Preparation of g-C3N4 quantum dot-TiO2/conductive attapulgite composites and their photocatalytic performance
-
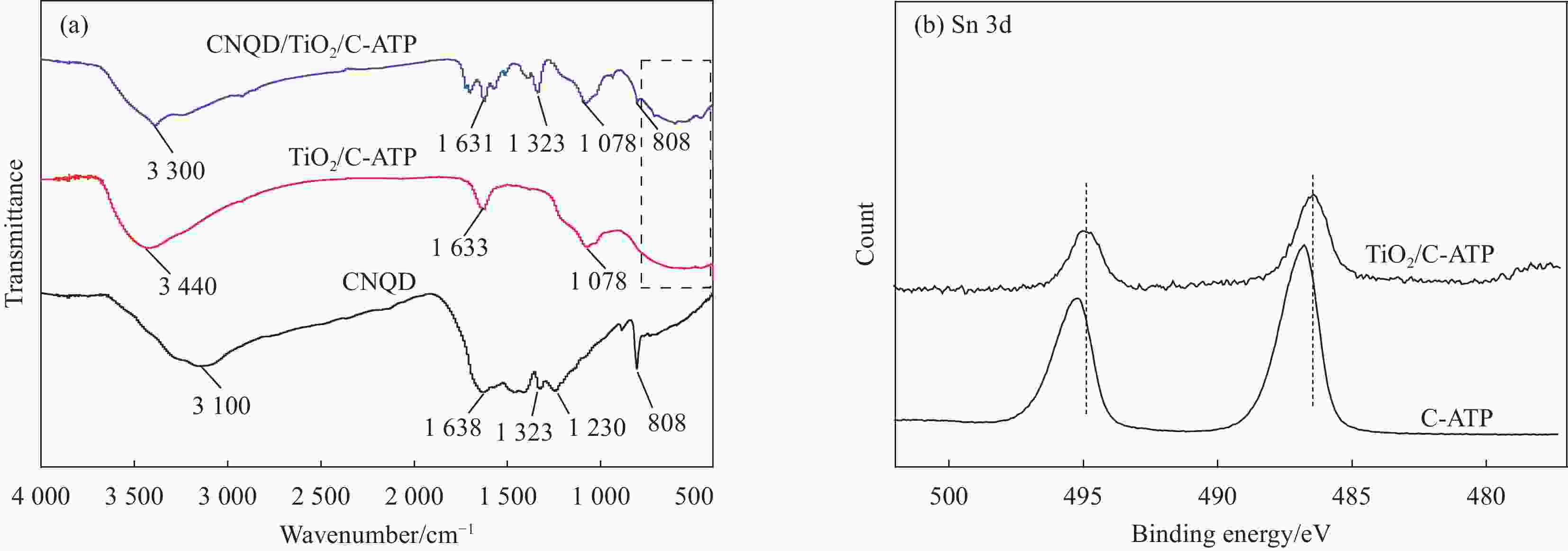
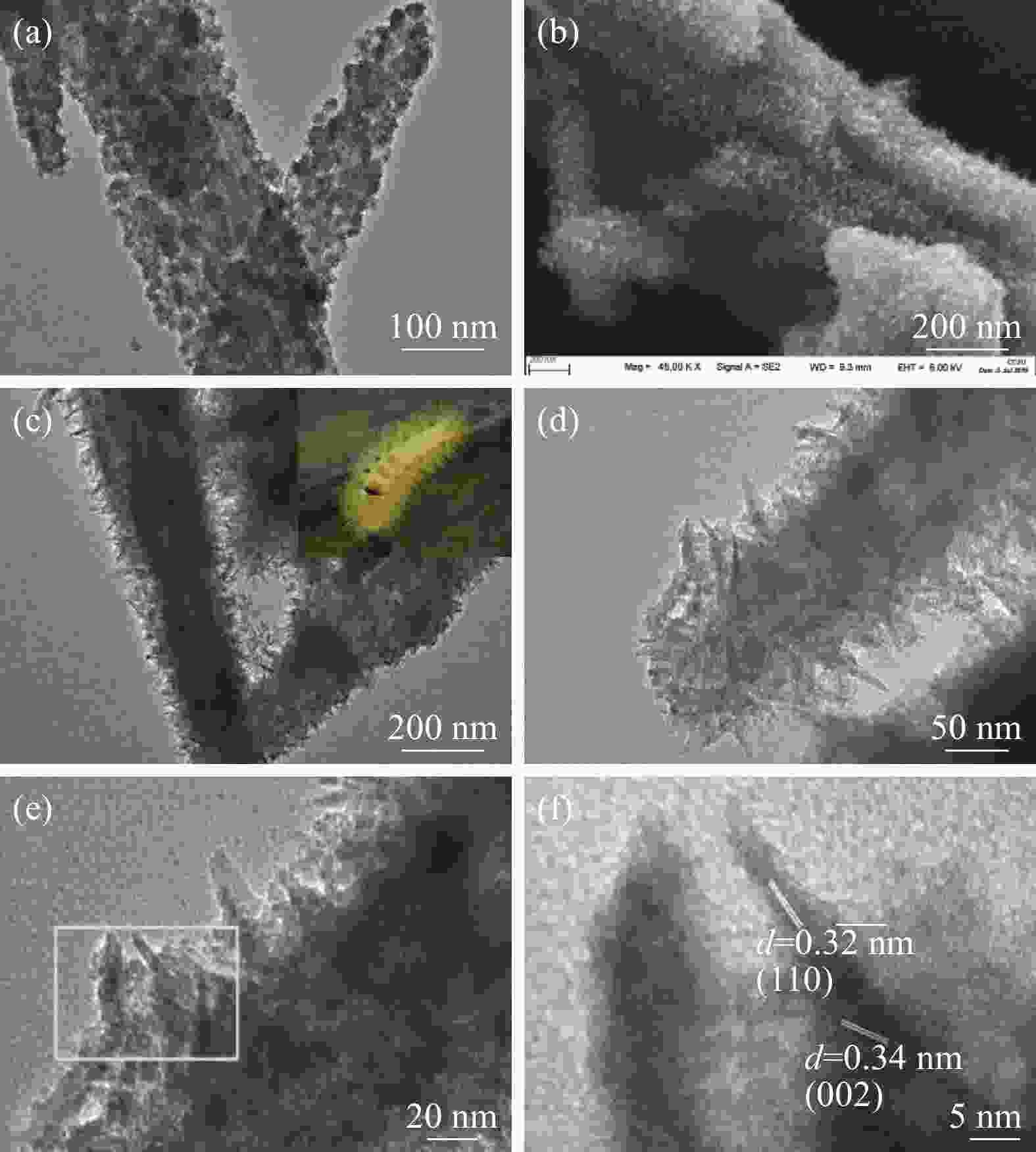
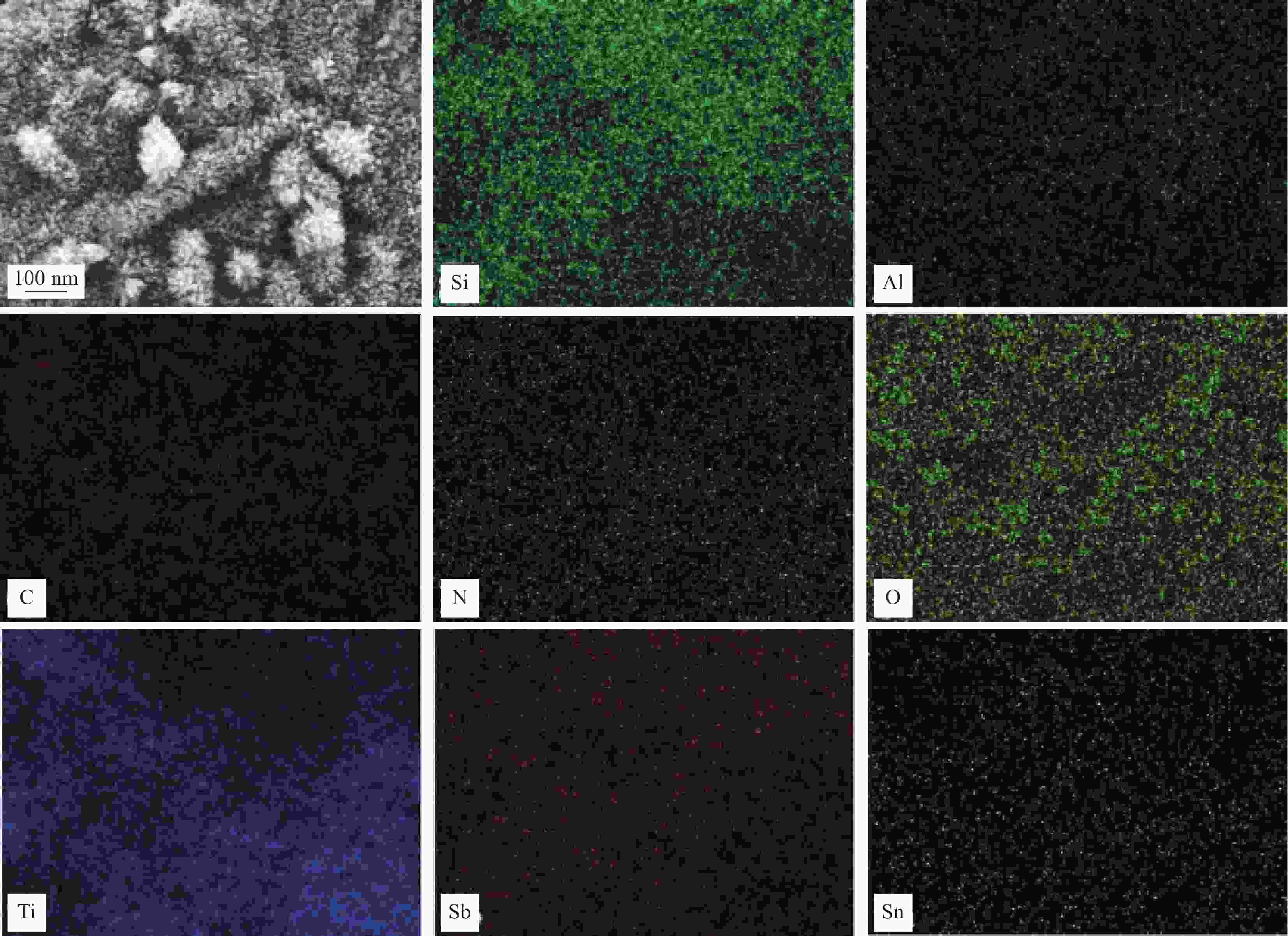
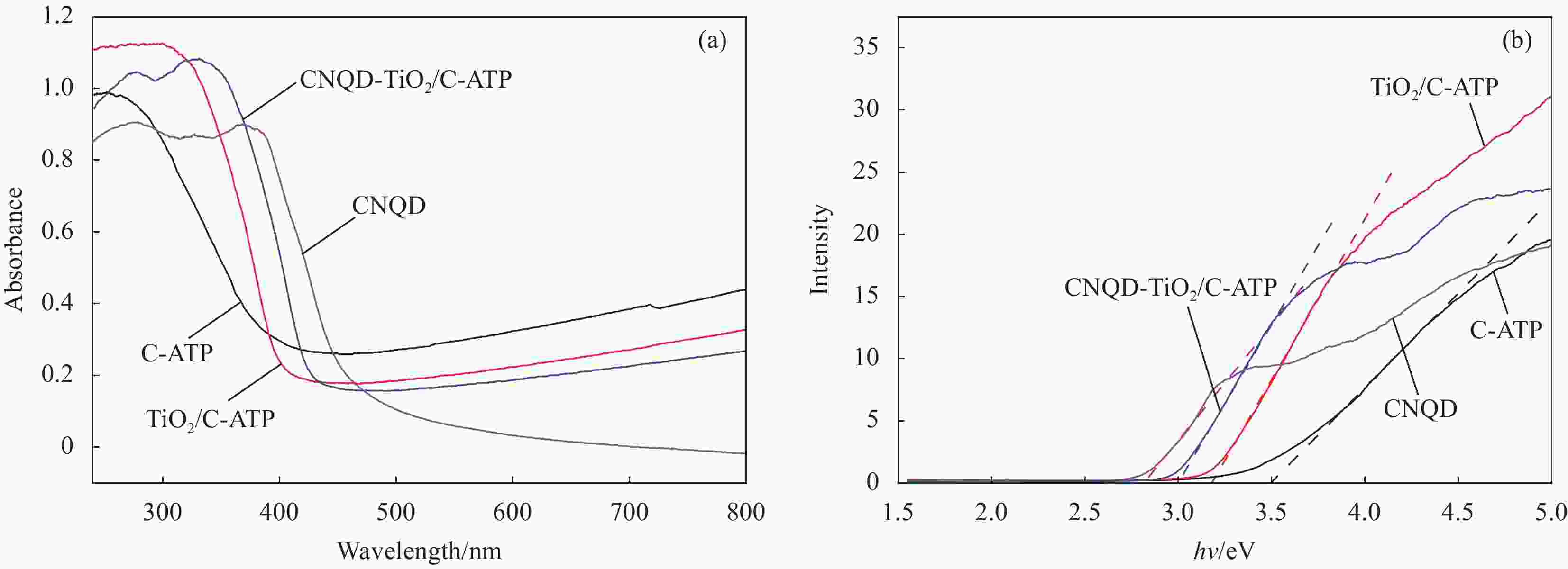
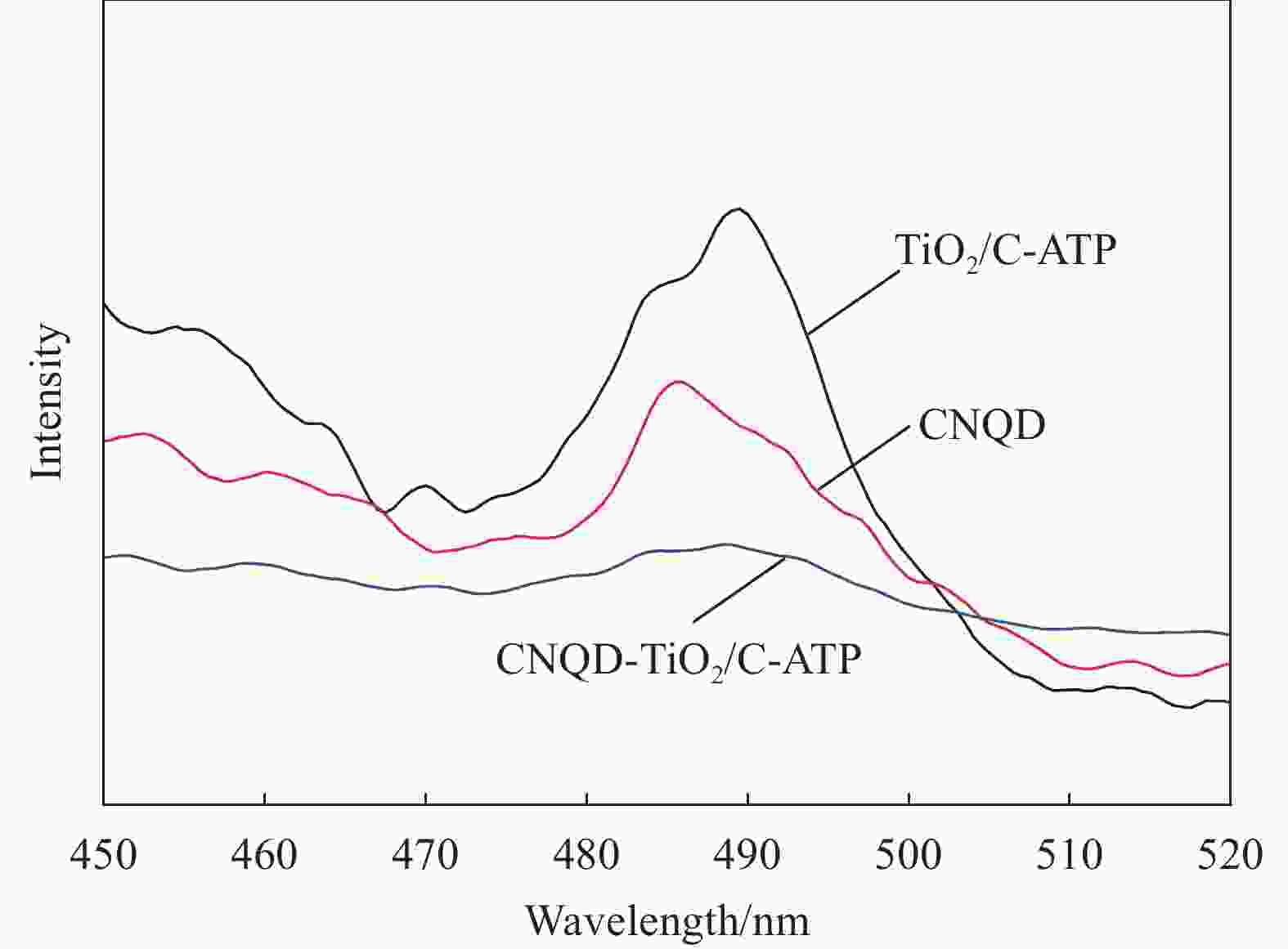
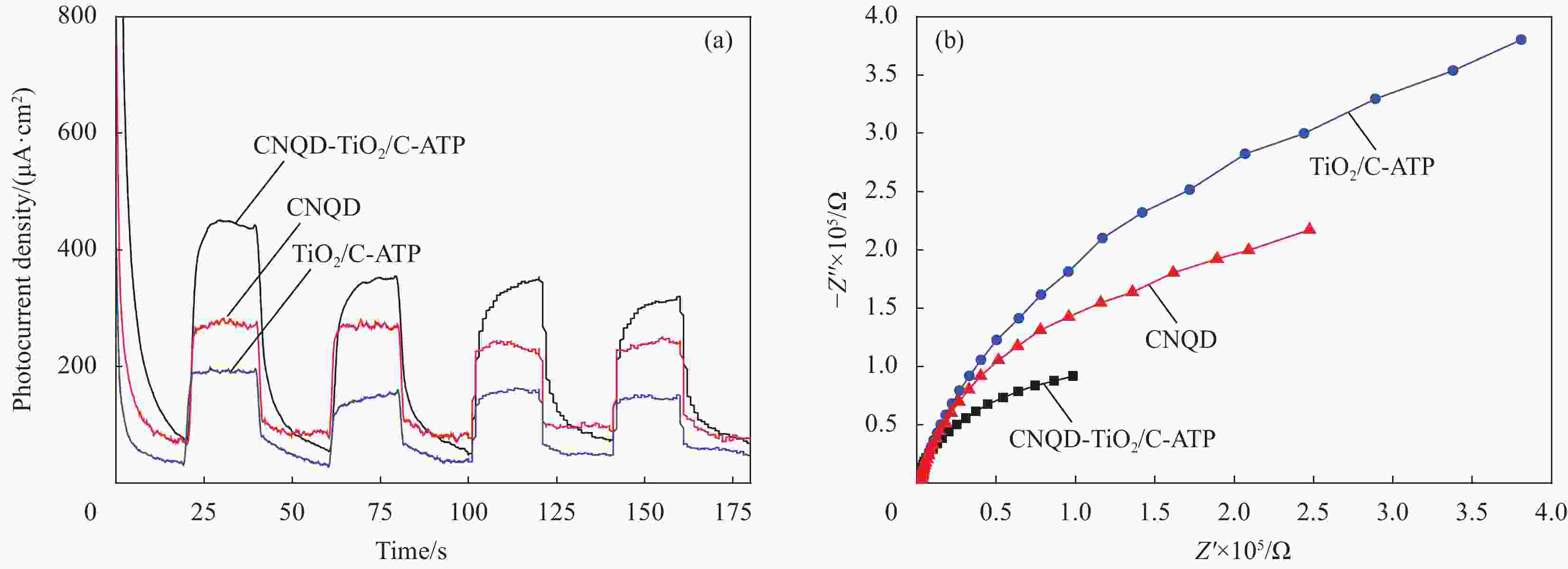
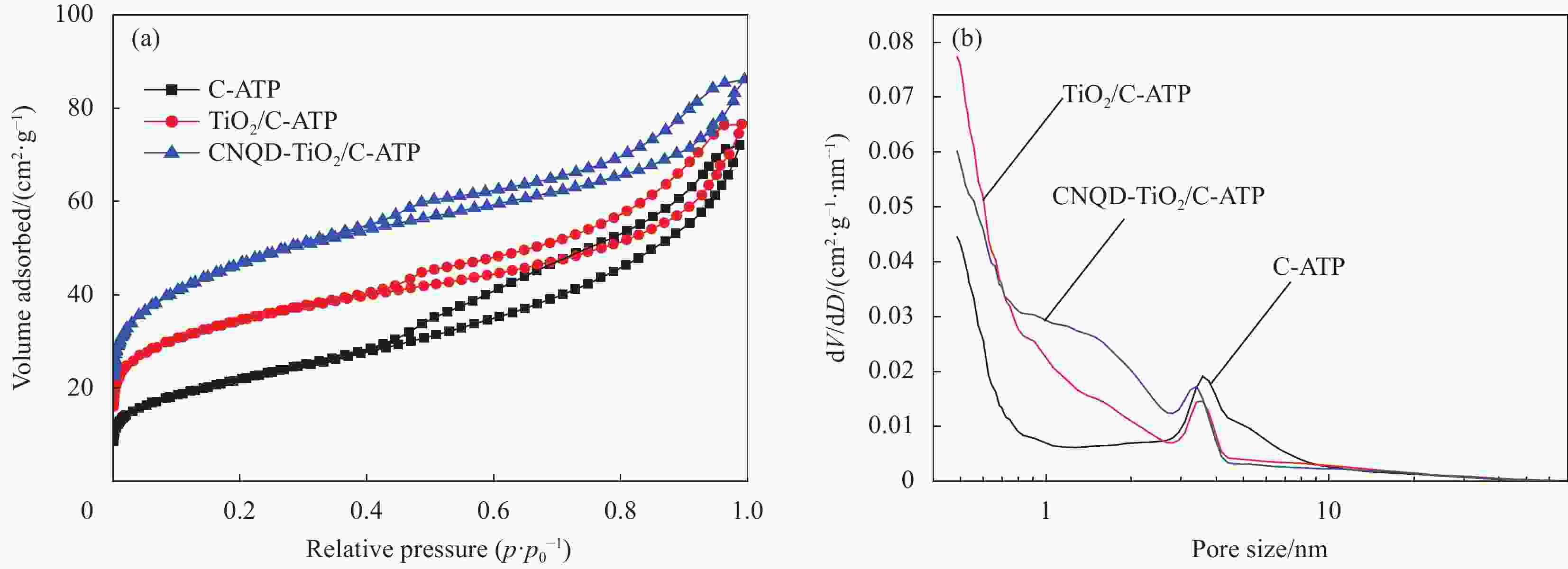
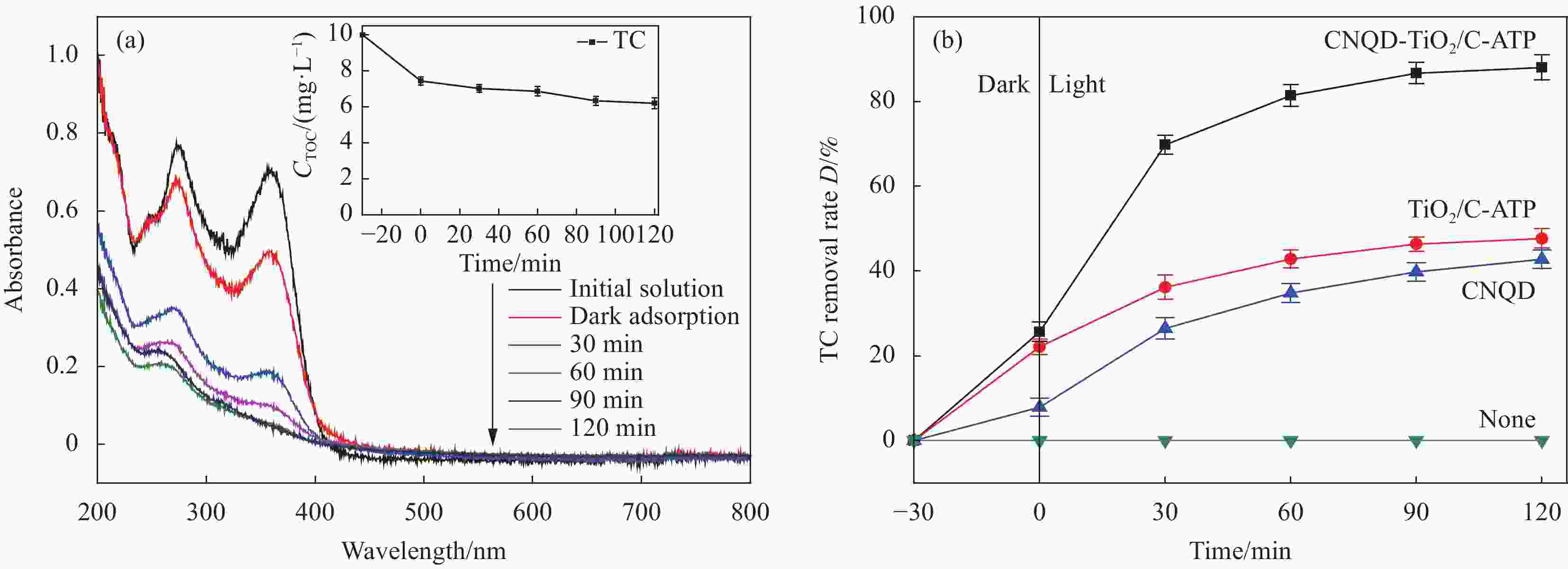
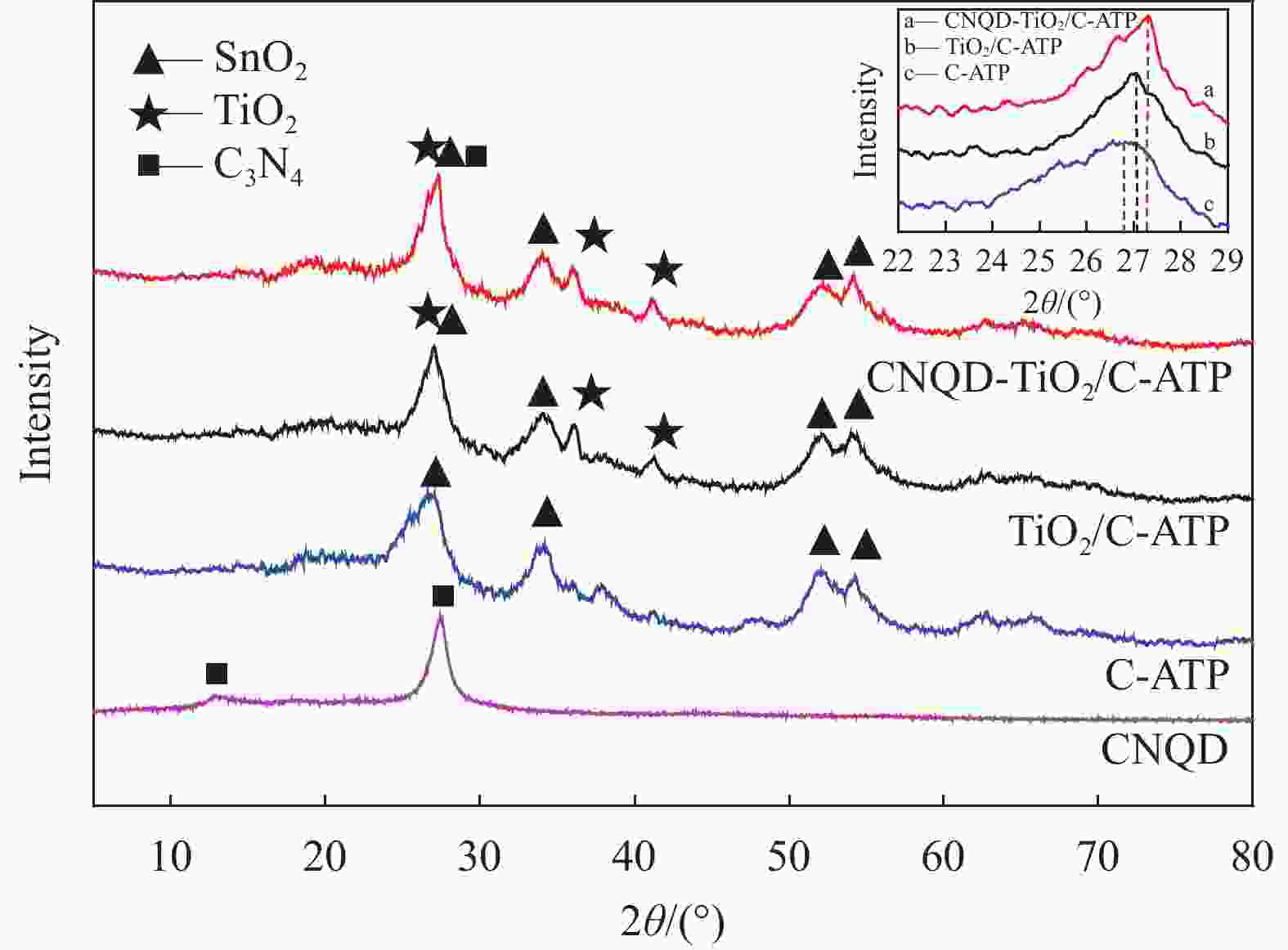
摘要: 通过水热法在导电凹凸棒石(C-ATP)表面原位生长TiO2纳米棒制得毛虫状结构的TiO2/C-ATP复合材料,然后以TiO2/C-ATP为载体,在TiO2纳米棒表面进一步复合g-C3N4量子点(CNQD)成功制备了多级结构的CNQD-TiO2/C-ATP异质结光催化材料。利用XRD、FTIR、SEM/TEM、紫外-可见吸收光谱(UV-Vis-DRS)、荧光发射光谱(PL)、BET比表面积分析仪和光电化学等技术对样品进行表征。在可见光照射下,考察了样品对盐酸四环素(TC)的光催化降解能力。结果表明:与TiO2/C-ATP和CNQD相比,CNQD-TiO2/C-ATP大幅提高了可见光响应、吸收能力和光生电子-空穴对的分离效率。当光照时间为120 min时,CNQD-TiO2/C-ATP对TC去除率可达88%。Abstract: TiO2/C-ATP composites consisting of palmerworm-like TiO2 nanorods in-situ growth on the surface of conductive attapulgite (C-ATP) were constructed via a hydrothermal approach. Then TiO2/C-ATP was used as the carrier to uniformly load carbon nitride quantum dots (CNQD) to successfully prepare hierarchical CNQD-TiO2/C-ATP heterojunction photocatalysts. The obtained samples were characterized by XRD, FTIR, SEM/TEM, ultraviolet-visible (UV-Vis-DRS), photoluminescence (PL), Brunauer-emmett-teller (BET) specific area analyzer and photoelectrochemistry. The degradation ability of the sample to tetracycline hydrochloride (TC) was investigated under visible light. The results show that CNQD-TiO2/C-ATP composites dramatically enhance the visible light response, the absorption capacity and the separation of photogenerated electron-hole pairs compared to TiO2/C-ATP and CNQD. After 120 min irradiation, the degradation rate of CNQD-TiO2/C-ATP to remove TC can achieve 88%.
-
图 9 不同时间的盐酸四环素(TC)光催化产物的UV-Vis-DRS图谱 (a) 和可见光下不同催化剂对TC的去除率曲线 (b)
Figure 9. UV-Vis-DRS spectra of tetracycline hydrochloride (TC) photocatalytic products at different time periods (a) and curves of TC removal rate of different catalysts under visible light (b)
CTOC—Concentration of total organic carbon
-
[1] LWIN H M, ZHAN W Q, SONG S X, et al. Visible-light photocatalytic degradation pathway of tetracycline hydrochloride with cubic structured ZnO/SnO2 heterojunction nanocatalyst[J]. Chemical Physics Letters,2019,736:136806. doi: 10.1016/j.cplett.2019.136806 [2] CAO Y, LEI X Y, CHEN Q L, et al. Enhanced photocatalytic degradation of tetracycline hydrochloride by novel porous hollow cube ZnFe2O4[J]. Journal of Photochemistry and Photobiology A: Chemistry,2018,364:794-800. doi: 10.1016/j.jphotochem.2018.07.023 [3] MA X, CHEN K Y, NIU B, et al. Preparation of BiOCl0.9I0.1/β-Bi2O3 composite for degradation of tetracycline hydrochloride under simulated sunlight[J]. Chinese Journal of Catalysis,2020,41(10):1535-1543. doi: 10.1016/S1872-2067(19)63486-8 [4] VIGNESH K, MATHEW S, BARTLETT J, et al. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances[J]. Applied Catalysis B: Environmental,2019,244:1021-1064. doi: 10.1016/j.apcatb.2018.11.080 [5] GUO Y, GUO T, CHEN J H, et al. Synthesis of C-N-S co-doped TiO2 mischcrystal with an isobandgap characteristic and its photocatalytic activity under visible light[J]. Catalysis Science & Technology,2018,8(16):4108-4121. [6] WANG Q, QIAO Z, JIANG P, et al. Hydrothermal synthesis and enhanced photocatalytic activity of mixed-phase TiO2 powders with controllable anatase/rutile ratio[J]. Solid State Sciences,2018,77:14-19. doi: 10.1016/j.solidstatesciences.2018.01.003 [7] WU J, MA X J, XU L M, et al. Fluorination promoted photoinduced modulation of Pt clusters on oxygen vacancy enriched TiO2/Pt photocatalyst for superior photocatalytic performance[J]. Applied Surface Science,2019,489:510-518. doi: 10.1016/j.apsusc.2019.05.304 [8] WANG C, CAO M H, WANG P F, et al. Preparation of graphene-carbon nanotube-TiO2 composites with enhanced photocatalytic activity for the removal of dye and Cr (VI)[J]. Applied Catalysis A-General,2014,473:83-89. doi: 10.1016/j.apcata.2013.12.028 [9] LI B, CHEN X G, ZHANG T Y, et al. Photocatalytic selective hydroxylation of phenol to dihydroxybenzene by BiOI/TiO2 p-n heterojunction photocatalysts for enhanced photocatalytic activity[J]. Applied Surface Science,2018,439:1047-1056. doi: 10.1016/j.apsusc.2017.12.220 [10] LIU J J, CHENG B, Yu JG. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure[J]. Physical Chemistry Chemical Physics,2016,18(45):31175-31183. doi: 10.1039/C6CP06147H [11] GUO Y R, XIAO L M, ZHANG M, et al. An oxygen-vacancy-rich Z-scheme g-C3N4/Pd/TiO2 heterostructure for enhanced visible light photocatalytic performance[J]. Applied Surface Science,2018,440:432-439. doi: 10.1016/j.apsusc.2018.01.144 [12] LIN X, LIU C, WANG J B, et al. Graphitic carbon nitride quantum dots and nitrogen-doped carbon quantum dots co-decorated with BiVO4 microspheres: A ternary heterostructure photocatalyst for water purification[J]. Separation and Purification Technology,2019,226:117-127. doi: 10.1016/j.seppur.2019.05.093 [13] JIANG G D, LIN Z F, ZHU L H. Preparation and photoelectrocatalytic properties of titania/carbon nanotube compo-site films[J]. Carbon,2010,48(12):3369-3375. doi: 10.1016/j.carbon.2010.05.029 [14] LI Y Y, WANG J G, SUN H H, et al. Heterostructured SnS2/SnO2 nanotubes with enhanced charge separation and excellent photocatalytic hydrogen production[J]. International Journal of Hydrogen Energy,2018,43(31):14121-14129. doi: 10.1016/j.ijhydene.2018.05.130 [15] YU Y T, WANG C Q, JIANG C, et al. Resistive switching behavior in memristors with TiO2 nanorod arrays of different dimensions[J]. Applied Surface Science,2019,485:222-229. doi: 10.1016/j.apsusc.2019.04.119 [16] SU J Y, ZHU L, CHEN G H. Ultrasmall graphitic carbon nitride quantum dots decorated self-organized TiO2 nanotube arrays with highly efficient photoelectrochemical activity[J]. Applied Catalysis B: Environmental,2016,186:127-135. doi: 10.1016/j.apcatb.2015.12.050 [17] LIU L, QI Y H, HU J S, et al. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core@shell Cu2O@g-C3N4 octahedra[J]. Applied Surface Science,2015,351:1146-1154. doi: 10.1016/j.apsusc.2015.06.119 [18] YADAV P, NISHANTHI S, PUROHIT B, et al. Metal-free visible light photocatalytic carbon nitride quantum dots as efficient antibacterial agents: An insight study[J]. Carbon,2019,152:587-597. doi: 10.1016/j.carbon.2019.06.045 [19] WANG H, LIANG Y H, LIU L, et al. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol[J]. Journal of Hazardous Materials,2018,344:369-380. doi: 10.1016/j.jhazmat.2017.10.044 [20] ZUO S X, YAO C, LIU W J, et al. Preparation of Ureido-palygorskite and its effect on the properties of urea-formaldehyde resin[J]. Applied Clay Science,2013,80-81:133-139. doi: 10.1016/j.clay.2013.06.031 [21] LIANG Z, HOU H L, FANG Z, et al. Hydrogenated TiO2 nanorod arrays decorated with carbon quantum dots toward efficient photoelectrochemical water splitting[J]. ACS Applied Materials & Interfaces,2019,11(21):19167-19175. [22] LIN X, XU D, ZHAO R, et al. Highly efficient photocatalytic activity of g-C3N4 quantum dots (CNQDs)/Ag/Bi2MoO6 nanoheterostructure under visible light[J]. Separation and Purification Technology,2017,178:163-168. doi: 10.1016/j.seppur.2017.01.020 [23] SU J Y, ZHU L, GENG P, et al. Self-assembly graphitic carbon nitride quantum dots anchored on TiO2 nanotube arrays: An efficient heterojunction or pollutants degradation under solar light[J]. Journal of Hazardous Materials,2016,316:159-168. doi: 10.1016/j.jhazmat.2016.05.004 [24] WANG Y P, LI YK, ZHAO J L, et al. g-C3N4/B doped g-C3N4 quantum dots heterojunction photocatalysts for hydrogen evolution under visible light[J]. International Journal of Hydrogen Energy,2019,44(2):618-628. doi: 10.1016/j.ijhydene.2018.11.067 [25] YIN S, CHEN Y, HU Q S, et al. In-situ preparation of iron(II) phthalocyanine modified bismuth oxybromide with enhanced visible-light photocatalytic activity and mechanism insight[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2019,575:339-345. [26] ZHANG Y G, WU M Y, KWORK Y, et al. In-situ synthesis of heterojunction TiO2/MnO2 nanostructure with excellent performance in vacuum ultraviolet photocatalytic oxidation of toluene[J]. Applied Catalysis B: Environmental,2019,259(15):118034. -





 下载:
下载: