Effect of Pt supported on the hybrid of porous carbon nanofibers and carbon black on oxygen reduction reaction activity and durability
-
摘要:
进一步提高Pt催化剂对氧还原反应(ORR)的催化活性和稳定性是促进质子交换膜燃料电池(PEMFC)商业化的关键。采用静电纺丝结合高温碳化的方法制备了直径约200 nm的多孔纳米碳纤维(PCNF),将其与炭黑(CB)混合作为Pt催化剂的复合载体,并使用乙二醇还原法制备了催化剂Pt/PCNF-CB,通过与商业Pt/C催化剂的对比,研究了Pt/PCNF-CB对ORR的催化活性与稳定性。当载体中CB含量为40wt%时,PCNF与CB能相互分散均匀,构建独特的三维贯通结构,以此混合载体制备的催化剂Pt/PCNF-CB-40在酸性电解液中表现出优良的ORR电催化活性,与Pt/C相比具有更高的起始电位(0.975 V)与半波电位(0.781 V)。同时,基于Pt/PCNF-CB-40的膜电极(MEA)表现出优良的输出性能,在铂载量较低的条件下其峰值功率密度高达599 mW·cm−2,较商业Pt/C催化剂提升19%,并且在加速应力测试(AST) (0.6 V和0.95 V)
30000 次循环后最大功率密度仅损失21%,而商业Pt/C催化剂损失了41%,证明了PCNF与CB形成的复合载体对提高催化剂活性和稳定性具有积极作用。Abstract:Further improving the activity and stability of Pt catalysts for oxygen reduction reaction (ORR) is the key to promote the commercialization of proton exchange membrane fuel cells (PEMFCs). In this paper, porous carbon nanofibers (PCNF) with a diameter of about 200 nm were prepared by electrospinning and followed carbonization, which were mixed with carbon black (CB) as a hybrid support for Pt catalysts, and the catalysts Pt/PCNF-CB were prepared by means of ethylene glycol reduction method. The electrocatalytic activity and stability of Pt/PCNF-CB for ORR was investigated by comparing it with commercial Pt/C. When the CB content in the support was 40wt%, PCNF and CB could disperse uniformly to construct a unique three-dimensional through structure, and the Pt/PCNF-CB-40 prepared from this hybrid support shows excellent ORR electrocatalytic activity in acidic electrolyte with higher onset potential (0.975 V) and half-wave potential (0.781 V) compared with commercial Pt/C. Meanwhile, the Pt/PCNF-CB-40-based membrane electrode assembly (MEA) exhibits higher output performance with a peak power density of up to 599 mW·cm−2 at low Pt loading, which is a 19% improvement over the commercial Pt/C, and the maximum power density is only lost by 21% after
30000 of accelerated stress test (AST) (0.6 V and 0.95 V), compared with the loss of the commercial Pt/C by 41%, demonstrating that the hybrid support formed by PCNF and CB has a positive effect on improving electrocatalytic activity and stability. -
-
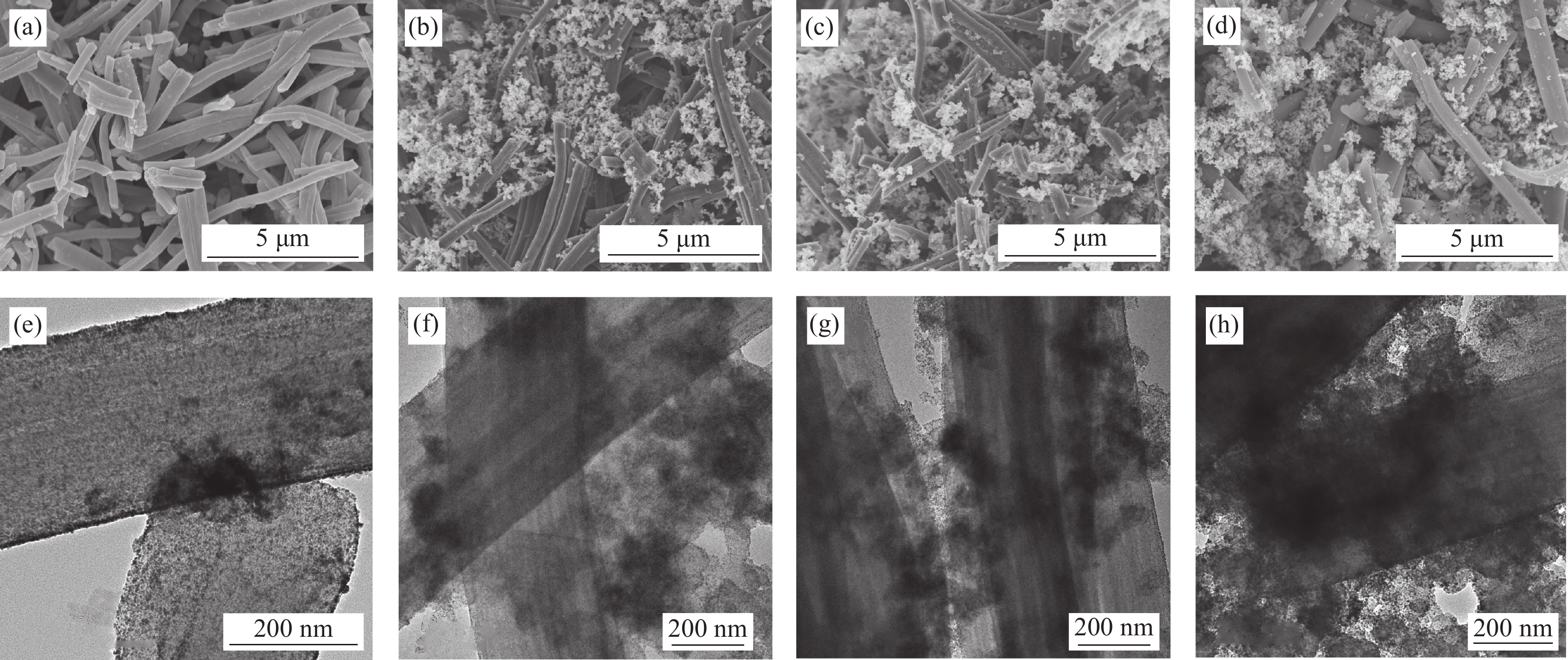
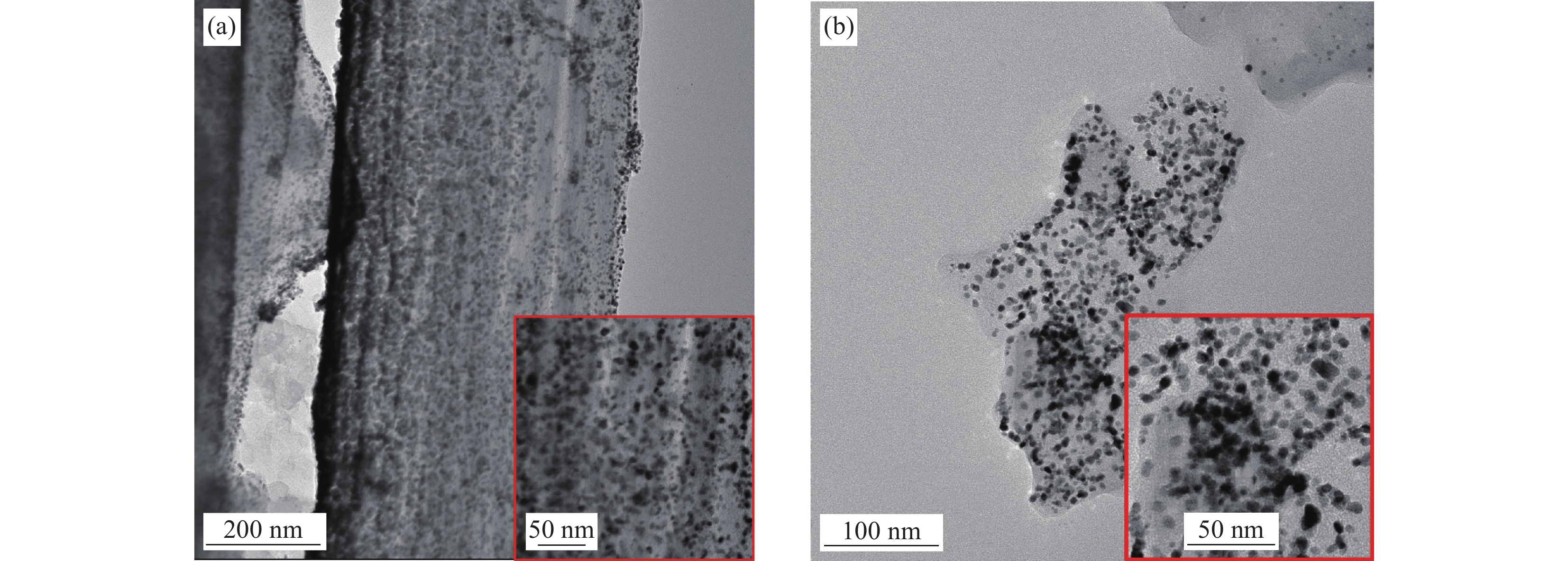
图 2 催化剂的SEM图像:Pt/PCNF (a),Pt/PCNF-CB-30 (b),Pt/PCNF-CB-40 (c),Pt/PCNF-CB-50 (d);催化剂的TEM图像:Pt/PCNF (e),Pt/PCNF-CB-30 (f),Pt/PCNF-CB-40 (g),Pt/PCNF-CB-50 (h)
Figure 2. SEM images of electrocatalysts: Pt/PCNF (a), Pt/PCNF-CB-30 (b), Pt/PCNF-CB-40 (c), Pt/PCNF-CB-50 (d); TEM images of electrocatalysts: Pt/PCNF (e), Pt/PCNF-CB-30 (f), Pt/PCNF-CB-40 (g), Pt/PCNF-CB-50 (h)
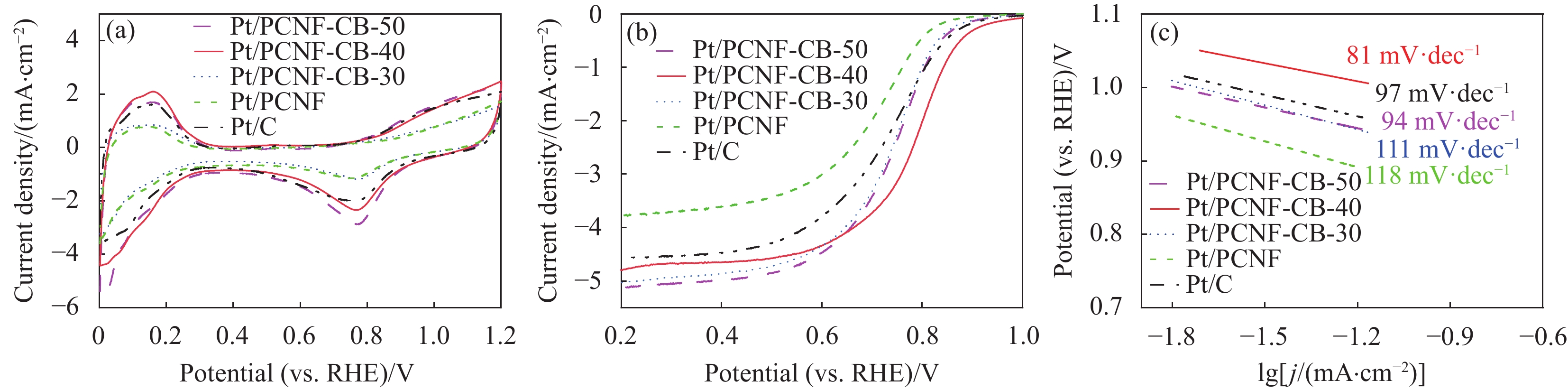
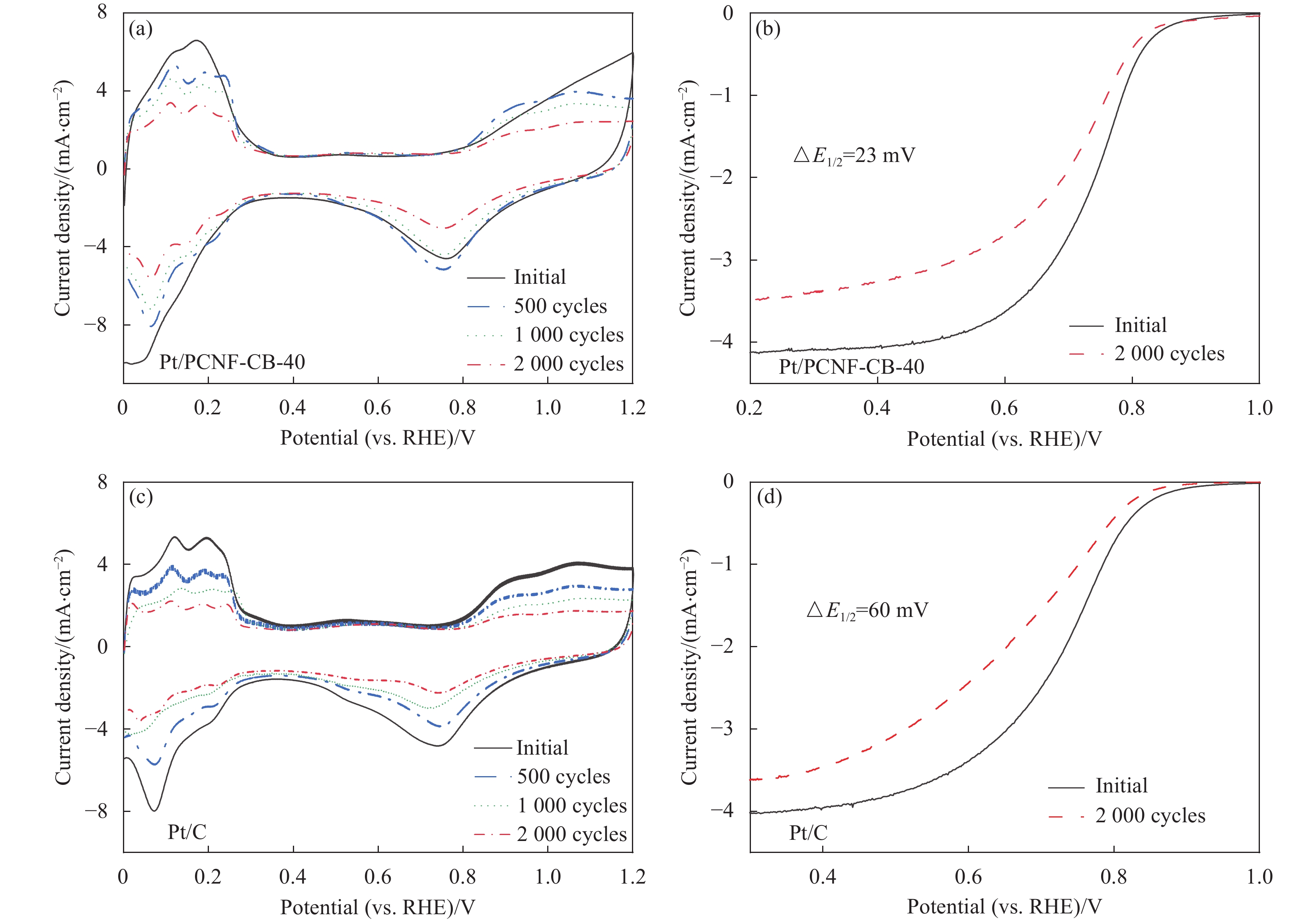
图 5 商业Pt/C与Pt/PCNF-CB在0.5 mol·L−1 H2SO4中的CV曲线(扫描速率为50 mV·s−1) (a)、LSV曲线(扫速为
1600 r/min) (b)与Tafel斜率(c)Figure 5. CV curves of commercial Pt/C and Pt/PCNF-CB series electrocatalysts in 0.5 mol·L−1 H2SO4 (under the scanning rate of 50 mV·s−1) (a), LSV curves of commercial Pt/C and Pt/PCNF-CB series electrocatalysts (under the
1600 r/min electrode rotation speed) (b) and Tafel plots (c)RHE—Reversible hydrogen electrode; j—Absolute value of current density
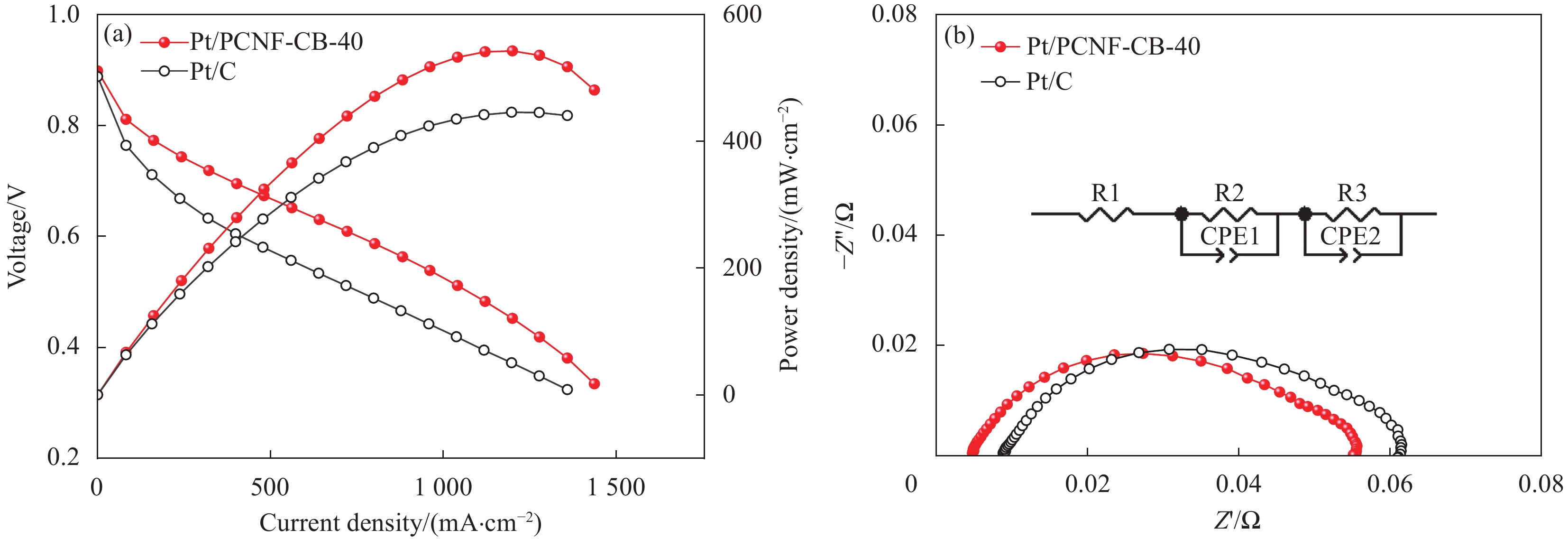
图 8 Pt/PCNF-CB-40与Pt/C分别作为阴极的膜电极(MEA)在H2/Air中的极化曲线与功率密度曲线(a)以及Pt/PCNF-CB-40与Pt/C在0.4 A·cm−2下的电化学阻抗谱(EIS)曲线(b)
Figure 8. Polarization and power density curves (a) and electrochemical impedance spectroscopy (EIS) curves (b) of membrane electrode assembly (MEA) of Pt/PCNF-CB-40 and Pt/C as cathodes respectively in H2/Air at 0.4 A·cm−2
Z'—The real part of impedance; Z''—The imaginary part of impedance; R1, R2, R3—Battery impedance; CPE1, CPE2—Constant phase element
表 1 各催化剂的组成
Table 1 Composition of catalysts
Catalyst Composition of the support Pt loading/wt% Pt/PCNF PCNF 20±1.5 Pt/PCNF-CB-30 PCNF∶CB=7∶3 Pt/PCNF-CB-40 PCNF∶CB=6∶4 Pt/PCNF-CB-50 PCNF∶CB=5∶5 Notes: PCNF—Porous carbon nanofibers; CB—Carbon black. 表 2 以Pt/PCNF-CB-40与Pt/C制备的膜电极(MEA)电化学性能对比
Table 2 Comparison of electrochemical properties of membrane electrode assembly (MEA) prepared with Pt/PCNF-CB-40 and Pt/C
Sample Maximum power density/(mW·cm−2) R0/(mΩ·cm−2) Rct/(mΩ·cm−2) Rmt/(mΩ·cm−2) Pt/PCNF-CB-40 599 0.934 4.078 6.565 Pt/C 502 1.815 10.632 8.005 Notes:R0 is the internal resistance of cell including the resistance of each component in cells and their interfaces; Rct is the charge transfer resistance; Rmt is the mass transport resistance. -
[1] LIU Y, YUE X, LI K, et al. PEM fuel cell electrocatalysts based on transition metal macrocyclic compounds[J]. Coordination Chemistry Reviews, 2016, 315: 153-177. DOI: 10.1016/j.ccr.2016.02.002
[2] CANO Z P, BANHAM D, YE S, et al. Batteries and fuel cells for emerging electric vehicle markets[J]. Nature Energy, 2018, 3(4): 279-289. DOI: 10.1038/s41560-018-0108-1
[3] ZHANG L, ZHANG J, WILKINSON D P, et al. Progress in preparation of non-noble electrocatalysts for PEM fuel cell reactions[J]. Journal of Power Sources, 2006, 156(2): 171-182. DOI: 10.1016/j.jpowsour.2005.05.069
[4] ANDÚJAR J M, SEGURA F. Fuel cells: History and updating. a walk along two centuries[J]. Renewable and Sustainable Energy Reviews, 2009, 13(9): 2309-2322. DOI: 10.1016/j.rser.2009.03.015
[5] YUSOFF Y N, SHAARI N. An overview on the development of nanofiber-based as polymer electrolyte membrane and electrocatalyst in fuel cell application[J]. International Journal of Energy Research, 2021, 45(13): 18441-18472. DOI: 10.1002/er.7020
[6] LIU M, ZHAO Z, DUAN X, et al. Nanoscale structure design for high-performance Pt-based ORR catalysts[J]. Advanced Materials, 2019, 31(6): 1802234. DOI: 10.1002/adma.201802234
[7] LAMIBRAC A, MARANZANA G, LOTTIN O, et al. Experimental characterization of internal currents during the start-up of a proton exchange membrane fuel cell[J]. Journal of Power Sources, 2011, 196(22): 9451-9458. DOI: 10.1016/j.jpowsour.2011.07.013
[8] PARRONDO J, HAN T, NIANGAR E, et al. Platinum supported on titanium-ruthenium oxide is a remarkably stable electrocatayst for hydrogen fuel cell vehicles[J]. Proceedings of the National Academy of Sciences, 2014, 111(1): 45-50. DOI: 10.1073/pnas.1319663111
[9] MENG Q H, HAO C, YAN B, et al. High-performance proton exchange membrane fuel cell with ultra-low loading Pt on vertically aligned carbon nanotubes as integrated catalyst layer[J]. Journal of Energy Chemistry, 2022, 71: 497-506. DOI: 10.1016/j.jechem.2022.03.018
[10] WANG X, LI Q, PAN H, et al. Size-controlled large-diameter and few-walled carbon nanotube catalysts for oxygen reduction[J]. Nanoscale, 2015, 7(47): 20290-20298. DOI: 10.1039/C5NR05864C
[11] LIU J, LIU S, YAN F, et al. Ultrathin nanotube structure for mass-efficient and durable oxygen reduction reaction catalysts in PEM fuel cells[J]. Journal of the American Chemical Society, 2022, 144(41): 19106-19114. DOI: 10.1021/jacs.2c08361
[12] HIGGINS D, ZAMANI P, YU A, et al. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress[J]. Energy & Environmental Science, 2016, 9(2): 357-390.
[13] PUMERA M. Graphene-based nanomaterials and their electrochemistry[J]. Chemical Society Reviews, 2010, 39(11): 4146. DOI: 10.1039/c002690p
[14] YARAR KAPLAN B, HAGHMORADI N, JAMIL E, et al. Platinum nanoparticles decorated carbon nanofiber hybrids as highly active electrocatalysts for polymer electrolyte membrane fuel cells[J]. International Journal of Energy Research, 2020, 44(13): 10251-10261. DOI: 10.1002/er.5646
[15] PARK J H, JU Y W, PARK S H, et al. Effects of electrospun polyacrylonitrile-based carbon nanofibers as catalyst support in PEMFC[J]. Journal of Applied Electrochemistry, 2009, 39(8): 1229-1236. DOI: 10.1007/s10800-009-9787-4
[16] WANG M, XU F, SUN H, et al. Nanoscale graphite-supported Pt catalysts for oxygen reduction reactions in fuel cells[J]. Electrochimica Acta, 2011, 56(5): 2566-2573. DOI: 10.1016/j.electacta.2010.11.019
[17] LU L, DENG H, ZHAO Z, et al. N-doped carbon nanotubes supported Pt nanowire catalysts for proton exchange membrane fuel cells[J]. Journal of Power Sources, 2022, 529: 231229. DOI: 10.1016/j.jpowsour.2022.231229
[18] KONG J, QIN Y H, WANG T L, et al. Photodeposition of Pt nanoparticles onto TiO2@CNT as high-performance electrocatalyst for oxygen reduction reaction[J]. International Journal of Hydrogen Energy, 2020, 45(3): 1991-1997. DOI: 10.1016/j.ijhydene.2019.11.016
[19] HAO C, MENG Q, YAN B, et al. Efficient catalyst layer with ultra-low Pt loading for proton exchange membrane fuel cell[J]. Chemical Engineering Journal, 2023, 472: 144945. DOI: 10.1016/j.cej.2023.144945
[20] PARK S, SHAO Y, WAN H, et al. Design of graphene sheets-supported Pt catalyst layer in PEM fuel cells[J]. Electrochemistry Communications, 2011, 13(3): 258-261. DOI: 10.1016/j.elecom.2010.12.028
[21] JI Z, CHEN J, PÉREZ-PAGE M, et al. Doped graphene/carbon black hybrid catalyst giving enhanced oxygen reduction reaction activity with high resistance to corrosion in proton exchange membrane fuel cells[J]. Journal of Energy Chemistry, 2022, 68: 143-153. DOI: 10.1016/j.jechem.2021.09.031
[22] FU K, WANG Y, MAO L, et al. Facile one-pot synthesis of graphene-porous carbon nanofibers hybrid support for Pt nanoparticles with high activity towards oxygen reduction[J]. Electrochimica Acta, 2016, 215: 427-434. DOI: 10.1016/j.electacta.2016.08.111
[23] MAO L, SHAN C, FU K, et al. Porous carbon nanofiber/carbon black composite as promising support for platinum catalyst to enhance oxygen reduction reaction in PEMFC[J]. ChemistrySelect, 2019, 4(35): 10555-10560. DOI: 10.1002/slct.201902857
[24] MAO L, FU K, JIN J, et al. PtFe alloy catalyst supported on porous carbon nanofiber with high activity and durability for oxygen reduction reaction[J]. International Journal of Hydrogen Energy, 2019, 44(33): 18083-18092. DOI: 10.1016/j.ijhydene.2019.05.058
[25] ZHOU T, ZHANG J, YANG S, et al. The oxygen reduction performance of Pt supported on the hybrid of porous carbon nanofibers and carbon black[J]. Materials, 2022, 15(13): 4560. DOI: 10.3390/ma15134560
[26] LI Y, LI Y, ZHU E, et al. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite[J]. Journal of American Chemical Society, 2012, 134(30): 12326-12329. DOI: 10.1021/ja3031449
[27] TIAN X L, WANG L, CHI B, et al. Formation of a tubular assembly by ultrathin Ti0.8Co0.2N nanosheets as efficient oxygen reduction electrocatalysts for hydrogen-/metal-air fuel cells[J]. ACS Catalysis, 2018, 8(10): 8970-8975. DOI: 10.1021/acscatal.8b02710
[28] ZHAO C, LIU J, LI B, et al. Multiscale construction of bifunctional electrocatalysts for long-lifespan rechargeable zinc-air batteries[J]. Advanced Functional Materials, 2020, 30(36): 2003619. DOI: 10.1002/adfm.202003619
[29] XIN L, YANG F, RASOULI S, et al. Understanding Pt nanoparticle anchoring on graphene supports through surface functionalization[J]. ACS Catalysis, 2016, 6(4): 2642-2653. DOI: 10.1021/acscatal.5b02722
[30] MEIER J C, GALEANO C, KATSOUNAROS I, et al. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start-stop conditions[J]. ACS Catalysis, 2012, 2(5): 832-843. DOI: 10.1021/cs300024h
[31] TAO L, QIAO M, JIN R, et al. Bridging the surface charge and catalytic activity of a defective carbon electrocatalyst[J]. Angewandte Chemie International Edition, 2019, 58(4): 1019-1024. DOI: 10.1002/anie.201810207
[32] CHENG Q, HU C, WANG G, et al. Carbon-defect-driven electroless deposition of Pt atomic clusters for highly efficient hydrogen evolution[J]. Journal of the American Chemical Society, 2020, 142(12): 5594-5601. DOI: 10.1021/jacs.9b11524
-
期刊类型引用(25)
1. 董连成,金圣豪. 损伤水泥混凝土路面非线性湿度翘曲应力分析. 公路交通科技. 2025(03): 78-86 .  百度学术
百度学术
2. 陆春华,朱学武,平安,杨钰婷. 含引气剂海工混凝土的抗冻性能及其梁受弯承载力. 硅酸盐通报. 2024(02): 418-427 .  百度学术
百度学术
3. 董连成,金圣豪,王金玉. 盐冻作用下水泥路面毛细吸水系数演化及应力状态分析. 黑龙江科技大学学报. 2024(01): 77-84 .  百度学术
百度学术
4. 王晨霞,周阳升,王高峰,刘涛,王晓云,曹芙波. 冻融环境下钢渣细骨料混凝土微观结构及损伤演化模型. 应用力学学报. 2024(03): 585-593 .  百度学术
百度学术
5. 袁立月,陈克政,彭飞,付翼博,孙剑飞,丁琳. 冻融循环作用下陶粒混凝土抗压性能研究. 黑龙江大学工程学报(中英俄文). 2023(04): 81-87 .  百度学术
百度学术
6. 张晓锋,卢浩,高渊. 冻融循环下混凝土动态力学性能试验研究. 混凝土. 2023(11): 12-16+19 .  百度学术
百度学术
7. 孙杰,孙明星,冯川,吴爽,马稳. 碳-玻璃混杂纤维改性橡胶混凝土抗冻性研究. 水利与建筑工程学报. 2022(02): 101-107+117 .  百度学术
百度学术
8. 甘磊,冯先伟,沈振中. 盐冻作用下水工混凝土强度演化模型. 水利水运工程学报. 2022(04): 131-139 .  百度学术
百度学术
9. 童彧斐,严鹏飞,张虎彪,延常玉,李宏波. 水泥粉煤灰稳定炉渣煤矸石混合料抗冻试验. 中国科技论文. 2022(10): 1078-1083 .  百度学术
百度学术
10. 蔡荨,杨妙帆,周克发. 考虑冻融作用的混凝土拱坝结构可靠度分析. 水电能源科学. 2021(04): 76-79+189 .  百度学术
百度学术
11. 张婵韬,段政彬. 可分散性乳胶粉对玻化微珠保温砂浆干湿循环下损伤影响研究. 混凝土世界. 2020(02): 63-67 .  百度学术
百度学术
12. 薛翠真,申爱琴,乔宏霞. 掺CWCPM混凝土的冻融损伤机理及演化模型. 华南理工大学学报(自然科学版). 2020(03): 136-144 .  百度学术
百度学术
13. 张卫东,董云,彭宁波,高延安. 冻融循环下透水再生混凝土力学性能损伤分析. 建筑材料学报. 2020(02): 292-296 .  百度学术
百度学术
14. 张经双,段雪雷,马冬冬. 氯盐和冻融耦合下水泥土的强度和破坏特征. 冰川冻土. 2020(02): 515-522 .  百度学术
百度学术
15. 程猛,张经双,段雪雷,朱建华. 冻融循环作用下混杂纤维粉煤灰混凝土力学性能试验. 科学技术与工程. 2020(27): 11288-11294 .  百度学术
百度学术
16. 赵小明,李奥阳,乔宏霞,李江川,王新科. 纤维混凝土抗冻性能及损伤劣化模型研究. 硅酸盐通报. 2020(10): 3196-3202 .  百度学术
百度学术
17. 张广泰,刘诗拓,耿天娇,彭瑞,陈勇. 基于Weibull分布的冻融循环下纤维混凝土损伤模型. 科学技术与工程. 2020(29): 12078-12084 .  百度学术
百度学术
18. 张兆文,周茗如,魏琴玲,谢国鑫. 基于冻融损伤下的高性能混凝土力学性能衰减规律研究. 混凝土与水泥制品. 2019(04): 16-19 .  百度学术
百度学术
19. 龙广成,杨振雄,白朝能,马昆林,谢友均. 荷载-冻融耦合作用下充填层自密实混凝土的耐久性及损伤模型. 硅酸盐学报. 2019(07): 855-864 .  百度学术
百度学术
20. 张经双,段雪雷. 冻融循环下不同龄期水泥土损伤特性和能量耗散. 硅酸盐通报. 2019(07): 2144-2151 .  百度学术
百度学术
21. 徐存东,李振,朱兴林,姚志鹏,王铭岩,张鹏. 盐冻侵蚀作用下混凝土力学性能变化研究. 人民黄河. 2019(09): 132-136 .  百度学术
百度学术
22. 姚贤华,冯忠居,王富春,管俊峰. 复合盐浸下多元外掺剂-混凝土抗干湿-冻融循环性能. 复合材料学报. 2018(03): 690-698 .  本站查看
本站查看
23. 王磊,刘欢,张旭辉. 不同介质冻融对高强混凝土的损伤及抗氯盐侵蚀性的影响. 长沙理工大学学报(自然科学版). 2018(02): 43-50 .  百度学术
百度学术
24. 张祺,裴向军,张佳兴,冉耀. 黏度时变浆液结石体冻融条件下力学特性试验研究. 工程地质学报. 2018(06): 1508-1515 .  百度学术
百度学术
25. 李英娜,张井财. 季节性冻土CFG桩复合地基冻融循环后承载力数值模拟. 低温建筑技术. 2017(11): 125-128 .  百度学术
百度学术
其他类型引用(24)
-
其他相关附件
-
目的
进一步提高Pt催化剂对氧还原反应(ORR)的催化活性和稳定性是促进质子交换膜燃料电池(PEMFC)商业化的关键。本文采用制备了直径约200 nm的多孔纳米碳纤维(PCNF),将其与炭黑(CB)混合作为Pt催化剂的复合载体制备了催化剂Pt/PCNF-CB,通过与商业Pt/C催化剂的对比,研究了Pt/PCNF-CB对ORR的催化活性与稳定性。
方法采用静电纺丝结合1000 ℃下高温碳化制备了直径约200 nm的PCNF。将PCNF与CB作为复合载体,以氯铂酸作为前驱体使用乙二醇还原法制备了催化剂Pt/PCNF-CB。利用场发射扫描电镜(SEM)研究了PCNF与CB复合载体的形貌,采用透射电子显微镜(TEM)检测了Pt纳米颗粒在纤维上的分散性与粒径大小。在旋转圆盘电极使用线性扫描伏安法(LSV)与循环伏安法(CV)完成催化剂的电化学表征,并通过加速老化测试(ADT)比较了催化剂的活性与耐久性。采用催化剂涂覆膜法制备阳极和阴极催化剂层,使用了热压机热压以获得膜电极(MEA)并使用燃料电池测试系统获得了MEA的极化曲线与功率密度曲线。膜电极(MEA)稳定性测试使用了0.6 V和0.95 V的方波电压循环,对比催化剂在5 k,10 k,30 k次循环后的最大功率密度衰减程度。
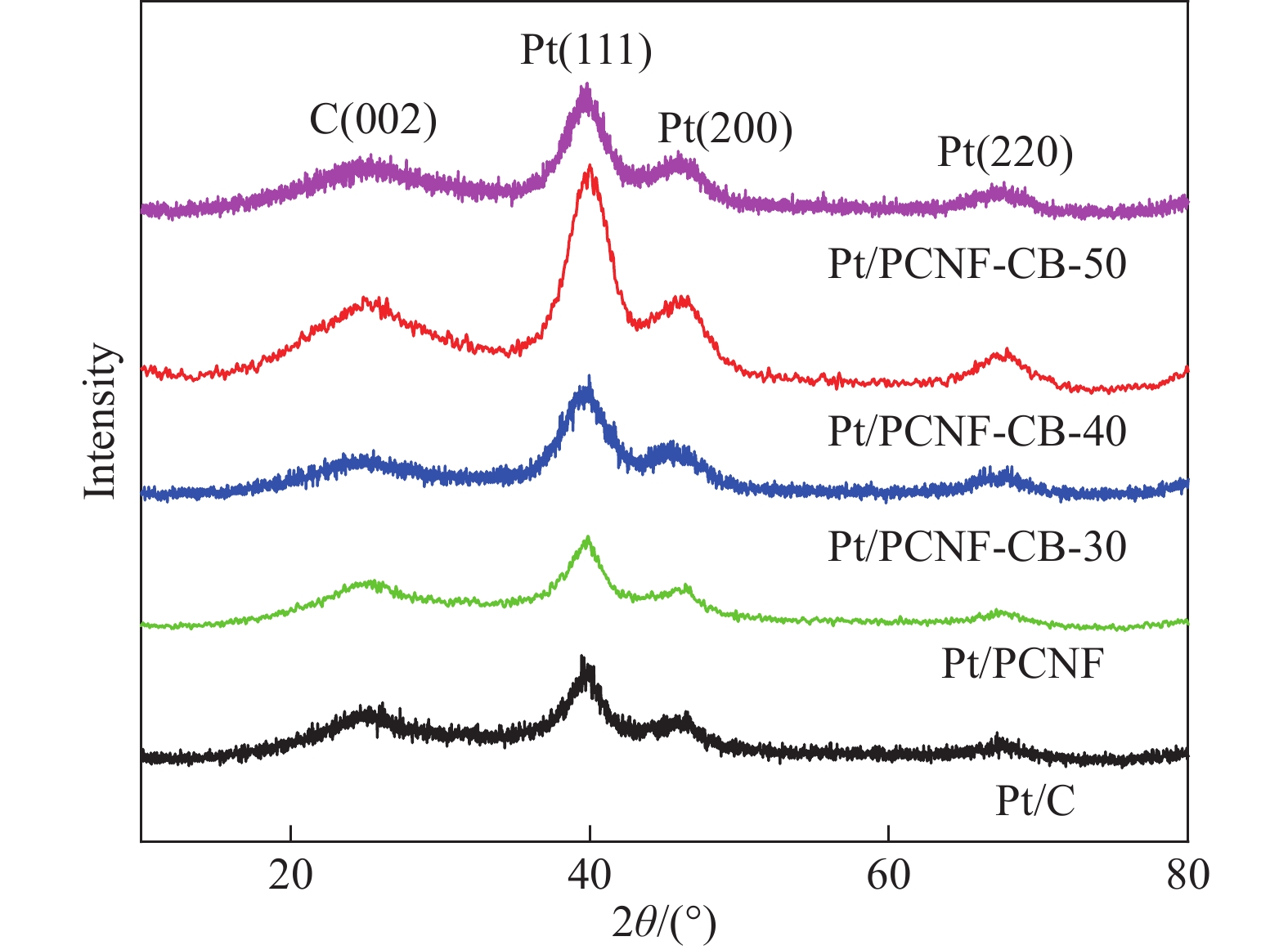
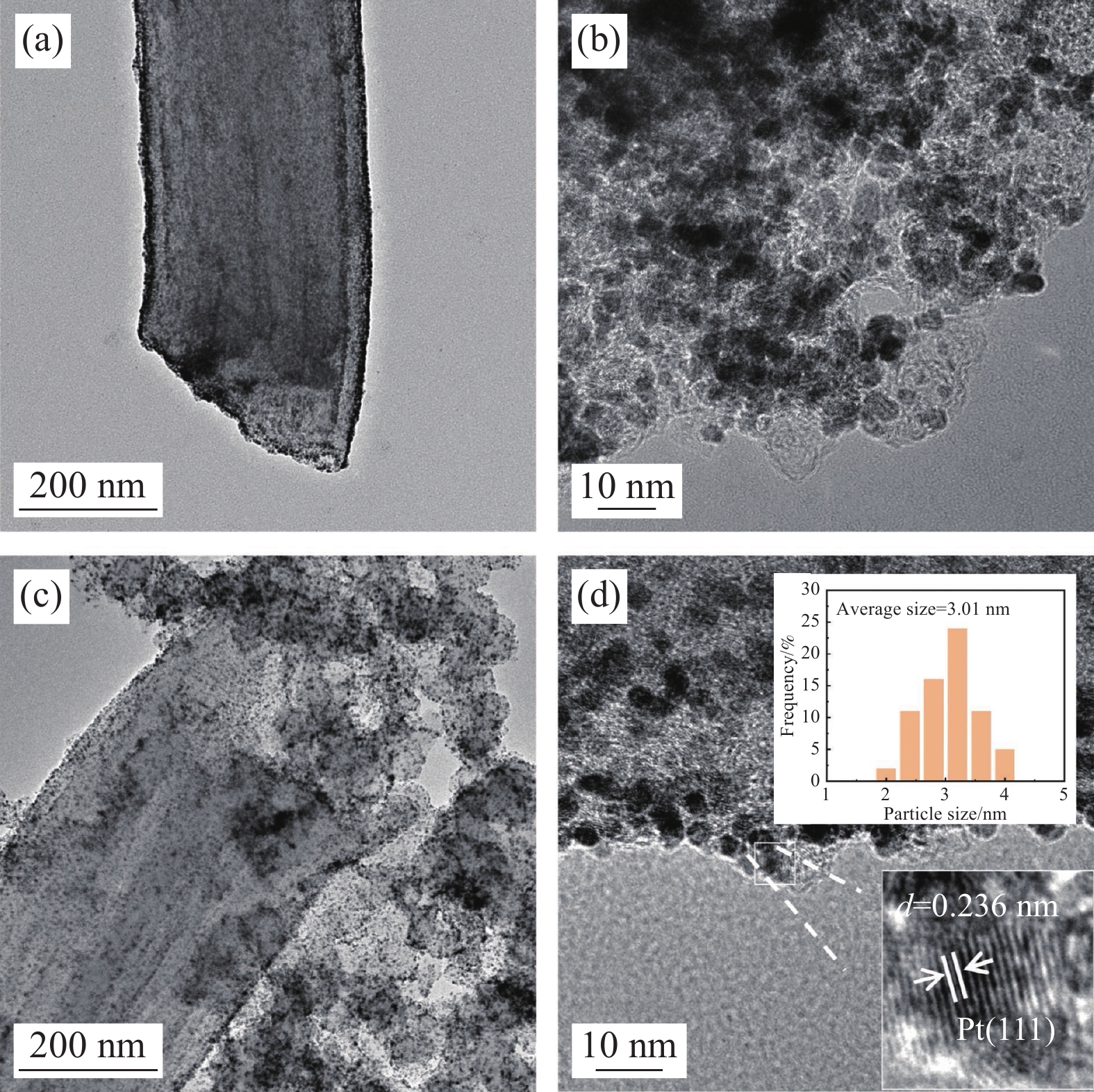
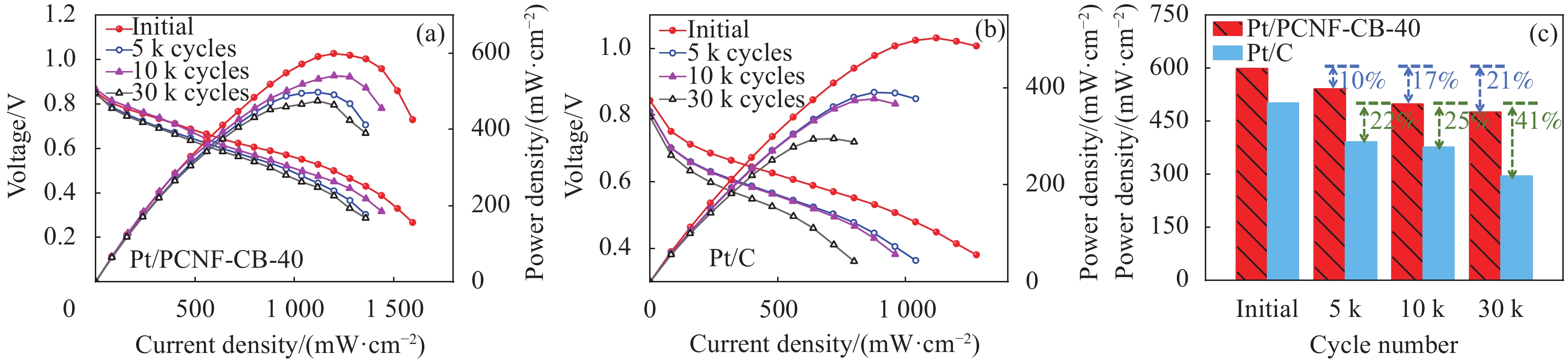
结果①当载体中CB含量40%时,PCNF和CB之间分散性更佳。根据催化剂形貌的TEM图像,可以看到Pt颗粒成功负载于复合载体上。其中负载于PCNF表面的Pt纳米粒子更加均匀,体现了多孔粗糙的PCNF表面对提高Pt颗粒分散性的积极作用。②催化剂Pt/PCNF-CB-40比商业的Pt/C具有更高的催化活性和稳定性,从得到的CV曲线计算得出Pt/PCNF-CB-40的氢脱附峰面积最大为86.3 m·g,高于商业Pt/C(71.7 m·g),而LSV曲线中Pt/PCNF-CB-40表现出更高的起始电位(0.975 V)与半波电位(0.781 V)。并且在2000次ADT后,Pt/PCNF-CB-40的性能衰减幅度小于商业Pt/C,半波电位下降了23 mV小于商业Pt/C(60 mV)。③由Pt/PCNF-CB-40组装的MEA比由Pt/C组装的MEA表现出更高的最大功率密度(599 mW·cm),较商业Pt/C催化剂提升19%,并在5 k,10 k与30 k次低电位循环后Pt/PCNF-CB-40的最大功率密度仅衰减了10%,17%以及21%,远低于商业Pt/C的22%,25%与41%的损耗。
结论Pt/PCNF-CB利用了两种载体不同的形貌特征,使PCNF与CB相互分散搭建了三维贯通的多孔结构,能提供更多三相反应界面,并减少传质阻力,以此混合载体制备的催化剂Pt/PCNF-CB-40在酸性电解液中表现出优良的ORR电催化活性,并在MEA测试中作为阴极催化剂表现了更高的输出性能。由于PCNF的粗糙多孔结构有利于Pt纳米粒子的均匀分散,在运行过程中由于复合载体中PCNF上丰富的缺陷位点与Pt粒子之间的强结合能有效抑制了Pt粒子的迁移,防止其从载体脱落与聚集提高了催化剂的耐久性,使Pt/PCNF-CB在三电极体系与燃料电池测试系统中均表现出了更佳的耐久性。PCNF与CB的协同作用提高了催化剂的活性与耐久性。
-
质子交换膜燃料电池(PEMFC)是一种通过电化学反应将化学能转换为电能的能源转换装置,具有功率密度高、结构简单、环境友好等优点。然而,PEMFC阴极氧还原反应(ORR)缓慢的动力学与高的过电位都需要依靠贵金属Pt基催化剂以加快反应,但Pt的高成本以及商业Pt/C的耐久性差制约了其商业化发展。
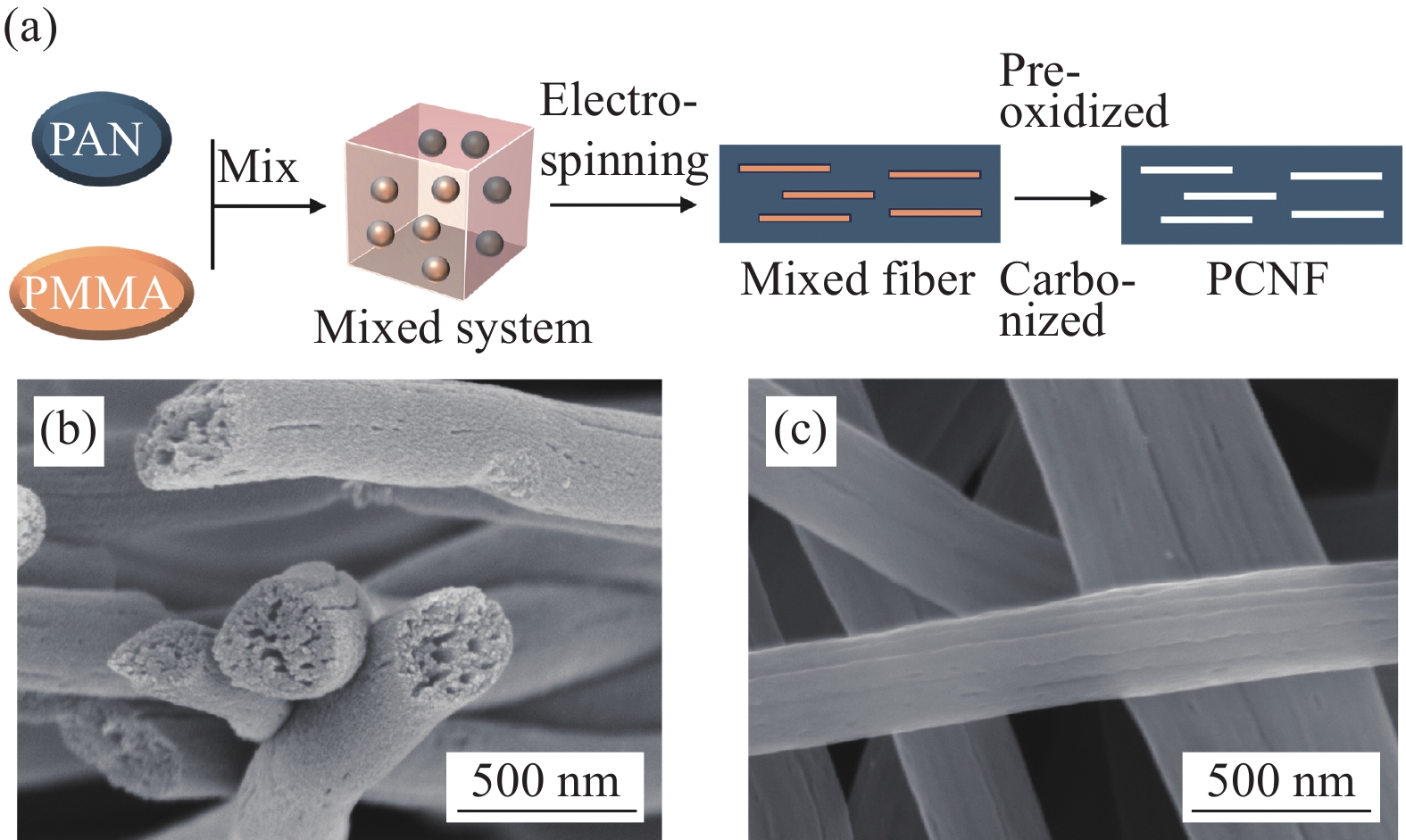
本文采用静电纺丝结合预氧化和碳化制备了多孔纳米碳纤维(PCNF),将其与炭黑(CB)混合作为复合载体负载Pt制备了催化剂Pt/PCNF-CB。PCNF的粗糙多孔结构不但有利于Pt纳米粒子的均匀分散,也增强了Pt纳米粒子与载体间的相互作用,使载体能够牢固地锚定Pt纳米粒子。此外,利用两种载体不同的形貌特征,PCNF与CB相互分散搭建了三维贯通的多孔结构,能提供更多三相反应界面,并减少传质阻力。正是两者的协同作用提高了催化剂的活性与耐久性。在低铂负载量下(阴、阳极负载量分别为0.15 mgPt·cm-2和0.1 mgPt·cm-2),由自制催化剂组装的膜电极最高输出功率密度可达599 mW·cm-2,高于商业Pt/C(502 mW·cm-2),尤其在低电位循环30 k圈后最大输出功率仅衰减21%,而相同条件下商业Pt/C衰减41%。
自制催化剂Pt/PCNF-CB-40与商业Pt/C组装的膜电极加速老化测试的极化曲线和功率密度曲线





 下载:
下载: