Preparation of carbon supported cerium doped zinc oxide composite material and its photocatalytic properties study in degradation of methylene blue dye
-
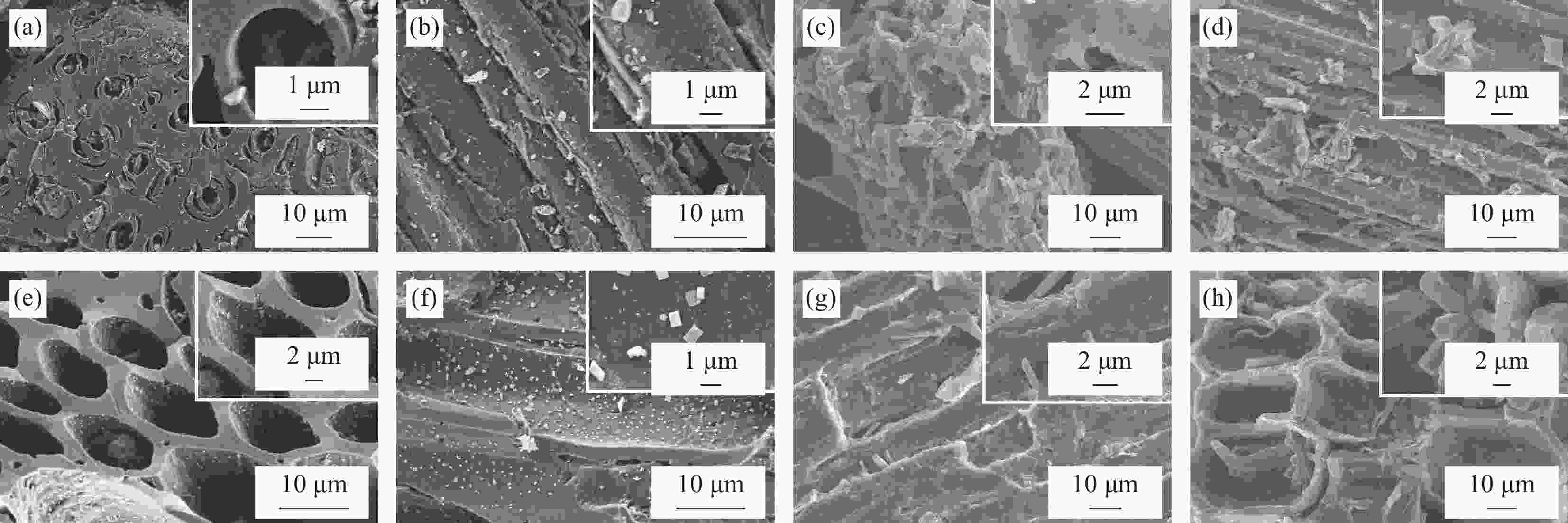
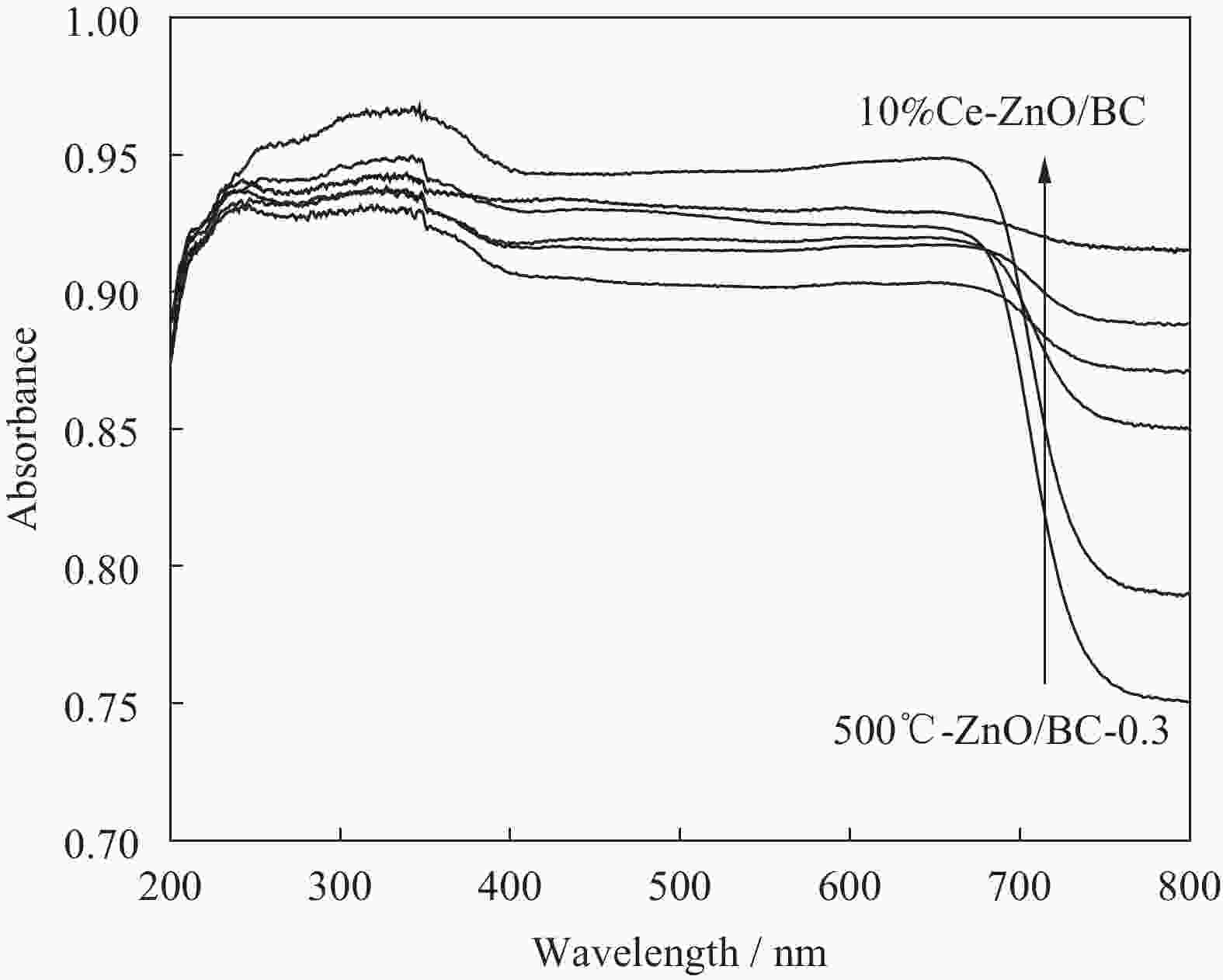
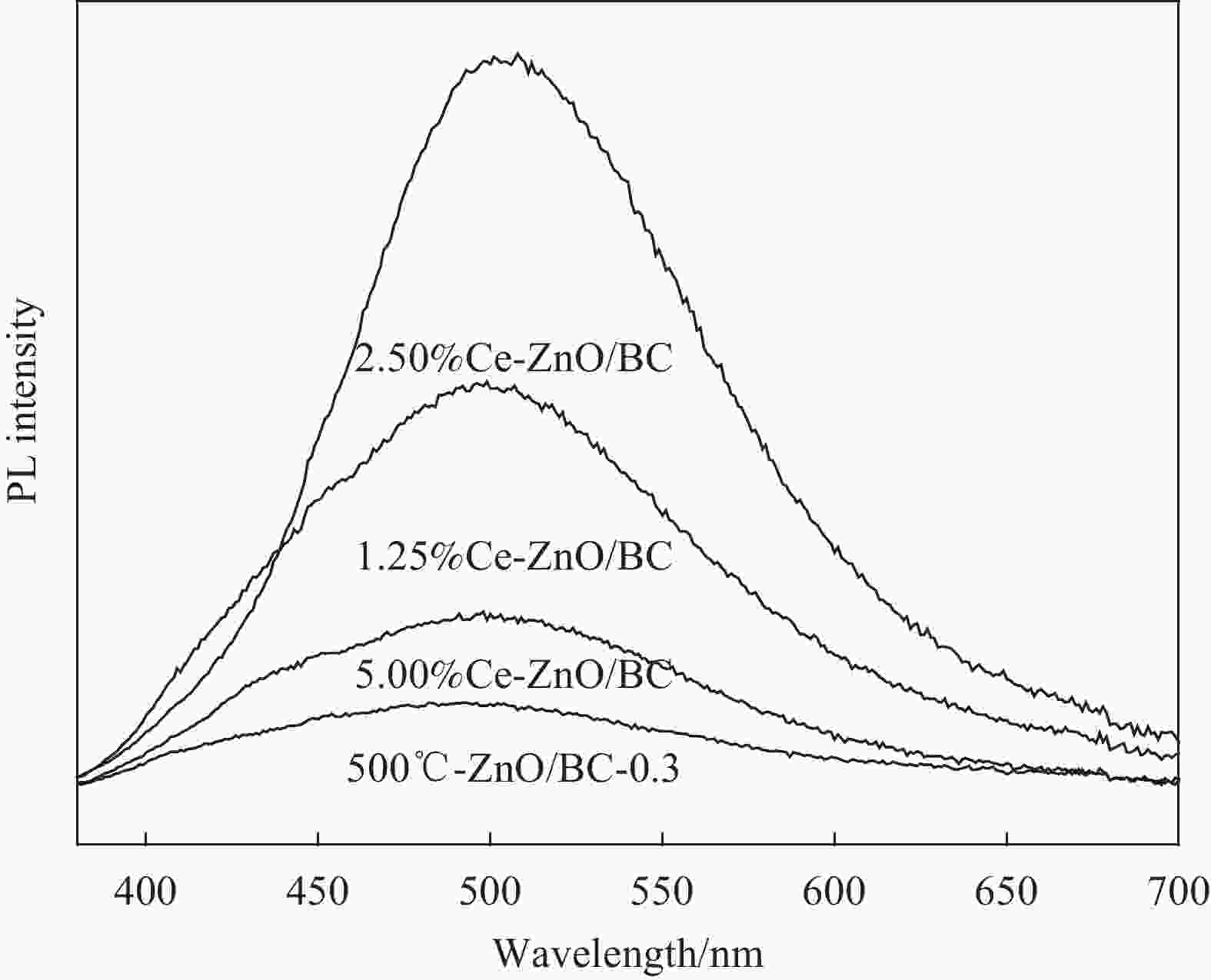
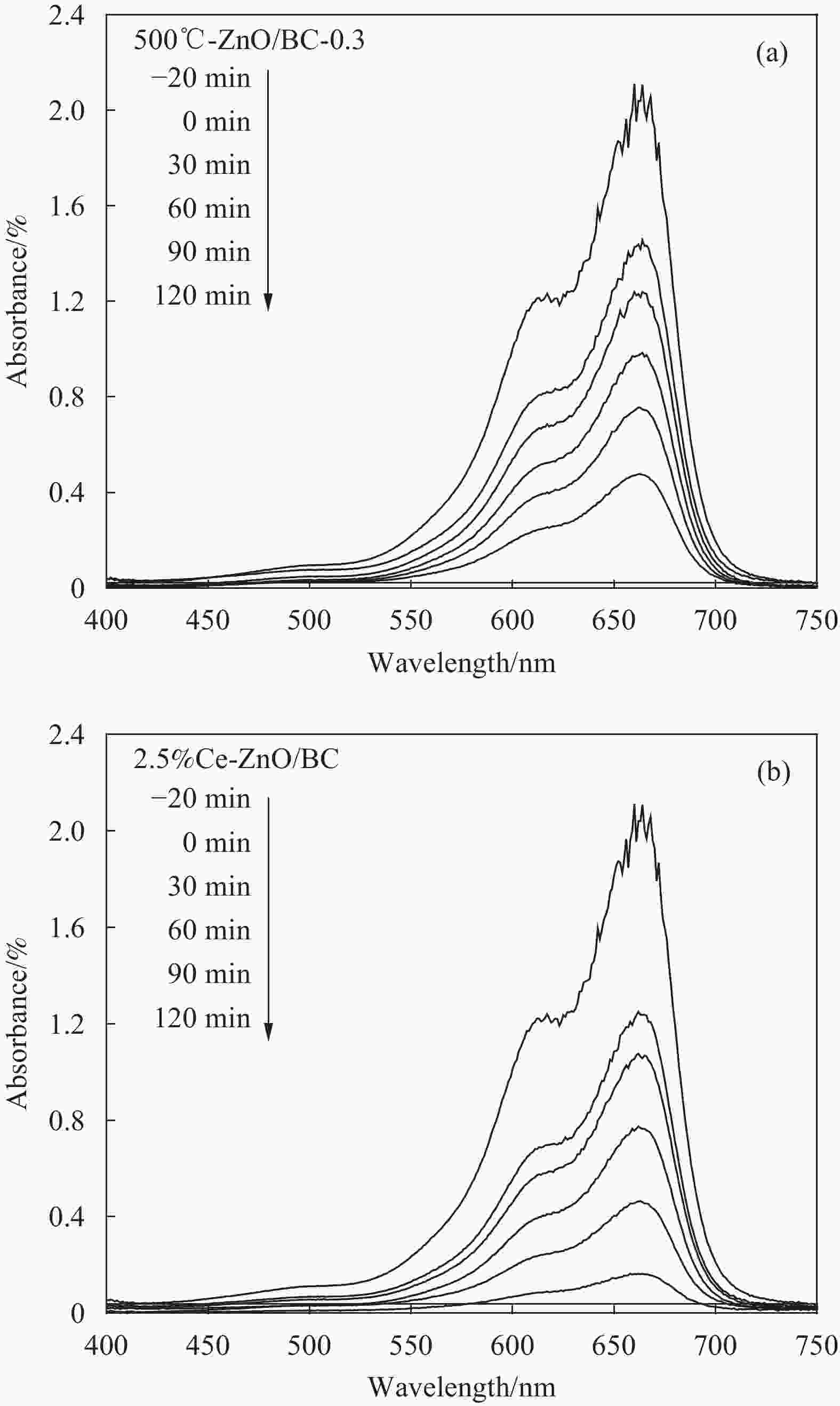
摘要: 以竹粉为碳源、ZnCl2为锌源、六水合硝酸铈(Ⅲ)(Ce(NO3)3·6H2O)为铈源,采用一锅法制备得到新型竹炭负载Ce掺杂ZnO复合材料(Ce-ZnO/BC)。利用XRD、FTIR、SEM、EDS、BET、XPS、紫外-可见光漫反射光谱(DRS)及光致荧光光谱(PL)对所制备复合材料进行全面表征,并对其光催化降解有机染料性能进行了研究。获得了催化材料的最佳制备条件:ZnCl2与竹粉浸渍比为3∶10,煅烧温度为500℃,Ce(NO3)3·6H2O加入量为ZnCl2的2.5%。所得材料分别以日光和紫外光为光源,在暗处吸附20 min,光照120 min、亚甲基蓝(MB)溶液50 mL(10 mg·L−1),催化剂用量为40 mg 条件下,对MB降解率分别为92.2%和93.7%。并且研究结果表明,其催化MB降解符合一级反应动力学原理,催化剂具有一定的重复使用性能。Abstract: Carbon supported cerium doped zinc oxide composite material was prepared using bamboo as carbon source, ZnCl2 as zinc source, Ce(NO3)3·6H2O as cerium source, in one-pot method. These materials were fully characterized by XRD, FTIR, SEM, EDS, BET, XPS, UV-Vis diffuse reflection spectrum (DRS) and photoluminescence spectroscopy (PL). Their properties in photocatalytic degradation of organic dyes were studied at the same time. The optimized preparing condition of the material was obtained as follows: impregnation ratio of ZnCl2 to bamboo is 3∶10 by mass, calcination temperature is 500℃ and the addition amount of ZnCl2 is 2.5% of bamboo. The degradation rates of methylene blue (MB) under sunlight and ultraviolet are 92.2% and 93.7%, respectively, under the following condition: dark adsorption time 20 min, illumination time 120 min, 50 mL MB (10 mg·L−1) and catalyst amount 40 mg. Moreover, kinetic studies of the reaction proved that the photocatalytic degradation of MB catalyzed by this material follows the first order reaction kinetics principle. And the catalyst can be reused several times.
-
Key words:
- bamboo charcoal /
- ZnO /
- composite /
- degradation /
- catalyst support
-
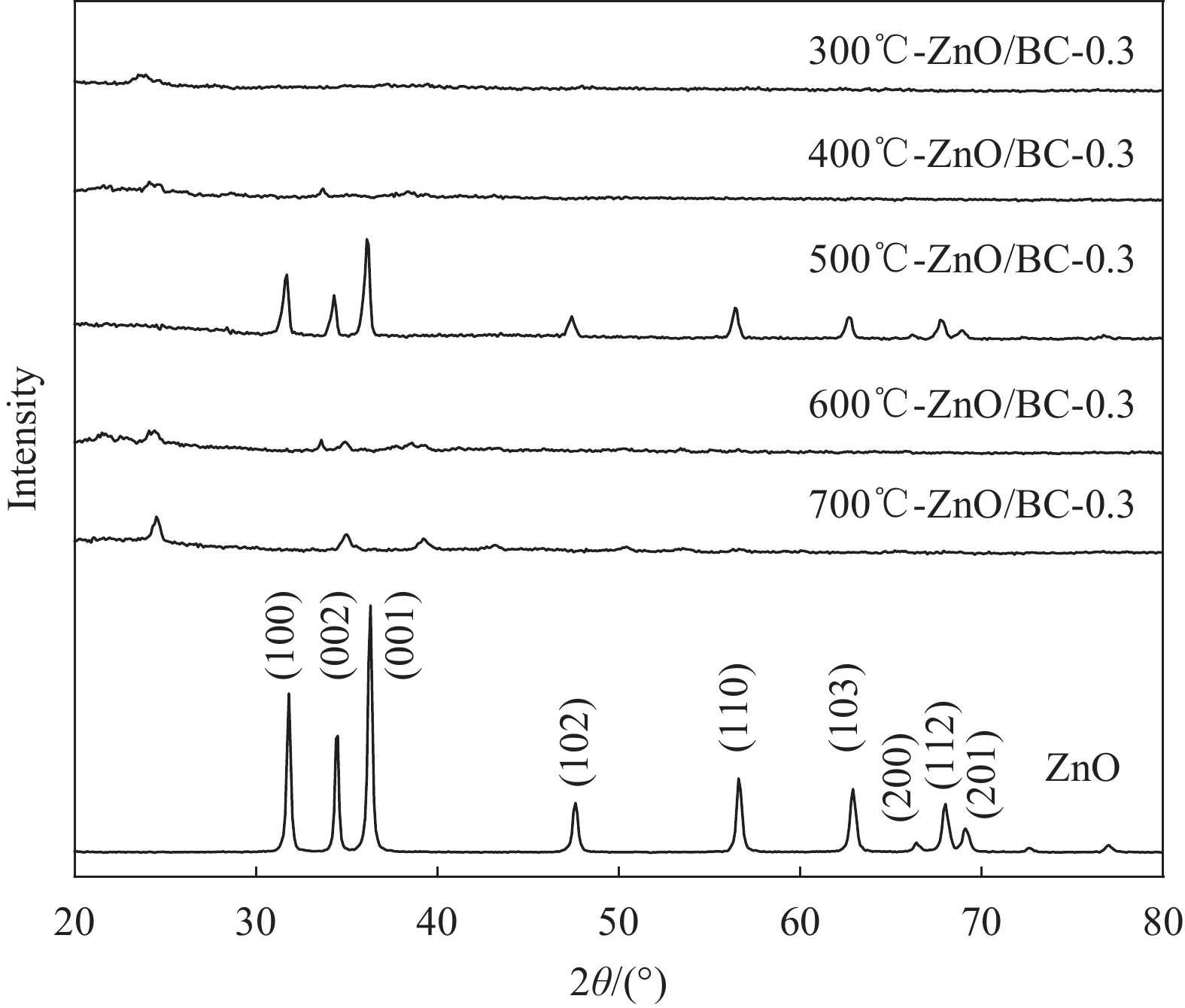
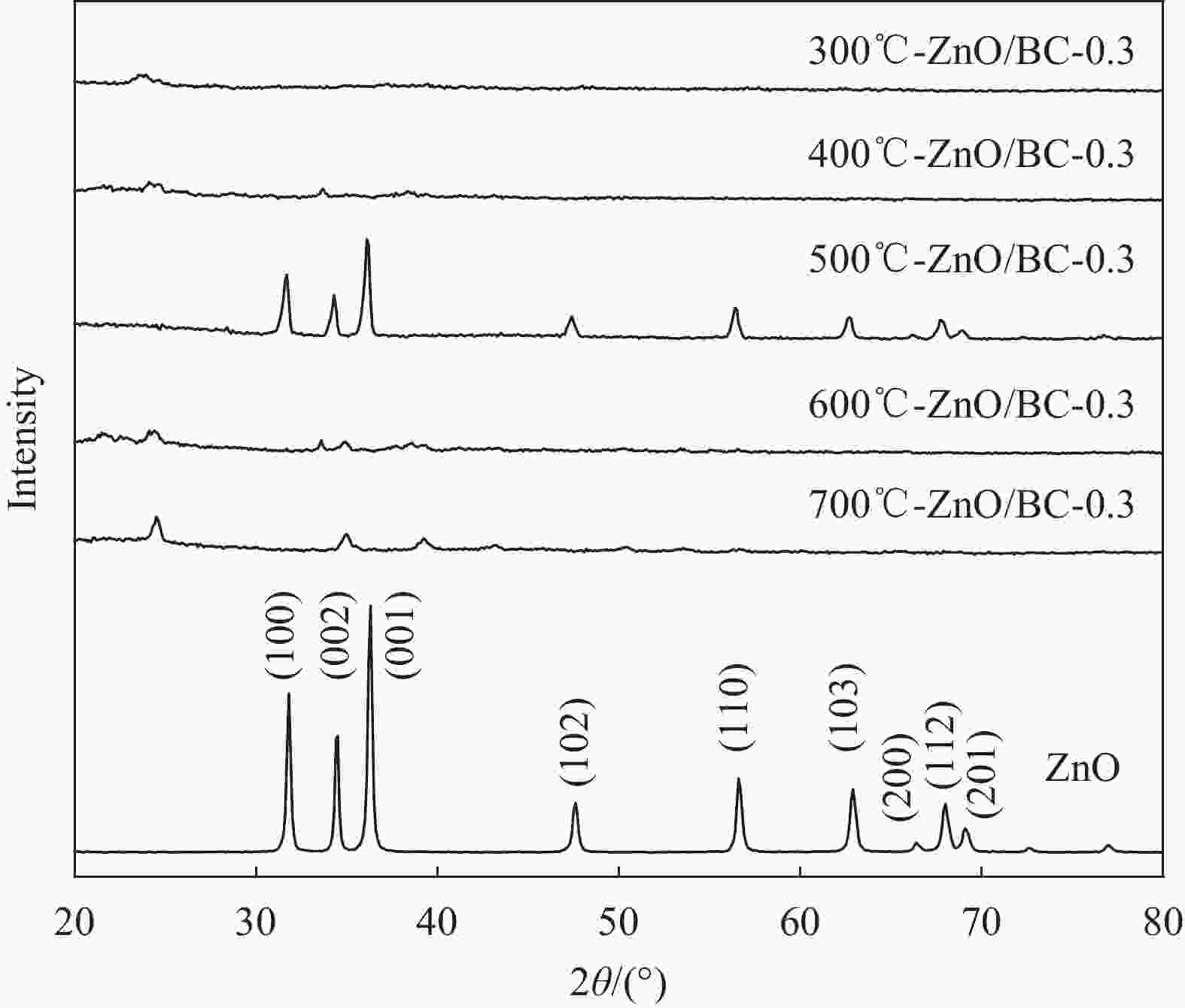
表 1 ZnO/BC和Ce-ZnO/BC材料制备及命名
Table 1. Preparation and nomenclature of ZnO/BC and Ce-ZnO/BC
Photocatalyst Temperature/℃ (mZnCl2/mBamboo)/% (mCe(NO3)3•6H2O/mZnCl2)/% 300 ℃-ZnO/BC-0.3 300 0.3 — 400 ℃-ZnO/BC-0.3 400 0.3 — 500 ℃-ZnO/BC-0.3 500 0.3 — 600 ℃-ZnO/BC-0.3 600 0.3 — 700 ℃-ZnO/BC-0.3 700 0.3 — 500 ℃-ZnO/BC-0.2 500 0.2 — 500 ℃-ZnO/BC-0.4 500 0.4 — 1.25%Ce-ZnO/BC 500 — 1.25 2.5%Ce-ZnO/BC 500 — 2.5 5%Ce-ZnO/BC 500 — 5 7.5%Ce-ZnO/BC 500 — 7.5 10%Ce-ZnO/BC 500 — 10 表 2 系列材料的EDS分析结果
Table 2. EDS characterization results of different materials
Entry Photocatalyst Atom fraction/at% C Zn Ce 1 300℃-ZnO/BC-0.3 75.84 0.38 — 2 400℃-ZnO/BC-0.3 84.78 2.97 — 3 500℃-ZnO/BC-0.3 87.31 5.73 — 4 600℃-ZnO/BC-0.3 87.64 1.51 — 5 700℃-ZnO/BC-0.3 90.16 1.41 — 6 500℃-ZnO/BC-0.2 92.10 2.06 — 7 500℃-ZnO/BC-0.4 79.21 15.09 — 8 1.25%Ce-ZnO/BC 89.69 4.37 0.03 9 2.5%Ce-ZnO/BC 84.42 5.46 0.16 10 5%Ce-ZnO/BC 83.11 5.24 0.29 11 7.5%Ce-ZnO/BC 83.17 6.29 0.49 12 10%Ce-ZnO/BC 82.79 6.11 0.61 表 3 Ce-ZnO/BC和ZnO/BC光催化剂在日光下的一级反应动力常数ka和R2
Table 3. First order reaction rate constant (ka) and R2 of photocatalysts under sunlight irradiation of Ce-ZnO/BC and ZnO/BC
Entry Photocatalyst Linear regression equation ka/min−1 R2 1 500℃-ZnO/BC-0.3 Y=0.0128X+0.0241 0.0128 0.981 2 1.25%Ce-ZnO/BC Y=0.0186X−0.075 0.0186 0.974 3 2.5%Ce-ZnO/BC Y=0.0205X+0.0929 0.0205 0.999 4 5%Ce-ZnO/BC Y=0.0164X+0.0593 0.0164 0.998 5 7.5%Ce-ZnO/BC Y=0.0141X+0.072 0.0141 0.987 6 10%Ce-ZnO/BC Y=0.0123X+0.0652 0.0123 0.996 表 4 Ce-ZnO/BC和ZnO/BC光催化剂在紫外光下的ka和R2
Table 4. ka and R2 of photocatalysts under UV irradiation of Ce-ZnO/BC and ZnO/BC
Entry Photocatalyst Linear regression equation ka/min−1 R2 1 500℃-ZnO/BC-0.3 Y=0.0079X+0.1978 0.0079 0.934 2 1.25%Ce-ZnO/BC Y=0.0106X+0.1235 0.0106 0.974 3 2.5%Ce-ZnO/BC Y=0.0231X−0.2397 0.0231 0.923 4 5%Ce-ZnO/BC Y=0.0119X+0.0652 0.0119 0.933 5 7.5%Ce-ZnO/BC Y=0.0083X+0.1693 0.0083 0.967 6 10%Ce-ZnO/BC Y=0.0084X+0.1683 0.0084 0.966 -
[1] NEENA D, KONDAMAREDDY K K, BIN H, et al. Enhanced visible light photodegradation activity of RhB/MB from aqueous solution using nanosized novel Fe-Cd co-modified ZnO[J]. Scientific Reports,2018,8:10691. doi: 10.1038/s41598-018-29025-1 [2] PASCARIU P, AIRINEI A, OLARU N, et al. Photocatalytic degradation of Rhodamine B dye using ZnO-SnO2 electrospun ceramic nanofibers[J]. Ceramics International,2016,42(6):6775–6781. [3] RAJENDRAN S, KHAN M. M, GRACIA F, et al. Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite[J]. Scientific Reports,2016,6:31641. doi: 10.1038/srep31641 [4] PHILLIPS R B, JAMES R R, MAGNUSON M L. Electrolyte selectionandmicrobial toxicity for electrochemical oxidative water treatment using a boron-doped diamond anode to support site specific contamination incident response[J]. Chemosphere,2018,197:135-141. doi: 10.1016/j.chemosphere.2018.01.007 [5] KATHERESAN V, KANSEDO J, LAU S Y. Efficiency of various recent wastewater dye removal methods: A review[J]. Journal of Environmental Chemical Engineering,2018,6(4):4676-4697. doi: 10.1016/j.jece.2018.06.060 [6] DAI M. Mechanism of adsorption for dyes on activated carbon[J]. Journal of Colloid and Interface Science,1998,198(1):6-10. doi: 10.1006/jcis.1997.5254 [7] ZULARISAM A W, ISMAIL A F, SALIM R. Behaviours of natural organic matter in membrane filtration for surface water treatment: A review[J]. Desalination,2006,194(1):211-231. [8] 钟伟, 夏颖帆, 翟杭玲, 等. 共沉淀法制备稀土Ce掺杂的纳米ZnO及其光催化降解染料的性能[J]. 无机化学学报, 2020, 36(1):40-52. doi: 10.11862/CJIC.2020.006ZHONG W, XIA Y F, ZHAI H L, et al. Preparation by Co-precipitation method and photocatalytic performances on the degradation of dyes of Ce3+-doped nano-ZnO[J]. Chinese Journal of Inorganic Chemistry,2020,36(1):40-52(in Chinese). doi: 10.11862/CJIC.2020.006 [9] LI D, HANEDA H. Morphologies of zinc oxide particles and their effects on photocatalysis[J]. Chemosphere,2003,51(2):129-137. doi: 10.1016/S0045-6535(02)00787-7 [10] YU X L, QIN A M, LIAO L, et al. Removal of organic dyes by nanostructure ZnO-bamboo charcoal composites with photocatalysis function[J]. Advances in Materials Science and Engineering,2015:1-6. [11] BYRAPPA K, SUBRAMANI A K, ANANDA S, et al. Impregnation of ZnO onto activated carbon under hydrothermal conditions and its photocatalytic properties[J]. Journal of Materials Science,2006,41(5):1355-1362. doi: 10.1007/s10853-006-7341-x [12] WU C H, SHR J F, WU C F, et al. Synthesis and photocatalytic characterization of titania-supported bamboo charcoals by using sol-gel method[J]. Journal of Materials Processing Technology,2008,203:326-332. doi: 10.1016/j.jmatprotec.2007.10.073 [13] 林文胜, 王欢, 杨东杰, 等. 木质素碳/氧化锌复合材料的制备及其光催化性能[J]. 高校化学工程学报, 2020, 36(1):40-52.LIN W S, WANG H, YANG D J, et al. Preparation of lignin-based carbon/zinc oxide composites and their photocatalytic performance[J]. Chinese Journal of Inorganic Chemistry,2020,36(1):40-52(in Chinese). [14] 金雯, 曲雯雯, 彭金辉, 等. 炭基氧化锌复合材料的研究进展[J]. 材料导报, 2012, 26(13):62-69. doi: 10.3969/j.issn.1005-023X.2012.13.013JIN W, QU W W, PENG J H, et al. Review of research in ZnO/AC composite materials[J]. Materials Review,2012,26(13):62-69(in Chinese). doi: 10.3969/j.issn.1005-023X.2012.13.013 [15] KHANITTA I, PONGSATON A, SUMETHA S, et al. Effect of Ag loading on activated carbon doped ZnO for bisphenol A degradation under visible light[J]. Advanced Powder Technology,2018,29(11):2608-2615. doi: 10.1016/j.apt.2018.07.006 [16] 张齐生. 重视竹材化学利用, 开发竹炭应用技术[J]. 竹子研究汇刊, 2001, 20(3):34-35.ZHANG Q S. Playing great attention on bamboo chemical processing and exploiting bamboo charcoal applying technique[J]. Journal of Bamboo Research,2001,20(3):34-35(in Chinese). [17] 朱丽虹, 蒋乐, 汪洋. 溶剂热法制备ZnO/竹炭及其光降解甲基橙性能[J]. 浙江理工大学学报, 2016, 35(6):826-831.ZHU L H, JIANG L, WANG Y. Preparation of ZnO/bamboo charcoal by solvothermal method and its photocatalytic degradation property on methyl orange[J]. Journal of Zhejiang Sci-Tech University,2016,35(6):826-831(in Chinese). [18] ZHOU Y L, HU Z B, TONG M X, et al. Preparation and photocatalytic performance of bamboo-charcoal-supported nano-ZnO composites[J]. Materials Science,2018,24(1):49-52. [19] 陈霞, 陆改玲, 计晶晶, 等. 稀土元素(镧、铈)掺杂TiO2复合材料的制备及其光催化性和抑菌性的研究[J]. 人工晶体学报, 2020, 49(1):62-66. doi: 10.3969/j.issn.1000-985X.2020.01.010CHEN X, LU G L, JI J J, et al. Photocatalytic and antibacterial activity of rare earth element (La, Ce)doped TiO2 composite[J]. Journal of Synthetic Crystals,2020,49(1):62-66(in Chinese). doi: 10.3969/j.issn.1000-985X.2020.01.010 [20] 黄丽礼, 赵深茂, 邓越全. 稀土钕掺杂氧化锌纳米材料的制备及对罗丹明B的降解研究[J]. 无机盐工业, 2019, 51(8):88-92.HUANG L L, ZHAO S M, DENG Y Q. Preparation of rare earth Nd-doped ZnO nano-materials and its degradation of Rhodamine B[J]. Inorganic Chamicals Industry,2019,51(8):88-92(in Chinese). [21] 张进, 姜婷婷, 蔡君一. Ce掺杂氧化锌的制备及应用研究[J]. 化工新型材料, 2019, 47(1):252-258.ZHANG J, JIANG T T, CAI J Y. Study on syntnesis and photocatalysis of Ce doped ZnO[J]. New Chamical Materials,2019,47(1):252-258(in Chinese). [22] 冯雅楠, 甘俊珍, 陈星辉, 等. 形貌的粒度对纳米二氧化铈光催化降解盐基品红的研究[J]. 应用化工, 2019, 48(1):14-17. doi: 10.3969/j.issn.1671-3206.2019.01.004FENG Y N, GAN J Z, CHEN X H, et al. Study of morphology and particle size of nano-CeO2 on basic fuchsine photocatalytic degradation[J]. Applied Chemical Industry,2019,48(1):14-17(in Chinese). doi: 10.3969/j.issn.1671-3206.2019.01.004 [23] MATTIA Costamagna, LUCA Ciacci, MARIA Cristina Paganini, et al. Combining the highest degradation efficiency with the lowest environmental impact in zinc oxide based photocatalytic systems[J]. Journal of Cleaner Production,2020,252:119762. [24] 陈林, 彭国良, 宋杰光, 等. Ce3+掺杂纳米ZnO的制备及其可见光光催化性能研究[J]. 化工新型材料, 2020, 48(1):200-206.CHEN L, PENG G L, SONG J G. Study on preparation and visible photocatalytic of Zn1-xCexO[J]. New Chamical Materials,2020,48(1):200-206(in Chinese). [25] 汪应灵, 谢友海, 薛载坤, 等. Ce掺杂ZnO纳米晶的光催化性能研究[J]. 人工晶体学报, 2011, 40(4):917-920, 931. doi: 10.3969/j.issn.1000-985X.2011.04.020WANG Y L, XIE Y H, XUE Z K, et al. Photocatalytic properties of Ce doped ZnO nanocrystalline[J]. Journal of Synthetic Crystals,2011,40(4):917-920, 931(in Chinese). doi: 10.3969/j.issn.1000-985X.2011.04.020 [26] 张会平, 叶李艺, 杨立春. 氯化锌活化法制备木质活性炭研究[J]. 材料科学与工艺, 2006, 14(1):42-45. doi: 10.3969/j.issn.1005-0299.2006.01.013ZHANG H P, YE L Y, YANG C L. Preparation of activated carbon from sawdust by chemical activation with zinc chloride[J]. Materials Science and Technology,2006,14(1):42-45(in Chinese). doi: 10.3969/j.issn.1005-0299.2006.01.013 [27] 王古平, 彭萍萍, 赵森, 等. Ce掺杂ZnO的晶体结构、发光和光催化性能的不同机理[J]. 化学工程师, 2019(4):13-17, 26.WANG G P, PENG P P, ZHAO S, et al. Different mechanisms of crystal structure, luminescent property and photocatalytic activity of Ce doped ZnO[J]. Chemical Engineer,2019(4):13-17, 26(in Chinese). [28] 翁诗甫. 傅里叶变换红外光谱分析[M]. 第2版. 北京: 化学工业出版社, 2012: 327-328.WENG Shifu. Fourier transform infrared spectrometry analysis[M]. 2nd Edition. Beijing: Chemical Industry Press, 2012: 327-328(in Chinese). [29] 刘峥, 韦梦琴, 杜弱莹, 等. 活化温度对桉树皮基活性炭的特性影响及吸附性能研究[J]. 生态与农村环境学报, 2020, 36(2):265-271.LIU Z, WEI M Q, DU Y Y, et a1. Effect of activation temperature on the properties and adsorption performance of eucalyptus robusta based activated carbon[J]. Journal of Ecology and Rural Environment,2020,36(2):265-271(in Chinese). [30] 李允超, 王贤华, 杨海平, 等. 竹炭表面结构及其对糠醛的吸附特性[J]. 农业工程学报, 2012, 28(12):257-263. doi: 10.3969/j.issn.1002-6819.2012.12.041LI Y C, WANG X H, YANG H P, et al. Surface structure of bamboo charcoal and its adsorption property on furfural[J]. Transactions of the Chinese Society of Agricultural Engineering,2012,28(12):257-263(in Chinese). doi: 10.3969/j.issn.1002-6819.2012.12.041 [31] AHMADPOUR A, DO D D. The preparation of activated carbon from macadamia nutshell by chemical activation[J]. Carbon,1997,35(12):1723-1732. doi: 10.1016/S0008-6223(97)00127-9 [32] SAPKOTA K P, LEE I, ABU H, et al. Solar-light-driven efficient ZnO-single-walled carbon nanotube photocatalyst for the degradationof a persistent water pollutant organic dye[J]. Catalysts,2019,9(498):1-14. [33] ZHANG X W, ZHANG X L, WANG X, et al. Enhancing the photocatalytic activity and photostability of zinc oxide nanorod arrays via graphitic carbon mediation[J]. Chinese Journal of Catalysis,2018,39(5):973-981. doi: 10.1016/S1872-2067(18)63010-4 [34] CERRATO E, GIONCO C, BERRUTI I, et al. R are earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment[J]. Journal of Solid State Chemistry,2018,264:42-47. doi: 10.1016/j.jssc.2018.05.001 [35] SENTHILKANNAN K, MALARKODI V, VENKATACHALAM K, et al. Fluorescence (FL) activities of Ce doped zinc oxide nano particles[J]. Materials Today: Proceedings,2020,33:2774-2775. [36] HEMANT K V, MAHAK V, MAURYA K K. Synthesis, characterization and sun light-driven photocatalytic activity of zinc oxide nanostructures[J]. Journal of Nanoscience and Nanotechnology,2020,6(20):3683-3692. [37] LI S Q, ZHOU P J, ZHANG W S, et al. Effective photocatalytic decolorization of methylene blue utilizing ZnO/rectorite nanocomposite under simulated solar irradiation[J]. Journal of Alloys and Compounds,2014,616:227-234. doi: 10.1016/j.jallcom.2014.07.102 -





 下载:
下载: