Degradation of bisphenol F by activated of peroxymonosulfate using sludge biochar loaded with cobalt iron bimetallic catalyst
-
摘要:
近年来,污水处理厂的大规模建设导致污泥产量逐年增加,污泥的处理面临严峻挑战,双酚F (BPF)被广泛应用于工业中化学添加剂,在地表水、土壤和污泥中被频繁检出。本文利用市政污泥负载钴铁双金属制备了钴铁双金属@污泥生物炭(CoFeO@SBC)复合材料,通过活化过一硫酸盐(PMS)降解BPF来探究其催化性能。采用SEM、比表面积测定(BET)、IR、XRD和XPS等表征分析所制备材料的理化性质;并考察了材料投加量、PMS投加量、初始pH和无机阴离子对CoFeO@SBC/PMS体系降解BPF效果的影响。结果表明:CoFeO与SBC复合后孔隙结构显著优化,比表面积增加了6.0倍,且具备更丰富的氧空位和还原性—OH官能团,产生了更多的Fe(II)和Co(II)。因此,CoFeO@SBC具有优异的催化活性,投加量为0.04 g/L时可以在10 min内几乎完全降解BPF(5 mg/L),降解速率与CoFeO相比提高了62%;Cl−和NO−3对体系降解效果影响较小,而HCO−3具有显著的抑制作用;通过EPR分析表明CoFeO@SBC/PMS体系存在羟基(•OH)和硫酸根(SO•−4)自由基及单线态氧(1O2)和超氧(O•−2),同时自由基淬灭实验证明,SO•−4是体系降解BPF的关键活性氧物种;最后通过液相色谱-质谱联用(LC-MS)对BPF的降解产物进行分析,揭示BPF在体系中的主要降解途径和机制。
Abstract:In recent years, the large-scale construction of wastewater treatment plants has led to an increase in the production of sludge year after year, and the treatment of sludge is facing a serious challenge. Bisphenol F (BPF), which is widely used as a chemical additive in the industry, has been frequently detected in surface water, soil and sludge. By using municipal sludge loaded with cobalt-iron bimetal, cobalt-iron bimetallic@sludge biochar (CoFeO@SBC) were prepared, and the catalytic performance was assessed by activating peroxymonosulfate (PMS) to degrade BPF. SEM, specific surface area determination (BET), IR, XRD, and XPS were used to characterise and analyse the physicochemical properties of the prepared materials. The effects of the dosage of the materials, the dosage of the PMS, the initial pH, and the inorganic anions on the degradation of the BPF by the CoFeO@SBC/PMS system were investigated. The results showed that the pore structure of CoFeO was significantly improved after compounding with SBC, and the specific surface area was increased by 6.0 times. Moreover, CoFeO@SBC composites showed richer oxygen vacancies and —OH functional groups, which led to higher production of Fe(II) and Co(II). Therefore, CoFeO@SBC composites had excellent catalytic activity, which could almost completely degrade BPF (5 mg/L) within 10 min at the dosage of 0.04 g/L, and the degradation rate was 62% higher than that of CoFeO; Cl− and NO−3 showed less influence on the degradation effect of the system, while HCO−3 had a significant inhibitory effect; EPR analysis shows that there are hydroxyl (•OH) and sulfate (SO•−4) free radicals as well as singlet oxygen (1O2) and superoxide (O•−2) free radicals in the CoFeO@SBC/PMS system. At the same time, the free radical quenching experiment, it is proved that SO•−4 is the key reactive oxygen species for the degradation of BPF in the system. Finally, the degradation products of BPF were identified by liquid chromatography-mass spectrometry (LC-MS), which could elucidate the primary degradation pathways and mechanisms in CoFeO@SBC/PMS system.
-
Keywords:
- bisphenol F /
- degradation /
- cobalt-iron bimetal /
- sludge biochar /
- peroxymonosulfate /
- radicals
-
近年来,内分泌干扰物的过度使用对人体健康和生态环境产生了严重威胁。双酚类化合物(BPs)是一类常见的内分泌干扰物[1],主要包括双酚A (BPA)、双酚F (BPF)和双酚S (BPS)等,其中BPA是工业中使用最广泛的化学添加剂之一,但由于其内分泌干扰特性和不良影响,越来越多的产品已限制或禁止使用BPA[2]。BPF具有与BPA相似的结构和性质,是其主要替代品,也逐渐成为生产环氧树脂、涂料和聚碳酸酯树脂等塑料制品的重要化学原料,被广泛应用于人类的日常生产活动中[3]。令人担忧的是,研究发现BPF也会带来一些新的环境问题,在雄激素、抗雄激素、雌激素和抗雌激素活性方面的影响与BPA相似,有时甚至更严重[4]。目前,BPF产量每年都在增加,导致其在地表水[5]、废水[6]、沉积物[7]和污水处理厂[8]中被频繁检出。因此迫切需要一种绿色而高效的技术方法来去除环境中的BPF。
过一硫酸盐(PMS)作为一种人们广泛关注的强氧化剂,因其能产生大量的自由基如羟基自由基(•OH)、硫酸根自由基(SO•−4)和超氧自由基(O•−2)而在高级氧化过程中发挥重要作用[9-11]。有研究表明[12-14],PMS可广泛应用于有机污染物的非选择性氧化,包括药物、挥发性有机物、内分泌干扰物和全氟化合物等。利用矿物、过渡金属、非金属化合物、电场和高强度光源等可以提高PMS的活化效率,综合考虑价格和活化效率,过渡金属活化是最常用的PMS催化方法[15]。其中,铁(Fe)是最常见的过渡金属,来源广泛、绿色环保,具有一定的PMS催化活性[16],另外钴(Co)也被认为是活化PMS最有效的过渡金属之一,由于其氧化还原电位与PMS相似,同时它也具有环保的特性和相对低廉的价格[17]。此外,还有研究发现双金属催化剂(Co-Fe)比单金属催化剂(Co或Fe)能更好地提高PMS利用效率[18]。然而,Co-Fe双金属催化剂仍不足以满足实际复杂废水应用的要求,且容易出现团聚等不良现象,其催化效率仍有提升的空间。
生物炭(BC)是生物质原料在无氧或限氧条件下通过热解或者水热碳化形成的多孔材料[19]。近年来,由于污水处理厂的大规模建设导致污泥产量逐年增加,采用市政污泥制备生物炭提供了一种经济环保的污泥处理方式[20-21]。研究表明,污泥生物炭(SBC)不仅具有较大的比表面积,可以作为催化剂载体,而且富含含氧官能团、C/O缺陷结构[22]。将污泥生物炭负载Co-Fe双金属,不仅可以优势互补,提高材料的理化特性,可以还可实现市政污泥变废为宝,具有重要的实际意义。
综上,本文采用某污水处理厂的脱水污泥(SBC)制备污泥生物炭并负载Co-Fe双金属(CoFeO),制备新型绿色高效的CoFeO@SBC催化剂,探究其活化PMS的性能并用于降解BPF。同时,目前基于SO•−4等自由基对BPF的降解途径和机制尚不清楚,不利于阐明它们的环境降解行为。因此,本文结合实验分析进一步探索其降解途径和机制,为环境真实污染水体的修复提供科学依据。
1. 材料与方法
1.1 原材料
过硫酸氢钾(KHSO5)、L-组氨酸(L-his)、叔丁醇(TBA)、九水硝酸铁(Fe(NO3)3·9H2O)和六水硝酸钴(Co(NO3)2·6H2O)购于阿拉丁试剂有限公司。双酚F(BPF,>99.5%)、无水乙醇(EtOH)和对苯醌(p-BQ)购于上海麦克林生化科技有限公司。甲醇(MeOH)和氯化钠(NaCl)购于德国CNW公司。碳酸氢钠(NaHCO3)和硝酸钠(NaNO3)购于国药集团化学试剂有限公司。尿素(CO(NH₂)₂)和氟化铵(NH4F)购于大茂化学试剂厂。所有试剂均为分析纯,使用时无需进一步提纯。实验溶液使用去离子水(18.2 MΩ)进行配制,使用0.1 mol/L NaOH和HCl溶液调节初始pH。
1.2 SBC、CoFeO和CoFeO@SBC的制备
污泥生物炭(SBC)制备方法如下:取适量脱水污泥(含水率35%)置于陶瓷坩埚然后移入马弗炉(SX2-4-10N,上海一恒科技有限公司)中,以26℃/min恒定速率加热至500℃,恒温热解120 min。冷却后研磨过100 μm筛网(承泽丝网制品加工厂),放入100 mL不锈钢高压釜中,在120℃下保温12 h制得污泥生物炭SBC。污泥生物炭负载钴铁双金属复合材料制备方法如下:以SBC为原料,采用水热法制备钴铁改性污泥生物炭复合材料。首先,将摩尔比1∶1的Co(NO3)2·6H2O和Fe(NO3)3·9H2O、CO(NH₂)₂、NH4F及0.1 g SBC加入到100 mL不锈钢高压釜中,在120℃下保温12 h,制得材料CoFeO@SBC,CoFeO材料的制备方法与上述步骤相同,但不投加SBC。
1.3 表征方法
采用场扫描电子显微镜(SEM,HITACHI SU8010,Hitachi)对样品的形貌进行表征;用X射线衍射仪(XRD,D8 ADVANCE,Bruker)对材料进行晶体结构鉴定;用Brunauer-Emmett-Teller (BET,ASAP 2460,美国麦克仪器) N2吸附-脱附等温线分析材料的比表面积及孔隙结构等参数;用红外光谱仪(IR,NICOLET IS10,Thermo Fisher Scientific)对样品表面的官能团进行分析;用X射线光电子能谱仪(XPS,Thermo ESCALAB 250XI,Thermo Fisher Scientific)分析样品表面的化学元素状态;用液相色谱-质谱联用仪(LC-MS,AB Sciex,API4000,Thermo Fisher Scientific)进行降解产物的鉴定。BPF的测定参考课题组之前的方法[23],质谱条件:选择反应监测(Selected reaction monitoring,SRM)扫描模式,电喷雾离子源(Electrospray ionization,ESI)。使用负离子电离模式,喷雾电压2.5 kV。离子源温度 350℃,鞘气20.7 kPa,辅助气5.18 kPa,离子传输毛细管温度350℃,碰撞气0.2 Pa。液相条件:Thermo Accucore Biphenyl 液相色谱柱(100 mm×2.1 mm,2.6 μm,美国Thermo公司),柱温为室温,流动相A为蒸馏水,流动相B为乙腈,流速0.3 mL/min,进样量为5 μL。
1.4 降解实验方法
用超纯水配制50 mL BPF溶液(5 mg/L)于锥形瓶中后置于磁力搅拌器上,在25℃、150 r/min条件下恒温搅拌。分别考察材料投加量、PMS投加量、初始pH和无机阴离子对体系中BPF的降解情况。在第0、2、5、8和10 min用注射器抽取1 mL溶液,并用0.5 mL无水乙醇淬灭,然后立即采用聚四氟乙烯(PTFE)针式过滤器(0.22 μm)过滤。
反应过程中的降解动力学常数可以采用一级动力学模型来研究,表观速率常数计算公式如下:
ln(Ct/C0)=−kobst (1) 式中:Ct为时间t (min)后BPF的浓度(mg/L);C0表示反应前BPF的浓度(mg/L);kobs代表反应速率常数。
2. 结果与分析
2.1 材料的表征结果
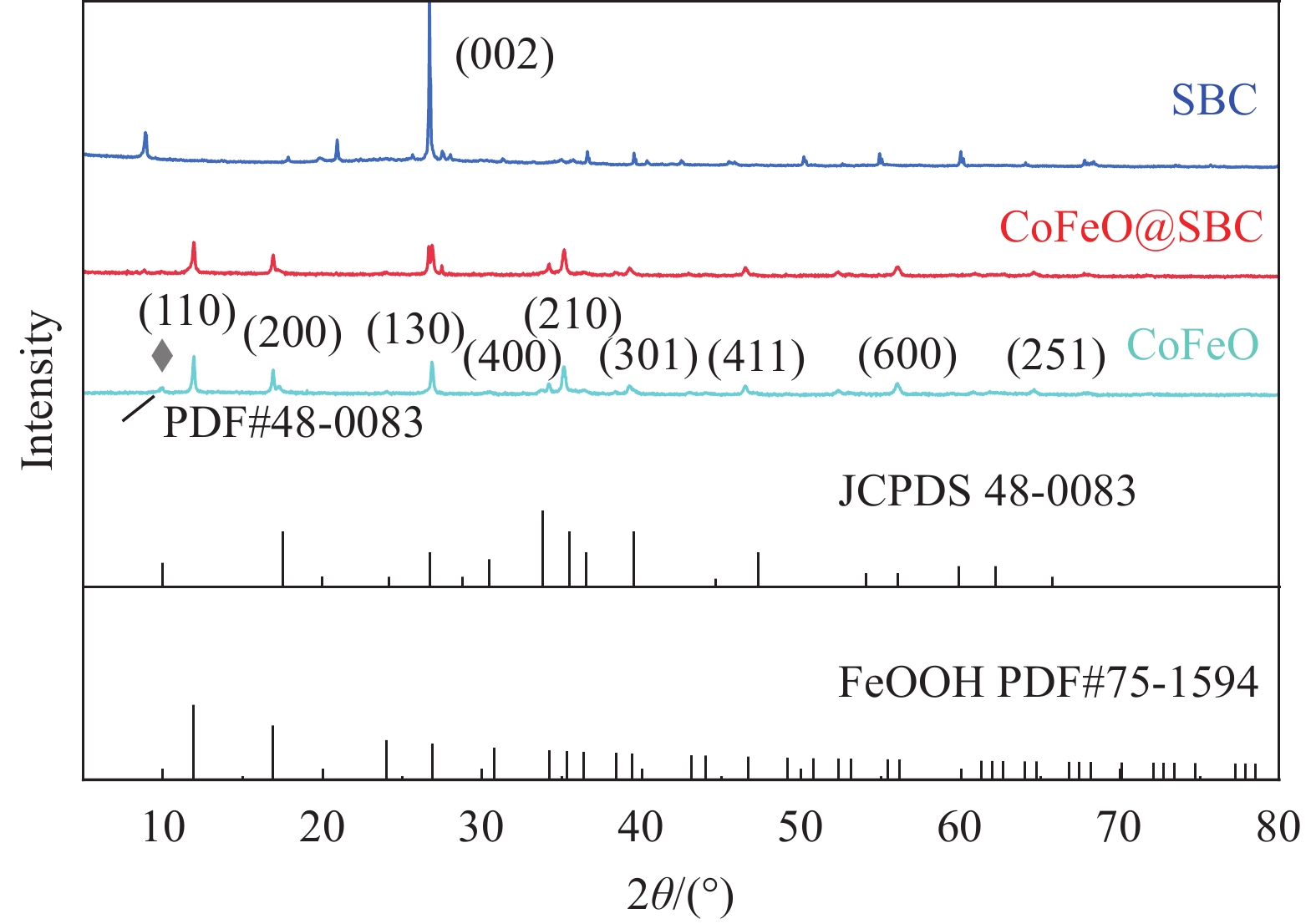
通过XRD图谱确定不同复合材料的晶体结构,不同样品的广角X射线衍射如图1所示,SBC、CoFeO@SBC和CoFeO的衍射峰尖锐而强烈,表明它们具有高度结晶的性质。SBC的衍射峰位于2θ=26.5°,属于典型的(002)平面相应的石墨无定形碳结构(JCPDS 41-1487)[24]。CoFeO复合材料在2θ值为12°、16.8°、26.9°、34.2°、35.2°、39.1°、46.5°、56.0°和64.5°有9个衍射峰,分别与FeOOH的(110)、(200)、(130)、(400)、(210)、(301)、(411)、(600)和(251)高度匹配(JCPDS 75-1594)[25]。位于9.8°衍射峰对为斜方碳酸钴氢氧化物水合物(Co(CO3)0.5(OH)·0.11H2O,JCPDS 48-0083)[26]。对于CoFeO@SBC,SBC的加入对四方FeOOH的晶体结构几乎没有变化。由以上结果可得,本实验成功制备了CoFeO@SBC催化剂,且催化剂具有较好的结晶度。
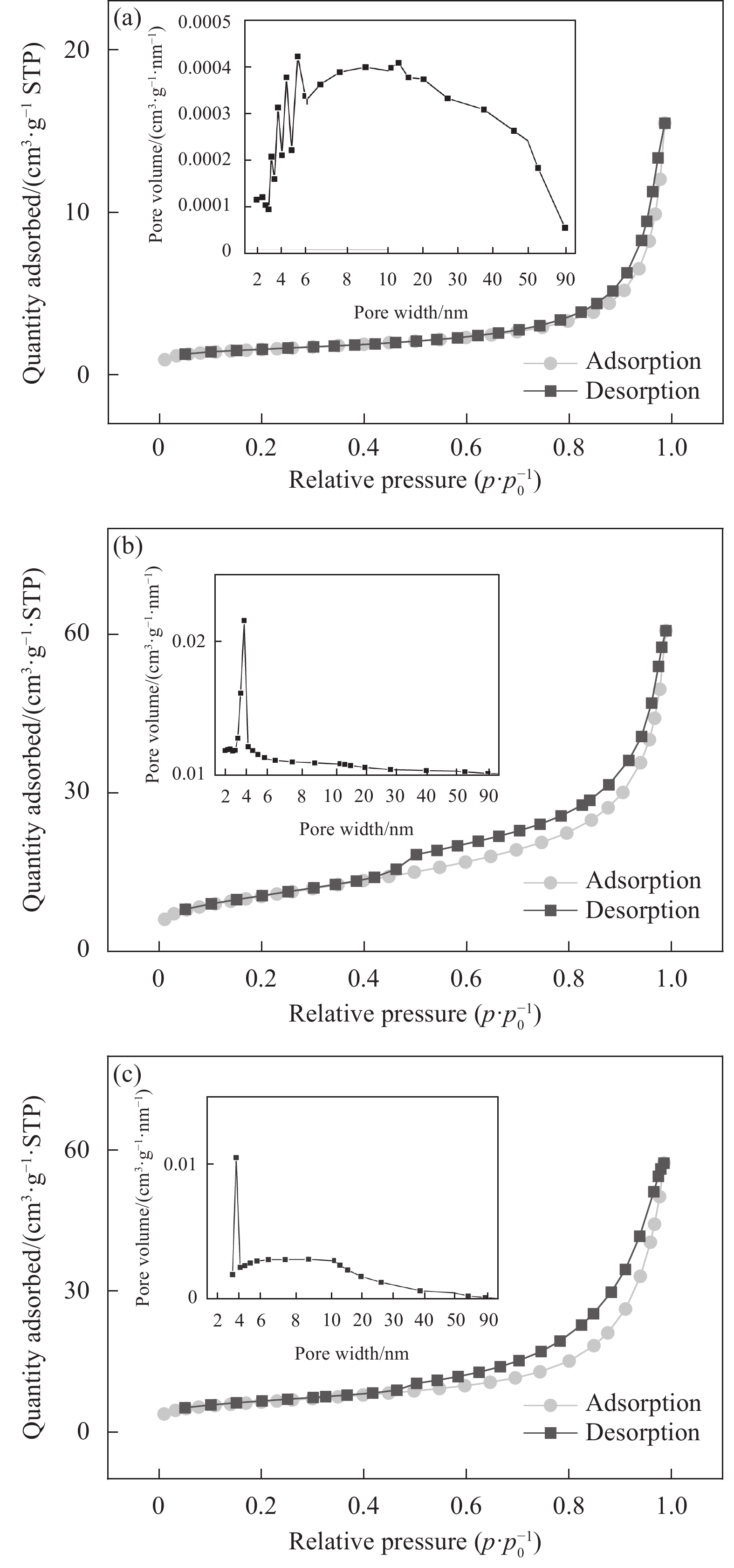
CoFeO、CoFeO@SBC和SBC的N2吸附-解吸等温线曲线如图2(a)~2(c)所示,图2(a)的吸附脱附曲线在相对压力(p/p0)为0.4~0.6较图2(b)和图2(c)更平滑,呈现出具有H1滞后环的IV型等温线,而图2(b)和图2(c)呈缓慢的上升趋势,呈台阶变化,属II型等温线,3种材料均具有典型的介孔结构,孔径分布主要在2~50 nm[27]。3种材料在p/p0=0.8附近存在突然的H2滞后环,在p/p0接近1时无限制吸附,通常被认为是板状颗粒聚集产生的狭缝孔[28]。如表1所示,CoFeO比表面积为5.2699 m²/g,与SBC复合后比表面积增加至37.1189 m²/g,增加了6.0倍。此外,尽管复合后平均孔径有所减少,但由于SBC的添加,其孔隙体积有显著增加。这些结果表明,与SBC复合后改善了CoFeO的孔隙结构,可以为PMS提供更多的活化位点,从而提高复合材料的PMS催化性。
表 1 不同材料的表面结构特征Table 1. Surface structure characterization of different materialsSample Surface area/
(m2·g−1)Average pore diameter/nm Pore volume/
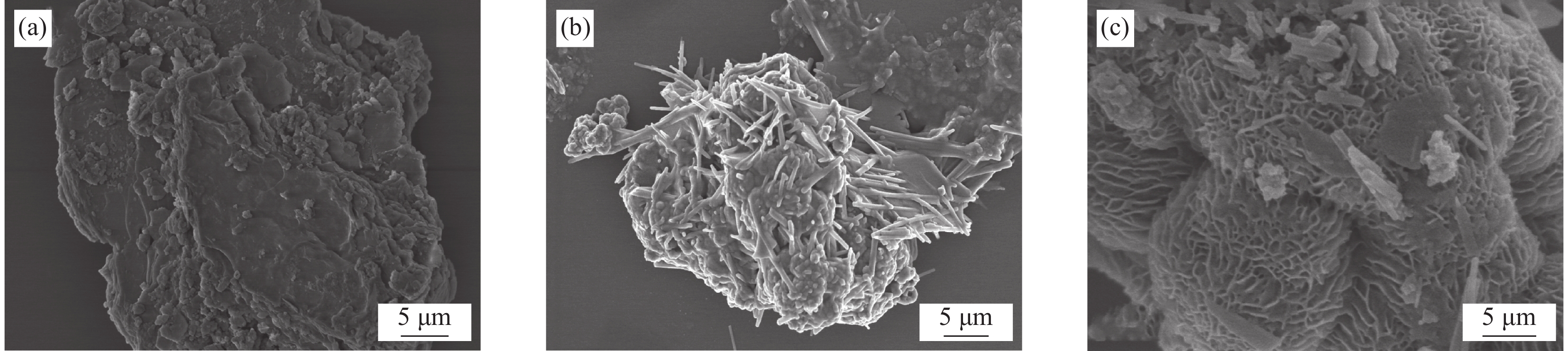
(cm3·g−1)SBC 22.0203 15.88640 0.088944 CoFeO 5.2699 17.45430 0.023892 CoFeO@SBC 37.1189 9.48554 0.093656 为了进一步明晰复合材料微观结构变化情况,通过SEM分析了SBC、CoFeO和CoFeO@SBC的形貌特征,如图3(a)~3(c)所示。SBC呈不规则片状结构,且表面存在少量碎屑,符合典型碳结构。CoFeO呈较长的针状,为典型的FeOOH形貌特征。然而,与SBC复合后,CoFeO@SBC形貌结构发生了明显的变化,出现了较多的规则且多孔的“珊瑚礁”形貌结构,说明SBC作为良好的催化剂载体,使Co-Fe双金属均匀分布在SBC的表面,尺寸显著减小,因此催化剂具有更多的反应位点,更有利于活化PMS,以上也很好地佐证了BET的结果。
为了研究CoFeO和CoFeO@SBC的表面官能团,采用IR分析在400~
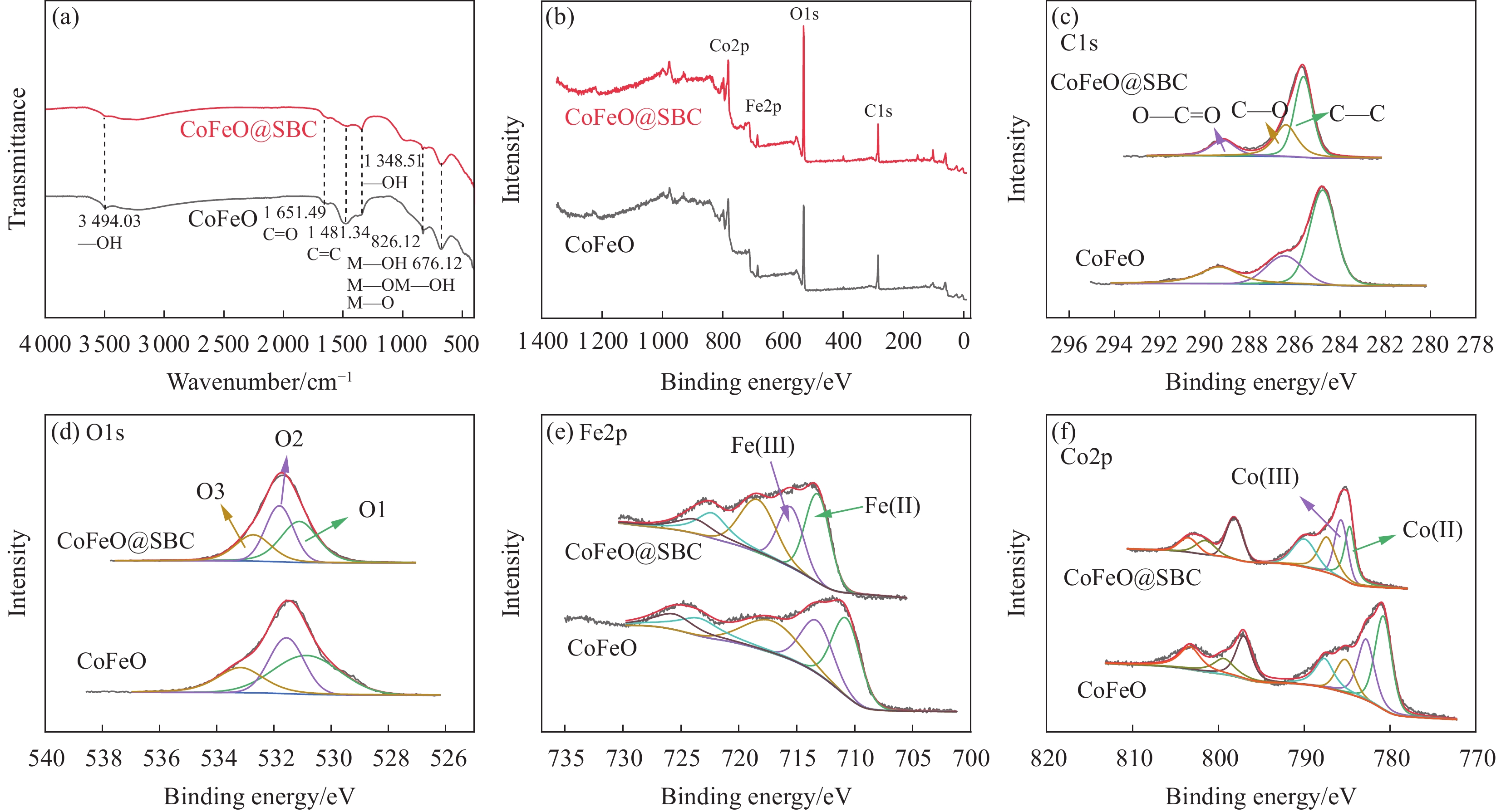
4000 cm−1波长范围的特征区域分析复合前后官能团的变化情况,如图4(a)所示。在波长3494.03 cm−1处的峰是由类氢氧化物层或嵌入水分子中的O—H基团的伸缩模式引起的,复合材料在波长1348.51 cm−1处出现了明显的特征峰,是由—OH的弯曲振动引起的[29]。在波长1651.49 cm−1和1481.34 cm−1处出现的特征峰归因于C=O和C=C的伸缩振动。位于826.12 cm−1和676.12 cm−1波长的吸收峰归因于金属羟基(Co—OH、Fe—OH)的振动[25]。以上结果表明,Co和Fe成功负载到CoFeO@SBC上,复合材料表面出现了更多的官能团,其中的—OH官能团具有还原性,有利于催化过程高价态过渡金属的还原。通过XPS进一步分析催化剂的官能团组成和元素含量,如图4(b)所示,材料复合后,CoFeO@SBC的XPS谱图中显示4个峰值较高的特征峰,285.63、533.04、718.52和782.51 eV的特征峰分别对应了C1s、O1s、Fe2p和Co2p,它们分别占了31.76%、50.18%、5.44%和12.62%。这些结果证明了催化剂中存在了C、O、Fe和Co元素。C1s高分辨谱(图4(c))可以分解为3种类型的峰(由自旋轨道分裂产生):C—C(284.65 eV)、C—O (285.65 eV)和O—C=O(289.30 eV),在添加SBC后材料的C—O含量显著增加,这与IR结果一致。CoFeO@SBC的O1s分峰拟合结果在530.88、531.68和532.75 eV处有3个峰(图4(d)),分别属于吸附氧(O1)、晶格氧(O2)和表面氧(O3)[30],材料复合后,O2含量增加了4%,这表明Co和Fe的添加让CoFeO@SBC材料引入更多的氧空位,有利于提高复合材料的催化能力。
Fe2p和Co2p的高分辨率图谱如图4(e)和图4(f)所示,通过定量分析XPS光谱发现(XPS分析数据如表2所示),CoFeO中Co(II)/Co(III)的比值约为0.7,而Fe(II)/Fe(III)为1.3,在负载SBC后Co(II)/Co(III)比值增加到0.98,Fe(II)/Fe(III)为1.5,这些结果表明,SBC可以促进Fe(III)和Co(III)向Fe(II)和Co(II)的转化,从而促进CoFeO@SBC中Fe(III)和Co(III)的还原,因此SBC可以延长CoFeO对PMS的激活,从而促进对BPF的降解[31],根据之前的研究[32-33],Fe(II)/Fe(III)或Co(II)/Co(III)比值的增加会增强表面氧空位的引入,从而导致更多的活性位点和 更好的电子传导性,进而促进PMS的激活。XPS的结果证明了CoFeO@SBC的成功合成及掺杂的Co和Fe在污泥生物炭上是多价态的,且进一步证实了生物炭中丰富的还原性官能团(如—OH)可以促进CoFeO高价态过渡金属的还原,进而有效提高材料的催化性能。
表 2 CoFeO和CoFeO@SBC的XPS分析数据Table 2. XPS analysis results of CoFeO and CoFeO@SBCSample Fe2+/Fe3+ Co2+/Co3+ C—O O CoFeO 1.3 0.7 22.59% 32.90% CoFeO@SBC 1.5 0.98 30.99% 36.31% 综上所述,本文成功制备出具有良好结晶度的CoFeO@SBC复合材料。SEM图像可以看出该材料呈现出孔隙结构丰富的“珊瑚礁”结构;BET分析表明材料具有较大的比表面积和孔隙。此外,IR表明CoFeO@SBC材料复合后具备更加丰富的官能团,促进CoFeO过渡金属的还原而产生更多的Fe(II)和Co(II),显著增强CoFeO@SBC复合材料对PMS的催化活性;XPS证明合成材料由钴、铁、碳和氧元素等组成,且具有丰富的氧空位和结构缺陷。后续的降解实验利用CoFeO@SBC降解BPF。
2.2 催化降解性能影响因素分析
2.2.1 不同体系及CoFeO@SBC投加量对BPF去除的影响
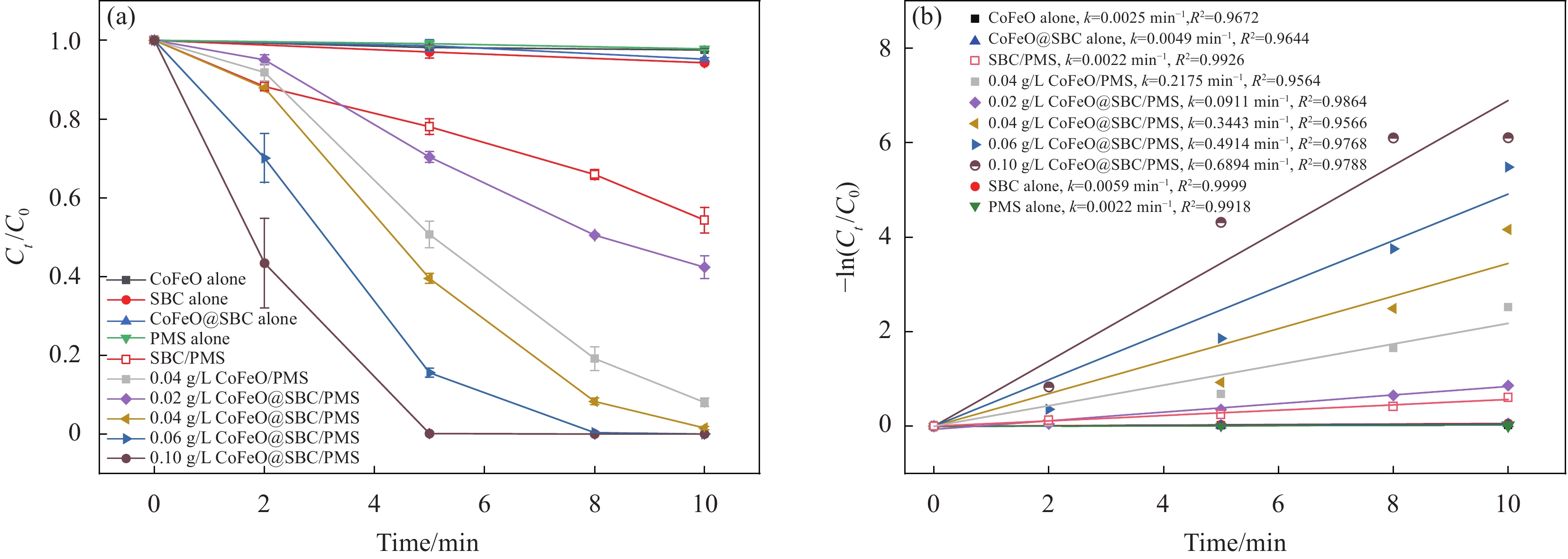
图5(a)为不同体系下及CoFeO@SBC投加量对BPF去除效率的影响,在降解实验中仅投加CoFeO、SBC、CoFeO@SBC和PMS时,10 min内BPF浓度基本不变,表明材料几乎不能吸附BPF,未经过催化的PMS也无法直接降解BPF,单独SBC催化PMS在10 min内降解48%的BPF。CoFeO活化PMS在10 min内降解了92%的BPF,降解效率低于相同浓度的CoFeO@SBC材料。随着CoFeO@SBC浓度的增加,BPF去除效率更快,在10 min内,0.04、0.06和0.10 g/L CoFeO@SBC均能去除98%以上的BPF,它们的催化降解速率常数kobs分别为
0.3443 min−1、0.4914 min−1和0.6894 min−1。以上结果表明,随着CoFeO@SBC用量的增加,为体系提供了更多的表面活性位点。但是在PMS用量不变的情况下,催化剂活化PMS产生的自由基数量会达到一个饱和值,因此仅增加催化剂投加量并不能继续提高BPF的降解率,0.04 g/L CoFeO@SBC在10 min内刚好降解99%的BPF,降解速率与CoFeO/PMS体系相比提高62%,因此选用其作为最佳投加量。![]() 图 5 (a) 不同体系及CoFeO@SBC投加量对双酚F (BPF)去除效果的影响;(b) 不同体系及材料投加量的速率常数图Figure 5. (a) Effects of different systems and CoFeO@SBC dosage on the removal of bisphenol F (BPF); (b) Rate constant diagrams for different systems and material dosagesPMS—Peroxymonosulfate; k—Speed constant; R2—Correlation coefficient; Ct—Concentration of BPF after t min; C0—Concentration of BPF before the reaction
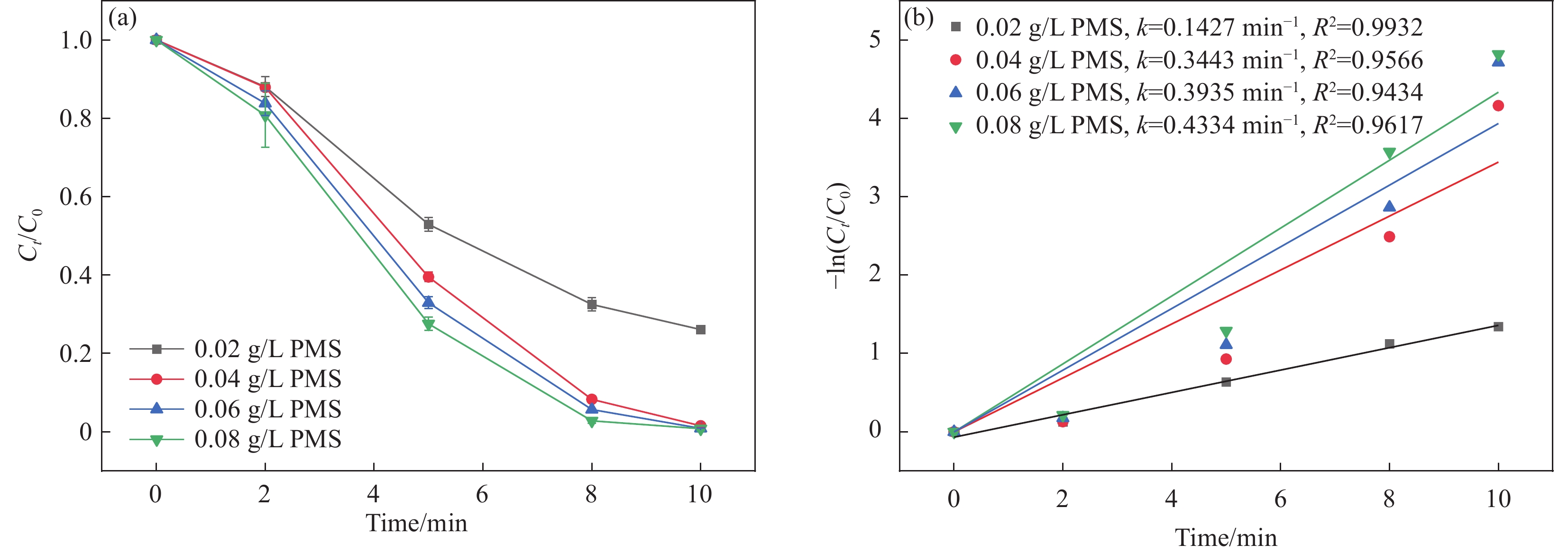
图 5 (a) 不同体系及CoFeO@SBC投加量对双酚F (BPF)去除效果的影响;(b) 不同体系及材料投加量的速率常数图Figure 5. (a) Effects of different systems and CoFeO@SBC dosage on the removal of bisphenol F (BPF); (b) Rate constant diagrams for different systems and material dosagesPMS—Peroxymonosulfate; k—Speed constant; R2—Correlation coefficient; Ct—Concentration of BPF after t min; C0—Concentration of BPF before the reaction2.2.2 PMS浓度的影响
图6显示了在反应10 min期间PMS浓度对BPF降解率的影响。实验结果表明,随着PMS浓度从0.02 g/L增加到0.08 g/L,BPF的去除率从73.8%增加到99.8%,催化降解速率常数kobs也从
0.1427 min−1增加到0.4334 min−1,这可以解释为PMS浓度越高,体系中活性物种越多[34]。然而,当PMS浓度进一步增加时,去除效率变化却不大,一方面是由于一定量的PMS被CoFeO@SBC表面的活性位点激活,产生活性物质,另一方面可能是过量的PMS导致了SO•−4和•OH发生自淬灭反应(式(2)~(3)),这也会影响BPF的降解效果[35]。因此,最佳PMS投加量为0.04 g/L。•OH+•OH⟶H2O2 (2) SO•−4+SO•−4→S2O2−8 (3) 2.2.3 初始pH的影响
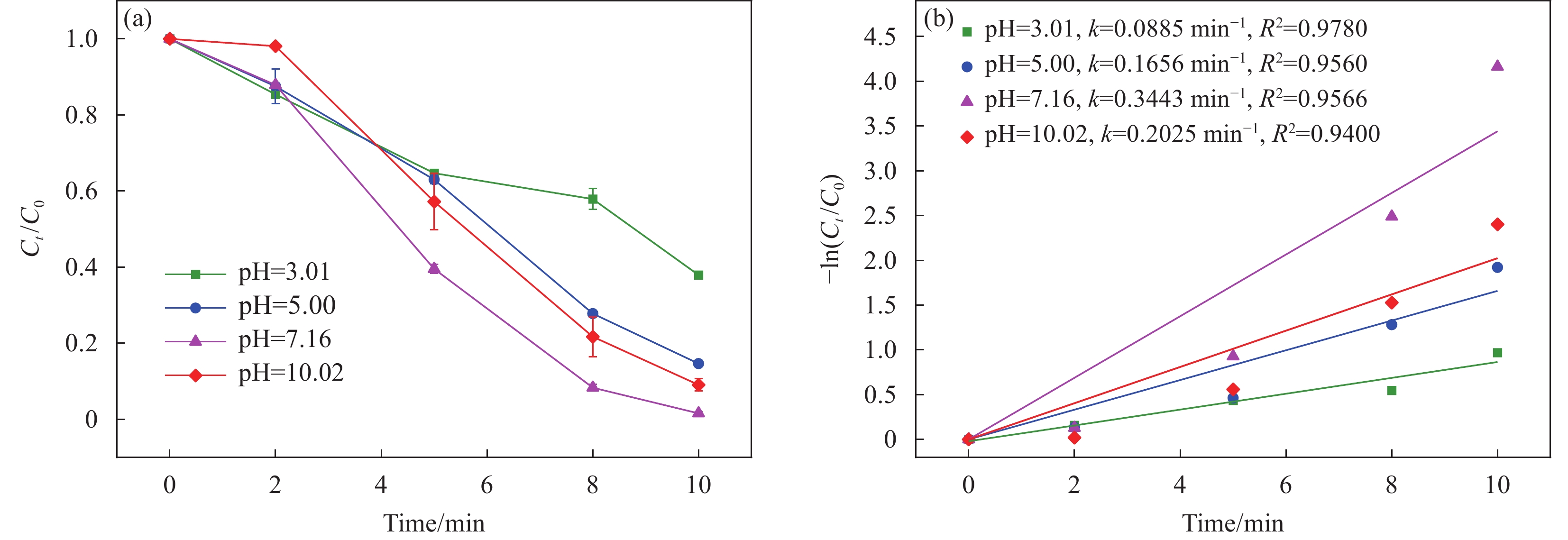
在高级氧化工艺中,pH是影响污染物降解效率的重要因素。图7研究了初始pH对体系中BPF去除的影响。结果表明,当pH从3.01增加到7.16(溶液初始pH)时,kobs从
0.0885 min−1增加到0.3443 min−1,pH增加至10.02时,kobs降低至0.2025 min−1。这些现象可以从以下几个方面解释,一是体系在低pH(<7)时,[H+]会与SO•−4和•OH发生反应(式(4)~(5)),从而影响BPF的降解效率[36];二是在碱性条件下BPF的去除效果更好,可能是由于碱性溶液中氢氧化物离子含量较高能激活PMS,产生更多活性物种。而当过量的OH−与SO•−4相互作用就会生成氧化能力较弱的•OH[37],从而减缓BPF的降解。考虑实际废水中pH为6~8,CoFeO@SBC/PMS体系pH为溶液初始pH,即7.16。H++•OH+e−⟶H2O (4) H++SO•−4+e−→HSO−4 (5) 2.2.4 环境应用
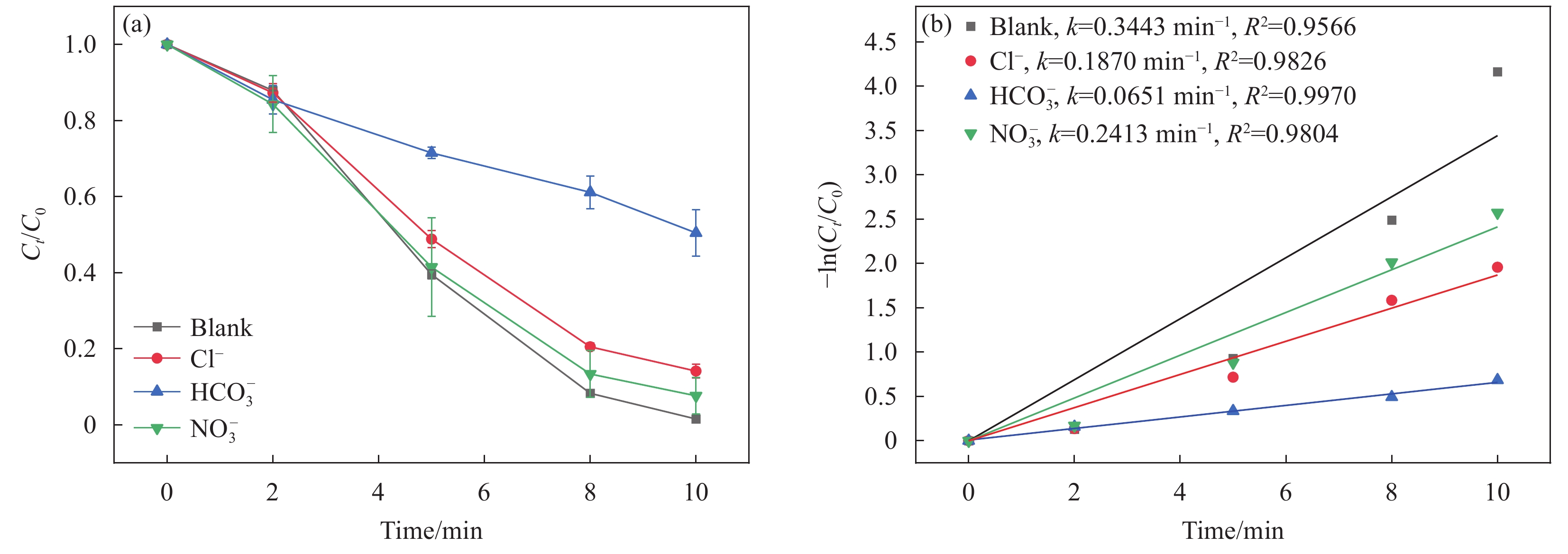
在实际废水体系中存在大量的无机阴离子,它们可能会与CoFeO@SBC/PMS体系中PMS反应,从而影响BPF的去除效果,结果如图8所示,在反应体系中存在Cl−和NO−3时,10 min内BPF的降解率仍保持在86.8%和92.4%,但反应速率常数 kobs从0.3443 min−1分别降至0.1870 min−1和0.2413 min−1,这表明Cl−和NO−3对体系的抑制作用较小,可能是由于Cl−和NO−3对于SO•−4和•OH来说是一种低效的淬灭剂[38]。当体系中存在5 mmol/L的HCO−3时,BPF的降解率降至49.5%,kobs也降至
0.0651 min−1,这可能是由于HCO−3与SO•−4和•OH反应生成氧化能力较弱的CO•−3(式(6)~(7))[39],从而影响BPF的降解率。HCO−3+SO•−4⟶CO•−3+SO2−4 (6) HCO−3+•OH⟶CO•−3+H2O (7) 2.3 CoFeO@SBC/PMS体系降解BPF的机制分析
2.3.1 自由基淬灭实验
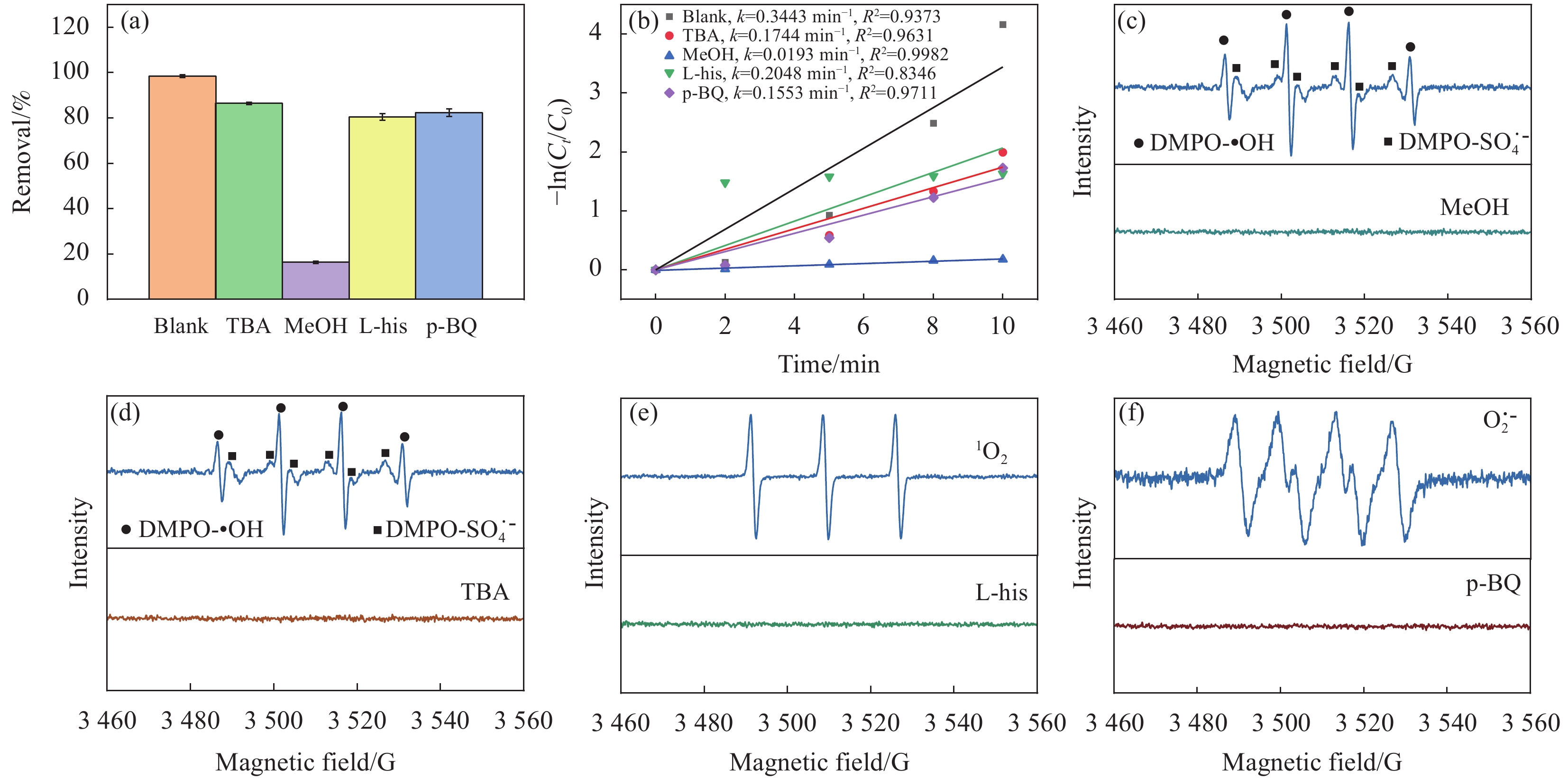
为了进一步明确活性氧物种(Reactive oxygen species,ROS)在反应体系中的作用,进行了自由基淬灭实验,测试了•OH、SO•−4、1O2和O•−2等自由基对BPF去除效率的影响。叔丁醇(TBA)、甲醇(MeOH)、L-组氨酸(L-his)和对苯醌(p-BQ)常被用作自由基淬灭剂,其中,MeOH被用作为SO•−4和•OH的主要淬灭剂,而TBA主要作为•OH的主要淬灭剂,对于SO•−4的反应性较低[40]。从图9可以看出,与空白组相比,TBA和MeOH处理下体系中BPF的去除率降低至86.4%(kobs=
0.1744 min−1)和16.3%(kobs=0.0193 min−1),这表明体系中存在SO•−4和•OH,其中SO•−4的抑制效果更加明显,说明SO•−4贡献度比•OH高。此外,当体系中添加L-his和p-BQ后,BPF的去除率分别降至80.5%(kobs=0.2048 min−1)和82.3%(kobs=0.1553 min−1),表明1O2和O•−2在BPF的降解过程中也发挥了一定作用,但是作用并不明显。综上而言,所测试的4种活性自由基在BPF的降解过程中都发挥了作用,其中SO•−4是最关键的自由基。![]() 图 9 (a) CoFeO@SBC/PMS体系自由基淬灭实验;(b) 自由基淬灭实验的速率常数图;不同活性氧物种(ROS)的鉴定:(c) 甲醇(MeOH);(d) 叔丁醇(TBA);(e) L-组氨酸(L-his);(f)对苯醌(p-BQ)Figure 9. (a) Radical quenching experiment in CoFeO@SBC/PMS system; (b) Rate constant plot for free radical quenching experiment; The identification of different reactive oxygen species (ROS): (c) Methanol (MeOH); (d) Tert butanol (TBA); (e) L-histidine (L-his); (f) p-benzoquinone (p-BQ)
图 9 (a) CoFeO@SBC/PMS体系自由基淬灭实验;(b) 自由基淬灭实验的速率常数图;不同活性氧物种(ROS)的鉴定:(c) 甲醇(MeOH);(d) 叔丁醇(TBA);(e) L-组氨酸(L-his);(f)对苯醌(p-BQ)Figure 9. (a) Radical quenching experiment in CoFeO@SBC/PMS system; (b) Rate constant plot for free radical quenching experiment; The identification of different reactive oxygen species (ROS): (c) Methanol (MeOH); (d) Tert butanol (TBA); (e) L-histidine (L-his); (f) p-benzoquinone (p-BQ)为进一步确认体系中活性物质的存在,利用5, 5-二甲基-1-吡咯啉-N-氧化物(DMPO)和2, 2, 6, 6-四甲基哌啶(TEMP)作为捕获剂,通过EPR分析对活性物质的鉴定及与无淬灭条件下的EPR谱峰强度做对比。结果如图9(c)~9(f)所示,在CoFeO@SBC/PMS体系中存在•OH、SO•−4、1O2和O•−2自由基,进一步佐证了自由基淬灭实验。
2.3.2 CoFeO@SBC/PMS体系的反应机制
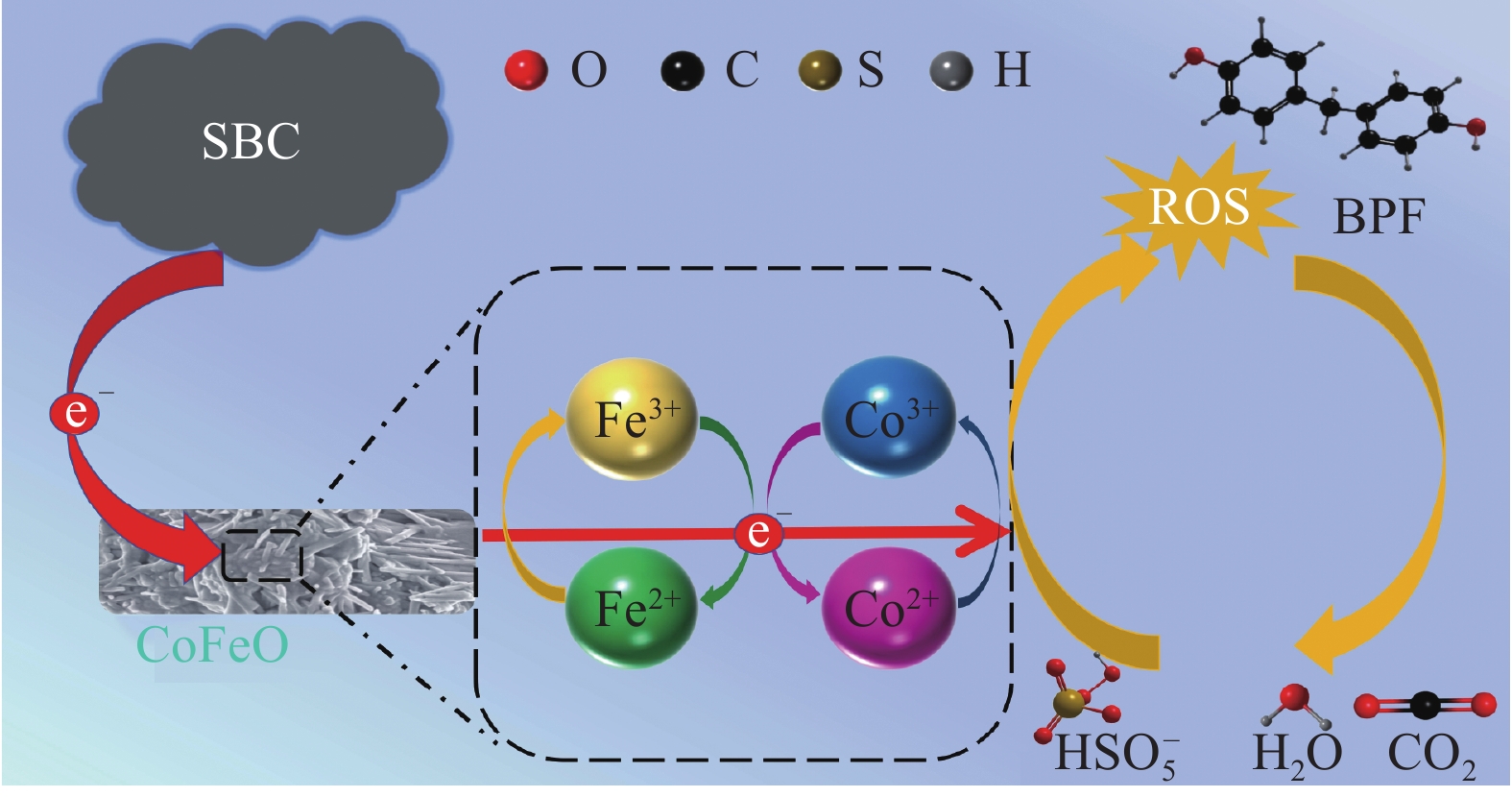
CoFeO@SBC活化PMS降解BPF的机制如图10所示,在CoFeO@SBC/PMS反应过程中,PMS的过氧化物键(—O—O—)可以通过接受来自低价态过渡金属Fe(II)和Co(II)的电子而被激活生成SO•−4和•OH(式(8)~(14))[40]。为了完成氧化还原循环,高价态过渡金属Fe(III)和Co(III)必须被HSO−5还原(式(15)~(16))。Co(III)/Co(II)氧化还原对的还原电位E0为1.83 V (式(17)),比HSO−5/SO•−4(2.5~3.1 V)更易还原,但比HSO−5/SO•−5(1.1 V)易氧化,这就使PMS体系中Co(III)/Co(II)循环在热力学上是可行的[41]。然而,Fe(III)/Fe(II)氧化还原对点位为0.77 V (式(18)),低于HSO−5/SO•−5(1.1 V),这对Fe(II)的热力学再生不利,从而影响了PMS的激活速率。此外,根据标准还原点位(式(19)),Fe(II)还原Co(III)在热力学上是可行的。复合SBC后,结合本文的表征结论,SBC的电子供体(如酚—OH)可以加速Fe(III)和Co(III)的还原(式(20)~(21)),产生更多的Fe(II)和Co(II)。因此,SBC增强了CoFeO的催化活性,提高了对PMS的活化效率并产生更多的ROS,ROS攻击BPF产生各种中间体,最终矿化为CO2和H2O (式(22))。
≡Co(II)+HSO−5→≡Co(III)+SO•−4+OH− (8) ≡Co(II)+HSO−5→≡Co(III)+SO2−4+OH• (9) ≡Fe(II)+HSO−5→≡Fe(III)+SO•−4+OH− (10) ≡Fe(II)+HSO−5⟶≡Fe(III)+SO2−4+OH− (11) SO•−4+H2O→HSO−5+OH• (12) HSO−5+SO•−4→SO•−5+HSO−4 (13) OH•+HSO−5→SO•−5+H2O (14) ≡Co(III)+HSO−5⟶≡Co(II)+SO•−5+H+ (15) ≡Fe(III)+HSO−5→≡Fe(II)+SO•−5+H+ (16) Co(III)+e−→Co (II) ,E0=1.83 V (17) Fe(III)+e−→Fe(II) ,E0=0.77 V (18) Fe(II)+Co(III)→Co(II)+Fe(III) (19) ≡Co(III)+OH⟶≡Co(II) (20) ≡Fe(III)+OH→≡Fe(II) (21) ROS+BPF⟶ Intermediates →CO2+H2O (22) 2.3.3 CoFeO@SBC/PMS体系降解路径分析
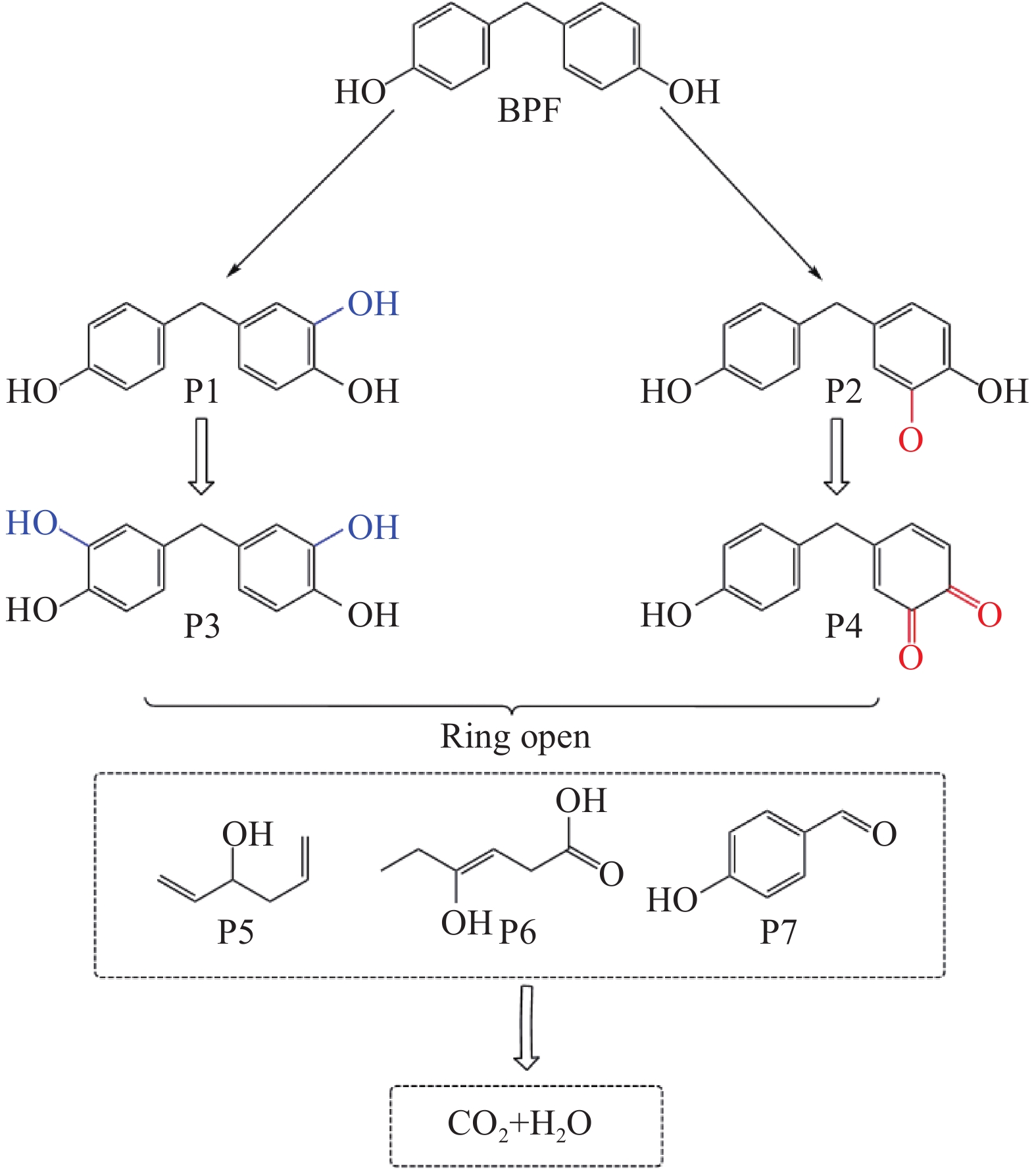
通过LC-MS测定了CoFeO@SBC/PMS体系中BPF的中间产物,并根据LC-MS结果和其他学者的研究[42-43]推断了主要的降解途径(图11)。途径1是CoFeO@SBC/PMS体系中的SO•−4等自由基破坏BPF(m/z=200)的C—C产生P1(m/z=216),进一步氧化成P3(m/z=232);途径2是BPF通过羟基化产生P2(m/z=215),氧化成为P4(m/z=214),然后苯环被自由基攻击开环并形成脂肪族化合物P5(m/z=98)、P6 (m/z=130)和P7(m/z=122)。最后,小分子的脂肪族化合物被矿化成H2O和CO2。
3. 结 论
(1) 本实验用Co和Fe双金属复合污泥生物炭制备新型催化剂钴铁双金属@生物炭复合材料(CoFeO@SBC),在过一硫酸盐(PMS)体系中10 min降解99%以上的双酚F (BPF)。
(2) SBC在多个方面显著改善了CoFeO的理化性质,首先与SBC复合后,CeFeO的形貌发生了改变,粒径更小,比表面积明显增大,为PMS提供了更多的活化位点,其次复合材料表面出现了更多含氧官能团,引入了更多的表面氧空位,有利于提高复合材料的催化能力。
(3) 在CoFeO@SBC/PMS体系降解BPF过程中•OH、SO•−4、1O2和O•−2这4种活性自由基都发挥了贡献,其中SO•−4起最主要作用。基于LC-MS分析,提出了BPF可能的两种降解途径。
-
图 5 (a) 不同体系及CoFeO@SBC投加量对双酚F (BPF)去除效果的影响;(b) 不同体系及材料投加量的速率常数图
Figure 5. (a) Effects of different systems and CoFeO@SBC dosage on the removal of bisphenol F (BPF); (b) Rate constant diagrams for different systems and material dosages
PMS—Peroxymonosulfate; k—Speed constant; R2—Correlation coefficient; Ct—Concentration of BPF after t min; C0—Concentration of BPF before the reaction
图 9 (a) CoFeO@SBC/PMS体系自由基淬灭实验;(b) 自由基淬灭实验的速率常数图;不同活性氧物种(ROS)的鉴定:(c) 甲醇(MeOH);(d) 叔丁醇(TBA);(e) L-组氨酸(L-his);(f)对苯醌(p-BQ)
Figure 9. (a) Radical quenching experiment in CoFeO@SBC/PMS system; (b) Rate constant plot for free radical quenching experiment; The identification of different reactive oxygen species (ROS): (c) Methanol (MeOH); (d) Tert butanol (TBA); (e) L-histidine (L-his); (f) p-benzoquinone (p-BQ)
表 1 不同材料的表面结构特征
Table 1 Surface structure characterization of different materials
Sample Surface area/
(m2·g−1)Average pore diameter/nm Pore volume/
(cm3·g−1)SBC 22.0203 15.88640 0.088944 CoFeO 5.2699 17.45430 0.023892 CoFeO@SBC 37.1189 9.48554 0.093656 表 2 CoFeO和CoFeO@SBC的XPS分析数据
Table 2 XPS analysis results of CoFeO and CoFeO@SBC
Sample Fe2+/Fe3+ Co2+/Co3+ C—O O CoFeO 1.3 0.7 22.59% 32.90% CoFeO@SBC 1.5 0.98 30.99% 36.31% -
[1] 王倩倩, 王永花, 汪贝贝, 等. 双酚F和双酚S联合暴露下的斑马鱼富集及神经毒性[J]. 中国环境科学, 2020, 40(2): 865-873. DOI: 10.3969/j.issn.1000-6923.2020.02.048 WANG Qianqian, WANG Yonghua, WANG Beibei, et al. Accumulation and neurotoxicity of bisphenol F and bisphenol S in zebrafish under combined exposure[J]. China Environmental Science, 2020, 40(2): 865-873(in Chinese). DOI: 10.3969/j.issn.1000-6923.2020.02.048
[2] GOMEZ-LASERNA O, LANZAFAME P, PAPANIKOLAOU G, et al. Analytical assessment to develop innovative nanostructured BPA-free epoxy-silica resins as multifunctional stone conservation materials[J]. Science of the Total Environment, 2018, 645: 817-826. DOI: 10.1016/j.scitotenv.2018.07.188
[3] MORALES M, DE LA FUENTE M, MARTIN-FOLGAR R. BPA and its analogues (BPS and BPF) modify the expression of genes involved in the endocrine pathway and apoptosis and a multi drug resistance gene of the aquatic midge Chironomus riparius (Diptera)[J]. Environmental Pollution, 2020, 265: 1148.
[4] ZENG D, LI P R, HU J W, et al. Fulvic acid enhanced peroxymonosulfate activation over Co-Fe binary metals for efficient degradation of emerging bisphenols[J]. Environmental Research, 2023, 231: 116041. DOI: 10.1016/j.envres.2023.116041
[5] HUANG Z, ZHAO J L, YANG Y Y, et al. Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: Urban rainfall runoff as a potential source for receiving rivers[J]. Environmental Pollution, 2020, 263: 114361. DOI: 10.1016/j.envpol.2020.114361
[6] ČESEN M, LENARCIC K, MISLEJ V, et al. The occurrence and source identification of bisphenol compounds in wastewaters[J]. Science of the Total Environment, 2018, 616-617: 744-752. DOI: 10.1016/j.scitotenv.2017.10.252
[7] STAOLES C, VAN DER H, CLARK K, et al. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data[J]. Chemosphere, 2018, 201: 448-458.
[8] PANG L, YANG H Q, LYU L N, et al. Occurrence and estrogenic potency of bisphenol analogs in sewage sludge from wastewater treatment plants in central China[J]. Archives of Environmental Contamination and Toxicology, 2019, 77(3): 461-470. DOI: 10.1007/s00244-019-00663-4
[9] YE Q Y, WU J Y, WU P X, et al. Enhancing peroxymonosulfate activation of Fe-Al layered double hydroxide by dissolved organic matter: Performance and mechanism[J]. Water Research, 2020, 185: 116246. DOI: 10.1016/j.watres.2020.116246
[10] LUO X W, YOU Y J, ZHONG M J, et al. Green synthesis of manganese-cobalt-tungsten composite oxides for degradation of doxycycline via efficient activation of peroxymonosulfate[J]. Journal of Hazardous Materials, 2022, 426: 127803. DOI: 10.1016/j.jhazmat.2021.127803
[11] GIANNAKIS S, LIN K A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate radical-based advanced oxidation processes (SR-AOPs)[J]. Chemical Engineering Journal, 2021, 406: 127083. DOI: 10.1016/j.cej.2020.127083
[12] 王俊辉, 陆彩妹, 李泽华, 等. 磁性氮掺杂杉木屑生物炭活化过一硫酸盐降解左氧氟沙星[J]. 复合材料学报, 2023, 40(11): 6383-6394. DOI: 10.13801/j.cnki.fhclxb.20230222.011ChemicalEngineeringJournal,2015,276:193-204 WANG Junhui, LU Caimei, LI Zehua, et al. Preparation of magnetic nitrogen-doped fir sawdust biochar to activate peroxymonosulfate for Levofloxacin degradation[J]. Acta Materiae Compositae Sinica, 2023, 40(11): 6383-6394(in Chinese). DOI: 10.13801/j.cnki.fhclxb.20230222.011ChemicalEngineeringJournal,2015,276:193-204
[13] FERNANDES A, MAKOS P, KHAN J A, et al. Pilot scale degradation study of 16 selected volatile organic compounds by hydroxyl and sulfate radical based advanced oxidation processes[J]. Journal of Cleaner Production, 2019, 208: 54-64. DOI: 10.1016/j.jclepro.2018.10.081
[14] NFODZO P, CHOI H. Sulfate radicals destroy pharmaceuticals and personal care products[J]. Environmental Engineering Science, 2011, 28(8): 605-609. DOI: 10.1089/ees.2011.0045
[15] SHEN M X, HUANG Z J, LUO X W, et al. Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis[J]. Chemical Engineering Journal, 2020, 396: 125238. DOI: 10.1016/j.cej.2020.125238
[16] FANG Y, LIU Q W, SONG Y, et al. Highly efficient in-situ purification of Fe(II)-rich high-arsenic groundwater under anoxic conditions: Promotion mechanisms of PMS on oxidation and adsorption[J]. Chemical Engineering Journal, 2023, 453: 139915. DOI: 10.1016/j.cej.2022.139915
[17] SUN C H, JIA Z R, XU S, et al. Synergistic regulation of dielectric-magnetic dual-loss and triple heterointerface polarization via magnetic MXene for high-performance electromagnetic wave absorption[J]. Journal of Materials Science & Technology, 2022, 113: 128-137.
[18] YUAN C, DAI Y D, CHEN Y C. Analysis of electric field efficacy and remediation performance of triclosan contaminated soil by Co-Fe/Al oxidation electrodes coupled with peroxymonosulfate (PMS) in an ECGO system with diversified electrode configurations[J]. Chemosphere, 2022, 307: 135841. DOI: 10.1016/j.chemosphere.2022.135841
[19] 张吉琛, 刘婷然, 董志强, 等. 污泥生物炭的催化机制及应用研究进展[J]. 环境化学, 2023, 42(6): 1-14. ZHANG Jichen, LIU Tingran, DONG Zhiqiang, et al. Mechanism and application for removal of contaminants by sludge derived biochar catalyst: A review[J]. Environmental Chemistry, 2023, 42(6): 1-14(in Chinese).
[20] GAO Y, CONG S B, YU H Y, et al. Investigation on microwave absorbing properties of 3D C@ZnCo2O4 as a highly active heterogenous catalyst and the degradation of ciprofloxacin by activated persulfate process[J]. Separation and Purification Technology, 2021, 262: 118330. DOI: 10.1016/j.seppur.2021.118330
[21] 吕鹏飞, 陈悫, 王佳程, 等. 层状双氢氧化物-生物炭复合材料在废水处理中的应用[J]. 复合材料学报, 2024, 41(7): 3467-3478. LYU Pengfei, CHEN Que, WANG Jiacheng, et al. Application of layered double hydroxide-biochar composite in wastewater treatment[J]. Acta Materiae Compositae Sinica, 2024, 41(7): 3467-3478(in Chinese).
[22] JI J Q, YUAN X Z, ZHAO Y L, et al. Mechanistic insights of removing pollutant in adsorption and advanced oxidation processes by sludge biochar[J]. Journal of Hazardous Materials, 2022, 430: 128375. DOI: 10.1016/j.jhazmat.2022.128375
[23] 贺德春, 胡嘉梧, 梁紫薇, 等. 同位素内标-高效液相色谱-串联质谱法测定畜禽粪便中6种雌激素[J]. 生态环境学报, 2021, 30(2): 383-390. HE Dechun, HU Jiawu, LIANG Ziwei, et al. Determination of six estrogens in livestock manures by isotope internal standard-high performance liquid chromatography coupled with tandem mass spectrometry[J]. Ecology and Environmental Science, 2021, 30(2): 383-390(in Chinese).
[24] LI Y, MA S L, XU S J, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4[J]. Chemical Engineering Journal, 2020, 387: 12094.
[25] WU J Y, XU Y J, WU P X, et al. Effects of different dissolved organic matter on peroxymonosulfate activation over Co-Fe binary metal: Experiments and density functional theory[J]. Chemical Engineering Journal, 2022, 450: 137770. DOI: 10.1016/j.cej.2022.137770
[26] GUAN Q, CHENG J, WANG B, et al. Needle-like Co3O4 anchored on the graphene with enhanced electrochemical performance for aqueous supercapacitors[J]. ACS Applied Materials and Interfaces, 2014, 6(10): 7626-7632. DOI: 10.1021/am5009369
[27] WU J Y, WANG Y H, WU Z X, et al. Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions[J]. Science of the Total Environment, 2020, 708: 134409. DOI: 10.1016/j.scitotenv.2019.134409
[28] JU L T, WU P X, YANG Q L, et al. Synthesis of ZnAlTi-LDO supported C60@AgCl nanoparticles and their photocatalytic activity for photo-degradation of bisphenol A[J]. Applied Catalysis B:Environmental, 2018, 224: 159-174. DOI: 10.1016/j.apcatb.2017.10.056
[29] CHEN L Y, WU P X, CHEN M Q, et al. Preparation and characterization of the eco-friendly chitosan/vermiculite biocomposite with excellent removal capacity for cadmium and lead[J]. Applied Clay Science, 2018, 159: 74-82. DOI: 10.1016/j.clay.2017.12.050
[30] LI Z, SUN Y Q, YANG Y, et al. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater[J]. Journal of Hazardous Materials, 2020, 383: 121240. DOI: 10.1016/j.jhazmat.2019.121240
[31] HAN S, XIAO P F. Catalytic degradation of tetracycline using peroxymonosulfate activated by cobalt and iron co-loaded pomelo peel biochar nanocomposite: Characterization, performance and reaction mechanism[J]. Separation and Purification Technology, 2022, 287: 120533. DOI: 10.1016/j.seppur.2022.120533
[32] JIA Q Q, ZHANG X J, LI Y Q, et al. Reductive dehalogenation in groundwater by Si-Fe(II) co-precipitates enhanced by internal electric field and low vacancy concentrations[J]. Water Research, 2023, 228: 119386. DOI: 10.1016/j.watres.2022.119386
[33] BAO C S, WANG H, WANG C Y, et al. Cooperation of oxygen vacancy and FeIII/FeII sites in H2-reduced Fe-MIL-101 for enhanced Fenton-like degradation of organic pollutants[J]. Journal of Hazardous Materials, 2023, 441: 129922. DOI: 10.1016/j.jhazmat.2022.129922
[34] LIU J W, DU Y F, SUN W Y, et al. A granular adsorbent-supported Fe/Ni nanoparticles activating persulfate system for simultaneous adsorption and degradation of ciprofloxacin[J]. Chinese Journal of Chemical Engineering, 2020, 28(4): 1077-1084. DOI: 10.1016/j.cjche.2019.12.019
[35] XIE H, XU W. Enhanced activation of persulfate by meso-CoFe2O4/SiO2 with ultrasonic treatment for degradation of chlorpyrifos[J]. ACS Omega, 2019, 4(17): 17177-17185. DOI: 10.1021/acsomega.9b01626
[36] HUANG Y M, LI G, LI M Z, et al. Kelp-derived N-doped biochar activated peroxymonosulfate for ofloxacin degradation[J]. Science of the Total Environment, 2021, 754: 141999. DOI: 10.1016/j.scitotenv.2020.141999
[37] HE J, XIAO Y, TANG J C, et al. Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect[J]. Science of the Total Environment, 2019, 690: 768-777. DOI: 10.1016/j.scitotenv.2019.07.043
[38] WANG X L, DING Y Z, DIONYSIOU D D, et al. Efficient activation of peroxymonosulfate by copper sulfide for diethyl phthalate degradation: Performance, radical generation and mechanism[J]. Science of the Total Environment, 2020, 749: 142387. DOI: 10.1016/j.scitotenv.2020.142387
[39] WANG S Z, LIU Y, WANG J L. Iron and sulfur co-doped graphite carbon nitride (FeOy/S-g-C3N4) for activating peroxymonosulfate to enhance sulfamethoxazole degradation[J]. Chemical Engineering Journal, 2020, 382: 122836. DOI: 10.1016/j.cej.2019.122836
[40] HU Y, CHEN D Z, ZHANG R, et al. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway[J]. Journal of Hazardous Materials, 2021, 419: 126495. DOI: 10.1016/j.jhazmat.2021.126495
[41] LOU X Y, WU L X, GUO Y G, et al. Peroxymonosulfate activation by phosphate anion for organics degradation in water[J]. Chemosphere, 2014, 117: 582-585. DOI: 10.1016/j.chemosphere.2014.09.046
[42] LI X N, WANG Z H, ZHANG B, et al. Fe xCo3− xO4 nanocages derived from nanoscale metal–organic frameworks for removal of bisphenol A by activation of peroxymonosulfate[J]. Applied Catalysis B: Environmental, 2016, 181: 788-799. DOI: 10.1016/j.apcatb.2015.08.050
[43] WANG Z W, LI Q, SU R D, et al. Enhanced degradation of bisphenol F in a porphyrin-MOF based visible-light system under high salinity conditions[J]. Chemical Engineering Journal, 2022, 428: 132106. DOI: 10.1016/j.cej.2021.132106
-
目的
近年来,污水处理厂的大规模建设导致污泥产量逐年增加,污泥的处理面临严峻挑战。双酚F(BPF)被广泛应用于工业中化学添加剂,是常见的塑化剂,在地表水,土壤和污泥中被频繁检出,但缺无法完全有效的去除。本文利用市政污泥负载钴铁双金属制备了生物炭-钴铁双金属复合材料(CoFeO@SBC),通过活化过一硫酸盐(PMS)降解BPF来探究其催化性能。
方法采用扫描电子显微镜(SEM)、比表面积测定(BET)、红外光谱(IR)、X射线衍射(XRD)和X射线光电子能谱(XPS)等表征分析所制备材料的理化性质;并考察了材料投加量、PMS投加量、初始pH和无机阴离子对CoFeO@SBC/PMS体系降解BPF效果的影响。用自由基淬灭实验确认反应体系中存在的自由基;通过液相色谱-质谱联用(LC-MS)对BPF的降解产物进行分析。
结果通过XRD图谱确定不同复合材料的晶体结构,SBC、CoFeO@SBC和CoFeO三种材料的衍射峰尖锐而强烈,表明它们具有高度结晶的性质; CoFeO的吸附脱附曲线在相对压力为0.4~0.6较SBC和CoFeO@SBC更平滑,呈现出具有H1滞后环的Ⅳ型等温线,而SBC和CoFeO@SBC呈缓慢的上升趋势,呈台阶变化,属II型等温线,三种材料均具有典型的介孔结构,孔径分布主要在2~50 nm。另外三种材料在P/P0=0.8附近存在突然的H2滞后环,在P/P0接近1时无限制吸附,通常被认为是板状颗粒聚集产生的狭缝孔;为了进一步明晰复合材料微观结构变化情况,通过SEM分析了三种材料的形貌特征,结果表明SBC呈不规则片状结构,且表面存在少量碎屑,符合典型碳结构。CoFeO呈较长的针状,为典型的FeOOH形貌特征。然而,与SBC复合后,CoFeO@SBC形貌结构发生了明显的变化,出现了较多的规则且多孔的“珊瑚礁”形貌结构;为了研究CoFeO和CoFeO@SBC的表面官能团,利用IR分析在400~4000 cm波长范围的特征区域分析复合前后官能团的变化情况,在波长3494 cm处的峰是由类氢氧化物层或嵌入水分子中的O-H基团的伸缩模式引起的,复合材料在波长1348 cm处出现了明显的特征峰,是由−OH的弯曲振动引起的,在波长1651 cm和1481 cm处出现的特征峰归因于C=O和C=C的伸缩振动。位于826 cm和676 cm波长的吸收峰归因于金属羟基(Co, Fe-OH)的振动; 通过XPS为了进一步分析催化剂的官能团组成和元素含量,通过定量分析XPS光谱发现,CoFeO中Co(Ⅱ)/Co(Ⅲ)的比值约为0.7而Fe(Ⅱ)/Fe(Ⅲ)为1.3,在负载SBC后Co(Ⅱ)/Co(Ⅲ)比值增加到0.98,Fe(Ⅱ)/Fe(Ⅲ)为1.5。通过材料和PMS投加量实验得出了材料和PMS的最佳投加量为0.04 g/L;Cl和NO对体系降解效果影响较小,而HCO具有显著的抑制作用;在初始pH为7.16时反应体系中BPF的去除率最高;EPR分析表明反应体系存在四种自由基,同时自由基淬灭实验证明SO•是体系降解BPF的关键活性氧物种。
结论本实验用Co和Fe双金属复合污泥生物炭制备新型催化剂CoFeO@SBC,在PMS体系中10 min降解99 %以上的BPF。SBC在多个方面显著改善了CoFeO的理化性质,首先与SBC复合后,CeFeO的形貌发生了改变,粒径更小,比表面积明显增大,为PMS提供了更多的活化位点,其次复合材料表面出现了更多含氧官能团,引入了更多的表面氧空位,有利于提高复合材料的催化能力。通过EPR分析和自由基淬灭实验证明SO•是体系降解BPF的关键活性氧物种。最后通过液相色谱-质谱联用(LC-MS)对BPF的降解产物进行分析,得出了BPF可能的两种降解途径。




 下载:
下载: