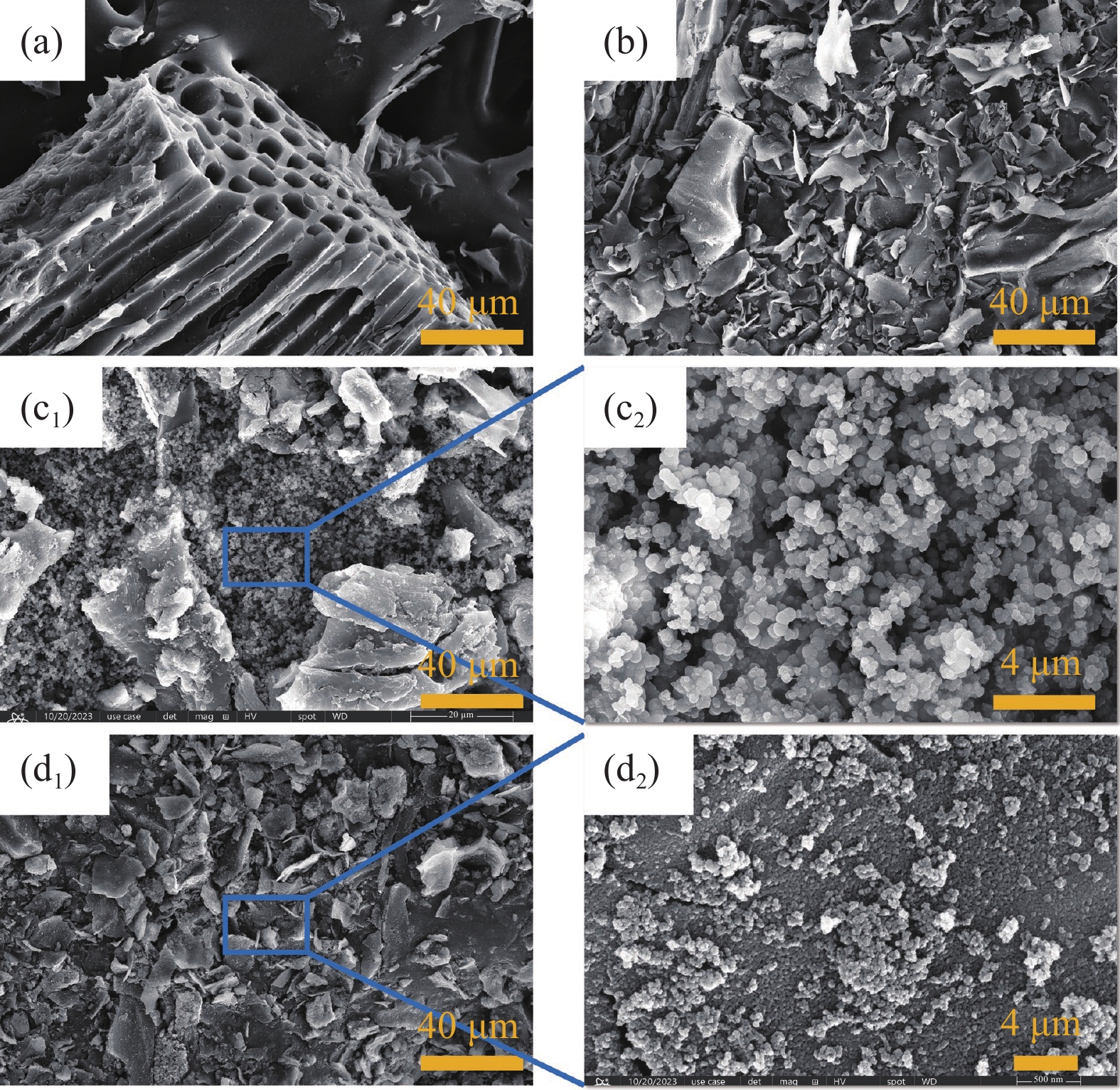
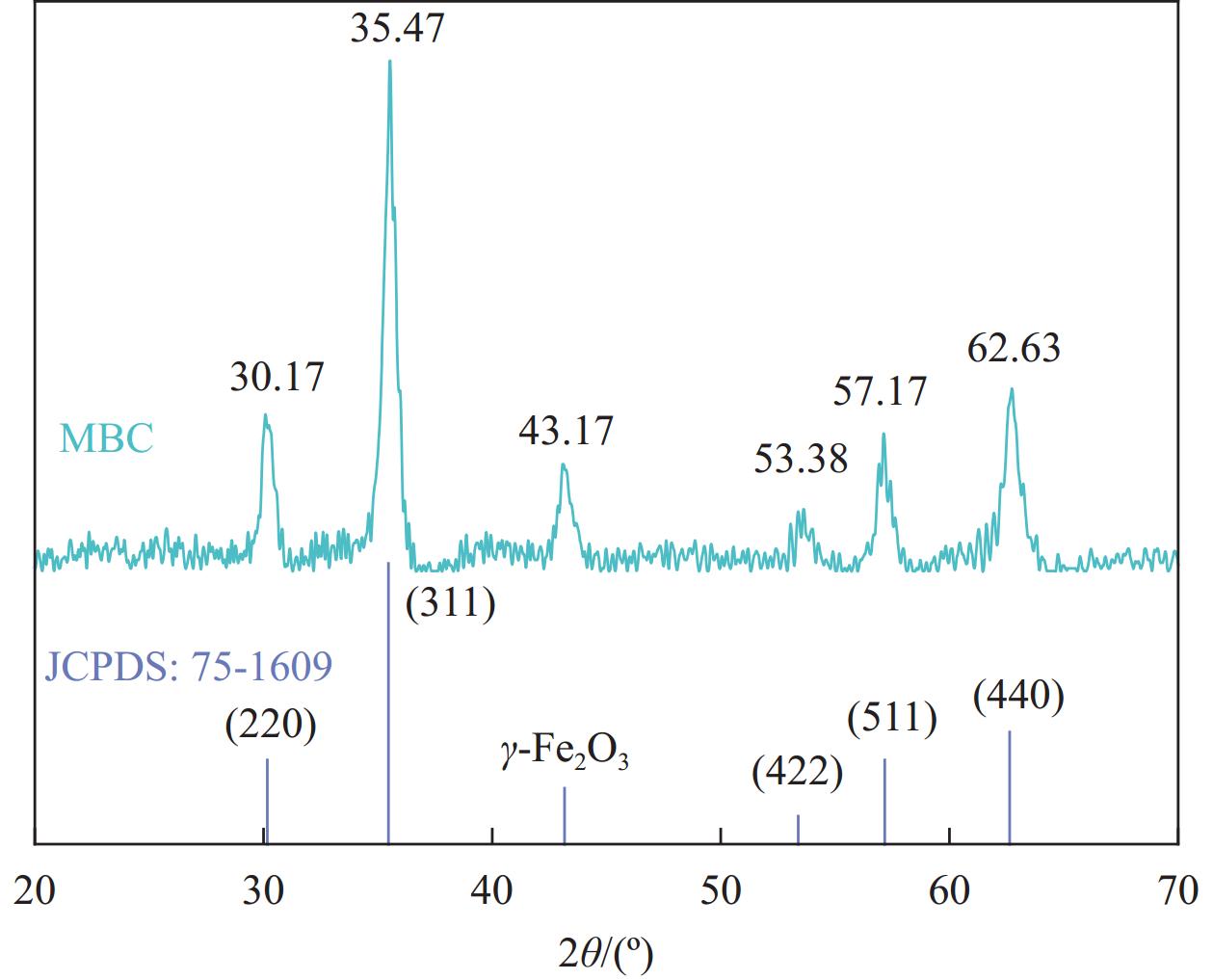
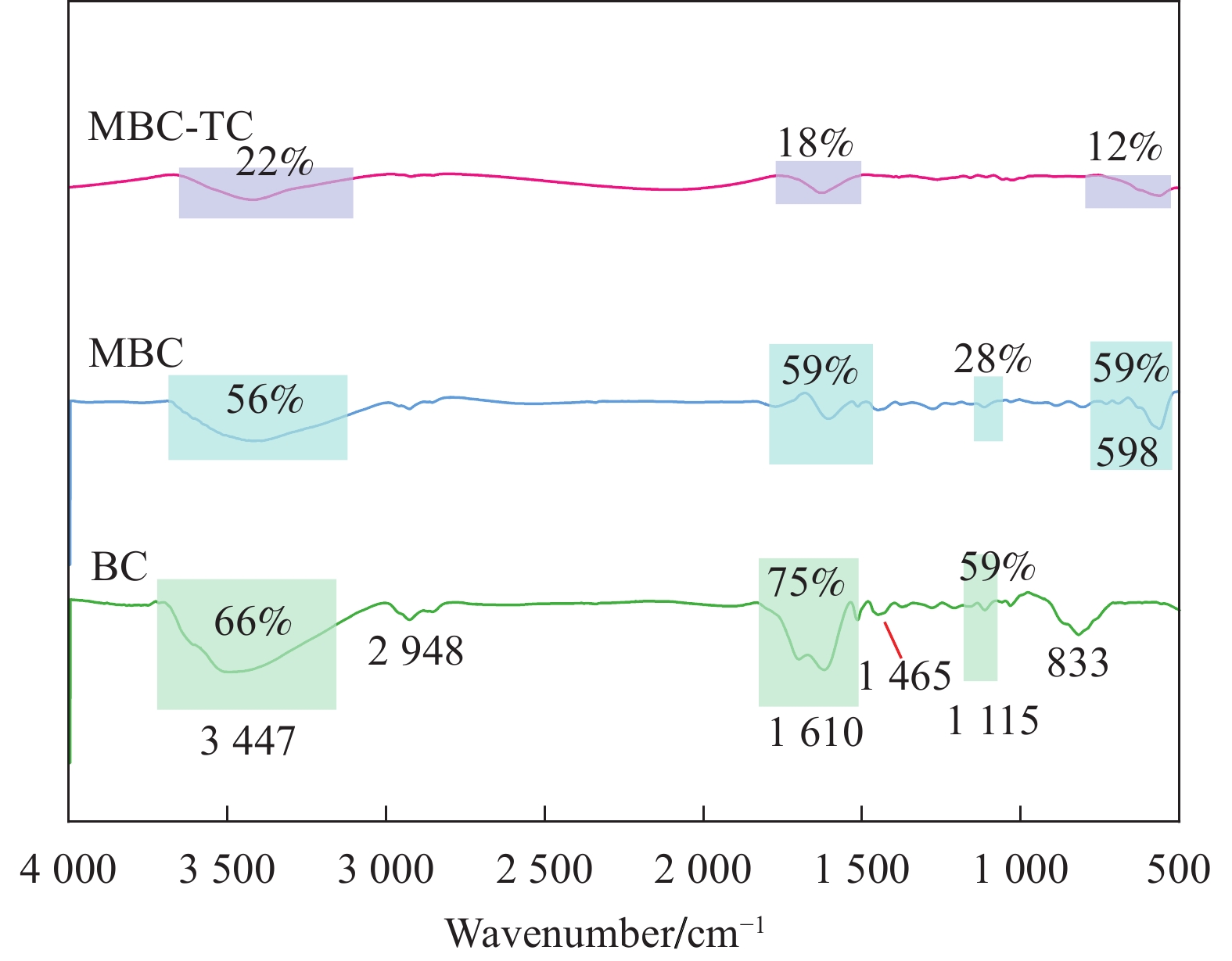
Preparation of magnetic coconut shell biochar and its removal of tetracycline from water
-
摘要:
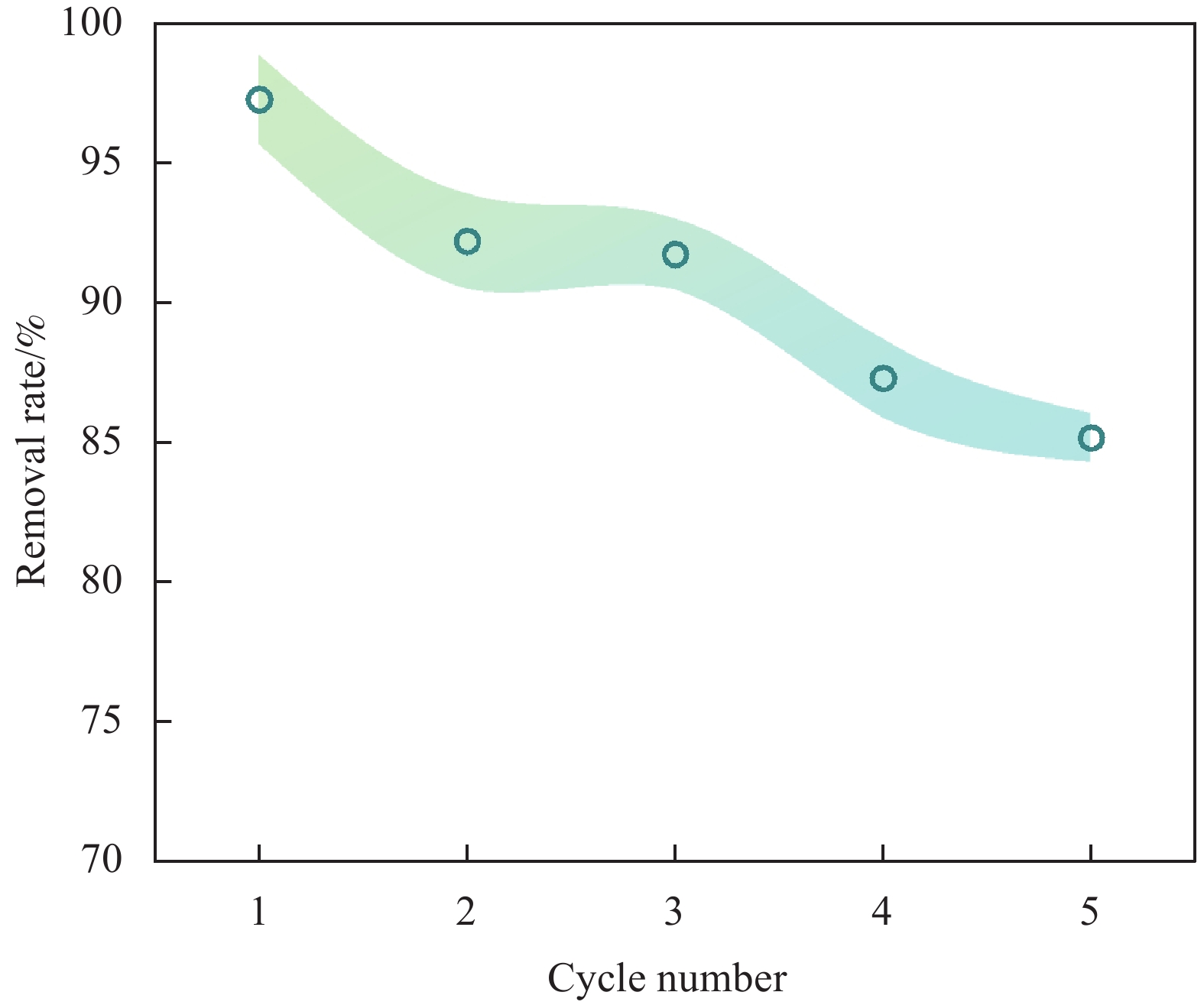
抗生素类药物在土壤和自然水体中的排放会危害到藻类及低等生物的生存,可能对生态环境带来持续性破坏。为此,本研究以四环素(TC)为研究对象,利用湛江本地特色农业废弃物椰壳为原料,以FeCl3和FeSO4为铁源,通过浸渍、共沉淀和水热合成等方法制备了磁性椰壳生物炭(MBC)。采用SEM、FTIR、XRD、BET、VSM、Zeta及XPS对样品表面形貌及化学结构进行了表征。研究了接触时间、初始TC浓度、pH值、反应温度及共存金属离子对MBC吸附TC的影响。结果表明,pH 4.0时,MBC对TC具有最佳吸附效果,且在24 h达到吸附饱和,饱和吸附量为36.40 mg·g−1,TC去除率达93.37%。高浓度Ca2+和Mg2+会显著降低了MBC对TC的吸附效果,而Na+的影响较小。吸附动力学符合拟二级动力学模型,等温吸附行为符合Langmuir模型,最大吸附容量在45℃时可达46.49 mg·g−1。温度升高有助于提高吸附容量,表明吸附过程为吸热反应。吸附机制主要涉及π-π相互作用、氢键和配位键的形成。MBC在重复利用性实验中表现出优良的稳定性,经过五次吸附-解吸循环后,去除率仍稳定在85.16%。表明磁性椰壳生物炭是一种高效、可重复利用的TC吸附材料,在养殖尾水、医药废水处理等领域具有较好的应用前景。
Abstract:The discharge of antibiotic drugs in soil and natural water bodies can endanger the survival of algae and lower organisms, and may bring continuous damage to the ecological environment. For this reason, in this study, magnetic coconut shell biochar (MBC) was prepared by impregnation, co-precipitation and hydrothermal synthesis using tetracycline (TC), a local speciality agricultural waste in Zhanjiang, as raw material, and FeCl3 and FeSO4 as iron sources. The surface morphology and chemical structure of the samples were characterised using SEM, FTIR, XRD, BET, VSM and XPS. The effects of contact time, initial TC concentration, pH, reaction temperature and different concentrations of metal ions on the adsorption of TC by MBC were investigated. The results showed that MBC had the best adsorption effect on TC at pH 4.0 and reached adsorption saturation at 24 h. The saturated adsorption amount was 36.40 mg·g−1, and the TC removal rate reached 93.37%. High concentrations of Ca2+ and Mg2+ significantly reduced the adsorption of TC by MBC, while the effect of Na+ was small. The adsorption kinetics conformed to the proposed secondary kinetic model, and the isothermal adsorption behaviour conformed to the Langmuir model, with the maximum adsorption capacity up to 46.49 mg·g−1 at 45℃. The increase in temperature contributed to the increase in the adsorption capacity, which indicated that the adsorption process was a heat-absorption reaction. The adsorption mechanism mainly involves π-π interactions, hydrogen bonding and ligand bonding formation.The MBC showed excellent stability in the reusability experiments, and the removal rate remained stable at 85.16% after five adsorption-desorption cycles. It is shown that magnetic coconut shell biochar is an efficient and reusable TC adsorbent material, which has a good application prospect in the fields of breeding tail water and pharmaceutical wastewater treatment.
-
Keywords:
- Magnetic /
- Coconut shell /
- Biochar /
- Adsorption /
- Tetracycline
-
海洋环境中氯离子含量高、穿透力及吸附力强,钢筋腐蚀问题尤为突出,是造成海洋混凝土基础设施性能劣化的首要因素。纤维增强复合材料 (Fiber Reinforced Polymer, FRP) 筋具有轻质高强、耐腐蚀与抗疲劳性能好、可设计性强的优点[1-6],采用FRP筋替换钢筋已成为海洋工程解决钢筋腐蚀问题的有效途径之一。
与钢筋混凝土结构类似,FRP筋混凝土结构应避免出现不利的剪切失效模式,而FRP箍筋是防止此类破坏的关键防线。传统FRP箍筋采用拉挤工艺制备,存在如下瓶颈问题:(1)受拉纤维不连续,采用搭接方式形成封闭箍筋,依靠搭接区段箍肢与混凝土的黏结来传递拉力,当保护层开裂或剥落后,搭接区段极易发生黏结锚固破坏[7-9],无法持续发挥FRP的高受拉强度;(2)箍筋在树脂热固前需要弯曲成型,使得角部弯曲段内层纤维产生大量褶皱,引起纤维的受力不均匀从而导致弯曲段受拉强度较低,仅为直线段受拉强度的30%~60%[10-12]。较低的受拉强度可能会造成FRP箍筋在受拉时过早断裂,大幅降低复材的利用效率。
为解决上述问题,课题组[9]提出了一种新型矩形截面连续纤维玻璃纤维增强树脂复合材料(GFRP)箍筋。制备工艺为在模具上逐层环向缠绕充分浸润树脂的连续纤维束,固化脱模后形成FRP管,并沿管的横截面方向进行切割,得到纤维连续、完全封闭、横截面为矩形的复材箍筋。相比于传统拉挤圆形截面FRP箍筋,连续纤维箍筋不存在搭接区段,因此避免了拉挤成型箍筋在箍肢间可能出现的黏结锚固破坏。连续纤维箍筋采用单层纤维束逐层缠绕的方式制备,在弯曲段不会发生纤维褶皱,弯曲段受拉强度将得到明显提高。需要指出的是,传统拉挤成型箍筋需要一定的搭接长度,当箍筋用量巨大时,搭接区段产生的纤维与树脂成本不容忽视,而连续纤维箍筋无需搭接区段,在经济性方面更具优势。
FRP箍筋弯曲段受拉强度是箍筋性能的关键控制参数,针对传统拉挤成型FRP箍筋,Ahmed等[12]采用ACI440.3 R-12[13]推荐的两种方法分别测试了CFRP与GFRP箍筋弯曲段的受拉强度。结果表明B.12方法由于没有考虑受拉时的反向摩擦
力,所测弯曲段受拉强度偏低,而B.5试验方法可以更准确地模拟箍筋在混凝土中的实际工作状态。Currier 等[14]考察了弯曲半径对拉挤箍筋弯曲段受拉强度的影响,当弯曲半径较小时,复材箍筋弯曲段受拉强度仅为直线段受拉强度的23%。Shehata 等[15]研究了FRP箍筋材料、弯曲半径和混凝土强度等因素对箍筋弯曲段受拉强度的影响,发现拉挤箍筋均在弯折处发生破坏,随着角部弯曲半径减小,FRP箍筋弯曲段受拉强度显著降低。El-Sayed等[11]测试了CFRP拉挤箍筋弯曲段受拉强度,同样表明箍筋弯曲段受拉强度仅为直线段的30%~59%,无法发挥CFRP的高受拉性能。李彪等[16]研究指出,由于拉挤箍筋弯曲段受拉强度较低,实际应用时需要很高的配箍用量,从而导致成本上升,限制了复材箍筋的实际应用。薛伟辰等[17]基于102个GFRP箍筋弯曲段受拉强度试验数据,提出了具有95%保证率的GFRP箍筋弯曲段受拉强度计算方法。为了克服拉挤复材箍筋弯曲段受拉强度较低的缺点,Lee等[18, 19] 通过手工缠绕CFRP条带的方式制作了连续纤维封闭箍筋,与拉挤箍筋对比发现,弯曲段受拉强度可达到直线段强度的60%~78%,大幅提升了材料利用效率。Spadea等[20]研究了手工缠绕CFRP箍筋的弯曲段受拉强度,根据试验结果修正了ACI规范,同时指出手工缠绕箍筋受拉性能离散性较大,不具备大规模应用的潜力。
此外,Zeng[21]等基于3D打印技术成功制备了FRP箍筋,通过优化制造参数使得FRP箍筋弯曲段纤维不会发生褶皱,但筋材的受拉强度和弹性模量都小于传统拉挤成型FRP筋材。目前3D打印复合材料力学性能远低于传统方法,有待进一步提高[22,23]。Jeong等[24]考察了CFRP网格箍筋混凝土构件的受剪性能,验证了网格箍筋的可行性。Dong等[25]研究了配置BFRP网格箍筋海水海砂混凝土短梁的剪切性能,发现网格箍筋的配置会导致混凝土有效面积减小,易使箍筋处产生竖向裂缝,改变拉压杆模型的传递机制,进而导致抗剪承载力的损失。原野等[26]初步开展了新型连续纤维箍筋增强混凝土梁的受剪性能试验,Dong等[9]研究了配置连续纤维GFRP箍筋混凝土柱的轴向受压性能,验证了连续纤维FRP箍筋的可行性。
目前尚缺乏关于这类连续纤维箍筋最优截面尺寸的研究工作,为进一步推动该类箍筋的实际应用,基于弯曲段受拉强度力学性能试验,提出力学性能最优的箍筋矩形截面尺寸是其应用的基础性工作。本文对9种不同截面尺寸的连续纤维GFRP箍筋开展弯曲段受拉性能试验,揭示箍筋截面尺寸对弯曲段受拉强度的影响规律,建立箍筋弯曲段受拉强度的预测模型,寻求矩形截面的最优宽厚比,为该类箍筋的实际应用提供指导依据。
1. 试验概况
1.1 试验装置
采用ACI440.3 R-12[13]建议的B.5试验方法测试箍筋的弯曲段受拉强度,试验方法如图1所示。箍筋内皮尺寸为250 mm×550 mm,一端锚固在混凝土块体中,另一端在混凝土块体中对箍筋直线段部分涂覆油脂,形成无黏结区段,避免箍筋直线段与混凝土的黏结对弯曲段受拉强度造成影响。在两个混凝土块体之间布置千斤顶和力传感器,试验前先进行预加载,比较两侧箍肢的应变数据,确保对中良好,正式加载时以5 kN为一级施加荷载,直至一侧箍筋弯曲段发生受拉破坏。
1.2 试验工况
为考察截面形状对箍筋弯曲段受拉性能的影响规律,依据直径为6 mm、8 mm、10 mm的圆形截面箍筋,采用等面积方法设计了9种矩形截面尺寸,如表1所示。作为对照,同时测试了直径为8 mm拉挤箍筋的弯曲段受拉强度。为减少强度离散性,每种工况设置了4个相同试件,共36个试件。试件命名包括两部分,第一部分字母表示箍筋形式,C表示连续纤维箍筋,P表示传统拉挤成型箍筋;第二部分表示矩形截面尺寸或直径。例如C-9×3表示宽度为9 mm,厚度为3 mm的矩形截面连续纤维箍筋。
表 1 箍筋弯曲段受拉性能试件工况与试验结果Table 1. Specimen design and test results of the bend testGroup Specimens Numbers N w/mm t/mm w/t Afv/mm2 ffb/MPa ffb,avg/MPa COV/% ffb/ffu υk/% Continuous fiber
GFRP rectangular
section stirrupsC-9×3 1 8 9 3 3 27.0 824.8 892.2 5.09 0.81 0.15 2 8 9 3 3 27.0 885.9 3 8 9 3 3 27.0 878.5 4 8 9 3 3 27.0 935.7 C-18×1.5 1 4 18 1.5 12 27.0 870.6 933.3 8.14 0.85 0.15 2 4 18 1.5 12 27.0 865.4 3 4 18 1.5 12 27.0 1009.1 4 4 18 1.5 12 27.0 988.3 C-9×6 1 16 9 6 1.5 54.0 765.8 726.5 3.96 0.66 0.14 2 16 9 6 1.5 54.0 700.5 3 16 9 6 1.5 54.0 728.9 4 16 9 6 1.5 54.0 710.5 C-18×3 1 8 18 3 6 54.0 727.2 784.6 6.68 0.72 0.15 2 8 18 3 6 54.0 796.4 3 8 18 3 6 54.0 763.9 4 8 18 3 6 54.0 850.8 C-12×4.5 1 12 12 4.5 2.7 54.0 742.5 760.6 2.90 0.69 0.15 2 12 12 4.5 2.7 54.0 763.2 3 12 12 4.5 2.7 54.0 790.6 4 12 12 4.5 2.7 54.0 745.8 C-14×3.75 1 10 14 3.75 3.7 52.5 778.3 773.3 2.92 0.71 0.15 2 10 14 3.75 3.7 52.5 800.5 3 10 14 3.75 3.7 52.5 768.2 4 10 14 3.75 3.7 52.5 746.1 C-14×4 1 10 14 4 3.5 56.0 787.5 768. 7 5.84 0.70 0.15 2 10 14 4 3.5 56.0 804.7 3 10 14 4 3.5 56.0 691.7 4 10 14 4 3.5 56.0 790.9 C-14×5 1 10 14 5 2.8 70.0 649.3 722.4 8.41 0.66 0.19 2 10 14 5 2.8 70.0 702.4 3 10 14 5 2.8 70.0 817.4 4 10 14 5 2.8 70.0 720.5 C-14×6 1 10 14 6 2.3 84.0 686.2 696.2 3.01 0.63 0.23 2 10 14 6 2.3 84.0 728.3 3 10 14 6 2.3 84.0 684.4 4 10 14 6 2.3 84.0 686.1 Pultruded stirrups P-8 1 — d=8 — 50.2 423.8 415.2 3.39 0.39 2.5 2 — d=8 — 50.2 398.6 3 — d=8 — 50.2 406.4 4 — d=8 — 50.2 392.0 Notes: N is the number of fiber winding layers of stirrups; w is the width of stirrups; t is the thickness of stirrups; d is the diameter of pultruded stirrups; Afv is the cross-sectional area of stirrups; ffb is the bend strength of stirrups; ffb,avg is the average value of the bend strength; COV is the coefficient of variation of the bend strength; υk is the volume fraction of the curling fibers. 1.3 材料力学性能
依据标准圆柱体受压试验,测得混凝土的轴心受压强度平均值为48.2 MPa。GFRP 箍筋中的增强材料采用 ECT 玻璃纤维,基体材料采用热固型环氧树脂。依据ASTM-D3039/3039M-00[27]方法测试矩形截面箍筋直线段的受拉强度,对于拉挤成型箍筋,依据纤维增强复合材料筋基本力学性能试验方法(GB/T30022-2013)[28]测试其直线段受拉强度。拉挤成型GFRP箍筋轴心受拉强度平均值为
1064.4 MPa,弹性模量为50.4 GPa。对于矩形截面GFRP 箍筋,不同截面尺寸试件轴心受拉强度差异较小,将直线段受拉试验中9种截面尺寸试件轴心受拉强度的平均值作为矩形截面GFRP箍筋直线段的受拉强度,连续纤维GFRP箍筋的轴心受拉强度平均值为1098.1 MPa,弹性模量为55 GPa。2. 结果分析
2.1 破坏模式
所有试件的破坏均发生在箍筋弯曲段,试验结束后未发现混凝土块体出现裂缝。图2给出了试件P-8、C-18×1.5、C-9×6的典型破坏模式。由拉挤箍筋P-8试件断裂面可知弯曲段外层纤维破坏严重,直线段基本保持完好。对于矩形截面连续纤维箍筋,试件C-9×6的箍筋厚度略小于拉挤箍筋直径,断裂面沿厚度方向平整,沿宽度方向的破断区域也相对集中,在直线段与弯曲段交界处发生局部纤维分层。试件C-18×1.5的宽厚比较大,在弯曲段破断前,箍筋直线段发生明显的纵向劈裂现象,且截面宽厚比越大,直线段纵向劈裂越明显,为避免实际应用中出现纤维纵向劈裂的现象,应限制矩形截面箍筋的宽厚比。
2.2 箍筋形式的影响
弯曲段受拉试验结果如表1所示,表中列出了各组试件受拉强度的平均值与变异系数,变异系数在2.90%~8.41%之间变化,表明试验结果的离散性较小。为了便于比较各种截面箍筋弯曲段受拉强度的利用效率,考察了弯曲段受拉强度ffb与直线段受拉强度ffu的比值,如图3所示。结果表明,连续纤维箍筋的弯曲段受拉强度相比于拉挤箍筋有显著提高,拉挤成型箍筋的ffb/ffu约为0.39,而连续纤维箍筋的ffb/ffu可达0.63~0.85,比前者提高了70%~118%,提高的原因在于纤维逐层缠绕缓解了弯曲段的纤维褶皱与蜷曲。
Lee[19]提出了箍筋弯曲段蜷曲纤维体积分数υk的计算方法,对于圆形截面的拉挤箍筋,其计算公式为
υk=13π⋅(10.5+r/d) (1) 对于矩形截面FRP箍筋,计算公式为:
υk=18N⋅(10.5+r/t) (2) 式中,r为箍筋的弯曲半径,其余符号与表1中的含义相同,各试件的蜷曲纤维体积分数汇总于表1。对连续纤维GFRP箍筋而言,当弯曲半径r相同时,截面宽度与厚度对于弯曲段蜷曲纤维体积分数的影响较小,所有连续纤维箍筋的蜷曲纤维体积分数介于0.14%~0.23%之间,而拉挤成型GFRP箍筋的蜷曲纤维体积分数达到了2.5%,是前者的10倍以上,这说明逐层环向缠绕的生产工艺有效减少了箍筋弯曲段的纤维蜷曲现象。此外,连续纤维GFRP箍筋为矩形截面形式,可缓解箍筋弯曲段的应力集中。当箍筋截面面积相同时,矩形截面连续纤维箍筋的厚度小于传统圆形截面拉挤箍筋的直径,弯曲段内外层纤维应力分布不均匀现象会随厚度的减小而缓解。
2.3 箍筋截面尺寸的影响
图4中的曲线为弯曲段受拉强度预测模型的分析结果,将在预测模型部分详细讨论。连续纤维GFRP箍筋截面厚度t对弯曲段受拉强度与直线段受拉强度之比ffb/ffu的影响如图4 (a)所示,由图中数据点可知,当截面宽度相同时,ffb/ffu随着截面厚度的增大而减小。当截面宽度为9 mm时,t由3 mm增加到6 mm,ffb/ffu减小了18.5%;当截面宽度为18 mm时,t由1.5 mm增加到3 mm,ffb/ffu减小了15.2%;当截面宽度为14 mm时,t由3.75 mm增加到6 mm,ffb/ffu减小了11.2%,且t由4 mm增到5 mm与t由5 mm增加到6 mm,ffb/ffu分别减小了5.7%和4.5%,下降幅度减缓。反之,随着截面厚度的减小,ffb/ffu呈增长趋势,且增幅逐渐增大。截面厚度影响弯曲段受拉强度的主要原因是随着截面厚度的增加,弯曲段内外层纤维应力分布不均匀加剧,进而导致弯曲段受拉强度降低。截面厚度对于弯曲段受拉性能的影响也体现在图2所示的弯曲段受拉破坏模式中,当截面厚度为6 mm时,由于弯曲段内外层纤维的应力分布不均匀,试件C-9×6的纤维拉断与分层主要集中在箍筋弯曲段,而当截面厚度为1.5 mm时,内外层纤维的应力差异较小,试件C-18×1.5弯曲段拉断时在临近的直线段发生了纤维劈裂破坏,说明当箍筋厚度较小时参与受拉的纤维更多。
图4(b)对比了当截面厚度相同时,箍筋宽度w对弯曲段受拉强度与直线段受拉强度之比ffb/ffu的影响。由数据点可知,当截面厚度为3 mm时,箍筋的截面宽度由9 mm增大到18 mm使得ffb/ffu下降了11.1%;当截面厚度为6 mm时,截面宽度由9 mm增大到14 mm使得ffb/ffu下降了1.5%,这表明连续纤维GFRP箍筋弯曲段受拉强度随着截面宽度的增加呈降低趋势。在连续纤维缠绕过程中,纤维丝束在平行于模具的轴线方向往复移动,不可避免地存在纤维丝束分布不均,气泡孔洞或纤维与树脂脱粘等材料缺陷将随着箍筋截面宽度的增大而增加[29],这是导致连续纤维GFRP箍筋弯曲段受拉强度降低的原因。
上述结果表明,弯曲段受拉强度与直线段受拉强度之比ffb/ffu随箍筋截面厚度减小而增大,随截面宽度增加而减小。因此,还应研究当连续纤维箍筋截面面积(Afv=w×t)一定时,截面的宽厚比w/t对ffb/ffu的影响规律,如图4(c)所示。对于截面面积为52.50 mm2的矩形截面GFRP箍筋(与直径8 mm圆型箍筋面积相当),在面积确定的前提下,截面宽度增加,则厚度必然减小,ffb/ffu受到宽度和厚度的综合影响。由图4(c)可知,宽厚比的增加,本质上是宽度增加(同时厚度减小)造成的。在宽厚比增长初期,厚度减小导致ffb/ffu增大的趋势将占据主导地位,ffb/ffu总体上呈增长趋势;随着宽度的不断增大,宽度增大导致ffb/ffu降低的趋势越来越明显。可以预见,随着宽厚比的不断增大,宽度增大导致ffb/ffu降低趋势将逐渐占据主导地位,ffb/ffu总体上会表现出下降趋势,故存在最优宽厚比的情况。
3. 弯曲段受拉强度预测模型
目前关于矩形截面连续纤维FRP箍筋的试验研究还很少,基于本文36个试验数据,首先讨论拉挤箍筋弯曲段受拉强度预测模型的适用性,相关模型如下:
(1) ACI440.1R-15[30]按下式计算FRP箍筋弯曲段受拉强度:
ffbffu=0.05rdfe+0.3dfe=√4Afπ (3) 式中r为箍筋的弯曲半径,d为FRP箍筋直径。对于矩形截面箍筋,采用等效直径dfe代替d,等效直径由等效截面面积确定,Af是FRP箍筋的横截面面积。
(2) Lee等[19]基于试验数据,提出了FRP箍筋弯曲段受拉强度预测模型,见下式:
ffbffu=0.02rdfi+0.47dfi=2t√π (4) Lee将矩形截面视为多个圆形截面的集合,将截面厚度t转化成等效直径dfi。
(3) Imjai等[31]基于Tsai-Hill破坏准则提出了FRP箍筋弯曲段受拉强度预测模型,见下式:
ffbffu=1√1+ξ(1r)+[ξ(1r)]2β2 (5) 采用截面因子ξ来考虑截面形状的影响,对于矩形截面,ξ为截面厚度。β为 FRP 筋纵向受拉强度与横向受压强度之比,其取值建议采用7.5。
(4) Spadea等[20]基于试验结果修正ACI模型,得到如下的弯曲段受拉强度预测模型:
ffbffu=0.03rdfe+0.35 (6) 各模型预测值与本文试验值的对比结果如图5(a)、5(b)、5(c)、5(d)所示,各模型的均方根误差(RMSE)、平均值(Mean)、标准偏差(SD)和变异系数(COV)如表2所示。Imjai模型单独考虑了矩形截面情况,与试验结果的拟合精度较其它模型更好,但变异系数较大,表明模型的离散程度依然较高。其次是Lee模型和Spadea模型,拟合精度略小;ACI模型拟合程度最为保守。
表 2 基于本文试验的弯曲段受拉强度预测模型的比较Table 2. Comparison of all prediction models for bend strength based on the tests in this paperPaper ACI Lee Imjai Spada RMSE 63.38 255.92 123.59 91.98 163.78 Mean 0.99 1.45 1.15 1.05 1.25 SD 0.08 0.12 0.11 0.13 0.15 COV/% 8.15 8.53 9.28 12.11 12.09 Notes: RMSE is Root Mean Squared Error; SD is Standard Deviation; COV is Coefficient of Variation. 需要指出的是,Lee模型与Spadea模型是基于手工缠绕的矩形截面FRP条带箍筋提出的,这种箍筋的弯曲段与本文连续纤维箍筋在形式上更为接近。由图5(b)、5(d)可知,模型预测值大多低于本文试验值,二者的预测平均值与试验平均值相比分别小了12.3%和17.6%,说明手工缠绕GFRP箍筋的弯曲段受拉强度低于本文连续纤维GFRP箍筋,也证明手工制作方式会大幅降低复材性能,不利于FRP材料的推广利用。
上述模型大多关注弯曲半径与箍筋直径的比值r/d,对于矩形截面箍筋,也多是按照面积来等效成圆形截面直径。试验结果表明了矩形截面的宽厚比w/t是箍筋弯曲段受拉强度的重要影响参数。基于本文试验结果,同时考虑了矩形截面宽厚比与弯曲半径的影响,建立了矩形截面连续纤维GFRP箍筋ffb/ffu的预测模型:
ffbffu=−0.01(wt)1.45+0.038rt+0.482 (7) 预测模型弯曲段受拉强度预测值与试验值的对比如图5(e)所示。可以看出,本文模型预测精度比其它现有模型精度更高,误差基本处于10%范围内,均方根误差和变异系数最小,相比其它模型离散程度更小。与文献[32]中19个手工缠绕GFRP箍筋试验数据进行比较可知,本文箍筋的弯曲段受拉强度高于手工缠绕箍筋,进一步说明了生产线缠绕工艺制作方式有利于复材箍筋力学性能的发挥。值得注意的是,式(7)中w/t与r/t不是完全独立的,二者都与箍筋厚度密切相关。
如前所述,图4中将模型预测曲线与试验结果进行了比较。由图4(a)曲线可知,当箍筋截面宽度一定时,ffb/ffu随箍筋厚度的减小而增大,且增长幅度逐渐增大,与试验数据吻合较好。由图4(b) 可以看出,当截面厚度一定时,ffb/ffu随截面宽度的增大而减小,且减小幅度逐渐增加。由图4(c)可知,当截面面积一定时, ffb/ffu与截面宽厚比呈抛物线关系,随着宽厚比的增加,ffb/ffu先增大后减小,存在最优宽厚比,验证了之前试验结果分析时的预想,截面宽度与厚度将耦合影响弯曲段受拉强度,同时说明本文强度预测模型中考虑宽厚比w/t的正确性。
由于直径为6 mm、8 mm和10 mm的传统拉挤成型箍筋在工程中应用较多,选取相近截面面积的矩形截面FRP箍筋,依据本文模型预测得到的箍筋在不同弯曲半径下的宽厚比与ffb/ffu的关系,如图6所示。本文模型考虑的最小宽厚比为1,即箍筋截面为正方形。由于试验中宽厚比为12的C-18×1.5试件出现了明显的纤维纵向劈裂破坏,为避免此情况,本文模型宽厚比最大值要求小于12。随着弯曲半径r的增大,弯曲段受拉强度的利用率不断提高,与拉挤箍筋研究结论一致。各曲线峰值点处对应的横坐标即为最优宽厚比,不同弯曲半径下的最优宽厚比与ffb/ffu近似成线性关系,如图6中公式所示。确定了最优宽厚比,则可得到连续纤维FRP箍筋的弯曲段受拉强度。
由图6可知,弯曲半径r影响最优宽厚比的取值,考虑到弯曲半径过小将导致FRP箍筋强度利用率降低,过大则使得弯曲段处的纵筋布置困难,且与纵筋不协调,因此弯曲半径考虑在10~40 mm之间变化。图7给出了基于本文模型的弯曲半径r与最优宽厚比的关系,两者近似成线性关系,依据弯曲半径,可以得到箍筋的最优宽厚比。
实际应用时,首先确定箍筋的截面面积,然后依据构造经验确定弯曲半径。由图7中的公式计算相应弯曲半径所对应的最优宽厚比w/t,结合截面面积(Afv=w×t) 即可确定出矩形截面箍筋的截面尺寸,同时依据图6中的公式,预测出矩形截面连续纤维FRP箍筋的弯曲段受拉强度与直线段受拉强度之比,从而为结构设计提供依据。例如,采用直径8 mm的拉挤箍筋面积 (Afv=50.24 mm2)为矩形箍筋截面面积,选择适中的弯曲半径为35 mm。依据图7中的公式,可知最优宽厚比(w/t) 为6.98,依据w×t=50.24 mm2,得到箍筋截面宽度为18.8 mm,厚度为2.7 mm,再根据图6 (b)中公式可得ffb/ffu为0.81,即弯曲段受拉强度为直线段受拉强度的81%,最后根据直线段受拉强度试验值即可确定箍筋弯曲段的受拉强度值。
由图6可以看出,弯曲半径在10~40 mm之间变化,当宽厚比由1增长到12时,最优宽厚比大约在2~8之间取值。为保证FRP材料的充分利用,弯曲段受拉强度与直线段受拉强度之比ffb/ffu建议大于0.7,对于面积为28.26 mm2(φ6)的矩形截面连续纤维FRP箍筋,弯曲半径建议在25~30 mm范围内取值;对于截面面积为50.24 mm2(φ 8)的箍筋,弯曲半径建议在25~40 mm范围内取值;对于截面面积为78.50 mm2(φ10)的矩形截面FRP箍筋,弯曲半径建议在35~40 mm范围内取值。
4. 结 论
(1) 连续纤维玻璃纤维增强树脂复合材料(GFRP)箍筋弯曲段受拉强度与直线段受拉强度之比可达到0.66~0.85,传统拉挤成型箍筋仅为0.39,连续纤维箍筋可显著提升复材的利用效率。
(2) 连续纤维GFRP箍筋弯曲段受拉强度与直线段受拉强度之比随截面厚度减小而增大,随截面宽度增大而减小。当截面面积一定时,截面宽度与厚度耦合影响弯曲段受拉强度,存在最优宽厚比的情况。
(3) 建立了连续纤维GFRP箍筋弯曲段受拉强度的预测模型,与试验结果吻合较好。建议矩形截面连续纤维GFRP箍筋的弯曲半径在25~40 mm范围内取值,并建议其最优宽厚比在2~8范围内取值。
-
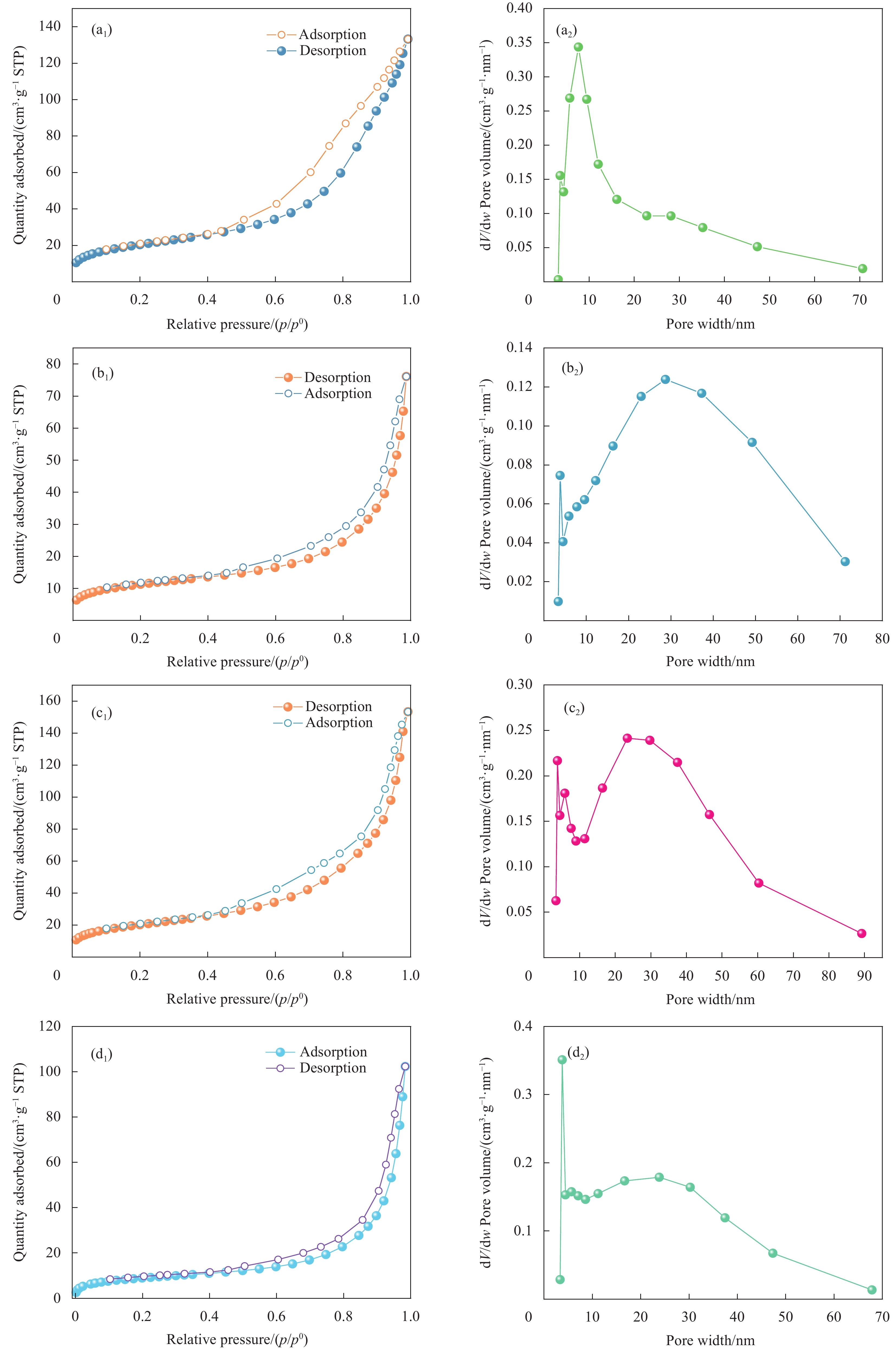
图 4 N2吸附-脱附曲线:(a1) BC;(b1) 吸附TC前的MBC;(c1) 吸附TC后的MBC;(d1) 吸附TC再生后的MBC与孔径分布图:(a2) BC;(b2) 吸附TC前的MBC;(c2) 吸附TC后的MBC;(d2) 吸附TC再生后的MBC
Figure 4. N2 adsorption-desorption curves: (a1) BC; (b1) MBC before adsorption of TC; (c1) MBC after adsorption of TC; (d1) MBC after regeneration. Pore size distribution: (a2) BC; (b2) MBC before adsorption of TC; (c2) MBC after adsorption of TC; (d2) MBC after TC regeneration.
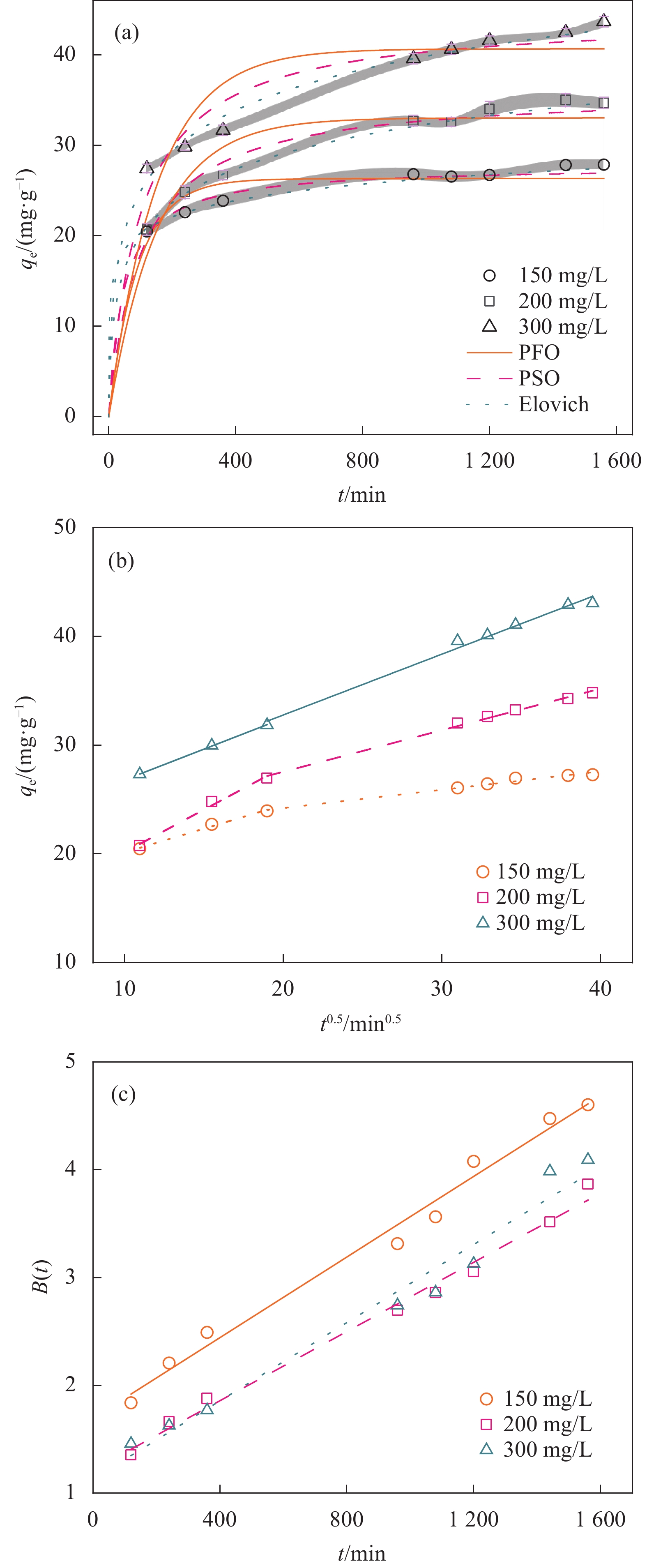
表 1 MBC吸附TC的动力学参数
Table 1 Kinetic parameters of TC adsorption onto MBC
Adsorption kinetics Parameters 150/(mg·L−1) 200/(mg·L−1) 300/(mg·L−1) Values R2 Values R2 Values R2 Experimental qe/(mg·g−1) 27.3 — 34.8 — 43.0 — PFO model k1/(min−1) 0.0109 0.981 0.00645 0.972 0.00655 0.947 qe/(mg·g−1) 26.3 33.0 40.8 PSO model k2/(g·mg−1·min−1) 7.67×10−4 0.998 2.74×10−4 0.994 2.31×10−4 0.981 qe/(mg·g−1) 27.7 36.0 44.3 Elovich model α/ (g·mg−1·min−2) 61.7 0.992 2.12 0.999 2.87 0.982 β/ (mg·g−1·min−1) 0.383 0.185 0.153 IPD model ki1/(mg−1·g−1min−0.5) 0.437 0.985 0.781 0.982 0.566 0.999 C1/(mg·g−1) 15.8 12.4 21.1 ki2/(mg−1·g−1min−0.5) 0.169 0.978 0.382 0.993 0.559 0.986 C2/(mg·g−1) 20.8 19.9 21.6 Notes: qe: Amount of TC adsorbate at equilibrium k1: Rate constant of the pseudo-first-order adsorption
k2: Rate constant of the pseudo-second-order adsorption α: Initial adsorption rate of the Elovich model
β: Desorption constant of the Elovich model ki1: Intraparticle diffusion rate constant at the first stage
C1: Boundary layer thickness in the intraparticle diffusion model at the first stage
ki2: Intraparticle diffusion rate constant at the second stage R2: Correlation coefficient
C2: Boundary layer thickness in the intraparticle diffusion model at the second stage表 2 MBC吸附TC的等温线参数
Table 2 Isotherm parameters of TC adsorption onto MBC at different temperatures
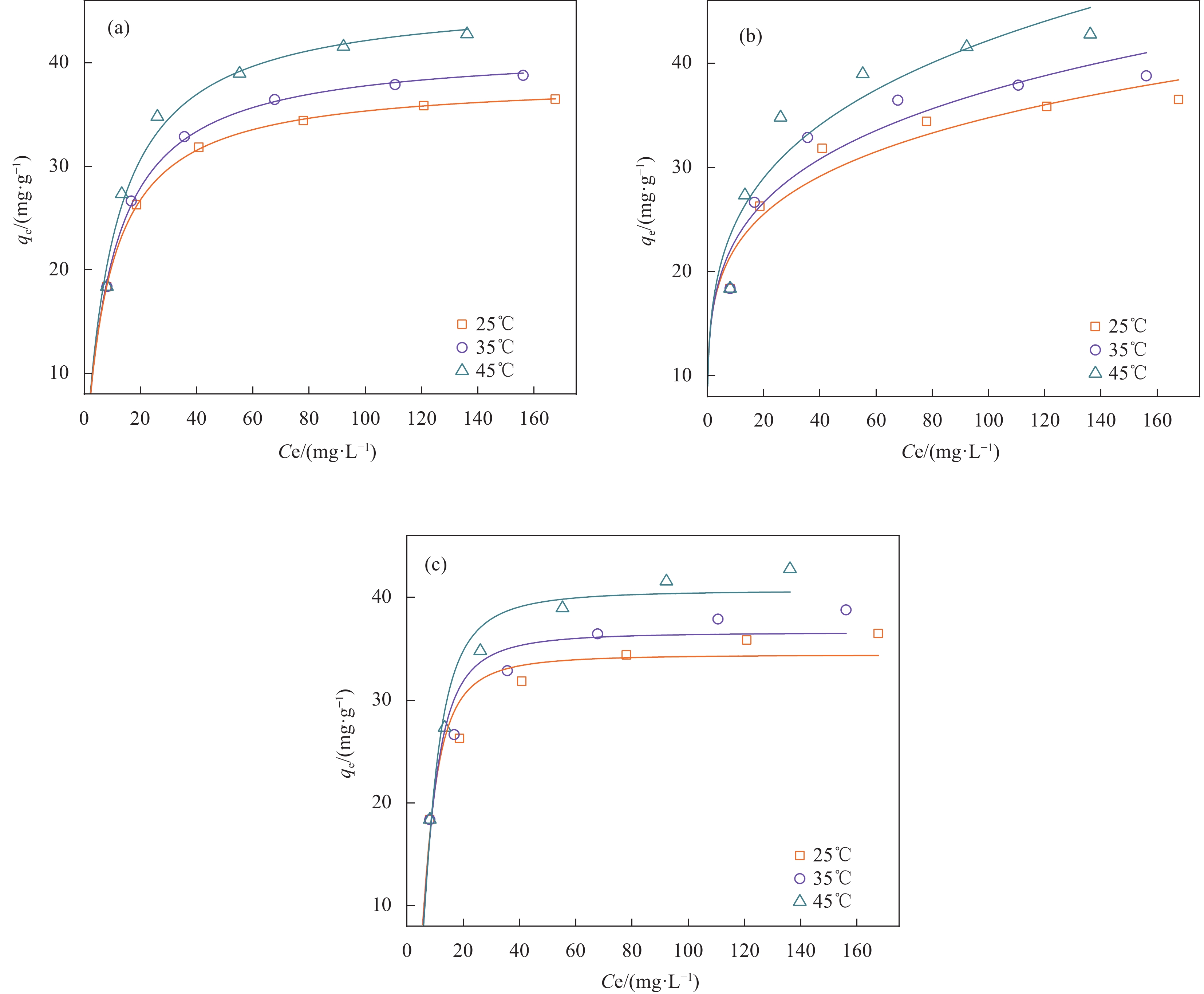
Adsorption isotherm Parameters 25℃ 35℃ 45℃ Values R2 Values R2 Values R2 Langmuir qm/(mg·g−1) 38.42 0.9999 41.42 0.9994 46.49 0.9933 KL/(L·mg−1) 0.1148 0.1036 0.0967 RL 0.5183 0.5438 0.5609 Freundlich n 5.194 0.9743 4.760 0.9683 4.286 0.9548 KF/[mg·g−1/(L·mg−1)n] 14.33 14.19 14.40 Dubinin-Radushkevich qm/(mg·g−1) 34.42 0.9721 36.58 0.9727 40.67 0.9853 KDR/(mol2·kJ−2) 8.455 8.857 9.328 E/(kJ·mol−1) 0.2432 0.2376 0.2315 Notes: Langmuir Model: qm: Theoretical maximum adsorption capacity, KL: Langmuir constant, RL: Langmuir separation factor; Freundlich Model: n: Freundlich intensity parameter, KF: Freundlich constant; Dubinin-Radushkevich Model: qm: Theoretical maximum adsorption capacity, KDR: Dubinin-Radushkevich constant, E: Mean free energy of adsorption. 表 3 MBC吸附TC的热力学参数
Table 3 Thermodynamic parameters of MBC adsorbed TC
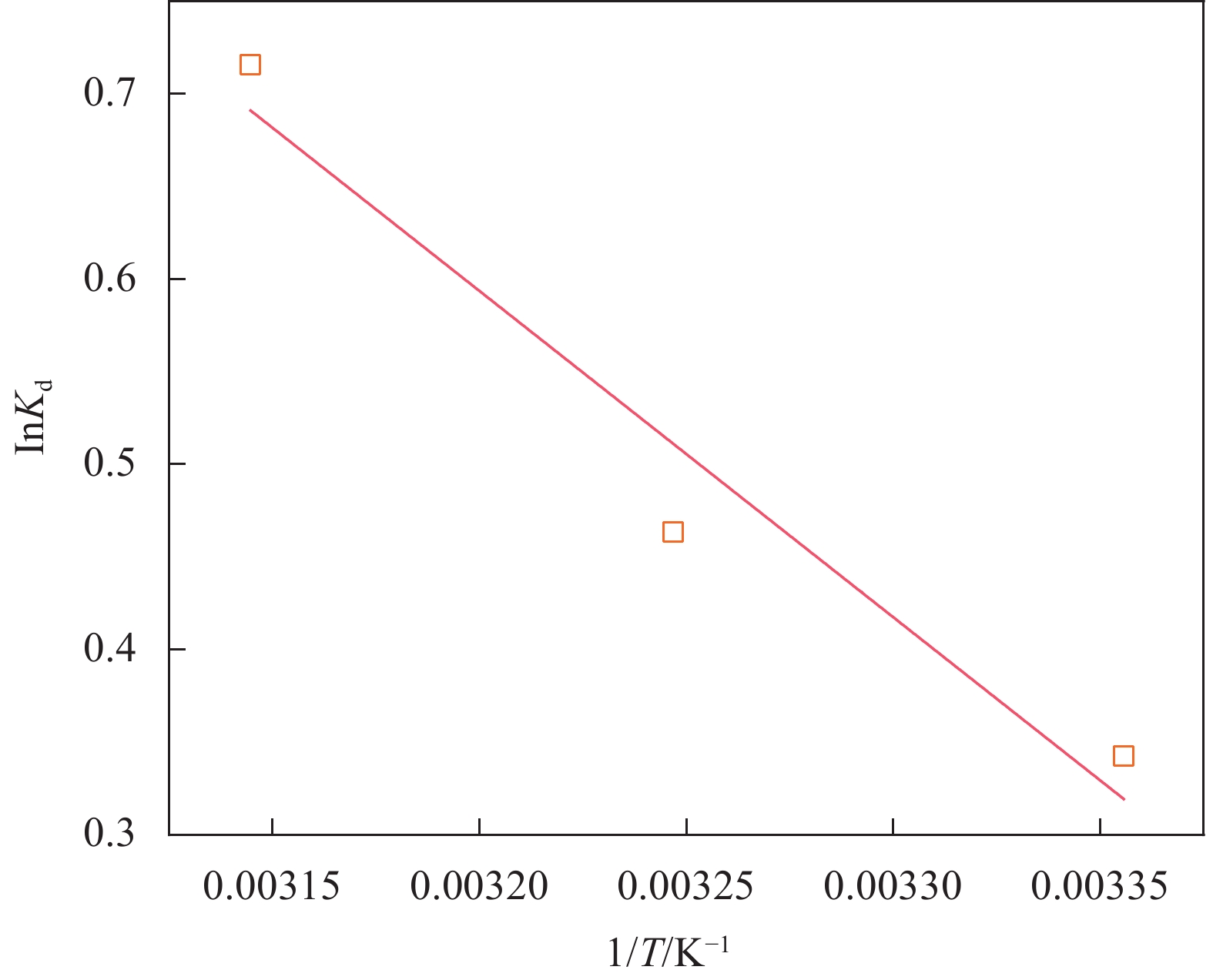
Temperature/K Kd ΔG/
(kJ·mol−1)ΔH/
(kJ·mol−1)ΔS
(J·K−1·mol−1)298 1.408 −0.85 14.65 51.80 308 1.589 −1.19 318 2.046 −1.89 Notes: Kd: Equilibrium constant of adsorption, ΔG: Gibbs free energy change, ΔH: Enthalpy change, ΔS: Entropy change -
[1] MOUSSAVI G, ALAHABADI A, YAGHMAEIAN K, et al. Preparation, characterization and adsorption potential of the NHCl-induced activated carbon for the removal of amoxicillin antibiotic from water[J]. Chemical Engineering Journal, 2013, 217: 119-128. DOI: 10.1016/j.cej.2012.11.069
[2] SECONDES M F N, NADDEO V, BELGIORNO V, et al. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation[J]. Journal of Hazardous Materials, 2014, 264: 342-349. DOI: 10.1016/j.jhazmat.2013.11.039
[3] SAMARGHANDI M R, AL-MUSAWI T J, MOHSENI-BANDPI A, et al. Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles[J]. Journal of Molecular Liquids, 2015, 211: 431-441. DOI: 10.1016/j.molliq.2015.06.067
[4] MIAO J, WANG F, CHEN Y, et al. The adsorption performance of tetracyclines on magnetic graphene oxide: A novel antibiotics absorbent[J]. Applied Surface Science, 2019, 475: 549-558. DOI: 10.1016/j.apsusc.2019.01.036
[5] IMWENE K O, NGUMBA E, KAIRIGO P K. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review[J]. Journal of Environmental Management, 2022, 322: 116065. DOI: 10.1016/j.jenvman.2022.116065
[6] KRASUCKA P, PAN B, SIK Ok Y, et al. Engineered biochar – A sustainable solution for the removal of antibiotics from water[J]. Chemical Engineering Journal, 2021, 405: 126926. DOI: 10.1016/j.cej.2020.126926
[7] Syranidou E, Kalogerakis N. Interaction of microplastics, antibiotics and antibiotic resistant genes within WWTPs[J]. Science of The Total Environment, 2022, 804: 150141. DOI: 10.1016/j.scitotenv.2021.150141
[8] XIONG W, ZENG G, YANG Z, et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent[J]. Science of The Total Environment, 2018, 627: 235-244. DOI: 10.1016/j.scitotenv.2018.01.249
[9] YANG H, YU H, WANG J, et al. Magnetic porous biochar as a renewable and highly effective adsorbent for the removal of tetracycline hydrochloride in water[J]. Environmental Science and Pollution Research, 2021, 28(43): 61513-61525. DOI: 10.1007/s11356-021-15124-6
[10] NGOC TRI N, HO D Q, TRAN GIA BAO N, et al. The adsorption of tetracycline, ciprofloxacin on reduced graphene oxide surfaces: Role of intermolecular interaction[J]. Chemical Physics, 2024, 579: 112207. DOI: 10.1016/j.chemphys.2024.112207
[11] WEI J, LIU Y, LI J, et al. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar[J]. Chemosphere, 2019, 236: 124254. DOI: 10.1016/j.chemosphere.2019.06.224
[12] GAO F, XU Z, DAI Y. Removal of tetracycline from wastewater using magnetic biochar: A comparative study of performance based on the preparation method[J]. Environmental Technology & Innovation, 2021, 24: 101916.
[13] ZHAO W, CUI Y, SUN X, et al. Corn stover biochar increased edible safety of spinach by reducing the migration of mercury from soil to spinach[J]. Science of The Total Environment, 2021, 758: 143883. DOI: 10.1016/j.scitotenv.2020.143883
[14] WANG Y, JIANG B, WANG L, et al. Hierarchically structured two-dimensional magnetic microporous biochar derived from hazelnut shell toward effective removal of p-arsanilic acid[J]. Applied Surface Science, 2021, 540: 148372. DOI: 10.1016/j.apsusc.2020.148372
[15] LIU L, FANG W, YUAN M, et al. Metolachlor-adsorption on the walnut shell biochar modified by the fulvic acid and citric acid in water[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106238. DOI: 10.1016/j.jece.2021.106238
[16] PANDEY D, SINGH S, DUTTA K, et al. Biobased Nanotechnology for Green Applications [M]. Cham: Springer Nature Switzerland AG, 2021: 619-639.
[17] YAO Y, GAO B, INYANG M, et al. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential[J]. Bioresource Technology, 2011, 102(10): 6273-6278. DOI: 10.1016/j.biortech.2011.03.006
[18] BOMBUWALA DEWAGE N, LIYANAGE A S, SMITH Q, et al. Fast aniline and nitrobenzene remediation from water on magnetized and nonmagnetized Douglas fir biochar[J]. Chemosphere, 2019, 225: 943-953. DOI: 10.1016/j.chemosphere.2019.03.050
[19] ZAHEDIFAR M, SEYEDI N, SHAFIEI S, et al. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb(ΙΙ) and Cd(ΙΙ)[J]. Materials Chemistry and Physics, 2021, 271: 124860 . DOI: 10.1016/j.matchemphys.2021.124860
[20] ZHOU J, HE Y, HUANG L, et al. Preparation of magnetic biochar from macadamia nutshell pretreated by FeCl3-assisted mechanochemical activation for adsorption of heavy metals FeCl3[J]. Journal of Environmental Chemical Engineering, 2024, 12(4): 113122. DOI: 10.1016/j.jece.2024.113122
[21] JIAO Y, WANG S, SUN B, et al. Adsorption efficiency and in-situ catalytic thermal degradation behaviour of microplastics from water over Fe-modified lignin-based magnetic biochar[J]. Separation and Purification Technology, 2025, 353: 128468. DOI: 10.1016/j.seppur.2024.128468
[22] HANG J, GUO Z, ZHONG C, et al. A super magnetic porous biochar manufactured by potassium ferrate-accelerated hydrothermal carbonization for removal of tetracycline[J]. Journal of Cleaner Production, 2024, 435: 140470. DOI: 10.1016/j.jclepro.2023.140470
[23] MEI Y, XU J, ZHANG Y, et al. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution[J]. Bioresource Technology, 2021, 325: 124732. DOI: 10.1016/j.biortech.2021.124732
[24] MELLITI E, TOUATI K, VAN DER BRUGGEN B, et al. Effect of Fe2+ ions on gypsum precipitation during bulk crystallization of reverse osmosis concentrates[J]. Chemosphere, 2021, 263: 127866. DOI: 10.1016/j.chemosphere.2020.127866
[25] HAN J, SONG Y, LI H, et al. Preparation of novel magnetic porous biochar and its adsorption mechanism on cerium in rare earth wastewater[J]. Ceramics International, 2023, 49(6): 9901-9908. DOI: 10.1016/j.ceramint.2022.11.165
[26] HANG J, GUO Z, ZHONG C, et al. A super magnetic porous biochar manufactured by potassium ferrate-accelerated hydrothermal carbonization for removal of tetracycline[J]. Journal of Cleaner Production, 2024, 435: 140470. DOI: 10.1016/j.jclepro.2023.140470
[27] YANG Y, YIN S, YANG D, et al. Carboxyl Fe3O4 magnetic nanoparticle-based SPE and HPLC method for the determination of six tetracyclines in water[J]. Analytical and Bioanalytical Chemistry, 2019, 411(2): 507-515. DOI: 10.1007/s00216-018-1475-y
[28] JAAFARZADEH N, KAKAVANDI B, TAKDASTAN A, et al. Powder activated carbon/Fe3O4 hybrid composite as a highly efficient heterogeneous catalyst for Fenton oxidation of tetracycline: degradation mechanism and kinetic[J]. RSC Advances, 2015, 5(103): 84718-84728. DOI: 10.1039/C5RA17953J
[29] HASANPOUR A, NIYAIFAR M, ASAN M, et al. Synthesis and characterization of FeO and ZnO nanocomposites by the sol-gel method[J]. Journal of Magnetism and Magnetic Materials, 2013, 334: 41-44. DOI: 10.1016/j.jmmm.2013.01.016
[30] ZHAO H, LANG Y. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 88: 152-160. DOI: 10.1016/j.jtice.2018.03.049
[31] MIMMO T, PANZACCHI P, BARATIERI M, et al. Effect of pyrolysis temperature on miscanthus (Miscanthus × giganteus) biochar physical, chemical and functional properties[J]. Biomass and Bioenergy, 2014, 62: 149-157. DOI: 10.1016/j.biombioe.2014.01.004
[32] WANG M, ZHAO Z, ZHANG Y. Magnetite-contained biochar derived from fenton sludge modulated electron transfer of microorganisms in anaerobic digestion[J]. Journal of Hazardous Materials, 2021, 403: 123972. DOI: 10.1016/j.jhazmat.2020.123972
[33] AYIANIA M, SMITH M, HENSLEY A J R, et al. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles[J]. Carbon, 2020, 162: 528-544. DOI: 10.1016/j.carbon.2020.02.065
[34] AHMED J, FAISAL M, JALALAH M, et al. An efficient amperometric catechol sensor based on novel polypyrrole-carbon black doped α-FeO nanocomposite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 619: 126469. DOI: 10.1016/j.colsurfa.2021.126469
[35] MA D, YANG Y, LIU B, et al. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation[J]. Chemical Engineering Journal, 2021, 408: 127992. DOI: 10.1016/j.cej.2020.127992
[36] WANG Q, ZHANG Z, XU G, et al. Magnetic porous biochar with nanostructure surface derived from penicillin fermentation dregs pyrolysis with KFeO activation: Characterization and application in penicillin adsorption[J]. Bioresource Technology, 2021, 327: 124818. DOI: 10.1016/j.biortech.2021.124818
[37] PAL D, MAITI S K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study[J]. Journal of Cleaner Production, 2019, 212: 986-996. DOI: 10.1016/j.jclepro.2018.12.087
[38] LI Z T, LIN B, JIANG L W, et al. Effective preparation of magnetic superhydrophobic FeO/PU sponge for oil-water separation[J]. Applied Surface Science, 2018, 427: 56-64. DOI: 10.1016/j.apsusc.2017.08.183
[39] AFZAL M Z, SUN X F, LIU J, et al. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads[J]. Science of The Total Environment, 2018, 639: 560-569. DOI: 10.1016/j.scitotenv.2018.05.129
[40] WANG Z, SHEN D, SHEN F, et al. Phosphate adsorption on lanthanum loaded biochar[J]. Chemosphere, 2016, 150: 1-7. DOI: 10.1016/j.chemosphere.2016.02.004
[41] TANG L, YU J, PANG Y, et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal[J]. Chemical Engineering Journal, 2018, 336: 160-169. DOI: 10.1016/j.cej.2017.11.048
[42] LIU S, XU W hua, LIU Y guo, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water[J]. Science of The Total Environment, 2017, 592: 546-553. DOI: 10.1016/j.scitotenv.2017.03.087
[43] MARTINS A C, PEZOTI O, CAZETTA A L, et al. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies[J]. Chemical Engineering Journal, 2015, 260: 291-299. DOI: 10.1016/j.cej.2014.09.017
[44] GAO Y, YUE Q, XU S, et al. Preparation and evaluation of adsorptive properties of micro-mesoporous activated carbon via sodium aluminate activation[J]. Chemical Engineering Journal, 2015, 274: 76-83. DOI: 10.1016/j.cej.2015.03.055
[45] PEZOTI O, CAZETTA A L, BEDIN K C, et al. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies[J]. Chemical Engineering Journal, 2016, 288: 778-788. DOI: 10.1016/j.cej.2015.12.042
[46] SEN GUPTA S, BHATTACHARYYA K G. Kinetics of adsorption of metal ions on inorganic materials: A review[J]. Advances in Colloid and Interface Science, 2011, 162(1): 39-58.
[47] ZHOU L, ZHANG G, ZENG Y, et al. Endogenous iron-enriched biochar derived from steel mill wastewater sludge for tetracycline removal: Heavy metals stabilization, adsorption performance and mechanism[J]. Chemosphere, 2024, 359: 142263. DOI: 10.1016/j.chemosphere.2024.142263
[48] ALHUJAILY A, MAO Y, ZHANG J, et al. Facile fabrication of Mg-Fe-biochar adsorbent derived from spent mushroom waste for phosphate removal[J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 117: 75-85. DOI: 10.1016/j.jtice.2020.11.034
[49] PATHAK P D, MANDAVGANE S A. Preparation and characterization of raw and carbon from banana peel by microwave activation: Application in citric acid adsorption[J]. Journal of Environmental Chemical Engineering, 2015, 3(4): 2435-2447. DOI: 10.1016/j.jece.2015.08.023
[50] CUI Z, XU G, ORMECI B, et al. A novel magnetic sludge biochar was prepared by making full use of internal iron in sludge combining KMnO4-NaOH modification to enhance the adsorption of Pb (Ⅱ), Cu (Ⅱ) and Cd(Ⅱ)[J]. Environmental Research, 2023, 236: 116470. DOI: 10.1016/j.envres.2023.116470
[51] SRIVASTAVA V, SHARMA Y C, SILLANPÄÄ M. Application of nano-magnesso ferrite (n-MgFeO) for the removal of Co ions from synthetic wastewater: Kinetic, equilibrium and thermodynamic studies[J]. Applied Surface Science, 2015, 338: 42-54. DOI: 10.1016/j.apsusc.2015.02.072
[52] LIU X J, LI M F, MA J F, et al. Chitosan crosslinked composite based on corncob lignin biochar to adsorb methylene blue: Kinetics, isotherm, and thermodynamics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 642: 128621. DOI: 10.1016/j.colsurfa.2022.128621
[53] ALIYU M, ABDULLAH A H, TAHIR M I B. Adsorption tetracycline from aqueous solution using a novel polymeric adsorbent derived from the rubber waste[J]. Journal of the Taiwan Institute of Chemical Engineers, 2022, 136: 104333. DOI: 10.1016/j.jtice.2022.104333
[54] WANG S, CHEN Y, GE S, et al. Adsorption characterization of tetracycline antibiotics on alkali-functionalized rice husk biochar and its evaluation on phytotoxicity to seed germination[J]. Environmental Science and Pollution Research, 2023, 30(58): 122420-122436. DOI: 10.1007/s11356-023-30900-2
[55] WANG K, YAO R, ZHANG D, et al. Tetracycline Adsorption Performance and Mechanism Using Calcium Hydroxide-Modified Biochars[J]. Toxics, 2023, 11(10): 841. DOI: 10.3390/toxics11100841
[56] JANG H M, KAN E. A novel hay-derived biochar for removal of tetracyclines in water[J]. Bioresource Technology, 2019, 274: 162-172. DOI: 10.1016/j.biortech.2018.11.081
[57] INYANG M, DICKENSON E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review[J]. Chemosphere, 2015, 134: 232-240. DOI: 10.1016/j.chemosphere.2015.03.072
[58] SAIKIA N, DEKA R C. Density functional study on noncovalent functionalization of pyrazinamide chemotherapeutic with graphene and its prototypes[J]. New Journal of Chemistry, 2014, 38(3): 1116-1128. DOI: 10.1039/c3nj00735a
[59] MAO H, WANG S, LIN J Y, et al. Modification of a magnetic carbon composite for ciprofloxacin adsorption[J]. Journal of Environmental Sciences, 2016, 49: 179-188. DOI: 10.1016/j.jes.2016.05.048
[60] MA D, YANG Y, LIU B, et al. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation[J]. Chemical Engineering Journal, 2021, 408: 127992. DOI: 10.1016/j.cej.2020.127992
[61] YANG X, LUO K, PI Z, et al. Insight to the mechanism of tetracycline removal by ball-milled nanocomposite CeO/FeO/Biochar: Overlooked degradation behavior[J]. Separation and Purification Technology, 2023, 307: 122703. DOI: 10.1016/j.seppur.2022.122703
[62] MOHAN D, SARSWAT A, OK Y S, et al. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent - A critical review[J]. Bioresource Technology, 2014, 160: 191-202. DOI: 10.1016/j.biortech.2014.01.120
[63] WANG J, CHEN Z, CHEN B. Adsorption of Polycyclic Aromatic Hydrocarbons by Graphene and Graphene Oxide Nanosheets[J]. Environmental Science & Technology, 2014, 48(9): 4817-4825.
-
其他相关附件
-
本文图文摘要
点击下载
-
-
目的
抗生素类药物(如,四环素(TC))在日常生产、生活中的广泛使用,导致了其在土壤和自然水体中的长期排放,对藻类及低等生物的生存构成了威胁,并可能对生态环境带来持续性破坏。传统的生物炭吸附材料在去除抗生素过程中普遍存在着分离效率低、易造成二次污染等缺陷,为此,本文制备了一种磁性椰壳生物炭多孔材料,以实现水中四环素的高效去除及材料的重复利用。
方法以湛江特色废弃物椰壳为生物质原料,经清洗、干燥、粉碎、过筛后与FeCl和FeSO混合,通过浸渍、氨水共沉淀及水热碳化等过程,制备了一种磁性椰壳生物炭(MBC)。利用SEM、FT-IR、XRD、BET、VSM、XPS和Zeta电位仪等对MBC的物理化学结构与性质进行了表征。利用吸附实验探究了pH值、温度及共存金属离子等因素对MBC吸附TC的影响,通过吸附动力学、吸附等温线和热力学实验考察了MBC对TC的吸附行为及相关机制,通过吸附-解析循环实验考察了MBC对TC的吸附稳定性与再生能力。
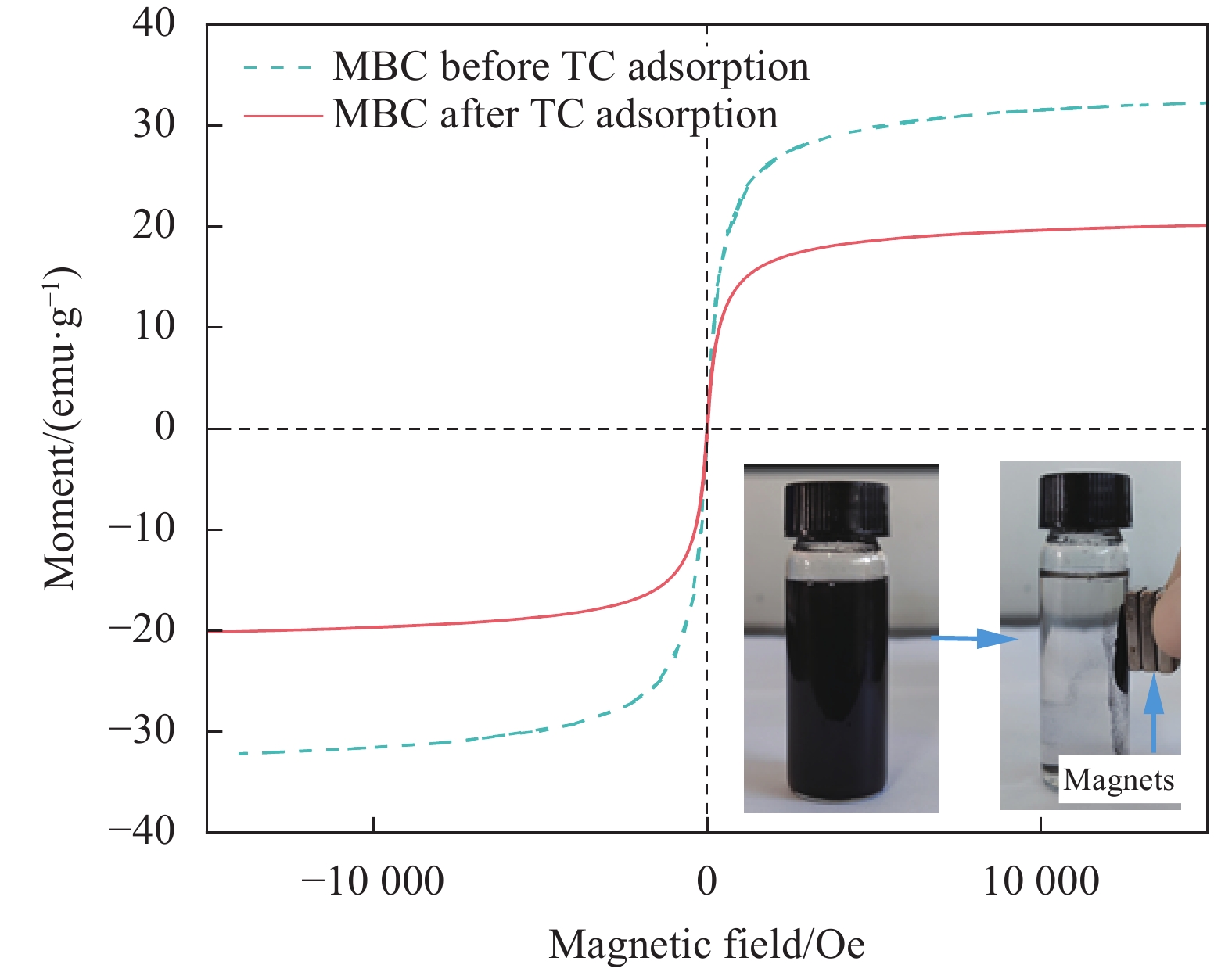
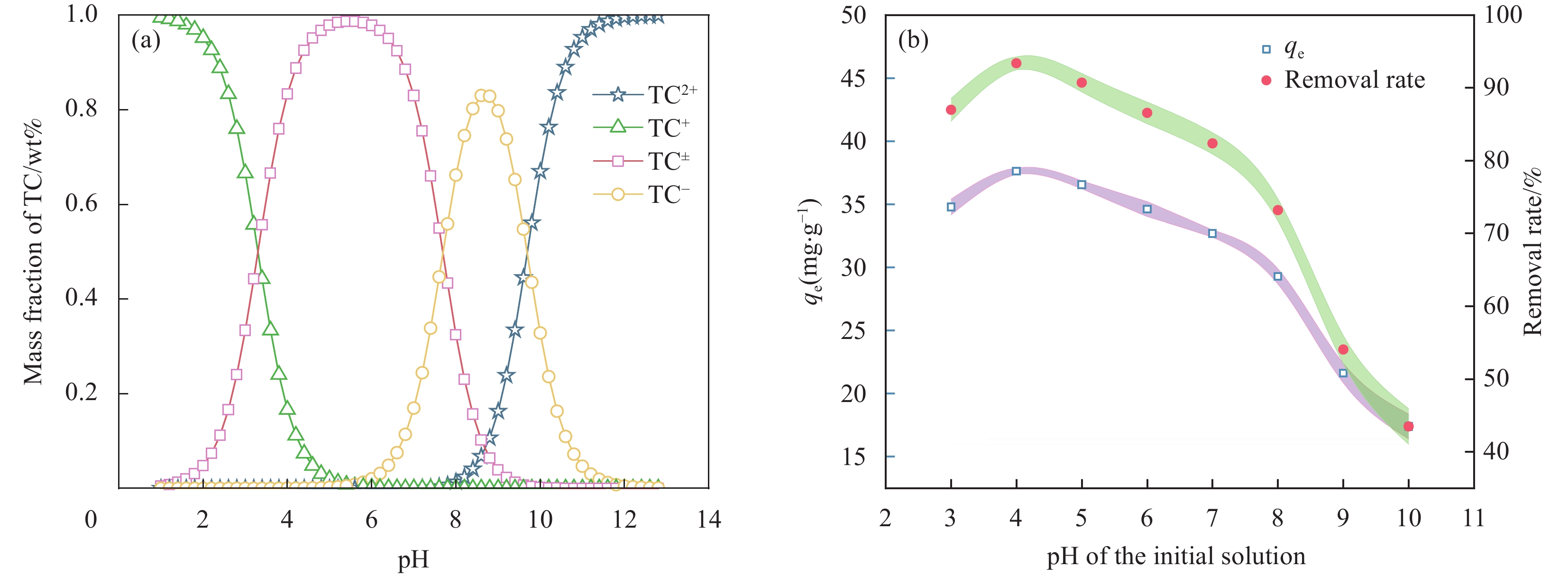
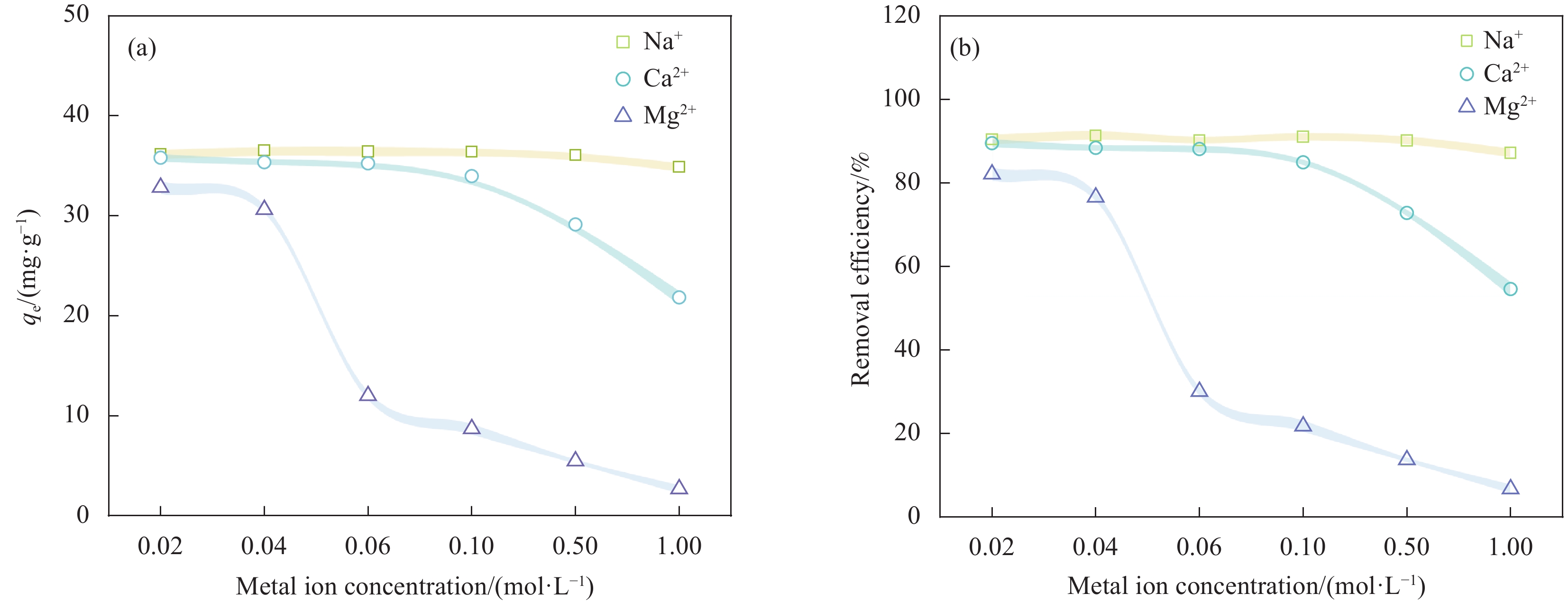
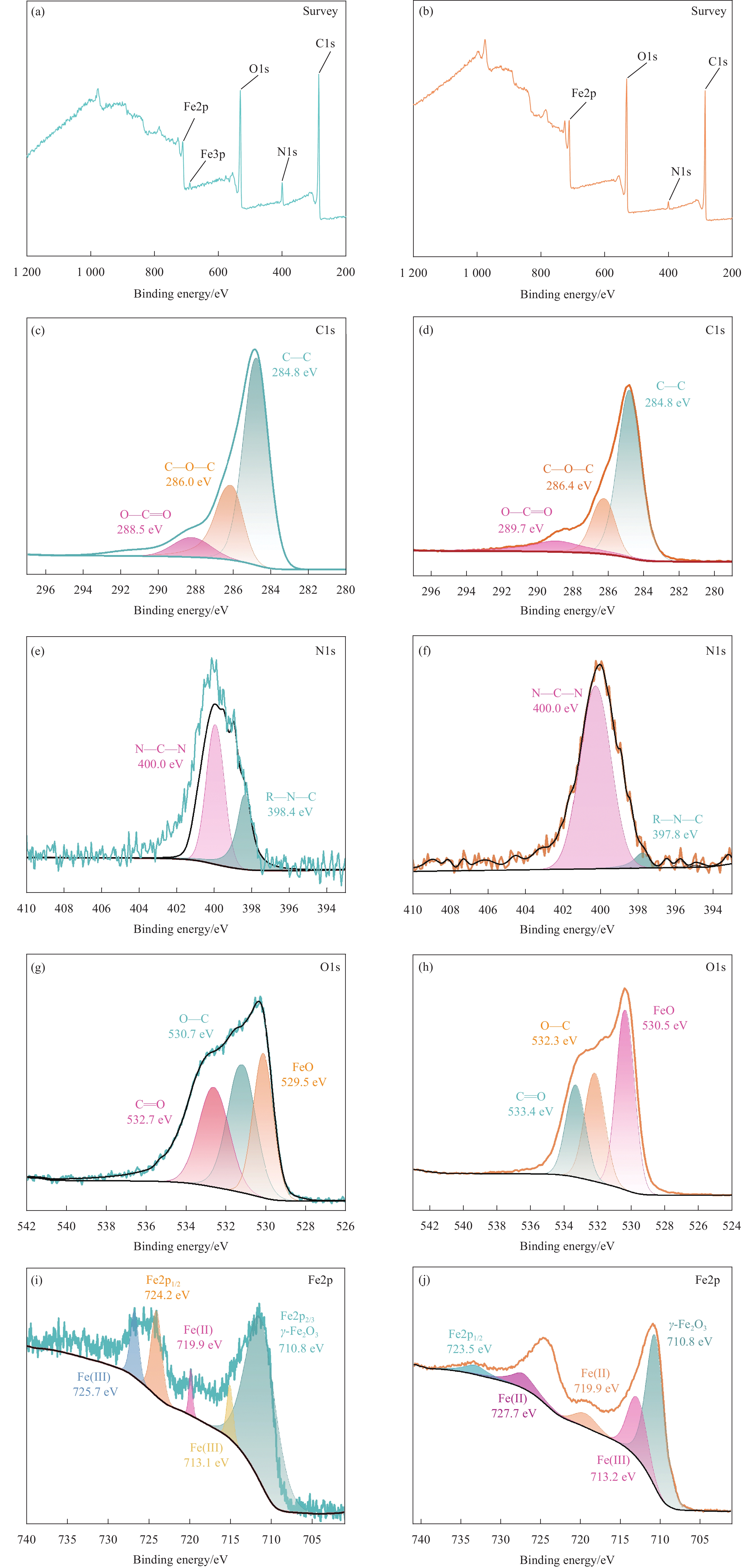
结果表征结果显示,MBC材料具有丰富的孔隙,MBC的总孔体积为0.006841 cm/g,比表面积为16.3638 m/g,并附着了大量磁性FeO和-FeO混晶,不仅为吸附提供了丰富的位点,也赋予了材料磁回收能力;材料表面Zeta电位低至-20.00±0.70 mV,具有对正电位点的吸附能力。吸附实验结果显示,pH 4.0时,MBC对TC的吸附效果最佳,在24 h可达到吸附饱和,饱和吸附量为36.40 mg·g,TC去除率达93.37%。因素探究实验结果显示,MBC对TC的吸附过程受多种因素影响:ϕ溶液pH越高,MBC对TC的吸附量越低。κ二价金属离子Ca和Mg的存在会显著降低MBC对TC的吸附效率。λ升高温度有利于提高MBC对TC的吸附量。动力学实验结果显示,MBC对TC的吸附行为符合伪二阶动力学模型,表明吸附主要受化学吸附控制。吸附等温实验结果显示,MBC对TC的吸附行为符合Langmuir等温线模型,表明吸附过程以单分子层吸附为主,MBC对TC的最大单分子层吸附容量可达到46.49 mg·g (45℃)。热力学实验结果显示,MBC对TC的吸附过程是自发的吸热反应,随着温度升高,自发性更显著;吸附会导致固-液界面无序度的增加。吸附-解析循环实验结果显示,MBC在经过5次吸附-解吸循环后,仍能保持85.16%的TC去除率,表明材料具有良好的稳定性和再生能力,吸附位点的不可逆损耗可能是导致材料吸附性能下降的主要原因。
结论磁性椰壳生物炭具有丰富的孔隙结构、大的比表面积和负Zeta电位,能高效吸附水中的TC,吸附过程以单分子层的化学吸附为主,是一种自发的吸热反应。吸附过程中存在受液膜扩散、内扩散、表面络合、氢键和π-π堆积等作用。磁性椰壳生物炭是一种高效、可重复利用的TC吸附材料,有望应用于养殖尾水与医药废水的净化。
-
抗生素类药物在土壤和自然水体中的排放会对生态环境造成严重危害,危及藻类及低等生物的生存,并可能对人类和动物健康带来长期风险。抗生素在水生环境中,即使在极低浓度下,也会对人类和动物产生急性和慢性毒性,导致细菌失衡、胃肠道不适,甚至肝损伤。因此,必须采取有效措施去除水中的抗生素,以降低其对生态环境和人类健康的潜在风险。
为解决这一问题,本研究创新性地利用湛江本地特色农业废弃物椰壳为原料,采用FeCl₃和FeSO₄作为铁源,通过浸渍、共沉淀和水热合成方法制备了磁性椰壳生物炭(MBC)。这种方法不仅有效利用了本地资源,展示了生态友好的原材料选择和制备过程的创新性。MBC对TC具有显著的吸附效果,尤其是在pH 4.0时吸附效果最佳,吸附过程符合Langmuir等温线和伪二阶动力学模型,最大吸附容量在45°C时可达46.49 mg·g-1。此外,MBC在重复利用性实验中表现出优良的稳定性,经过五次吸附-解吸循环后,去除率仍稳定在85.16%。
通过本研究,不仅展示了MBC在水中TC去除方面的巨大潜力,还为农业废弃物的高值化利用提供了新的思路。这些创新性研究和亮点展示为未来水处理技术的发展和推广奠定了坚实的基础。研究表明,磁性椰壳生物炭是一种高效、可重复利用的TC吸附材料,在养殖尾水、医药废水处理等领域具有广阔的应用前景。
(a) TC的型体分布;(b) pH对MBC吸附TC的影响






 下载:
下载: