Research progress on doping modified tin oxide electron transport layer in perovskite solar cells
-
摘要:
自从制备出第一件钙钛矿太阳能电池器件以来,钙钛矿太阳能电池的光电转换效率已从3.8%飞跃至26.1%,是下一代商用太阳能电池的有力竞争者。近十年来,SnO2因其适宜的能带结构、较好的电子传输性能、简单的制备工艺及良好的化学稳定性成为n-i-p型钙钛矿太阳能电池电子传输层材料的首选。虽然SnO2电子传输层优点众多,但还存在电子传输性能较差、传输层与钙钛矿层之间能级偏移、界面缺陷造成光生载流子大量损失及成膜性能较差容易出现针孔等问题。鉴于此,本文总结了上述问题形成的主要原因,并通过金属离子掺杂、卤素离子掺杂、有机分子掺杂、纳米颗粒掺杂等不同溶液掺杂工艺研究结果的分析,阐明了不同掺杂工艺在解决溶液法制备的SnO2薄膜缺陷及在钙钛矿电池器件中应用的优点与缺点,并针对钙钛矿器件掺杂SnO2传输层性能优化做出展望。
Abstract:Since the preparation of the first perovskite solar cell device, the photoelectric conversion efficiency of perovskite solar cells has jumped from 3.8% to 26.1%, making them a favorable competitor for the next generation of commercial solar cells. In the past decade, tin oxide has become the preferred electron transport layer material for n-i-p perovskite solar cells due to its suitable band structure, good electron transfer performance, simple preparation process, and good chemical stability. Although tin oxide electron transport layer has many advantages, there are still issues that need to be improved in terms of electron transport performance, such as energy level shift between the transport layer and the perovskite layer, interface defects causing significant loss of photo generated carriers, and poor film-forming performance that is prone to pinholes. In view of this, this article summarizes the main reasons for the formation of the above problems, and analyzes the research results of different solution doping processes such as metal ion doping, halogen ion doping, organic molecule doping, and nanoparticle doping. It elucidates the advantages and disadvantages of different doping processes in solving the defects of solution based tin oxide thin films and their applications in perovskite battery devices, and makes prospects for optimizing the performance of doped tin oxide transport layers in perovskite devices.
-
Keywords:
- SnO2 /
- perovskite solar cells /
- doping /
- interfacial defects /
- energy level alignment
-
双稳态复合材料层板具有两种稳定状态,两种稳定状态之间的转换只需要一个较小激励便可获得较大变形,且无需持续的能量输入维持其稳定构型,在可变形结构和能量收集领域得到了高度关注。
Hyer[1]首次在实验中发现了非对称铺层的复合材料层板在固化后会呈现两个圆柱形稳定状态,与经典层板理论获得的马鞍形构型不同,随后建立了双稳态层板理论预报模型[2-3],开始了双稳态层板研究的先河。目前,国内外研究学者在双稳态层板的改进理论预报模型[4-6]、跳变过程分析[7-10]、驱动方法[11-14]与可变形结构应用[15-17]等方面展开了大量的研究。
在双稳态层板的基础上,发展复合材料层板的多稳态特性可以拓宽其在可变形结构中的应用范围。目前主要的实现途径分为两类,一类是分段铺层的变刚度设计,Mattioni等[18]提出了一种由对称与非对称两种不同铺层顺序组成的变刚度层板,并建立了层板的数值计算模型,验证了多稳态铺层设计。Sousa等[19]针对对称与非对称铺层区域相交处纤维不连续性所产生的应力集中问题,引入弯曲纤维来提高结构的稳定性。Arrieta等[20]提出了分段式铺层的多稳态结构,通过串联连续的复合铺层,在机翼结构中嵌入可变形单元来获得变刚度特性。Cui等[21]将多稳态层板的应用扩展至二维空间,设计了一种由9个双稳态方形单元组合而成的曲面层板,运用特定的铺层顺序减小变形单元连接处由于弯曲方向不一致而产生的几何不兼容现象。Wang等[22]引入了对称铺层的过渡单元设计来缓解各变形单元之间的几何兼容问题,提高了多稳态层板的可设计性与可变形性。Zhang等[23]受此启发,将不对称铺层层板应用于过渡单元,发现该方法不仅能减小过渡单元的面积,并且对各类变形单元都有较好的适应性。Annamalai[24]运用分布式铺层方法,对如十字形等各类不同几何外形的层板进行了多稳态结构的有限元设计,但该方法仍存在几何不兼容的问题。
第二类是使用组合叠加的方法,该方法能够优化分段铺层的几何兼容性问题,同时具有大变形与可设计性等优势。Dai等[25]采用螺栓固定的方法制备了一种多稳态晶格结构,利用刚性连接来获得相交处的几何兼容性,并且通过拼接可以使该结构具有各种变形形状。Zhang等[26]以机械连接形式将双稳态层板进行组合获得了具有多种稳态的捕蝇草结构系统。Panesar等[27]利用丝束转向技术制造了一种多稳态混合襟翼,确保了纤维在层板面内的连续性。Algmuni等[28]通过在变形单元之间连接柔性带,以提高周期性结构的稳定性。Risso等[29]提出了一种将预拉伸薄膜与条带状复合材料层板相结合的多稳态结构,通过条带状层板的调整,能够实现正多边形的多稳态结构设计。Phanendra等[30]从理论与仿真两方面对由多个矩形层板叠加铺层得到的星形多稳态层板进行了研究,但是没有考虑制备的可行性。组合叠加得到的层板,结构较复杂且需要额外的机械紧固件连接。
本文通过将两块矩形层板以交叉铺设的形式连接并采用热压罐共固化成型,期望获得多稳态层板。然而这种交叉连接的方式会引起中心胶接区域产生较大刚度,从而导致结构失去多稳态特性。因此,本文对十字形层板引入切口设计的方法削弱中心区域刚度,并获得层板的多稳态特性。通过有限元和试验手段研究了胶接面积、切口角度和层板纵横比等参数对新型十字形多稳态层板稳定构型的影响规律,并根据层板的跳变行为揭示其影响机制,为含切口十字形多稳态层板的构型调控奠定基础。
1. 含切口矩形非对称层板的双稳态特性
选用S4C9/SY-24型玻璃纤维增强环氧树脂复合材料(中航复合材料有限公司),材料属性如表1所示。对矩形层板进行切口设计,如图1所示,采用[902/02]非对称铺层,单层厚度为0.11 mm,固化温度135℃。令层板总长L=250 mm、宽度W=75 mm,中心区域边长a=25 mm、切口角度θ=45°,首先从仿真与实验两方面分析该切口设计对矩形层板稳定构型的影响情况。
表 1 S4C9/SY-24型玻璃纤维增强环氧树脂复合材料的材料属性Table 1. Material properties of S4C9/SY-24 glass fiber reinforced polymer compositeMaterial property E1/GPa E2/GPa ν12 G12/GPa G13/GPa G23/GPa α11/℃−1 α12/℃−1 α13/℃−1 Value 54.6 10.5 0.33 5.5 5.5 3.9 6.7×10−6 2.9×10−5 2.9×10−5 Notes: E1—Longitudinal modulus; E2—Transverse modulus; ν12—Poisson's ratio; G12—In-plane shear modulus; G13, G23—Inter-laminar shear modulus; α11—Longitudinal thermal expansion coefficient; α12, α13—Transverse thermal expansion coefficient. 在ABAQUS中采用隐式静态几何非线性分析方法,对矩形层板的几何中心点施加一个固定约束,并在设定的固化温度条件下获得层板的第一稳态,随后在矩形的四角点上添加方向与第一稳态弯曲方向相反的位移载荷,以获得其第二稳态。在网格划分中,主要选取SR4缩减积分壳单元,切口处填充S3单元,划分完成后得到
2673 个单元网格。仿真结果如图2(a)所示,矩形层板具有传统的双稳态变形特点,在第一稳态中,矩形层板首先沿长度方向发生变形,向90°铺层面进行弯曲(沿z轴正向),宽度方向上的纤维基本不发生改变,维持直线状态。在施加位移载荷后获得的第二稳态中,层板沿宽度方向发生反向弯曲(沿z轴反向),在长度方向上发生回弹。在图2(b)中,含切口层板的第一稳态与矩形层板具有几乎相同的弯曲变形,但是其第二稳态构型发生了不同的变形情况,层板发生了翘曲现象,最大翘曲位置出现在层板两端中点处,如图中红色标点所示。
采用热压罐成型工艺制备了普通及含切口的矩形非对称铺层层板,如图3所示。为获得层板的稳态构型及其面外最大位移值,使用示波器(美国Tektronix,MDO3024数字混合域示波器)、滑台、滑轨与激光测距仪(日本KEYENCE,IL-100)等搭建了双稳态试件稳定构型测量平台,如图4所示。将试件放置于平整表面,操纵滑台与滑轨以控制试件在水平面内的匀速平移,采用激光测距仪沿层板对称轴连续测量试件各点的面外相对位移,并通过示波器绘制构型曲线,曲线拐点所对应的值即面外最大位移。
为提高实验数据的准确性,对矩形及含切口矩形层板均制备了至少3个有效试件,实验结果通过计算均值与添加误差棒的形式表现。仿真与实验结果对比显示:矩形层板与含切口矩形层板的第一稳定构型几乎完全一致,如图5所示,切口设计对矩形层板的第一稳态影响甚微。第二稳态则出现了明显的差异,含切口矩形层板的两端出现了翘曲现象,通过对面外最大位移进行测量,得到矩形层板的面外最大位移均值仅为4.39 mm,含切口矩形层板的面外最大翘曲高度均值为11.96 mm,翘曲高度远大于纤维弯曲变形所产生的位移变化量,如图6所示。
2. 含切口十字形非对称层板的多稳态特性
2.1 十字形层板多稳态特性
将两块尺寸为250 mm×75 mm,铺层均为[902/02]的矩形层板在几何中心处交叉铺设,采用共固化成型工艺,预期获得的十字形层板具有多种稳态,且相较于传统的采用机械连接的双稳态层板组合叠加设计方案,简化了工艺步骤,降低了结构的复杂程度,拓展了多稳态层板在结构可重构领域内的实际应用价值。
然而通过实验发现,交叉铺设得到的十字形结构仅能获得第一稳态,层板跳变无法得到第二稳态。一是由于层板第二稳态以交叉形式连接时,上下胶接面的变形方向不同造成了几何不兼容问题,使共固化后胶接区域内会产生较大约束力;二是共固化引起胶接区域内的铺层厚度增大,并且铺层顺序改变为对称铺层[902/02]S,使中心区域刚度加强,二者阻碍了跳变发生,导致第二稳态失去稳定性。这一现象在仿真中表现为获得的第二稳态稳定性极差,施加的位移载荷发生微小改变就会大幅影响最终的计算结果。因此,对矩形层板进行切口设计,通过削弱中心区域的刚度减少胶接区域对第二稳态产生的影响,如图7所示。
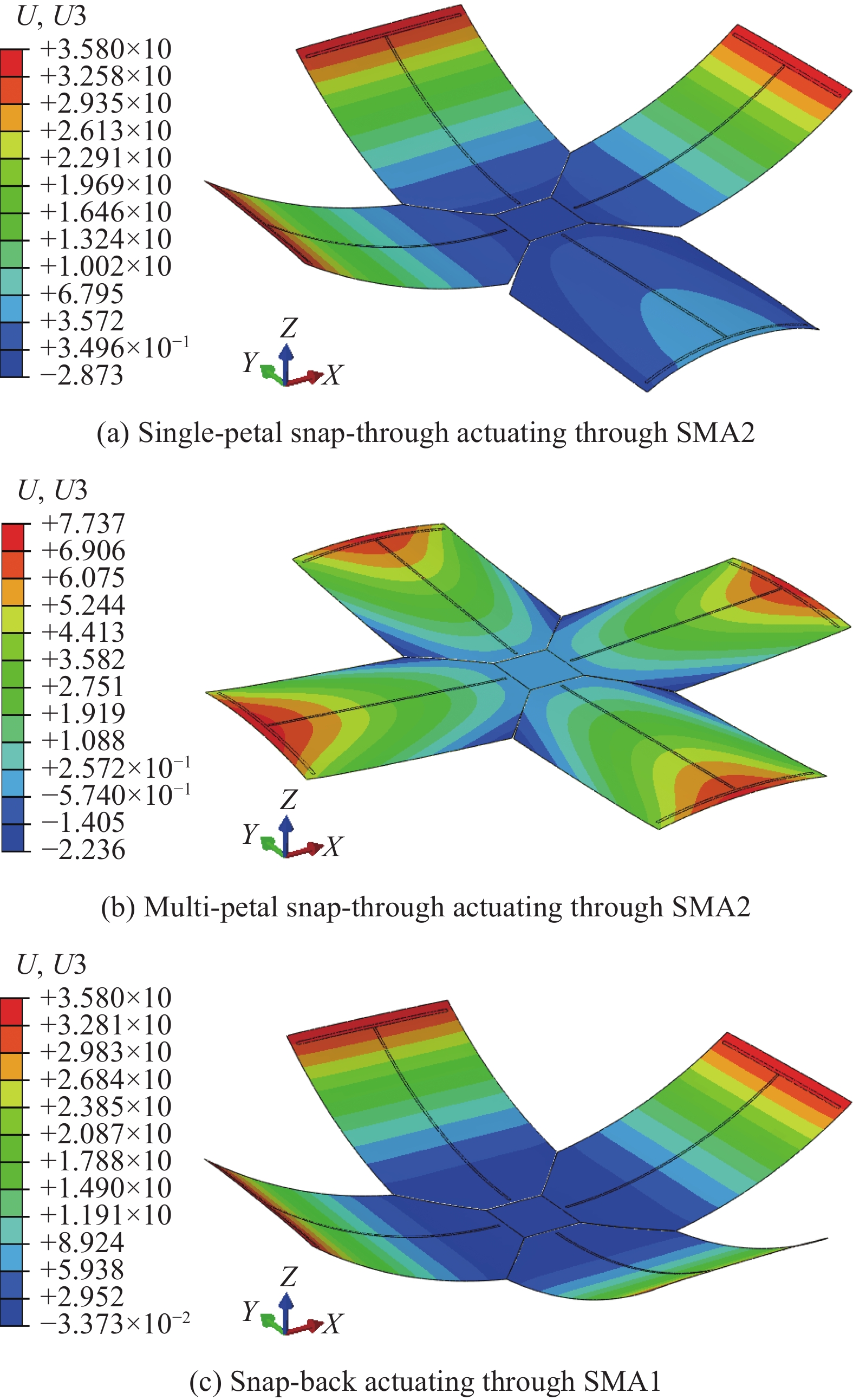
在ABAQUS中对两个非对称矩形层板进行单独建模,在胶接区域内施加TIE约束条件连接层板模拟共固化成型,获得含切口设计的十字形多稳态层板结构,如图8所示。在图8(a)中,第一稳态发生了较大变形,随后在十字形四边的8个角点上施加垂直向下的位移载荷,层板发生跳变并获得了如图8(b)中所示的第二稳态,十字形层板的四边都发生了一定程度的翘曲现象,且翘曲高度大于矩形层板自身第二稳态发生的弯曲变形,这与含切口矩形层板第二稳态的变化情况具有相似性。此时十字形层板第二稳态的面外最大位移不再取决于宽度方向上纤维产生的弯曲变形,而是由四边翘曲高度决定。此外,单独对十字形四瓣中的其中一瓣或多瓣施加位移载荷,能够使十字形层板出现多种不同的稳定状态,将双稳态特性进一步拓展至多稳态的特性研究,如图8(c)所示。同时对含切口设计的十字形层板进行实验制备,获得的试件具有与仿真结果相同的多稳态特性,证明了通过切口设计实现十字形多稳态复合材料层板的可行性。
在多稳态层板的有限元研究中,通常使用外力加载的方式实现层板构型变换的表征,但在实际应用中,则需要通过驱动方法来实现层板多稳态构型的转变。为此,本文设计了一种基于形状记忆合金(SMA)的多稳态驱动方案,利用形状记忆合金在温度作用下形状收缩的特点实现层板多稳态构型的转变。
在ABAQUS中,由于仅利用了形状记忆合金的形状收缩特性,因此可以对仿真模型进行简化,将形状记忆合金的形状收缩等效为热膨胀材料由于温度降低引起的形状收缩,赋予材料热膨胀系数α=
0.0008 ,其余材料属性取母相奥氏体,弹性模量E=81 GPa,泊松比ν=0.3。采用片状SMA驱动器,其厚度为0.05 mm,宽度为1 mm,加载方式如图9(a)所示,驱动元件与层板之间使用TIE刚性连接,驱动元件SMA1加载于90°铺层面上,控制层板由第二稳态向第一稳态发生转变,SMA2则加载于0°铺层面上,控制层板由第一稳态向第二稳态发生转变。在网格划分中,对驱动元件进行网格加密,最终得到9893 个单元网格,如图9(b)所示。仿真结果如图10所示,对于形状记忆合金驱动的十字形多稳态层板,通过控制SMA2的形状收缩,能够实现单瓣或多瓣的跳变驱动,使层板从第一稳态向第二稳态发生转变;同样,通过控制SMA1,也可以使层板由第二稳态向第一稳态发生单瓣或多瓣的跳回现象。通过对形状记忆合金的调控,实现了对十字形多稳态层板结构变形的主动控制。同时,对比形状记忆合金加载前后十字形层板多种稳态的稳定构型与面外位移,可以发现,由于采用的片状SMA驱动器尺寸很小,因此驱动元件的加载对十字形层板的构型影响甚微。
此外,研究发现,外力施加与形状记忆合金驱动下的十字形多稳态层板,单瓣片均具有独立变形能力,即对于未施加位移扰动的瓣片,其面外位移保持相对稳定,相较于第一稳态没有显著变化。而对于已经施加了位移扰动并完成了跳变的瓣片,其最大翘曲程度又与第二稳态具有的翘曲现象相当。
这一多稳态变形特点取决于胶接区域的大刚度特性。胶接区域的较大刚度导致了当一部分瓣片受到载荷作用时,其变形不会对其他未受载荷的瓣片产生显著影响,这种变形独立性体现了含切口十字形层板在设计上的优势,尤其是在需要精确控制局部变形的应用场景中。
2.2 胶接面积影响
对含切口十字形多稳态层板进行参数影响研究。保持层板尺寸L=250 mm、W=75 mm不变,当中心胶接区域为正方形(θ=45°)时,就能够在不改变几何外形为十字形的前提下,通过改变胶接区域边长a,研究不同胶接面积对十字型多稳态层板稳定构型的影响规律。
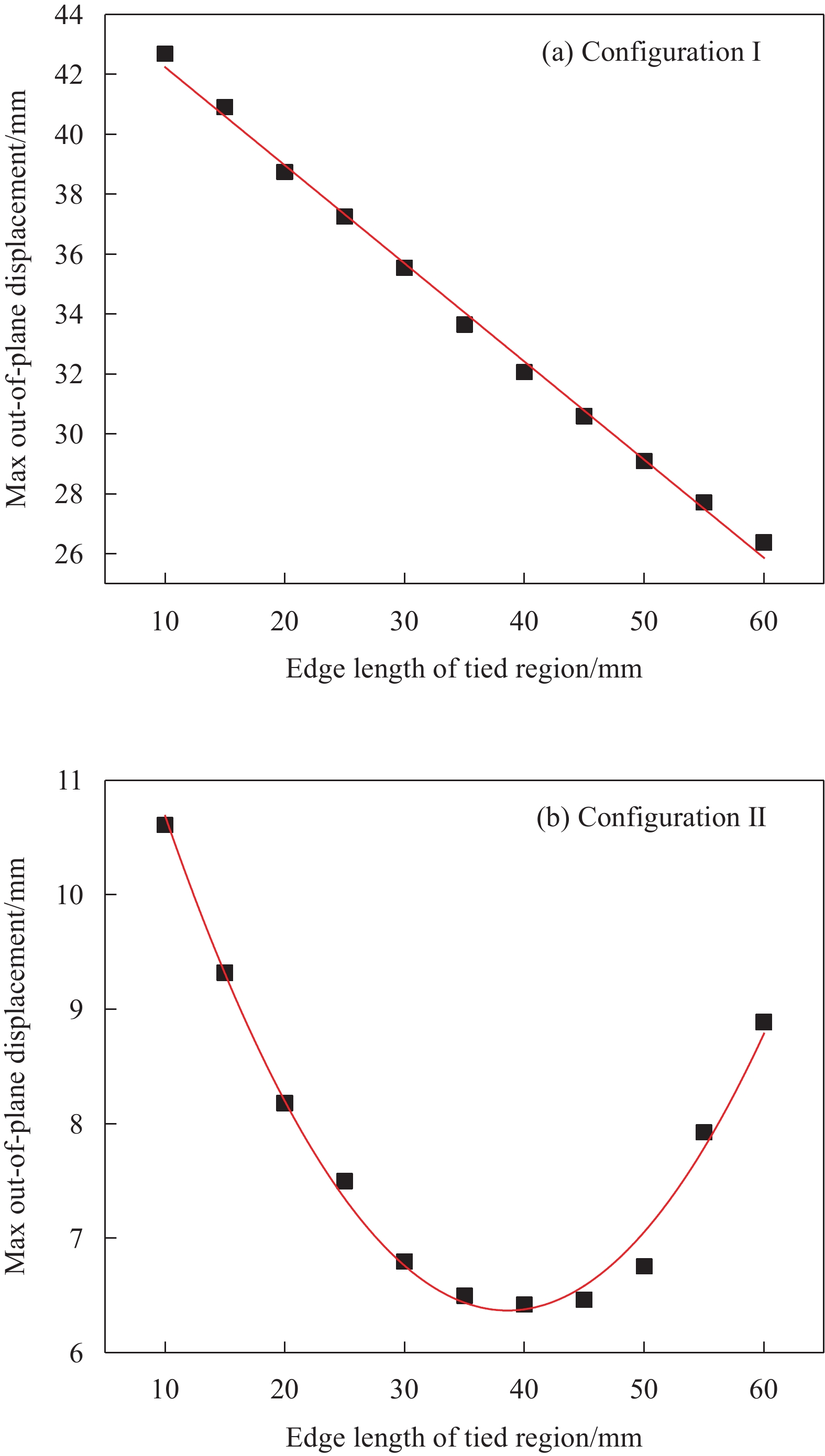
令胶接区域边长a在10~60 mm范围内逐渐增大,单次增幅∆a为5 mm,不同胶接面积与层板面外最大位移之间的变化关系曲线,如图11所示。由图可知,第一稳态面外位移大小随胶接面积的增大而减小,且呈线性关系,这是由于胶接面积的增大使层板参与弯曲变形的长度缩小导致的。而第二稳态中面外最大位移的变化情况较复杂,如图11(b)所示,首先当a在10~40 mm的范围内增大时,面外最大位移减小,随后a继续在40~60 mm区间内增大时,面外最大位移则出现上升趋势。
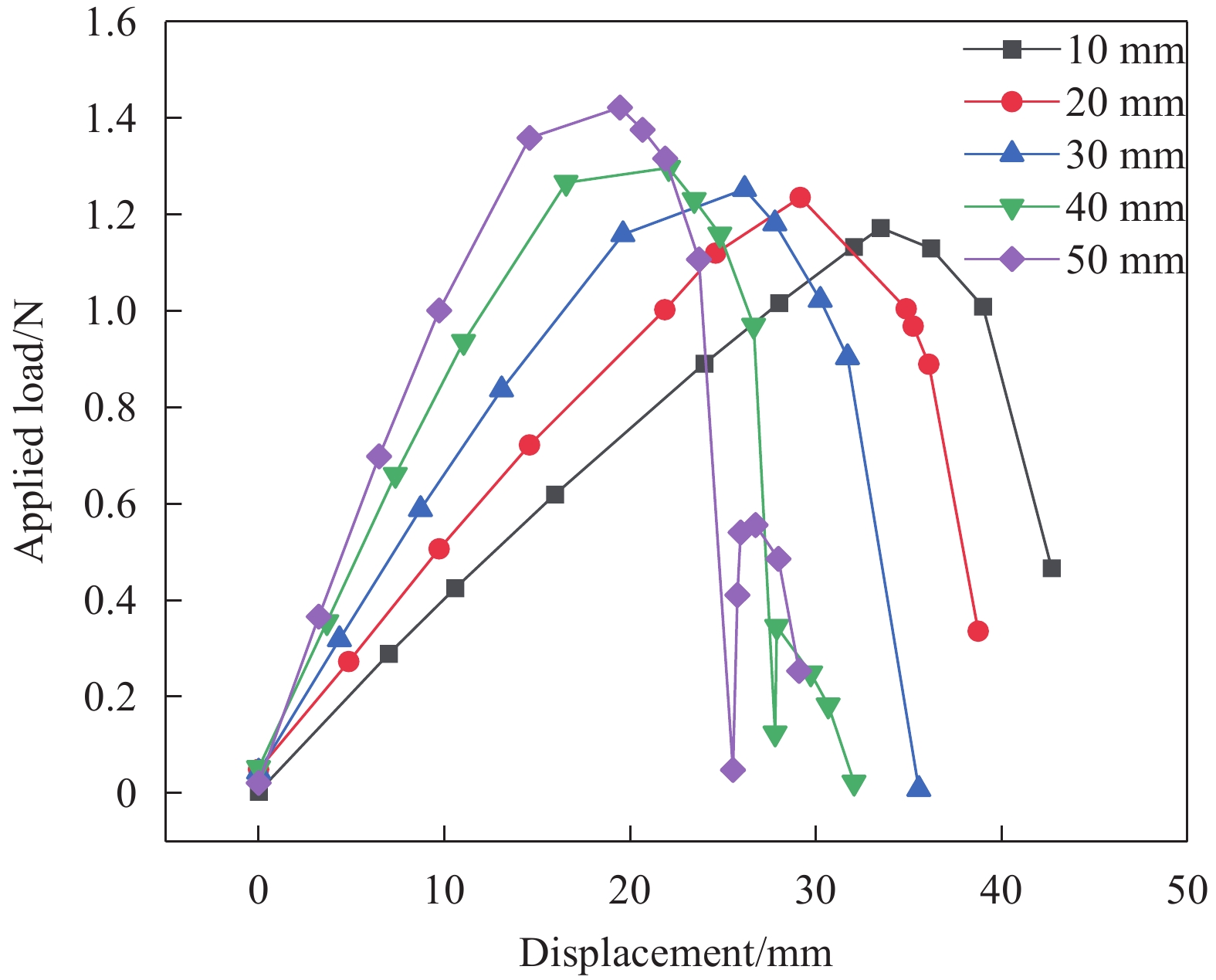
依次选择参数a=10、20、30、40、50 mm,研究不同胶接面积对层板跳变行为的影响情况,如图12所示。将仿真中层板固定端支反力,等效为十字形层板跳变所需的外部加载,获得了位移-载荷曲线图。从图中能够观察到层板的跳变行为:载荷首先随层板面外位移的增大线性增加,层板发生弹性形变;当载荷上升至峰值时,层板开始跳变,曲线的最大值即跳变载荷大小;随后载荷迅速降低,载荷的减小量随胶接面积的增大而增大。当a=30 mm时,维持层板变形所需的载荷大小降为0。这意味着对于a=30 mm的含切口十字形层板,当施加的外部载荷达到结构的跳变载荷后无需继续加载,即可使层板完成向第二稳态的转变。对于a=40 mm、50 mm的十字形层板,则表现出负刚度行为,载荷出现了回升,对应着图11(b)中a=40 mm处曲线出现拐点的现象,第二稳态的面外最大位移变化趋势发生改变。
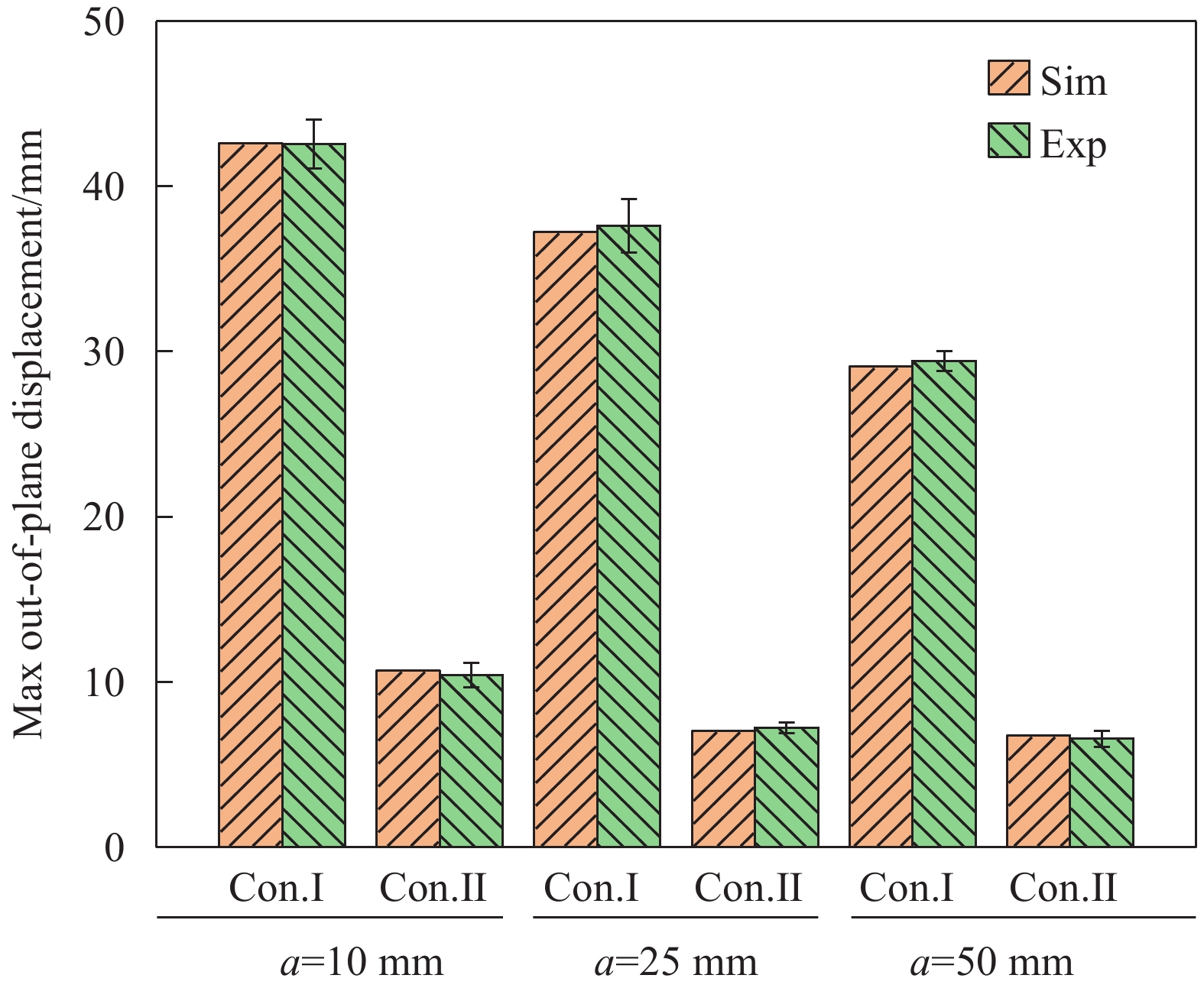
试验验证了不同胶接面积对十字形层板稳定构型的影响规律,如表2所示,分别制备了边长a=10、25、50 mm的3种十字形层板(红色框选表示实际的胶接区域),层板的两种稳态均具有良好的稳定性,且与仿真中两种稳定构型的变形情况吻合,第一稳态发生较大变形,第二稳态产生了四边翘曲现象,翘曲高度决定了层板的面外最大位移。同时,结构的稳定性还体现在,重复变换不同稳态多次后,结构仍然可以使用,层板的稳定构型与多稳态特性几乎不受到影响。
表 2 不同胶接面积下十字形多稳态层板的两种稳定构型Table 2. Two stable configurations of cruciform multistable laminates with different co-curing areaCo-curing area/(mm×mm) Configuration I Configuration II 10×10 

25×25 

50×50 

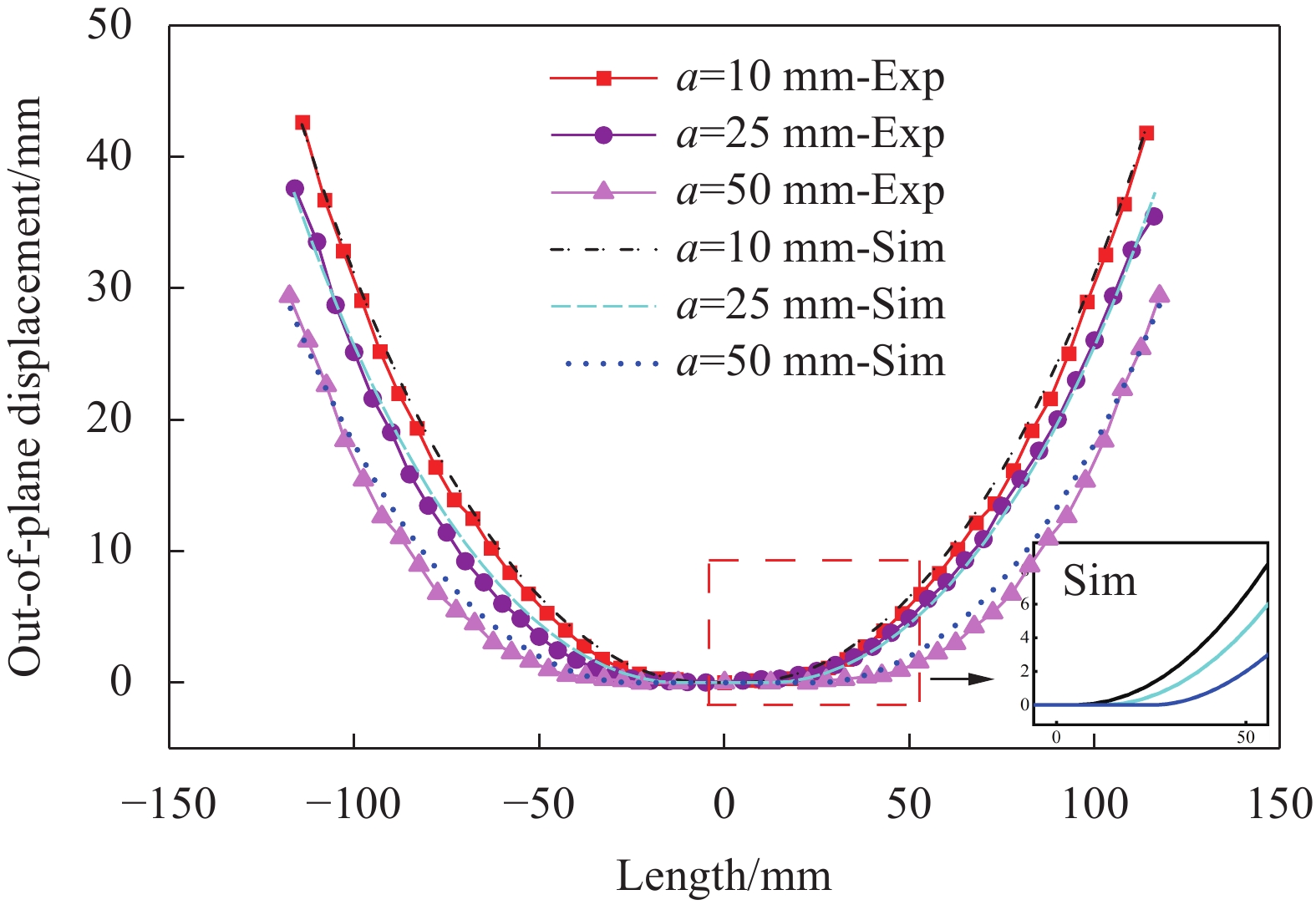
采用激光测距仪对层板的稳定构型与面外最大位移进行测量,如图13、图14所示,实验结果与有限元结果之间吻合良好,最大误差不超过5%。对比图13中所示的层板第一稳态构型情况,可以发现:虽然层板第一稳态的面外最大位移会随着胶接面积的改变发生线性变化,但层板在胶接区域外发生的弯曲变形曲率基本一致,胶接面积的改变对构型曲率产生较小影响。
2.3 切口角度影响
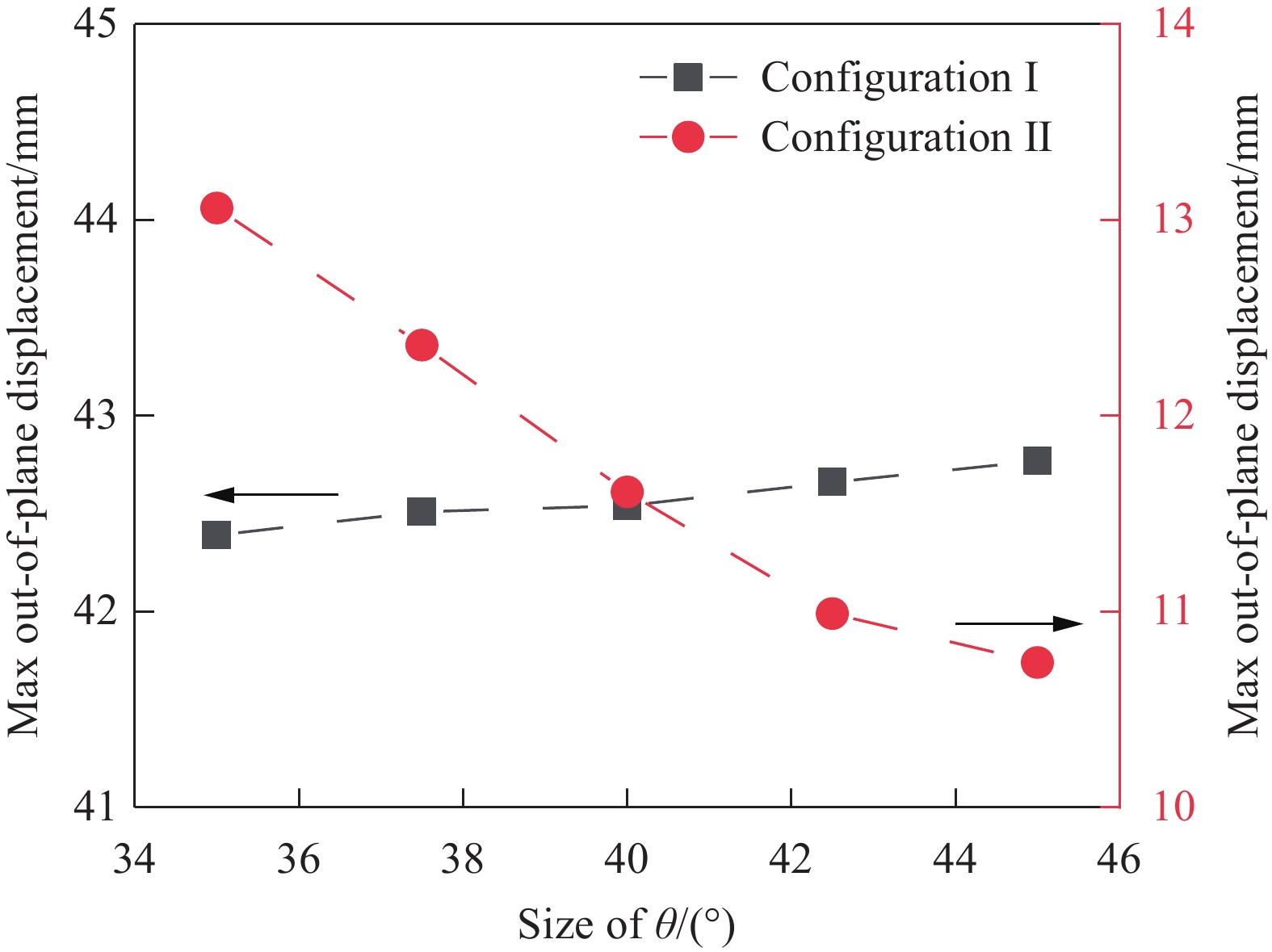
为了获得在相同胶接面积下不同切口角度对十字形多稳态层板稳定构型的影响情况,保持胶接区域边长a=10 mm不变,改变切口角度θ。为了保证结构的稳定性,结构参数需要满足W≤L1及防止过度胶接切口角度需小于45°。
令切口角度分别为θ=35°、37.5°、40°、42.5°和45°,切口角度对面外最大位移的影响情况如图15所示。可知:随着切口角度θ的改变,第一稳态的面外最大位移基本不受影响,改变量仅为0.38 mm,百分比变化仅为0.8%;第二稳态的面外最大位移则随θ的减小而增大,总增量为2.32 mm,百分比变化为21.6%。
切口角度θ的减小,使胶接区域周边的纤维面积缩减。十字形层板第二稳态的面外最大位移受到中心区域内作用力影响,作用力主要取决于胶接区域内阻碍跳变发生的约束力及层板内部激发双稳态变形的热残余应力。在胶接面积不发生改变的条件下,引起层板四边翘曲的约束力大小保持不变,但切口角度与纤维面积的缩减减小了参与矩形层板第二稳态弯曲变形的热应力大小,在二者的相互作用下,导致翘曲现象更加明显,第二稳态面外最大位移增大。
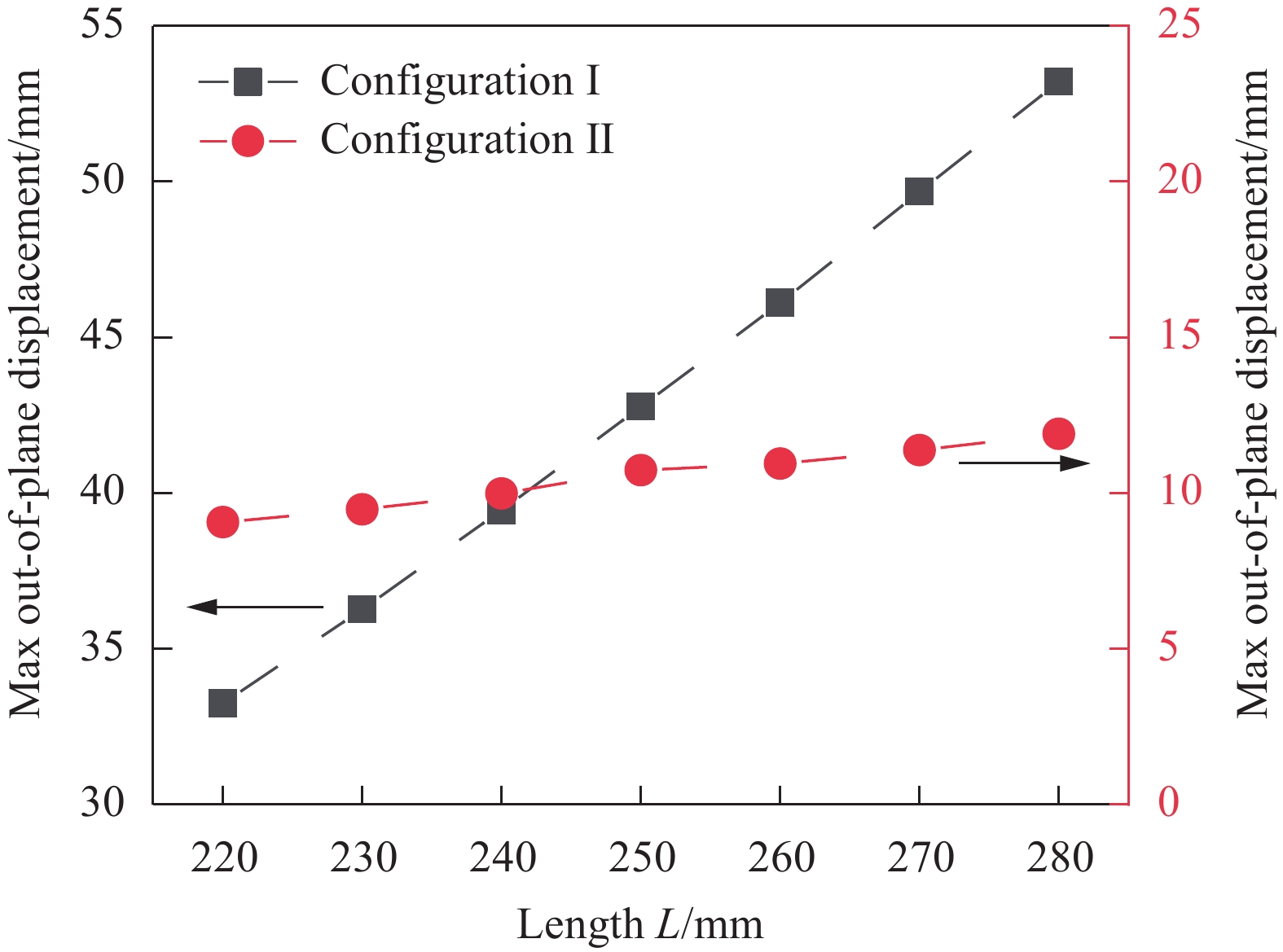
2.4 纵横比影响
改变矩形层板纵横比,由于矩形宽度的改变会带动切口角度发生变化,因此保持宽度不变,令层板长度逐渐增大(L=220、230、240、250、260、270、280 mm),参数对面外最大位移的影响如图16所示。可知:随着长度L增大,第一稳态与第二稳态的面外最大位移均随之增大,并且L对第一稳态构型的影响远大于第二稳态,第一稳态的面外最大位移总增量为19.96 mm,百分比变化为46.7%;而第二稳态总增量仅为2.83 mm,百分比变化为26.4%。
十字形层板两种稳态的构型变化规律与矩形层板基本相同,长度L与面外最大位移呈线性关系,这是由于纵横比L/W的增大,使层板内部沿长度方向上的纤维长度增加,结构发生了更大的弯曲变形。
3. 结 论
(1)通过仿真与试验手段研究了普通及含切口矩形双稳态层板的稳定构型,获得了二者的稳定构型与变形规律。结果表明切口设计对层板第二稳定构型影响较大,含切口的矩形层板第二稳定状态两边产生了翘曲现象,且翘曲高度大于纤维自身所产生的弯曲变形量,面外最大位移变为由翘曲高度决定。
(2)通过将两块矩形双稳态层板交叉铺设共固化成型并引入切口设计,提出了一种新的十字形多稳态复合材料层板,通过有限元和试验验证了切口设计的可行性。
(3)胶接面积与十字形层板第一稳定构型面外最大位移呈线性减小关系;第二稳定构型面外最大位移随胶接面积的增加呈现出先减小后增大的趋势。
(4)研究了相同胶接面积下不同切口角度与纵横比对十字形层板稳定构型的影响规律,切口角度主要影响十字形层板的第二稳态,而纵横比则对第一稳态起重要作用。
-
图 2 (a) SnO2中本征点缺陷形成能[37];(b) SnO2中缺陷转变水平的示意图[41];(c) 60 nm膜厚的TiO2与SnO2膜与FTO衬底的透射图谱[43]
VO—Oxygen vacancy; VSn—Tin vacancy; Sni—Interstitial tin; Oi—Interstitial oxygen; SnO—Oxidation states of tin; ECBM—Conduction band edge; EVBM—Valence band maximum; HO—H impurity; FTO—Fluorine doped tin oxide
Figure 2. (a) Eigenpoint defect formation energy in SnO2[37]; (b) Schematic diagram of defect transformation level in SnO2[41]; (c) Transmission spectra of 60 nm thick TiO2 and SnO2 films and FTO substrate[43]
图 4 (a)能级悬崖结构;(b)能级尖峰结构[51]
ETL—Electron transport layer; Ec—Conduction band edge; Ev—Valence band maximum; EFN—Fermi level of n-type semiconductor; EFP—Fermi level of p-type semiconductor; ΔEc—Potential barrier of conduction band edge; qV—Potential barrier
Figure 4. (a) Energy level cliff structure; (b) Energy level spike structure[51]
图 7 SnO2 (a)和 SnO2-Cl (b)膜的SEM图像;SnO2 (c)和SnO2-Cl (d) 膜的AFM图像;SnO2和SnO2-Cl薄膜的透射光谱(e)和吸收光谱(f);(g)单载流子器件的电流密度-电压特性曲线;(h)器件光电流的演变(偏置电压为0.8 V)[76]
RMS—Root mean square
Figure 7. SEM images of SnO2 (a) and SnO2-Cl films (b); AFM images of SnO2 (c) and SnO2-Cl films (d); Transmission spectra (e) and absorption spectra (f) of SnO2 and SnO2-Cl films; (g) Current density-voltage characteristic curves of single carrier devices; (h) Evolution of device photocurrent with bias voltage of 0.8 V [76]
图 8 (a) 乳酸钾、苹果酸钾和柠檬酸钾的钙钛矿太阳能电池(PSCs)结构和化学组成示意图;(b)本工作中SnO2的原位钝化策略[81]
Spiro-OMeTAD—2, 2', 7, 7'-tetra kis[N, N-di(4-methoxyphenyl)amino]-9, 9'-spirobifluorene; FTO—F-doped tin oxide; FA—Formamidine
Figure 8. (a) Schematic diagram of the structure and chemical composition of perovskite solar cells (PSCs) in potassuim lactate, potassuim malate, and potassuim citrate; (b) In situ passivation strategy of SnO2 in this work [81]
图 9 (a) 聚丙烯酰胺(PAM)改性SnO2的原理示意图;(b) SnO2、SnO2:PAM和PAM薄膜的XPS光谱;(c) 薄膜中锡元素的高分辨率XPS光谱;(d) SnO2和SnO2:PAM溶液的电势[85]
Figure 9. (a) Illustration of perovskite films on pristine SnO2 and polyacrylamide (PAM)-modified SnO2; (b) XPS spectra for the SnO2, SnO2 : PAM and PAM films; (c) High-resolution XPS spectra for Sn in the films; (d) Potential of SnO2 and SnO2 : PAM solution[85]
表 1 钙钛矿太阳能电池中常见金属氧化物电子传输层(ETL)的导带能级与体相迁移率
Table 1 Conduction band energy levels and bulk phase mobility of common metal oxide electron transport layers (ETL) in perovskite solar cells
表 2 SnO2电子传输层基钙钛矿太阳能电池中常见的添加剂及其对太阳能电池性能的贡献
Table 2 Dopants and their contribution to the improvement of PSCs based on SnO2 ETL
Device structure VOC/V JSC/(mA·cm−2) FF PCE/% Ref. FTO/SnO2/FAPbI3/spiro-OMeTAD/Au 1.144 21.43 0.752 19.69 [65] Nb-doped 1.157 22.77 0.747 20.47 ITO/SnO2/FAPbI3/spiro-OMeTAD/Au 1.158 21.65 0.777 19.48 [66] Ta-doped 1.161 22.79 0.786 2.80 FTO/SnO2/MAPbI3/spiro-OMeTAD/Au 1.000 16.80 0.530 9.02 [63] Al-doped 1.030 19.40 0.580 12.10 ITO/SnO2/(FAPbI3)0.95(MAPbBr3)0.05/spiro-OMeTAD/Au 1.060 24.80 0.66 17.30 [67] Zr-doped 1.080 25.30 0.72 19.54 FTO/SnO2/MAPbI3/spiro-OMeTAD/Au 1.030 18.60 0.610 11.69 [68] Y-doped 1.070 21.80 0.670 15.60 FTO/SnO2/CsFAMA/spiro-OMeTAD/Ag 1.078 23.20 0.771 19.43 [70] Cu-doped 1.108 24.20 0.790 21.35 AZO/SnO2/Csx(FAMA)100-x/spiro-OMeTAD/Au 0.997 22.10 0.570 12.50 [76] Ga-doped 1.070 22.80 0.786 22.80 FTO/SnO2/(FAPbI3)0.85(MAPbBr3)0.15/spiro-OMeTAD/Au 1.020 21.00 0.590 15.07 Cl-doped 1.110 23.00 0.690 18.10 ITO/SnO2/(FA0.98MA0.02)0.95Cs0.05Pb(I0.95Br0.05)3/spiro-MeOTAD/Au 1.060 24.46 0.759 19.72 [74] K-doped 1.080 24.47 0.769 20.40 Rb-doped 1.100 24.51 0.776 20.84 Cs-doped 1.060 24.37 0.802 20.64 ITO/SnO2/FA0.95MA0.05PbI3/spiro-OMeTAD/Au 1.060 23.23 0.708 17.43 [79] MES-doped 1.120 23.88 78.69 21.05 ITO/SnO2/FA0.95MA0.05PbI3/spiro-OMeTAD/Au 1.090 24.05 79.15 20.78 [80] DMAPAI2-doped 1.17 24.20 82.19 23.20 FTO/SnO2/(FAPbI3)0.96(MAPbBr3)0.04/spiro-OMeTAD/Au 1.136 24.89 0.811 22.92 [81] PC-doped 1.188 24.98 0.821 24.36 ITO/SnO2/CsFAMA/spiro-OMeTAD/Ag 1.070 21.80 0.740 18.74 [82] PEIE-doped 1.140 23.80 0.760 20.61 ITO/SnO2/Cs0.04FA0.74MA0.22/spiro-OMeTAD/Au 1.070 22.60 0.771 18.60 [84] PEG-doped 1.110 22.70 0.818 20.80 ITO/SnO2/FA0.95MA0.05PbI2.95Br0.05/spiro-OMeTAD/Au 1.103 23.16 0.792 20.22 [85] PAM-doped 1.122 24.82 0.811 22.59 ITO/SnO2/Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3/spiro-OMeTAD/Au 1.030 22.30 0.746 17.13 [88] RCQ-doped 1.140 23.30 0.826 22.51 ITO/SnO2/FA0.95MA0.05PbI3/PCBM/Au 1.125 22.93 0.743 19.17 [31] GYD-doped 1.137 23.32 0.796 21.11 Notes: VOC—Open circuit voltage; JSC—Short-circuit current; PCE—Photoelectric conversion efficiency; FF—Fill factor; MES—4-morpholine ethane sulfonic acid sodium salt; DMAPAI2—N, N-dimethyl-1, 3-propanediamine dihydroiodide; PC—Potassium citrate; PEIE—Polyethylenimine-ethoxylated; PEG—Polyethylene glycol; PAM—Polyacrylamide; RCQ—Red-carbon quantum dots; GYD—Graphdiyne; PCBM—(6, 6)-phenyl C61 butyric acid methyl ester; FA—Formamidine; MA—Methyalamino group. -
[1] The National Renewable Energy Laboratory (NREL). Best research-cell efficiency chart [EB/OL]. (2024-04-08).
[2] KOJIMA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells[J]. Journal of the American Chemical Society, 2009, 131(17): 6050-6051. DOI: 10.1021/ja809598r
[3] LIANG Z, ZHANG Y, XU H, et al. Homogenizing out-of-plane cation composition in perovskite solar cells[J]. Nature, 2023, 624(7992): 557-563. DOI: 10.1038/s41586-023-06784-0
[4] KIM H S, LEE C R, IM J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%[J]. Scientific Reports, 2012, 2(1): 591. DOI: 10.1038/srep00591
[5] LIU M, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467): 395-398. DOI: 10.1038/nature12509
[6] CHEN Y, MENG Q, ZHANG L, et al. SnO2-based electron transporting layer materials for perovskite solar cells: A review of recent progress[J]. Journal of Energy Chemistry, 2019, 35: 144-167. DOI: 10.1016/j.jechem.2018.11.011
[7] KIM J Y, LEE J W, JUNG H S, et al. High-efficiency perovskite solar cells[J]. Chemical Reviews, 2020, 120(15): 7867-7918. DOI: 10.1021/acs.chemrev.0c00107
[8] XING G, MATHEWS N, SUN S, et al. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3[J]. Science, 2013, 342(6156): 344-347. DOI: 10.1126/science.1243167
[9] NI Y, LI H Y, LI H M, et al. Strong connection between PVK and ETL induced by an anti-allergic agent interface for high-quality PSCs[J]. Journal of Materials Science: Materials in Electronics, 2022, 33(9): 6456. DOI: 10.1007/s10854-022-07817-6
[10] GUO Z, GAO L, ZHANG C, et al. Low-temperature processed non-TiO2 electron selective layers for perovskite solar cells[J]. Journal of Materials Chemistry A, 2018, 6(11): 4572-4589. DOI: 10.1039/C7TA10742K
[11] WANG Y, ZHANG X, WANG Q, et al. Investigating PCE of PSCs with N-based groups doped TiO2 ETLs prepared by sol-gel and sputtering technologies[J]. Materials Letters, 2022, 327: 133055. DOI: 10.1016/j.matlet.2022.133055
[12] WEI J, ZHANG C, JI G, et al. Roll-to-roll printed stable and thickness-independent ZnO: PEI composite electron transport layer for inverted organic solar cells[J]. Solar Energy, 2019, 193: 102-110. DOI: 10.1016/j.solener.2019.09.037
[13] DONG J, WU J, JIA J, et al. Annealing-free Cr2O3 electron-selective layer for efficient hybrid perovskite solar cells[J]. ChemSusChem, 2018, 11(3): 619-628. DOI: 10.1002/cssc.201701864
[14] GHENO A, THU PHAM T T, DI BIN C, et al. Printable WO3 electron transporting layer for perovskite solar cells: Influence on device performance and stability[J]. Solar Energy Materials and Solar Cells, 2017, 161: 347-354. DOI: 10.1016/j.solmat.2016.10.002
[15] JIANG Q, ZHANG X, YOU J. SnO2: A wonderful electron transport layer for perovskite solar cells[J]. Small, 2018, 14(31): 1801154. DOI: 10.1002/smll.201801154
[16] FERNANDES S L, VÉRON A C, NETO N F A, et al. Nb2O5 hole blocking layer for hysteresis-free perovskite solar cells[J]. Materials Letters, 2016, 181: 103-107. DOI: 10.1016/j.matlet.2016.06.018
[17] THAMPY S, ZHANG B, HONG K H, et al. Altered stability and degradation pathway of CH3NH3PbI3 in contact with metal oxide[J]. ACS Energy Letters, 2020, 5: 1147-1152. DOI: 10.1021/acsenergylett.0c00041
[18] HAN J, KWON H, KIM E, et al. Interfacial engineering of a ZnO electron transporting layer using self-assembled monolayers for high performance and stable perovskite solar cells[J]. Journal of Materials Chemistry A, 2020, 8(4): 2105-2113. DOI: 10.1039/C9TA12750J
[19] LIU P, WANG W, LIU S, et al. Fundamental understanding of photocurrent hysteresis in perovskite solar cells[J]. Advanced Energy Materials, 2019, 9(13): 1803017. DOI: 10.1002/aenm.201803017
[20] JI J, LIU X, JIANG H, et al. Two-stage ultraviolet degradation of perovskite solar cells induced by the oxygen vacancy-Ti4+ states[J]. iScience, 2020, 23(4): 101013. DOI: 10.1016/j.isci.2020.101013
[21] CHEN B, YANG M, PRIYA S, et al. Origin of J-V hysteresis in perovskite solar cells[J]. The Journal of Physical Chemistry Letters, 2016, 7(5): 905-917. DOI: 10.1021/acs.jpclett.6b00215
[22] DENG H X, LI S S, LI J. Quantum confinement effects and electronic properties of SnO2 quantum wires and dots[J]. The Journal of Physical Chemistry C, 2010, 114(11): 4841-4845. DOI: 10.1021/jp911035z
[23] REN X, YANG D, YANG Z, et al. Solution-processed Nb: SnO2 electron transport layer for efficient planar perovskite solar cells[J]. ACS Applied Materials & Interfaces, 2017, 9(3): 2421-2429.
[24] LIU X, TSAI K W, ZHU Z, et al. A low-temperature, solution processable tin oxide electron-transporting layer prepared by the dual-fuel combustion method for efficient perovskite solar cells[J]. Advanced Materials Interfaces, 2016, 3(13): 1600122. DOI: 10.1002/admi.201600122
[25] KE W, FANG G, LIU Q, et al. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells[J]. Journal of the American Chemical Society, 2015, 137(21): 6730-6733. DOI: 10.1021/jacs.5b01994
[26] SONG J, ZHENG E, BIAN J, et al. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells[J]. Journal of Materials Chemistry A, 2015, 3(20): 10837-10844. DOI: 10.1039/C5TA01207D
[27] JIANG Q, ZHAO Y, ZHANG X, et al. Surface passivation of perovskite film for efficient solar cells[J]. Nature Photonics, 2019, 13(7): 460-466. DOI: 10.1038/s41566-019-0398-2
[28] MIN H, LEE D Y, KIM J, et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes[J]. Nature, 2021, 598(7881): 444-450. DOI: 10.1038/s41586-021-03964-8
[29] AHMAD W, LIU D, AHMAD W, et al. Physisorption of oxygen in SnO2 nanoparticles for perovskite solar cells[J]. IEEE Journal of Photovoltaics, 2019, 9: 200-206. DOI: 10.1109/JPHOTOV.2018.2877002
[30] YU M, GUO Y, YUAN S, et al. The influence of the electron transport layer on charge dynamics and trap-state properties in planar perovskite solar cells[J]. RSC Advances, 2020, 10(21): 12347-12353. DOI: 10.1039/D0RA00375A
[31] ZHANG S, SI H, FAN W, et al. Graphdiyne: Bridging SnO2 and perovskite in planar solar cells[J]. Angewandte Chemie International Edition, 2020, 59(28): 11573-11582. DOI: 10.1002/anie.202003502
[32] JARZEBSKI Z, MORTON. Physical properties of SnO2 materials: III . Optical properties[J]. Journal of the Electrochemical Society, 1976, 123: 333C.
[33] ANARAKI E H, KERMANPUR A, STEIER L, et al. Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide[J]. Energy & Environmental Science, 2016, 9(10): 3128-3134.
[34] TIWANA P, DOCAMPO P, JOHNSTON M B, et al. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells[J]. ACS Nano, 2011, 5(6): 5158-5166. DOI: 10.1021/nn201243y
[35] ZHENG H, TACHIBANA Y, KALANTAR-ZADEH K. Dye-sensitized solar cells based on WO3[J]. Langmuir, 2010, 26(24): 19148-19152. DOI: 10.1021/la103692y
[36] YOON S, KIM S J, KIM H S, et al. Solution-processed indium oxide electron transporting layers for high-performance and photo-stable perovskite and organic solar cells[J]. Nanoscale, 2017, 9(42): 16305-16312. DOI: 10.1039/C7NR05695H
[37] KILIÇ Ç, ZUNGER A. Origins of coexistence of conductivity and transparency in SnO2[J]. Physical Review Letters, 2002, 88(9): 095501. DOI: 10.1103/PhysRevLett.88.095501
[38] WANG Z P, LI R, ZHANG M, et al. Interface modification and performance optimization of SnO2 based perovskite solar cells[J]. Chinese Journal of Engineering, 2023, 45(2): 263-277.
[39] YANG G, CHEN C, YAO F, et al. Effective carrier-concentration tuning of SnO2 quantum dot electron-selective layers for high-performance planar perovskite solar cells[J]. Advanced Materials, 2018, 30(14): 1706023. DOI: 10.1002/adma.201706023
[40] GANOSE A M, SCANLON D O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaics[J]. Journal of Materials Chemistry C, 2016, 4(7): 1467-1475. DOI: 10.1039/C5TC04089B
[41] PARK S Y, ZHU K. Advances in SnO2 for efficient and stable n–i–p perovskite solar cells[J]. Advanced Materials, 2022, 34(27): 2110438. DOI: 10.1002/adma.202110438
[42] XIONG L, GUO Y, WEN J, et al. Review on the application of SnO2 in perovskite solar cells[J]. Advanced Functional Materials, 2018, 28(35): 1802757. DOI: 10.1002/adfm.201802757
[43] DONG Q, SHI Y, WANG K, et al. Insight into perovskite solar cells based on SnO2 compact electron-selective layer[J]. The Journal of Physical Chemistry C, 2015, 119(19): 10212-10217. DOI: 10.1021/acs.jpcc.5b00541
[44] SUBBIAH A S, MATHEWS N, MHAISALKAR S G, et al. Novel plasma-assisted low-temperature-processed SnO2 thin films for efficient flexible perovskite photovoltaics[J]. ACS Energy Letters, 2018, 3(7): 1482-1491. DOI: 10.1021/acsenergylett.8b00692
[45] DONG Q, SHI Y, ZHANG C, et al. Energetically favored formation of SnO2 nanocrystals as electron transfer layer in perovskite solar cells with high efficiency exceeding 19%[J]. Nano Energy, 2017, 40: 336-344. DOI: 10.1016/j.nanoen.2017.08.041
[46] JIANG Q, ZHANG L, WANG H, et al. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells[J]. Nature Energy, 2016, 2(1): 16177. DOI: 10.1038/nenergy.2016.177
[47] ZHANG J, BAI C, DONG Y, et al. Batch chemical bath deposition of large-area SnO2 film with mercaptosuccinic acid decoration for homogenized and efficient perovskite solar cells[J]. Chemical Engineering Journal, 2021, 425: 131444. DOI: 10.1016/j.cej.2021.131444
[48] TONG G, ONO L K, LIU Y, et al. Up-scalable fabrication of SnO2 with multifunctional interface for high performance perovskite solar modules[J]. Nanomicro Letters, 2021, 13(1): 155.
[49] CORREA BAENA J P, STEIER L, TRESS W, et al. Highly efficient planar perovskite solar cells through band alignment engineering[J]. Energy & Environmental Science, 2015, 8(10): 2928-2934.
[50] SHAO S, LOI M A. The role of the interfaces in perovskite solar cells[J]. Advanced Materials Interfaces, 2020, 7(1): 1901469. DOI: 10.1002/admi.201901469
[51] PAN H, SHAO H, ZHANG X L, et al. Interface engineering for high-efficiency perovskite solar cells [J]. Journal of Applied Physics, 2021, 129(13): 130904.
[52] HOANG HUY V P, NGUYEN T M H, BARK C W. Recent advances of doped SnO2 as electron transport layer for high-performance perovskite solar cells[J]. Materials, 2023, 16(18): 6170. DOI: 10.3390/ma16186170
[53] ZHAO P, LIN Z, WANG J, et al. Numerical simulation of planar heterojunction perovskite solar cells based on SnO2 electron transport layer[J]. ACS Applied Energy Materials, 2019, 2(6): 4504-4512. DOI: 10.1021/acsaem.9b00755
[54] BOEHM H P. Acidic and basic properties of hydroxylated metal oxide surfaces[J]. Discussions of the Faraday Society, 1971, 52: 264-275. DOI: 10.1039/df9715200264
[55] YUAN Y, WANG Y, WANG M, et al. Effect of unsaturated Sn atoms on gas-sensing property in hydrogenated SnO2 nanocrystals and sensing mechanism[J]. Scientific Reports, 2017, 7(1): 1231. DOI: 10.1038/s41598-017-00891-5
[56] YOO J J, SEO G, CHUA M R, et al. Efficient perovskite solar cells via improved carrier management[J]. Nature, 2021, 590(7847): 587-593. DOI: 10.1038/s41586-021-03285-w
[57] HAN D, JIANG B, FENG J, et al. Photocatalytic self-doped SnO2−x nanocrystals drive visible-light-responsive color switching[J]. Angewandte Chemie International Edition, 2017, 56(27): 7792-7796. DOI: 10.1002/anie.201702563
[58] 刘贤哲, 张旭, 陶洪, 等. 溶胶-凝胶法制备氧化锡基薄膜及薄膜晶体管的研究进展[J]. 物理学报, 2020, 69(22): 228102. DOI: 10.7498/aps.69.20200653 LIU Xianzhe, ZHANG Xu, TAO Hong, et al. Research progress of tin oxide-based thin films and thin-film transistors prepared by sol-gel method[J]. Acta Physica Sinica, 2020, 69(22): 228102(in Chinese). DOI: 10.7498/aps.69.20200653
[59] WANG H, LIU H, YE F, et al. Hydrogen peroxide-modified SnO2 as electron transport layer for perovskite solar cells with efficiency exceeding 22%[J]. Journal of Power Sources, 2021, 481: 229160. DOI: 10.1016/j.jpowsour.2020.229160
[60] XU Z, ZHOU X, LI X, et al. Polymer-regulated SnO2 composites electron transport layer for high-efficiency n–i–p perovskite solar cells[J]. Solar RRL, 2022, 6(8): 2200092. DOI: 10.1002/solr.202200092
[61] HALVANI ANARAKI E, KERMANPUR A, MAYER M T, et al. Low-temperature Nb-doped SnO2 electron-selective contact yields over 20% efficiency in planar perovskite solar cells[J]. ACS Energy Letters, 2018, 3(4): 773-778. DOI: 10.1021/acsenergylett.8b00055
[62] CHEN Y, WANG Q, YAO Y, et al. Synergistic transition metal ion co-doping and multiple functional additive passivation for realizing 25.30% efficiency perovskite solar cells[J]. Energy & Environmental Science, 2023, 16(11): 5243-5254.
[63] CHEN H, LIU D, WANG Y, et al. Enhanced performance of planar perovskite solar cells using low-temperature solution-processed Al-doped SnO2 as electron transport layers[J]. Nanoscale Reseach Letters, 2017, 12(1): 238. DOI: 10.1186/s11671-017-1992-1
[64] ROOSE B, JOHANSEN C M, DUPRAZ K, et al. A Ga-doped SnO2 mesoporous contact for UV stable highly efficient perovskite solar cells[J]. Journal of Materials Chemistry A, 2018, 6(4): 1850-1857. DOI: 10.1039/C7TA07663K
[65] GUO R, ZHAO Y, ZHANG Y, et al. Significant performance enhancement of all-inorganic CsPbBr3 perovskite solar cells enabled by Nb-doped SnO2 as effective electron transport layer[J]. Energy & Environmental Materials, 2021, 4(4): 671-680.
[66] LIU Q, ZHANG X, LI C, et al. Effect of tantalum doping on SnO2 electron transport layer via low temperature process for perovskite solar cells[J]. Applied Physics Letters, 2019, 115(14): 143903. DOI: 10.1063/1.5118679
[67] NOH Y W, LEE J H, JIN I S, et al. Tailored electronic properties of Zr-doped SnO2 nanoparticles for efficient planar perovskite solar cells with marginal hysteresis[J]. Nano Energy, 2019, 65: 104014. DOI: 10.1016/j.nanoen.2019.104014
[68] SONG J, ZHANG W, WANG D, et al. Colloidal synthesis of Y-doped SnO2 nanocrystals for efficient and slight hysteresis planar perovskite solar cells[J]. Solar Energy, 2019, 185: 508-515. DOI: 10.1016/j.solener.2019.04.084
[69] QUY H V, BARK C W. Ni-doped SnO2 as an electron transport layer by a low-temperature process in planar perovskite solar cells[J]. ACS Omega, 2022, 7(26): 22256-22262. DOI: 10.1021/acsomega.2c00965
[70] ZHOU X, ZHANG W, WANG X, et al. Solution-processed Cu-doped SnO2 as an effective electron transporting layer for high-performance planar perovskite solar cells[J]. Applied Surface Science, 2022, 584: 152651. DOI: 10.1016/j.apsusc.2022.152651
[71] CHENG N, LI W, ZHENG D, et al. Enhance the efficiency of perovskite solar cells using W doped SnO2 electron transporting layer[J]. ChemPhotoChem, 2024, 8(6): e202300275. DOI: 10.1002/cptc.202300275
[72] WANG E, CHEN P, YIN X, et al. Tailoring electronic properties of SnO2 quantum dots via aluminum addition for high-efficiency perovskite solar cells[J]. Solar RRL, 2019, 3(5): 1900041. DOI: 10.1002/solr.201900041
[73] WANG J, QIN M, TAO H, et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer[J]. Applied Physics Letters, 2015, 106(12): 121104. DOI: 10.1063/1.4916345
[74] HOANG M T, YANG Y, CHIU W H, et al. Unraveling the mechanism of alkali metal fluoride post-treatment of SnO2 for efficient planar perovskite solar cells[J]. Small Methods, 2024, 8(2): 2300431. DOI: 10.1002/smtd.202300431
[75] HUANG Y, LI S, WU C, et al. Introduction of LiCl into SnO2 electron transport layer for efficient planar perovskite solar cells[J]. Chemical Physics Letters, 2020, 745: 137220. DOI: 10.1016/j.cplett.2020.137220
[76] GONG W, GUO H, ZHANG H, et al. Chlorine-doped SnO2 hydrophobic surfaces for large grain perovskite solar cells[J]. Journal of Materials Chemistry C, 2020, 8(33): 11638-11646. DOI: 10.1039/D0TC00515K
[77] LI Z, WANG L, LIU R, et al. Spontaneous interface ion exchange: Passivating surface defects of perovskite solar cells with enhanced photovoltage[J]. Advanced Energy Materials, 2019, 9(38): 1902142. DOI: 10.1002/aenm.201902142
[78] ZHANG S, GU H, CHEN S C, et al. KF-doped SnO2 as an electron transport layer for efficient inorganic CsPbI2Br perovskite solar cells with enhanced open-circuit voltages[J]. Journal of Materials Chemistry C, 2021, 9(12): 4240-4247. DOI: 10.1039/D1TC00277E
[79] MENG X, DENG J, SUN Q, et al. High-efficiency planar heterojunction perovskite solar cell produced by using 4-morpholine ethane sulfonic acid sodium salt doped SnO2[J]. Journal of Colloid and Interface Science, 2022, 609: 547-556. DOI: 10.1016/j.jcis.2021.11.051
[80] MA H, WANG M, WANG Y, et al. Asymmetric organic diammonium salt buried in SnO2 layer enables fast carrier transfer and interfacial defects passivation for efficient perovskite solar cells[J]. Chemical Engineering Journal, 2022, 442: 136291. DOI: 10.1016/j.cej.2022.136291
[81] DONG W, ZHU C, BAI C, et al. Low-cost hydroxyacid potassium synergists as an efficient in situ defect passivator for high performance tin-oxide-based perovskite solar cells[J]. Angewandte Chemie International Edition, 2023, 62(25): e202302507. DOI: 10.1002/anie.202302507
[82] ANEFNAF I, AAZOU S, SCHMERBER G, et al. Polyethylenimine-ethoxylated interfacial layer for efficient electron collection in SnO2-based inverted organic solar cells[J]. Crystals, 2020, 10(9): 731. DOI: 10.3390/cryst10090731
[83] WANG D, CHEN S C, ZHENG Q. Poly(vinylpyrrolidone)-doped SnO2 as an electron transport layer for perovskite solar cells with improved performance[J]. Journal of Materials Chemistry C, 2019, 7(39): 12204-12210. DOI: 10.1039/C9TC04269E
[84] WEI J, GUO F, WANG X, et al. SnO2-in-polymer matrix for high-efficiency perovskite solar cells with improved reproducibility and stability[J]. Advanced Materials, 2018, 30(52): 1805153. DOI: 10.1002/adma.201805153
[85] DONG H, WANG J, LI X, et al. Modifying SnO2 with polyacrylamide to enhance the performance of perovskite solar cells[J]. ACS Applied Materials & Interfaces, 2022, 14(29): 34143-34150.
[86] GONG H, WANG Y J, TEO S C, et al. Interaction between thin-film tin oxide gas sensor and five organic vapors[J]. Sensors and Actuators B: Chemical, 1999, 54(3): 2325.
[87] CHEN J, DONG H, ZHANG L, et al. Graphitic carbon nitride doped SnO2 enabling efficient perovskite solar cells with PCEs exceeding 22%[J]. Journal of Materials Chemistry A, 2020, 8(5): 2644-2653. DOI: 10.1039/C9TA11344D
[88] HUI W, YANG Y, XU Q, et al. Red-carbon-quantum-dot-doped SnO2 composite with enhanced electron mobility for efficient and stable perovskite solar cells[J]. Advanced Materials, 2020, 32(4): 1906374. DOI: 10.1002/adma.201906374
-
期刊类型引用(2)
1. 郭小杰,杜丽勇. 尿素掺杂CH_3NH_3PbI_3薄膜及其钙钛矿太阳能电池性能研究. 功能材料. 2024(01): 1086-1091 .  百度学术
百度学术
2. 张晨亮. 溴化锂材料浓度对太阳能电池蓄能力影响的计算机动态分析. 信息记录材料. 2023(02): 158-160 .  百度学术
百度学术
其他类型引用(5)
-
目的
随着钙钛矿太阳能技术的发展,SnO材料因其良好的光稳定性、高透射率、良好的电子迁移率、相对于普通钙钛矿的合适的能级位置、低温加工工艺等优点成为钙钛矿太阳能电池电子传输层材料的重要选择。但溶液法制备的SnO薄膜很难形成良好的氧化锡晶相,这将导致氧化锡薄膜出现迁移率显著下降、界面缺陷增多、能级不匹配、成膜性较差等问题。因此本文聚焦于SnO薄膜出现问题的原因,重点论述了掺杂工程改善SnO薄膜的方案。
方法本文将从氧化锡的晶体结构与物理性质出发,基于溶液法氧化锡薄膜的缺陷特点及其作为钙钛矿电池器件中电子传输材料的要求,分析并总结了当前仍存在的导电性能仍需提高、界面能级需要更加匹配、界面缺陷需要钝化、成膜性能(尤其是大面积制备)不足等问题并针对金属离子掺杂、卤素离子掺杂、有机分子掺杂、纳米颗粒掺杂等不同的溶液掺杂工艺,分析了其在解决溶液法氧化锡薄膜缺陷以及在钙钛矿电池器件中应用的优点与缺点;最后对溶液法掺杂氧化锡的研究方向与发展趋势做出展望。
结果氧化锡晶体的缺陷按照其存在的位置大概可以分为内部缺陷与界面缺陷两类。其中晶体内部的V、Sn是氧化锡晶体具有优异导电性能的原因,但由于缺陷产生的自发性,很难通过控制手段来重复制备具有相同自掺杂程度的氧化锡薄膜,这就导致氧化锡导电能力与能级位置容易发生改变。而溶液制备的氧化锡薄膜在晶体表面会存在大量的悬空键与悬挂羟基。这些悬空键会在氧化锡层与钙钛矿层之间形成大量的氧空位缺陷,形成的缺陷能级将会捕获光生电子,成为电子陷阱,使得器件效率大量降低。为了改善薄膜性能,可以通过掺杂工程进行改性,常用的掺杂剂可分为金属离子、卤素离子、有机分子以及碳量子点四种。在氧化锡薄膜中采用金属离子掺杂主要通过提高SnO 薄膜的载流子迁移率,使导带能级向更有利的方向偏移,钝化界面缺陷以及减少钙钛矿内部缺陷的方式来提升器件性能。但是目前金属离子的掺杂一般都维持在较低浓度,这是因为如果掺杂浓度过高会引起氧化锡严重的晶格畸变,导致整体器件性能下降。因此金属离子掺杂对氧化锡某些性能的改善有限,只能用于微调。卤素离子不仅可以通过氢键和静电相互作用有效钝化晶体表面的悬空键,还能改善钙钛矿膜的成膜情况,此外还可以通过离子交换或离子扩散到钙钛矿层中同时钝化钙钛矿的缺陷。但是由于卤素离子一般较小,可以扩散到钙钛矿层中,这会使得钙钛矿层的构成更加复杂,使得原本就很复杂的混合卤素钙钛矿成膜及添加剂研究变得更加困难。有机分子与碳量子点的官能团可以有效钝化缺陷且复杂多样的有机物分子可以更加准确的调配氧化锡层能级,实现与钙钛矿的最优匹配。除了官能团之外,长链结构的聚合物可以有效提升氧化锡层与钙钛矿层界面的亲和性,改善钙钛矿的成膜性能。但是有机分子与碳量子点载流子迁移率一般较低并且其稳定性与致密性和无机材料相比都较差,这些都会使得器件的寿命降低,这就为钙钛矿太阳能电池的封装工艺提出了新的挑战。
结论氧化锡的性能提升与长期稳定性的改善仍然是目前钙钛矿太阳能电池研发环节中的一项重要内容。目前来看,在提升氧化锡传输层性能的道路上选择复合掺杂的道路不可避免,选择协同掺杂策略可以实现不同掺杂材料的优势互补。虽然复合掺杂道路上困难重重,但将会是解决问题的有效手段。




 下载:
下载: