Research progress of superhydrophobic coatings in the field of metal corrosion protection
-
摘要:
金属材料凭借其优异的力学性能,在航空航天、海洋工程、交通运输等众多领域具有广泛的应用。然而,金属腐蚀问题仍是制约其在工业领域广泛应用的关键因素之一。研究人员从自然中汲取灵感,通过研究荷叶等动植物表面的微结构,成功设计并开发出具有特殊润湿性能的超疏水表面,将其应用于金属表面后,展现出卓越的抗腐蚀性能。本文回顾了近年来关于超疏水涂层在金属防腐蚀领域的研究成果,归纳了超疏水涂层的耐蚀性和制备技术,阐述了基本润湿理论以及腐蚀防护机制。最后,总结了超疏水涂层在金属防腐蚀领域的研究现状和存在的问题,并对其在金属防腐蚀领域的未来发展趋势和应用前景做了展望。
Abstract:With its excellent mechanical properties, metal materials have a wide range of applications in aerospace, marine engineering, transportation and many other fields. However, metal corrosion is still one of the key factors restricting its widespread use in the industrial field. Drawing inspiration from nature, the researchers have successfully designed and developed superhydrophobic surfaces with special wetting properties by studying the microstructure of animal and plant surfaces such as lotus leaves. When applied to metal surfaces, it exhibits excellent corrosion resistance. In this paper, we review the research results of superhydrophobic coatings in the field of metal corrosion protection in recent years, summarize the corrosion resistance and preparation technology of superhydrophobic coatings, and expound the basic wetting theory and corrosion protection mechanism. Finally, the research status and existing problems of superhydrophobic coatings in the field of metal anti-corrosion are summarized, and the future development trend and application prospect of superhydrophobic coatings in the field of metal anti-corrosion are prospected.
-
金属材料以其强度高、导电性好、导热率优异等优点,在交通运输、建筑业、能源开采、航天航空和医疗器械等领域均具有广泛应用,几乎覆盖了所有关键的工业生产流程[1-2]。同时,金属材料在环境中长期暴露会不可避免地发生腐蚀,其腐蚀本质是金属原子或离子与环境中的氧气、水或其他化学物质发生反应,从而导致金属表面的损坏[3]。常见的腐蚀类型包括均匀腐蚀、点蚀、晶间腐蚀等[4],见图1。
金属材料的腐蚀问题不仅对材料的使用性能和寿命造成了严重影响,还造成了巨大的经济损失,对环境和人类安全构成潜在威胁[5]。例如:香港咸水管道腐蚀爆裂事故[6],中国管道腐蚀泄漏事故[7]。为了避免腐蚀引起的重大事故,开发低成本、高耐蚀、环境适应能力强的防腐涂层或材料,仍是业内普遍关注的重点问题。
常规的金属防腐措施包括缓蚀剂防腐[8]、电化学保护[9]以及金属镀层保护[10]等,然而其防腐成效尚待提高[11]。近年来,超疏水材料的出现为金属表面防腐蚀提供了新的思路[12]。受自然界“荷叶效应”启发,科学家通过复制植物表面的微纳粗糙结构,成功制备出具备荷叶疏水特性的超疏水涂层[13]。该涂层表面可使水珠保持近似球形,并能够轻松滚动或滑落,可有效隔断金属与腐蚀介质的直接接触,从而达到较好的防腐蚀目的[14-15]。目前,尽管超疏水涂层的基础理论、制备技术以及单一金属的耐蚀性已有显著进展[16-17]。然而,本文的核心在于深入探究不同金属表面在各种环境条件下的超疏水防腐蚀机制,分析其在不同条件下的性能表现,并探讨如何实现高效经济的表面制备方法。这些研究不仅对于金属防腐技术具有重要意义,而且有助于推动相关领域的技术创新和发展。
同时,本文主要聚焦于涂层性能的稳定性、机械耐久性以及其在极端环境中的实际防腐应用。本文通过系统梳理超疏水涂层在金属防腐蚀领域的主要研究现状,归纳了固体表面的基本湿润理论以及超疏水涂层的防腐蚀机制。文章重点综述了防蚀性超疏水聚合物涂层、耐蚀性纳米超疏水涂层以及耐蚀性超疏水陶瓷涂层的研究进展,为读者提供了全面的研究视角。
此外,本文还深入分析了超疏水涂层在金属防腐蚀领域应用中面临的关键问题和挑战,揭示了其在实际应用中的局限性。同时,总结了目前常用的制备方法,并对不同制备方法的特点进行了细致的分析,为研究人员提供了实用的参考。
最后,本文展望了超疏水涂层在防腐蚀应用领域的未来发展趋势,指出了未来可能的研究方向和创新点,为超疏水涂层的进一步研究与应用提供了有价值的指导。
1. 基本润湿理论
固体表面的润湿,主要指液体与固体接触时,液体沿该表面扩展的现象。其表面的湿润性决定了液滴在两者接触表面上的形态和润湿行为[18]。
1.1 Young's方程
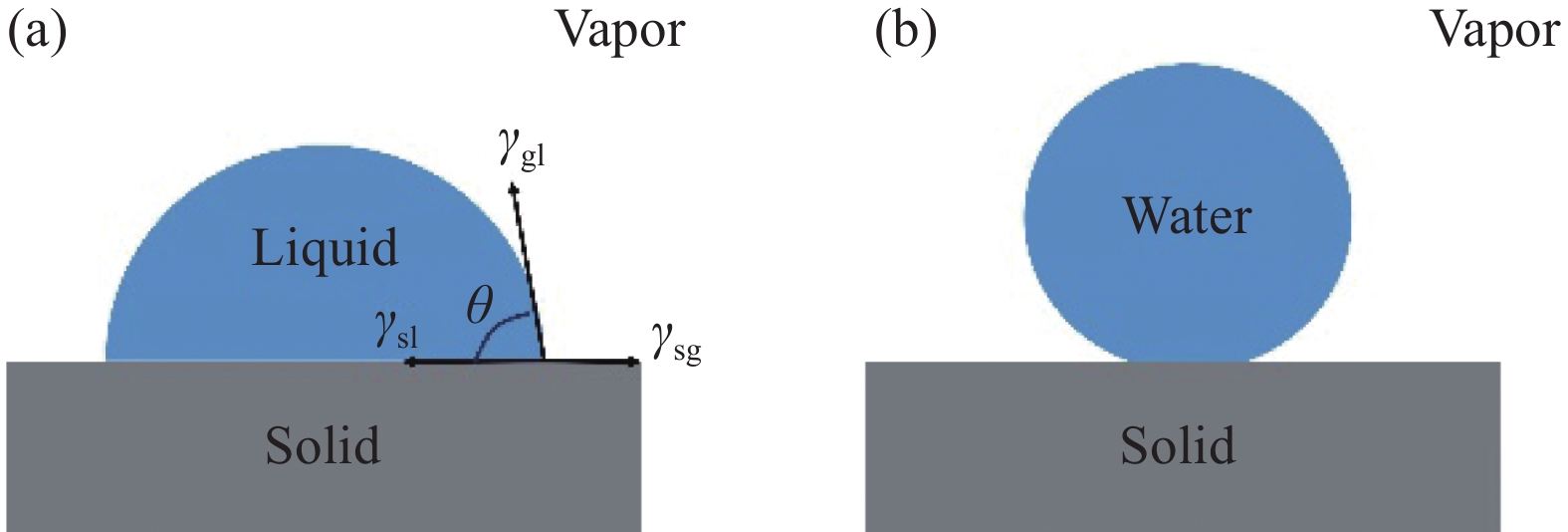
Young's方程是表面科学领域中描述固体表面液体铺展情况的基本方程之一,如图2所示,该方程假设固体表面完全光滑,且界面张力为常数,其模型方程为[19]
cosθ=γsg−γslγgl (1) 其中:θ表示液滴在固体表面的本征接触角;γsg为固-气界面张力;γsl是固-液界面张力;γgl是液-气界面张力。该方程实际上是由刚性固体表面张力和流体在固体表面的作用力相平衡得出的。然而现实中很少存在完全光滑的理想表面。因此该方程的适用性需要进一步完善。
![]() 图 2 Young's方程[19];(a)固-液-气三者之间的关系;(b)液滴在固体表面的Young's状态Figure 2. Young's equation[19]: (a) Relationship between solids, liquids and gases; (b) Young's state of droplets on solid surfacesθ—Intrinsic contact angle of a liquid droplet on a solid surface; γsg—Solid-gas interfacial tension; γsl—Solid-liquid interfacial tension; γgl—Liquid-gas interfacial tension
图 2 Young's方程[19];(a)固-液-气三者之间的关系;(b)液滴在固体表面的Young's状态Figure 2. Young's equation[19]: (a) Relationship between solids, liquids and gases; (b) Young's state of droplets on solid surfacesθ—Intrinsic contact angle of a liquid droplet on a solid surface; γsg—Solid-gas interfacial tension; γsl—Solid-liquid interfacial tension; γgl—Liquid-gas interfacial tension1.2 Wenzel模型
鉴于现实中的固体表面都具有一定的粗糙结构,不可能完全光滑。因此Wenzel[20]在Young's方程的基础上引入表面粗糙因子(r≥1),解释了粗糙度对实际固体表面润湿特性的影响,并提出了著名的Wenzel模型,见图3(a)。Wenzel模型认为,当水滴接触固体表面时,液滴可能进入粗糙结构的凹槽中,形成所谓的“湿接触”。达到平衡后,液滴在粗糙表面形成的实际接触角θw与Young's方程中的本征接触角θ之间存在一定关系,其表达式为[19-20]
cosθw=r(γsg−γsl)γgl=rcosθ (2) 式中,r代表固体表面的粗糙度因子,它表示粗糙表面的实际面积与几何投影面积之比。由于在粗糙表面上,固体的实际面积总是大于几何投影面积,因此r值恒大于等于1,这意味着无论是亲水表面(θ<90°),还是疏水表面(θ>90°),它都会使得粗糙表面的实际接触角θw更远离90°,即使亲水表面更亲水,疏水表面更疏水。但如果表面非均匀,且由多种不同的化学成分组成时,Wenzel方程就不再适用,因此,该方程还需进一步改进。
1.3 Cassie模型
Cassie模型是在Wenzel模型基础上提出的另一种描述液体滴在粗糙或异质表面上润湿性行为的模型。该模型更好地解释了液体不完全浸润粗糙表面或由不同材料构成的表面时的情况,如图3(b)所示。其模型公式为[19, 21-22]
cosθCB=fscosθ−(1−fs)=fscosθ+fs−1 (3) 式中:θCB为液滴在Cassie模型表面上的表观接触角;fs是液体与固体接触部分面积占固体表面投影面积的比例。当引入固体表面润湿部分的粗糙度因子rf,便可得到更普适性的Cassie模型方程[23]:
cosθCB=rffscosθ+fs−1 (4) 根据公式(4)可以看出,当面积分数fs=1时,即水滴完全进入凹槽,凹槽内不存在空气,方程就会变为Wenzel模型。然而,一旦凹槽内存在空气,表观接触角就会有所上升,亲水表面就可能会向疏水表面转变,这种现象是Wenzel模型无法解释的。因此,Cassie模型对Wenzel模型进行了修正,适用于描述复合接触的粗糙表面。表1总结了润湿理论在金属防腐蚀方面的潜在优势[24-27]。
表 1 润湿理论在金属防腐蚀方面的潜在优势Table 1. Potential advantages of wetting theory in metal corrosion protectionPerformance Principle Ref. Enhanced corrosion resistance Adjusting the wetting properties of metal surfaces can reduce the direct contact between corrosive mediums (such as water, saline solutions, etc.) and the metal surface, thereby
delaying the corrosion process of the metal surface[24] Self-cleaning ability Superhydrophobic surfaces can achieve self-cleaning through the lotus effect, thereby
reducing the accumulation of dirt and microorganisms, both of which are factors
that promote metal corrosion[25] Extended service life By improving the wetting properties of metal surfaces, the durability and service life of metal structures in harsh environments can be enhanced [26] Sustainability Strategies involving the modification of surface wetting properties using physical or chemical methods can be environmentally friendly, providing a sustainable alternative to
reducing the use of traditional corrosion inhibitors[27] 2. 超疏水涂层在金属表面的防腐蚀机制
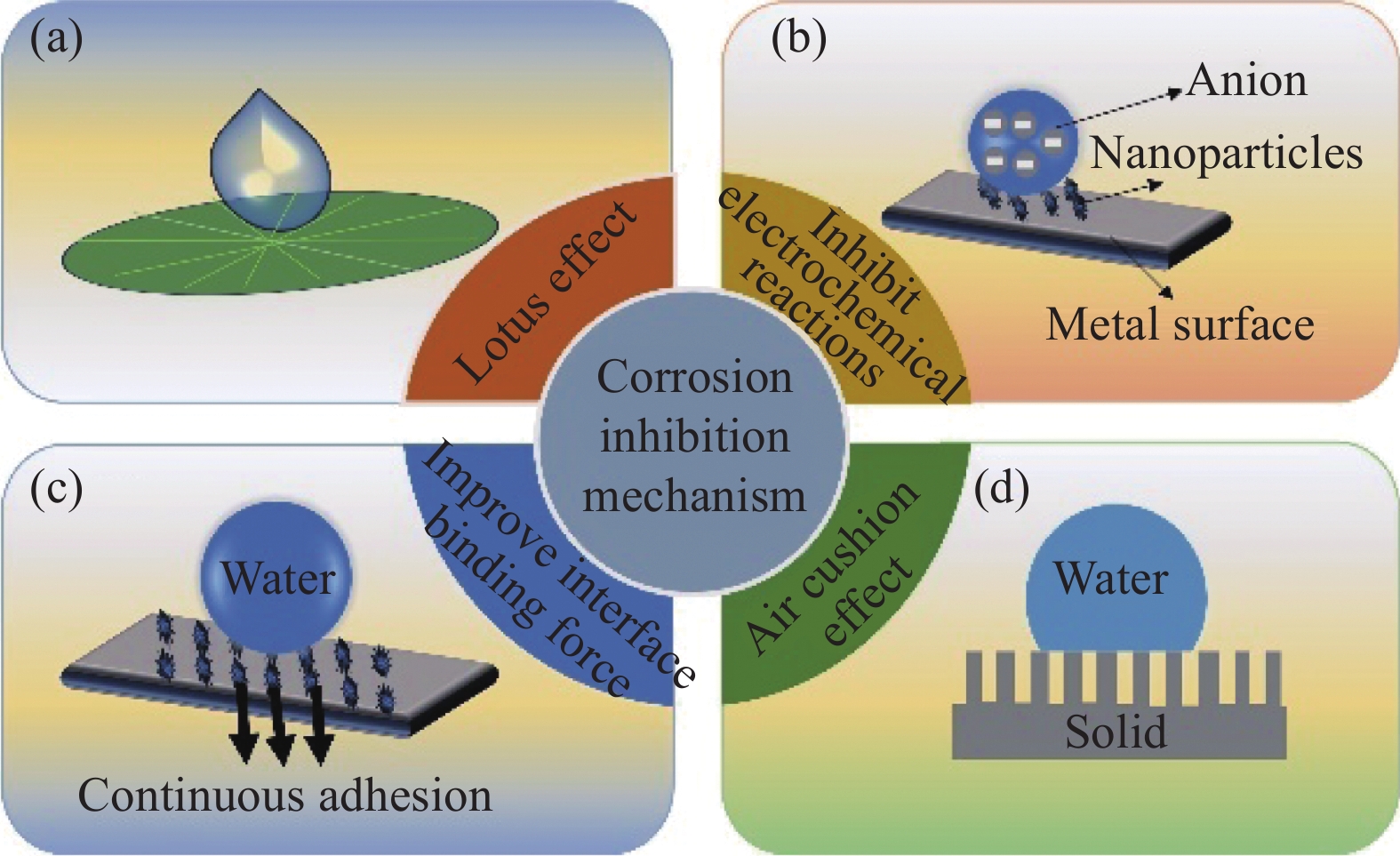
超疏水涂层依靠其较低的表面自由能和独特的微纳米粗糙结构,不仅改变了金属表面的润湿性质,还引入了新的腐蚀防护机制[28]。相较于传统的腐蚀方法(如添加缓蚀剂等),超疏水涂层在金属表面的防腐蚀效果更佳,被广泛应用于各个领域[29-30]。例如,Xia等[31]通过将十六烷基三甲氧基硅烷修饰的二氧化硅颗粒与聚二甲基硅氧烷粘合剂相混合的方式,在Al片上制备出超疏水涂层,其接触角为158.5°,缓蚀效率为98.9%,且该涂层具有取代传统有机树脂防腐涂料的潜力。近年来,超疏水涂层的防腐蚀机制主要包括:Lotus效应[32]、阻碍电化学腐蚀[33]、提高界面结合力[34]以及气垫效应[35]等,如图4所示。
2.1 Lotus效应
Lotus效应,即荷叶效应,指水滴落在类似荷叶表面时形成接近完美的球形,并轻松滚落,同时带走粘附的污垢和颗粒的现象[36]。这一效应通过基体表面微结构和低附着力,有效带走表面污物和腐蚀介质,从而保持长期的腐蚀防护效果。图5为Lotus效应的超疏水涂层在金属表面的防腐蚀过程[37]。受荷叶效应的启发,Cui等[38]采用纳秒激光烧蚀技术在锆基金属玻璃表面构建出分层微纳米结构,随后将其放置在空气中进行热处理获得超疏水表面,该分层微纳米结构表面的超疏水性是由于在热处理过程中吸收了低表面能物质(C—C/C—H),使得其表面能降低。其接触角达到168.2°±1.5°。与抛光锆基金属玻璃表面相比,该表面的腐蚀电位从(−231±35) mV增加到(−123±24) mV,其腐蚀电流减少了约一个数量级,具有优异的耐蚀性。同样地,Wang等[39]在镁合金表面采用简单的原位生长和低能量改性的方式制备出具有交叉型咪唑酸盐骨架结构的超疏水涂层,涂层的接触角为159.71°,将其置于碱性溶液中浸泡112 h后仍保持超疏水状态,展示出优异的耐碱性。此外,低附着力测试还显示出水滴能带走涂层表面的粉煤灰层,表现出优异的自清洁性能,可以保护镁基底免受部分腐蚀物质污染。尽管自清洁性能可以带走部分腐蚀物质,但由于腐蚀物质的多样性,为了达到防护目的,超疏水涂层更依赖于其极低的表面能来降低腐蚀物质的粘附强度[40]。 刘战剑等[41]对防腐阻垢领域的研究发现,超疏水涂层以其极低的表面能可以极大地降低腐蚀介质和结垢离子与该表面的接触面积,降低其粘附强度,从而减缓金属表面腐蚀速率。由此可见,不管是通过自清洁带走腐蚀介质,还是降低腐蚀介质的粘附强度,Lotus效应的关键在于微纳米结构以及对金属表面的改性处理。然而考虑到实际腐蚀介质的多样性,超疏水涂层为金属表面的防腐蚀提供了一种可行的防腐蚀方案,其深层机制仍需进一步研究和完善。
2.2 阻碍电化学腐蚀
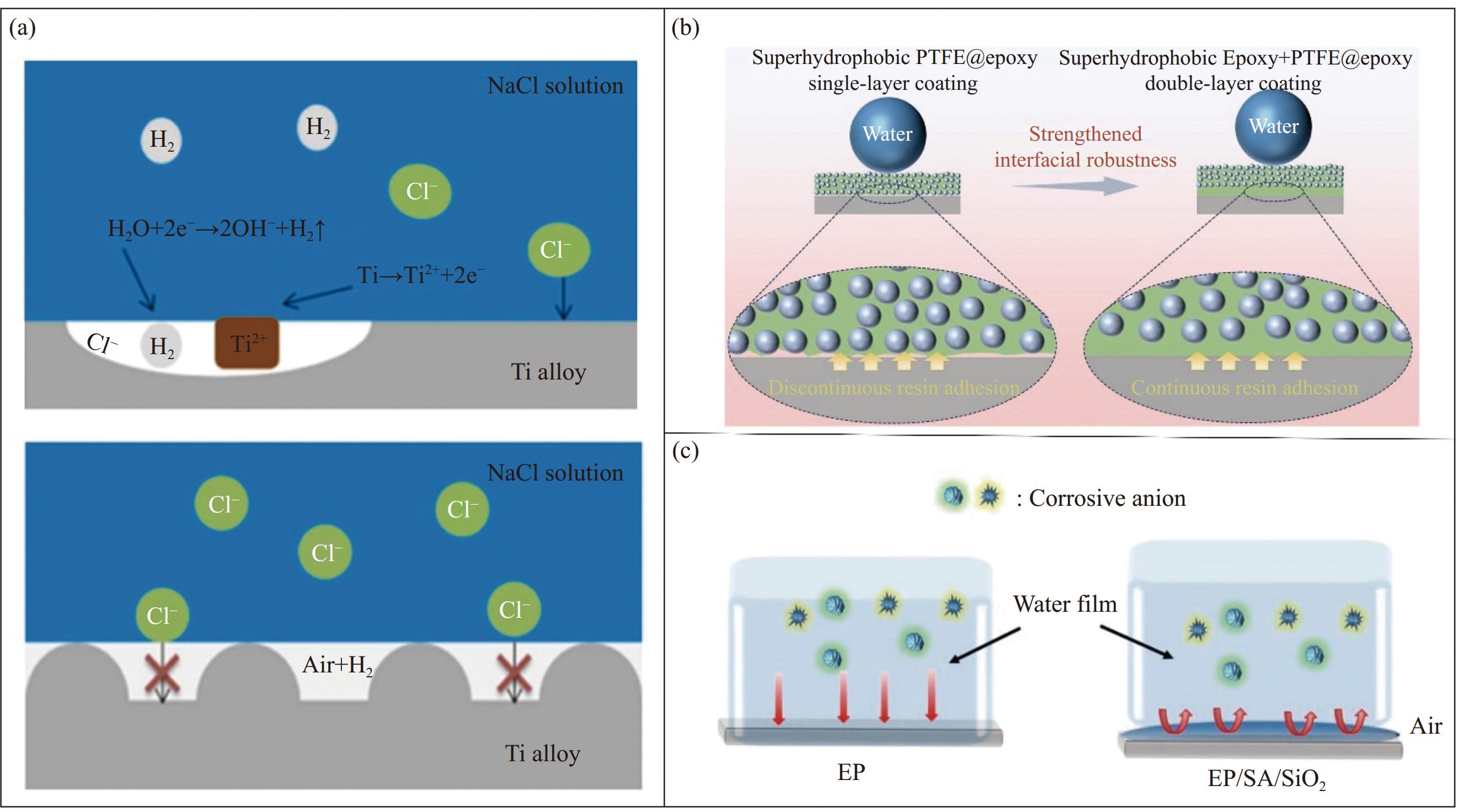
电化学腐蚀是金属常见的腐蚀形式之一,通常发生在金属和电解质溶液(水)中,由于超疏水涂层的高接触角,使得水滴与金属表面的实际接触面积较少,从而降低腐蚀微电池的形成,阻断电化学腐蚀过程的发生,提高金属表面的耐腐蚀性[42]。尹晓丽等[43]采用水热反应法,在泡沫镍基体表面直接生长Ni3S2微纳米复合结构,经过十四酸修饰反应后,获得性能优良的超疏水表面,其水接触角达到160.28°,与泡沫镍基体相比,该结构试样的自腐蚀电位升高,但基体的自腐蚀电位显著降低。说明该表面可有效阻止水分子进入基体表面,具有良好的耐电化学腐蚀特性。另外,Liu等[44]采用激光-热复合处理技术制备出超湿钛合金表面,其接触角为156.2°,与未处理的钛合金表面相比,该表面在NaCl溶液中有更小的腐蚀电流密度和更大的电位,具有较好的耐蚀性。由于超疏水涂层的微纳米结构形成的空腔,有效减少了固液接触面积,从而降低了腐蚀微电池的形成(图6(a))。Wang等[45]利用硬脂酸(SA)对钛酸钾晶须(PTW)进行改性,在低碳钢表面制备出超疏水磷化转化涂层,其接触角为153°,当SA-PTW的浓度为0.8 g/L时,其腐蚀速率最低,且通过实验验证,该涂层在3.5wt%的NaCl溶液中浸泡20天后,腐蚀效率仍保持最低,对低碳钢的长期腐蚀能力增强。这是由于超疏水涂层有效地阻止腐蚀介质的侵入和热量的积累,降低腐蚀微电池的形成,从而阻碍电化学腐蚀。可见,该机制通常是在金属表面通过材料反应构建超疏水膜层,这对材料的性质以及含量都有严格要求,该机制还需进一步完善,拓宽其应用范围。
![]() Figure 6. (a) Corrosion mechanism diagram of Ti alloy in NaCl solution[44]; (b) Mechanism and schematic diagram of enhanced interface robustness of superhydrophobic double-layer coatings[47]; (c) Schematic diagram of corrosion mechanism[51]PTFE—Polytetrafluoroethylene; EP—Epoxy resin; SA—Sodium alginate
Figure 6. (a) Corrosion mechanism diagram of Ti alloy in NaCl solution[44]; (b) Mechanism and schematic diagram of enhanced interface robustness of superhydrophobic double-layer coatings[47]; (c) Schematic diagram of corrosion mechanism[51]PTFE—Polytetrafluoroethylene; EP—Epoxy resin; SA—Sodium alginate2.3 提高界面结合力
金属常常也会因为摩擦、冲击、振动等而导致腐蚀,由于超疏水材料不仅具备良好的憎水性,在特定情况下,该涂层与基体之间还具有很强的结合力,能够抵抗机械磨损,降低由物理损伤导致的腐蚀问题[46]。Zhang等[47]利用聚四氟乙烯纳米粒子和水性环氧树脂设计制备出超疏水单层涂层和超疏水双层涂层进行对比发现,超疏水双层中引入的环氧树脂中间层,能够提升该复合涂层的界面结合力,并通过电化学测试表明,复合涂层与裸露的Q235碳钢基底相比,其阻抗值和低频模量值提高7~8个数量级,展示出优异的耐腐蚀性(图6(b))。中间层的引入能够提高界面结合力,过渡层也有类似的作用。例如,赵明欣等[48]采用阳极氧化工艺以及喷涂的方式在Ti6A14V钛合金表面制备具有微纳米表面结构且接触角为151.6°的超疏水膜层。经测试发现,该膜层利用阳极氧化膜作为过渡层,使超疏水膜层与钛合金基底牢固结合,从而表现出良好的机械稳定性。此外,通过提高防腐底层和超疏水表层的界面结合力还能使防腐效率高达90%以上。于元昊等[49]利用聚二甲基硅氧烷(PDMS)为疏水层,纳米SiO2为填料构筑粗糙表面,通过条件控制实现防腐底层和超疏水表层的界面融合,从而引入稳定的SiO2/PDMS超疏水涂层。电化学测试发现,阻抗提升了2个数量级,涂层对Q235碳钢的防腐效率高达99.8%,展示出优异的耐腐蚀性能。可见,该腐蚀机制生效的关键在于超疏水膜层与金属基体界面结合力的强弱,从而抵抗外界物理损伤导致金属基体的腐蚀失效。然而,由物理因素引起金属腐蚀的可能性很小,因此,该机制的适用范围受到特定条件的限制。
2.4 气垫效应
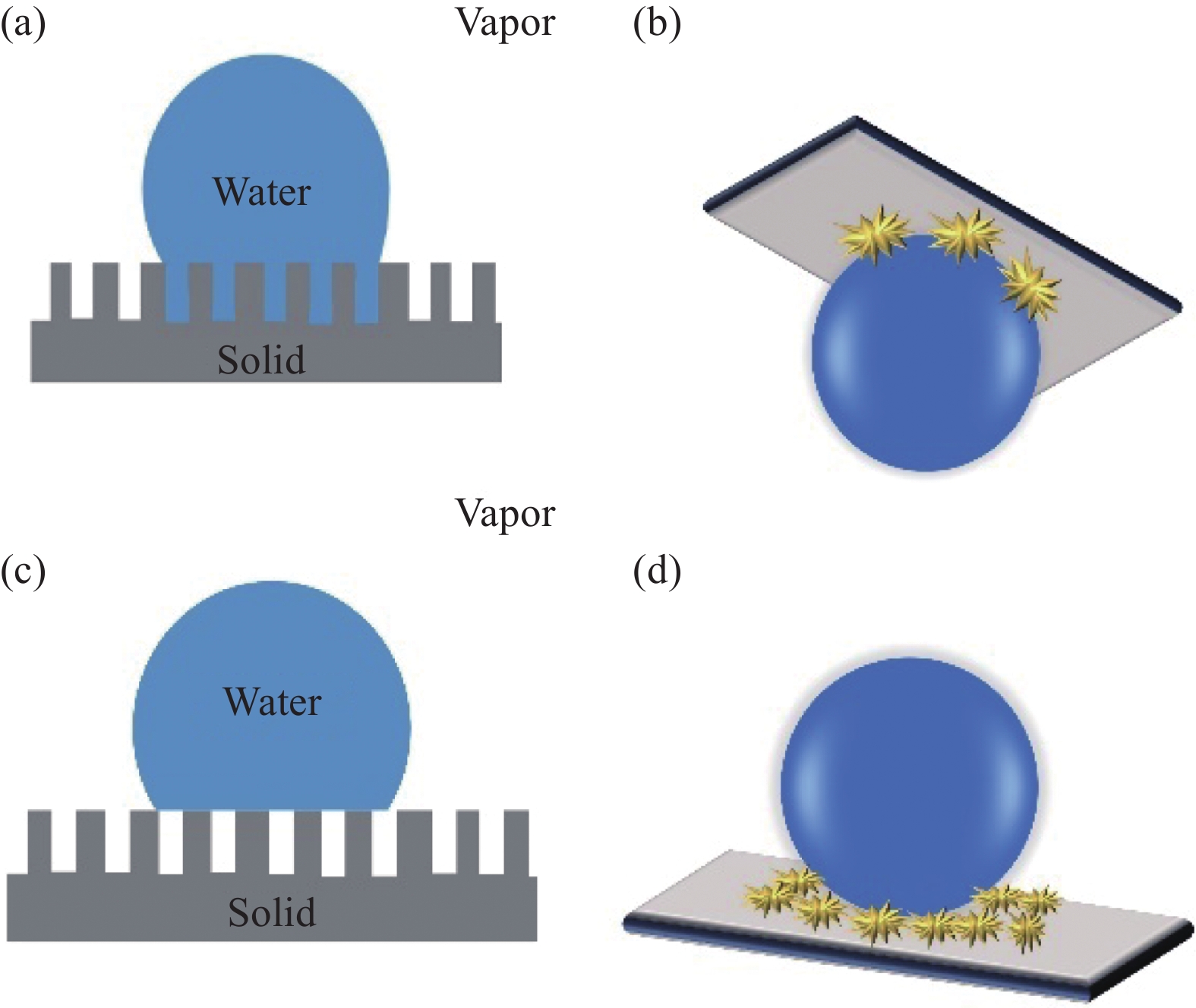
气垫效应主要是指当水滴或液滴接触到超疏水表面时,由于表面的特殊结构和化学性质,会导致水珠无法完全附着在表面上,从而形成半悬浮状态[50]。在这种情况下,液滴就会与表面形成气体薄膜,因此产生类似气垫的现象。其在金属防腐蚀方面的作用是通过气垫的形成减少了侵蚀元素直接接触金属而引发腐蚀的必要性。Guo等[51]采用环氧树脂/海藻酸钠乳酸相分离的方法制备出涂层体,再利用PDMS/气相SiO2为材料制备出超疏水SiO2涂层。研究发现,该涂层的阻抗值比环氧涂层高两个数量级,具有优异的抗腐蚀性能。这是由于表面Cassie-Baxter模型的微纳米结构,空气能够被困在涂层的固-液接触面上,减少侵蚀元素与金属的直接接触(图6(c))。可见,只要有气垫效应的存在,就能够减缓金属基体的腐蚀,但其稳定性是影响该基体腐蚀程度的关键因素。然而,Bico等[52]发现疏水表面在粗糙表面的空气层处于亚稳态的条件是:
cosθ∗<∅s−1r−∅s (5) 即[52]:
90∘<θ∗<cos−1(∅s−1r−∅s) (6) 其中, ∅s为液滴在固-液界面所占的比例。由公式可知,当θ∗>90°时,空气将被包裹于表面微纳米二元结构中,并处于一个相对稳定的状态。此外,He等[53]通过电化学测试表明,只要满足该亚稳定状态条件,空气膜就可以稳定存在于超疏水表面的粗糙结构中。因此,该效应在一定条件下能够极大地减缓金属基体的腐蚀。
相较于前3种机制,气垫效应也是金属防腐蚀机制中被广泛接受的理论,由于该理论简单有效,并且所有超疏水涂层都具备这样的气垫保护层。
3. 金属表面超疏水涂层的耐蚀特性
随着润湿理论和防腐蚀机制的不断完善,超疏水涂层在金属防腐蚀领域受到了广泛关注。在实际应用中,涂层的耐腐蚀性能直接影响金属制品的使用寿命以及性能的稳定性[54]。为了更全面地了解不同超疏水涂层在金属表面的耐蚀性,通过对涂层材料细致地分类,并深入讨论其在化学环境中的性能表现,从而更好地评估各类涂层的优缺点,为该领域的研究与应用提供指导。近几年,依据涂层材料,可以分为防蚀性超疏水聚合物涂层[55]、耐蚀性纳米超疏水涂层[56]、耐蚀性超疏水陶瓷涂层[57]三大类。
3.1 防蚀性超疏水聚合物涂层
防蚀性超疏水聚合物涂层的制备通常涉及特定的功能性物质,如防腐蚀剂、添加剂和纳米颗粒等,这不仅提升了涂层的防腐蚀性能,还增强了其疏水特性,从而阻止腐蚀介质的侵入,为金属基材提供更优良的耐腐蚀保护[58]。例如,Moradi等[59]在低碳钢表面制备出超疏水聚丙烯(PP)涂层,通过将氧化石墨烯、聚丙烯接枝马来酸酐等添加剂添加到PP基体中,实验发现,当两者添加剂的含量分别为2 mg和1.5 g时,其PP涂层具有最高的耐腐蚀性(比纯PP高800倍),展现出良好的耐腐蚀性。适量的添加剂能够提升超疏水涂层的耐腐蚀性,同时一些防腐填料通过填补涂层的微小空隙,也能进一步提升其防腐性能。安然等[60]在羟基化的六方氮化硼表面原位聚合生长聚苯胺(PANI),获得聚苯胺/氮化硼纳米颗粒(PBN),将PBN作为防腐填料来增强水性环氧涂层的防腐性。经测试发现,其粘结强度达到6.05 MPa,且电流密度降低约3个数量级,能够显著提高该表面的防腐蚀性能,这是由于该纳米颗粒能够均匀分散在环氧树脂中,填补涂层的微小空隙并提升对腐蚀介质的阻隔作用。
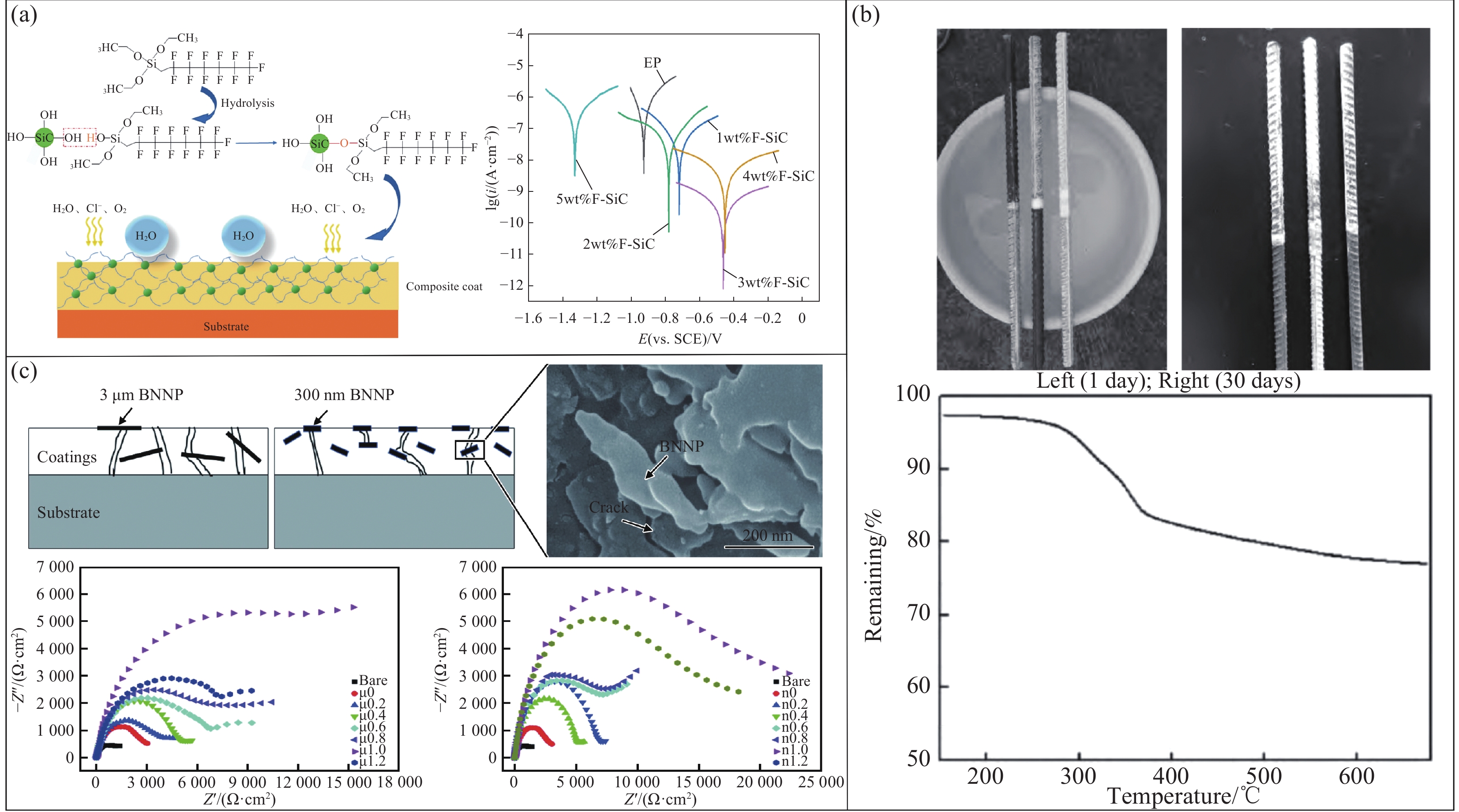
制备防蚀性超疏水聚合物涂层的关键在于是否含有功能性物质,但其耐腐蚀性能的优异却取决于涂层结构的设计以及相关成分的含量及配比[61]。吕艾娟等[62]通过3,4-乙烯二氧噻吩(EDOT)与含氟丙烯酸酯共聚得到EDOT-含氟聚合物涂层(PFET),该涂层的接触角可达到115°,通过调控两者物质的量之比,观察其耐腐蚀性能,发现当EDOT与含氟丙烯酸酯物质的量之比为0.3∶1时,与PF涂层相比,涂层阻抗增加两个数量级,该涂层的耐蚀性最佳。Zhang等[63]采用含氟有机物对SiC进行改性,并在环氧树脂(EP)基体中添加不同含量的氟改性碳化硅(F-SiC)纳米颗粒,发现添加F-SiC后,涂层的疏水性和耐腐蚀性都显著提高,并分析了不同含量F-SiC复合涂层的耐腐蚀性能,发现当添加量为3wt%时,与EP涂层相比,其腐蚀电流降低了3个数量级,该涂层的耐蚀性最佳(图7(a))。聚合物涂层不仅能通过调整其配比来获得优异的耐蚀性,还可以通过改变表面粗糙度来影响其耐蚀性。例如Robert等[64]通过制备Al-6061合金超疏水(SH)聚合物纳米复合涂层(PNC)研究发现,调整ZnO纳米颗粒的浓度可以提高干膜的表面平均粗糙度,以提高涂层的疏水性。当该ZnO纳米颗粒的浓度为2.5%时,其水接触角达到154°,且在该表面镀PNC-5后,Al-6061合金的腐蚀速率从0.60325降低到0.00572262 mm/年,显著提高涂层的耐蚀性。
![]() 图 7 (a)制备碳化硅及其复合涂层的实验方法以及不同涂层的塔菲尔图[63];(b)超疏水纳米涂层材料涂刷在钢筋的盐雾腐蚀试验和超疏水纳米涂层材料的热重曲线[70];(c)氮化硼纳米片(BNNP)强化抗腐蚀机制图和各涂层(μ为3 μm片径的BNNP,n为300 nm片径的BNNP,字母后数字指代添加相在涂层中的质量分数)的阻抗谱[79]Figure 7. (a) Experimental method for preparing silicon carbide and its composite coating and Tafel diagram of different coatings[63]; (b) Salt spray corrosion test and thermogravimetric curve of superhydrophobic nano-coating material applied to steel bar[70]; (c) Boron nitride nanoplate (BNNP) enhanced corrosion resistance mechanism diagram and the impedance spectra of each coating (μ represents BNNP with a flake-diameter of 3 μm, n represents BNNP with a flake-diameter of 300 nm, and the number after the letter refers to the mass fraction of the added phase in the coating)[79]i—Current density; E—Potential; SCE— Saturated calomel electrode; Z'—Real part of impedance; Z''—Imaginary part of impedance
图 7 (a)制备碳化硅及其复合涂层的实验方法以及不同涂层的塔菲尔图[63];(b)超疏水纳米涂层材料涂刷在钢筋的盐雾腐蚀试验和超疏水纳米涂层材料的热重曲线[70];(c)氮化硼纳米片(BNNP)强化抗腐蚀机制图和各涂层(μ为3 μm片径的BNNP,n为300 nm片径的BNNP,字母后数字指代添加相在涂层中的质量分数)的阻抗谱[79]Figure 7. (a) Experimental method for preparing silicon carbide and its composite coating and Tafel diagram of different coatings[63]; (b) Salt spray corrosion test and thermogravimetric curve of superhydrophobic nano-coating material applied to steel bar[70]; (c) Boron nitride nanoplate (BNNP) enhanced corrosion resistance mechanism diagram and the impedance spectra of each coating (μ represents BNNP with a flake-diameter of 3 μm, n represents BNNP with a flake-diameter of 300 nm, and the number after the letter refers to the mass fraction of the added phase in the coating)[79]i—Current density; E—Potential; SCE— Saturated calomel electrode; Z'—Real part of impedance; Z''—Imaginary part of impedance从对防蚀性超疏水聚合物涂层的研究可见,该涂层主要由聚合物构成,通过改变表面能量使液体形成球状,从而达到迅速滚落而不粘附的效果,以此提高基体的耐蚀性。然而,该类涂层在应用中也将面临一些挑战。一方面,一些超疏水聚合物涂层可能在长期使用后耐久性减弱,导致耐腐蚀性能下降;另一方面,涂层可能会在外界力作用下脱落,无法有效保护基材免遭腐蚀。因此,研究人员将侧重点倾向于在具有良好耐蚀性的情况下,提高超疏水涂层的稳定性。
3.2 耐蚀性纳米超疏水涂层
耐蚀性纳米超疏水涂层利用纳米材料与其他材料的结合形成微纳米级结构表面,这种结构不仅能抵御化学腐蚀,还能让水滴形成球状以自清洁表面,减少污染物附着,从而加强金属的防腐蚀能力并延长材料的使用寿命[65]。Sharma等[66]采用三乙氧基辛基硅烷对SiO2颗粒进行改性,在黄铜表面制备出耐腐蚀超疏水涂层,研究表明,该涂层与未涂覆黄铜相比具有较高的阻抗值以及较低的电流密度,其耐腐蚀性良好。尽管单一涂层能够减缓金属表面的腐蚀速率,但它对金属腐蚀防护效果不佳,而二维纳米材料具有大的径厚比和优异的物理阻隔性能,能够有效改善长期防腐蚀性能,提高涂层的稳定性[67]。曹怀杰等[68]利用浸泡法制备的氮化硼/硅烷复合涂层,通过电化学实验表明,该涂层的阻抗值提高,腐蚀电流密度明显降低,且利用长期浸泡实验证实该涂层具有优异的长效防护性能,进一步验证了二维纳米材料能够显著提高单一涂层的防腐蚀性。虽然二维纳米材料具有优异的物理阻隔性,提高了涂层的耐蚀性,但对于涂层稳定性的问题却未能很好地解决。近几年,研究人员发现纳米颗粒能够增加涂层的表面积和机械强度,从而提高涂层的稳定性[69]。高旭超[70]制备的超疏水纳米涂层材料,通过中性盐雾环境测试发现,放置30天后,未涂的钢筋表面出现大面积的锈斑,而涂有超疏水纳米涂层材料的表面出现轻微锈点。证实该涂层能有效减缓钢筋腐蚀生锈,增加涂层的表面积,延长其使用寿命,且还具有良好的热稳定性(图7(b))。李健鹏等[71]在镁合金表面制备不含与含有SiC和CeO2纳米颗粒的3种镁合金微弧氧化(MAO)涂层,通过实验发现,含有CeO2纳米颗粒的涂层具有较小的孔隙率且厚度大,有效地改善了涂层的耐蚀性,使其具有最高的低频阻抗值,还显著降低该涂层的腐蚀电流密度。而含有SiC纳米颗粒的MAO涂层则增大了涂层的稳定电流,提高了等离子体放电强度,导致纳米颗粒的填充作用不明显,使涂层孔隙率升高,对涂层的耐蚀性改善不充分,但两者都能进一步提高ZM5镁合金微弧氧化涂层的耐蚀性能,这都归因于纳米颗粒独特的结构特点。另外,含有纳米颗粒的双层复合涂层也展示出优异的耐蚀性,腐蚀效率达到90%以上。张思等[72]以苯胺单体和纳米SiO2为原料,十二烷苯磺酸(DBSA)为掺杂剂,利用化学氧化聚合法分别制备了聚苯胺(PANI)和PANI/SiO2复合材料,通过实验发现,该双层涂层的腐蚀保护效率高达98.82%。
耐蚀性纳米超疏水涂层相比防蚀性超疏水聚合物涂层在耐蚀性和稳定性上表现更出色,这归因于纳米材料独特的性质以及与其他材料的协同作用,能够制备出合适孔隙大小的涂层[73]。另外,制备超疏水聚合物涂层通常使用含氟试剂,可能对环境和人体健康造成危害[74]。相比之下,纳米超疏水涂层不仅更环保,而且其使用的试剂也更为友好[75]。但该类涂层在实际应用时也将面临许多挑战,如较高的制备成本可能限制其在工业中的广泛应用,涂层的稳定性也需进一步加强。
3.3 耐蚀性超疏水陶瓷涂层
耐蚀性超疏水陶瓷涂层结合了特殊的微纳米结构和表面改性技术,赋予基体表面强大的腐蚀抵抗力和防污染能力,能够更有效地保护金属材料免受腐蚀与污染[76]。Fu等[77]采用等离子体电解氧化和化学气相沉积的方法在铝表面制备出陶瓷基超疏水涂层,其接触角为160.5°,与裸铝相比,该涂层的腐蚀电流密度减小了3个数量级,缓蚀效率高达99.76%。这是由于制备的超疏水涂层具有网状结构的SiO2纳米颗粒和凹坑的多孔陶瓷。同样地,王丽等[78]通过等离子体电解氧化表面改性技术,在镁合金表面成功获得特殊微纳米级结构,经测试表明,该涂层为锐钛矿TiO2陶瓷涂层,其腐蚀电流提高7个数量级,显著提高了镁合金的耐腐蚀性能。特殊的微纳米级结构往往能够给涂层提供疏水性,减缓腐蚀发生,但其原料之间的配比、烧结温度、升温速度等因素将直接影响其耐腐蚀性能的好坏。李东升等[79]通过在304不锈钢表面制备不同片径的氮化硼纳米片(BNNP)以增强氧化铝胶粘陶瓷涂层的性能,经测试发现,涂层的疏水性随着BNNP含量的增加而提高,当BNNP含量达到1.0wt%时,涂层展现出优异的疏水特性,且添加片径为300 nm BNNP的涂层,其低频阻抗和自腐蚀电位分别达到最高值,具有更好的耐腐蚀性能(图7(c))。同样地,刘富等[80]采用涂覆法在304钢表面制备复合陶瓷涂层,发现在其原料SiO2、Al2O3、MgO骨料(质量比65∶20∶15)与水玻璃质量比为1∶5、烧结温度为750℃、升温速度为2℃/min、烧结时间为30 min的条件下,该涂层表面质量良好、无裂纹、成分均匀,且由两者在NaCl溶液中浸泡的失重曲线可知,整体上不锈钢曲线要比涂层曲线略陡,即不锈钢基体的腐蚀速率要比涂覆涂层的不锈钢略快,且随着NaCl浓度提高,陶瓷涂层的腐蚀越来越严重,因此,涂层的耐碱性还需进一步提高。
相较于前两种涂层,耐蚀性超疏水陶瓷涂层通常具有更佳的防腐蚀性[81]。这是由于该类涂层不仅能防止化学腐蚀对基材的侵蚀,减少腐蚀的可能性。此外,陶瓷材料本身优异的耐腐蚀性和硬度,与超疏水技术的结合,提供了更可靠和持久的防腐蚀保护。表2总结了不同涂层类型的耐腐蚀性能及其防腐蚀机制[82-88]。
表 2 不同涂层类型的耐腐蚀性能及其防腐蚀机制Table 2. Corrosion resistance of different coating types and its anti-corrosion mechanismType Material Evaluation method Contact angle (CA) Corrosion mechanism Ref. Corrosion resistant superhydrophobic polymer coating Zn-coated carbon steel CA, SEM, EIS 159.8° Resistance to electrochemical corrosion [82] Magnesium alloy CA, SEM 152.6° Resistance to electrochemical corrosion [83] Corrosion resistant nano superhydrophobic coating Aluminum alloy CA, EIS 162.4° Air cushion effect [84] Galvanized steel CA, XRD 150° Improve interface binding force [85] Steel SEM, EIS 155.4° Lotus effect [86]
Corrosion resistant superhydrophobic ceramic coatingGlass ceramics CA, sanding with sandpaper 158° Air cushion effect [87] Rare-earth oxide ceramics CA, SEM 160° Air cushion effect [88] 4. 金属表面超疏水涂层的制备方法
超疏水表面因其独特的浸润性,逐渐成为解决金属材料耐蚀性差的新思路[89]。因此,超疏水涂层的表面制备方法显得极为关键,它决定了是否能在金属材料表面实现超疏水防腐蚀性能[90]。近年来,超疏水涂层制备的主要原理可以归纳为在粗糙表面修饰低表面能物质,或在低表面能物质表面构造粗糙结构[91-92]。在过去的几十年里,研究人员提出了多种制备超疏水涂层的方法,主要包括自组装法[93]、溶胶凝胶法[94]、化学气相沉积法[95]和激光刻蚀法[96]等,为金属基体超疏水表面的制备及应用提供了多种技术方案选择。
4.1 自组装法
自组装法通常依靠分子间作用力如氢键、范德华力和静电吸引力等,使分子或纳米颗粒根据其内在性质形成有序、重复的微纳米级结构[97]。Feng等[98]在铜网表面采用全氟十硫醇自组装的方法制备出纳米级粗糙结构,电化学实验表明,与原铜网相比,其阻抗值降低了约一个数量级,且在NaCl、NaOH、HCl水溶液中处理后的防腐效率明显提高。另外,该方法制备的超疏水膜的耐蚀性还与反应物的协同效应有关。Wang等[99]利用1-羟乙基-1,1-二膦酸(HEDP)分子在铝合金表面制备反应性保护膜,通过盐雾试验验证该膜的缓蚀率为99.99%,并且讨论了磷酸盐协同效应对6061铝合金自组装超疏水复合膜耐腐蚀性能的影响。
自组装法通常适用于具有一定晶体结构或特定表面特性的金属材料。这些材料能够通过自组装形成有序结构,如金属纳米颗粒或自组装单分子膜[100]。特别是在需要精确控制纳米级结构时,自组装能够提供高度有序的排列[101]。此外,自组装方法还能够改变金属表面的化学性质和结构[102]。例如增强表面的生物相容性或电化学性能。因此,自组装方法适合于需要在纳米尺度上精确控制结构和表面性能的情况。尽管自组装法简便快捷,高效精准,但控制难度、结构稳定性和规模生产化仍是挑战。
4.2 溶胶凝胶法
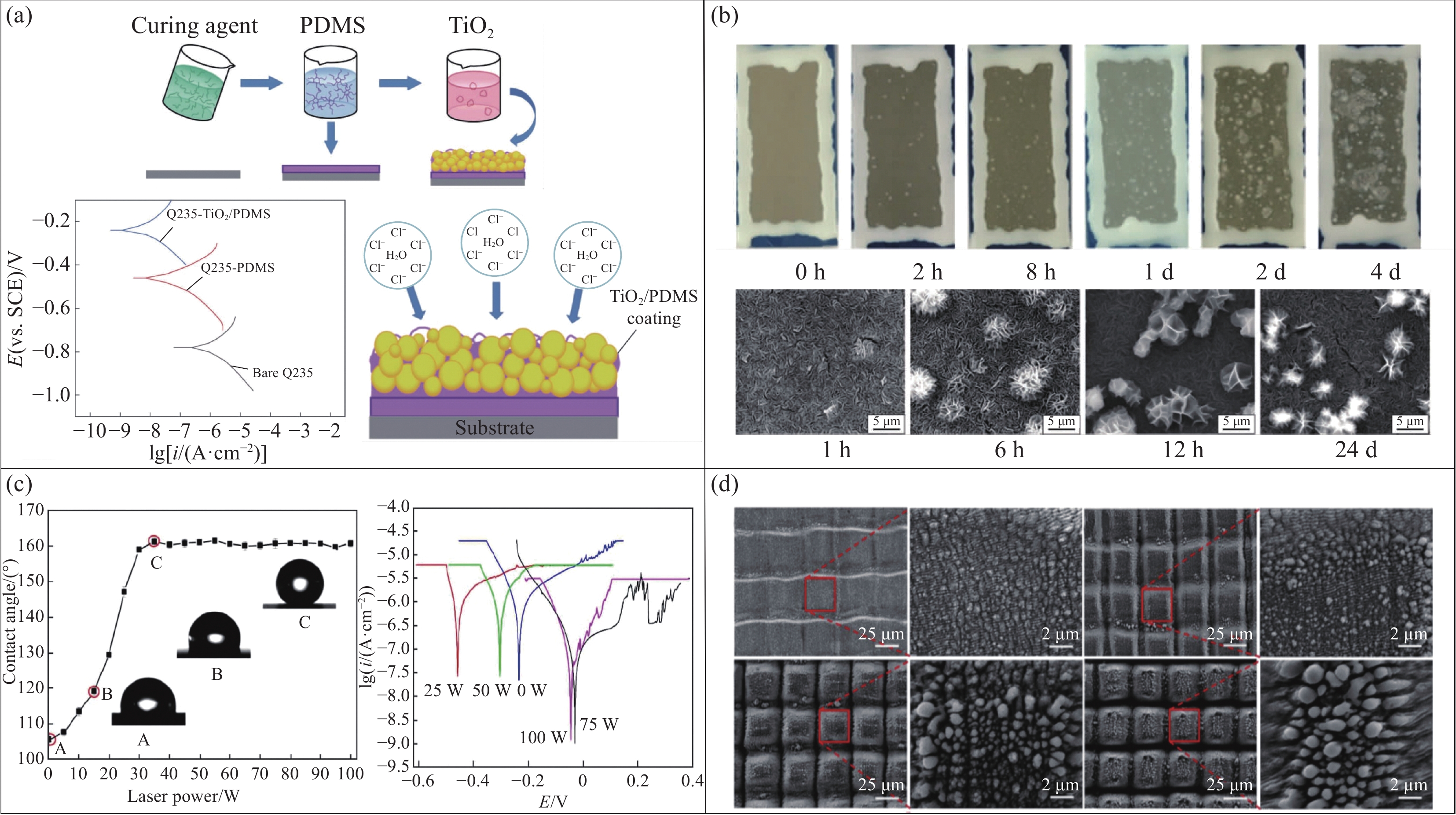
溶胶凝胶法是一种将溶液中固体的前驱体通过特定的控制手段转变成凝胶状态,随后通过干燥或热处理来形成具有目标结构的薄膜或涂层的过程[103-104]。通过精细控制溶胶-凝胶的变化和调整质量配比等因素,能够调控表面微结构以实现超疏水特性[105]。刘富等[106]选用304不锈钢为基体,采用溶胶凝胶法制备陶瓷涂层,将其浸泡在NaCl溶液中5~7天时,浸泡实验表明,304不锈钢基体的质量变化比陶瓷涂层的质量变化大,说明该涂层的耐蚀性比不锈钢基体好1.5~2倍。此外,他们还分析了溶胶原料的配比、溶胶剂的加入量和不同烧结温度对陶瓷涂层表面形貌和耐腐蚀性能的影响。另一方面,采用该方法,通过添加过渡层,也能获得优异的耐腐蚀性能。薛鑫宇等[107]在Q235钢表面运用同样的方法制备出有PDMS过渡层的TiO2/PDMS超疏水涂层,该涂层表面具有独特的微纳结构,其接触角达到154.3°,且腐蚀电流密度下降两个数量级,展现出卓越的防腐性能(图8(a))。另外,由于基板表面粗糙度的大小将会影响凝胶铺展成膜,因此选择合适的基板粗糙度对获得性能良好的超疏水膜至关重要。例如,贠柯等[108]利用溶胶凝胶法在钢基板表面制备TiO2-SiO2复合薄膜。研究发现,基板表面粗糙度太大,不利于凝胶铺展,反之,太小则会导致膜层破裂,当基板表面粗糙度为1.379 µm时,该复合薄膜成膜质量最高且具有的腐蚀电位最高、腐蚀电流最小,拥有较高的耐蚀性。
![]() 图 8 (a) TiO2/聚二甲基硅氧烷(PDMS)超疏水涂层的制备流程示意图和不同试样在3.5wt% NaCl溶液中的Tafel曲线以及超疏水涂层防腐蚀机制原理图[107];(b)盐雾腐蚀不同时间改性后的镁铝水滑石膜层(LDH-F)的宏观形貌和经不同时间改性后十钒酸根插层镁铝水滑石膜层(LDH-V-F)的SEM形貌[110];(c)不同激光功率刻蚀不锈钢试样表面的接触角和不锈钢电极在NaCl溶液中的极化曲线[117];(d) EL-1、EL-5、EL-10和EL-15(即经过1次、5次、10次和15次激光加工的EH40钢)的SEM图像[120]Figure 8. (a) Preparation process diagram of TiO2/polydimethylsiloxane (PDMS) superhydrophobic coating, the Tafel curves of different samples in 3.5wt% NaCl solution, and the schematic diagram of the anti-corrosion mechanism of the superhydrophobic coating[107]; (b) Macroscopic morphology of the modified magnesium-aluminum hydrotalcite film (LDH-F) after salt spray corrosion for different durations, and the SEM morphology of the decavanadate-intercalated magnesium-aluminum hydrotalcite film (LDH-V-F) after modification for different time[110]; (c) Contact angle of stainless steel specimen surface and the polarization curves of stainless steel electrode in NaCl solution were etched by different laser powers[117]; (d) SEM images of EL-1, EL-5, EL-10 and EL-15 ( EH40 steel processed by laser for 1 time, 5 times, 10 times and 15 times)[120]
图 8 (a) TiO2/聚二甲基硅氧烷(PDMS)超疏水涂层的制备流程示意图和不同试样在3.5wt% NaCl溶液中的Tafel曲线以及超疏水涂层防腐蚀机制原理图[107];(b)盐雾腐蚀不同时间改性后的镁铝水滑石膜层(LDH-F)的宏观形貌和经不同时间改性后十钒酸根插层镁铝水滑石膜层(LDH-V-F)的SEM形貌[110];(c)不同激光功率刻蚀不锈钢试样表面的接触角和不锈钢电极在NaCl溶液中的极化曲线[117];(d) EL-1、EL-5、EL-10和EL-15(即经过1次、5次、10次和15次激光加工的EH40钢)的SEM图像[120]Figure 8. (a) Preparation process diagram of TiO2/polydimethylsiloxane (PDMS) superhydrophobic coating, the Tafel curves of different samples in 3.5wt% NaCl solution, and the schematic diagram of the anti-corrosion mechanism of the superhydrophobic coating[107]; (b) Macroscopic morphology of the modified magnesium-aluminum hydrotalcite film (LDH-F) after salt spray corrosion for different durations, and the SEM morphology of the decavanadate-intercalated magnesium-aluminum hydrotalcite film (LDH-V-F) after modification for different time[110]; (c) Contact angle of stainless steel specimen surface and the polarization curves of stainless steel electrode in NaCl solution were etched by different laser powers[117]; (d) SEM images of EL-1, EL-5, EL-10 and EL-15 ( EH40 steel processed by laser for 1 time, 5 times, 10 times and 15 times)[120]溶胶凝胶法通常适用于制备金属氧化物(如TiO2)和半导体材料(如SiO2)。这些材料通过溶胶凝胶法形成均匀的凝胶体系,随后经过热处理获得所需的纳米结构或多孔结构。特别是在需要精确控制孔隙结构时,溶胶凝胶法表现出最佳效果。尽管溶胶凝胶法具有制备过程均匀、适用范围广、操作简便的特点。但严苛的制备条件、较高的成本和复杂的工艺限制了其在金属防腐蚀领域的广泛应用。
4.3 化学气相沉积法
化学气相沉积法通常是由气态或蒸汽态物质在气相或气固界面发生化学反应,形成固态沉积物[109]。这一过程主要通过选择适当的前驱体气体、反应条件和沉积参数,实现在目标表面制备具有特定性质的薄膜或结构,使其获得超疏水性。杨文广等[110]在铝基表面制备镁铝水滑石膜层(LDH)和十钒酸根插层水滑石膜层(LDH-V)两种类水滑石膜层,随后通过气相沉积法对两类水滑石膜层进行不同时间的表面改性得到超疏水膜层(LDH-F膜和LDH-V-F膜),实验发现,改性1 h即可使类水滑石膜层获得超疏水性能,且随着改性时间的延长,超疏水膜层的接触角基本不变。12 h为最佳改性时间,获得接触角为152°~158°的超疏水膜层,由电化学阻抗谱可知,两种超疏水膜层的阻抗值都高于裸材的阻抗水平,能够极大提高基体的耐蚀性(图8(b))。此外,化学气相法制备过程简单,能够不使用催化剂就制备出超疏水涂层。Zhang等[111]采用无催化剂化学气相沉积技术,在Al表面直接制备出致密的ZnO纳米棒阵列,接触角高达157.9°,电化学测量结果表明,缓蚀效率高达90%以上,具有良好的耐蚀性,还分析了温度对其结构的影响。
由于化学气相法是通过化学反应在材料表面形成特殊结构的薄膜,如果控制不当,不可避免地会产生污染环境的物质。近年来,研究人员发现石墨烯在金属基体防腐蚀方面具有较好的作用,因此采用化学气相沉积法等技术制备高质量、大面积的石墨烯逐渐受到业内研究者的普遍重视[112]。陈清新等[113]利用化学气相法在镍钛形状记忆合金表面原位生长出石墨烯,在人工唾液环境中测试发现,镍钛合金主要组成元素在浸泡试验中的释放速率减慢,即基材的腐蚀速率减慢,这是由于石墨烯在金属基体和腐蚀介质中可以起到物理屏蔽作用,降低金属腐蚀发生速率。另外,耐腐蚀性能的优异还取决于石墨烯的层数,但并非层数越多,腐蚀效果越好。例如,李思仪等[114]采用化学气相沉积法在铜表面制备石墨烯薄膜,系统研究了裸铜表面、少数石墨烯薄膜修饰的铜表面以及多层数石墨烯薄膜修饰的铜表面的腐蚀行为。测试结果表明,在腐蚀初期,石墨烯薄膜的阻抗值高于铜基体,说明石墨烯对铜具有一定的防腐蚀作用,但在腐蚀中后期,铜基体表面产生了大量腐蚀产物,修饰少层石墨烯薄膜的铜表面腐蚀产物较为稀疏,而修饰多层石墨烯的铜表面腐蚀产物较为密集。上述结果说明,在铜表面修饰少层适量的石墨烯薄膜,其防腐蚀效果反而优于表面修饰多层石墨烯的铜表面。
化学气相沉积法通常适用于制备金属薄膜和半导体材料,尤其在追求薄膜厚度与结构精确控制时,其优势尤为显著[115]。该方法通过气相中的化学反应,生成沉积物,进而形成均匀、稳定的薄膜或结构。随后,这些薄膜或结构可通过热处理等多种后处理手段,进一步实现材料特性的优化。化学气相沉积法在微观结构控制、薄膜生长和材料表面改性等领域表现出良好的应用潜力。但是,该方法用于制备纯度和均匀度要求较高的薄膜时,其制备条件要求较为严格,成本较高,同时在环保方面也存在一定的挑战。
4.4 激光刻蚀法
激光刻蚀法是通过激光功率调整表面的形态和性质,从而在材料表面产生微纳米结构,再结合其他表面修饰方法形成具有超疏水性能的涂层[116]。激光刻蚀法通常是与热处理技术相结合制备超疏水涂层。王帅等[117]采用纳秒激光在316L不锈钢表面进行刻蚀,随后进行热处理,获得接触角大于160°的超疏水不锈钢表面,其表面的缓蚀效率达到89.75%,具有良好的耐蚀性(图8(c))。另外,激光刻蚀法与其他工艺相结合,也能够获得耐蚀性更佳的超疏水涂层。刘祁文等[118]通过激光刻蚀法结合化学浸泡处理对镁合金制备了超疏水表面,当激光能量密度为20 J/cm2时,接触角达到158.14°,并展示出良好的耐久性,且通过动电位极化实验证明,超疏水处理后的镁合金缓蚀率达到97.2%,具有优异的耐腐蚀性能。同样地,徐雷秋等[119]利用盐酸-激光烧蚀+十八烷酸(SA)修饰工艺在抛光态AZ91镁合金表面制备出超疏水结构,将其浸泡在3.5wt% NaCl溶液中24 h,未发现明显腐蚀,且通过极化实验,与抛光态镁合金相比,该表面的自腐蚀电位提高,自腐蚀电流密度下降,显示出良好的耐蚀性。此外,通过激光加工技术调控材料表面微纳结构的尺寸,能够实现润湿行为的转变,从强水附着力的Wenzel状态过渡到弱水附着力的Cassie-Baxter状态,以实现更好的耐蚀性。Chen等[120]利用皮秒激光加工与室温真空加工的协同作用,在低温钢DH36、EH40和FH36制备具有不同水附着力的超疏水表面(EL-10和EL-15,即经过10次和15次激光加工的EH40钢)。证实了激光加工可以调节低温钢表面微纳结构的尺寸,真空加工可以控制激光加工表面的化学成分,导致其润湿行为从超亲水状态过渡到具有强水附着力的Wenzel状态,最后过渡到具有弱水附着力的Cassie-Baxter状态。而具有Cassie-Baxter状态的超疏水表面与钢表面和Wenzel状态的超疏水表面相比,自腐蚀电流密度最低,自腐蚀电位最高,耐腐蚀性能最好(图8(d))。
激光刻蚀法通常适用于各种金属材料,如镁合金、铜、钛、钨等。这种方法能够精确控制微米或纳米级结构,适合在金属表面进行精细加工和结构调控,以满足不同领域的特定应用需求。通过激光的高能量聚焦,可以在材料表面进行精确的刻蚀和雕刻,以形成所需的结构或图案[121]。激光刻蚀法在微米或纳米结构制备、表面改性和功能化,以及材料加工和模板制备方面表现出良好的应用潜力。尽管激光刻蚀法能够精准控制基材表面的微纳米结构,但其微纳米结构仅仅是构建超疏水涂层的基础,后续还需要结合其他表面修饰方法,制备工艺复杂。并且还存在成本较高、控制复杂度等问题。表3总结了不同制备方法的优缺点以及在金属表面的表征[122-129]。
表 3 不同制备方法的优缺点以及在金属表面的表征Table 3. Advantages and disadvantages of different preparation methods and characterization on metal surfacesProcessing method Strength/Weakness Material CA Ref. Self-assembly method Simplicity, low cost, versatility, self-healing ability, scalability;
Structural control difficulties, stability issues, complexity and predictability, scale limitations6082-T6 aluminum alloy 180° [122] Sol-gel method Low temperature processes, uniformity and purity, controllable microstructure, diversity, coating and film preparation;
Drying and heat treatment, shrinkage and cracking,
reaction time, complexity, costCu 155° [123] Chemical vapor
depositionHigh-quality films, wide applicability, suitable for complex
shapes, good adhesion and interfacial quality,
controllable chemical composition;
High-temperature processes, complex equipment and control, precursor selection and cost, environmental concerns, limited deposition ratesAluminum 158° [124] Laser etching Non-contact process, high accuracy and resolution, speed and flexibility, multiple material suitability;
Heat affected zone, equipment cost, processing time,
possibility of reprocessing3Cr13 stainless steel 164° [125] Chemical etching High precision, wide range of application, high performance;
Poor selectivity, environmental impact, etching control difficultyStainless-steel 151.6° [126] Electrochemical etching method Good selectivity, wide range of applications, environmentally friendly;
Batch processing restriction, current distribution problem, equipment and technical requirementsAluminum alloy 152.3°±4.5° [127] Spraying method Simple and practicable, low cost, rapid production, wide applicability;
Limited accuracy, coating thickness control is difficult, coating thickness control is difficultQ235 steel plate 163.9° [128] Electrochemical
deposition methodGood uniformity, environmentally friendly, precise shape control;
High equipment requirements, limited to conductive substrates, the deposition rate is slowStainless-steel 160.6° [129] 4.5 其他方法
除上述的自组装法[130]、溶胶凝胶法[131]、化学气相沉积法[132]、激光刻蚀法[133]外,根据微纳结构的减材或增材制备方式,还可以将金属表面制备超疏水涂层的常用方法分为减材和增材两类。减材方式包括化学刻蚀[134]和电化学刻蚀[135]等。Rodic等[136]利用HCl和H2O2化学刻蚀,接枝正辛基三甲氧基硅烷(AS-8)和1H,1H,2H,2H-全氟辛基三甲氧基硅烷(FAS-8),制备了超疏水铝表面。其表面接触角大于150°,且浸入0.1 mol/L NaCl溶液时具有高效的耐腐蚀性,根据标准ASTM B117-19[137],即使在盐雾室中暴露2周后,经过FAS-8处理的表面仍保持不变。可见,化学刻蚀法利用化学溶液或蚀剂局部溶解或去除金属表面的部分,可形成微纳级的粗糙结构,从而实现超疏水性能[138]。该类方法适用于能被常见蚀剂如酸性或碱性溶液所溶解的金属材料,并且在需要大面积表面处理的情况下使用较合适。而电化学刻蚀则是通过在特定电解质溶液中施加电流,控制金属表面的局部腐蚀,形成所需的微纳结构,以达到超疏水效果[139]。例如,Ma等[140]通过在中性NaCl溶液中电化学刻蚀和低表面能改性,在Al-Mg基体上成功制备出超疏水表面。通过稳定性、摩擦力和电化学性能测试表明,制备的表面具有优异的耐腐蚀性和耐磨性。电化学刻蚀法主要适用于能够在电解质溶液中发生电化学反应的金属材料,该方法与化学刻蚀均适用于在金属表面大面积制备微纳结构的场合。另一方面,增材方式则包括喷涂法[141]和电化学沉积法[142]等。Yang等[143]考虑到超疏水涂层耐磨性以及含有毒氟物质等缺点,采用刮擦法和喷涂法制备了一种不含氟的双层涂层,其接触角为159.5°。由于分散良好的氧化石墨烯和疏水表面的协同作用,喷涂法可以在不同基底材料表面进行实施,适合在金属表面形成保护性涂层或增强特定功能的情况,如防腐蚀涂层、耐磨涂层等。同时,喷涂法操作简便,适用于快速涂覆需求。而电化学沉积则是利用化学反应中产生的沉积物,形成均匀的超疏水薄膜或结构[144]。Liu等[145]采用电化学沉积方法在铝合金表面制备了荷叶状超疏水膜,其接触角达到159.3°,且其电化学阻抗谱结果表明,该涂层的耐腐蚀性能较好。可见,电化学沉积法是一种能够在电解质溶液中进行,且能精确控制金属沉积结构和性能的方法。该方法与电化学刻蚀技术有所不同,后者是通过电解反应去除材料,属于减材方式,而电化学沉积法则是增加材料厚度或形成特定结构。
5. 超疏水涂层在金属防腐蚀领域的应用
金属因其优异的力学性能已被广泛应用于各领域中,然而,金属材料的耐久性却因腐蚀而受到严峻挑战。金属材料的腐蚀问题不仅会严重影响其使用性能和寿命,还会带来巨大的经济损失,并对环境和人类安全构成潜在威胁[146]。因此,对金属腐蚀问题的深入研究和有效防护显得尤为重要。超疏水涂层因其优异的憎水特性和特殊的界面性,已在金属防腐蚀领域取得显著成效[147]。在实际应用方面,该类涂层还提供了防锈、抗腐蚀、耐磨损和抗菌防污等多方面的保护[148]。近几年,超疏水涂层已被广泛应用于铜[149]、铝及其合金[150]、镁合金[151]、钢铁及其合金[152]等金属材料的腐蚀防护。
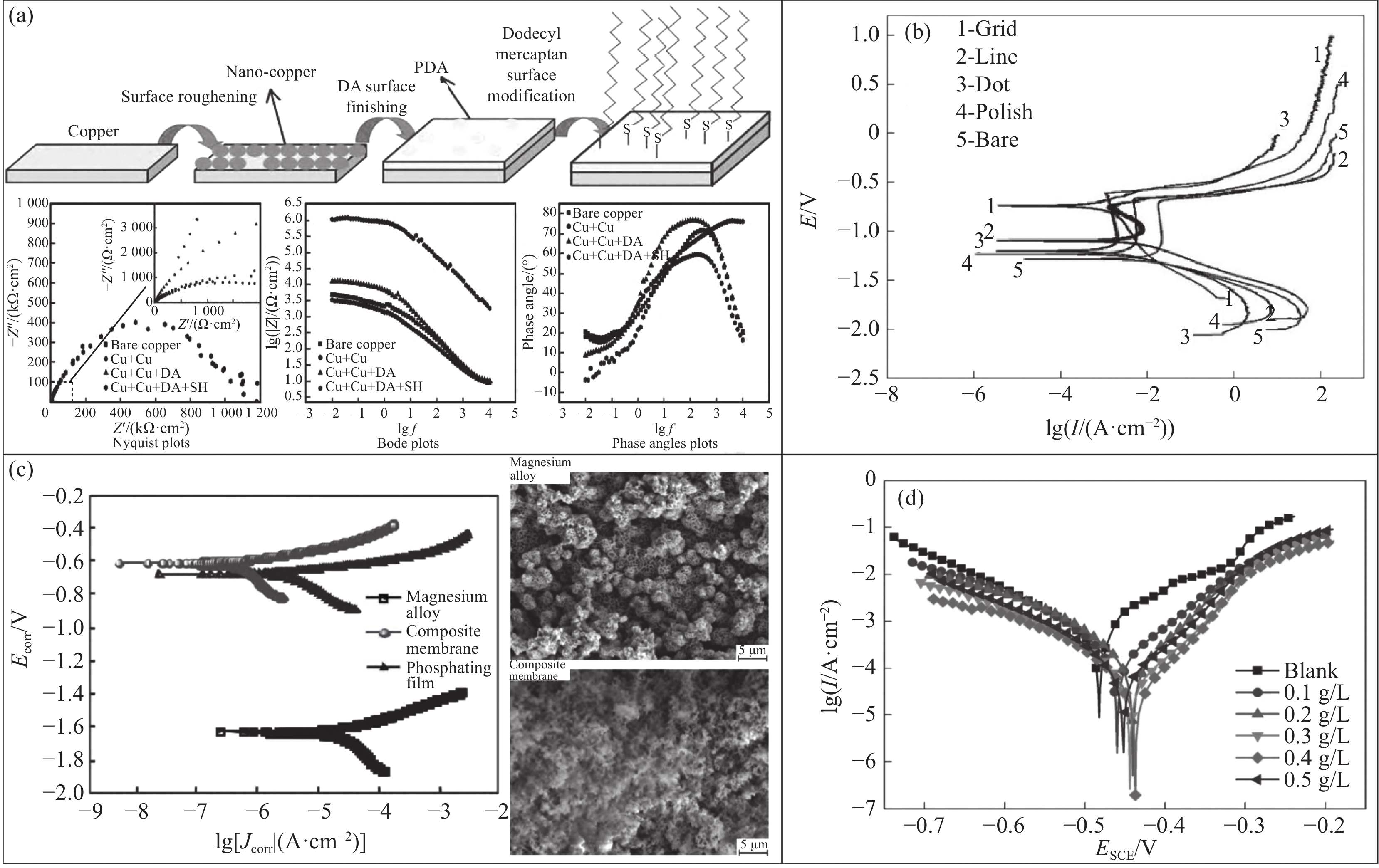
铜基表面超疏水涂层大多使用电沉积制备。曹怀杰等[153]在铜基表面通过电沉积获得微纳米粗糙化铜结构,再利用多巴胺键联低表面能物质(十二硫醇)进行修饰,最终制备出超疏水膜层,其接触角可达150°以上,且低频阻抗比裸铜低2.4个数量级,阳极极化电流降低到1.02×10−8 A/cm2,展示出较好的抗腐蚀能力,放置在模拟海洋潮差区经过28个干湿交替周期后,仍具有抗腐蚀性(图9(a))。该工艺为铜基超疏水表面的制备提供了一种有效的方法。蒋翔等[154]以NaOH和(NH4)2S2O8的混合水溶液为氧化剂、十七氟癸基三甲氧基硅烷(FAS-17)为低表面能改性剂,在铜表面制备超疏水涂层,该涂层的接触角大于160°,通过Tafel极化曲线和EIS谱图分析样品在3.5wt% NaCl溶液中的防腐蚀特性,相比裸铜基板,该涂层展示出较低的腐蚀电流密度且抑制腐蚀效率达到99.65%。可见,其能有效阻止海水中腐蚀性物质进入铜基表面,这为超疏水涂层在海水环境中的实际应用提供了一种有效的途径。另外,Wang等[155]采用水热法以及后改性技术相结合的工艺,在铜网表面制备了超疏水-超亲油-光热涂层,通过试验发现,该涂层具有良好的自清洁能力、机械耐久性、化学稳定性和环境适应性,同时还具有理想的防腐蚀性能(腐蚀防护效率η=97.7%)、被动防冰性能(冻结时间延长
1800 %)和油水分离性能。不仅解决了铜基材料的耐腐蚀问题,这种涂层还具有出色的机械耐久性和化学稳定性,同时具备防冰和油水分离的功能,极大地拓宽了铜在各种应用领域的应用潜力。![]() 图 9 (a)超疏水膜形成示意图以及电化学测试阻抗图[153];(b)试样在海水中的极化曲线[157];(c)镁合金、磷化膜和复合膜在3.5wt% NaCl溶液中浸泡24 h后的极化曲线以及镁合金和复合膜在3.5wt% NaCl溶液中浸泡24 h后的腐蚀形貌[162];(d)吸附了不同浓度LLE聚集材料的Q235钢电极在1 mol/L HCl溶液中的动电位极化曲线[166]Figure 9. (a) Schematic diagram of superhydrophobic film formation and impedance diagram of electrochemical test[153]; (b) Polarization curves of sample in seawater[157]; (c) Polarization curves of magnesium alloy, phosphating film and composite film after soaking in 3.5wt% NaCl solution for 24 h and corrosion morphology of magnesium alloy and composite film after soaking in 3.5wt% NaCl solution for 24 h[162]; (d) Potentiodynamic polarization curves of Q235 steel electrodes adsorbed with different concentrations of LLE aggregates in 1 mol/L HCl solution[166]DA—Dopamine; HS—1-dodecanethiol; f—Frequency; E—Electrode potential; Ecorr—Corrosion potential; Jcorr—Corrosion current; ESCE—Electrode potential of the saturated calomel electrode; I—Current density
图 9 (a)超疏水膜形成示意图以及电化学测试阻抗图[153];(b)试样在海水中的极化曲线[157];(c)镁合金、磷化膜和复合膜在3.5wt% NaCl溶液中浸泡24 h后的极化曲线以及镁合金和复合膜在3.5wt% NaCl溶液中浸泡24 h后的腐蚀形貌[162];(d)吸附了不同浓度LLE聚集材料的Q235钢电极在1 mol/L HCl溶液中的动电位极化曲线[166]Figure 9. (a) Schematic diagram of superhydrophobic film formation and impedance diagram of electrochemical test[153]; (b) Polarization curves of sample in seawater[157]; (c) Polarization curves of magnesium alloy, phosphating film and composite film after soaking in 3.5wt% NaCl solution for 24 h and corrosion morphology of magnesium alloy and composite film after soaking in 3.5wt% NaCl solution for 24 h[162]; (d) Potentiodynamic polarization curves of Q235 steel electrodes adsorbed with different concentrations of LLE aggregates in 1 mol/L HCl solution[166]DA—Dopamine; HS—1-dodecanethiol; f—Frequency; E—Electrode potential; Ecorr—Corrosion potential; Jcorr—Corrosion current; ESCE—Electrode potential of the saturated calomel electrode; I—Current density在铝及其合金表面制备超疏水涂层大多应用于船用材料领域,防止离子和微生物等接触金属表面[156]。连峰等[157]利用激光刻蚀和聚合物基纳米复合材料在船用铝合金表面构建具有微纳双层结构的超疏水表面,该表面接触角可达157.8°,且铝合金的腐蚀阻抗能够提高2个数量级(图9(b))。铝合金构成的舰船材料容易受到海水腐蚀的问题得到了较好的解决。然而,实际应用中该类涂层的长期稳定性仍然是一个热点问题。Tran等[158]采用激光织构、沸水处理和硅油热处理共同制备超疏水铝表面,经一系列表面表征技术、动电位极化测试和电化学阻抗谱测试,与未处理的表面相比,该表面的防腐效率可达99.40%,展示出优异的耐腐蚀性能,长时间暴露在空气、淡水、海水和高温环境中也具有良好的稳定性。尽管该类涂层的长期稳定性较好,但其耐磨性也不可忽视。Cheng等[159]采用浸涂法在2024铝合金表面制备出纳米SiO2超疏水杂化涂层,其接触角可达161.6°。且该涂层经过100次磨损后仍保持超疏水性,进一步拓展了铝在其他领域的应用。
在镁合金表面制备超疏水涂层的研究也取得了比较理想的结果。周垲杰等[160]利用喷涂法在AZ31B镁合金基体上制备稳定的超疏水涂层,该涂层的接触角可达157.4°且放入海水浸泡30天后,未经表面处理的镁合金基体表面有明显的腐蚀痕迹,而超疏水涂层的表面仅发生局部腐蚀,具备良好的耐腐蚀性。为了拓宽镁基材料的应用范围,环境因素不可忽视。因此,在一定的条件下,该涂层还需要具有较好的化学稳定性。刘金玉等[161]在AZ91D镁合金表面制备3种不同超疏水涂层(MZS-1、MZS-2和ZnO@ZIF-8)在5wt% NaCl溶液中,通过极化曲线测试和盐雾处理24 h后,3种涂层的腐蚀电流密度仍比金属基体降低1个数量级,表现出较好的耐蚀性。在确保长期稳定性的同时,提高涂层的化学稳定性可以显著增强金属基体的应用潜力。张晓菲[162]采用磷化工艺在AZ31B镁合金表面制备锌系磷化膜,通过改性硅溶液固化后与该膜形成复合膜。膜表面的静态接触角大于150°且在3.5wt% NaCl溶液中浸泡24 h后,与镁合金基体相比,其腐蚀电流密度降低近两个数量级,极化电阻为镁合金的3.3倍,具有良好的耐久性和耐蚀性(图9(c))。这是由于复合膜具有微纳米分级结构且表面能低,对纯水和水性介质都表现出强疏水作用,能有效阻止腐蚀介质进入基体表面。同样地,Cai等[163]采用激光加工、水热处理和浸没法相结合的工艺,在镁合金表面制备出复合型拒水表面,研究了环境温度的变化对该表面耐蚀性的影响,当环境温度为180℃时,其阻抗值最大,具有良好的耐腐蚀性能,且证实该表面即使在严酷的盐雾试验中,失去了超疏水性,仍具有良好的耐腐蚀性,进一步拓宽镁合金的应用环境。
钢铁及其合金是使用率较高的金属材料,但在使用过程中,腐蚀是钢铁不可避免的问题[164]。因此,提升钢铁在腐蚀环境中的稳定性成为了一项重大挑战,而超疏水涂层的发展对提高钢铁的耐蚀性以及扩展其应用范围具有重要的意义[165]。罗为平等[166]利用荷叶提取物(LEE)在Q235钢样品表面产生聚集制备超疏水的吸附层,电化学结果表明,该吸附层在0.4 g/L浓度下,缓蚀效率高达93.14%,表现出优异的耐蚀性(图9(d)),拓宽了钢铁基体的应用范围。Tang等[167]采用一步喷涂的方法制备了具有凹凸鳞片状微纳米结构的多功能集成涂层,其接触角达到153.9°。由于环氧树脂和硅树脂具有良好的附着力和疏水性,能够作为复合粘连剂将涂料附着在基材上,故所制备的涂层具有低附着力的超疏水性,此外,还表现出良好的自清洁和耐腐蚀性能、化学稳定性和环境耐久性。在保持良好的耐蚀性的同时,增强涂层与基底的结合力,其机械稳定性也不可忽视。刘艳等[168]利用电沉积工艺与喷涂法结合,在45#钢表面制备Ni-W/ZnO超疏水复合涂层,该涂层的接触角可达151.4°,且经过20次胶带提拉、20次砂粒冲击和20个周期的砂纸摩擦、耐蚀性分析,其接触角仍大于150°,该复合涂层能稳定地保持超疏水性能并展示出良好的机械稳定性,通过电化学实验发现,其腐蚀电流密度仅为6.79×10−7 A/cm2,极化电阻达到3.25×104 Ω·cm2,具备优异的耐蚀性。其良好的机械稳定性拓宽了钢铁的应用场景。另外,王萃等[169]采用一步快速电沉积方法在45钢表面制备超疏水结构,通过接触角和滚动角分析确定最优工艺,对45钢试样表面进行超疏水处理后,其自腐蚀电位更高,说明该表面对3.5wt% NaCl溶液具有更强的耐蚀性,还能同时提升阴极与阳极的耐蚀能力,有效地解决了钢铁在不同腐蚀环境中的腐蚀问题,并扩大了其应用范围。
6. 总结与展望
超疏水涂层凭借其优异的疏水特性,在金属防腐蚀领域具备较好的应用前景。本文综述了超疏水涂层在金属防腐蚀领域的最新研究进展,总结了固体表面基本润湿理论,归纳了超疏水涂层的基本特性、防腐蚀机制以及金属表面超疏水涂层的制备方法。虽然超疏水涂层在理论上能显著提高金属的耐腐蚀性,但在性能持久性、涂层与基底结合力、制备成本、环境适应性等方面依旧存在挑战,仍需开展进一步深入研究。
(1)尽管超疏水涂层能有效提高金属表面的耐腐蚀性,但该类涂层在实际应用时会受到环境因素的影响,导致涂层稳定性下降和防腐蚀性能减弱。尤其在强酸、强碱或高湿度环境中如何维持涂层的防腐特性,仍需进一步研究。
(2)研发低成本、易于批量化制备应用、环保、耐磨的高效防腐涂层,仍是当前面临的重要挑战。尤其在面临机械磨损及高温高压场合,涂层的超疏水性能容易失效。因此,研发经济、环保、耐磨的超疏水涂层,在金属防腐蚀领域具有重要的现实意义。
(3)相比于自组装法、化学气相法、激光加工等技术手段,溶胶凝胶法等涂层技术相对更容易实现规模化制备和应用,且可通过喷涂、浸涂等方式进行施工,将是未来在金属防腐蚀领域的重点关注方向。但涂层与基底表面的粘附强度、机械稳定性,尤其在光滑金属表面的稳固粘附,将是后续重点关注的研究课题。
(4)在面向航海等复杂环境,超疏水涂层不仅需要具备优异的防腐蚀特性,而且还需要赋予更多的功能性,如抗生物粘附、减阻、耐高渗透压等性能。因此,进一步研发面向具体环境的超疏水防腐涂层,也是未来发展的重要趋势。
综上,通过超疏水涂层提高金属材料的防腐特性,是近年来受到普遍关注且行之有效的技术手段。但在成本控制、规模化应用、耐磨性提高、环境适应性等方面仍存在问题需要解决,开发经济、环保、高效、易于批量化制备应用的多功能耐腐蚀超疏水涂层,是推进超疏水表面工业化应用的未来发展方向。
-
图 2 Young's方程[19];(a)固-液-气三者之间的关系;(b)液滴在固体表面的Young's状态
Figure 2. Young's equation[19]: (a) Relationship between solids, liquids and gases; (b) Young's state of droplets on solid surfaces
θ—Intrinsic contact angle of a liquid droplet on a solid surface; γsg—Solid-gas interfacial tension; γsl—Solid-liquid interfacial tension; γgl—Liquid-gas interfacial tension
图 4 超疏水在金属表面的防腐蚀机制:(a) Lotus效应[32];(b)阻碍电化学反应的过程[33];(c)提高界面结合力[34];(d)气垫效应保护层[35]
Figure 4. Corrosion protection mechanism of superhydrophobic metal surface: (a) Lotus effect[32]; (b) Processes that hinder electrochemical reactions[33]; (c) Improve interface binding force[34]; (d) Protective layers of air cushion effect[35]
图 6 (a) Ti合金在NaCl溶液中的腐蚀机制示意图[44];(b)超疏水双层涂层界面鲁棒性增强的机制以及示意图[47];(c)腐蚀机制示意图[51]
Figure 6. (a) Corrosion mechanism diagram of Ti alloy in NaCl solution[44]; (b) Mechanism and schematic diagram of enhanced interface robustness of superhydrophobic double-layer coatings[47]; (c) Schematic diagram of corrosion mechanism[51]
PTFE—Polytetrafluoroethylene; EP—Epoxy resin; SA—Sodium alginate
图 7 (a)制备碳化硅及其复合涂层的实验方法以及不同涂层的塔菲尔图[63];(b)超疏水纳米涂层材料涂刷在钢筋的盐雾腐蚀试验和超疏水纳米涂层材料的热重曲线[70];(c)氮化硼纳米片(BNNP)强化抗腐蚀机制图和各涂层(μ为3 μm片径的BNNP,n为300 nm片径的BNNP,字母后数字指代添加相在涂层中的质量分数)的阻抗谱[79]
Figure 7. (a) Experimental method for preparing silicon carbide and its composite coating and Tafel diagram of different coatings[63]; (b) Salt spray corrosion test and thermogravimetric curve of superhydrophobic nano-coating material applied to steel bar[70]; (c) Boron nitride nanoplate (BNNP) enhanced corrosion resistance mechanism diagram and the impedance spectra of each coating (μ represents BNNP with a flake-diameter of 3 μm, n represents BNNP with a flake-diameter of 300 nm, and the number after the letter refers to the mass fraction of the added phase in the coating)[79]
i—Current density; E—Potential; SCE— Saturated calomel electrode; Z'—Real part of impedance; Z''—Imaginary part of impedance
图 8 (a) TiO2/聚二甲基硅氧烷(PDMS)超疏水涂层的制备流程示意图和不同试样在3.5wt% NaCl溶液中的Tafel曲线以及超疏水涂层防腐蚀机制原理图[107];(b)盐雾腐蚀不同时间改性后的镁铝水滑石膜层(LDH-F)的宏观形貌和经不同时间改性后十钒酸根插层镁铝水滑石膜层(LDH-V-F)的SEM形貌[110];(c)不同激光功率刻蚀不锈钢试样表面的接触角和不锈钢电极在NaCl溶液中的极化曲线[117];(d) EL-1、EL-5、EL-10和EL-15(即经过1次、5次、10次和15次激光加工的EH40钢)的SEM图像[120]
Figure 8. (a) Preparation process diagram of TiO2/polydimethylsiloxane (PDMS) superhydrophobic coating, the Tafel curves of different samples in 3.5wt% NaCl solution, and the schematic diagram of the anti-corrosion mechanism of the superhydrophobic coating[107]; (b) Macroscopic morphology of the modified magnesium-aluminum hydrotalcite film (LDH-F) after salt spray corrosion for different durations, and the SEM morphology of the decavanadate-intercalated magnesium-aluminum hydrotalcite film (LDH-V-F) after modification for different time[110]; (c) Contact angle of stainless steel specimen surface and the polarization curves of stainless steel electrode in NaCl solution were etched by different laser powers[117]; (d) SEM images of EL-1, EL-5, EL-10 and EL-15 ( EH40 steel processed by laser for 1 time, 5 times, 10 times and 15 times)[120]
图 9 (a)超疏水膜形成示意图以及电化学测试阻抗图[153];(b)试样在海水中的极化曲线[157];(c)镁合金、磷化膜和复合膜在3.5wt% NaCl溶液中浸泡24 h后的极化曲线以及镁合金和复合膜在3.5wt% NaCl溶液中浸泡24 h后的腐蚀形貌[162];(d)吸附了不同浓度LLE聚集材料的Q235钢电极在1 mol/L HCl溶液中的动电位极化曲线[166]
Figure 9. (a) Schematic diagram of superhydrophobic film formation and impedance diagram of electrochemical test[153]; (b) Polarization curves of sample in seawater[157]; (c) Polarization curves of magnesium alloy, phosphating film and composite film after soaking in 3.5wt% NaCl solution for 24 h and corrosion morphology of magnesium alloy and composite film after soaking in 3.5wt% NaCl solution for 24 h[162]; (d) Potentiodynamic polarization curves of Q235 steel electrodes adsorbed with different concentrations of LLE aggregates in 1 mol/L HCl solution[166]
DA—Dopamine; HS—1-dodecanethiol; f—Frequency; E—Electrode potential; Ecorr—Corrosion potential; Jcorr—Corrosion current; ESCE—Electrode potential of the saturated calomel electrode; I—Current density
表 1 润湿理论在金属防腐蚀方面的潜在优势
Table 1 Potential advantages of wetting theory in metal corrosion protection
Performance Principle Ref. Enhanced corrosion resistance Adjusting the wetting properties of metal surfaces can reduce the direct contact between corrosive mediums (such as water, saline solutions, etc.) and the metal surface, thereby
delaying the corrosion process of the metal surface[24] Self-cleaning ability Superhydrophobic surfaces can achieve self-cleaning through the lotus effect, thereby
reducing the accumulation of dirt and microorganisms, both of which are factors
that promote metal corrosion[25] Extended service life By improving the wetting properties of metal surfaces, the durability and service life of metal structures in harsh environments can be enhanced [26] Sustainability Strategies involving the modification of surface wetting properties using physical or chemical methods can be environmentally friendly, providing a sustainable alternative to
reducing the use of traditional corrosion inhibitors[27] 表 2 不同涂层类型的耐腐蚀性能及其防腐蚀机制
Table 2 Corrosion resistance of different coating types and its anti-corrosion mechanism
Type Material Evaluation method Contact angle (CA) Corrosion mechanism Ref. Corrosion resistant superhydrophobic polymer coating Zn-coated carbon steel CA, SEM, EIS 159.8° Resistance to electrochemical corrosion [82] Magnesium alloy CA, SEM 152.6° Resistance to electrochemical corrosion [83] Corrosion resistant nano superhydrophobic coating Aluminum alloy CA, EIS 162.4° Air cushion effect [84] Galvanized steel CA, XRD 150° Improve interface binding force [85] Steel SEM, EIS 155.4° Lotus effect [86]
Corrosion resistant superhydrophobic ceramic coatingGlass ceramics CA, sanding with sandpaper 158° Air cushion effect [87] Rare-earth oxide ceramics CA, SEM 160° Air cushion effect [88] 表 3 不同制备方法的优缺点以及在金属表面的表征
Table 3 Advantages and disadvantages of different preparation methods and characterization on metal surfaces
Processing method Strength/Weakness Material CA Ref. Self-assembly method Simplicity, low cost, versatility, self-healing ability, scalability;
Structural control difficulties, stability issues, complexity and predictability, scale limitations6082-T6 aluminum alloy 180° [122] Sol-gel method Low temperature processes, uniformity and purity, controllable microstructure, diversity, coating and film preparation;
Drying and heat treatment, shrinkage and cracking,
reaction time, complexity, costCu 155° [123] Chemical vapor
depositionHigh-quality films, wide applicability, suitable for complex
shapes, good adhesion and interfacial quality,
controllable chemical composition;
High-temperature processes, complex equipment and control, precursor selection and cost, environmental concerns, limited deposition ratesAluminum 158° [124] Laser etching Non-contact process, high accuracy and resolution, speed and flexibility, multiple material suitability;
Heat affected zone, equipment cost, processing time,
possibility of reprocessing3Cr13 stainless steel 164° [125] Chemical etching High precision, wide range of application, high performance;
Poor selectivity, environmental impact, etching control difficultyStainless-steel 151.6° [126] Electrochemical etching method Good selectivity, wide range of applications, environmentally friendly;
Batch processing restriction, current distribution problem, equipment and technical requirementsAluminum alloy 152.3°±4.5° [127] Spraying method Simple and practicable, low cost, rapid production, wide applicability;
Limited accuracy, coating thickness control is difficult, coating thickness control is difficultQ235 steel plate 163.9° [128] Electrochemical
deposition methodGood uniformity, environmentally friendly, precise shape control;
High equipment requirements, limited to conductive substrates, the deposition rate is slowStainless-steel 160.6° [129] -
[1] 常坤, 梁恩泉, 张韧, 等. 金属材料增材制造及其在民用航空领域的应用研究现状[J]. 材料导报, 2021, 35(3): 3176-3182. DOI: 10.11896/cldb.19100153 CHANG Kun, LIANG Enquan, ZHANG Ren, et al. Status of metal additive manufacturing and its application research in the field of civil aviation[J]. Materials Reports, 2021, 35(3): 3176-3182(in Chinese). DOI: 10.11896/cldb.19100153
[2] CHU F, WU X. Fabrication and condensation characteristics of metallic superhydrophobic surface with hierarchical micro-nano structures[J]. Applied Surface Science, 2016, 371: 322-328. DOI: 10.1016/j.apsusc.2016.02.208
[3] 王杨松, 王英丹, 于帅, 等. 金属防腐及其防腐蚀措施的研究[J]. 辽宁化工, 2020, 49(3): 315-318. DOI: 10.3969/j.issn.1004-0935.2020.03.031 WANG Yangsong, WANG Yingdan, YU Shuai, et al. Research on metal corrosion prevention and corrosion prevention measures[J]. Liaoning Chemical Industry, 2020, 49(3): 315-318(in Chinese). DOI: 10.3969/j.issn.1004-0935.2020.03.031
[4] 孙小舟. 浅析金属腐蚀的防护技术[J]. 当代化工研究, 2022(7): 123-125. DOI: 10.3969/j.issn.1672-8114.2022.07.041 SUN Xiaozhou. Analysis on the protection technology of metal corrosion[J]. Modern Chemical Research, 2022(7): 123-125(in Chinese). DOI: 10.3969/j.issn.1672-8114.2022.07.041
[5] HOU B, LI X, MA X, et al. The cost of corrosion in China[J]. Materials Degradation, 2017, 1(1): 4. DOI: 10.1038/s41529-017-0005-2
[6] XING J, ZAYED T, MA S. Corrosion -based failure analysis of steel saltwater pipes: A Hong Kong case study[J]. Engineering Failure Analysis, 2024, 161: 108266. DOI: 10.1016/j.engfailanal.2024.108266
[7] WANG J, ZHANG M, XIAN H. 24Model-based comparative analysis of two catastrophic hazardous chemical pipeline accidents[J]. International Journal of Occupational Safety and Ergonomics, 2024, 30(2): 549-558. DOI: 10.1080/10803548.2024.2325258
[8] 柳泽邦, 冉博元, 裴恒, 等. 金属铝用复配缓蚀剂协同缓蚀作用研究[J]. 中国腐蚀与防护学报, 2024, 44(2): 312-322. DOI: 10.11902/1005.4537.2023.186 LIU Zebang, RAN Boyuan, PEI Heng, et al. Synergistic corrosion inhibition effect of a compound inhibitor for aluminum[J]. Journal of Chinese Society for Corrosion and Protection, 2024, 44(2): 312-322(in Chinese). DOI: 10.11902/1005.4537.2023.186
[9] HU P, LI S, JIANG N, et al. Effect of applied current density on corrosion behavior and protection efficiency of hanger steel wire for suspension bridge in marine rainfall environment[J]. Ocean Engineering, 2024, 308: 118286. DOI: 10.1016/j.oceaneng.2024.118286
[10] HABIB S, QURESHI A, SAJJAD S, et al. TiO2-mesoporous ceria carrier modified with sodium benzoate: An innovative polyurethane matrix for enhanced corrosion protection of steel[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 697: 134471.
[11] 智鹏飞. 金属材料的腐蚀与防腐技术研究[J]. 山西冶金, 2023, 46(2): 63-64, 81. ZHI Pengfei. Study on corrosion and anti-corrosion technology of metallic materials[J]. Shanxi Metallurgy, 2023, 46(2): 63-64, 81(in Chinese).
[12] HAN X, REN L, MA Y, et al. A mussel-inspired self-repairing superhydrophobic coating with good anti-corrosion and photothermal properties[J]. Carbon, 2022, 197: 27-39. DOI: 10.1016/j.carbon.2022.05.056
[13] 刘双平, 俞熹. 荷叶效应的研究[J]. 大学物理, 2011, 30(9): 50-54, 61. DOI: 10.3969/j.issn.1000-0712.2011.09.015 LIU Shuangping, YU Xi. A study on lotus effect[J]. College Physics, 2011, 30(9): 50-54, 61(in Chinese). DOI: 10.3969/j.issn.1000-0712.2011.09.015
[14] 陈耀峰, 邵文鹏, 赵广宾, 等. 金属基体表面超疏水涂层材料的制备及应用研究进展[J]. 材料研究与应用, 2024, 18(1): 106-115. CHEN Yaofeng, SHAO Wenpeng, ZHAO Guangbin, et al. Research progress on the preparation and application of superhydrophobic coating materials on metal substrate surface[J]. Materials Research and Application, 2024, 18(1): 106-115(in Chinese).
[15] ZENG Q, MIN X, LUO Z, et al. In-situ preparation of superhydrophobic Zn-Al layered double hydroxide coatings for corrosion protection of aluminum alloy[J]. Materials Letters, 2022, 328: 133077. DOI: 10.1016/j.matlet.2022.133077
[16] 徐乾坤, 张玉林, 解承东, 等. 铝基表面彩色超疏水膜层的制备与耐蚀性研究[J]. 稀有金属, 2022, 46(12): 1580-1588. XU Qiankun, ZHANG Yulin, XIE Chengdong, et al. Preparation and corrosion resistance of color superhydrophobic films on aluminum base surface[J]. Chinese Journal of Rare Metals, 2022, 46(12): 1580-1588(in Chinese).
[17] 刘明明, 侯媛媛, 陈唐建, 等. 超疏水防/除冰材料的基础理论和制备技术研究进展[J]. 材料保护, 2023, 56(5): 40-62. LIU Mingming, HOU Yuanyuan, CHEN Tangjian, et al. Research progress of basic theory and preparation technology of superhydrophobic anti/de-icing materials[J]. Materials Protection, 2023, 56(5): 40-62(in Chinese).
[18] SHARMA J, BHANDARI A, KHATRI N, et al. A brief review of transitional wetting regimes for superhydrophobic surfaces[J]. Journal of the Brazilian Society of Mechanical Sciences and Engineering, 2024, 46(5): 273. DOI: 10.1007/s40430-024-04844-8
[19] YOUNG T. An essay on the cohesion of fluids[J]. Philosophical Transactions of the Royal Society of London, 1805, 95: 65-87.
[20] WENZEL R N. Resistance of solid surfaces to wetting by water[J]. Industrial & Engineering Chemistry, 1936, 28(8): 988-994.
[21] CASSIE A, BAXTER S. Wettability of porous surfaces[J]. Transactions of the Faraday Society, 1944, 40: 546-551. DOI: 10.1039/tf9444000546
[22] CASSIE A. Contact angles[J]. Discussions of the Faraday, 1948(3): 11-16.
[23] 柯冲, 李中发, 朱志平, 等. 超疏水涂层的制备及其在金属防腐领域的应用研究进展[J]. 材料保护, 2022, 55(2): 145-159, 194. KE Chong, LI Zhongfa, ZHU Zhiping, et al. Research progress on preparation of superhydrophobic coatings and its application in metal anti-corrosive field[J]. Materials Protection, 2022, 55(2): 145-159, 194(in Chinese).
[24] CHOBAOMSUP V, METZNER M, BOONYONGMANEERAT Y. Superhydrophobic surface modification for corrosion protection of metals and alloys[J]. Journal of Coatings Technology and Research, 2020, 17(3): 583-595. DOI: 10.1007/s11998-020-00327-2
[25] DEEPA M J, ARUNIMA S R, SHIBLI S M A. Hydrophobic and corrosion-resistant composite (BiVO4/TiO2) hot-dip zinc coating with enhanced self-cleaning ability[J]. Journal of Alloys and Compounds, 2022, 924: 166522. DOI: 10.1016/j.jallcom.2022.166522
[26] SUBESHAN B, ASMATULU R. Corrosion mitigation of metals and alloys via superhydrophobic coatings with plasma surface and heat treatment processes[J]. Engineering Failure Analysis, 2022, 139: 106437. DOI: 10.1016/j.engfailanal.2022.106437
[27] YU M, ZHANG M, SUN J, et al. Facile electrochemical method for the fabrication of stable corrosion-resistant superhydrophobic surfaces on Zr-based bulk metallic glasses[J]. Molecules (Basel, Switzerland), 2021, 26(6): 1558. DOI: 10.3390/molecules26061558
[28] SABZAVAR S, GHAHARI M, ROSTAMI M, et al. Preparation of active-passive anticorrosion antistatic epoxy nanocomposite coatings loaded with CeO2, CeO2@C, and CHS particles[J]. Journal of Coatings Technology and Research, 2024, 21(4): 1263-1279. DOI: 10.1007/s11998-023-00890-4
[29] JIANG L, YANG J, WU C, et al. Fabrication of a robust superhydrophobic coating exhibiting superior corrosion resistance via spray application technique[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 687: 133497.
[30] XU J, CAI Q, LIAN Z, et al. Research progress on corrosion-resistance of magnesium alloys with bio-inspired water-repellent properties: A review [J]. Journal of BionicEngin-eering, 2021, 18(4): 735-763.
[31] XIA R, ZHANG B, DONG K, et al. HD-SiO2/SiO2 Sol@PDMS superhydrophobic coating with good durability and anti-corrosion for protection of Al sheets[J]. Materials, 2023, 16(9): 3532. DOI: 10.3390/ma16093532
[32] YU Y, WEI Y, GUO Y, et al. Electrodeposition of nanotubes for corrosion inhibition: Dual role as superhydrophobic matrix and nanocontainer for storing-releasing corrosion inhibitor[J]. Applied Surface Science, 2023, 640: 158377. DOI: 10.1016/j.apsusc.2023.158377
[33] LIU L, LEI J, LI L, et al. Robust rare-earth-containing superhydrophobic coatings for strong protection of magnesium and aluminum alloys[J]. Advanced Materials Interfaces, 2018, 5(16): 1800213. DOI: 10.1002/admi.201800213
[34] ZHANG C, LI C, SI X, et al. Mechanical durable ceria superhydrophobic coating fabricated by simple hot-press sintering[J]. Applied Surface Science, 2020, 529: 147113. DOI: 10.1016/j.apsusc.2020.147113
[35] MIAO C, LI C, HUANG X, et al. A robust anticorrosive coating derived from superhydrophobic, superoleophobic, and antibacterial SiO2@POS/N+ composite materials[J]. Materials Today Communications, 2023, 35: 105566. DOI: 10.1016/j.mtcomm.2023.105566
[36] 张邦维. 纳米材料与荷叶效应[J]. 科学中国, 2007(10): 38-41. ZHANG Bangwei. Nanomaterials and the lotus leaf effect[J]. Science, 2007(10): 38-41(in Chinese).
[37] XIN G, WU C, LIU W, et al. Anti-corrosion superhydrophobic surfaces of Al alloy based on micro-protrusion array structure fabricated by laser direct writing[J]. Journal of Alloys and Compounds, 2021, 881: 160649. DOI: 10.1016/j.jallcom.2021.160649
[38] CUI M, HUANG H, WU H, et al. Achieving superhydrophobicity of Zr-based metallic glass surface with anti-corrosion and anti-icing properties by nanosecond laser ablation and subsequent heat treatment[J]. Surface and Coatings Technology, 2023, 475: 130159. DOI: 10.1016/j.surfcoat.2023.130159
[39] WANG J, YU S, YIN X, et al. Fabrication of cross-like ZIF-L structures with water repellency and self-cleaning property via a simple in-situ growth strategy[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 623: 126731.
[40] LI B, OUYANG Y, HAIDER Z, et al. One-step electrochemical deposition leading to superhydrophobic matrix for inhibiting abiotic and microbiologically influenced corrosion of Cu in seawater environment[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 616: 126337.
[41] 刘战剑, 付雨欣, 任丽娜, 等. 超疏水涂层在防腐阻垢领域研究进展[J]. 化工进展, 2023, 42(6): 2999-3011. LIU Zhanjian, FU Yuxin, REN Lina, et al. New research progress of superhydrophobic coatings in the field of anti-corrosion and anti-scaling[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2999-3011(in Chinese).
[42] 张颖怀, 许立宁, 路民旭, 等. 用电化学阻抗谱(EIS)研究环氧树脂涂层的防腐蚀性能[J]. 腐蚀与防护, 2007, 28(5): 227-230, 234. DOI: 10.3969/j.issn.1005-748X.2007.05.004 ZHANG Yinghuai, XU Lining, LU Minxu, et al. An EIS study of the anticorrosion performance of epoxy resin coating[J]. Corrosion and Protection, 2007, 28(5): 227-230, 234(in Chinese). DOI: 10.3969/j.issn.1005-748X.2007.05.004
[43] 尹晓丽, 于思荣, 胡锦辉. Ni3S2微纳米结构超疏水表面的制备及耐蚀性能[J]. 材料导报, 2019, 33(20): 3372-3376. DOI: 10.11896/cldb.18080178 YIN Xiaoli, YU Sirong, HU Jinhui. Fabrication of Ni3S2 micro-nanostructure superhydrophobic surface with anti-corrosion property[J]. Materials Reports, 2019, 33(20): 3372-3376(in Chinese). DOI: 10.11896/cldb.18080178
[44] LIU C, TONG S, YUE Y, et al. Laser-based fabrication of superwetting titanium alloy with enhanced corrosion and erosion-corrosion resistance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 688: 133648.
[45] WANG W, TIAN Y, SHEN H, et al. Modified potassium titanate whiskers for preparation of enhanced corrosion-resistant phosphating conversion coatings with high NIR reflectivity on mild steel[J]. Journal of Alloys and Compounds, 2023, 955: 170247. DOI: 10.1016/j.jallcom.2023.170247
[46] FIRDAVS Sultonzoda, 王晶, 周瑾萱, 等. 超疏水铝合金表面的制备、耐腐蚀及防污性能[J]. 中国有色金属学报, 2020, 30(10): 2316-2321. DOI: 10.11817/j.ysxb.1004.0609.2020-39298 FIRDAVS Sultonzoda, WANG Jing, ZHOU Jinxuan, et al. Fabrication, anti-corrosion and antifouling performance of superhydrophobic aluminum alloy surface[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(10): 2316-2321(in Chinese). DOI: 10.11817/j.ysxb.1004.0609.2020-39298
[47] ZHANG B, YANG G, FAN X, et al. Intermediate-layer strengthened superhydrophobic coating with stable corrosion resistance, delayed icing and long-term weatherability[J]. Journal of Industrial and Engineering Chemistry, 2023, 127: 331-342. DOI: 10.1016/j.jiec.2023.07.018
[48] 赵明欣, 赵旭, 郎小尘. 钛合金表面超疏水膜的制备及其耐蚀性与机械稳定性[J]. 电镀与精饰, 2024, 46(2): 44-51. DOI: 10.3969/j.issn.1001-3849.2024.02.007 ZHAO Mingxin, ZHAO Xu, LANG Xiaochen. Preparation and corrosion resistance and mechanical stability of superhydrophobic film on titanium alloy[J]. Plating and Finishing, 2024, 46(2): 44-51(in Chinese). DOI: 10.3969/j.issn.1001-3849.2024.02.007
[49] 于元昊, 董玉花, 邢静, 等. Q235碳钢表面SiO2/PDMS超疏水涂层的制备及防腐性能研究[J]. 表面技术, 2023, 52(9): 209-219. YU Yuanhao, DONG Yuhua, XING Jing, et al. Preparation and anti-corrosion properties of SiO2/PDMS super-hydrophobic coating on Q235 carbon steel[J]. Surface Technology, 2023, 52(9): 209-219(in Chinese).
[50] 张凯, 文邦伟, 谭勇. 超疏水膜层防腐蚀机理及气相法制备技术研究进展[J]. 腐蚀科学与防护技术, 2018, 30(4): 441-448. DOI: 10.11903/1002.6495.2017.153 ZHANG Kai, WEN Bangwei, TAN Yong. Current status of research on anticorrosion mechanism and vapor phase preparation technique of uperhydrophobic film on metallic materials[J]. Corrosion Science and Protection Technology, 2018, 30(4): 441-448(in Chinese). DOI: 10.11903/1002.6495.2017.153
[51] GUO H, YANG C, WANG C. Sodium alginate/epoxy resin was separated by emulsion phase to fabricate a strong superhydrophobic coating[J]. Surfaces and Interfaces, 2024, 45: 103941. DOI: 10.1016/j.surfin.2024.103941
[52] BICO J, THIELE U, QUERE D. Wetting of textured surfaces[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 206(1-3): 41-46. DOI: 10.1016/S0927-7757(02)00061-4
[53] HE T, WANG Y, ZHANG Y, et al. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater[J]. Corrosion Science, 2009, 51(8): 1757-1761. DOI: 10.1016/j.corsci.2009.04.027
[54] 王鑫, 王兵兵, 杨威, 等. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. WANG Xin, WANG Bingbing, YANG Wei, et al. Anti-scale and anti-corrosion properties of PDA/PTFE superhydrophobic coating on metal surface[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4315-4321(in Chinese).
[55] KLIMOV V V, KOLYAGANOVA O V, BRYUZGIN E V, et al. Investigation of the mechanical and chemical stability of superhydrophobic coatings based on reactive copolymers of glycidyl methacrylate and fluoroalkyl methacrylates[J]. Colloid Journal, 2024, 86(1): 52-63. DOI: 10.1134/S1061933X23601208
[56] SHEN X, MAO T, LI C, et al. Durable superhydrophobic coatings based on CNTs-SiO2 gel hybrids for anti-corrosion and thermal insulation[J]. Progress in Organic Coatings, 2023, 181: 107602. DOI: 10.1016/j.porgcoat.2023.107602
[57] ZHANG D, ZHANG X, WEI E, et al. Construction of superhydrophobic film on the titanium alloy welded joint and its corrosion resistance study[J]. Anti-corrosion Methods and Materials, 2023, 70(6): 328-340. DOI: 10.1108/ACMM-05-2023-2812
[58] WANG H, LIU L, FEI G, et al. Enhancement of anticorrosion resistance of a fluorinated polyimide matrix by incorporating self-fixing POSS-GO[J]. Progress in Organic Coatings, 2024, 187: 108135. DOI: 10.1016/j.porgcoat.2023.108135
[59] MORADI M, REZAEI M. Construction of highly anti-corrosion and super-hydrophobic polypropylene/graphene oxide nanocomposite coatings on carbon steel: Experimental, electrochemical and molecular dynamics studies[J]. Construction and Building Materials, 2022, 317: 126136. DOI: 10.1016/j.conbuildmat.2021.126136
[60] 安然, 韩飞, 兰柯, 等. 聚苯胺-氮化硼纳米颗粒复合水性环氧涂层的制备与防腐性能研究[J]. 材料保护, 2024, 57(2): 18-27. AN Ran, HAN Fei, LAN Ke, et al. Preparation and anti-corrosion properties of polyaniline-boron nitride nanoparticle composite waterborne epoxy coating[J]. Materials Protection, 2024, 57(2): 18-27(in Chinese).
[61] DU J, WU P, KOU H, et al. Self-healing superhydrophobic coating with durability based on EP+PDMS/SiO2 double-layer structure design[J]. Progress in Organic Coatings, 2024, 190: 108359. DOI: 10.1016/j.porgcoat.2024.108359
[62] 吕艾娟, 孙俊涛, 胡青青, 等. EDOT-含氟聚合物涂层的制备及其防腐蚀性能[J]. 浙江师范大学学报(自然科学版), 2023, 46(4): 408-415. LYU Aijuan, SUN Juntao, HU Qingqing, et al. Preparation of EDOT-fluoropolymer coatings and their anti-corrosion properties[J]. Journal of Zhejiang Normal University (Natural Sciences), 2023, 46(4): 408-415(in Chinese).
[63] ZHANG Z, ZHAO N, QI F, et al. Reinforced superhydrophobic anti-corrosion epoxy resin coating by fluorine-silicon-carbide composites[J]. Coatings, 2020, 10(12): 1244. DOI: 10.3390/coatings10121244
[64] ROBERT R B J, HIKKU G S, JEYASUBRAMANIAN K, et al. ZnO nanoparticles impregnated polymer composite as superhydrophobic anti-corrosive coating for Aluminium-6061 alloy[J]. Materials Research Express, 2019, 6(7): 075705. DOI: 10.1088/2053-1591/ab153f
[65] GUO F, DUAN S, WU D, et al. Micro-nano structure constructed AA7055 superhydrophobic surface with long service life and high corrosion resistance[J]. Journal of Applied Polymer Science, 2023, 140(14): 53702. DOI: 10.1002/app.53702
[66] SHARMA K, MALIK M K, CHAWLA A, et al. Development of corrosion-resistant superhydrophobic coating on brass using modified silica nanoparticles[J]. Journal of Sol-gel Science and Technology, 2023, 105(3): 701-708.
[67] 赵明月, 裴晓园, 王维, 等. 二维纳米材料/环氧树脂复合涂层在腐蚀防护中的应用[J]. 复合材料学报, 2022, 39(5): 2049-2059. ZHAO Mingyue, PEI Xiaoyuan, WANG Wei, et al. Application of two-dimensional nanomaterial/epoxy composite coating in corrosion protection[J]. Acta Materiae Compositae Sinica, 2022, 39(5): 2049-2059(in Chinese).
[68] 曹怀杰, 徐群杰. 二维纳米材料增强复合涂层设计及防腐性能研究[C]//2021第八届海洋材料与腐蚀防护大会暨2021第二届钢筋混凝土耐久性与设施服役安全大会论文集. 贵阳: 中国腐蚀与防护学会, 2021: 037442. CAO Huaijie, XU Qunjie. Design and anti-corrosion property of composite coating incorporated with two-dimensional materials[C]//Proceedings of the 8th Marine Materials and Corrosion Protection Conference in 2021 & the 2nd Durability of Reinforced Concrete and Service Safety of Facilities Conference in 2021. Guiyang: Chinese Society for Corrosion and Protection, 2021: 037442(in Chinese).
[69] HUANG W, JIANG X, ZHANG Y, et al. Robust superhydrophobic silicone/epoxy functional coating with excellent chemical stability and self-cleaning ability[J]. Nanoscale, 2023, 15(44): 17793-17807. DOI: 10.1039/D3NR04062C
[70] 高旭超. 超疏水纳米涂层材料的制备及其在钢筋防锈中的应用[J]. 当代化工研究, 2023(8): 53-55. GAO Xuchao. Preparation of superhydrophobic nano-coating and its application in rust prevention of reinforcement[J]. Modern Chemical Research, 2023(8): 53-55(in Chinese).
[71] 李健鹏, 万红霞, 涂小慧, 等. 纳米颗粒对ZM5镁合金微弧氧化涂层耐磨和耐蚀性能的影响[J]. 表面技术, 2022, 51(12): 131-141. LI Jianpeng, WAN Hongxia, TU Xiaohui, et al. Effect of nanoparticles on the wear and corrosion resistance of MAO coatings on ZM5 Mg alloy[J]. Surface Technology, 2022, 51(12): 131-141(in Chinese).
[72] 张思, 傅海丰, 张欣, 等. 聚苯胺-二氧化硅/锌双层涂层的制备与防腐蚀性能[J]. 电镀与涂饰, 2023, 42(14): 55-63. ZHANG Si, FU Haifeng, ZHANG Xin, et al. Preparation and anticorrosion property of PANI–SiO2/Zn double-layer coating[J]. Electroplating & Finishing, 2023, 42(14): 55-63(in Chinese).
[73] WANG H, DI D, ZHAO Y, et al. A multifunctional polymer composite coating assisted with pore-forming agent: Preparation, superhydrophobicity and corrosion resistance[J]. Progress in Organic Coatings, 2019, 132: 370-378. DOI: 10.1016/j.porgcoat.2019.04.027
[74] LI Y, ZHANG X, CUI Y, et al. Anti-corrosion enhancement of superhydrophobic coating utilizing oxygen vacancy modified potassium titanate whisker[J]. Chemical Engineering Journal, 2019, 374: 1326-1336. DOI: 10.1016/j.cej.2019.06.028
[75] 李雪伍, 段世龙, 石甜, 等. 无氟铜基超疏水表面的制备及其耐腐蚀性能研究[J]. 材料保护, 2023, 56(6): 22-26. LI Xuewu, DUAN Shilong, SHI Tian, et al. Study on preparation and corrosion resistance of fluorine free copper based superhydrophobic surface[J]. Materials Protection, 2023, 56(6): 22-26(in Chinese).
[76] FARHADI S S, ALIOFKHAZRAEI M, DARBAND G B, et al. Corrosion and wettability of PEO coatings on magnesium by addition of potassium stearate[J]. Journal of Magnesium and Alloys, 2017, 5(2): 210-216. DOI: 10.1016/j.jma.2017.06.002
[77] FU J, SUN Y, JI Y, et al. Fabrication of robust ceramic based superhydrophobic coating on aluminum substrate via plasma electrolytic oxidation and chemical vapor deposition methods[J]. Journal of Materials Processing Technology, 2022, 306: 117641. DOI: 10.1016/j.jmatprotec.2022.117641
[78] 王丽, 孙倩倩, 付文. AZ31镁合金疏水性TiO2陶瓷涂层的制备及其性能研究[J]. 热加工工艺, 2020, 49(12): 100-104. WANG Li, SUN Qianqian, FU Wen. Research on preparation of hydrophobic TiO2 ceramic coating on AZ31 alloy and its property[J]. Hot Working Technology, 2020, 49(12): 100-104(in Chinese).
[79] 李东升, 杨网, 王永光, 等. 氮化硼纳米片增强胶黏陶瓷涂层的耐腐蚀行为[J]. 材料工程, 2023, 51(3): 105-112. LI Dongsheng, YANG Wang, WANG Yongguang, et al. Corrosion resistance of boron nitride nanoplatelet reinforced chemically bonded ceramic coatings[J]. Journal of Materials Engineering, 2023, 51(3): 105-112(in Chinese).
[80] 刘富, 相珺, 孙丽月, 等. 304钢表面陶瓷涂层的制备条件优化及其性能表征[J]. 稀有金属与硬质合金, 2019, 47(4): 65-69. LIU Fu, XIANG Jun, SUN Liyue, et al. Optimization of preparation conditions and performance characterization of ceramic coatings on 304 steel surface[J]. Rare Metals and Cemented Carbides, 2019, 47(4): 65-69(in Chinese).
[81] LI X, DU S, MA C, et al. Nano-SiO2 based anti-corrosion superhydrophobic coating on Al alloy with mechanical stability, anti-pollution and self-cleaning properties[J]. Ceramics International, 2024, 50(6): 9469-9478. DOI: 10.1016/j.ceramint.2023.12.264
[82] HU C, KWAN K, XIE X, et al. Superhydrophobic polyaniline/TiO2 composite coating with enhanced anticorrosion function[J]. Reactive & Functional Polymers, 2022, 179: 105381.
[83] CAO K, YU Z, ZHU L, et al. Fabrication of superhydrophobic layered double hydroxide composites to enhance the corrosion-resistant performances of epoxy coatings on Mg alloy[J]. Surface & Coatings Technology, 2021, 407: 126763.
[84] YU Y, DONG Y, NING H, et al. A robust superhydrophobic coating with multi-dimensional micro-nano structure on 5052 aluminum alloy[J]. Surface & Coatings Technology, 2023, 465: 129564.
[85] CAO L, WAN Y, LI Y, et al. Corrosion-resistant and friction-reducing performance of super-hydrophobic coating on hot-dip galvanised steel in a 3.5% NaCl solution[J]. Lubrication Science, 2021, 33(6): 325-334. DOI: 10.1002/ls.1555
[86] LI J, LIU Z, WANG Z. Study of nano-ZnO improvement of the mechanical properties and corrosion resistance of modified-SiO2/PTFE superhydrophobic nanocomposite coatings by one-step spraying[J]. New Journal of Chemistry, 2023, 47(13): 6246-6257. DOI: 10.1039/D2NJ06376J
[87] ZHONG W, WU M, XIONG B, et al. High stability superhydrophobic glass-ceramic surface with micro–nano hierarchical structure[J]. Ceramics International, 2022, 48(16): 23527-23535. DOI: 10.1016/j.ceramint.2022.04.350
[88] AZIMI G, KWON H, VARANASI K K. Superhydrophobic surfaces by laser ablation of rare-earth oxide ceramics[J]. MRS Communications, 2014, 4(3): 95-99. DOI: 10.1557/mrc.2014.20
[89] SONG X G, LIANG Z H, WANG H J, et al. Fabrication of functional surfaces of aluminum alloy with a transition from superhydrophilic to superhydrophobic by nanosecond laser irradiation[J]. Journal of Coatings Technology and Research, 2023, 20(6): 1897-1912. DOI: 10.1007/s11998-023-00785-4
[90] GE-ZHANG S, YANG H, NI H, et al. Biomimetic superhydrophobic metal/nonmetal surface manufactured by etching methods: A mini review[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 958095. DOI: 10.3389/fbioe.2022.958095
[91] WANG X, LI X, LEI Q, et al. Fabrication of superhydrophobic composite coating based on fluorosilicone resin and silica nanoparticles[J]. Royal Society Open Science, 2018, 5(7): 180598. DOI: 10.1098/rsos.180598
[92] 姜丹, 黄国胜, 马力, 等. 仿生表面/涂层在金属腐蚀防护中的研究进展[J]. 表面技术, 2022, 51(6): 180-193. JIANG Dan, HUANG Guosheng, MA Li, et al. Research progress of bionic surface/coating in metal corrosion protection[J]. Surface Technology, 2022, 51(6): 180-193(in Chinese).
[93] SOLMAZ R, DUESUN Y A, SAHIN E A, et al. Bingol propolis self-assembled monolayer films: Preparation, characterization and application as corrosion inhibitors for copper protection in NaCl environment[J]. Materials Chemistry and Physics, 2024, 315: 128956. DOI: 10.1016/j.matchemphys.2024.128956
[94] HATTAB M, HASSEN S B, SPRIANO S, et al. Ce-doped MgO films on AZ31 alloy substrate for biomedical applications: Preparation, characterization and testing[J]. Biomedical Materials, 2024, 19(2): 025013. DOI: 10.1088/1748-605X/ad1dfa
[95] WANG G, GUO L, RUAN Y, et al. Improved wear and corrosion resistance of alumina alloy by MAO and PECVD[J]. Surface & Coatings Technology, 2024, 479: 130556.
[96] JIANG P, WANG G, WU Y, et al. Microstructure evolution, tribological and corrosion properties of amorphous alloy strengthening stainless steel fabricated by selective laser melting in NaCl solution[J]. Acta Metallurgica Sinica-English Letters, 2024, 37(5): 825-839. DOI: 10.1007/s40195-024-01665-5
[97] 代学玉, 于娇娇, 汪永丽. 层层自组装法制备超疏水表面的研究进展[J]. 山东化工, 2020, 49(12): 44-45. DOI: 10.3969/j.issn.1008-021X.2020.12.018 DAI Xueyu, YU Jiaojiao, WANG Yongli. Research progress on the preparation of superhydrophobic surfaces by layer-by-layer self-assembly method[J]. Shandong Chemical Industry, 2020, 49(12): 44-45(in Chinese). DOI: 10.3969/j.issn.1008-021X.2020.12.018
[98] FENG J, CHEN F, CHAO J, et al. Anti-corrosion property of superhydrophobic copper mesh with one-step self-assembled perfluorothiolate monolayers[J]. Surface and Interface Analysis, 2022, 54(10): 1087-1097. DOI: 10.1002/sia.7135
[99] WANG L, LU S, XIE F, et al. Influence of synergistic effect of phosphate on corrosion resistance of self-assembled superhydrophobic composite film on 6061 aluminum alloy surfaces[J]. Journal of Industrial and Engineering Chemistry (Seoul, Korea), 2023, 128: 335-345. DOI: 10.1016/j.jiec.2023.07.068
[100] MA C, LIU J, ZHANG Z, et al. Preparation and properties of micro-arc oxidation/self-assembly coatings with different hydrophobicities on magnesium alloy[J]. Advanced Engineering Materials, 2022, 24(12): 2200741. DOI: 10.1002/adem.202200741
[101] YANG S, ZOU P, WANG Y, et al. Novel method for protecting copper: An in-situ click-assembly film on copper surface[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 677: 132355.
[102] FENG L, REN X, FENG Y, et al. Self-assembly of new O- and S-heterocycle-based protective layers for copper in acid solution[J]. Physical Chemistry Chemical Physics, 2020, 22(8): 4592-4601. DOI: 10.1039/C9CP06910K
[103] CHWATAL S, ZAZIMAL F, BURSIKOVA V, et al. Modification of silicon-polyurethane-based sol-gel coatings through diverse plasma technologies: Investigation of impact on surface properties[J]. New Journal of Chemistry, 2024, 48(12): 5232-5246. DOI: 10.1039/D3NJ05986C
[104] WANG S, GUO X, XIE Y, et al. Preparation of superhydrophobic silica film on Mg-Nd-Zn-Zr magnesium alloy with enhanced corrosion resistance by combining micro-arc oxidation and sol-gel method[J]. Surface & Coatings Technology, 2012, 213: 192-201.
[105] SUN B, YAN L, GAO K. Hydrophobicity and improved corrosion resistance of weathering steel via a facile sol-gel process with a natural rust film[J]. ACS Applied Materials & Interfaces, 2023, 15(39): 46400-46407.
[106] 刘富, 相珺, 张越, 等. 溶胶凝胶法制备304钢表面陶瓷涂层的耐蚀性研究[J]. 表面技术, 2017, 46(12): 233-237. LIU Fu, XIANG Jun, ZHANG Yue, et al. Corrosion resistance of ceramic coating on 304 steel in sol-gel method[J]. Surface Technology, 2017, 46(12): 233-237(in Chinese).
[107] 薛鑫宇, 尹正生, 蒋永锋, 等. 碳钢表面防腐超疏水TiO2/PDMS涂层的制备及性能[J]. 中国表面工程, 2021, 34(4): 53-59. DOI: 10.11933/j.issn.1007-9289.20210227001 XUE Xinyu, YIN Zhengsheng, JIANG Yongfeng, et al. Preparation and properties of TiO2/PDMS anticorrosion superhydrophobic coating on carbon steel[J]. China Surface Engineering, 2021, 34(4): 53-59(in Chinese). DOI: 10.11933/j.issn.1007-9289.20210227001
[108] 贠柯, 张澄, 杨旭, 等. 基板表面粗糙度对TiO2-SiO2复合薄膜制备及耐蚀性影响研究[J]. 涂层与防护, 2023, 44(7): 35-40. YUN Ke, ZHANG Cheng, YANG Xu, et al. Effect of substrate surface roughness on preparation and corrosion resistance of TiO2-SiO2 composite films[J]. Coating and Protection, 2023, 44(7): 35-40(in Chinese).
[109] BOEKE F, GINER I, KELLER A, et al. Plasma-enhanced chemical vapor deposition (PE-CVD) yields better hydrolytical stability of biocompatible SiO x thin films on implant alumina ceramics compared to rapid thermal evaporation physical vapor deposition (PVD)[J]. ACS Applied Materials & Interfaces, 2016, 8(28): 17805-17816.
[110] 杨文广, 刘振红, 朱梅婷, 等. 铝合金表面超疏水缓蚀自修复膜的制备及其耐蚀性[J]. 腐蚀与防护, 2021, 42(5): 1-7. DOI: 10.11973/fsyfh-202105001 YANG Wenguang, LIU Zhenhong, ZHU Meiting, et al. Preparation of super-hydrophobic corrosion-inhibited self-repairing film on aluminum alloy surface and its corrosion resisatnce[J]. Corrosion & Protection, 2021, 42(5): 1-7(in Chinese). DOI: 10.11973/fsyfh-202105001
[111] ZHANG M, ZHOU T, LI H, et al. UV-durable superhydrophobic ZnO/SiO2 nanorod arrays on an aluminum substrate using catalyst-free chemical vapor deposition and their corrosion performance[J]. Applied Surface Science, 2023, 623: 157085. DOI: 10.1016/j.apsusc.2023.157085
[112] 李小兵, 余雄, 胡顺保, 等. 一步电沉积法制备铜表面稀土镧/石墨烯超疏水复合涂层及其耐腐蚀性能[J]. 材料保护, 2024, 57(5): 152-157. LI Xiaobing, YU Xiong, HU Shunbao, et al. Preparation and corrosion resistance performance of rare earth lanthanum/graphene superhydrohobic composite coatings on copper surface by one-step electrodeposition method[J]. Materials Protection, 2024, 57(5): 152-157(in Chinese).
[113] 陈清新, 单广程, 周希, 等. 石墨烯对镍钛形状记忆合金耐腐蚀性能的影响[J]. 化学通报, 2017, 80(1): 104-107. CHEN Qingxin, SHAN Guangcheng, ZHOU Xi, et al. Effects of graphene on anticorrosion properties of nickel titanium shape memory alloys[J]. Chemistry, 2017, 80(1): 104-107(in Chinese).
[114] 李思仪, 董玉华, 周琼, 等. 石墨烯及石墨烯/环氧复合涂层的防腐蚀性能[J]. 腐蚀与防护, 2015, 36(8): 748-753. DOI: 10.11973/fsyfh-201508012 LI Siyi, DONG Yuhua, ZHOU Qiong, et al. Corrosion performance of graphene and graphene/epoxy composite coating[J]. Corrosion & Protection, 2015, 36(8): 748-753(in Chinese). DOI: 10.11973/fsyfh-201508012
[115] ABDOLMALEKI M, ALLAHGHOLIPOUR G R, TAHZIBI H, et al. Fabrication of superhydrophobic aluminum with enhanced anticorrosive property[J]. Materials Chemistry and Physics, 2024, 313: 128711. DOI: 10.1016/j.matchemphys.2023.128711
[116] 郑博源, 底月兰, 王海斗, 等. 激光加工制备金属基体超疏水表面的研究进展[J]. 材料导报, 2020, 34(23): 23109-23120. DOI: 10.11896/cldb.19090218 ZHENG Boyuan, DI Yuelan, WANG Haidou, et al. Research progress in preparation of super-hydrophobic surface of metal matrix by laser processing[J]. Materials Reports, 2020, 34(23): 23109-23120(in Chinese). DOI: 10.11896/cldb.19090218
[117] 王帅, 于世胜, 于庆华, 等. 纳秒激光诱导超疏水316L不锈钢表面的制备及其耐腐蚀性能研究[J]. 热加工工艺, 2023, 52(8): 108-112. WANG Shuai, YU Shisheng, YU Qinghua, et al. Preparation of superhydrophobic 316L stainless steel surface by nanosecond laser-induced and its corrosion resistance[J]. Hot Working Technology, 2023, 52(8): 108-112(in Chinese).
[118] 刘祁文, 刘国东, 李子航, 等. 纳秒激光制备镁合金超疏水表面及其性能研究[J]. 激光与光电子学进展, 2022, 59(5): 214-221. LIU Qiwen, LIU Guodong, LI Zihang, et al. Preparation and properties of superhydrophobic surface of magnesium alloy by nanosecond laser[J]. Laser & Optoelectronics Progress, 2022, 59(5): 214-221(in Chinese).
[119] 徐雷秋, 万晓峰, 董菁, 等. 盐酸-激光复合刻蚀+SA修饰制备镁合金表面超疏水结构的耐腐蚀性能[J]. 机械工程材料, 2019, 43(10): 6-10. DOI: 10.11973/jxgccl201910002 XU Leiqiu, WAN Xiaofeng, DONG Jing, et al. Corrosion resistance of superhydrophobic structure on magnesium alloy surface prepared by hydrochloric acid-laser composite etching and SA modification[J]. Materials for Mechanical Engineering, 2019, 43(10): 6-10(in Chinese). DOI: 10.11973/jxgccl201910002
[120] CHEN X, SUN S, WANG D, et al. Construction of superhydrophobic surfaces with different water adhesion on the low-temperature steels by picosecond laser processing[J]. Surface & Coatings Technology, 2024, 477: 130340.
[121] WU Z, LIU Y, ZHANG Y, et al. The effect of micro/nanostructures formed by laser ablation on the superhydrophobicity of AZ31B magnesium alloy[J]. Journal of Materials Research, 2024, 39(5): 850-863. DOI: 10.1557/s43578-023-01275-4
[122] KHASKHOUSSI A, CALABRESE L, PROVERBIO E. Superhydrophobic self-assembled silane monolayers on hierarchical 6082 aluminum alloy for anti-corrosion applications[J]. Applied Sciences-basel, 2020, 10(8): 2656. DOI: 10.3390/app10082656
[123] RAO A V, LATTHE S S, MAHADIK S A, et al. Mechanically stable and corrosion resistant superhydrophobic sol-gel coatings on copper substrate[J]. Applied Surface Science, 2011, 257(13): 5772-5776. DOI: 10.1016/j.apsusc.2011.01.099
[124] ALZADJALI S, MATOUK Z, ALSHEHHI A, et al. Simple, scalable route to produce transparent superhydrophobic/hydrophilic film surfaces[J]. Applied Sciences, 2023, 13(3): 1707. DOI: 10.3390/app13031707
[125] LAN L, WANG H, ZHU L, et al. Preparation and wetting mechanism of laser-etched composite self-assembled 1H,1H,2H,2H-perfluorodecyltriethoxysilane superhydrophobic surface coating[J]. Physica Status Solidi A: Applications and Materials Science, 2022, 219(3): 2100568.
[126] ZHANG Z, CHEN Y, GU Q, et al. Preparation and corrosion resistance of 304 super-hydrophobic stainless-steel surface[C]//2nd International Conference on Frontiers of Materials Synthesis and Processing. Sanya: ICFM, 2019, 493: 012057.
[127] 赵树国, 陈阳, 马宁, 等. 电化学刻蚀法制备铝合金超疏水表面及其润湿性转变[J]. 表面技术, 2018, 47(3): 115-120. ZHAO Shuguo, CHEN Yang, MA Ning, et al. Superhydrophobic surface of aluminum alloy prepared by electrochemical etching and wettability transition[J]. Surface Technology, 2018, 47(3): 115-120(in Chinese).
[128] QIN X, KONG L, WANG J, et al. Double-layer with electroactive and superhydrophobicity based on polymer nanotubes to improve robustness and anti-corrosion performances[J]. Chemical Engineering Journal, 2024, 488: 150928. DOI: 10.1016/j.cej.2024.150928
[129] LI S, LIU Y, TIAN Z, et al. Biomimetic superhydrophobic and antibacterial stainless-steel mesh via double-potentiostatic electrodeposition and modification[J]. Surface & Coatings Technology, 2020, 403: 126355.
[130] FAN H, LU P, ZHU X, et al. Development of superhydrophobic and corrosion resistant coatings on carbon steel by hydrothermal treatment and fluoroalkyl silane self-assembly[J]. Materials Chemistry and Physics, 2022, 290: 126569. DOI: 10.1016/j.matchemphys.2022.126569
[131] PILLADO B, MATYKINA E, OLIVIER M, et al. Functionalization of plasma electrolytic oxidation/sol-gel coatings on AZ31 with organic corrosion inhibitors[J]. Coatings, 2024, 14(1): 84. DOI: 10.3390/coatings14010084
[132] ZHENG X, YANG Y, XIAN Y, et al. In situ grown vertically oriented graphene coating on copper by plasma-enhanced CVD to form superhydrophobic surface and effectively protect corrosion[J]. Nanomaterials, 2022, 12(18): 3202. DOI: 10.3390/nano12183202
[133] ZHANG Q, ZHANG H. Corrosion resistance and mechanism of micro-nano structure super - hydrophobic surface prepared by laser etching combined with coating process[J]. Anti-corrosion Methods and Materials, 2019, 66(3): 264-273. DOI: 10.1108/ACMM-07-2018-1964
[134] YANG H, GAO Y, QIN W, et al. A robust superhydrophobic surface on AA3003 aluminum alloy with intermetallic phases in-situ pinning effect for corrosion protection[J]. Journal of Alloys and Compounds, 2022, 898: 163038. DOI: 10.1016/j.jallcom.2021.163038
[135] KALGUDI S, PAVITHRA G P, PRABHU K N, et al. Effect of surface treatment on wetting behavior of copper[J]. Materials Today Proceedings, 2021, 35: 295-297. DOI: 10.1016/j.matpr.2020.01.379
[136] RODIC P, KAPUN B, MILOSEV I. Superhydrophobic aluminium surface to enhance corrosion resistance and obtain self-cleaning and anti-icing ability[J]. Molecules, 2022, 27(3): 1099. DOI: 10.3390/molecules27031099
[137] ASTM. Standard practice for operating salt spray (fog) apparatus: ASTM B117—19[S]. West Conshohocken: ASTM, 2019.
[138] KHASKHOUSSI A, CALABRESE L, PROVERBIO E. Anticorrosion superhydrophobic surfaces on AA6082 aluminum alloy by HF/HCl texturing and self-assembling of silane monolayer[J]. Materials, 2022, 15(23): 8549. DOI: 10.3390/ma15238549
[139] 李纬, 马宁, 张亚雄. 电化学法制备TA15钛合金超疏水表面及性能研究[J]. 机械设计与制造工程, 2022, 51(10): 37-42. LI Wei, MA Ning, ZHANG Yaxiong. Study on superhydrophobic surface and properties of TA15 titanium alloy prepared by electrochemical method[J]. Machine Design and Manufacturing Engineering, 2022, 51(10): 37-42(in Chinese).
[140] MA N, CHEN Y, ZHAO S, et al. Preparation of super-hydrophobic surface on Al-Mg alloy substrate by electrochemical etching[J]. Surface Engineering, 2019, 35(5): 394-402. DOI: 10.1080/02670844.2017.1421883
[141] BEN J, WU P, WANG Y, et al. Preparation and characterization of modified ZrO2/SiO2 /silicone-modified acrylic emulsion superhydrophobic coating[J]. Materials, 2023, 16(24): 7621. DOI: 10.3390/ma16247621
[142] LIN Z, ZHANG W, XU L, et al. Fabrication of Ni-Co/Cu super-hydrophobic coating with improved corrosion resistance[J]. Materials Chemistry and Physics, 2022, 277: 125503. DOI: 10.1016/j.matchemphys.2021.125503
[143] YANG J, CUI H, ZHANG Y, et al. A double-layer multifunctional superhydrophobic coating with excellent anti-corrosion performance based on fluorine-free chemicals[J]. Progress in Organic Coatings, 2024, 187: 108150. DOI: 10.1016/j.porgcoat.2023.108150
[144] MA D, LIN H, ZHENG K, et al. Rose-like Cr-Fe robust super-hydrophobic surfaces with high adhesion and corrosion resistance[J]. Journal of Materials Science, 2022, 57(39): 18640-18654. DOI: 10.1007/s10853-022-07724-5
[145] LIU T, CUI Y, LI X, et al. Effect of crystalline water molecules on the preparation and growth of superhydrophobic films via electrodeposition[j]. Surface Review and Letters, 2022, 29(4): 2250051. DOI: 10.1142/S0218625X22500512
[146] 尤航, 彭毅, 杨冲, 等. 金属超疏水涂层制备及其耐腐蚀性能研究进展[J]. 中国有色金属学报, 2024, 34(3): 703-724. DOI: 10.11817/j.ysxb.1004.0609.2023-44428 YOU Hang, PENG Yi, YANG Chong, et al. Research progress on preparation of metal superhydrophobic surface coatings and their corrosion resistance[J]. The Chinese Journal of Nonferrous Metals, 2024, 34(3): 703-724(in Chinese). DOI: 10.11817/j.ysxb.1004.0609.2023-44428
[147] 李玉峰, 高文博, 史凌志, 等. 超疏水涂层的制备及其对Mg-Li合金的防腐蚀性能[J]. 中国表面工程, 2020, 33(5): 1-9. DOI: 10.11933/j.issn.1007-9289.20200813001 LI Yufeng, GAO Wenbo, SHI Lingzhi, et al. Preparation of superhydrophobic coating and its corrosion resistance to Mg-Li alloy[J]. China Surface Engineering, 2020, 33(5): 1-9(in Chinese). DOI: 10.11933/j.issn.1007-9289.20200813001
[148] ZHANG W, LI S, WEI D, et al. Fluorine-free, robust and self-healing superhydrophobic surfaces with anticorrosion and antibacterial performances[J]. Journal of Materials Science & Technology, 2024, 186: 231-243.
[149] MAMGAIN H P, SAMANTA K K, BRAJPURIYA R, et al. Fabrication of nanostructured corrosion-resistant superhydrophobic coating on copper by electrodeposition: A comprehensive critical review[J]. ECS Journal of Solid State Science and Technology, 2024, 13(4): 043010. DOI: 10.1149/2162-8777/ad3c25
[150] FU J, SUN Y, WANG J, et al. Fabrication of fluorine-free superhydrophobic surface on aluminum substrate for corrosion protection and drag reduction[J]. Journal of Marine Science and Engineering, 2023, 11(3): 520. DOI: 10.3390/jmse11030520
[151] YANG C, WANG C, ZHAO X, et al. Superhydrophobic surface on MAO-processed AZ31B alloy with zinc phosphate nanoflower arrays for excellent corrosion resistance in salt and acidic environments[J]. Materials & Design, 2024, 239: 112769.
[152] MEENA M K, TUDU B K, KUMAR A, et al. Development of polyurethane-based superhydrophobic coatings on steel surfaces[J]. Philosophical Transactions of the Royal Society A: Mathematical Physical and Engineering Sciences, 2020, 378(2167): 20190446. DOI: 10.1098/rsta.2019.0446
[153] 曹怀杰, 陈守刚, 刘盈. 铜基超疏水膜的制备及其在干湿交替环境下的抗腐蚀行为研究[J]. 功能材料, 2016, 47(11): 11226-11230. DOI: 10.3969/j.issn.1001-9731.2016.11.044 CAO Huaijie, CHEN Shougang, LIU Ying. Preparation of superhydrophobic film on copper substrate and its anticorrosion behaviors under dry/wet alternative environment[J]. Journal of Functional Materials, 2016, 47(11): 11226-11230(in Chinese). DOI: 10.3969/j.issn.1001-9731.2016.11.044
[154] 蒋翔, 田蒙蒙, 雷瑜, 等. 铜基超疏水表面的制备及防腐蚀特性[J]. 华南理工大学学报(自然科学版), 2020, 48(4): 87-94. JIANG Xiang, TIAN Mengmeng, LEI Yu, et al. Fabrication and corrosion resistance of copper-based superhydrophobic surface[J]. Journal of South China University of Technology (Natural Science Edition), 2020, 48(4): 87-94(in Chinese).
[155] WANG Y, LIU P, LUO R, et al. Design and fabrication of superhydrophobic photothermal coating on copper mesh and its applications on anti-corrosion, anti-icing and oil-water separation[J]. Progress in Organic Coatings, 2024, 188: 108243. DOI: 10.1016/j.porgcoat.2024.108243
[156] LIU X, ZHAN T, ZHANG B. Attapulgite-based superhydrophobic coating on aluminum alloy substrate with self-cleaning, anti-corrosion and robustness[J]. Journal of Industrial and Engineering Chemistry, 2024, 130: 357-367. DOI: 10.1016/j.jiec.2023.09.039
[157] 连峰, 王增勇, 张会臣. 超疏水船用铝合金表面微结构对耐海水腐蚀性能的影响[J]. 材料热处理学报, 2014, 35(12): 179-183. LIAN Feng, WANG Zengyong, ZHANG Huichen. Effect of micro-structure of super-hydrophobic warship aluminum alloy surface on its corrosion resistance in seawater[J]. Transactions of Materials and Heat Treatment, 2014, 35(12): 179-183(in Chinese).
[158] TRAN N G, CHUN D, ABD-ELRAHIM A G. Superhydrophobic aluminum surfaces with nano-micro hierarchical composite structures: A novel and sustainable approach to corrosion protection[J]. Journal of Alloys and Compounds, 2023, 960: 170907. DOI: 10.1016/j.jallcom.2023.170907
[159] CHENG Y, TANG X, LI Z, et al. Preparation technology of nano-SiO2 super-hydrophobic hybrid coating with low cost and high abrasion-resistant[J]. Materials Letters, 2024, 355: 135453. DOI: 10.1016/j.matlet.2023.135453
[160] 周垲杰, 辛蕾, 黄小文, 等. 镁合金基底超疏水涂层的制备及其防污防腐性能研究[J]. 材料保护, 2023, 56(5): 71-75. ZHOU Kaijie, XIN Lei, HUANG Xiaowen, et al. Preparation of superhydrophobic coating on magnesium alloy substrate and its anti-fouling and anti-corrosion properties[J]. Materials Protection, 2023, 56(5): 71-75(in Chinese).
[161] 刘金玉, 张志远, 王东, 等. 镁合金表面不同MOF超疏水涂层的耐蚀行为[J]. 材料工程, 2024, 52(4): 138-145. LIU Jinyu, ZHANG Zhiyuan, WANG Dong, et al. Corrosion resistance behavior of different MOF superhydrophobic coatings on magnesium alloy surface[J]. Journal of Materials Engineering, 2024, 52(4): 138-145(in Chinese).
[162] 张晓菲. 镁合金表面制备耐久性超疏水复合膜及其性能[J]. 兵器材料科学与工程, 2022, 45(6): 80-86. ZHANG Xiaofei. Preparation of durable superhydrophobic composite film on magnesium alloy and its performance[J]. Ordnance Material Science and Engineering, 2022, 45(6): 80-86(in Chinese).
[163] CAI Q, XU J, XU A, et al. Magnesium alloy composite water-repellent surface with durable corrosion resistance and self-cleaning behavior[J]. Advanced Engineering Materials, 2024, 26(7): 2301936. DOI: 10.1002/adem.202301936
[164] YANG F, ZHANG J, PAN J, et al. Preparation of superhydrophobic coating on X80 steel and its corrosion resistance in oilfield produced water[J]. Langmuir, 2024, 40(19): 10250-10260. DOI: 10.1021/acs.langmuir.4c00687
[165] EMELYANENKO K A, EMELYANENKO A M, BOINOVICH L B. Laser obtained superhydrophobic state for stainless steel corrosion protection, a review[J]. Coatings, 2023, 13(1): 194. DOI: 10.3390/coatings13010194
[166] 罗为平, 罗雪, 石悦婷, 等. Q235钢表面的超疏水吸附层形成与缓蚀研究[J]. 中国腐蚀与防护学报, 2022, 42(6): 903-912. DOI: 10.11902/1005.4537.2021.296 LUO Weiping, LUO Xue, SHI Yueting, et al. Preparation and corrosion inhibition of superhydrophobic adsorption film of lotus leaf extract on mild steel[J]. Journal of Chinese Society for Corrosion and Protection, 2022, 42(6): 903-912(in Chinese). DOI: 10.11902/1005.4537.2021.296
[167] TANG Z, GAO M, ZHANG Z, et al. Biomimetic construction of green, fire-proof and super-hydrophobic multifunctionality-integrated coatings via one-step spraying method for steel structures[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 683: 133056.
[168] 刘艳, 冀杰. 45#钢表面Ni-W/ZnO超疏水复合涂层的制备及性能研究[J]. 电镀与精饰, 2023, 45(5): 26-33. DOI: 10.3969/j.issn.1001-3849.2023.05.004 LIU Yan, JI Jie. Preparation and properties of Ni-W/ZnO superhydrophobic composite coating on 45# steel[J]. Plating and Finishing, 2023, 45(5): 26-33(in Chinese). DOI: 10.3969/j.issn.1001-3849.2023.05.004
[169] 王萃, 刘传辉. 建筑45钢结构表面超疏水结构的制备及其耐蚀性能分析[J]. 材料保护, 2022, 55(1): 165-169. WANG Cui, LIU Chuanhui. Preparation of superhydrophobic structure on the surface of building 45 steel structure and its corrosion resistance analysis[J]. Materials Protection, 2022, 55(1): 165-169(in Chinese).
-
目的
自然界中的超疏水现象在金属表面的防腐蚀行为方面引起了表界面科学、微纳制造以及复合涂层等多学科领域研究者的广泛关注,展现出了巨大的应用前景。超疏水涂层的防腐蚀性能与其润湿性及表面微纳结构密切相关,本文将聚焦于不同超疏水涂层的防腐性能以及其腐蚀机制,综述超疏水涂层在防腐性能、制备方法和金属表面应用等方面的发展现状。
方法本文在梳理超疏水涂层的润湿理论后,探讨了超疏水涂层引入的新型腐蚀防护机制。根据涂层材料的不同,分析并比较了各类涂层的防腐性能及其防腐机制。此外,还归纳总结了金属表面不同的制备方法,并分析了它们各自最合适的应用场景。最后,聚焦于超疏水涂层在金属表面的应用前景,分析了超疏水涂层的主要应用现状,并总结了目前存在的不足和缺陷。
结果润湿理论包括Young模型、Wenzel模型、Cassie-Baxter模型在内的固体表面基本润湿理论。低附着力、屏蔽作用、高界面结合力和空气层的四种界面行为在超疏水涂层防腐蚀方面起到了关键作用。其中,Lotus效应使水滴在类似荷叶的表面上形成近乎完美的球形,通过基体表面微结构和低附着力,有效带走表面污物和腐蚀介质,从而保持长期的腐蚀防护效果;阻碍电化学腐蚀通过超疏水涂层的高接触角减少水滴与金属表面的实际接触面积,从而阻碍腐蚀微电池的形成;提高界面结合力主要抵抗由物理因素引起的金属腐蚀;气垫效应则通过超疏水涂层的特殊结构和化学性质使水珠无法完全附着,从而形成类似空气层的半悬浮状态,阻碍腐蚀性物质与金属直接接触。超疏水涂层根据涂层材料可以将其分为防蚀性超疏水聚合物涂层、耐蚀性纳米超疏水涂层、耐蚀性超疏水陶瓷涂层三大类,其在金属表面的防腐蚀行为各有高低。其中,防蚀性聚合物涂层主要涉及到特定的功能性物质,如防腐蚀剂、添加剂和纳米颗粒等,其含量以及相关成分配比都会影响该超疏水涂层的防腐性能,在实际应用时也将面临耐久性减弱、容易脱落的问题;耐蚀性纳米超疏水涂层得益于纳米材料的独特性能,将其与其它材料的结合形成微纳米级结构表面,不仅能够抵御化学腐蚀还能减少污染物附着,以此来加强金属的防腐蚀能力并延长材料的使用寿命。该类涂层在耐蚀性以及稳定性方面比防蚀性超疏水聚合物涂层表现得更出色;耐蚀性超疏水陶瓷涂层结合了特殊的微纳米结构和表面改性技术,在金属防腐蚀领域展现出极大地应用前景。其环境因素、设计成分配比都将影响涂层的防腐蚀性能,并且由于陶瓷材料本身的耐腐蚀性和硬度,该类涂层的耐腐蚀性能优于上述两种涂层。金属表面超疏水涂层的制备方法主要包括自组装法、溶胶凝胶法、化学气相沉积法、激光刻蚀法。其中,自组装法利用分子间的作用力自动排列形成有序结构,适用于制备纳米级结构;溶胶凝胶法通过化学反应将液态前驱体转变为固态凝胶,再经过热处理形成涂层,适用于大面积涂覆;化学气相沉积法在气相中通过化学反应沉积前驱体,适用于高精度涂层;激光刻蚀法利用激光刻蚀材料,形成所需的微纳结构,适用于需要高精度微结构的场景。此外,根据微纳结构的减材或增材制备方式,还可以将金属表面制备超疏水涂层的方法分为减材和增材两类。减材方式主要包括化学刻蚀和电化学刻蚀,化学刻蚀适用于能被常见蚀剂所溶解的金属材料,而电化学刻蚀则是通过在特定电解质溶液中施加电流,控制金属表面的局部腐蚀,形成所需的微纳结构,两者均适用于在金属表面大面积制备微纳结构的场合;电化学沉积和喷涂法则属于增材制备方法,其中,喷涂法可以在不同基底材料表面进行实施,适合在金属表面形成保护性涂层或增强特定功能的情况,如防腐蚀涂层、耐磨涂层等。同时,喷涂法操作简便,适用于快速涂覆需求。而电化学沉积则是利用化学反应中产生的沉积物,形成均匀的超疏水薄膜或结构,并且该方法与电化学刻蚀技术有所不同,后者是通过电解反应去除材料,属于减材方式,而电化学沉积法则是增加材料厚度或形成特定结构。超疏水涂层在金属防腐蚀领域主要广泛应用于铜、铝及其合金、镁合金以及钢铁及其合金方面。铜基材料的应用扩展到防冰和油水分离;铝及其合金主要用于船用材料,提升耐腐蚀稳定性;镁合金的应用则关注在特定条件下提高耐蚀性;钢铁及其合金由于使用广泛,超疏水涂层可以有效提高耐蚀性,拓宽应用范围。不同性能的超疏水涂层,如低附着力、良好的自清洁性和环境耐久性,有效解决了钢铁在不同腐蚀环境中的腐蚀问题。
结论随着科学技术的发展,超疏水涂层在金属防腐蚀领域取得了显著进展。未来应重点开发经济、环保、高效且多功能的超疏水涂层,以推动其在工业中的广泛应用。目前的现状可以归纳为以下四点:尽管超疏水涂层能显著提高金属的耐腐蚀性,但在实际应用中,环境因素如强酸、强碱或高湿度可能影响涂层的稳定性和防腐蚀性能;‚研发低成本、环保且耐磨的高效涂层仍然是主要挑战,特别是在高温高压或机械磨损环境中;ƒ相比其他技术,溶胶凝胶法易于规模化制备,但需提高涂层与基底的黏附强度和机械稳定性;„面向复杂环境,涂层不仅需具备防腐蚀性能,还需具备抗生物黏附、减阻等功能。




 下载:
下载: