Synthesis and electrocatalytic oxygen evolution performances of FeOOH-Ni(OH)2 composites
-
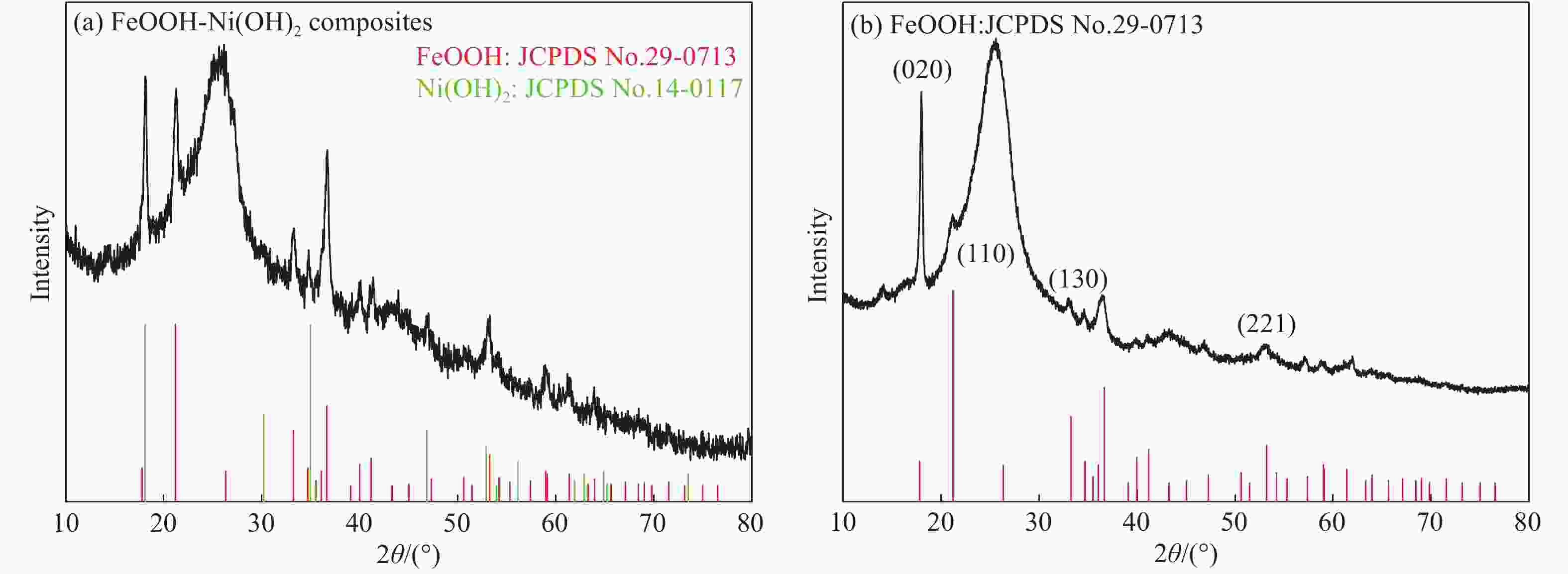
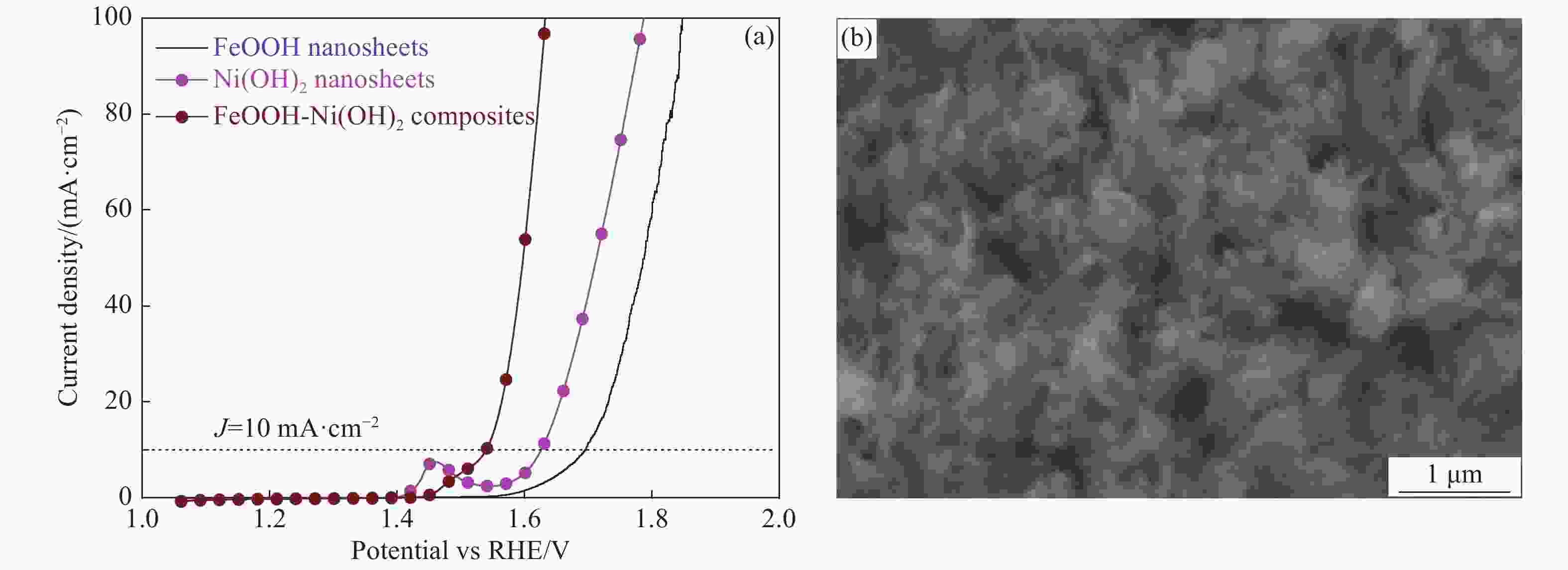
摘要: 以碳纤维布(CFC)为基底,通过两步法(恒电流电沉积法、溶剂热法)成功制备了FeOOH-Ni(OH)2复合材料。与FeOOH和Ni(OH)2相比,该FeOOH-Ni(OH)2复合材料作为电催化剂时,电催化析氧反应(OER)活性显著提高。在1 mol/L KOH电解质溶液中,达到10 mA·cm−2电流密度时所需要的过电位仅为270 mV,Tafel斜率为78 mV/dec,电化学阻抗谱进一步揭示了电解过程中良好的动力学特性。FeOOH-Ni(OH)2复合材料在碱性介质中具有优异的稳定性,其在高电流密度下(50 mA·cm−2)的过电势经过连续24 h的测试之后几乎没有发生明显变化。FeOOH和Ni(OH)2之间的强电子相互作用和协同效应有效提高了电导性,促进了电荷转移;此外,这种核壳结构有效增强了电催化活性面积,进而增强了其电催化析氧性能。
-
关键词:
- FeOOH-Ni(OH)2复合材料 /
- 核壳结构 /
- 协同效应 /
- 电催化 /
- 析氧反应
Abstract: FeOOH-Ni(OH)2 composites were successfully prepared on carbon fiber cloth (CFC) by electrochemical deposition and hydrothermal method. The electrocatalytic oxygen evolution activity of the FeOOH-Ni(OH)2 composite is significantly improved compared with FeOOH and Ni(OH)2. The FeOOH-Ni(OH)2 electrodes require an overpotential as low as 270 mV and the tafel slope to deliver 10 mA·cm−2 for oxygen evolution reaction (OER) in 1 mol/L KOH. The electrochemical impedance spectroscopy further reveals the favorable kinetic during electrolysis. Moreover, The FeOOH-Ni(OH)2 composite has excellent stability in the alkaline medium, and its overpotential remains stable during the 24 h test at high current density(50 mA·cm−2). The strong electron interaction and synergistic reaction between FeOOH and Ni(OH)2 are enhanced effectively. The conductivity promotes the charge transfer, and the core-shell structure effectively enhances the electrocatalytic activity area, and further enhances its oxygen evolution properties. -
图 2 FeOOH纳米片(a)、FeOOH-Ni(OH)2复合材料(b)和Ni(OH)2纳米片(j)的SEM图像以及FeOOH纳米片(c)和FeOOH-Ni(OH)2复合材料(d)的TEM图像、FeOOH-Ni(OH)2复合材料的HRTEM图像(e)、FeOOH-Ni(OH)2复合材料的元素分布图((f)~(i))
Figure 2. SEM images of FeOOH(a), FeOOH-Ni(OH)2 composites(b)and Ni(OH)2(j) and TEM images of FeOOH(c) and FeOOH-Ni(OH)2 composites(d), HRTEM image of FeOOH-Ni(OH)2 composites(e) and elemental mapping images of FeOOH-Ni(OH)2 composites((f)-(i))
图 5 FeOOH纳米片、Ni(OH)2纳米片及FeOOH-Ni(OH)2复合材料的极化曲线(a)、taifel斜率(b)、电化学阻抗谱(c)、双层电容曲线(d)及FeOOH-Ni(OH)2复合材料连续循环3000圈前后的极化曲线(e)、在碱性条件下电流密度50 mA·cm−2的计时-电压曲线(f)
Figure 5. Polarization curves(a), tafel plots(b), nyquist plots(c), the capacitive current densities plotted against scan rate(d) of the FeOOH-Ni(OH)2 composites, Ni(OH)2 sheets and FeOOH sheets and polarization curves of FeOOH-Ni(OH)2 composites before and after 3 000 CV cycles(e), chronopotentiometric curve of FeOOH-Ni(OH)2 composite at a constant current density of 50 mA·cm−2(f)
-
[1] GRAY H B. Powering the planet with solar fuel[J]. Nature Chemistry,2009,1(1):7. doi: 10.1038/nchem.141 [2] SHI Y, ZHANG B. Correction: Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction[J]. Chemical Society Reviews,2016,45(6):1781. doi: 10.1039/C6CS90013E [3] MORALES C G, STERN L, HU X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution[J]. Chemical Society Reviews,2014,43(18):6555-6569. doi: 10.1039/C3CS60468C [4] YAN J, YAO Z, JARONIEC M, et al. Design of electrocatalysts for oxygen and hydrogen involving energy conversion reactions[J]. Chemical Society Reviews,2015,46(25):2060-2086. [5] LU W, LIU T, XIE L, et al. In situ derived CoB nanoarray: A high-efficiency and durable 3D bifunctional electrocatalyst for overall alkaline water splitting[J]. Small,2017,13(32):1700805. doi: 10.1002/smll.201700805 [6] XIA C, LIANG H, ZHU J, et al. Active edge sites engineering in nickel cobalt selenide solid solutions for highly efficient hydrogen evolution[J]. Advanced Energy Materials,2017,7(9):1602089. doi: 10.1002/aenm.201602089 [7] XU K, CHENG H, LIU L, et al. Promoting active species generation by electrochemical activation in alkaline media for efficient electrocatalytic oxygen evolution in neutral media[J]. Nano Letters,2017,17(1):578–583. [8] FENG Y F , XU C Y, HU E L et al. Construction of hierarchical FeP/Ni2P hollow nanospindles for efficient oxygen evolution[J]. Journal of Materials Chemistry A,2018,6:14103-14111. [9] YU L, XIA B Y, WANG X, et al. General formation of M-MoS<sub>3</sub> (M = Co, Ni) hollow structures with enhanced electrocatalytic activity for hydrogen evolution[J]. Advanced Materials,2016,28(1):92-97. doi: 10.1002/adma.201504024 [10] LI X, LI C, YOSHIDA A, et al. Facile fabrication of CuO microcube@Fe-Co<sub>3</sub>O<sub>4</sub> nanosheet array as a high-performance electrocatalyst for the oxygen evolution reaction[J]. Journal of Materials Chemistry A,2017,5(41):21740-21749. doi: 10.1039/C7TA05454H [11] ESPOSITO D V, HUNT S T, STOTTLEMYER A L, et al. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates[J]. Angewandte Chemie,2010,49(51):9859-9862. [12] LIANG H, GANDI A N, ANJUM D H, et al. Plasma-assisted synthesis of NiCoP for efficient overall water splitting[J]. Nano Letters,2016,16(12):7718-7725. doi: 10.1021/acs.nanolett.6b03803 [13] CHEN P, ZHOU T, ZHANG M, et al. 3D Nitrogen-anion-decorated nickel sulfides for highly efficient overall water splitting[J]. Advanced Materials,2017,29(30):1701584. doi: 10.1002/adma.201701584 [14] HAN L, DONG S, WANG E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction[J]. Advanced Materials,2016,28(42):9266-9291. doi: 10.1002/adma.201602270 [15] LI X M, HAO X G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects[J]. Journal of Materials Chemistry A,2016,4:11973-12000. doi: 10.1039/C6TA02334G [16] LUO W, JIANG C, LI Y, et al. Highly crystallized α-FeOOH for a stable and efficient oxygen evolution reaction[J]. Journal of Materials Chemistry A,2016(5):2021-2028. [17] CHI J, YU H M. QIN B W, et al. Vertically aligned FeOOH/NiFe layered double hydroxides electrode for highly efficient oxygen evolution reaction[J]. Acs Applied Materials <italic>&</italic> Interfaces,2017(9):464-471. [18] FENG J, YE S, XU H, et al. Design and synthesis of FeOOH/CeO<sub>2</sub> heterolayered nanotube electrocatalysts for the oxygen evolution reaction[J]. Advanced Materials,2016,28(23):4698-4703. doi: 10.1002/adma.201600054 [19] WANG H, CASALONGUE H S, LIANG Y, et al. Ni(OH)<sub>2</sub> nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials[J]. Journal of the American Chemical Society,2010,132(21):7472-7477. doi: 10.1021/ja102267j [20] JI J Y, ZHANG L L, JI H X, et al. Nanoporous Ni(OH)<sub>2</sub> thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor[J]. ACS Nano,2013,7(7):6237-6243. doi: 10.1021/nn4021955 -






 下载:
下载: