Effect of reactor length diameter ratio on chemical vapor deposition SiC deposition kinetics
-
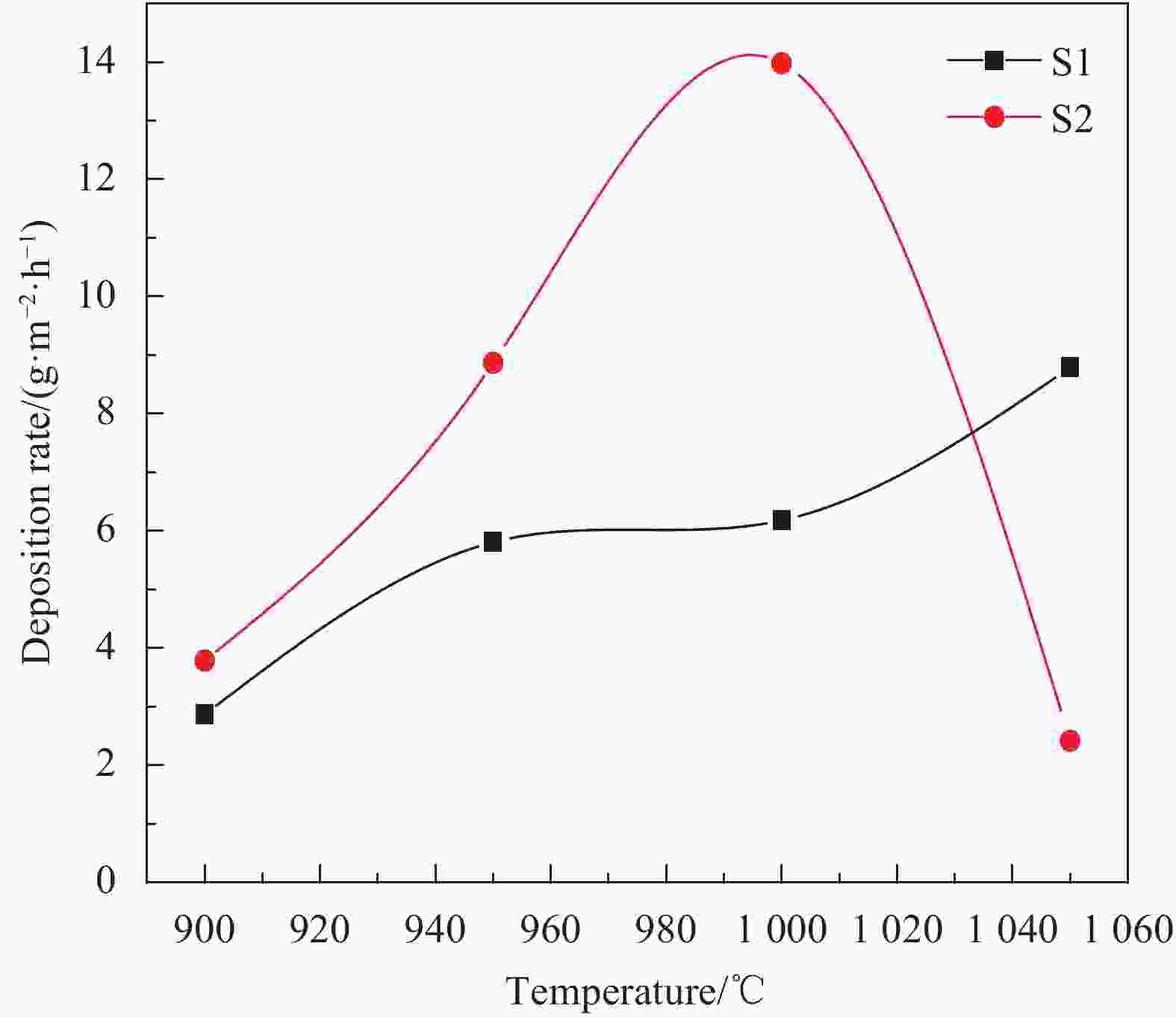
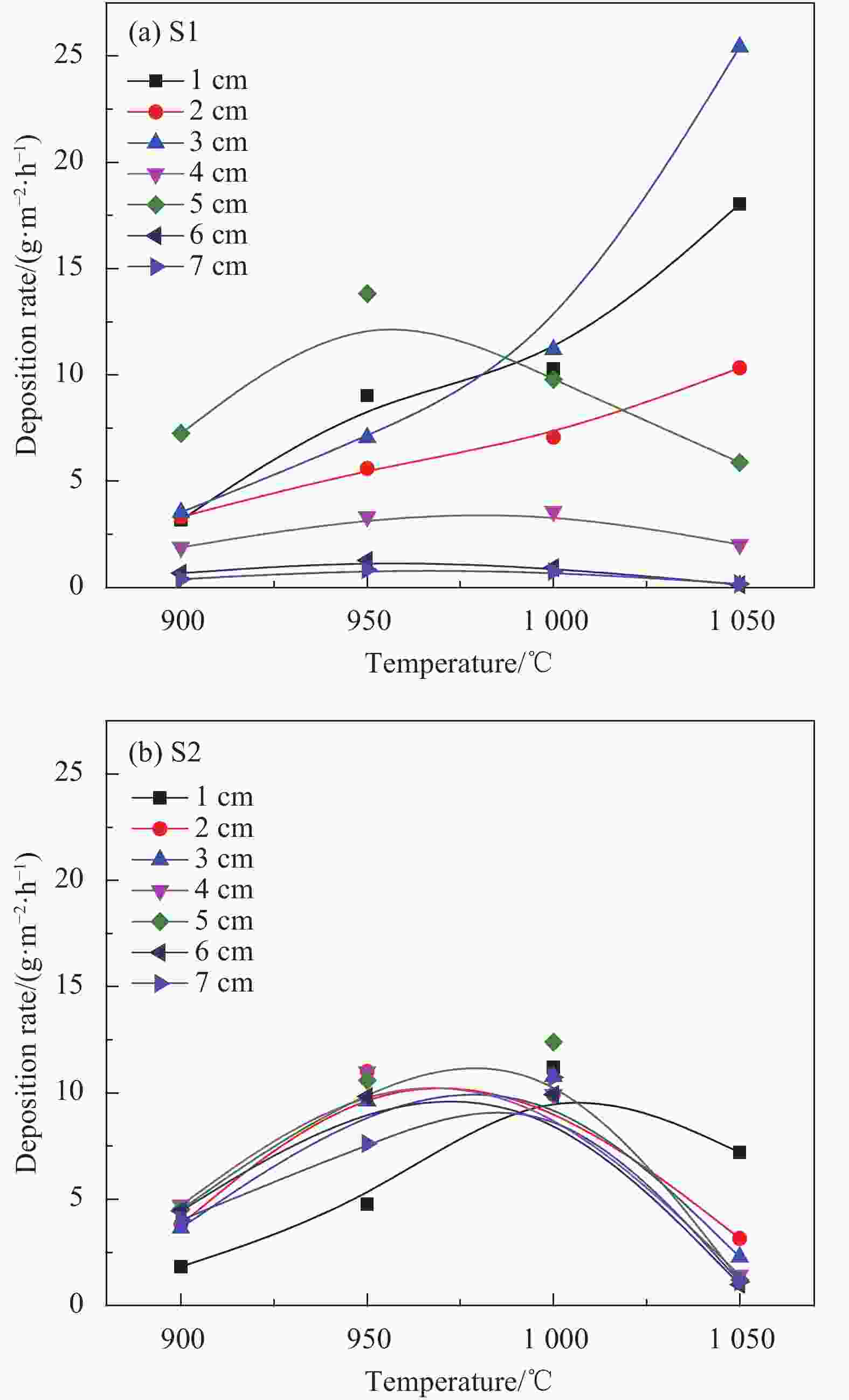
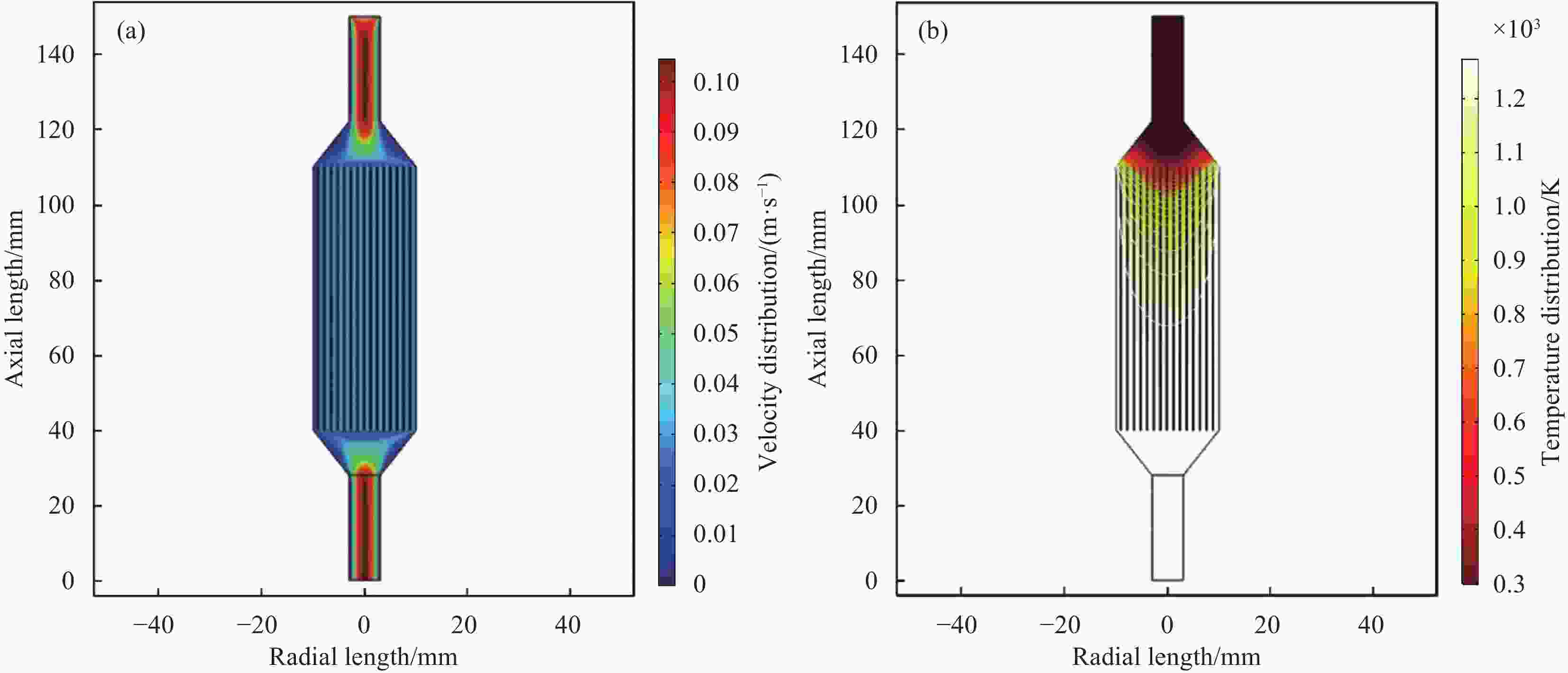
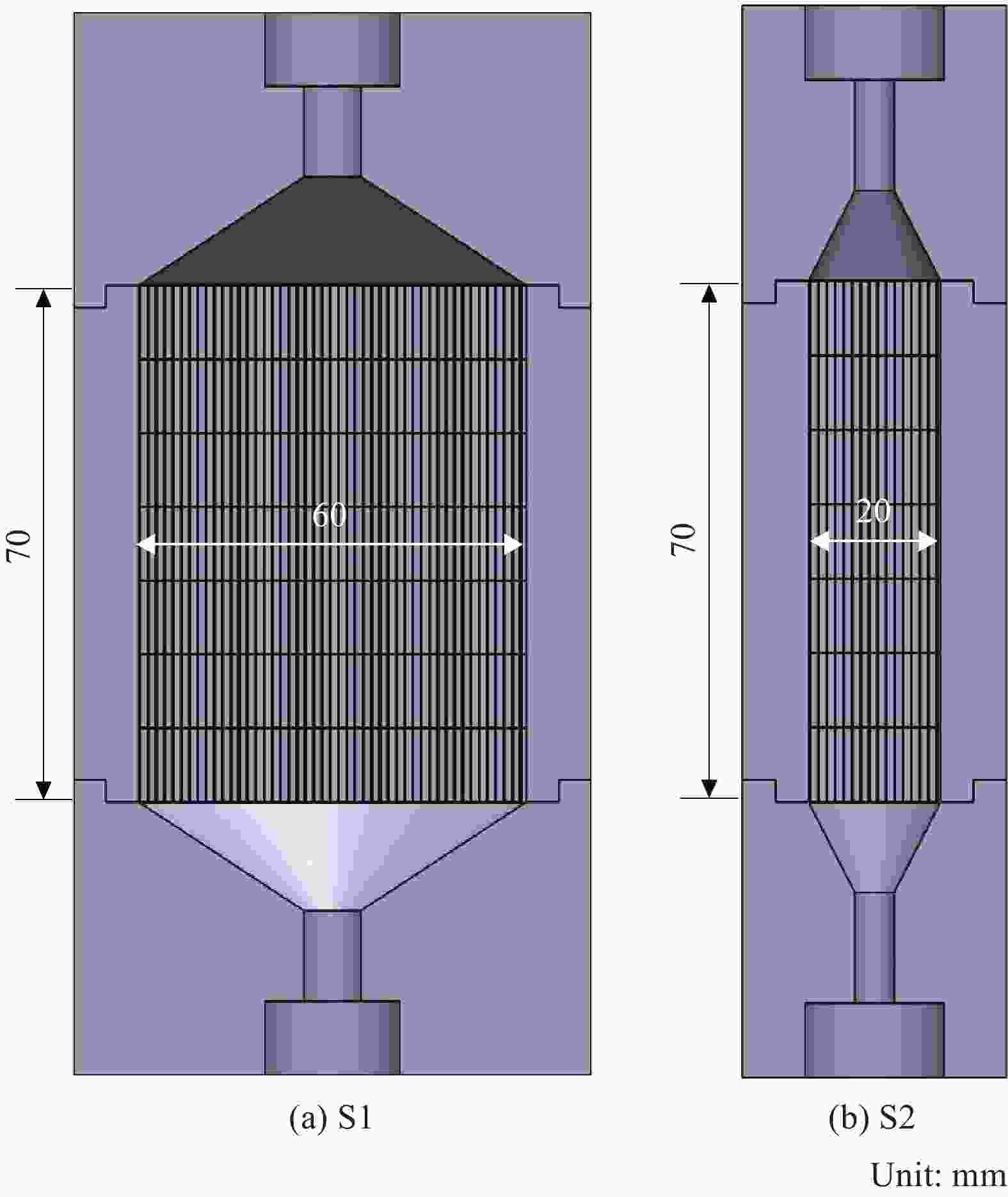
摘要: 以三氯甲基硅烷(MTS)和H2为前驱体,在沉积温度900~1 050℃,H2和MTS摩尔比为4~20和滞留时间0.4~1 s下,采用化学气相沉积(CVD)工艺研究沉积反应器长径比分别为7∶6和7∶2时的碳化硅(SiC)沉积动力学。结果发现,不同尺寸反应器中SiC沉积速率随工艺参数变化的规律性差异明显。长径比7∶6的反应器中SiC平均沉积速率随着温度的增加而增加,而长径比7∶2的反应器中SiC平均沉积速率随着温度先增加后降低,且长径比7∶6的沉积反应器中沿程SiC沉积存在多重稳态的特征。不同H2/MTS摩尔比下SiC沉积速率变化规律在两种反应器中基本一致,尽管长径比7∶6的反应器中出现了SiC沿程的多重择优沉积位置,但整体来说H2对SiC沉积的抑制作用远大于反应器尺寸效应所带来的影响。长径比7∶6反应器中SiC平均沉积速率随滞留时间的增加而降低,但沿程沉积速率受反应器尺寸效应并没有出现单调降低的规律;长径比7∶2反应器中SiC平均沉积速率和沿程沉积速率均随滞留时间增加而降低后趋于稳定。利用COMSOL软件对两种长径比反应器的流场和温度场进行了数值模拟分析发现,长径比7∶6的反应器产生明显的径向流速差,而且轴向和径向流速差和温差较大,而长径比7∶2的沉积反应器流场和温度场较为均匀,这种反应器尺寸效应引起的实际工艺参数和理论工艺参数之间的偏差,正是实验中不同长径比反应器中SiC沉积动力学规律差异的原因。
-
关键词:
- 化学气相沉积(CVD) /
- 碳化硅(SiC) /
- 沉积动力学 /
- 长径比 /
- 数值模拟
Abstract: Using trichloromethylsilane (MTS) and H2 as precursors, the deposition kinetics of silicon carbide (SiC) was studied by chemical vapor deposition (CVD) process at 900-1 050℃, H2/MTS mole ratio of 4-20 and residence time of 0.4-1 s. The results show that the variation of SiC deposition rate with process parameters in different reactors is obviously different. The average deposition rate of SiC in the reactor with length diameter ratio of 7∶6 increases with the increase of temperature, while the average deposition rate of SiC in the reactor with length diameter ratio of 7∶2 first increases and then decreases with the increase of temperature. Moreover, the deposition of SiC along the reactor with length diameter ratio of 7∶6 has the characteristics of multiple steady states. The variation of SiC deposition rate at different H2/MTS mole ratios is basically consistent in the two reactors. Although multiple preferential deposition positions along the SiC path appear in the reactor with length diameter ratio of 7∶6, the inhibition effect of H2 on SiC deposition is greater than that caused by reactor size effect. The results show that the average deposition rate of SiC in the reactor with length diameter ratio of 7∶6 decreases with the increase of residence time, but the deposition rate along the path does not decrease monotonically due to the effect of reactor size; both the average deposition rate and the deposition rate along the path of the reactor with length diameter ratio of 7∶2 decrease with the increase of residence time and then tend to be stable. The flow field and temperature field of two kinds of length diameter ratio reactors were simulated by COMSOL software. It is found that the reactor with length diameter ratio of 7∶6 produces obvious radial velocity difference, and the axial and radial temperature difference are large, while the flow field and temperature field of deposition reactor with length diameter ratio of 7∶2 are more uniform. The actual process parameters and theory caused by the size effect of the reactor were analyzed. The deviation of process parameters is the reason for the difference of SiC deposition kinetics in different length diameter ratio reactors. -
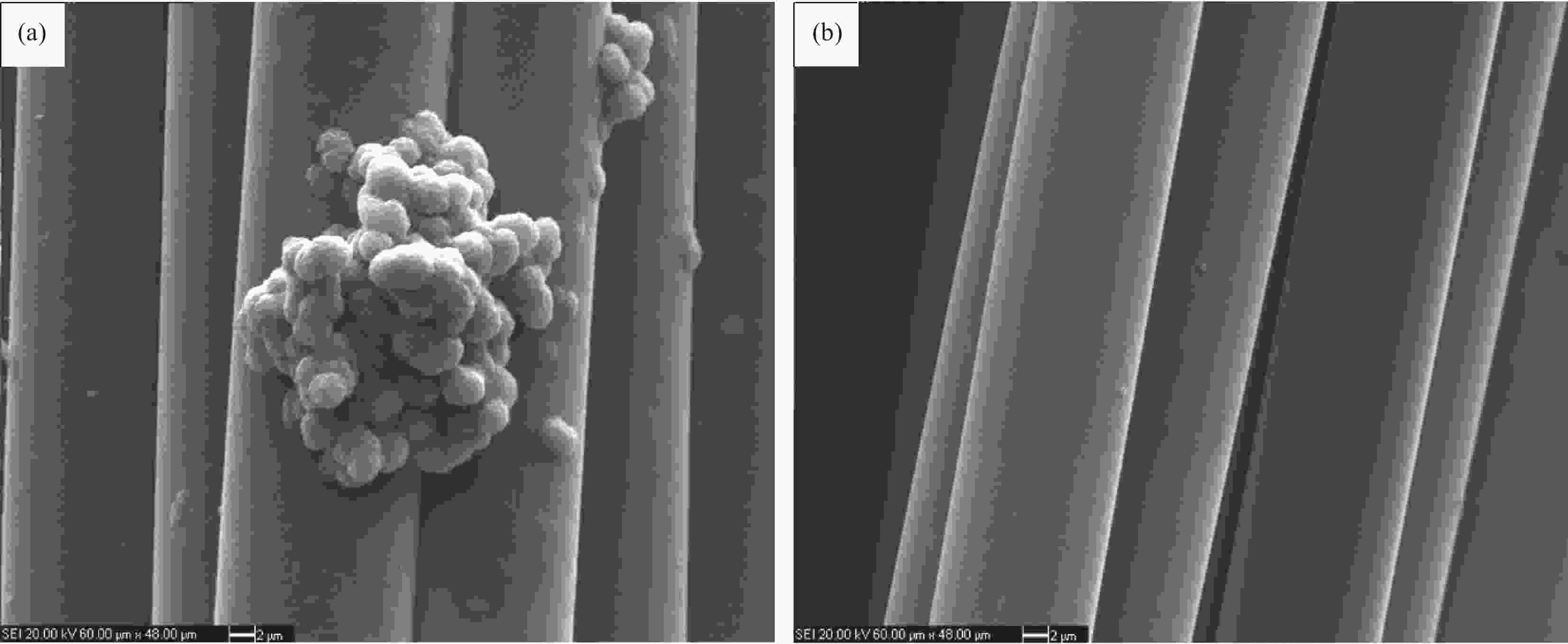
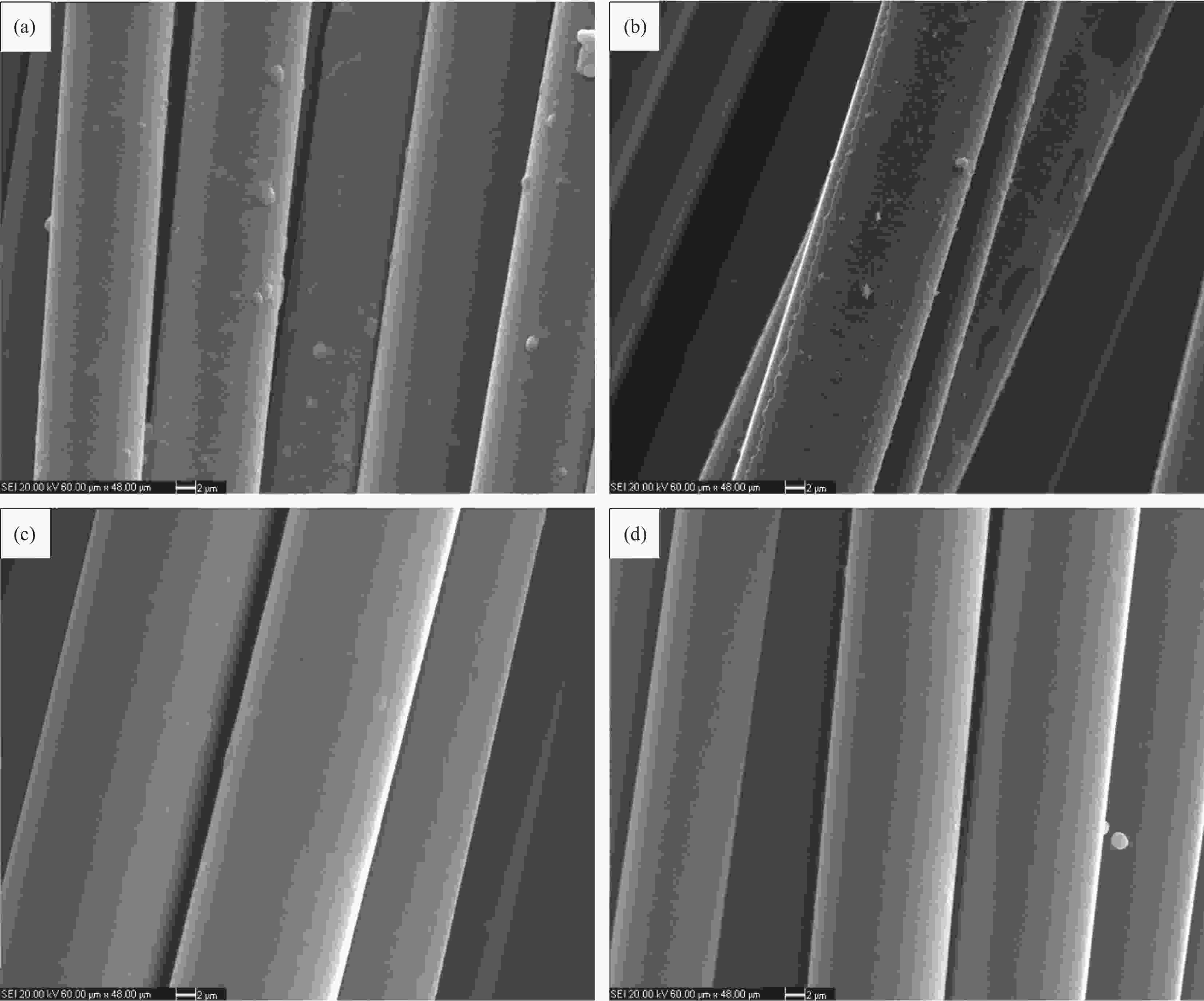
图 12 S1反应器中径向中心位置 (a)、S1反应器中径向边缘位置 (b)、S2反应器中径向中心位置 (c) 和S2反应器中径向边缘位置处距进气口0 cm处 (d) SiC涂层形貌
Figure 12. Morphologies of SiC coatings at 0 cm distance from gas inlet at the radial center position of reactor S1 (a), the radial edge position of reactor S1 (b), the radial center position of reactor S2 (c) and the radial edge position of reactor S2 (d)(1 000℃, 10 kPa, 0.2 s)
表 1 堇青石多孔陶瓷片的几何特征
Table 1. Geometric characteristics of cordierite porous ceramic plates
Substrate diameter/
mmPore density/
cpsiWall thickness/
mmFree volume/
103 mm3Surface area/
103 mm2Specific surfaces
area/mm−1Porosity/
%S400 60 400 0.1781 0.415 37 3.2 74.0 Note: cpsi—Channels per square inch. -
[1] KEE R J, COLTRIN M E, GLARBORG P. Chemically reacting flow: Theory and practice[M]. Manhattan: Wiley, 2005. [2] GE Y B, GORDON M S, BATTAGLIA F, et al. Theoretical study of the pyrolysis of methyltrichlorosilane in the gas phase. 1. Ther-modynamics[J]. The Journal of Physical Chemistry. A,2007,111(8):1462-1474. doi: 10.1021/jp065453q [3] WANG X, SU K H, DENG J L, et al. Initial decomposition of methyltrichlorosilane in the chemical vapor deposition of silicon-carbide[J]. Computational & Theoretical Chemistry,2011,967(2-3):265-272. [4] FISCHMAN G S, PETUSKEY W T. Thermo-dynamic analysis and kinetic implications of chemical vapor deposition of SiC from Si-C-Cl-H gas systems[J]. Journal of the American Ceramic Society,1985,68(4):185-190. [5] DENG J L, SU K H, ZENG Q F, et al. Thermodynamics of the production of condensed phases in the CVD of methyltrichlorosilane pyrolysis[J]. Chemical Vapor Deposition,2010,15(10-12):281-290. [6] LOUMAGNE F, LANGLAIS F, NASLAIN R, et al. Physicochemical properties of SiC-based ceramics deposited by low pressure chemical vapor deposition from CH3SiCl3-H2[J]. Thin Solid Films,1995,254(1-2):75-82. doi: 10.1016/0040-6090(94)06237-F [7] CHIN J, GANTZEL P K, HUDSON R G. The structure of chemical vapor deposited silicon carbide[J]. Thin Solid Films,1977,40:57-72. doi: 10.1016/0040-6090(77)90103-1 [8] 王子梁, 刘荣正, 刘马林, 等. 致密SiC包覆层低温流化床化学气相沉积制备及形成机制[J]. 复合材料学报, 2016, 33(8):1777-1784.WANG Ziliang, LIU Rongzheng, LIU Malin, et al. Low temperature synthesis and formation mechanism of dense SiC coating layer by fluidized bed chemical vapor deposition[J]. Acta Materiae Compositae Sinica,2016,33(8):1777-1784(in Chinese). [9] KOSTJUHIN I M, SOTIRCHOS S V. Codepo-sition of SiC and C from mixtures of methyl-trichlorosilane and ethylene in hydrogen[J]. Industrial & Engineering Chemistry Research,2001,40(12):2586-2596. [10] RAMOS A, FILTVEDT W O, LINDHOLM D, et al. Deposition reactors for solar grade silicon: A comparative thermal analysis of a Siemens reactor and a fluidized bed reactor[J]. Journal of Crystal Growth,2015,431:1-9. doi: 10.1016/j.jcrysgro.2015.08.023 [11] 张伟刚. 化学气相沉积: 从烃类气体到固体碳[M]. 北京: 科学出版社, 2007: 1-277.ZHANG Weigang. Chemical vapor deposition: From gasous hydrocarbon to solid carbon[M]. Beijing: Science Press, 2007: 1-277(in Chinese). [12] MOLLICK P K, VENUGOPALAN R, SRIVASTAVA D. CFD coupled kinetic modeling and simulation of hot wall vertical tubular reactor for deposition of SiC crystal from MTS[J]. Journal of Crystal Growth,2017,475:97-109. doi: 10.1016/j.jcrysgro.2017.06.004 [13] JI W, LOFGREN P M, HALLIN C, et al. Computational modeling of SiC epitaxial growth in a hot wall reactor[J]. Journal of Crystal Growth,2000,220(4):560-571. doi: 10.1016/S0022-0248(00)00843-5 [14] VELENYI L J, SONG Y, FAGLEY J C. Carbon deposition in ethane pyrolysis reactors[J]. Industrial& Engineering Chemistry Research,1991,30(8):1708-1712. [15] DANCKWERTS P V. Continuous flow systems. Distribution of residence times[J]. Chemical Engineering Science,1953,2(1):1-13. doi: 10.1016/0009-2509(53)80001-1 [16] PAPASOULIOTIS G D, SOTIRCHOS S V. On the homogeneous chemistry of the thermal decomposition of methyltrichlorosilane[J]. Journal of the Electrochemical Society,1994,141(6):1599-1611. doi: 10.1149/1.2054969 [17] SUN G D, LI H J, FU Q G, et al. Finite element simulation of the effects of process parameters on deposition uniformity of chemical-vapor-deposited silicon carbide[J]. Computational Materials Science,2009,46(4):1002-1006. doi: 10.1016/j.commatsci.2009.05.005 [18] PAPASOULIOTIS G D, SOTIRCHOS S V. Experimental study of atmospheric pressure chemical vapor deposition of silicon carbide from methyltrichlorosilane[J]. Journal of Materials Research,1999,14(8):3397-3409. doi: 10.1557/JMR.1999.0460 [19] HLAVACEK V, PUSZYNSKI J A. Chemical engineering aspects of advanced ceramic materials[J]. Industrial & Engineering Chemistry Research,1996,35(2):349-377. [20] BESMANN T M, SHELDON B W, MOSS Ⅲ T S, et al. Depletion effects of silicon carbide deposition from methyltrichlorosilane[J]. Journal of the American Ceramic Society,1992,75(10):2899-2903. doi: 10.1111/j.1151-2916.1992.tb05529.x [21] SOTIRCHOS S V, PAPASOULIOTIS G D. Kinetic modelling of the deposition of SiC from methyltrichlorosilane[J]. Mrs Proceedings,1991,250:35-40. doi: 10.1557/PROC-250-35 [22] KAUSHAL A, PRAKASH J, DASGUPTA K, et al. Simulation and experimental study of CVD process for low temperature nanocrystalline silicon carbide coating[J]. Nuclear Engineering and Design,2016,303:122-131. doi: 10.1016/j.nucengdes.2016.04.009 [23] MEZIERE J, UCAR M, BLANQUET E, et al. Modeling and simulation of SiC CVD in the horizontal hot-wall reactor concept[J]. Journal of Crystal Growth,2004,267(3-4):436-451. doi: 10.1016/j.jcrysgro.2004.04.038 [24] MISHRA P, VERMA N. A CFD study on a vertical chemical vapor deposition reactor for growing carbon nanofibers[J]. Chemical Engineering Research and Design,2012,90(12):2293-2301. doi: 10.1016/j.cherd.2012.05.006 -





 下载:
下载: