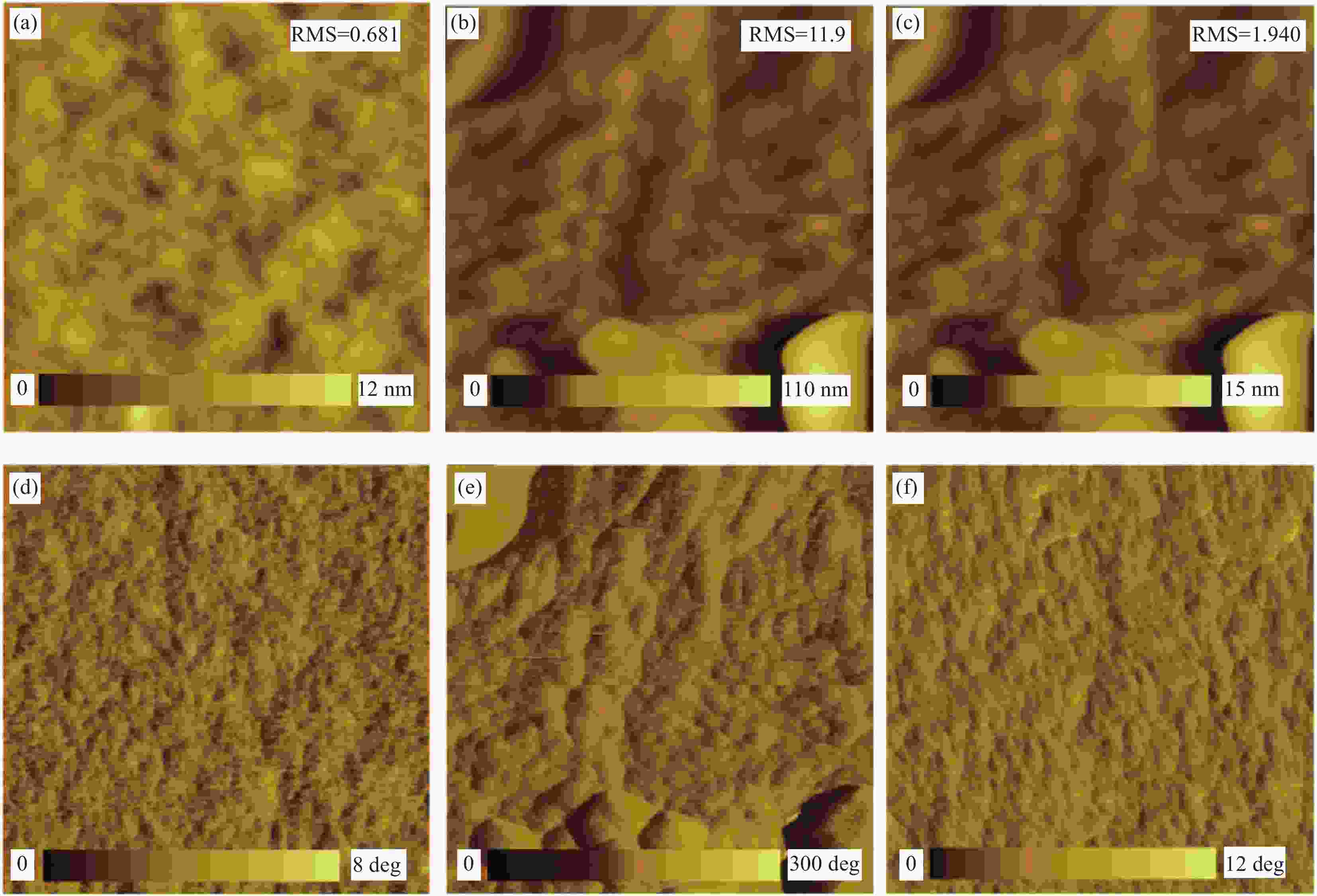
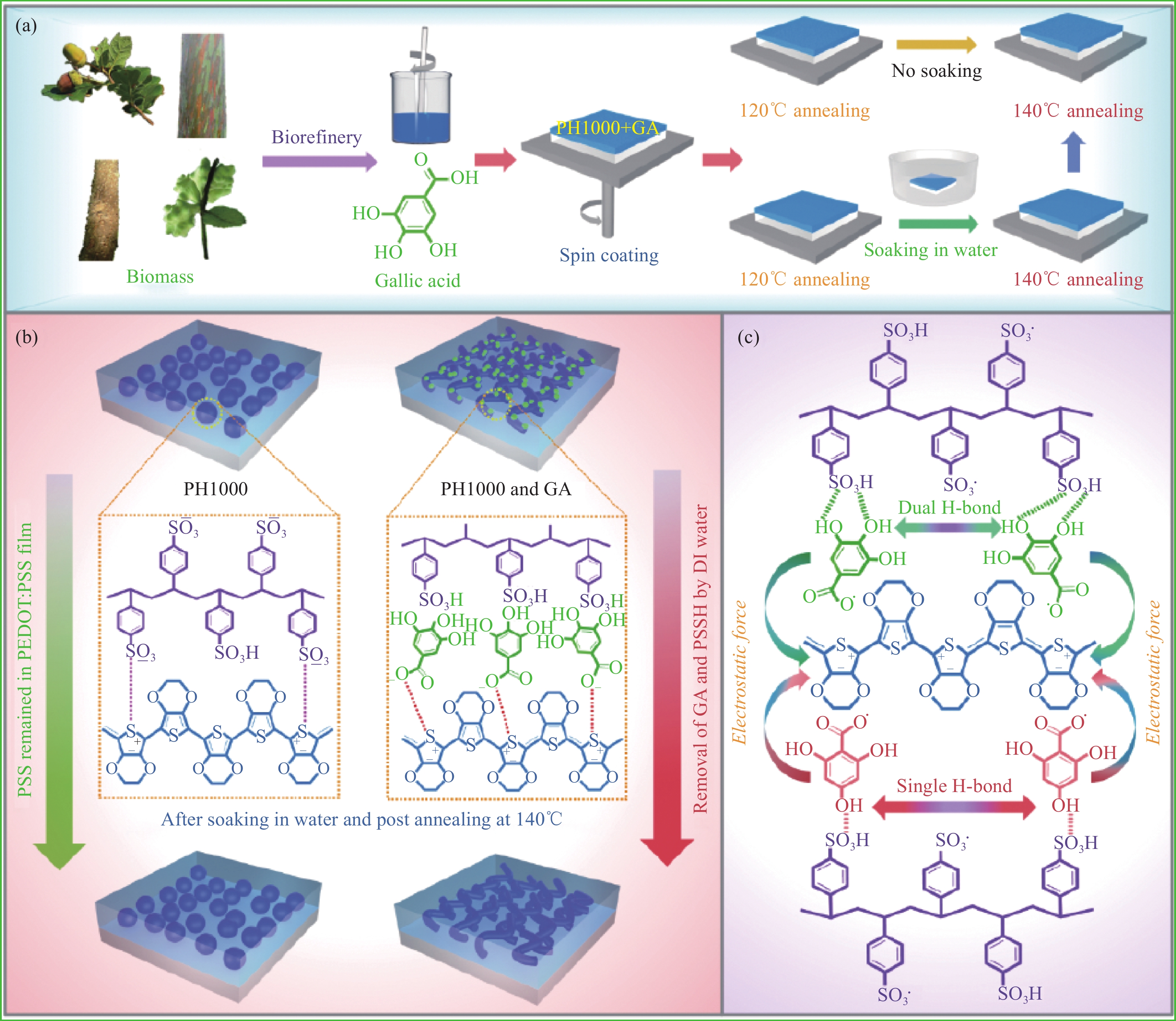
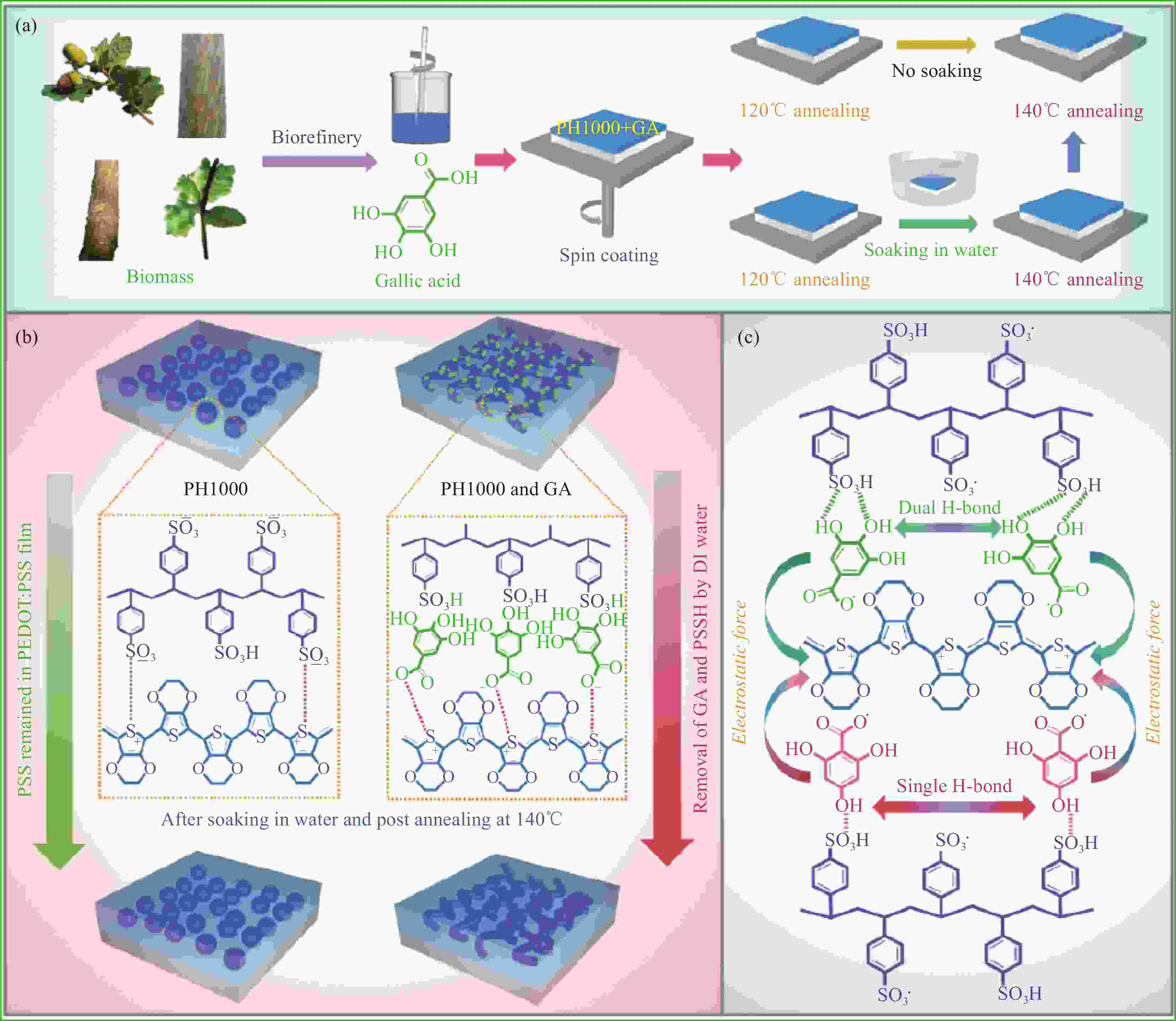
图 1 生物质基GA掺杂制备高导电PH1000纳米膜的路径(a)、GA掺杂与去离子水后处理对导电膜聚集结构的作用机制(b)以及GA、2,4,6-三羟基苯甲酸与PEDOT、PSS/PSSH间的静电与氢键作用(c)
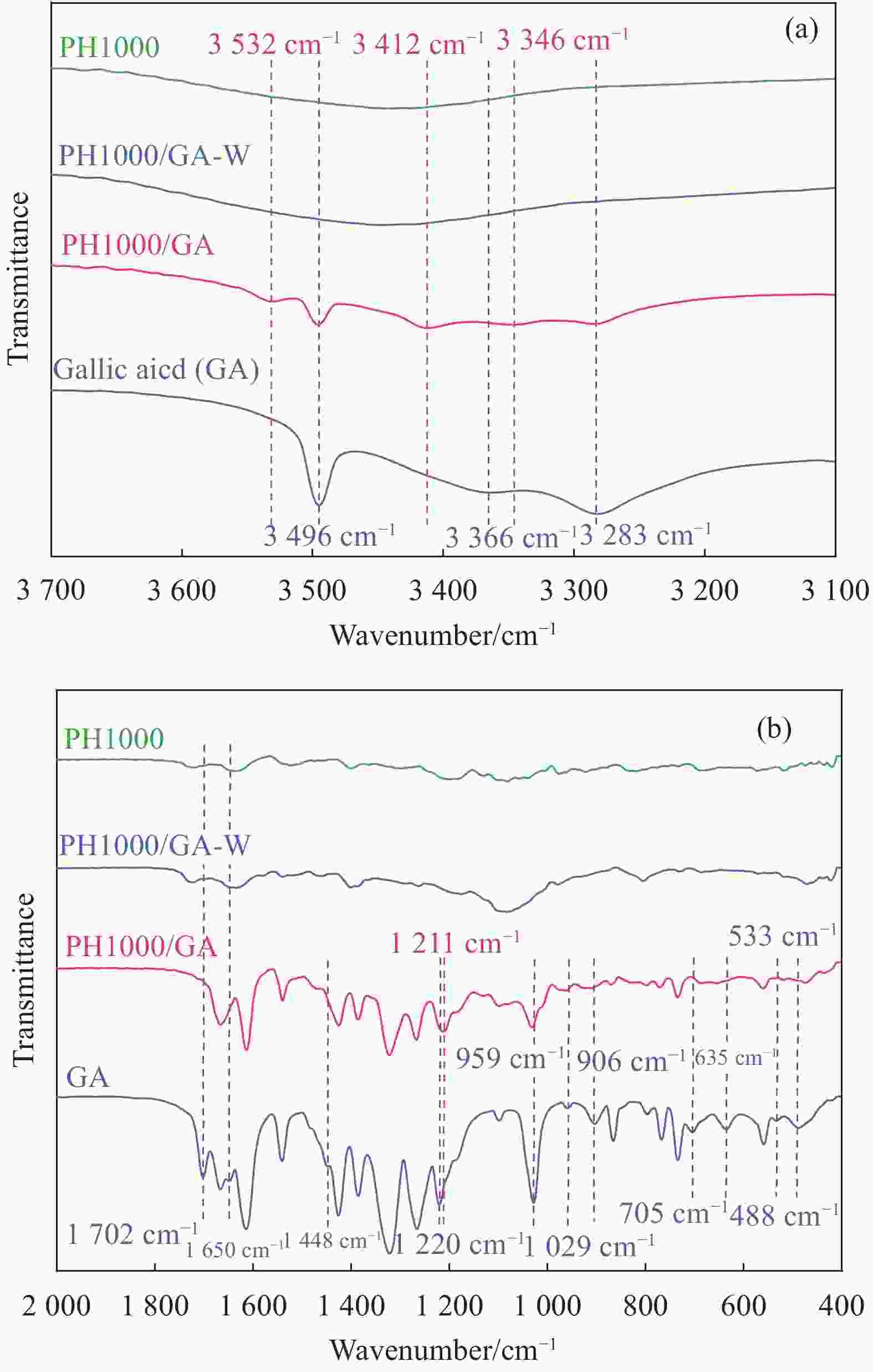
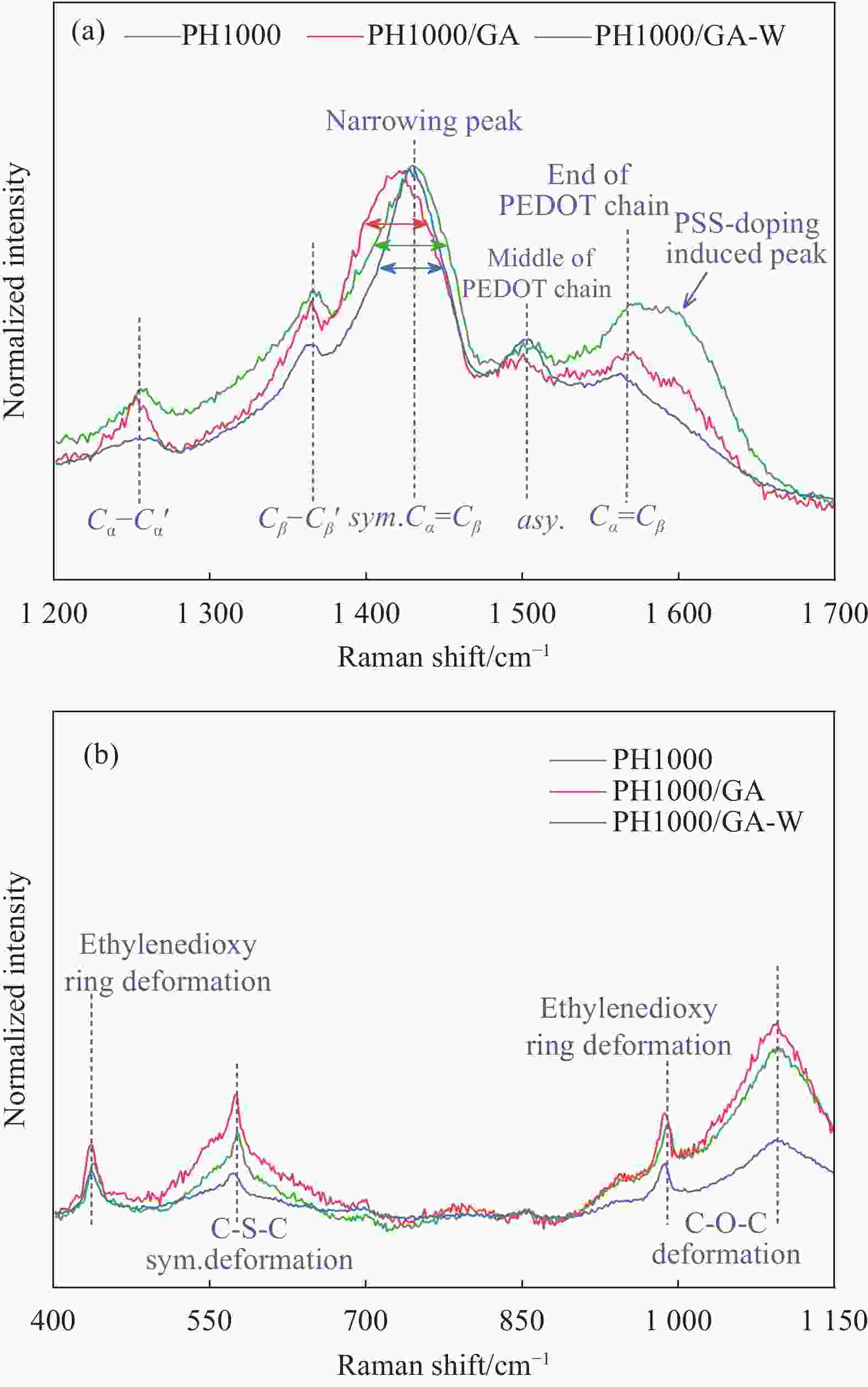
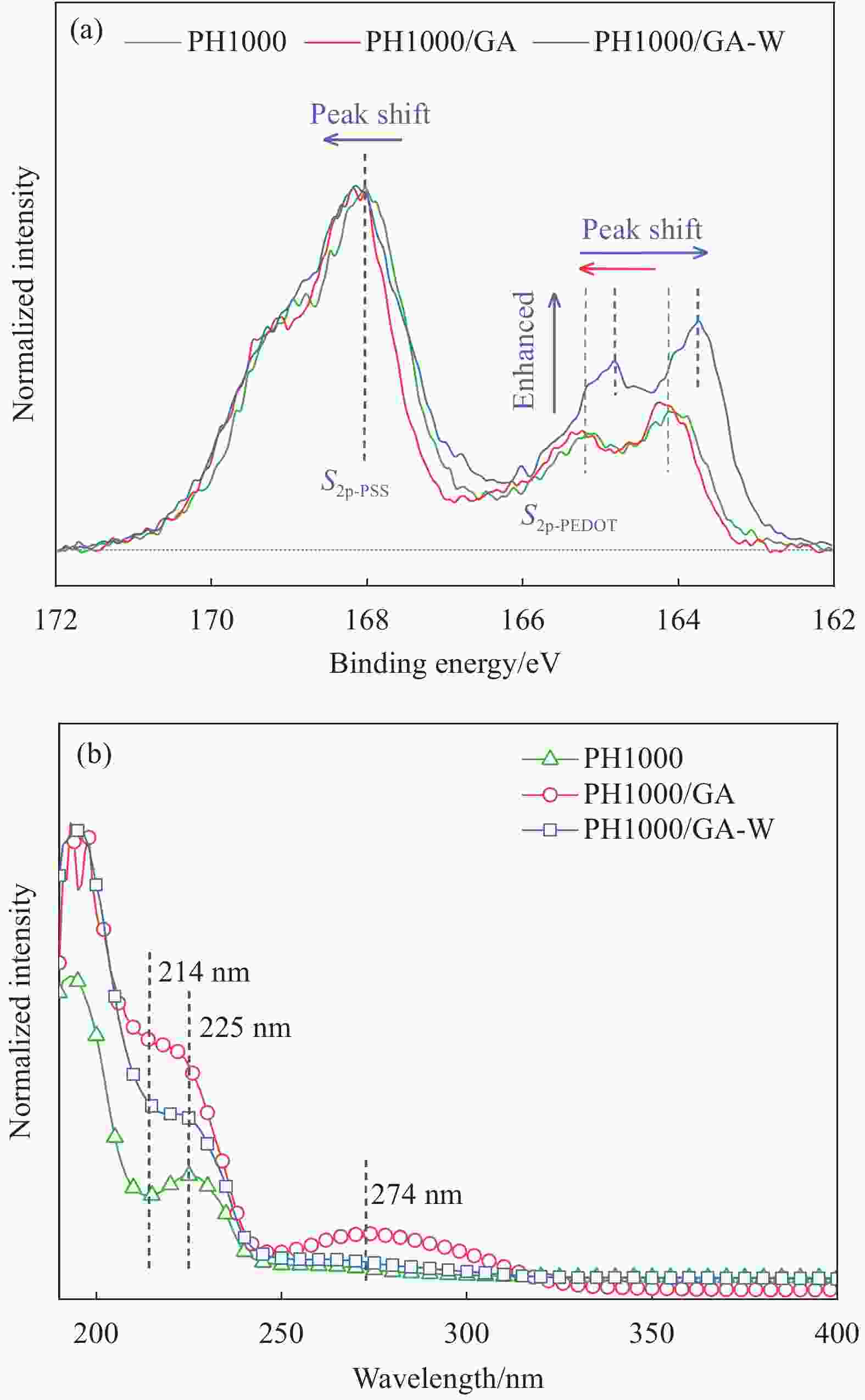
Figure 1. Pathway of high-conductive PH 1000 nanofilm prepared by doping with bio-based GA (a), interaction mechanism of GA doping and DI water post-treatment on aggregation structure of conductive film (b), electrostatic and hydrogen bond interaction of GA, 2,4,6-THBA with PEDOT and PSSH/PSS (c)
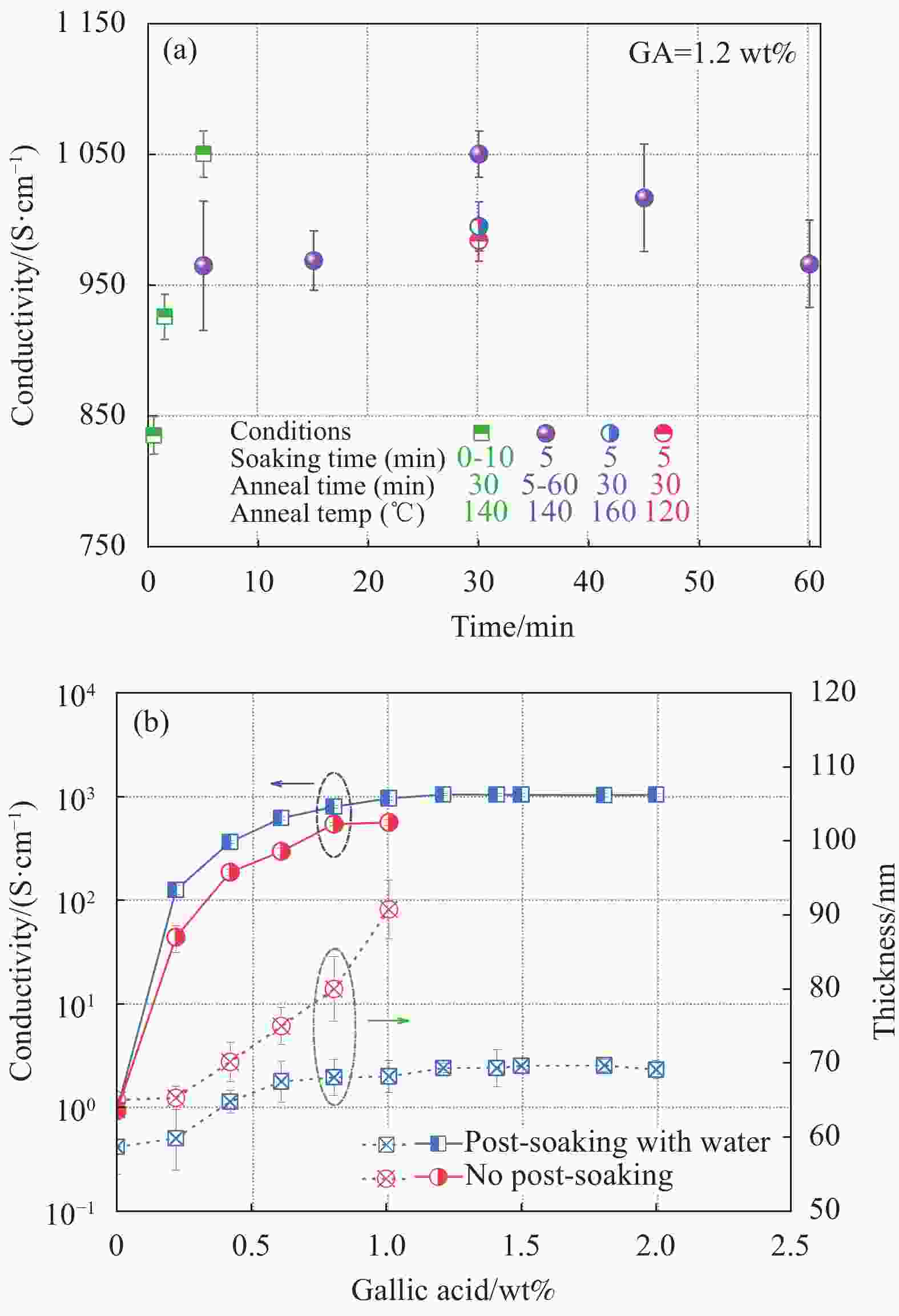
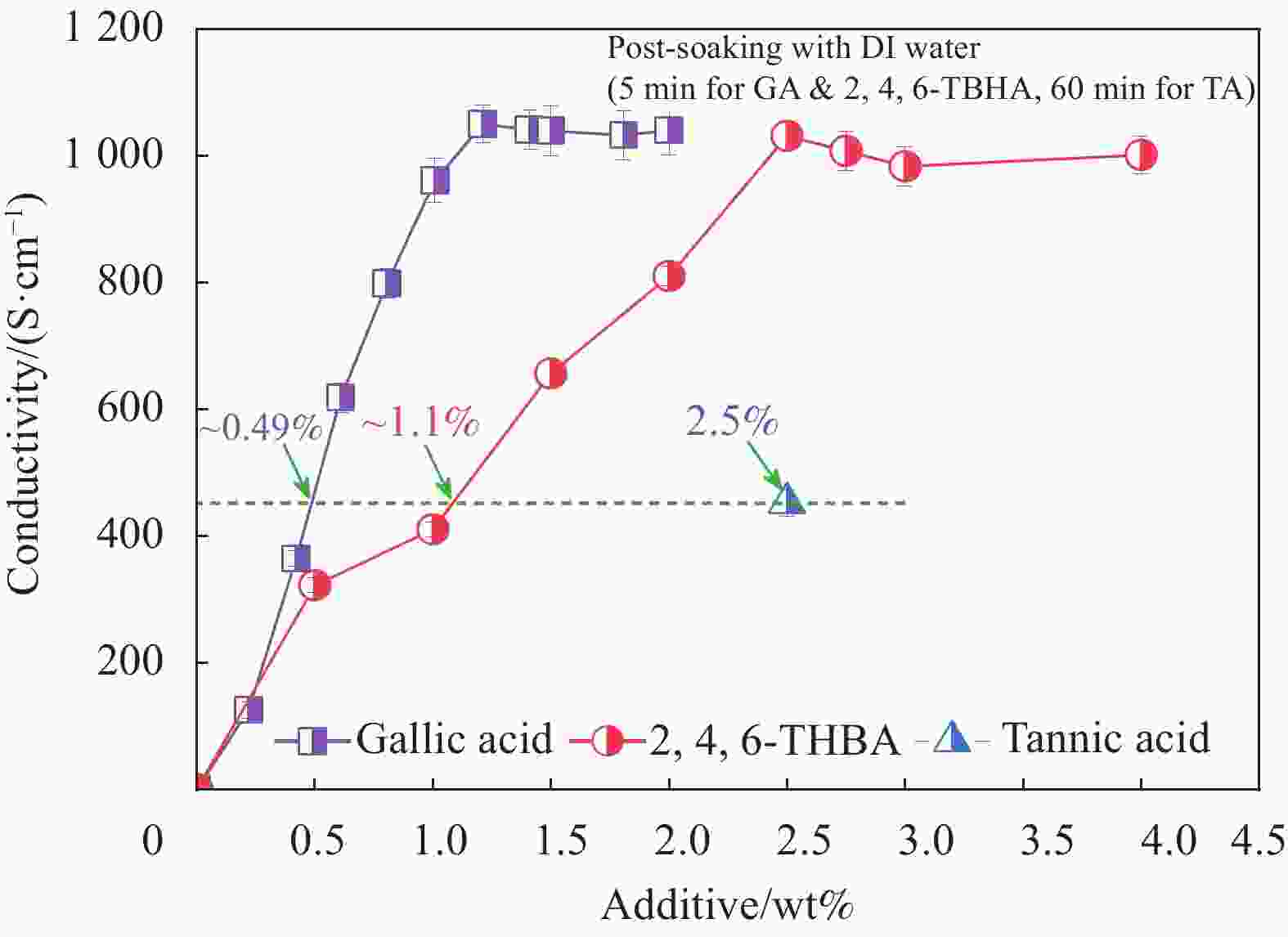
图 2 退火温度、时间与去离子水后处理时间对PEDOT:PSS导电膜电导率的影响(a)以及没食子酸掺杂与去离子水后处理对PEDOT:PSS膜电导率和厚度的影响(b)
Figure 2. Effect of DI water post-treatment time, annealing time and temperature on conductivity of PEDOT:PSS conductive film (a), andinfluence of GA doping and DI water post-treatment on its conductivity and thickness (b)
表 1 没食子酸、2,4,6-三羟基苯甲酸和单宁酸掺杂制备纳米膜的导电性能对比
Table 1. Comparison on conductivity of nanofilms prepared by doping with GA, 2,4,6-THBA, and tannin acid.
Additives Melting
point/℃pKa Addition Post-soaking
with DI waterThickness /
nmσ/(S·cm−1) Tannic acid 218 6 1.0% No[14] 135.9±1.2 2.48±0.6 5 min 67.6±2.7 65.7±2.5 2.5% No[14] 250.2±3.5 25.0±28 60 min[14] 110.0±1.3 453.0±21 Gallic acid 252 4.41 1.0% No 90.8±4.0 563±30 5 min 68.2±2.2 961±32 1.2% 5 min 69.3±0.6 1050±27 2,4,6-THBA 210 1.68 1.0% 5 min 46.5±1.3 411±12 表 2 典型结合结构的氢键作用与分子结合能
Table 2. Hydrogen bonds and molecular binding energies of typical binding structure
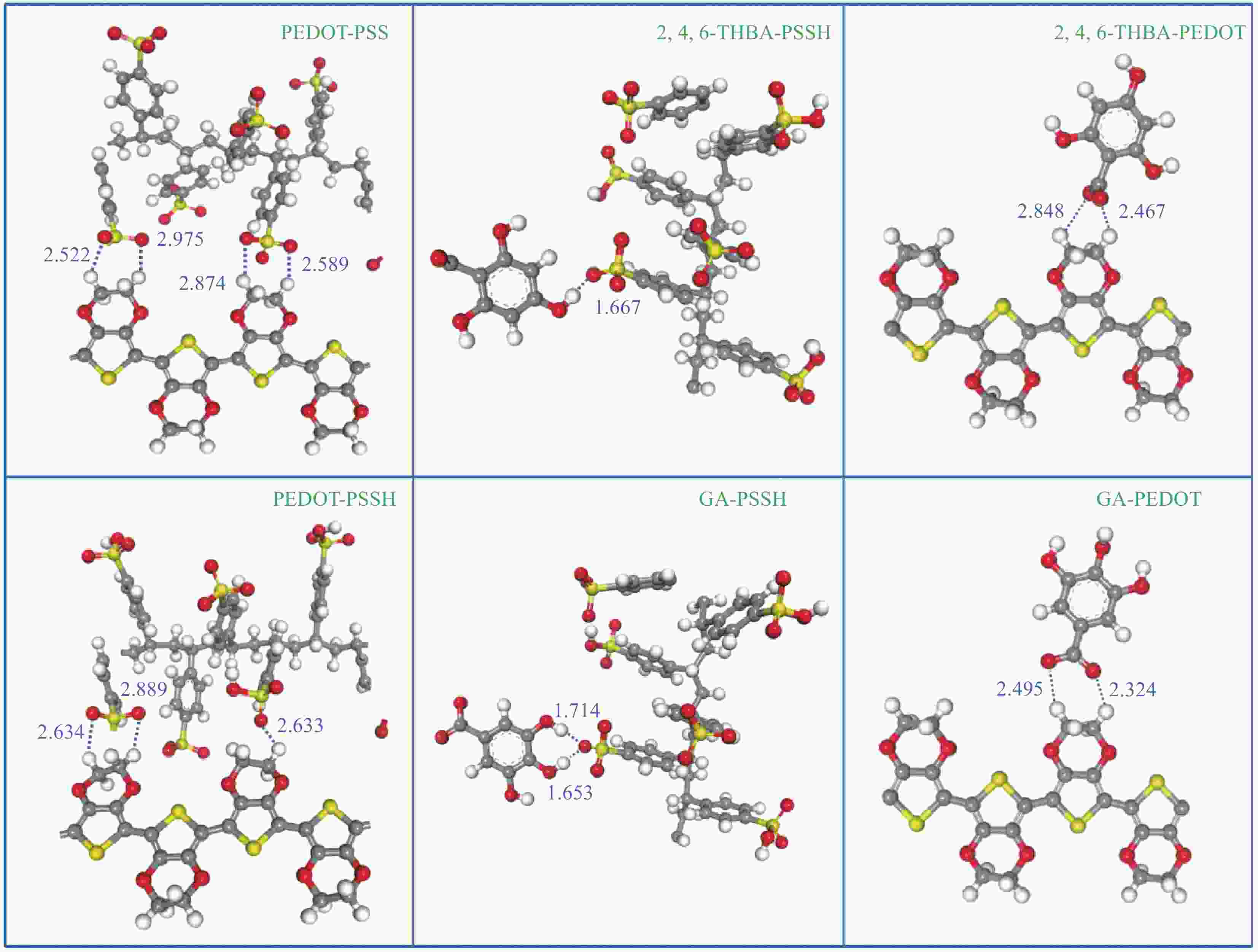
Binding structure H-bond distance /10-1nm Coulomb force /nN Binding energy /eV PEDOT PSSH 2.633 0.192 −0.425 2.634 (2.889) 0.272 (0.201) PSS 2.874 (2.589) 0.213 (0.271) −0.872 2.522 (2.975) 0.292 (0.187) 2,4,6-THBA PSSH 1.667 1.511 −0.787 PEDOT 2.848 (2.467) 0.234 (0.369) −0.343 GA PSSH 1.714 (1.653) 1.530 (1.574) −1.168 PEDOT 2.495 (2.324) 0.350 (0.396) −0.314 表 S1 添加或不添加去离子水后处理后的GA掺杂导电膜的厚度和电导率
Table S1. Thickness and conductivity of GA-doping conductive films with or without DI water post-treatment
Content of GA/% PH1000/GA PH1000/GA-W Thickness/nm Conductivity/(S·cm−1) Thickness/nm Conductivity/(S·cm−1) 0.0 65.0±0.62 0.93±0.02 58.7±3.64 0.93±0.07 0.2 65.3±1.59 43.9±1.02 59.8±4.31 125.8±11.57 0.4 70.1±2.68 186.3±4.63 64.8±1.57 364.8±11.62 0.6 75.0±2.49 296.3±14.53 67.5±2.77 618.9±21.19 0.8 80.1±4.37 543.5±35.36 68.1±2.43 799.2±20.89 1.0 90.7±3.97 563.4±29.9 68.2±2.16 961.5±32.04 1.2 – – 69.3±0.60 1050.5±27.31 1.4 – – 69.3±2.48 1041.6±28.49 1.5 – – 69.6±1.06 1039.9±35.69 1.8 – – 69.7±0.8 1032.7±35.66 2.0 – – 69.1±1.26 1040.9±36.22 表 S2 2,4,6-THBA掺杂的DI水后处理导电膜的厚度和电导率
Table S2. Thickness and conductivity of 2,4,6-THBA-doping conductive films with DI water post-treatment
2,4,6-TBHA/% Thickness/nm Conductivity/(S·cm−1) 0.0 58.7±3.64 0.93±0.02 0.5 50.6±1.71 322.6±14.1 1.0 46.5±1.33 411.0±4.12 1.5 44.0±1.70 657.1±6.91 2.0 40.9±1.31 810.6±5.09 2.5 45.3±1.72 1031.6±8.84 2.75 45.9±2.65 1007.9±30.7 3.0 45.6±1.85 983.5±30.8 4.0 46.1±1.87 1001.7±30.1 -
[1] PENG R, WAN Z, SONG W, et al. Improving performance of non-fullerene organic solar cells over 13% by employing silver nanowires doped PEDOT: PSS composite interface[J]. ACS Appl Mater Interfaces,2019,11:42447. doi: 10.1021/acsami.9b16404 [2] LEE C, KANG D J, KANG H, et al. Simultaneously enhancing light extraction and device stability of organic light-emitting diodes using a corrugated polymer nanosphere templated PEDOT: PSS layer[J]. Adv Energy Mater.,2014,4:1301345. doi: 10.1002/aenm.201301345 [3] FAN X, NIE W, TSAI H, et al. PEDOT: PSS for flexible and stretchable electronics: modifications, strategies, and applications[J]. Adv Sci,2019,6:1900813. doi: 10.1002/advs.201900813 [4] YAN H, JO T, OKUZAKI H. Highly conductive and transparent Poly (3, 4-ethylenedioxythiophene)/ Poly (4-styrenesulfonate) (PEDOT/PSS) thin films[J]. Polym J.,2009,41:1028. doi: 10.1295/polymj.PJ2009143 [5] LIM K, JUNG S, LEE S, et al. The enhancement of electrical and optical properties of PEDOT: PSS using one-step dynamic etching for flexible application[J]. Org Electron.,2014,15:1849. doi: 10.1016/j.orgel.2014.04.014 [6] FAN X, WANG J. Z, WANG H. B, et al. Bendable ITO-free organic solar cells with highly conductive and flexible PEDOT: PSS electrodes on plastic substrates[J]. ACS Appl Mater Interfaces,2015,7:16287. doi: 10.1021/acsami.5b02830 [7] ZHAO D, ZHANG Q, CHEN W, et al. Highly flexible and conductive cellulose-mediated PEDOT: PSS/ MWCNT composite films for supercapacitor electrodes[J]. ACS Appl Mater Interfaces,2017,9(15):13213. doi: 10.1021/acsami.7b01852 [8] XIA Y, SUN K, OUYANG J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices[J]. Adv Mater,2012,24:2436. doi: 10.1002/adma.201104795 [9] YEON C, YUN S J, KIM J, et al. PEDOT: PSS films with greatly enhanced conductivity via nitric acid treatment at room temperature and their application as Pt/TCO-free counter electrodes in dyesensitized solar cells[J]. Adv Electron Mater,2015,1(10):1500121. doi: 10.1002/aelm.201500121 [10] NA S. I, KIM S. S, JO J. D, et al. Efficient and flexible ito-free organic solar cells using highly conductive polymer anodes[J]. Adv Mater,2008,20:4061. doi: 10.1002/adma.200800338 [11] OUYANG J. Solution-processed PEDOT: PSS films with conductivities as indium tin oxide through a treatment with mild and weak organic acids[J]. ACS Applied Material Interfaces,2013,5(24):13082-13088. doi: 10.1021/am404113n [12] LI Y, TANIGAWA R, OKUZAKI H. Soft and flexible PEDOT/PSS films for applications to soft actuators[J]. Smart Mater Struct,2014,23(7):074010. doi: 10.1088/0964-1726/23/7/074010 [13] HE H, ZHANG L, GUAN X, et al. Biocompatible conductive polymers with high conductivity and high stretchability[J]. ACS Appl Mater Interfaces,2019,11(29):26185-26193. doi: 10.1021/acsami.9b07325 [14] YI Z, ZHAO Y, LI P, et al. The effect of tannic acids on the electrical conductivity of PEDOT: PSS films[J]. Applied Surface Science,2018,448:583-588. doi: 10.1016/j.apsusc.2018.04.168 [15] KIM J, PATEL R, JUNG B J, et al. Simultaneous improvement of performance and stability in PEDOT: PSS–Sorbitol composite based flexible thermoelectric modules by novel design and fabrication process[J]. Macromol Res,2018,26(1):61-65. doi: 10.1007/s13233-018-6008-1 [16] YANG E, KIM J, JUNG B J, et al. Enhanced thermoelectric properties of sorbitol-mixed PEDOT: PSS thin films by chemical reduction[J]. J Mat Sci: Mater Electron,2015,26(5):2838-2843. doi: 10.1007/s10854-015-2766-0 [17] BOLES J S, CRERAR D A, GRISSOM G, et al. Aqueous thermal degradation of gallic acid[J]. Geochim Cosmochim Acta,1988,52(2):341-344. doi: 10.1016/0016-7037(88)90089-0 [18] KIM N, KANG H, LEE J. H, et al. Highly conductive all-plastic electrodes fabricated using a novel chemically controlled transfer-printing method[J]. Adv Mater,2015,27:2317. doi: 10.1002/adma.201500078 [19] 洪伟良, 刘剑洪, 赵凤起, 等. 纳米Pb(II)-没食子酸配合物的合成及其燃烧催化性能[J]. 化学学报, 2005(3):249-253+178. doi: 10.3321/j.issn:0567-7351.2005.03.014HONG W L, LIU J H, ZHAO F Q, et al. Synthesis of nano-Pb (II)-gallic acid complexes and their combustion catalytic properties[J]. Acta Chemica Sinica,2005(3):249-253+178(in Chinese). doi: 10.3321/j.issn:0567-7351.2005.03.014 [20] KRILOV A, HOLMGREN A, GREF R, et al. Effects of gallic acid on metals: An FT-IR study of complexes between gallic acid and sawblade steel[J]. Holzforschung,1993,47(3):239-246. doi: 10.1515/hfsg.1993.47.3.239 [21] BOI M, GORGIEVA S, KOKOL V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties[J]. Carbohydr Polym,2012,87(4):2388-2398. doi: 10.1016/j.carbpol.2011.11.006 [22] YU Z, XIA Y, DU D, et al. PEDOT: PSS films with metallic conductivity through a treatment with common organic solutions of organic salts and their application as a transparent electrode of polymer solar cells[J]. ACS Appl Mater Interfaces,2016:11629. [23] LIU J, LU J F, KAN J, et al. Synthesis of chitosan-gallic acid conjugate: structure characterization and in vitro anti-diabetic potential[J]. Int J Biol Macromol,2013,62(Complete):321-329. [24] YU Z, XIA Y, DU D, et al. PEDOT: PSS films with metallic conductivity through a treatment with common organic solutions of organic salts and their application as a transparent electrode of polymer solar cells[J]. ACS Applied Material Interfaces,2016:11629. [25] REYES-REYES M, CRUZ-CRUZ I, LÓPEZ-SANDOVAL R. Enhancement of the electrical conductivity in PEDOT: PSS Films by the addition of dimethyl sulfate[J]. J Phys Chem C,2010,114(47):20220-20224. doi: 10.1021/jp107386x [26] TRAN-VAN F, GARREAU, SÉBASTIEN, LOUARN G, et al. Fully undoped and soluble oligo(3, 4-Ethylenedioxythiophene)S: spectroscopic study and electrochemical characterization[J]. J Mater Chem,2001,11(5):1378-1382. doi: 10.1039/b100033k [27] XIONG S, FU J, LI Z, et al. Modulating the electrochromic performances of transmissive and reflective devices using N,N-Dimethyl formamide modified poly (3, 4-Ethylenedioxythiophene)/Poly (Styrene Sulfonate) blend as active layers[J]. J Macromol Sci Part B-Phys,2015,54(7):799-810. doi: 10.1080/00222348.2015.1037203 [28] CHIU W W, TRAVAS-SEJDIC J, COONEY R P, et al. Studies of dopant effects in Poly(3, 4-ethylenedi-oxythiophene) using raman spectroscopy[J]. J Raman Spectrosc,2010,37:1354-1361. [29] SINGH V, ARORA S, ARORA M, et al. Characterization of doped PEDOT: PSS and its influence on the performance and ddegradation of organic solar cells[J]. Semicond Sci Technol,2014,29(4):045020. doi: 10.1088/0268-1242/29/4/045020 [30] XU B, GOPALAN S A, GOPALAN A I, et al. Functional solid additive modified PEDOT: PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells[J]. Sci Rep,2017,7:45079. doi: 10.1038/srep45079 [31] XIA Y, OUYANG J. Salt-induced charge screening and significant conductivity enhancement of conducting Poly(3, 4-ethylenedioxythiophene): Poly (styrenesulfonate)[J]. Macromolecules,2009,42(12):4141-4147. doi: 10.1021/ma900327d [32] XIA Y, SUN K, OUYANG J. Highly conductive Poly(3, 4-ethylenedioxythiophene): Poly(styrene sulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells[J]. Energy & Environ Sci,2012,5(1):5325-5332. [33] FAN Z, LI P, DU D, et al. Significantly enhanced thermoelectric properties of PEDOT: PSS films through sequential post-treatments with common acids and bases[J]. Adv Energy Mater,2017,7(8):1602116. doi: 10.1002/aenm.201602116 [34] CRISPIN X, JAKOBSSON F L E, CRISPIN A, et al. The origin of the high conductivity of Poly(3, 4-ethylenedioxythiophene) Poly(styrenesulfonate) (PEDOT PSS) plastic electrodes[J]. Chem Mat,2006,18(18):4354-4360. doi: 10.1021/cm061032+ [35] PETTERSSON L A A, GHOSH S, INGANAES O. Optical anisotropy in thin films of Poly(3, 4-ethylenedioxythiophene)–Poly(4-styrenesulfonate)[J]. Org Electron,2002,3(3/4):143-148. [36] DELLEY, B. From molecules to solids with the DMol[sup3] approach[J] J Chem Phys 2000, 113(18): 7756-0. [37] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett,1996,77(18):3865-3868. doi: 10.1103/PhysRevLett.77.3865 [38] DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. J Chem Phys,1990,92(1):508-517. doi: 10.1063/1.458452 [39] DOLG M, WEDIG U, STOLL H, et al. Energy-adjusted ab initio pseudopotentials for the first row transition elements[J]. J Chem Phys,1987,86(2):866-872. doi: 10.1063/1.452288 [40] BERGNER A, DOLG M, KÜCHLE W, et al. Ab initio energy-adjusted pseudopotentials for elements of groups 13-17[J]. Mol Phys,1993,80(6):1431-1441. doi: 10.1080/00268979300103121 [41] DELLEY B. The conductor-like screening model for polymers and surfaces[J]. Mol Simu,2006,32(2):117-123. doi: 10.1080/08927020600589684 [42] KLAMT A, SCHUEUERMANN G J. Cosmo: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient[J]. J Chem Soc Perkin Trans,1993,2(5):799-805. [43] GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. J Chem Phys,2010,132(15):154104. doi: 10.1063/1.3382344 -





 下载:
下载: