Manganese and phosphorus co-doped corn stover biochar to activate peroxymonosulfate for degradation of norfloxacin
-
摘要:
抗生素的大规模使用对自然环境及人类健康造成极大威胁,因此急需探寻一种高效、绿色的降解方法。本研究制备了Mn、P掺杂玉米秸秆生物炭(Mn/P-C)用于活化过一硫酸盐(PMS)降解诺氟沙星(NOR)。对比纯生物炭(BC)、P掺杂生物炭(P-C),Mn/P-C具有更大的缺陷结构及丰富的表面含氧官能团。在pH为2.84、PMS为3 mmol/L、催化剂投加量为1 g/L的条件下,80 min反应时间内,NOR降解率达到94%,体系降解反应速率为0.034 min−1。催化剂表征、淬灭实验和电子顺磁共振(EPR)实验表明,在Mn/P-C活化PMS体系中,NOR主要通过SO4•−、O2•−自由基以及催化剂表面产生的1O2非自由基途径得到降解。此外,Mn/P-C在较宽的pH范围内均有效,并且具有较高的可重复利用性和稳定性,由于其良好的磁性,不会对环境造成二次污染。本研究证实了掺杂Mn、P可以有效提高生物炭活化PMS降解NOR的效能,为碳基材料的优化以及其在过硫酸盐活化中的应用提供了新的思路。
Abstract:The large-scale use of antibiotics poses a significant threat to the natural environment and human health, so there is an urgent need to explore an efficient and green degradation method. In this study, Mn/P-doped corn stover biochar (Mn/P-C) was prepared for the degradation of norfloxacin (NOR) by activated permonosulfate (PMS). Compared with pure biochar (BC), P-doped biochar (P-C), Mn/P-C has a larger defect structure and abundant surface oxygen-containing functional groups. Under the conditions of pH=2.84, PMS=3 mmol/L, and catalyst dosing 1 g/L, the NOR removal reached 94% within 80 min reaction time, and the degradation reaction rate of the system was 0.034 min−1. The catalyst characterization, quenching experiments, and electron paramagnetic resonance (EPR) experiments demonstrated that, in the Mn/P-C-activated PMS system, the NOR was mainly removed via SO4•− and O2•− radicals as well as the 1O2 non-radical pathway generated on the catalyst surface were degraded. In addition, Mn/P-C is effective in a wide pH range, has high reusability and stability, and does not cause secondary pollution to the environment due to its good magnetic properties. This study confirms that doping Mn and P can effectively improve the efficacy of biochar-activated PMS for NOR degradation, which provides a new idea for the optimization of carbon-based materials as well as their application in persulfate activation.
-
Keywords:
- manganese/phosphorus doping /
- biochar /
- advanced oxidation /
- peroxymonosulfate /
- norfloxacin
-
聚合物电介质薄膜凭借其低介电损耗、易加工成型、高击穿强度等优点,已广泛应用于医用除颤设备、柔性电子器件、脉冲功率系统、摩擦纳米发电机等[1-2]。随着混合电动汽车、油气勘探技术、航天电力系统的发展及应用环境的复杂化,对聚合物基电介质薄膜宽温域内的介电性能和击穿强度提出更高要求[3-4]。目前广泛使用的聚合物电介质薄膜为双向拉伸聚丙烯(BOPP),但由于BOPP的热稳定性欠佳,高温下的介电稳定性和击穿强度急剧下降,无法满足上述应用需求[5]。
为了制备高温、强电场等极端环境中具有良好稳定性的聚合物电介质薄膜,有学者选择具有高玻璃化转变温度(Tg)的芳香族聚合物,如聚酰亚胺(PI)、聚醚酰亚胺(PEI)、聚芳醚酮(PEEK)、聚芳醚脲(PEEU)等制备了高温电介质薄膜,但研究发现虽然其在高温、强电场环境中的介电性能保持稳定,但击穿强度迅速下降[6]。这可归因于温度场-电场耦合环境中芳香族聚合物分子结构中苯环的π-π耦合作用引起的高漏电流密度[7]。为了降低漏电流对芳香族聚合物电介质薄膜的影响,Duan等[8]将交联结构引入到PEI分子结构中制备不同交联度的c-PEI,交联结构在增加PEI内部电子陷阱能级和陷阱密度的同时,打破了分子结构的规整性,降低了π-π耦合效应,抑制了高温、强电场环境中漏电流的形成,所制备c-PEI高温下的击穿强度较非交联PEI显著提升。此外,采用密度泛函理论(DFT)分析发现,PI分子结构中酸酐上的苯环带有正电性,PEI分子中连接醚键的苯环带有负电性,因此,Zhang等[9]将PI和PEI共混,利用分子链间静电作用降低了PI和PEI分子链间距以及内部自由体积,所制备的PI-PEI共混薄膜的最高击穿强度超过
1000 MV/m。但需要指出的是,由于聚合物的击穿强度(E)与其介电常数(ε)存在内禀矛盾关系(E~1/ε0.65),即击穿强度的提升往往伴随着介电常数的下降,进而影响到聚合物电介质薄膜储能特性的改善[10]。因此,如何制备同时具有高介电常数和高击穿强度的聚合物电介质薄膜是目前的研究热点。为了打破介电常数与击穿强度间的内禀矛盾,有学者基于不同聚合物功能层(极化层、绝缘层、过渡层等),通过调控空间组装工艺构筑了多层聚合物电介质薄膜[11]。在多层结构中,特殊的空间电场分布机制赋予绝缘层更高的电场强度,而极化层和多尺度界面结构则通过偶极子极化和Maxwell-Wagner-Sillars (MWS)界面极化提升了介电常数[12-13]。Wang等[14]通过PEI和聚(偏氟乙烯-三氟乙烯-三氟氯乙烯)(PTVC)构筑了顺式三层结构和反式三层结构的全有机聚合物电介质薄膜,研究发现顺式三层结构的最大击穿强度达到504 MV/m,并且介电常数在室温−100℃范围内保持稳定。但遗憾的是,目前多层结构电介质薄膜的研究大多局限于铁电聚合物,无法满足高温应用需求[11, 15]。
近期,Su等[16]采用去质子化法制备了芳纶纳米纤维(ANF)并抽滤得到ANFm,研究发现ANFm具有较高的介电常数和优异的高温稳定性,所制备的ANFm能够满足高温环境的应用需求,但由于ANFm表面粗糙度较高,易诱导空间电荷聚集,导致击穿强度较低。Vu等[17]基于ANF与氟化石墨烯(GF)制备了ANFm-GF电介质薄膜,结果表明,由于GF的高本征击穿强度(~
1000 MV/m) ANFm-GF电介质薄膜在室温下最大击穿强度提升至507 MV/m。但在高温下,电极处注入的电子以及空间电荷在ANFm表面缺陷处的聚集诱导了电树枝的形成并引发电击穿,引起ANFm击穿强度迅速降低(<300 MV/m)[18]。因此,改善ANFm的表面粗糙度有助于提升其高温击穿强度。本文选用ANFm和可溶性PI,采用浸渍提拉法构筑了具有三明治结构的全有机PI-ANFm-PI (P-A-P)复合薄膜。ANFm具有较高的介电常数以及出众的热学稳定性能;PI具有极高的击穿强度和玻璃化温度,能够满足高温电介质材料的应用需求。研究结果发现,ANFm表面粗糙度的降低以及P-A-P复合薄膜内部电子-空穴对的构建有效抑制了漏电流的形成;同时ANFm的高极化率可为P-A-P复合薄膜提供高介电常数;本文通过分析P-A-P复合薄膜的介电性能、电导损耗和击穿强度以期为制备新型高温电介质薄膜提供新思路和新方法。
1. 实验材料及方法
1.1 原材料
芳纶(PPTA),日本帝人芳纶公司;二甲基亚砜(DMSO),分析纯,天津市科密欧化学试剂有限公司;聚酰亚胺(PI),型号P84,美国杜邦公司;KOH,纯度98%,阿拉丁试剂(上海)有限公司;N-甲基吡咯烷酮(NMP),分析纯,上海凌峰化学试剂有限公司。上述试剂直接使用,无需提纯。
1.2 试样的制备
首先将剪切得到的PPTA短纤(长度约1 mm)分别用丙酮、乙醇超声处理30 min,以除去表面污染物;随后将0.08 g芳纶短纤分散于含有0.16 g KOH的40 mL DMSO∶H2O(体积比为25∶1)混合溶液中,室温下超声处理4 h后得到暗红色ANF/DMSO溶液。将适量去离子水加入到ANF/DMSO溶液中,高速搅拌后形成ANF胶体悬浮液,随后采用真空抽滤方法制备ANFm,并在80℃下干燥12 h。
将PI粉末溶解于NMP中分别制备1wt%、3wt%、5wt%、7wt%和10wt%的PI溶液,随后将ANFm垂直浸渍于PI溶液中,并采用浸渍5 min,提拉静置1 min的方式循环5次;将浸渍得到的ANFm至于100℃中干燥12 h,并在10 MPa,180℃条件下热压5 min。为了方便描述所制备的PI-ANFm-PI (P-A-P)复合薄膜,根据溶液中PI的质量分数分别将P-A-P复合薄膜命名为P-A-P-1、P-A-P-3、P-A-P-5、P-A-P-7、P-A-P-10 (表1);单层ANF薄膜命名为ANFm。所制备的ANFm和P-A-P复合薄膜的厚度约为15 μm,PI单层厚度为0~0.2 μm。此外,需要指出的是,对比发现P-A-P-10中PI层的厚度反而略低于P-A-P-7,这可能是由于PI溶液浓度过高后,分子链间缠结点增加,导致黏附或进入ANFm的PI减少。ANFm和P-A-P复合薄膜的制备流程如图1所示。
表 1 材料参数Table 1. Materials parametersSamples outer
layerMiddle
layerThickness of
sample/μmConcentration of
PI solution/wt%P-A-P-1 PI ANF 14.7 1 P-A-P-3 14.9 3 P-A-P-5 15.2 5 P-A-P-7 15.0 7 P-A-P-10 14.6 10 Notes: PI—Polyimide; ANF—Aramid nanofiber. 1.3 结构表征与性能测试
红外测试:采用Nicolet 6700型傅里叶变换红外光谱仪(FTIR,美国赛默飞世尔科技公司),测试波数范围为
4000 ~500 cm−1。X射线衍射(XRD)测试:D/max-2550PC型,日本理学公司,靶材Cu,管电压40 V,管电流40 mA,扫描范围为5°~90°,波长0.154 nm。AFM测试:Dimension FastScan型,德国布鲁克公司,采用敲击模式,扫描范围2 μm×2 μm。SEM测试:S4800型场发射扫描电子显微镜,日本Hitachi公司。TEM测试:JEM-2100型透射电子显微镜,日本电子株式会社。介电性能测试:Concept 40型宽频介电阻抗谱仪,德国Novocontrol公司,频率范围101~106 Hz,测试温度分别为25℃和150℃。击穿强度测试:CS9916BX型程控超高压分析仪,南京长盛公司,每个样品测试12次,并通过Weibull分布拟合得到Weibull击穿强度。1.4 模拟计算
采用Gaussview5.0和Gaussian09 W计算了ANF和PI的电子结构和能级分布。在密度泛函理论(DFT)计算中,所用基组为B3LYP/6-31G(d),并且仅使用ANF和PI分子结构中的一个结构单元进行计算。通过Multiwfn程序分析了ANF和PI的静电势(ESP)分布[19-21]。
2. 结果与讨论
2.1 结构表征
图2(a)为去质子化过程中ANF/DMSO分散液的光学图片,从图中可以看到,随着时间的增加,ANF/DMSO溶液的颜色逐渐变深,4 h后变为暗红色均相溶液。这是由于在KOH作用下,PPTA分子链上的氢原子逐渐去质子化,削弱了分子链间的氢键作用,PPTA纤维逐渐转变为ANF。Yang等[22]研究发现,由于PPTA分子链中π-π堆叠效应及分子链间范德华力相互作用,PPTA纤维无法完全溶解于DMSO,而是以纳米纤维的形式存在。从图2(b)中可以看到,所制备的ANF具有高长径比。上述结果表明,通过调控DMSO和H2O的比例能够在短时间内制备得到ANF,比仅采用纯DMSO溶剂制备ANF的方法更高效[16]。对比ANF与PPTA的FTIR谱图发现(图2(c)),ANF和PPTA中特征峰位置基本相同,表明采用去质子化方法制备的ANF化学结构没有发生明显变化,这有利于保持其高强度、高绝缘和高温稳定性能。图2(d)为ANF和PPTA的XRD图谱。在PPTA的XRD谱图中2θ=21.1°、23.5°和28.5°的特征峰分别对应(110)、(200)和(004)晶面;在ANF的XRD谱图中,只在2θ=21.1°处出现了(110)晶面的特征衍射峰,而(200)和(004)晶面的衍射峰强度显著下降,表明ANF内部晶体结构与PPTA一致,只是晶粒尺寸发生了变化[23]。采用谢乐公式(D=0.89λ/(βcosθ),其中D为晶粒尺寸,λ为波长,β为半峰宽,θ为衍射角)计算了PPTA和ANF的晶粒尺寸,结果发现PPTA中(110)晶面对应的晶粒尺寸为5.27 nm,而ANF中(110)晶面对应的晶粒尺寸降低至1.46 nm。晶粒尺寸的降低可归因于去质子化过程中分子链间氢键网络的破坏扰乱了PPTA分子链的规整排列,进而引起分子链从有序结构转变为无序结构[24]。
2.2 微观形貌
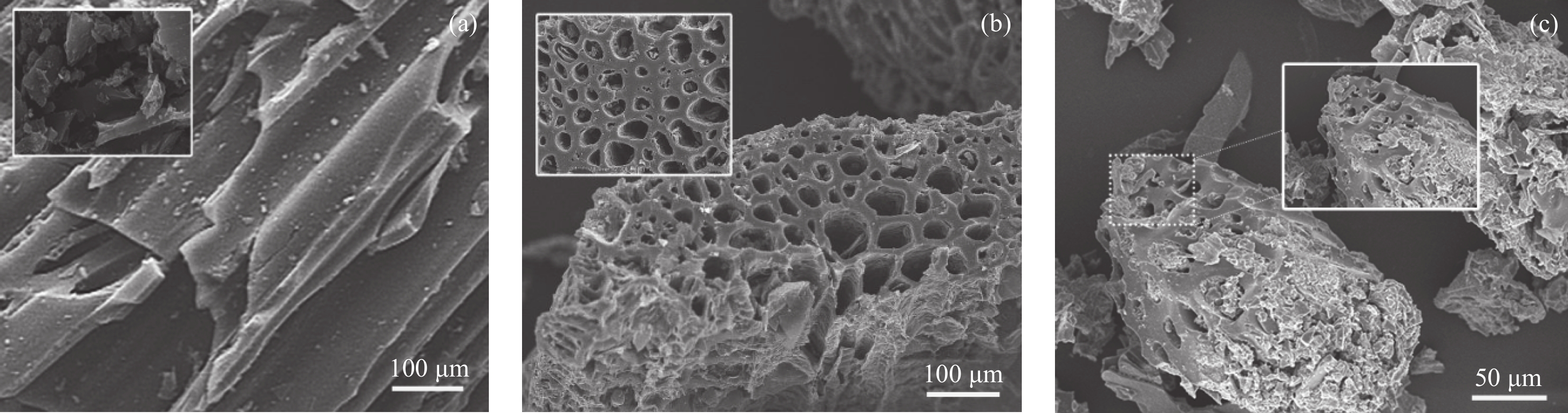
图3(a)展示了ANFm横截面形貌,从图中可以看到,ANFm呈现致密的珍珠层状结构,而P-A-P-3 (图3(b))和P-A-P-7 (图3(c))具有明显的三层结构,其中上下层为PI (箭头所示),中间层为ANFm,并且PI层和ANFm层结合紧密,没有明显的孔隙。图3(d)~3(f)分别为ANFm、P-A-P-3和P-A-P-7的表面形貌,其中,ANFm表面凹凸不平,纤维堆积结构明显;而随着PI溶液浓度的增加,P-A-P-3和P-A-P-7的表面逐渐光滑平整,缺陷明显减少。从ANFm、P-A-P-3和P-A-P-7 (图3(g)~3(i))的光学图片可知,随着PI溶液浓度的增加,薄膜的颜色逐渐加深,间接表明PI层的厚度逐渐增大。Luo等[25]研究发现,当电介质薄膜表面粗糙程度较高时,空间电荷以及越过电极/电介质界面势垒的电子会聚集在电介质薄膜的缺陷处,长时间累积后诱导电击穿的发生。因此,减少电介质薄膜的表面缺陷,有助于阻碍电极中电子的注入以及电树枝的形成与发展,在提升击穿强度的同时,降低内部漏电流密度。
![]() 图 3 横截面形貌:ANFm (a)、P-A-P-3 (b)、P-A-P-7 (c);表面形貌:ANFm (d)、P-A-P-3 (e)、P-A-P-7 (f);光学图片:ANFm (g)、P-A-P-3 (h)、P-A-P-7 (i)Figure 3. Cross-sectional morphologies of ANFm (a), P-A-P-3(b), and P-A-P-7 (c); Surface morphologies of ANFm (d), P-A-P-3 (e), and P-A-P-7 (f); Digital photos of ANFm (g), P-A-P-3 (h), and P-A-P-7 (i)
图 3 横截面形貌:ANFm (a)、P-A-P-3 (b)、P-A-P-7 (c);表面形貌:ANFm (d)、P-A-P-3 (e)、P-A-P-7 (f);光学图片:ANFm (g)、P-A-P-3 (h)、P-A-P-7 (i)Figure 3. Cross-sectional morphologies of ANFm (a), P-A-P-3(b), and P-A-P-7 (c); Surface morphologies of ANFm (d), P-A-P-3 (e), and P-A-P-7 (f); Digital photos of ANFm (g), P-A-P-3 (h), and P-A-P-7 (i)2.3 电学性能
击穿强度是影响聚合物电介质薄膜储能特性的关键参数之一。采用威布尔分布函数分析了ANFm和P-A-P复合薄膜在25℃和150℃时的击穿强度,如图4(a)和4(b)所示。可以看到,在宽温域范围内,三层结构复合薄膜的击穿强度均优于单层ANFm,表明PI层有助于提升ANFm的击穿强度。在图4(c)中,P-A-P复合薄膜在25℃和150℃时击穿强度分别为259.8 MV/m和242.3 MV/m,而P-A-P-7复合薄膜在相同温度下的击穿强度达411.6 MV/m和350.7 MV/m,相较于ANFm提升了58.4%和44.7%。研究表明,在多层电介质材料中,绝缘层承担更高的电场强度,极化层提供高介电常数[26]。在本文中,上下PI层为绝缘层,ANFm层为极化层,当三层结构形成后,PI层承担更高的电场强度,ANFm层上的电场强度迅速下降。由于聚合物的击穿机制主要包括电-机械击穿、热击穿、电击穿等[27]。因此,提升聚合物的杨氏模量、导热性能和绝缘性能均有助于改善其击穿强度。从图4(d)中可知,随着PI层溶液浓度的增加,P-A-P复合薄膜的杨氏模量从ANFm的1.59 GPa增加至P-A-P-7的2.87 GPa,而P-A-P-10杨氏模量下降的原因可归因于PI层厚度的降低。由于聚合物击穿强度与其杨氏模量成正比关系,即E=0.606(Y/(εrε0))1/2 (E为击穿强度,εr为聚合物本征介电常数,ε0为真空介电常数,Y为杨氏模量)[28]。因此,杨氏模量的提升有助于抑制电-机械击穿的发生。从图4(e)可知,P-A-P复合薄膜的漏电流密度也随着PI浓度的增加逐渐降低,这不但抑制了P-A-P复合薄膜内部电击穿的发生,同时降低了内部漏电流引起的热效应,避免了热击穿的发生。图4(f)为ANFm、P-A-P-3和P-A-P-7击穿强度、漏电流密度和杨氏模量的雷达图,可以看到,P-A-P-7的杨氏模量和击穿强度最高,漏电流密度最低,表明PI层厚度的增加有助于优化ANFm的电学性能。
![]() 图 4 ANFm和P-A-P复合薄膜25℃ (a)和150℃ (b)的击穿强度威布尔分布、25℃和150℃的击穿强度对比图(c)、力学性能(d)和漏电流密度(e);(f) ANFm、P-A-P-3和P-A-P-7薄膜击穿强度、漏电流密度和杨氏模量的雷达图Figure 4. Weibull distribution of breakdown strength at 25℃ (a) and 150℃ (b), comparison of breakdown strength at 25℃ and 150℃ (c), mechanical properties (d), and leakage current density (e) for ANFm and P-A-P composite films; (f) Radar chart of breakdown strength, leakage current density, and Young's modulus for ANFm, P-A-P-3, and P-A-P-7 filmsE—Breakdown strength; P—Polarization intensity
图 4 ANFm和P-A-P复合薄膜25℃ (a)和150℃ (b)的击穿强度威布尔分布、25℃和150℃的击穿强度对比图(c)、力学性能(d)和漏电流密度(e);(f) ANFm、P-A-P-3和P-A-P-7薄膜击穿强度、漏电流密度和杨氏模量的雷达图Figure 4. Weibull distribution of breakdown strength at 25℃ (a) and 150℃ (b), comparison of breakdown strength at 25℃ and 150℃ (c), mechanical properties (d), and leakage current density (e) for ANFm and P-A-P composite films; (f) Radar chart of breakdown strength, leakage current density, and Young's modulus for ANFm, P-A-P-3, and P-A-P-7 filmsE—Breakdown strength; P—Polarization intensity为了进一步分析ANFm和P-A-P复合薄膜漏电流密度变化的内在机制,采用密度泛函理论(DFT)分析了PI和ANF的电子轨道能级和静电势(ESP)分布。PI和ANF的ESP分布如图5(a)和5(b)所示。可以看出,ANF的最高静电势达到45,而PI最高仅为20,表明ANF具有更强的吸引电子的能力,可以作为电子陷阱位点捕获电极处注入以及内部形成的自由电子[29]。在图5(c)中,PI的最高占据分子轨道(HOMO)能级为−6.01 eV,最低占据分子轨道(LUMO)能级为−3.27 eV,禁带宽度为2.74 eV;ANF的HOMO能级为−5.63 eV,LUMO能级为−1.99 eV,禁带宽度为3.64 eV。虽然PI的禁带宽度低于ANF,高温下易形成自由电子,但由于PI的LUMO能级与ANF的HOMO能级差别较小(2.36 eV),PI层的电子与ANF的空穴在库仑力的作用下形成电子-空穴对(图5(d)),并作为电子陷阱捕获空间电荷[30-31]。
![]() 图 5 PI (a)和ANF (b)的静电势分布及各静电势范围内的面积百分比;(c) PI和ANF的分子轨道能级示意图;(d)电子-空穴对的形成与作用机制Figure 5. Electrostatic potential (ESP) distributions and normalized ESP area distribution statistics of PI (a) and ANF (b); (c) Molecular orbital energy levels of PI and ANF; (d) Formation and mechanism of action of electron-hole pairsLUMO—Lowest unoccupied molecular orbital; HOMO—Highest occupied molecular orbital
图 5 PI (a)和ANF (b)的静电势分布及各静电势范围内的面积百分比;(c) PI和ANF的分子轨道能级示意图;(d)电子-空穴对的形成与作用机制Figure 5. Electrostatic potential (ESP) distributions and normalized ESP area distribution statistics of PI (a) and ANF (b); (c) Molecular orbital energy levels of PI and ANF; (d) Formation and mechanism of action of electron-hole pairsLUMO—Lowest unoccupied molecular orbital; HOMO—Highest occupied molecular orbital2.4 介电性能
ANFm和P-A-P复合薄膜的介电性能如图6所示。在图6(a)中,ANFm的介电常数高达7.2(102 Hz),这归因于ANF表面丰富的极性基团以及内部高偶极矩酰胺键(~3.7 D)的存在。此外,P-A-P复合薄膜的介电常数对频率的依赖性明显降低。由于聚合物介电常数主要源于空间电荷极化,偶极子极化,原子极化和离子极化;其中原子极化和离子极化发生在高频率范围内(>108 Hz)[32-33]。因此,本文中P-A-P复合薄膜介电常数主要源于空间电荷极化(<104 Hz)和偶极子极化(104~106 Hz)。在低频率范围内,PI层的形成不但抑制了界面处空间电荷的聚集,同时PI和ANF内部电子-空穴对以及分子链间氢键网络的构建阻碍了载流子的迁移,降低了P-A-P复合薄膜的空间电荷密度,因此,P-A-P复合薄膜在低频率范围内的空间电荷极化强度随着PI溶液浓度的增加逐渐降低。同时,PI层的形成还引起P-A-P复合薄膜介电常数的降低。另外,PI较低的介电常数也会引起P-A-P复合薄膜介电常数的下降[34]。介电损耗会将电介质电容器储存的电能转化为焦耳热,降低电介质薄膜的使用寿命和效率。图6(b)为ANFm和P-A-P复合薄膜介电损耗与频率的关系。可以看到,在频率范围内,随着PI溶液浓度的增加,P-A-P复合薄膜的介电损耗逐渐降低,表明PI层的形成有助于降低P-A-P复合薄膜服役过程中能量的损耗以及抑制热效应的形成。图6(c)为ANFm和P-A-P复合薄膜在频率范围内的交流电导率。ANFm和P-A-P复合薄膜的交流电导率均与测试频率呈良好的线性关系,表明其均具有优异的绝缘性能[35]。此外,10 Hz时,ANFm和P-A-P复合薄膜的交流电导率随着PI层厚度的增加不断下降,如样品的交流电导率从2.88×10−13 S/cm(ANFm)降至3.63×10−14 S/cm (P-A-P-7),说明PI层的形成有助于进一步提升P-A-P复合薄膜的绝缘性能。
此外,在25℃和150℃时对比分析了P-A-P-7的介电性能。在图6(d)中,P-A-P-7在150℃时的介电常数均高于25℃时的介电常数,尤其是在102~103 Hz范围内提升显著。这是由于随着温度的升高,从电极处注入的电子以及被束缚的电子热激发形成自由电子引起空间电荷极化强度增大;同时,PI和ANF分子链段的运动能力也随着温度的升高逐渐增加,进而增强了偶极子的取向极化。在图6(e)中,当频率低于104 Hz时,P-A-P-7的电导损耗在150℃时增加显著,这主要源于空间电荷的增加。在25℃时,电极处的电子无法越过电极/电介质间的界面势垒进入电介质,同时PI和ANF形成的电子-空穴对以及分子链间的氢键网络均会抑制空间电荷的迁移;但150℃时,电极处的电子吸收热能越过界面势垒,同时被电子-空穴对束缚的电荷热激发形成自由电子,引起电导损耗迅速增大。在图6(f)中,10 Hz时,P-A-P-7的电导率从25℃时的3.63×10−14 S/cm增加至150℃时的1.45×10−12 S/cm,也进一步表明高温下漏电流密度的增加。但需要指出的是,虽然P-A-P-7在150℃时介电损耗和电导率均有所增大,但依然保持在较低的范围,满足电介质薄膜的使用要求。
3. 结 论
本文基于芳纶纳米纤维薄膜(ANFm)和聚酰亚胺(PI)溶液,采用浸渍提拉法构筑了具有三明治结构的全有机PI-ANFm-PI (P-A-P)复合薄膜,并研究了宽温域内P-A-P复合薄膜的击穿强度、电导损耗和介电性能,主要结论如下:
(1) ANFm表面粗糙度的降低以及PI与ANF形成的电子-空穴对有助于降低P-A-P复合薄膜的漏电流密度,减低电导损耗;
(2)随着PI浓度的增加以及内部漏电流密度的降低,P-A-P复合薄膜的在25℃和150℃下的击穿强度达411.6 MV/m和350.7 MV/m,较ANF薄膜分别提升了58.4%和44.7%;
(3) PI层的形成提升了P-A-P复合薄膜的介电稳定性,并且介电损耗随着PI溶液浓度的增加逐渐降低,绝缘性能随着PI溶液浓度的增加逐渐增大。
-
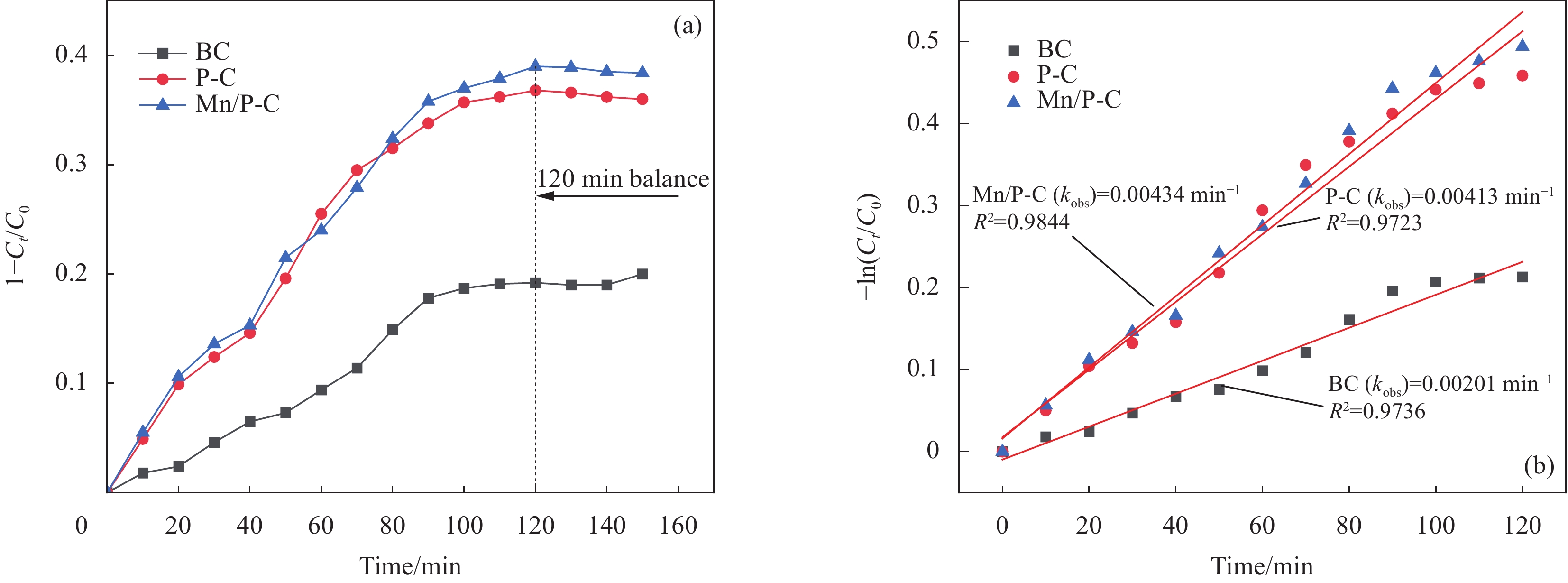
图 3 BC、P-C和Mn/P-C对诺氟沙星(NOR)的吸附去除率(a)、对应的吸附反应速率常数(b) (反应条件:过一硫酸盐(PMS)=1.5 mmol/L;BC、P-C、Mn/P-C=1 g/L;NOR=10 mg/L;pH=6)
Figure 3. Adsorption removal rate of NOR (a) and the corresponding adsorption reaction rate constants (b) caused by BC, P-C and Mn/P-C (Reaction conditions: Permonosulfate (PMS)=1.5 mmol/L; BC, P-C, Mn/P-C=1 g/L; Norfloxacin (NOR)=10 mg/L; pH=6)
Ct—; C0—; kobs—; R2—
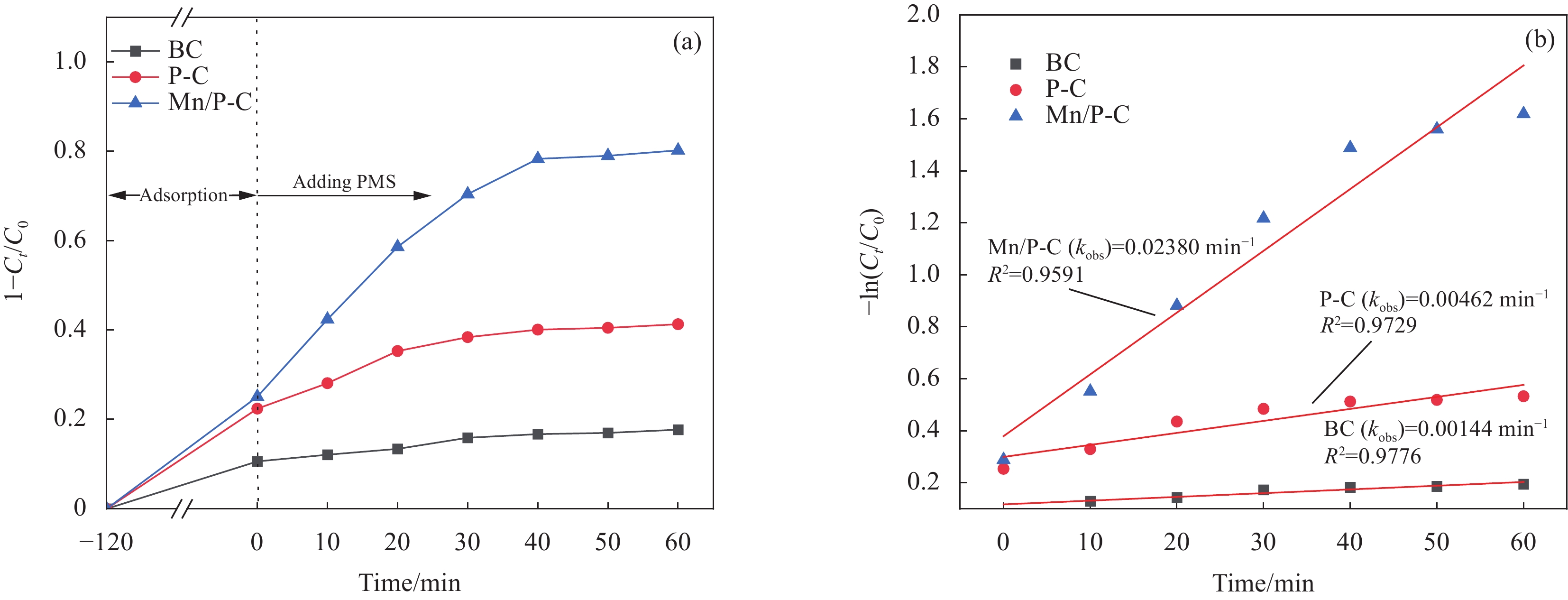
图 4 BC、P-C和Mn/P-C对NOR的降解去除率(a)及相应的降解反应速率常数(b) (反应条件:PMS=1.5 mmol/L;BC、P-C、Mn/P-C=0.5 g/L;NOR=10 mg/L;pH=6)
Figure 4. Degradation removal rate of NOR (a) and the corresponding degradation reaction rate constants (b) caused by BC, P-C and Mn/P-C (Reaction conditions: PMS=1.5 mmol/L; BC, P-C, Mn/P-C=0.5 g/L; NOR=10 mg/L; pH=6)
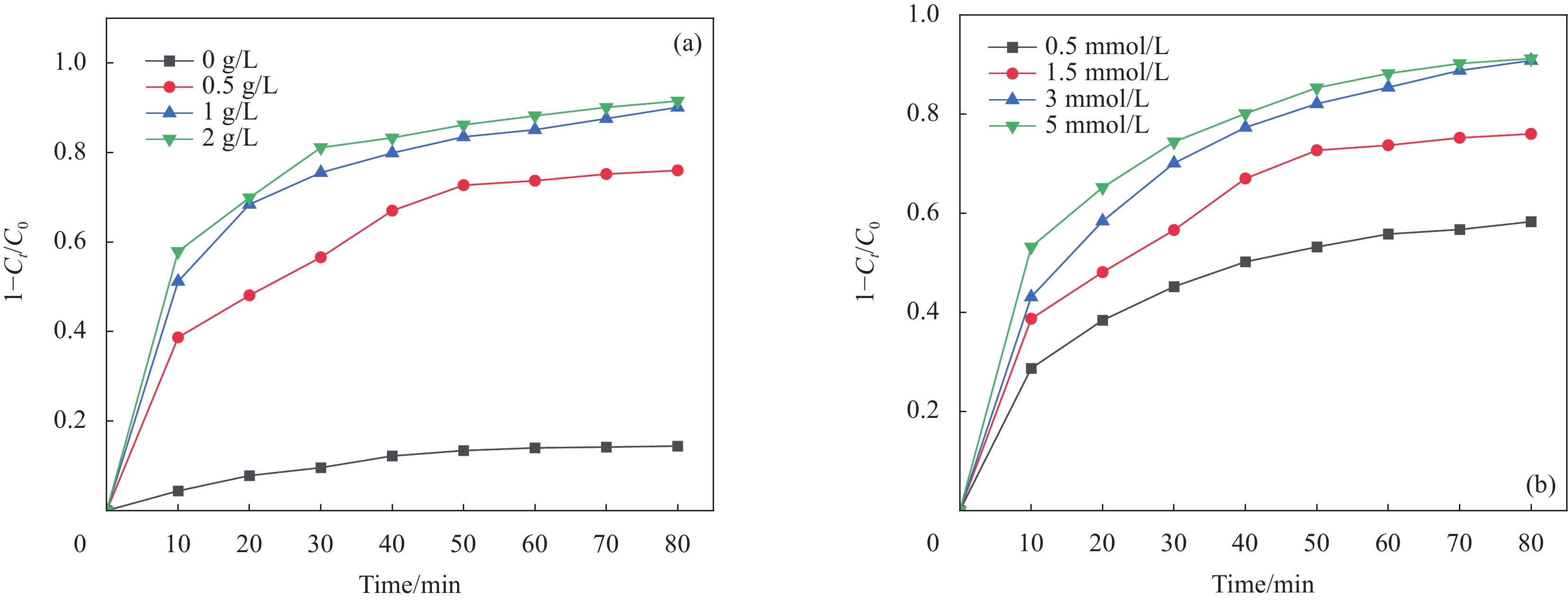
图 5 (a) Mn/P-C 投加量对NOR降解的影响(反应条件:PMS=1.5 mmol/L、pH=6、NOR=10 mg/L);(b) PMS浓度对NOR降解的影响(反应条件:Mn/P-C=0.5 g/L、pH=6、NOR=10 mg/L)
Figure 5. (a) Effect of Mn/P-C dosage for NOR degradation (Reaction conditions: PMS=1.5 mmol/L, pH=6, NOR=10 mg/L); (b) PMS concentrations for NOR degradation (Reaction conditions: Mn/P-C=0.5 g/L, pH=6, NOR=10 mg/L)
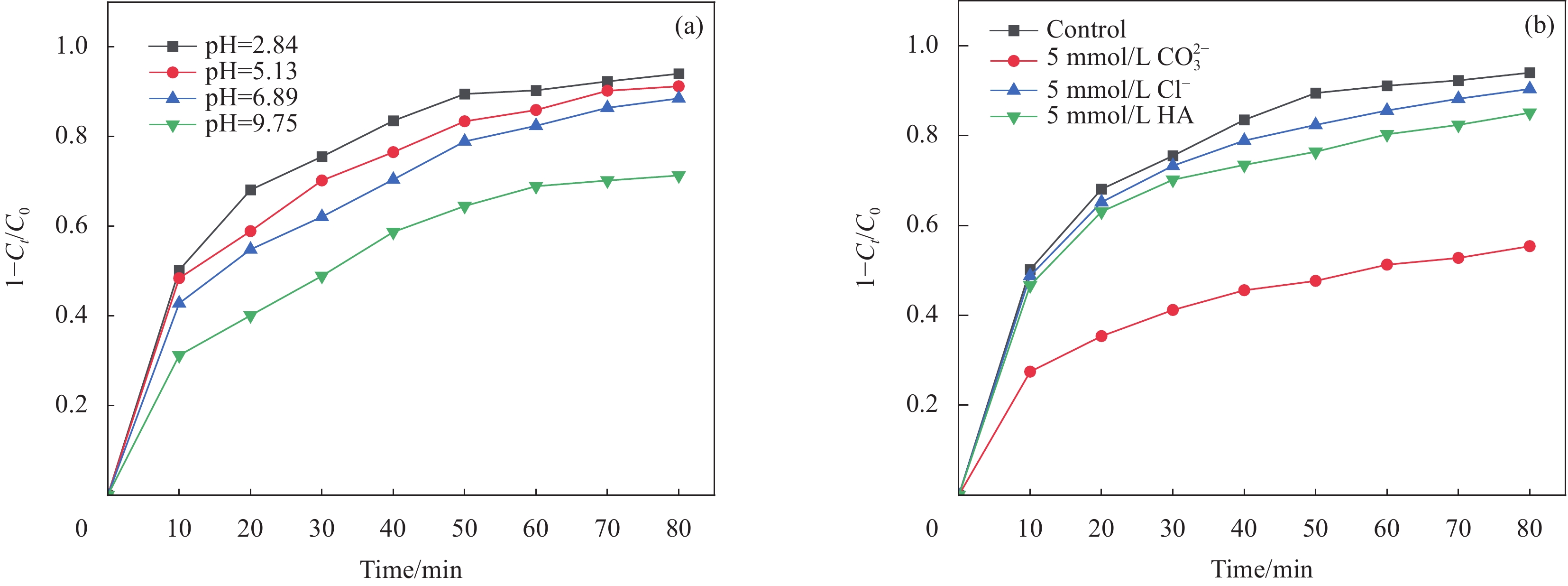
图 6 (a) pH值对NOR降解的影响(反应条件:Mn/P-C=1 g/L、PMS=3 mmol/L、NOR=10 mg/L);(b)阴离子和腐殖酸(HA)对NOR 降解的影响(反应条件:Mn/P-C=1 g/L、PMS=3 mmol/L、NOR=10 mg/L、Cl−=5 mmol/L、CO32−=5 mmol/L、HA=5 mmol/L)
Figure 6. (a) Effect of pH for NOR degradation (Reaction conditions: Mn/P-C=1 g/L, PMS=3 mmol/L, NOR=10 mg/L); (b) Anions and humic acid (HA) for NOR degradation (Reaction conditions: Mn/P-C=1 g/L, PMS=3 mmol/L, NOR=10 mg/L, Cl−=5 mmol/L, CO32−=5 mmol/L, HA=5 mmol/L)
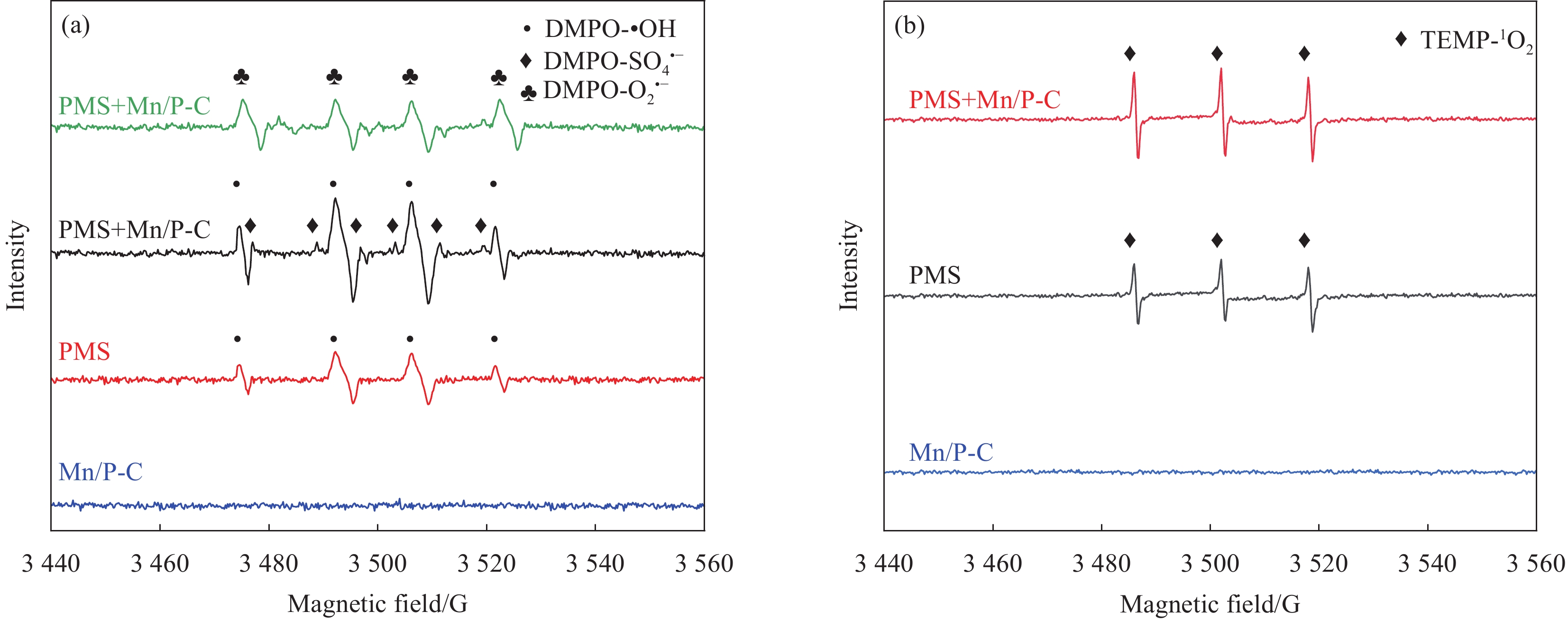
图 8 (a)以甲基-1-氧化吡咯琳(DMPO)为捕获剂的Mn/P-C、PMS和Mn/P-C活化PMS体系中SO4•−、•OH、O2•−的电子顺磁共振(EPR)图谱;(b)以2,2,6,6-四甲基-4-哌啶酮盐酸盐(TEMP)为捕获剂的Mn/P-C、PMS和Mn/P-C活化PMS体系中1O2的EPR图谱(实验条件: PMS=3 mmol/L、Mn/P-C=1 g/L,NOR=10 mg/L,pH=6)
Figure 8. (a) Electron paramagnetic resonance (EPR) spectra of SO4•−, •OH, O2•− radicals in Mn/P-C, PMS and Mn/P-C to activate PMS system with DMPO; (b) EPR spectra of 1O2 radical in Mn/P-C, PMS and Mn/P-C to activate PMS system with TEMP (Experimental conditions: PMS=3 mmol/L, Mn/P-C=1 g/L, NOR=10 mg/L, pH=6)
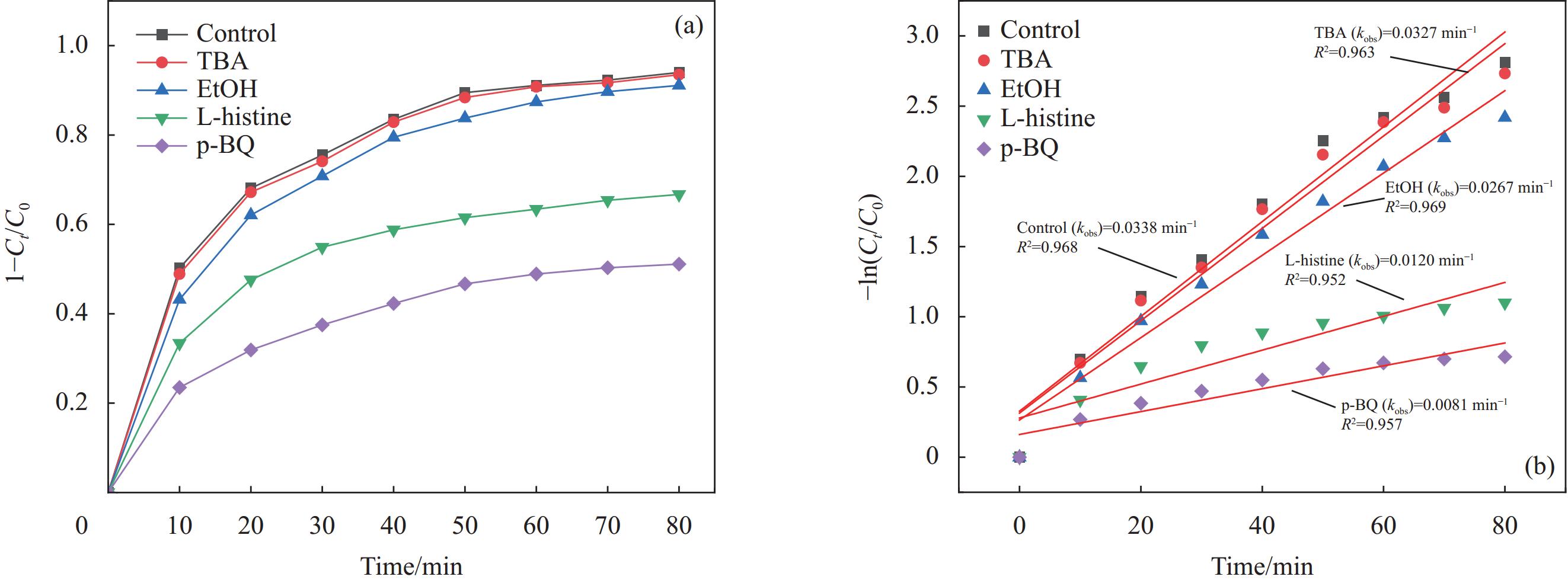
图 9 不同抑制剂无水乙醇(EtOH)、叔丁醇(TBA)、组氨酸(L-histidine)和对苯醌(p-BQ)对NOR的降解动力学(a)及相应的反应速率常数(b) (反应条件:PMS=3 mmol/L、Mn/P-C=1 g/L、NOR=10 mg/L、pH=6)
Figure 9. Degradation kinetics (a) and the corresponding degradation reaction rate constants (b) of NOR by different scavengers EtOH, TBA, L-histidine and p-BQ (Reaction conditions: PMS=3 mmol/L, Mn/P-C=1 g/L, NOR=10 mg/L, pH=6)
-
[1] JIANG X, GUO Y, ZHANG L, et al. Catalytic degradation of tetracycline hydrochloride by persulfate activated with nano Fe0 immobilized mesoporous carbon[J]. Chemical Engineering Journal, 2018, 341: 392-401. DOI: 10.1016/j.cej.2018.02.034
[2] XU M, DENG J, CAI A, et al. Comparison of UVC and UVC/persulfate processes for tetracycline removal in water[J]. Chemical Engineering Journal, 2020, 384: 123320. DOI: 10.1016/j.cej.2019.123320
[3] SCARIA J, ANUPAMA K V, NIDHEESH P V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management[J]. Science of the Total Environment, 2021, 771: 145291. DOI: 10.1016/j.scitotenv.2021.145291
[4] ZHANG X, CAI T, ZHANG S, et al. Contamination distribution and non-biological removal pathways of typical tetracycline antibiotics in the environment: A review[J]. Journal of Hazardous Materials, 2024, 463: 132862. DOI: 10.1016/j.jhazmat.2023.132862
[5] SONG Z, MA Y, LI C. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite[J]. Science of the Total Environment, 2019, 651: 580-590. DOI: 10.1016/j.scitotenv.2018.09.240
[6] LI Y, ZHANG G, LIANG D, et al. Tetracycline hydrochloride degradation in polarity inverted microbial fuel cells: Performance, mechanisms and microbiology[J]. Chemosphere, 2024, 349: 140902. DOI: 10.1016/j.chemosphere.2023.140902
[7] ZHANG Z, CHEN Y, WANG Z, et al. Effective and structure-controlled adsorption of tetracycline hydrochloride from aqueous solution by using Fe-based metal-organic frameworks[J]. Applied Surface Science, 2021, 542: 148662. DOI: 10.1016/j.apsusc.2020.148662
[8] XIANG W, ZHANG X, LUO J, et al. Performance of lignin impregnated biochar on tetracycline hydrochloride adsorption: Governing factors and mechanisms[J]. Environmental Research, 2022, 215: 114339. DOI: 10.1016/j.envres.2022.114339
[9] PENG H, WANG H, WANG L, et al. Efficient adsorption-photocatalytic removal of tetracycline hydrochloride over La2S3-modified biochar with S, N-codoping[J]. Journal of Water Process Engineering, 2022, 49: 103038. DOI: 10.1016/j.jwpe.2022.103038
[10] ZHENG J, XU Z, XIN S, et al. Low-temperature molten salt synthesis of Na, K-codoped g-C3N4 Fenton-like catalyst with remarkable TCH degradation performance in a wide pH range[J]. Materials Letters, 2022, 325: 132912. DOI: 10.1016/j.matlet.2022.132912
[11] ZHANG X, YAO Z, ZHOU Y, et al. Theoretical guidance for the construction of electron-rich reaction microcenters on C—O—Fe bridges for enhanced Fenton-like degradation of tetracycline hydrochloride[J]. Chemical Engineering Journal, 2021, 411: 128535. DOI: 10.1016/j.cej.2021.128535
[12] SHI X, WANG L, ZUH A, et al. Photo-Fenton reaction for the degradation of tetracycline hydrochloride using a FeWO4/BiOCl nanocomposite[J]. Journal of Alloys and Compounds, 2022, 903: 163889. DOI: 10.1016/j.jallcom.2022.163889
[13] LIU Y, LI J, WU L, et al. Synergetic adsorption and Fenton-like degradation of tetracycline hydrochloride by magnetic spent bleaching earth carbon: Insights into performance and reaction mechanism[J]. Science of the Total Environment, 2021, 761: 143956. DOI: 10.1016/j.scitotenv.2020.143956
[14] REN W, CHENG C, SHAO P, et al. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes[J]. Environmental Science & Technology, 2021, 56(1): 78-97.
[15] USHANI U, LU X, WANG J, et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the-art review[J]. Chemical Engineering Journal, 2020, 402: 126232. DOI: 10.1016/j.cej.2020.126232
[16] ZHOU Z, LIU X, SUN K, et al. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review[J]. Chemical Engineering Journal, 2019, 372: 836-851. DOI: 10.1016/j.cej.2019.04.213
[17] SONG W, LI J, WANG Z, et al. A mini review of activated methods to persulfate-based advanced oxidation process[J]. Water Science and Technology, 2019, 79(3): 573-579. DOI: 10.2166/wcc.2018.168
[18] LI F, DUAN F, JI W, et al. Biochar-activated persulfate for organic contaminants removal: Efficiency, mechanisms and influencing factors[J]. Ecotoxicology and Environmental Safety, 2020, 198: 110653. DOI: 10.1016/j.ecoenv.2020.110653
[19] MISERLI K, KOGOLA D, PARASCHOUDI I, et al. Activation of persulfate by biochar for the degradation of phenolic compounds in aqueous systems[J]. Chemical Engineering Journal Advances, 2022, 9: 100201. DOI: 10.1016/j.ceja.2021.100201
[20] OUYANG D, CHEN Y, YAN J, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. DOI: 10.1016/j.cej.2019.03.235
[21] YUAN T, TAHMASEBI A, YU J. Comparative study on pyrolysis of lignocellulosic and algal biomass using a thermogravimetric and a fixed-bed reactor[J]. Bioresource Technology, 2015, 175: 333-341. DOI: 10.1016/j.biortech.2014.10.108
[22] LIU X, RAO L, YAO Y, et al. Phosphorus-doped carbon fibers as an efficient metal-free bifunctional catalyst for removing sulfamethoxazole and chromium(VI)[J]. Chemosphere, 2020, 246: 125783. DOI: 10.1016/j.chemosphere.2019.125783
[23] HUANG D, ZHANG Q, ZHANG C, et al. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline[J]. Chemical Engineering Journal, 2020, 391: 123532. DOI: 10.1016/j.cej.2019.123532
[24] HUANG P, ZHANG P, WANG C, et al. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar[J]. Applied Catalysis B: Environmental, 2022, 303: 120926. DOI: 10.1016/j.apcatb.2021.120926
[25] ZHONG Q, LIN Q, HUANG R, et al. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar[J]. Chemical Engineering Journal, 2020, 380: 122608. DOI: 10.1016/j.cej.2019.122608
[26] WANG C, HOLM P E, ANDERSEN M L, et al. Phosphorus doped cyanobacterial biochar catalyzes efficient persulfate oxidation of the antibiotic norfloxacin[J]. Bioresource Technology, 2023, 388: 129785. DOI: 10.1016/j.biortech.2023.129785
[27] 陈思良, 孙雯, 洪耀良. 氮掺杂生物炭负载CuS活化过硫酸盐去除橙黄G[J]. 中国环境科学, 2024, 44(5): 2483-2494. DOI: 10.3969/j.issn.1000-6923.2024.05.011 CHEN Siliang, SUN Wen, HONG Yaoliang. Removal of orange G by nitrogen-doped biochar loaded with CuS activated persulfate[J]. China Environmental Science, 2024, 44(5): 2483-2494(in Chinese). DOI: 10.3969/j.issn.1000-6923.2024.05.011
[28] XU L, FU B, SUN Y, et al. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practival application[J]. Chemical Engineering Journal, 2020, 400: 125870.
[29] 黄仕元, 林森焕, 董雯, 等. 锰氮共掺杂稻壳生物炭活化过二硫酸盐降解酸性橙[J]. 复合材料学报, 2023, 40(2): 1071-1084. HUANG Shiyuan, LIN Senhuan, DONG Wen, et al. Manganese-nitrogen co-doped rice husk biochar activated peroxydisulfate to degrade acid orange[J]. Acta Materiae Compositae Sinica, 2023, 40(2): 1071-1084(in Chinese).
[30] SHI C, HU K, NIE L, et al. Degradation of acetaminophen using persulfate activated with P-doped biochar and thiosulfate[J]. Inorganic Chemistry Communications, 2022, 146: 110160. DOI: 10.1016/j.inoche.2022.110160
[31] HUNG C, CHEN C, HUANG C, et al. Metal-free single heteroatom (N, O, and B)-doped coconut-shell biochar for enhancing the degradation of sulfathiazole antibiotics by peroxymonosulfate and its effects on bacterial community dynamics[J]. Environmental Pollution, 2022, 311: 119984. DOI: 10.1016/j.envpol.2022.119984
[32] ZHONG S, PAN J, TIAN K, et al. Efficient degradation of p-chlorophenol by N, S-codoped biochar activated perxymonosulfate[J]. Process Safety and Environmental Protection, 2023, 169: 437-446. DOI: 10.1016/j.psep.2022.10.081
[33] QIU X, YANG S, DZAKPASU M, et al. Attenuation of BPA degradation by SO4•− in a system of peroxymonosulfate coupled with Mn/Fe MOF-templated catalysts and its synergism with Cl− and bicarbonate[J]. Chemical Engineering Journal, 2019, 372: 605-615. DOI: 10.1016/j.cej.2019.04.175
[34] GAO L, GUO Y, ZHAN J, et al. Assessment of the validity of the quenching method for evaluating the role of reactive species in pollutant abatement during the persulfate-based process[J]. Water Research, 2022, 221: 118730. DOI: 10.1016/j.watres.2022.118730
[35] GAO Y, WANG Q, JI G, et al. Degradation of antibiotic pollutants by persulfate activated with various carbon materials[J]. Chemical Engineering Journal, 2022, 429: 132387. DOI: 10.1016/j.cej.2021.132387
[36] HUSSAIN I, LI M, ZHANG Y, et al. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol[J]. Chemical Engineering Journal, 2017, 311: 163-172. DOI: 10.1016/j.cej.2016.11.085
[37] WU Y, GUO J, HAN Y, et al. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline[J]. Chemosphere, 2018, 200: 373-379. DOI: 10.1016/j.chemosphere.2018.02.110
[38] LIU Y, GUO H, ZHANG Y, et al. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of rhodamine B[J]. Chemical Physics Letters, 2016, 653: 101-107. DOI: 10.1016/j.cplett.2016.04.069
[39] LUO R, LI M, WANG C, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition[J]. Water Research, 2019, 148: 416-424. DOI: 10.1016/j.watres.2018.10.087
[40] YANG S, GUO, X, WANG Z, et al. Significance of B-site cobalt on bisphenol A degradation by MOFs-templated Co xFe3− xO4 catalysts and its severe attenuation by excessive cobalt-rich phase[J]. Chemical Engineering Journal, 2019, 359: 552-563. DOI: 10.1016/j.cej.2018.11.187
[41] WANG B, LI Y, WANG L. Metal-free activation of persulfates by corn stalk biochar for the degradation of antibiotic norfloxacin: Activation factors and degradation mechanism[J]. Chemosphere, 2019, 237: 124454. DOI: 10.1016/j.chemosphere.2019.124454
[42] WANG L, LAN X, PENG W, et al. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review[J]. Journal of Hazard Materials, 2021, 408: 124436. DOI: 10.1016/j.jhazmat.2020.124436
[43] ZHANG J, HAN J, WANG M, et al. Fe3O4/PANI/MnO2 core-shell hybrids as advanced adsorbents for heavy metal ions[J]. Journal of Materials Chemistry A, 2017, 5(8): 4058-4066. DOI: 10.1039/C6TA10499A
[44] SHAO P, TIAN J, YANG F, et al. Identification and regulation of active sites on nanodiamonds: Establishing a highly efficient catalytic system for oxidation of organic contaminants[J]. Advanced Functional Materials, 2018, 28(13): 17052.
[45] TAN L, TONG Y, YANG Y, et al. Degradation of tetracycline using persulfate activated by Mn, Ce co-doped g-C3N4 composites under visible light[J]. Diamond and Related Materials, 2023, 140: 110522. DOI: 10.1016/j.diamond.2023.110522
-
期刊类型引用(1)
1. 刘晓军,战丽,邹爱玲,李志坤,赵俨梅,王绍宗. 纤维增强复合材料层间增韧技术研究进展. 复合材料科学与工程. 2022(01): 117-128 .  百度学术
百度学术
其他类型引用(1)
-
目的
近年来随着工业和城市快速发展,抗生素类药物在国内外被广泛使用,对于自然环境和人类健康而言,过度使用会带来基因抗药性,并且排放后的抗生素会随着生物链不断累积,对生态环境造成危害。因此加强抗生素的生态环境危害机制研究,深化末端治理,降低抗生素等新污染物的环境风险成为了国内外废水处理研究的热点。本研究制备了Mn、P掺杂玉米秸秆生物炭(Mn/P-C)用于活化过一硫酸盐(PMS)降解诺氟沙星(NOR),优化生物炭催化剂在活化过一硫酸盐中的应用,并为水体中抗生素类有机污染物的降解提供可行策略。
方法以NOR为目标污染物,以玉米秸秆、磷酸三丁酯(CH)PO和四水合氯化锰MnCl·4HO为原料,通过热解法制备Mn/P-C,利用SEM、XRD、FTIR等表征方法对改性生物炭催化剂的结构特征进行了分析,结合反应前后XPS化学元素变化、淬灭实验和电子顺磁共振(EPR)检测,探讨了Mn/P-C活化PMS体系降解NOR的潜在机制。此外,还研究了不同PMS浓度,催化剂用量,初始pH值和实际水体常见无机阴离子、腐殖酸对降解效能的影响。
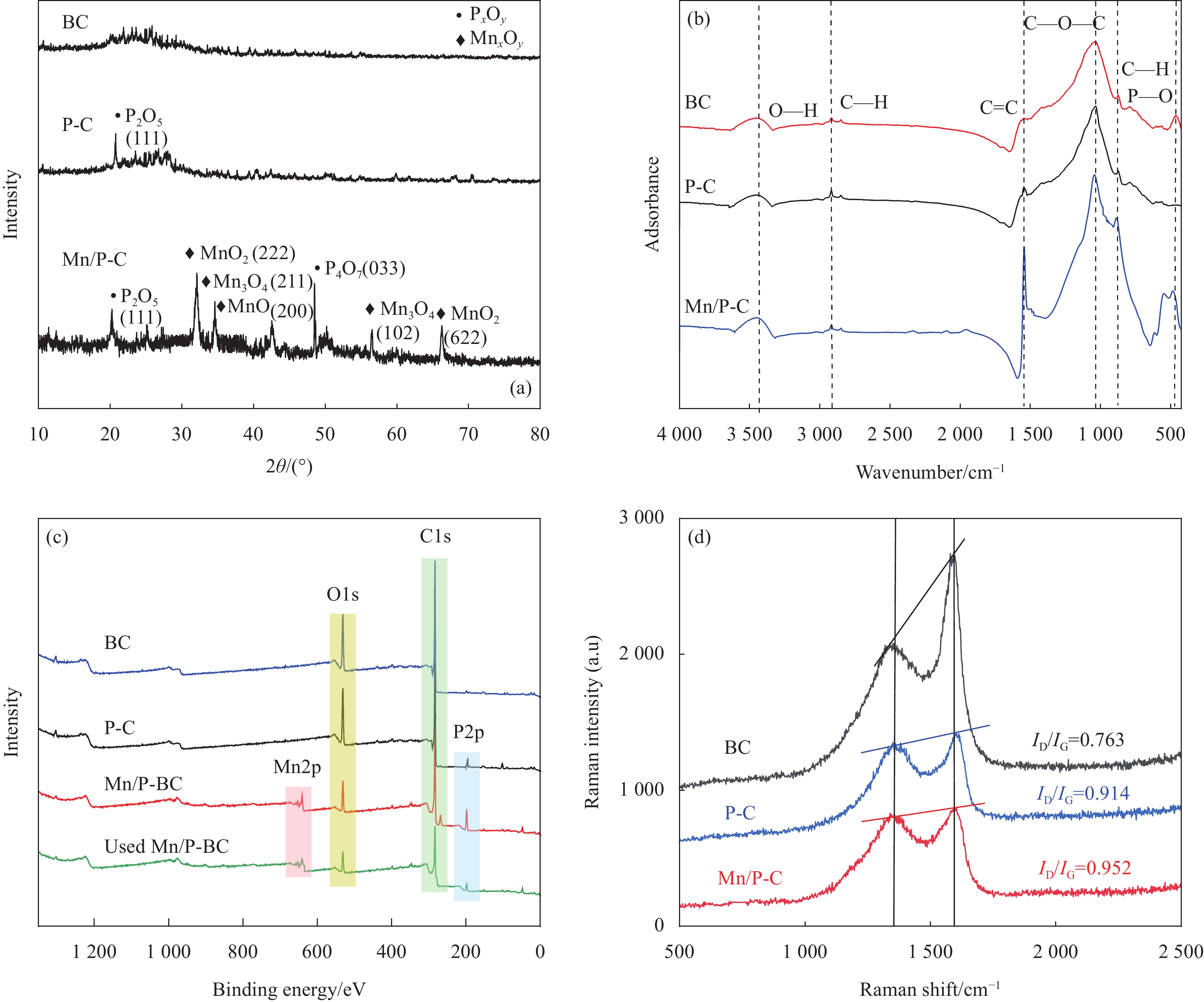
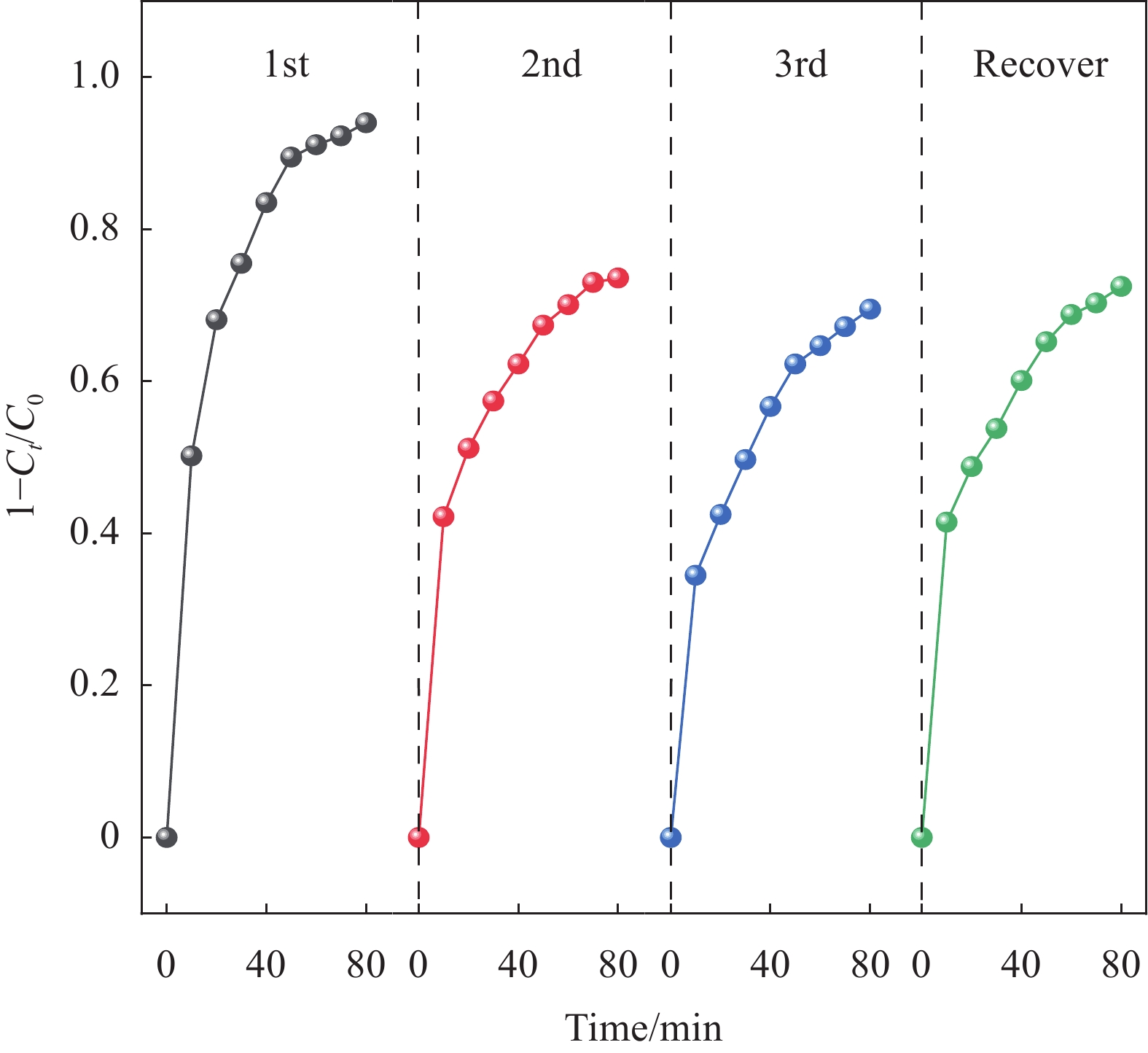
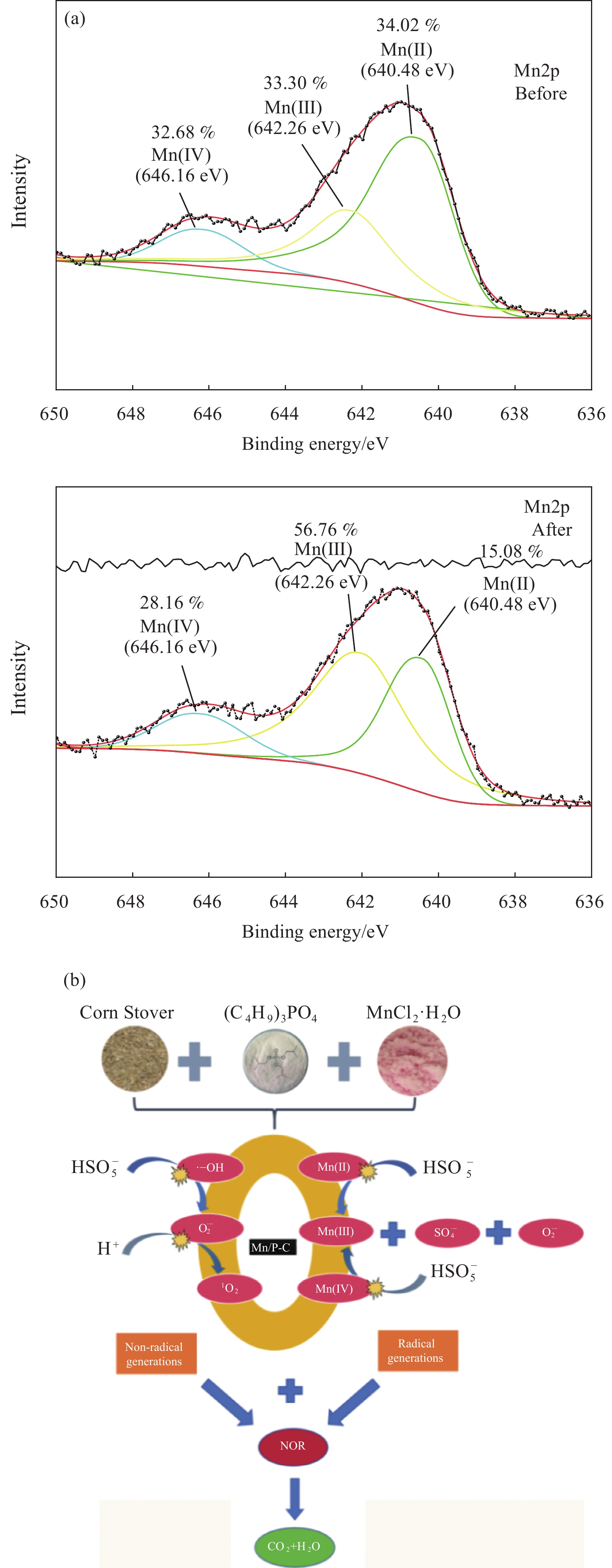
结果本研究成功制备了Mn、P共掺杂生物炭(Mn/P-C),并将其作为催化剂活化过一硫酸盐(PMS)降解诺氟沙星(NOR)。总结如下:(1)相较于原始生物炭(BC)和P掺杂生物炭(P-C),Mn/P-C具有更好的吸附和降解性能。(2)从Mn/P-C的表征结果可以看出Mn、P成功掺杂后,催化剂的形貌发生了巨大变化,可以观察到堆叠复杂的褶皱片状,使得催化剂具有更大的缺陷结构和丰富的表面含氧官能团,为NOR的降解提供了更多的活化位点。(3)在催化降解过程中,存在Mn(II)向Mn(III)的转化以及Mn(IV)向Mn(III)的转化,金属元素Mn的掺杂改变了生物炭催化剂的晶型组成及元素分布,使得催化剂活性位点增加,电子传导能力提高,降解性能提高。(4)综合EPR检测以及淬灭反应,在Mn/P-C活化PMS降解NOR过程中存在自由基及非自由基的共同作用,发挥主要作用的活性氧化物质是SO、O和O。(5)此外,Mn/P-C催化剂在较宽的pH范围内均有效,具有较高的可重复利用性和稳定性。水体中常见的CO、Cl以及腐殖酸HA均抑制了Mn/P-C的催化性能。
结论根据“以废治废”的目的,以天然废弃物为原料制备获得的生物炭催化剂在活化过硫酸盐降解抗生素类废水领域吸引了越来越多的研究,本文成功制备的Mn/P-C具有催化性能高、自由基产生持久、催化剂可回收性强等特点,可以有效优化生物炭催化剂在活化过一硫酸盐中的应用,并为水体中抗生素类有机污染物的降解提供可行策略。本研究为制备更加高效清洁的催化剂活化过硫酸盐降解NOR提供了系统、全面的研究,探讨了Mn/P-C活化PMS体系存在的降解机制,提出了一种新的电子转移路径。




 下载:
下载: