Research progress of PEO-based composite solid-state electrolytes for lithium batteries
-
摘要:
固态锂离子电池能量密度高、安全性强,是突破电池技术瓶颈的关键,受到了学术界和工业界的广泛关注。固态电解质是固态电池的核心,其中聚氧化乙烯(PEO)基聚合物固态电解质在改善电极界面相容性方面具有优势,是最有潜力的电解质材料之一。本文系统阐述了PEO与无机填料间的协同作用及其对复合固态电解质的离子传输性和界面相容性的影响机制。首先对PEO基复合固态电解质做出概述,并探讨离子传输相关机制,然后分别综述了PEO-惰性填料和PEO-活性填料复合固态电解质体系的设计、制备、性能及机制,最后对复合固态聚合物电解质的未来发展和优化设计做出展望。
-
关键词:
- 固态锂电池 /
- 聚氧化乙烯 /
- 复合固态聚合物电解质 /
- 离子传输机制 /
- 无机填料
Abstract:Featured with high energy density and sound safety, solid-state lithium-ion battery is the key to breaking through the bottleneck shackling battery technology, gathering wide attention from academia and industry. Solid-state electrolytes are the core of solid-state batteries, among which polyethylene oxide (PEO) polymer solid-state electrolyte excels at improving electrode interface compatibility and is one of the most potential electrolyte materials. This paper systematically elucidated the synergistic effect of PEO with inorganic modified fillers and its influence on ion transport and interfacial compatibility of composite solid-state electrolytes. Following an overview of the PEO-based composite solid-state electrolyte, the mechanism of ion transport was discussed. Then, the design, preparation, performance, and mechanism of PEO-inert filler and PEO-active filler composite solid-state electrolyte systems were reviewed respectively. Finally, an outlook on the future development and optimization design of composite solid-state polymer electrolytes was presented.
-
为缓解世界能源、气候与环境危机,我国提出了力争2030年前实现“碳达峰”、2060年前实现“碳中和”的可持续发展目标,旨在促进清洁能源转型[1]。锂离子电池(LIBs)是最主要的清洁能源载体,具有工作电压高、能量密度高、自放电少和循环性能好等优点[2-4]。然而,基于液体电解质(LEs)的传统LIBs已逐渐难以满足能源市场对能量密度和安全性越来越高的要求[5]。以石墨和过渡金属氧化物分别为阳极和阴极时,LIBs的能量密度已接近其理论极限300 W·h/kg[6]。用锂金属代替石墨可极大提高电池能量密度,但电解液和锂之间的连续界面反应会产生不稳定的固态电解质界面膜,导致LEs和锂持续消耗,进而使电池失效。另外,LEs中的高挥发性和易燃的有机溶剂,极易造成火灾和爆炸等安全问题[7]。为了解决这些问题,固态电解质(SSEs)应运而生。
与传统电解液相比,SSEs具有可燃性低、热稳定性高、无泄漏及爆炸风险低等优点[8]。此外,固态电解质具有优异的机械强度和化学中性,更易稳定界面、减少锂副反应、抑制锂枝晶生长[9],可以显著提高电池安全性、优化电化学性能。固态电解质主要分为三类:无机固态电解质(ISEs)、固态聚合物电解质(SPEs)和复合固态聚合物电解质(CSPEs)[10] ,如图1所示。ISEs在安全性、室温离子电导率(>10−3 S·cm−1)、锂离子迁移数(≈1)和阻燃性方面具有优势[11],然而其电极界面兼容性差、脆性高,严重限制了其实际应用。SPEs灵活性高、可加工性好、电极兼容性好,但也受到如下关键限制:①室温离子电导率差;②离子迁移数低;③电化学稳定性低;④机械强度和热稳定性差;⑤界面相容性差[12]。CSPEs兼具了SPEs和ISEs的优点,在灵活性、可加工性、界面相容性方面具有明显优势,具有更强的应用前景。在聚合物固态电解质中加入无机填料,制备复合固态聚合物电解质,表现出良好的电化学性能。聚氧化乙烯(PEO)可以溶解多种不同种类的锂盐[13] ,易于构建有机-无机复合固态聚合物电解质,可以同时改善机械强度、室温离子电导率、电化学稳定窗口、电极界面相容性和锂枝晶抑制能力等关键指标,显著提高电池安全性和耐久性[14]。
本文从协同效应角度综述了无机填料在PEO基复合固态电解质(CPEs)的研究进展。首先对PEO基复合固态电解质进行了概述,并深入探讨了复合固态聚合物电解质内部的离子传输机制。进而,全面综述了近年来关于PEO基复合固态电解质体系的前沿研究成果,主要聚焦于填料-聚合物和填料-锂盐的相互作用,前者主要影响聚合物的聚集态结构,可以用CPEs的结晶度(Xc)、玻璃化转变温度(Tg)和球晶形貌的变化来表征;后者则涉及到锂离子化学环境的改变,主要反映在离子电导率和离子迁移数的变化上,这些因素都对离子电导率具有一定影响。最后,本文对当前CSPEs领域的进展进行总结,针对该领域现存挑战提出见解,同时对未来发展方向做出分析与展望。
1. PEO基复合固态电解质概述
1.1 PEO基固态电解质
PEO基固态聚合物材料中的离子输运现象被发现于1973年[15],固态离子学从此突破了无机材料的限制,并逐渐成为研究重点。其中发展较成熟的是不含增塑剂的聚醚碱金属盐复合物,其离子传导依赖极性聚合物网络中的离子,PEO是这类材料的典型代表[16],是研究最早、最广泛的材料。此外,聚丙烯腈(PAN)[17]、聚甲基丙烯酸甲酯(PMMA)[18]、聚偏氟乙烯(PVDF)[19]和聚偏氟乙烯-六氟丙烯(PVDF-HFP)[20]等聚合物也被广泛研究[21]。PEO的化学式为H(OCH2CH2)nOH,具有水溶性,室温下为半结晶状态,分子量分布广泛。分子量大于
20000 的白色聚合物粉末被称为PEO,分子量小于20000 的黏性液体被称为聚乙二醇(PEG)[22]。PEO因其特殊结构而具有许多优点,如强供电子醚氧(EO)基团、软大分子主链、良好的热稳定性和力学性能等[23]。PEO基固态电解质对锂盐的解离能力强,与电极的相容性好且电化学稳定性好,但是室温下结晶度高(70%~80%)、离子电导率低(10−8~10−6 S·cm−1)等问题限制了其实际应用[13, 24-27]。近年来,在改性PEO基体结构方面取得了重大进展,但在导电性方面仍面临巨大挑战[28]。由聚合物、锂盐和无机填料组成的CSPEs不仅继承了SPEs的灵活性和可加工性的优势,还提高了SSEs的离子电导率。这是由于无机填料可以降低聚合物的Xc,促进锂盐的解离,并且能够构建新的快速离子传输通道[29]。在PEO基固态电解质中添加惰性或活性的无机填料,均能抑制PEO再结晶,增加非晶区域的比例,提高CSPEs室温离子电导率。
1.2 PEO中的离子传输机制
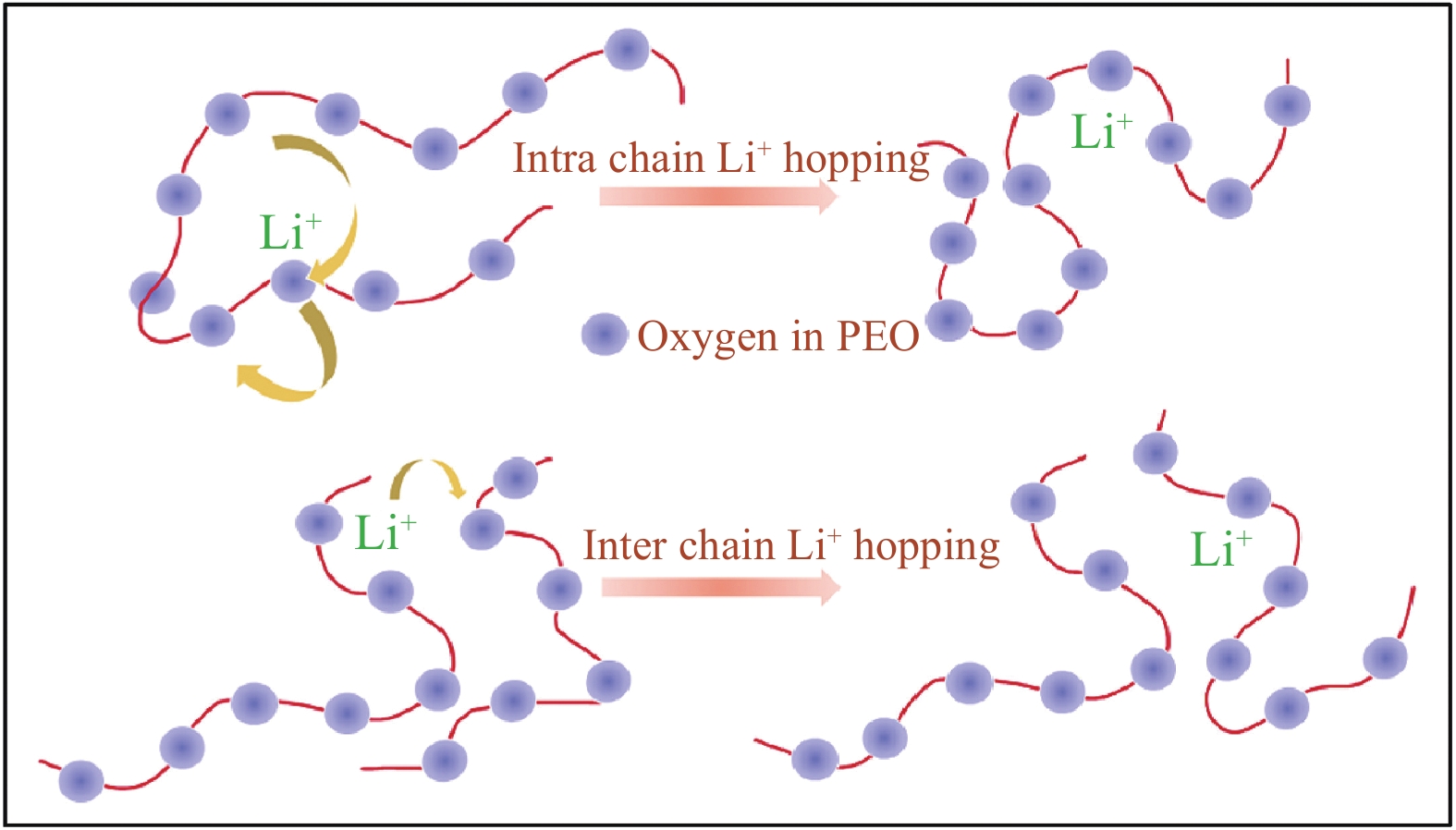
PEO基固态电解质对锂盐具有良好的溶解度,Li+输运遵循Vogel-Tammann-Fulcher (VTF)方程,PEO链段上的醚氧键(—C—O—C—)中氧原子孤对电子通过库仑作用与Li+发生配位,促使锂盐阴、阳离子解离,通过链段运动使Li—O键的Li+不断耦合/解耦[30-31],Li+在一条链上不同的配位点间及不同链的配位点间实现输运[32],基本原理如图2所示。由于分子链的运动主要发生在非晶态区域,因此Li+在PEO中的输运也主要发生于这些区域,这意味着离子电导率受温度影响较大,且结晶区离子电导率很低。因此,PEO的离子电导率主要取决于非晶态区域的比例。另一方面,Tg是节段运动开始的阈值,Tg越低,PEO室温离子电导率越高。此外,Li+与EO基团络合并在分子链上移动,EO/Li比值显著影响Li+的结合和输运速度,EO/Li过低则电解质中Li+载流子数量不足,EO/Li过高会限制Li+的浓度[23]。以上两种情况下PEO-LiX电解质的离子电导率都受到限制,一般EO/Li=12~16时的离子电导率最高[33-34]。此外,聚合物的端基也影响Li+的输运[23]。
1.3 CSPEs中的离子传输机制
CSPEs由聚合物基体、无机填料和锂盐组成,其中无机填料可以降低PEO基体的Xc和Tg,加速锂盐解离,有效提高Li+的迁移率[5]。在PEO-LiX体系中,无机填料会破坏晶体相,增加离子导电的非晶相体积分数,提升CSPEs整体离子电导率[35]。复合固态电解质的离子输运能力改善,得益于填料的以下功能:①与聚合物相互作用,降低聚合物的Tg和Xc[36-37];②与锂盐相互作用,促进Li+解离,使游离Li+数量增加[38-39];③通过特殊官能团与Li+和聚合物配位,削弱Li+-聚合物相互作用,降低Li+在聚合物链段运动的能量势垒[40];④增加Li+传输通道,包括填料-聚合物界面相和活性填料等[41]。
CSPEs中的Li+传输路径主要有以下3种:①无机填料;②聚合物基体链段运动[42];③无机填料-聚合物链界面相[43-44]。图3(a)总结了Li+的传输路径和特性[45],优先传输路径不同,离子电导率差异较大。如图3(b)所示,Zheng等[46]利用高分辨率固态核磁共振系统地研究了CSPEs中锂离子的局部结构环境和动力学,发现Li7La3Zr2O12 (LLZO)含量小于20wt%时,Li+更倾向于在PEO基体中转移,LLZO含量为50wt%时,大量Li+在LLZO中转移,表明PEO-C2F6LiNO4S2 (LiTFSI)-LLZO CSPEs中的Li+迁移路径取决于LLZO浓度。而Shi等[47]则认为Li+迁移路径是由热力学扩散(吸附能)和动力学迁移(迁移能垒)共同决定的,他们对比了SiO2和LLZO填料,提出Li+在CSPEs中吸附-传导-迁移的输运机制,指出无论是否含有锂盐,LLZO都是PEO-LLZO基SPEs中Li+的主要输运通道。离子传输通道的连续化是提高CSPEs离子电导率的有效策略,如图3(c)所示,Liu等[48]报道了纳米颗粒(NPs)、随机纳米线(NWs)和排列纳米线在离子传输行为上的差异,传输路径不连续的NPs基CSPEs的Li+电导率较低,随机NWs基CSPEs的Li+电导率有所提高,沿电极方向排列的NWs基CSPEs界面中的Li+电导率最高,主要得益于较短且没有交叉的快速离子传导路径。尽管探索CSPEs锂离子输运机制挑战很大,但相关研究至关重要。
![]() 图 3 (a) Li+在聚氧化乙烯(PEO)相中、PEO相和PEO/陶瓷界面、PEO相和陶瓷相及PEO/陶瓷界面的传导途径示意图[45];(b) 锂离子在Li7La3Zr2O12 (LLZO) (5wt%) -PEO (LiTFSI)、LLZO (20wt%) -PEO (LiTFSI) 和LLZO (50wt%) -PEO (LiTFSI)复合电解质中的路径示意图[46];(c) 纳米颗粒(NPs)、无序纳米线(NWs)和排列NWs在复合聚合物电解质中的锂离子传导途径[48];(d) 填料、聚合物和锂盐之间的路易斯酸碱相互作用示意图[36]EO—Ether oxygenFigure 3. (a) Schematic illustration of the Li+ conduction pathways in the polyoxyethylene (PEO) phase, in the PEO phase and at PEO/ceramic interface, and in the PEO phase and ceramic phase and at PEO/ceramic interface[45]; (b) Schematic of Li-ion pathways within Li7La3Zr2O12 (LLZO) (5wt%)-PEO (LiTFSI), LLZO (20wt%)-PEO (LiTFSI) and LLZO (50wt%)-PEO (LiTFSI) composite electrolytes[46]; (c) Li-ion conduction pathways in composite polymer electrolytes with nanoparticles (NPs), random nanowires (NWs) and aligned NWs[48]; (d) Illustration of Lewis acid-base interaction between fillers, polymer, and Li salt[36]
图 3 (a) Li+在聚氧化乙烯(PEO)相中、PEO相和PEO/陶瓷界面、PEO相和陶瓷相及PEO/陶瓷界面的传导途径示意图[45];(b) 锂离子在Li7La3Zr2O12 (LLZO) (5wt%) -PEO (LiTFSI)、LLZO (20wt%) -PEO (LiTFSI) 和LLZO (50wt%) -PEO (LiTFSI)复合电解质中的路径示意图[46];(c) 纳米颗粒(NPs)、无序纳米线(NWs)和排列NWs在复合聚合物电解质中的锂离子传导途径[48];(d) 填料、聚合物和锂盐之间的路易斯酸碱相互作用示意图[36]EO—Ether oxygenFigure 3. (a) Schematic illustration of the Li+ conduction pathways in the polyoxyethylene (PEO) phase, in the PEO phase and at PEO/ceramic interface, and in the PEO phase and ceramic phase and at PEO/ceramic interface[45]; (b) Schematic of Li-ion pathways within Li7La3Zr2O12 (LLZO) (5wt%)-PEO (LiTFSI), LLZO (20wt%)-PEO (LiTFSI) and LLZO (50wt%)-PEO (LiTFSI) composite electrolytes[46]; (c) Li-ion conduction pathways in composite polymer electrolytes with nanoparticles (NPs), random nanowires (NWs) and aligned NWs[48]; (d) Illustration of Lewis acid-base interaction between fillers, polymer, and Li salt[36]2. PEO与惰性填料的兼容性设计
根据是否具有传输离子的能力,无机填料可分为两类:惰性填料和活性填料[21]。惰性填料是锂离子绝缘体,本身不能传输锂离子,但是它可以提高基体力学性能、抑制PEO结晶、降低Tg[49],从而提高离子传输效率;其次其表面化学基团与锂盐阳离子/阴离子间存在较强的路易斯酸碱相互作用,可以促进锂盐的解离和离子输运[29];此外,得益于氢键相互作用、正空位-盐相互作用和偶极-偶极相互作用等路易斯酸碱相互作用,填料还可以增强PEO基CSPEs的电化学稳定性[36, 50],如图3(d)所示。近几十年来,Al2O3、SiO2、TiO2、ZrO2等[51-54]惰性填料因易于合成、粒径可控和物理化学稳定等优势,获得了广泛研究与应用[3]。
2.1 PEO-Al2O3体系
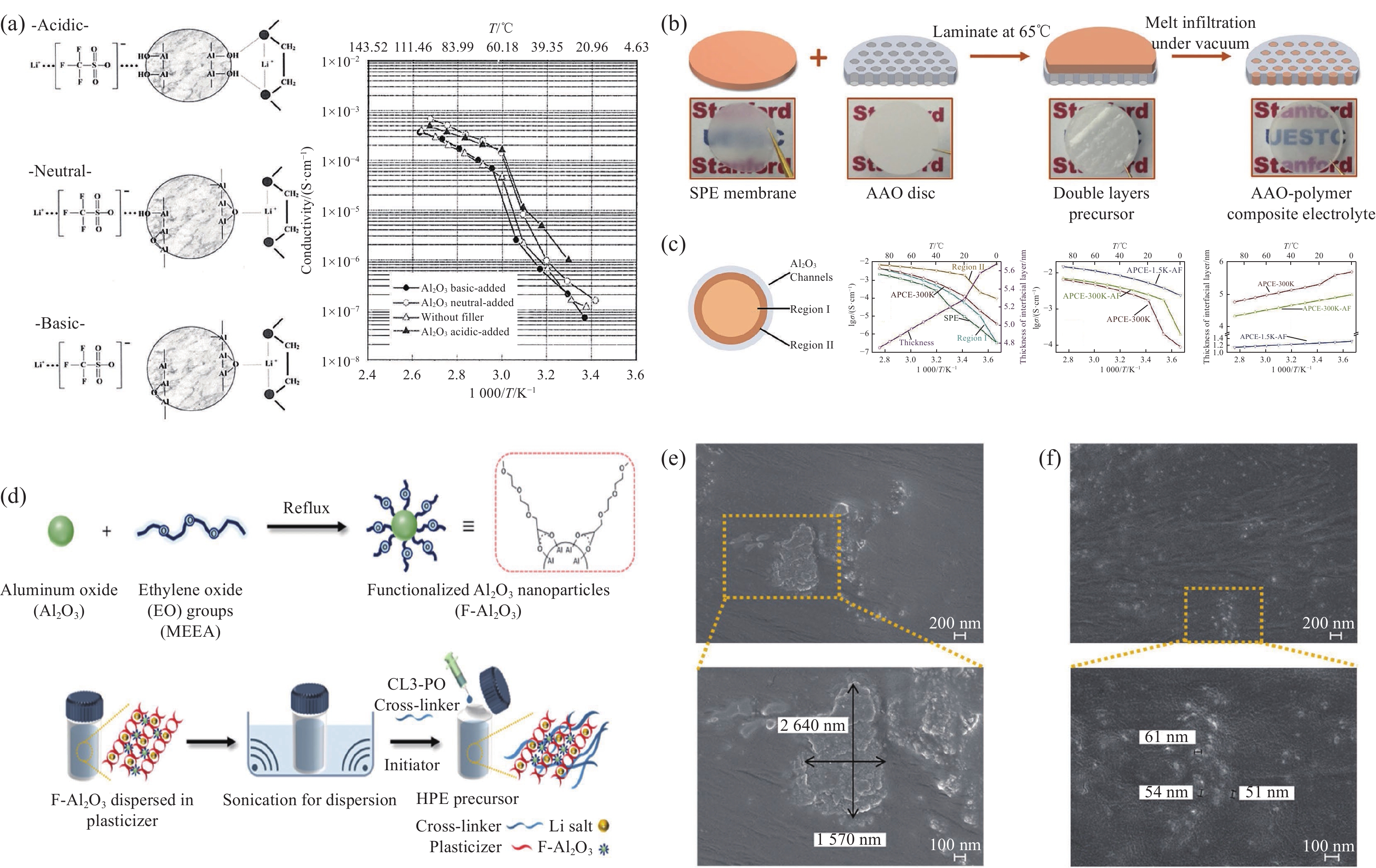
Al2O3价格低廉、热稳定性好,是最早的填料之一。1989年Wieczorek等[55]首次提出,添加Al2O3可以使PEO基SPEs离子电导率提高一个数量级以上。如图4(a)所示,2001年Croce等[56]研究了3种不同Al2O3颗粒在PEO-LiSO3CF3基CSPEs中增强离子电导率的机制,发现了Al2O3与PEO间的3种不同表面相互作用,其中酸性表面Al2O3的—OH基团通过氢键与锂盐阴离子和PEO的相互作用,因此离子电导率最高。随后,Park等[57]设计了3种不同酸碱性Al2O3作为PEO-LiClO4的填料,发现Al2O3与ClO4−之间没有相互作用,表明表面化学性质对表面相互作用至关重要。通过设计填料/聚合物理想界面,可以优化Li+的快速传输路径。如图4(b)所示,Zhang等[58]以阳极氧化铝(AAO)多孔模板为陶瓷骨架、PEO为离子传输介质,在复合固态电解质中构筑了高密度、垂直连续规则排布的异质界面阵列,Li+可沿连续的陶瓷/聚合物界面在电极间快速传输,显著提高离子电导率,如图4(c)所示。如图4(d)所示,Bae等[59]合成了具有EO基团的表面功能化的氧化铝(F-Al2O3),EO基团可以使F-Al2O3纳米颗粒在PEO基SPEs中均匀分散,提高了离子电导率和电化学稳定性,如图4(e)和图4(f)所示。通过改变陶瓷相的表面化学性质、聚合物相的组成或特定的界面面积,可有效提升CSPEs的离子电导率。此外,通过优化陶瓷相的几何结构和聚合物-锂盐-陶瓷组分中的材料选择,有望将其离子电导率提高到10−3 S·cm−1以上,适用于工业化应用。
![]() 图 4 (a) 3种不同类型Al2O3与PEO表面相互作用和Al2O3-PEO复合固态聚合物电解质(CSPEs)的电导率图[56];(b) 阳极氧化铝(AAO)-聚合物复合电解质制备工艺示意图[58];(c) AAO圆盘中单个纳米通道中聚合物电解质示意图、各组分的离子电导率、界面离子电导率及AAO-聚合物复合电解质(APCE)界面层厚度[58];(d) 功能化Al2O3 (F-Al2O3)的合成及其制备杂化聚合物电解质(HPE)[59];分散在硅片上PEO薄膜中颗粒的SEM图像: (e) Al2O3;(f) F-Al2O3[59]T—Temperature; APCE-1.5K-AF—It was prepared by infiltrating polymer electrolyte based on poly(ethylene glycol) (MW 1500) and LiTFSI into AlF3 modified AAO; APCE-300K-AF—The AAO-polymer composite electrolyte with AlF3 coated AAO discs and PEO (MW 300 000); APCE-300K—The AAO-polymer composite electrolyte without AlF3 coating AAO discs and PEO (MW 300 000); MW—Molecular weight; CL3-linker—Cross-linker; HPE—Hybrid polymer electrolyteFigure 4. (a) Surface interactions between three diferent type Al2O3 and PEO and conductivity plots of Al2O3-PEO composite solid polymer electrolyte (CSPEs)[56]; (b) Schematics of fabrication procedures of anodized aluminum oxide (AAO)-polymer composite electrolyte[58]; (c) Schematics of polymer electrolyte in individual nanochannel of the AAO disc, the ionic conductivities of each component, the interface ion conductivities, and the thickness of interfacial layer of AAO-polymer composite electrolyte (APCE), respectively[58]; (d) Synthesis of functionalized Al2O3 (F-Al2O3) and preparation of hybrid polymer electrolyte (HPE) using F-Al2O3[59]; SEM images of the particles dispersed in PEO film on a Si wafer: (e) Al2O3; (f) F-Al2O3[59]
图 4 (a) 3种不同类型Al2O3与PEO表面相互作用和Al2O3-PEO复合固态聚合物电解质(CSPEs)的电导率图[56];(b) 阳极氧化铝(AAO)-聚合物复合电解质制备工艺示意图[58];(c) AAO圆盘中单个纳米通道中聚合物电解质示意图、各组分的离子电导率、界面离子电导率及AAO-聚合物复合电解质(APCE)界面层厚度[58];(d) 功能化Al2O3 (F-Al2O3)的合成及其制备杂化聚合物电解质(HPE)[59];分散在硅片上PEO薄膜中颗粒的SEM图像: (e) Al2O3;(f) F-Al2O3[59]T—Temperature; APCE-1.5K-AF—It was prepared by infiltrating polymer electrolyte based on poly(ethylene glycol) (MW 1500) and LiTFSI into AlF3 modified AAO; APCE-300K-AF—The AAO-polymer composite electrolyte with AlF3 coated AAO discs and PEO (MW 300 000); APCE-300K—The AAO-polymer composite electrolyte without AlF3 coating AAO discs and PEO (MW 300 000); MW—Molecular weight; CL3-linker—Cross-linker; HPE—Hybrid polymer electrolyteFigure 4. (a) Surface interactions between three diferent type Al2O3 and PEO and conductivity plots of Al2O3-PEO composite solid polymer electrolyte (CSPEs)[56]; (b) Schematics of fabrication procedures of anodized aluminum oxide (AAO)-polymer composite electrolyte[58]; (c) Schematics of polymer electrolyte in individual nanochannel of the AAO disc, the ionic conductivities of each component, the interface ion conductivities, and the thickness of interfacial layer of AAO-polymer composite electrolyte (APCE), respectively[58]; (d) Synthesis of functionalized Al2O3 (F-Al2O3) and preparation of hybrid polymer electrolyte (HPE) using F-Al2O3[59]; SEM images of the particles dispersed in PEO film on a Si wafer: (e) Al2O3; (f) F-Al2O3[59]2.2 PEO-SiO2体系
SiO2熔点高、硬度高、稳定性好,是一种理想的惰性填料,纳米SiO2在大规模工业化生产方面具有巨大优势。如图5(a)所示,Lin等[60]在PEO溶液中原位水解正硅酸四乙酯(TEOS),制备了PEO-单分散超细SiO2(MUSiO2)复合固态聚合物电解质,原位水解在SiO2和PEO链之间构建了化学键合和机械包裹等强相互作用机制,极大降低Xc,提高离子电导率。如图5(b)所示,Yu等[61]通过Li2SO4修饰静电纺丝和煅烧工艺,成功开发了均匀Li+掺杂的分层介孔SiO2纳米纤维网络结构,提高了SiO2与PEO间的润湿性,促进了路易斯酸对阴离子的吸附作用,显著提高了Li+电导率。为避免因添加大量锂盐导致的锂金属-锂盐不良反应,Wang等[53]调整EO∶Li 至30∶1,基于TEOS碱性水解反应,制备了一种氢键增强的CSPEs (图5(c)),SiO2表面的羟基与C、N、F形成氢键,实现了PEO、LiTFSI和琥珀腈形成互联网络,降低了PEO结晶度,改善了Li+传输。与传统的PEO基CSPEs相比,CSPEs的各组分通过氢键连接、化学键合等方法可增强PEO与无机填料之间的相互作用,提高Lewis酸碱相互作用的有效表面积,从而降低PEO的结晶度,提高锂盐的解离度,实现高离子电导率。
![]() 图 5 (a) PEO链与单分散超细SiO2(MUSiO2)的原位水解过程及相互作用机制示意图[60];(b) SiO2-Li2SO4-PEO CSPEs的制备工艺示意图及由Li2SO4衍生的SiO2纤维CSPEs实现Li+快速传导的原理图[61];(c) 原位水解制备CSPEs的示意图[53];(d) 不同成分聚合物电解质的离子电导率[64];(e) PEO-LiTFSI电解质和PEO-LiTFSI-3wt%TiO2@PDA复合电解质的DSC曲线[64];(f) CPEs中缺氧TiO2表面相互作用示意图[65];TiO2样品的XPS光谱(g)和Ti2p的高分辨率光谱(h)[66]TEOS—Tetraethyl orthosilicate; PVA—Poly(vinyl alcohol); LiTFSI—Lithium bis(trifluoromethanesulfonyl)imide; DC—Direct current; PDA—Polydopamine; Tg—Glass transition temperature; Tm—Crystalline melting temperatureFigure 5. (a) Schematic figures showing the procedure of in situ hydrolysis and interaction mechanisms among PEO chains and monodisperse ultrafine SiO2 (MUSiO2)[60]; (b) Schematic diagram of preparation process of SiO2/Li2SO4/PEO CSPEs and schematics of fast Li+ conduction enabled by the Li2SO4-derived SiO2 fibers CSPEs [61]; (c) Schematic diagram of CPSEs prepared by in situ hydrolysis[53]; (d) Ionic conductivities of the polymer electrolytes with different compositions[64]; (e) DSC curves of the PEO-LiTFSI electrolyte and PEO-LiTFSI-3wt%TiO2@PDA composite electrolyte[64]; (f) Schematic illustrations of surface interaction of oxygen-deficient TiO2 in CPEs[65]; XPS survey spectra of the TiO2 sample (g) and high-resolution spectra of the Ti2p (h)[66]
图 5 (a) PEO链与单分散超细SiO2(MUSiO2)的原位水解过程及相互作用机制示意图[60];(b) SiO2-Li2SO4-PEO CSPEs的制备工艺示意图及由Li2SO4衍生的SiO2纤维CSPEs实现Li+快速传导的原理图[61];(c) 原位水解制备CSPEs的示意图[53];(d) 不同成分聚合物电解质的离子电导率[64];(e) PEO-LiTFSI电解质和PEO-LiTFSI-3wt%TiO2@PDA复合电解质的DSC曲线[64];(f) CPEs中缺氧TiO2表面相互作用示意图[65];TiO2样品的XPS光谱(g)和Ti2p的高分辨率光谱(h)[66]TEOS—Tetraethyl orthosilicate; PVA—Poly(vinyl alcohol); LiTFSI—Lithium bis(trifluoromethanesulfonyl)imide; DC—Direct current; PDA—Polydopamine; Tg—Glass transition temperature; Tm—Crystalline melting temperatureFigure 5. (a) Schematic figures showing the procedure of in situ hydrolysis and interaction mechanisms among PEO chains and monodisperse ultrafine SiO2 (MUSiO2)[60]; (b) Schematic diagram of preparation process of SiO2/Li2SO4/PEO CSPEs and schematics of fast Li+ conduction enabled by the Li2SO4-derived SiO2 fibers CSPEs [61]; (c) Schematic diagram of CPSEs prepared by in situ hydrolysis[53]; (d) Ionic conductivities of the polymer electrolytes with different compositions[64]; (e) DSC curves of the PEO-LiTFSI electrolyte and PEO-LiTFSI-3wt%TiO2@PDA composite electrolyte[64]; (f) Schematic illustrations of surface interaction of oxygen-deficient TiO2 in CPEs[65]; XPS survey spectra of the TiO2 sample (g) and high-resolution spectra of the Ti2p (h)[66]2.3 PEO-TiO2体系
TiO2具有高介电常数(ε>180)和较强的路易斯酸碱作用,能与锂盐阴离子和PEO醚氧原子反应,增加非晶态区域,促进锂盐解离。此外,TiO2易于制备成高体积比的一维纳米结构材料,扩大填料/PEO接触面积,促进固态电解质的离子传导,是制备CSPEs的理想材料[62-63]。Zhao等[64]将聚多巴胺(PDA)涂在TiO2纳米纤维表面,制备出了PEO-LiTFSI-TiO2@PDA复合固态电解质,降低了PEO结晶度,利用Lewis酸碱相互作用改善LiTFSI的解离,提高载流子的浓度,改善离子电导率,如图5(d)、图5(e)所示。如图5(f)所示,Luo等[65]制备出富含氧空位的一维TiO2,增加了PEO非晶相,其长径比为强阴离子吸附提供了长程且连续的相互作用表面,缓解离子聚集和电位梯度,改善了界面稳定性。Li等[66]制备了表面改性的Ti3+掺杂TiO2纳米线,Ti3+掺杂TiO2纳米线中大量可电离的氧空位作为路易斯酸位点,与阴离子强烈相互作用并释放更多Li+,表面支链和交联网络结构有效抑制了PEO结晶,增强PEO链迁移率,为Li+提供更多传输途径,如图5(g)、图5(h)所示。为解决纳米颗粒填料团聚削弱填料-锂盐路易斯酸碱相互作用的问题[67-68],Chen等[69]采用PEO前驱体简单固化策略,制备了一种原位三维CSPEs,3D-TiO2的高孔隙率增大了PEO体积比,高表面积提供了更多的路易斯酸碱相互作用,从而大大提高离子电导率。与零维纳米颗粒和二维纳米片填料相比,一维结构填料因其长径比,可以提供更长、更连续的界面和Li+输运通道,提高离子电导率。而相较于一维填料,原位制备三维CSPEs,不仅可以保证良好的润湿性、紧凑的阴极||电解质界面接触,还可以建立良好的离子运输途径。
2.4 PEO-ZrO2体系
ZrO2具有良好的化学稳定性、热稳定性和Lewis酸性,能够吸引锂盐中的阴离子,促进锂盐解离,释放出更多Li+[70]。Croce等[71]将酸处理纳米ZrO2粉末与PEO、LiBF4混合,ZrO2与阴离子和PEO链间特定的路易斯酸碱相互作用提高了离子输运性能,Li+迁移数从0.42增加到0.68。Dam等[72]利用宽带介电光谱研究了PEO-三氟甲磺酸锂(LiCF3SO3)-纳米ZrO2复合固态电解质在宽频率和宽温度范围内的导电机制,证实了加入纳米ZrO2后聚合物-盐的络合和复合,电导率弛豫时间及链段弛豫时间以下的VTF方程表明离子传导过程与聚合物链段弛豫过程有很强的相关性。Mohd-Yasin等[73]发现锂盐、增塑剂和纳米ZrO2的加入使PEO的熔融温度、玻璃化转变温度和结晶度不断降低,这主要由非晶相体积分数增加引起,PEO-纳米ZrO2之间存在较强相互作用,CSPEs中明确的多边形边界表明无定形化增加,热稳定性和电导率获得增强。为了全面了解CSPEs中的离子传输活性,以便更好地理解导电机制,重点关注离子-离子和离子-聚合物相互作用、自由离子和无机填料相互作用、聚合物弛豫过程和玻璃化转变现象等过程是至关重要的。
综上所述,惰性填料通常能有效地增强聚合物链的非晶态程度,从而促进聚合物链段的运动,但离子的导电性仍与可移动Li+的数量密切相关。而活性填料作为离子导电无机物,不仅保留了惰性填料的一般功能,还可以通过形成完全渗透的填料相和建立填料-聚合物界面相的锂离子输运途径参与离子传导过程,从而进一步提高离子电导率。
3. PEO与活性填料的兼容性设计
活性填料是本身含有Li+且具备离子传输能力的无机陶瓷材料,也称为快离子导体,可直接用作锂离子电池固态电解质,通常展现出较高的离子电导率,主要原因包括:①内部连续缺陷结构提供低活化能的Li+跳跃通道;②释放大量Li+,提升活性填料/聚合物界面处的自由Li+浓度,提高离子电导率。这些快离子导体除了固有的高Li+电导率外,还具备无电解质泄漏、电化学/热稳定性高及不易燃等优点,能够有效解决电池安全问题。常见的活性填料种类涵盖石榴石型(如Li7La3Zr2O12,LLZO)、NASICON型(如LixAlxTi2-x(PO4)3,LATP;Li1+xAlxGe2-x(PO4)3,LAGP等)、钙钛矿型(如Li0.33La0.56TiO3)、硫化物型等[74-77]。它们不仅可以抑制PEO结晶,还能形成Li+传输通道[78]。随着锂电池研究的深入,将活性填料与柔性PEO基体结合,构建新型CSPEs成为了近年来的研究热点。
3.1 PEO-石榴石型体系
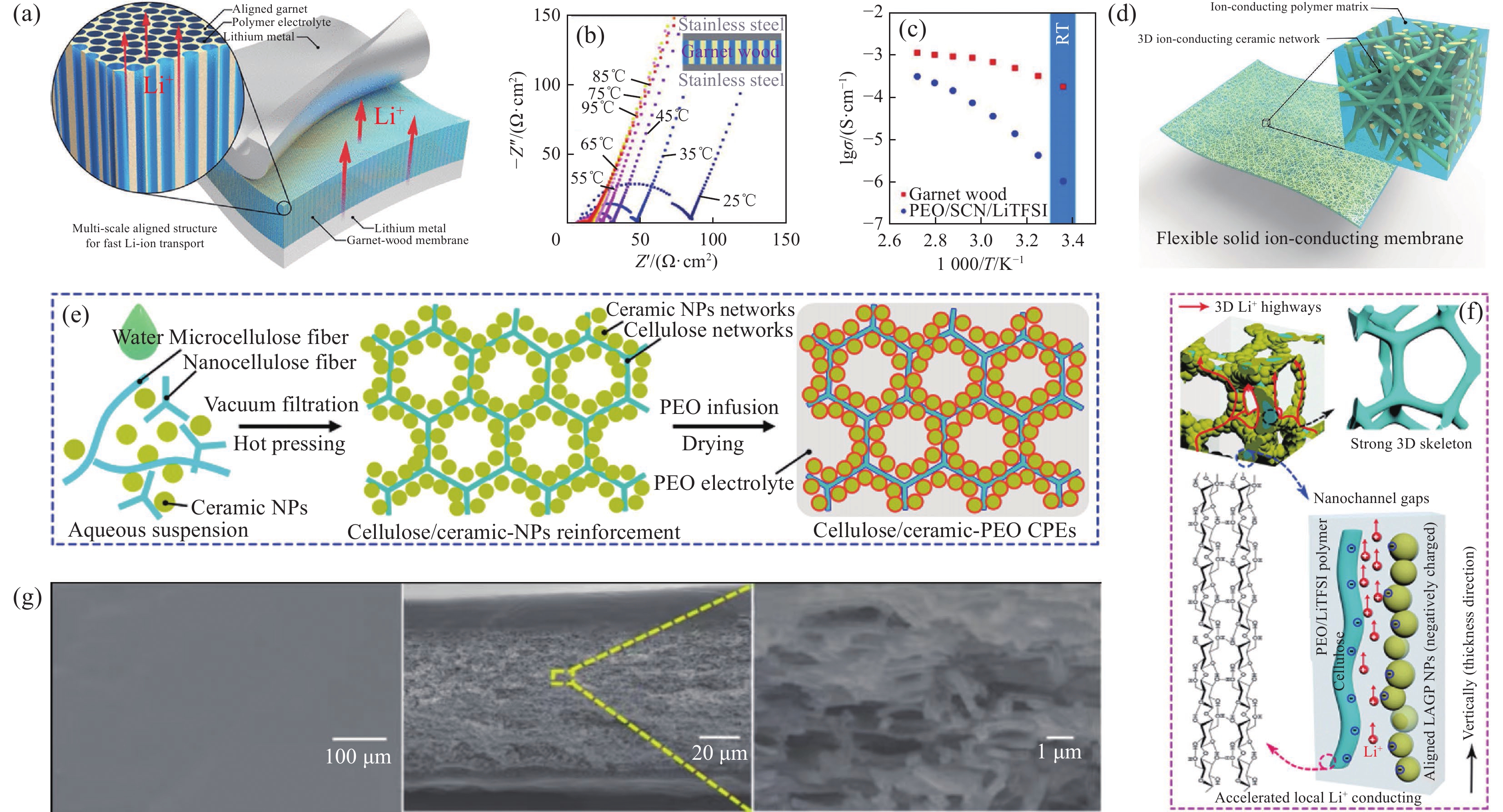
石榴石型陶瓷电解质一般结构为A3B2(XO4)3 (A 为 Ca、Mg、Y或La;B为Al、Fe、Ga、Ge、Mn、Ni或V;X为Si、Ge或Al)[21]。其中,Li7La3Zr2O12 (LLZO)及其衍生物离子电导率高达10−4 S·cm−1,活化能低至0.31 eV,电化学稳定窗口超过5 V (vs. Li /Li+),使它们在固态锂电池领域具有巨大应用潜力[79]。如图6(a)所示,Dai等[80]制备了基于PEO-Li6.4La3Zr2Al0.2O12 (LLZO)的柔性CPEs,利用圆柱形微通道木模板技术构建垂直排列的LLZO纳米结构,并与柔韧的PEO基体相互整合,改变了PEO链的动力学特性,优化Li+输运,有效提升了离子电导率,如图6(b)、图6(c)所示。对填料表面进行聚合物改性或官能团接枝,能够显著改善填料-聚合物基体相互作用和界面相容性[74]。Liu等[81]借助硅烷偶联剂,在Li6.4La3Zr1.4Ta0.6O12 (LLZTO)颗粒表面接枝锂单离子聚合物(LiSTFSI),制备出PEO-Li@LLZTO材料,陶瓷颗粒表面锂单离子聚合物层既可以抑制阴离子与Li+间的离子配对,调节表面电荷分布,提高LLZTO-PEO界面相容性,抑制锂枝晶生长。如图6(d)所示,Fu等[82]采用静电纺丝及高温退火工艺,构建了3D-LLZO纳米纤维网络,进而制备由PEO和3D-LLZO纳米线组成的PEO-LLZO膜,随机分布的三维互联LLZO纳米线形成了连续的Li+传导网络,避免了纳米颗粒团聚,增强了力学性能。创建具有相互连接的远程离子传输的连续纳米级网络和控制最小/无填料团聚是设计高离子导电复合固态聚合物电解质的主要方向。
![]() 图 6 (a) 锂对称电池中多尺度排列的介孔石榴石Li6.4La3Zr2Al0.2O12 (LLZO)膜与聚合物电解质结合示意图[80];(b) 石榴石-木膜阻抗随温度升高而下降的Nyquist图(插图为测试单元的结构)[80];(c) 石榴石木和PEO聚合物电解质在不同温度下的离子电导率对比示意图[80];(d) 以陶瓷石榴石纳米纤维为增强层、锂离子导电聚合物为基体的复合固态电解质示意图[82];(e) 纤维素衍生的CPEs的制备工艺示意图[87];(f) 纤维素/陶瓷增强CPEs中Li+导电机制示意图[87];(g) PEO|PEO-钙钛矿|PEO复合电解质的表面SEM图像、横截面SEM图像及横截面形貌详细视图[89]–Z''—Imaginary part of the impedance; Z'—Real part of the impedance; σ—Ionic conductivity; SCN—Succinonitrile; RT—Room temperature; NP—Nanoparticle; LAGP— Li1.5Al0.5Ge1.5(PO4)3Figure 6. (a) Schematic of multi-scale aligned mesoporous garnet Li6.4La3Zr2Al0.2O12 (LLZO) membrane incorporated with polymer electrolyte in a lithium symmetric cell[80]; (b) Nyquist plot showing the decrease in the impedance of the garnet-wood membrane with increasing temperature (The inset schematic shows the structure of the testing cell)[80]; (c) Comparison of the ionic conductivity of the garnet-wood and PEO based polymer electrolyte at different temperatures[80]; (d) Schematic of the composite solid electrolyte, where ceramic garnet nanofibers function as the reinforcement and lithium-ion conducting polymer functions as the matrix[82]; (e) Schematic illustration of fabrication procedures for the cellulose derived CPEs[87]; (f) Schematic of proposed Li+ conducting mechanism in the cellulose/ceramic reinforced CPEs[87]; (g) SEM image of the surface, cross-sectional SEM image and detailed view of the cross-section morphology of the PEO|PEO-perovskite|PEO composite electrolyte[89]
图 6 (a) 锂对称电池中多尺度排列的介孔石榴石Li6.4La3Zr2Al0.2O12 (LLZO)膜与聚合物电解质结合示意图[80];(b) 石榴石-木膜阻抗随温度升高而下降的Nyquist图(插图为测试单元的结构)[80];(c) 石榴石木和PEO聚合物电解质在不同温度下的离子电导率对比示意图[80];(d) 以陶瓷石榴石纳米纤维为增强层、锂离子导电聚合物为基体的复合固态电解质示意图[82];(e) 纤维素衍生的CPEs的制备工艺示意图[87];(f) 纤维素/陶瓷增强CPEs中Li+导电机制示意图[87];(g) PEO|PEO-钙钛矿|PEO复合电解质的表面SEM图像、横截面SEM图像及横截面形貌详细视图[89]–Z''—Imaginary part of the impedance; Z'—Real part of the impedance; σ—Ionic conductivity; SCN—Succinonitrile; RT—Room temperature; NP—Nanoparticle; LAGP— Li1.5Al0.5Ge1.5(PO4)3Figure 6. (a) Schematic of multi-scale aligned mesoporous garnet Li6.4La3Zr2Al0.2O12 (LLZO) membrane incorporated with polymer electrolyte in a lithium symmetric cell[80]; (b) Nyquist plot showing the decrease in the impedance of the garnet-wood membrane with increasing temperature (The inset schematic shows the structure of the testing cell)[80]; (c) Comparison of the ionic conductivity of the garnet-wood and PEO based polymer electrolyte at different temperatures[80]; (d) Schematic of the composite solid electrolyte, where ceramic garnet nanofibers function as the reinforcement and lithium-ion conducting polymer functions as the matrix[82]; (e) Schematic illustration of fabrication procedures for the cellulose derived CPEs[87]; (f) Schematic of proposed Li+ conducting mechanism in the cellulose/ceramic reinforced CPEs[87]; (g) SEM image of the surface, cross-sectional SEM image and detailed view of the cross-section morphology of the PEO|PEO-perovskite|PEO composite electrolyte[89]3.2 PEO-NASICON型体系
NASICON型快锂离子导体的通式为LiT2(PO4)3,其中T为Ti、Ge、Zr等元素,采用Al、Cr、Ga等三价离子取代部分T,可生成结构类似的Li1+xMxTi2−x(PO4)3,其中Li1+xAlxTi2−x(PO4)3 (LATP)在室温下表现出高离子电导率(10−4至10−3 S·cm−1)、高化学稳定性、高氧化电压(~6 V)及低成本,受到广泛关注[83-84]。Zhao等[85]研究了LATP粒径对PEO-LATP-LiTFSI电解质的影响,发现过小的LATP颗粒易团聚,减少快速离子迁移通道,过大的LATP颗粒与PEO基体接触不足,限制Li+传输。Wang等[86]利用模板法构建三维互联多孔LATP框架,多孔LATP骨架与 PEO形成渗透网络,提供了一个长程连续的Li+传输通道,力学强度好,在60℃时实现高达7.47×10−4 S·cm−1的离子电导率。如图6(e)所示,Wang等[87]以天然纤维素和Li+导电陶瓷纳米颗粒为基础,成功开发了具有3D纤维素-陶瓷网络结构的CSPEs,相互连接的聚合物-填料界面提供了连续的3D Li+传输路径,有序排列的LAGP纳米颗粒限制了更多的PEO链,促进了Lewis酸碱相互作用,进而吸附TFSI−并释放Li+,如图6(f)所示。填料是否具有Li+的传导能力,是影响CSPEs整体性能的重要因素。相较于惰性填料,活性填料可以降低渗透阈值,其内部的Li+可以被聚合物有效吸附,从而增加移动电荷载流子的浓度。
3.3 PEO-钙钛矿型体系
钙钛矿型固态电解质材料(Li3xLa2/3−xTiO3)具有卓越的性能[29],其中x=0.1的LLTO尤为突出,其室温离子电导率高达1×10−3 S·cm−1,与正极间存在良好的界面稳定性[88]。然而,类似于LATP,LLTO中的Ti4+易被金属锂还原,不能接触锂负极(Ti4++Li→Ti3++Li+)。为解决这一问题,Liu等[89]构筑了PEO|PEO-钙钛矿|PEO三明治结构(图6(g)),使LLTO纳米纤维框架避免了钙钛矿与锂金属的不良反应,LLTO纳米纤维网络提供了快速连续的Li+传输通道,显著增强了PEO-LLTO界面的离子传导速率。Zhu等[90]采用静电纺丝技术制备出高长径比LLTO纳米纤维,它们提供了连续离子传输路径,扩大了LLTO-PEO导电接触面积,限制了PEO再结晶,从而大幅度提升了CPEs的整体离子电导性和综合性能。Teng等[91]制备出基于一维Li0.33La0.557TiO3纳米管(LLTO NTs)的LLTO-PEO-LiTFSI CSEs膜,在三维网状LLTO NTs内部和PEO/LLTO NTs界面构筑了连续高效的Li+传输路径,显著提高了Li+电导率。Wu等[37]通过向PEO中引入两种商用的Li+绝缘氧化物萤石型Gd0.1Ce0.9O1.95(GDC)和钙钛矿型La0.8Sr0.2Ga0.8-Mg0.2O2.55(LSGM)制备CSPEs,复合聚合物电解质中GDC/LSGM的表面氧空位与TFSI−间的强相互作用改变了Li+在两个局部环境中的分布,可有效提高Li+电导率。通过精密调控无机填料的形态以顺应渗透机制,可以在CSPEs中创建长距离的Li+导电路径。此外,添加与SPEs具有强相互作用的无机填料来改变局部Li+环境以激活聚合物中更多可移动的Li+,是提高CSPEs的Li+电导率及促进聚合物内Li+高效输运的有效策略。
3.4 PEO-硫化物型体系
硫化物电解质是将氧化物电解质中的氧原子替换为硫原子,以形成更大的离子传导通道,硫化物超锂离子导体以Li10GeP2S12 (LGPS)和Thio-LISICON型(如Li3.25Ge0.25P0.75S4等)为代表,其中LGPS因其独特的三维框架结构和极高的Li+电导性能而备受瞩目,室温离子电导率高达1.2×10−2 S·cm−1 [92]。Li等[93]发现PEO-LGPS复合材料老化过程中Li+的配位作用可能引发LGPS电子态和化学键的演变,导致LGPS结构“Li+耗尽区”中的PS4单元聚合,原因可能是PEO高亲核性及其对Li+良好的溶解性,有利于增强LGPS中的Li+与PEO链有效配位结合。Zhao等[94]向PEO基体中引入LGPS,并用差示扫描量热法深入分析相变行为,发现LGPS的路易斯酸性会与Li+的路易斯酸性竞争,与PEO链形成配合物,从而降低聚合物链的Tg,改善与电极间的界面接触。受到H键和Li键相似性的启发,Pan等[95]采用(3-氯丙基)三甲氧基硅烷(CTMS)原位偶联反应,制备出均匀的柔性CPEs,揭示了LGPS无机相与有机基体(如PEO/PEG)间的化学键对提升离子电导率和Li+迁移数的关键作用。建立化学键是优化CSPEs与电极间的界面相容性的一种新策略,通过化学结合相互作用,有利于无机填料均匀分散到聚合物基体中,从而形成具有高导电性和电化学稳定性的柔性CSPEs。
与惰性填料不同,活性填料易于提供更多的Li+传输途径,因此,离子电导率不受聚合物链的节段运动限制。然而,在使用锂金属阳极时,许多活性填料存在化学不稳定性,并且在环境空气中不稳定。虽然将活性填料与聚合物基体复合可以在一定程度上减少这一问题,但通过掺杂、包覆或表面改性等方法来提高活性填料的性能仍然是至关重要的。特别是硫化物填料的超高离子电导率具有广阔的应用前景,但在CSPEs中提高硫化物的空气稳定性对其商业化至关重要。因此,设计有前景的锂导电陶瓷和硫化物填料将是下一代CSPEs的关键方向。
4. 总结与展望
综上所述,本文系统探讨了聚氧化乙烯基固态电解质的基础特性,并深入解析了聚氧化乙烯在复合固态聚合物电解质中的离子传输机制。核心部分详尽综述了聚氧化乙烯与惰性/活性填料间的协同效应机制及其对复合固态聚合物电解质电化学性能的影响,相关研究进展如表1 所示。将惰性填料添加到聚氧化乙烯基体中,可以增加聚氧化乙烯的非晶相,降低玻璃化转变温度,此外,通过其表面化学基团与锂盐阳离子/阴离子间较强的路易斯酸碱相互作用,可以促进锂盐解离和离子输运,增强复合固态聚合物电解质的电化学稳定性;而活性填料不仅保留了惰性填料的一般功能,还可以在活性填料区域和填料-聚合物界面构建额外高效的Li+输运通道参与离子传导过程,提高离子电导率。尽管复合固态聚合物电解质展现出巨大的性能优势和应用潜力,但仍面临多项亟待解决的关键挑战:
表 1 聚氧化乙烯(PEO)基复合固态电解质文献报道汇总Table 1. Summary of literature reports on polyethylene oxide (PEO)-based composite solid electrolytePolymer matrix Lithium salt Filler Ionic conductivity/(S·cm−1) ESW (vs. Li/Li+)/V tLi+ Ref. PEO LiTFSI Al2O3 5.82×10−4 (RT) — — [58] PEO LiTFSI Al2O3 9.6×10–4 (25℃) 5 0.81 [96] PEO LiTFSI SiO2 1.8×10–4 (30℃) 5.3 0.42 [53] PEO LiClO4 SiO2 1.2×10–3 (60℃) >5.5 — [97] PEO LiClO4 SiO2 1.1×10–4 (30℃) >4.8 — [98] PEO LiClO4 TiO2 1×10–5 (30℃) 5 0.3 [99] PEO LiTFSI TiO2@PDA 4.36×10–4 (55℃) 5 0.19 [64] PEO LiTFSI Ti3+-TiO2 1×10–4 (30℃) 5.5 0.36 [66] PEO LiBF4 ZrO2 4.4×10–4 (80℃) — 0.68 [71] PEO LiCF3SO3 ZrO2 1.38×10–4 (RT) — — [73] PEO LiTFSI LLZTO 1.9×10–4 (40℃) 5.1 0.67 [100] PEO LiTFSI LLZTO 3.03×10–4 (55℃) 4.5 0.117 [101] PEO LiTFSI LLZTO 5.6×10–4 (60℃) 4.75 0.46 [102] PEO LiTFSI LLZO 5.5×10–4 (30℃) >5.7 0.21 [103] PEO LiTFSI LLZO 2.39×10–4 (25℃) >5.5 — [104] PEO LiTFSI LATP 4×10–4 (60℃) 4.7 — [105] PEO LiTFSI LATP 3.61×10–4 (60℃) 4.8 — [76] PEO LiTFSI LAGP 6.76×10–4 (60℃) 5.3 0.378 [106] PEO LiTFSI LAGP 1.6×10–5 (20℃) — — [107] PEO LiTFSI LLTO 2.04×10–4 (25℃) 4.7 — [108] PEO LiTFSI LLTO 2.3×10–4 (RT) 4.5 — [109] PEO LiTFSI LLTO 1.8×10–4 (RT) 4.5 0.33 [110] PEO LiTFSI LGPS 1.21×10–3 (80℃) 5.7 0.26 [94] PEG-PEO LiTFSI LGPS 9.83×10–4 (RT) — 0.68 [95] Notes: ESW—Electrochemical stability window; tLi+—Lithium-ion transference number; RT—Room temperature; PDA—Polydopamine; LLZTO—Li6.4La3Zr1.4Ta0.6O12/Li6.75La3Zr1.75Ta0.25O12; LLZO—Li7La3Zr2O12; LATP—Li1.4Al0.4Ti1.6(PO4)3; LAGP—Li1.5Al0.5Ge1.5(PO4)3; LLTO—Li0.33La0.557TiO3; LGPS—Li10GeP2S12; PEG—Polyethylene glycol. (1)目前室温下复合固态聚合物电解质的离子电导率仅为10−4 S·cm−1左右,无法满足实际应用所需的10−3 S·cm−1标准。这一问题可通过以下3种策略进行改善:①优化电解质体相以诱导均匀的Li+沉积,构建高效且均一的离子输运网络;②采用具有三维框架结构、纳米线或纳米片形态的无机填料提升离子迁移速度;③增加填料的表面缺陷改变聚合物/锂盐的相互作用、Li+的局部环境及Li+传输的活化能;
(2)复合固态聚合物电解质中离子传输的具体机制尚未阐明,这直接阻碍了离子电导率水平的进一步提升。将理论计算与先进表征技术相结合,特别是原位测试技术,通过研究Li+在复合固态聚合物电解质内的局部结构环境和动力学行为,验证实验结果并阐明离子传导增强机制有望解决这一难题;
(3)复合固态聚合物电解质与电极间的界面问题仍是限制固态电解质性能提升的关键难题之一。强化复合固态聚合物电解质与电极间的有效接触、改善锂亲和力及提高力学性能是改善界面相容性和抑制枝晶生长的有效途径。此外,利用填料原位生成Li+-导电界面层作为电极-电解质界面相,能有效降低界面阻抗;
(4)复合固态聚合物电解质电化学稳定性和兼容性有待进一步提升,固态电解质在与电极接触时易分解,其电化学稳定性窗口决定了能否匹配高压电极材料。基于路易斯酸碱相互作用等思路,调整聚合物最高占据分子轨道(Highest occupied molecular orbital,HOMO能级),可以增强界面稳定性,Al2O3等特定无机填料与丁二腈等小分子增塑剂可能成为改善上述性能的理想选择;
(5)无机填料-聚合物的相互作用有待深入研究。惰性填料(如Al2O3、SiO2、TiO2等)和活性填料(如Li6.4La3Zr1.4Ta0.6O12、Li7La3Zr2O12、Li1.4Al0.4Ti1.6(PO4)3等)种类对复合固态聚合物电解质离子电导率和锂离子迁移数的影响仍缺乏系统研究,明确这些相关机制虽充满挑战,但对优选适用于复合固态聚合物电解质的无机填料具有重大意义。
尽管存在上述挑战,但复合固态聚合物电解质仍被视为提升锂电池性能和安全性的最重要发展方向。先进的表征技术和模拟计算方法显著推动了这一领域的进步。未来,系统地优化各组分相互作用将是推动复合固态聚合物电解质实用化进程的核心环节。随着研究不断深入,复合固态聚合物电解质有望实现应用,这对突破电池能量密度和安全性瓶颈具有极为重要的意义。
-
图 3 (a) Li+在聚氧化乙烯(PEO)相中、PEO相和PEO/陶瓷界面、PEO相和陶瓷相及PEO/陶瓷界面的传导途径示意图[45];(b) 锂离子在Li7La3Zr2O12 (LLZO) (5wt%) -PEO (LiTFSI)、LLZO (20wt%) -PEO (LiTFSI) 和LLZO (50wt%) -PEO (LiTFSI)复合电解质中的路径示意图[46];(c) 纳米颗粒(NPs)、无序纳米线(NWs)和排列NWs在复合聚合物电解质中的锂离子传导途径[48];(d) 填料、聚合物和锂盐之间的路易斯酸碱相互作用示意图[36]
EO—Ether oxygen
Figure 3. (a) Schematic illustration of the Li+ conduction pathways in the polyoxyethylene (PEO) phase, in the PEO phase and at PEO/ceramic interface, and in the PEO phase and ceramic phase and at PEO/ceramic interface[45]; (b) Schematic of Li-ion pathways within Li7La3Zr2O12 (LLZO) (5wt%)-PEO (LiTFSI), LLZO (20wt%)-PEO (LiTFSI) and LLZO (50wt%)-PEO (LiTFSI) composite electrolytes[46]; (c) Li-ion conduction pathways in composite polymer electrolytes with nanoparticles (NPs), random nanowires (NWs) and aligned NWs[48]; (d) Illustration of Lewis acid-base interaction between fillers, polymer, and Li salt[36]
图 4 (a) 3种不同类型Al2O3与PEO表面相互作用和Al2O3-PEO复合固态聚合物电解质(CSPEs)的电导率图[56];(b) 阳极氧化铝(AAO)-聚合物复合电解质制备工艺示意图[58];(c) AAO圆盘中单个纳米通道中聚合物电解质示意图、各组分的离子电导率、界面离子电导率及AAO-聚合物复合电解质(APCE)界面层厚度[58];(d) 功能化Al2O3 (F-Al2O3)的合成及其制备杂化聚合物电解质(HPE)[59];分散在硅片上PEO薄膜中颗粒的SEM图像: (e) Al2O3;(f) F-Al2O3[59]
T—Temperature; APCE-1.5K-AF—It was prepared by infiltrating polymer electrolyte based on poly(ethylene glycol) (MW 1500) and LiTFSI into AlF3 modified AAO; APCE-300K-AF—The AAO-polymer composite electrolyte with AlF3 coated AAO discs and PEO (MW 300 000); APCE-300K—The AAO-polymer composite electrolyte without AlF3 coating AAO discs and PEO (MW 300 000); MW—Molecular weight; CL3-linker—Cross-linker; HPE—Hybrid polymer electrolyte
Figure 4. (a) Surface interactions between three diferent type Al2O3 and PEO and conductivity plots of Al2O3-PEO composite solid polymer electrolyte (CSPEs)[56]; (b) Schematics of fabrication procedures of anodized aluminum oxide (AAO)-polymer composite electrolyte[58]; (c) Schematics of polymer electrolyte in individual nanochannel of the AAO disc, the ionic conductivities of each component, the interface ion conductivities, and the thickness of interfacial layer of AAO-polymer composite electrolyte (APCE), respectively[58]; (d) Synthesis of functionalized Al2O3 (F-Al2O3) and preparation of hybrid polymer electrolyte (HPE) using F-Al2O3[59]; SEM images of the particles dispersed in PEO film on a Si wafer: (e) Al2O3; (f) F-Al2O3[59]
图 5 (a) PEO链与单分散超细SiO2(MUSiO2)的原位水解过程及相互作用机制示意图[60];(b) SiO2-Li2SO4-PEO CSPEs的制备工艺示意图及由Li2SO4衍生的SiO2纤维CSPEs实现Li+快速传导的原理图[61];(c) 原位水解制备CSPEs的示意图[53];(d) 不同成分聚合物电解质的离子电导率[64];(e) PEO-LiTFSI电解质和PEO-LiTFSI-3wt%TiO2@PDA复合电解质的DSC曲线[64];(f) CPEs中缺氧TiO2表面相互作用示意图[65];TiO2样品的XPS光谱(g)和Ti2p的高分辨率光谱(h)[66]
TEOS—Tetraethyl orthosilicate; PVA—Poly(vinyl alcohol); LiTFSI—Lithium bis(trifluoromethanesulfonyl)imide; DC—Direct current; PDA—Polydopamine; Tg—Glass transition temperature; Tm—Crystalline melting temperature
Figure 5. (a) Schematic figures showing the procedure of in situ hydrolysis and interaction mechanisms among PEO chains and monodisperse ultrafine SiO2 (MUSiO2)[60]; (b) Schematic diagram of preparation process of SiO2/Li2SO4/PEO CSPEs and schematics of fast Li+ conduction enabled by the Li2SO4-derived SiO2 fibers CSPEs [61]; (c) Schematic diagram of CPSEs prepared by in situ hydrolysis[53]; (d) Ionic conductivities of the polymer electrolytes with different compositions[64]; (e) DSC curves of the PEO-LiTFSI electrolyte and PEO-LiTFSI-3wt%TiO2@PDA composite electrolyte[64]; (f) Schematic illustrations of surface interaction of oxygen-deficient TiO2 in CPEs[65]; XPS survey spectra of the TiO2 sample (g) and high-resolution spectra of the Ti2p (h)[66]
图 6 (a) 锂对称电池中多尺度排列的介孔石榴石Li6.4La3Zr2Al0.2O12 (LLZO)膜与聚合物电解质结合示意图[80];(b) 石榴石-木膜阻抗随温度升高而下降的Nyquist图(插图为测试单元的结构)[80];(c) 石榴石木和PEO聚合物电解质在不同温度下的离子电导率对比示意图[80];(d) 以陶瓷石榴石纳米纤维为增强层、锂离子导电聚合物为基体的复合固态电解质示意图[82];(e) 纤维素衍生的CPEs的制备工艺示意图[87];(f) 纤维素/陶瓷增强CPEs中Li+导电机制示意图[87];(g) PEO|PEO-钙钛矿|PEO复合电解质的表面SEM图像、横截面SEM图像及横截面形貌详细视图[89]
–Z''—Imaginary part of the impedance; Z'—Real part of the impedance; σ—Ionic conductivity; SCN—Succinonitrile; RT—Room temperature; NP—Nanoparticle; LAGP— Li1.5Al0.5Ge1.5(PO4)3
Figure 6. (a) Schematic of multi-scale aligned mesoporous garnet Li6.4La3Zr2Al0.2O12 (LLZO) membrane incorporated with polymer electrolyte in a lithium symmetric cell[80]; (b) Nyquist plot showing the decrease in the impedance of the garnet-wood membrane with increasing temperature (The inset schematic shows the structure of the testing cell)[80]; (c) Comparison of the ionic conductivity of the garnet-wood and PEO based polymer electrolyte at different temperatures[80]; (d) Schematic of the composite solid electrolyte, where ceramic garnet nanofibers function as the reinforcement and lithium-ion conducting polymer functions as the matrix[82]; (e) Schematic illustration of fabrication procedures for the cellulose derived CPEs[87]; (f) Schematic of proposed Li+ conducting mechanism in the cellulose/ceramic reinforced CPEs[87]; (g) SEM image of the surface, cross-sectional SEM image and detailed view of the cross-section morphology of the PEO|PEO-perovskite|PEO composite electrolyte[89]
表 1 聚氧化乙烯(PEO)基复合固态电解质文献报道汇总
Table 1 Summary of literature reports on polyethylene oxide (PEO)-based composite solid electrolyte
Polymer matrix Lithium salt Filler Ionic conductivity/(S·cm−1) ESW (vs. Li/Li+)/V tLi+ Ref. PEO LiTFSI Al2O3 5.82×10−4 (RT) — — [58] PEO LiTFSI Al2O3 9.6×10–4 (25℃) 5 0.81 [96] PEO LiTFSI SiO2 1.8×10–4 (30℃) 5.3 0.42 [53] PEO LiClO4 SiO2 1.2×10–3 (60℃) >5.5 — [97] PEO LiClO4 SiO2 1.1×10–4 (30℃) >4.8 — [98] PEO LiClO4 TiO2 1×10–5 (30℃) 5 0.3 [99] PEO LiTFSI TiO2@PDA 4.36×10–4 (55℃) 5 0.19 [64] PEO LiTFSI Ti3+-TiO2 1×10–4 (30℃) 5.5 0.36 [66] PEO LiBF4 ZrO2 4.4×10–4 (80℃) — 0.68 [71] PEO LiCF3SO3 ZrO2 1.38×10–4 (RT) — — [73] PEO LiTFSI LLZTO 1.9×10–4 (40℃) 5.1 0.67 [100] PEO LiTFSI LLZTO 3.03×10–4 (55℃) 4.5 0.117 [101] PEO LiTFSI LLZTO 5.6×10–4 (60℃) 4.75 0.46 [102] PEO LiTFSI LLZO 5.5×10–4 (30℃) >5.7 0.21 [103] PEO LiTFSI LLZO 2.39×10–4 (25℃) >5.5 — [104] PEO LiTFSI LATP 4×10–4 (60℃) 4.7 — [105] PEO LiTFSI LATP 3.61×10–4 (60℃) 4.8 — [76] PEO LiTFSI LAGP 6.76×10–4 (60℃) 5.3 0.378 [106] PEO LiTFSI LAGP 1.6×10–5 (20℃) — — [107] PEO LiTFSI LLTO 2.04×10–4 (25℃) 4.7 — [108] PEO LiTFSI LLTO 2.3×10–4 (RT) 4.5 — [109] PEO LiTFSI LLTO 1.8×10–4 (RT) 4.5 0.33 [110] PEO LiTFSI LGPS 1.21×10–3 (80℃) 5.7 0.26 [94] PEG-PEO LiTFSI LGPS 9.83×10–4 (RT) — 0.68 [95] Notes: ESW—Electrochemical stability window; tLi+—Lithium-ion transference number; RT—Room temperature; PDA—Polydopamine; LLZTO—Li6.4La3Zr1.4Ta0.6O12/Li6.75La3Zr1.75Ta0.25O12; LLZO—Li7La3Zr2O12; LATP—Li1.4Al0.4Ti1.6(PO4)3; LAGP—Li1.5Al0.5Ge1.5(PO4)3; LLTO—Li0.33La0.557TiO3; LGPS—Li10GeP2S12; PEG—Polyethylene glycol. -
[1] JIN B. Research on performance evaluation of green supply chain of automobile enterprises under the background of carbon peak and carbon neutralization[J]. Energy Reports, 2021, 7: 594-604. DOI: 10.1016/j.egyr.2021.10.002
[2] HU Y, XIE X, LI W, et al. Recent progress of polymer electrolytes for solid-state lithium batteries[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(4): 1253-1277.
[3] YANG X, LIU J, PEI N, et al. The critical role of fillers in composite polymer electrolytes for lithium battery[J]. Nano-Micro Letters, 2023, 15(74): 1-37. DOI: 10.1007/s40820-023-01051-3
[4] CHEN X, LI X, LUO L, et al. Practical application of all-solid-state lithium batteries based on high-voltage cathodes: Challenges and progress[J]. Advanced Energy Materials, 2023, 13(35): 2301230. DOI: 10.1002/aenm.202301230
[5] XIAO Z, LONG T, SONG L, et al. Research progress of polymer-inorganic filler solid composite electrolyte for lithium-ion batteries[J]. Ionics, 2021, 28: 15-26.
[6] CHEN S, DAI F, CAI M. Opportunities and challenges of high-energy lithium metal batteries for electric vehicle applications[J]. ACS Energy Letters, 2020, 5(10): 3140-3151. DOI: 10.1021/acsenergylett.0c01545
[7] XI G, XIAO M, WANG S, et al. Polymer-based solid electrolytes: Material selection, design, and application[J]. Advanced Functional Materials, 2021, 31(9): 2007598. DOI: 10.1002/adfm.202007598
[8] TANG S, GUO W, FU Y. Advances in composite polymer electrolytes for lithium batteries and beyond[J]. Advanced Energy Materials, 2021, 11(2): 2000802. DOI: 10.1002/aenm.202000802
[9] YANG Y, ZHOU H, XIE J, et al. Organic fast ion-conductor with ordered Li-ion conductive nano-pathways and high ionic conductivity for electrochemical energy storage[J]. Journal of Energy Chemistry, 2022, 66: 647-656. DOI: 10.1016/j.jechem.2021.09.011
[10] NGUYEN A G, PARK C J. Insights into tailoring composite solid polymer electrolytes for solid-state lithium batteries[J]. Journal of Membrane Science, 2023, 675: 121552. DOI: 10.1016/j.memsci.2023.121552
[11] FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18(12): 1278-1291. DOI: 10.1038/s41563-019-0431-3
[12] FAN P, LIU H, MAROSZ V, et al. High performance composite polymer electrolytes for lithium-ion batteries[J]. Advanced Functional Materials, 2021, 31(23): 2101380. DOI: 10.1002/adfm.202101380
[13] HOU W, OU Y, LIU K. Progress on high voltage PEO-based polymer solid electrolytes in lithium batteries[J]. Chemical Research in Chinese Universities, 2022, 38(3): 735-743. DOI: 10.1007/s40242-022-2065-2
[14] SUN J, LIU C, LIU H, et al. Advances in ordered architecture design of composite solid electrolytes for solid-state lithium batteries[J]. The Chemical Record, 2023, 23(6): e202300044. DOI: 10.1002/tcr.202300044
[15] MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 1-16.
[16] MEYER W H. Polymer electrolytes for lithium-ion batteries[J]. Advanced Materials, 1998, 10(6): 439-448. DOI: 10.1002/(SICI)1521-4095(199804)10:6<439::AID-ADMA439>3.0.CO;2-I
[17] LIU X, BI Z, WAN Y, et al. Composition regulation of polyacrylonitrile-based polymer electrolytes enabling dual-interfacially stable solid-state lithium batteries[J]. Journal of Colloid and Interface Science, 2024, 665: 582-591. DOI: 10.1016/j.jcis.2024.03.166
[18] ZHANG W, KOVERGA V, LIU S, et al. Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries[J]. Nature Energy, 2024, 9(4): 386-400.
[19] WU Y, LI Y, WANG Y, et al. Advances and prospects of PVDF based polymer electrolytes[J]. Journal of Energy Chemistry, 2022, 64: 62-84. DOI: 10.1016/j.jechem.2021.04.007
[20] LI Z, ZHANG S, JIANG Z, et al. Deep eutectic solvent-immobilized PVDF-HFP eutectogel as solid electrolyte for safe lithium metal battery[J]. Materials Chemistry and Physics, 2021, 267: 124701. DOI: 10.1016/j.matchemphys.2021.124701
[21] SU X, XU X P, JI Z Q, et al. Polyethylene oxide-based composite solid electrolytes for lithium batteries: Current progress, low-temperature and high-voltage limitations, and prospects[J]. Electrochemical Energy Reviews, 2024, 7(2): 1-38. DOI: 10.1007/s41918-023-00204-7
[22] ZHANG D, LI L, WU X, et al. Research progress and application of PEO-based solid state polymer composite electrolytes[J]. Frontiers in Energy Research, 2021, 9: 726738. DOI: 10.3389/fenrg.2021.726738
[23] FENG J, WANG L, CHEN Y, et al. PEO based polymer-ceramic hybrid solid electrolytes: A review[J]. Nano Convergence, 2021, 8: 1-12. DOI: 10.1186/s40580-020-00251-6
[24] 周伟东, 黄秋, 谢晓新, 等. 固态锂电池聚合物电解质研究进展[J]. 储能科学与技术, 2022, 11(6): 1788-1805. ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, et al. Research progress of polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805(in Chinese).
[25] SZCZĘSNA-CHRZAN A, MARCZEWSKI M, SYZDEK J, et al. Lithium polymer electrolytes for novel batteries application: The review perspective[J]. Applied Physics A, 2022, 129(37): 1-20.
[26] WANG H, SHENG L, YASIN G, et al. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2020, 33: 188-215. DOI: 10.1016/j.ensm.2020.08.014
[27] DING Z, LI J, LI J, et al. Review-interfaces: Key issue to be solved for all solid-state lithium battery technologies[J]. Journal of the Electrochemical Society, 2020, 167(7): 070541. DOI: 10.1149/1945-7111/ab7f84
[28] ZHENG Y, LI X, LI C Y. A novel de-coupling solid polymer electrolyte via semi-interpenetrating network for lithium metal battery[J]. Energy Storage Materials, 2020, 29: 42-51. DOI: 10.1016/j.ensm.2020.04.002
[29] LIU S, LIU W, BA D, et al. Filler-integrated composite polymer electrolyte for solid-state lithium batteries[J]. Advanced Materials, 2022, 35(2): 2110423.
[30] ROLLO-WALKER G, MALIC N, WANG X, et al. Development and progression of polymer electrolytes for batteries: Influence of structure and chemistry[J]. Polymers, 2021, 13(23): 4127. DOI: 10.3390/polym13234127
[31] YUE L, MA J, ZHANG J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries[J]. Energy Storage Materials, 2016, 5: 139-164. DOI: 10.1016/j.ensm.2016.07.003
[32] KUNDU S, EIN-ELI Y. A review on design considerations in polymer and polymer composite solid-state electrolytes for solid Li batteries[J]. Journal of Power Sources, 2023, 553: 232267. DOI: 10.1016/j.jpowsour.2022.232267
[33] MARZANTOWICZ M, DYGAS J R, KROK F, et al. Influence of crystalline complexes on electrical properties of PEO: LiTFSI electrolyte[J]. Electrochimica Acta, 2007, 53(4): 1518-1526. DOI: 10.1016/j.electacta.2007.03.032
[34] MARZANTOWICZ M, DYGAS J R, KROK F, et al. Crystalline phases, morphology and conductivity of PEO: LiTFSI electrolytes in the eutectic region[J]. Journal of Power Sources, 2006, 159(1): 420-430. DOI: 10.1016/j.jpowsour.2006.02.044
[35] GRUNDISH N S, GOODENOUGH J B, KHANI H. Designing composite polymer electrolytes for all-solid-state lithium batteries[J]. Current Opinion in Electrochemistry, 2021, 30: 100828. DOI: 10.1016/j.coelec.2021.100828
[36] ZHOU Q, MA J, DONG S, et al. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries[J]. Advanced Materials, 2019, 31(50): 1902029. DOI: 10.1002/adma.201902029
[37] WU N, CHIEN P H, QIAN Y, et al. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte[J]. Angewandte Chemie International Edition, 2020, 59(10): 4131-4137. DOI: 10.1002/anie.201914478
[38] ROJAEE R, CAVALLO S, MOGURAMPELLY S, et al. Highly-cyclable room-temperature phosphorene polymer electrolyte composites for Li metal batteries[J]. Advanced Functional Materials, 2020, 30(32): 1910749. DOI: 10.1002/adfm.201910749
[39] JEON Y M, KIM S, LEE M, et al. Polymer-clay nanocomposite solid-state electrolyte with selective cation transport boosting and retarded lithium dendrite formation[J]. Advanced Energy Materials, 2020, 10(47): 2003114. DOI: 10.1002/aenm.202003114
[40] XU S, SUN Z, SUN C, et al. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature[J]. Advanced Functional Materials, 2020, 30(51): 2007172. DOI: 10.1002/adfm.202007172
[41] LIU M, CHENG Z, GANAPATHY S, et al. Tandem interface and bulk Li-ion transport in a hybrid solid electrolyte with microsized active filler[J]. ACS Energy Letters, 2019, 4(9): 2336-2342. DOI: 10.1021/acsenergylett.9b01371
[42] 宋鑫, 高志浩, 骆林, 等. 全固态锂电池有机-无机复合电解质研究进展[J]. 复合材料学报, 2023, 40(4): 1857-1878. SONG Xin, GAO Zhihao, LUO Lin, et al. Research progress of organic-inorganic composite electrolytes for all-solid-state lithium batteries[J]. Acta Materiae Compositae Sinica, 2023, 40(4): 1857-1878(in Chinese).
[43] YANG T, WANG C, ZHANG W, et al. A critical review on composite solid electrolytes for lithium batteries: Design strategies and interface engineering[J]. Journal of Energy Chemistry, 2023, 84: 189-209. DOI: 10.1016/j.jechem.2023.05.011
[44] CHEN H, ZHENG M, QIAN S, et al. Functional additives for solid polymer electrolytes in flexible and high-energy-density solid-state lithium-ion batteries[J]. Carbon Energy, 2021, 3(6): 929-956. DOI: 10.1002/cey2.146
[45] SU Y, XU F, ZHANG X, et al. Rational design of high-performance PEO/ceramic composite solid electrolytes for lithium metal batteries[J]. Nano-Micro Letters, 2023, 15(82): 1-35. DOI: 10.1007/s40820-023-01055-z
[46] ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4113-4120.
[47] SHI C, SONG J, ZHANG Y, et al. Revealing the mechanisms of lithium-ion transport and conduction in composite solid polymer electrolytes[J]. Cell Reports Physical Science, 2023, 4(3): 101321.
[48] LIU W, LEE S W, LIN D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires[J]. Nature Energy, 2017, 2(5): 1-7.
[49] SHEN Z, CHENG Y, SUN S, et al. The critical role of inorganic nanofillers in solid polymer composite electrolyte for Li+ transportation[J]. Carbon Energy, 2021, 3(3): 482-508. DOI: 10.1002/cey2.108
[50] SEN S, TREVISANELLO E, NIEMÖLLER E, et al. The role of polymers in lithium solid-state batteries with inorganic solid electrolytes[J]. Journal of Materials Chemistry A, 2021, 9(35): 18701-18732. DOI: 10.1039/D1TA02796D
[51] ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23): 8790-8839. DOI: 10.1039/D0CS00305K
[52] LI J, JING M X, LI R, et al. Al2O3 fiber-reinforced polymer solid electrolyte films with excellent lithium-ion transport properties for high-voltage solid-state lithium batteries[J]. ACS Applied Polymer Materials, 2022, 4(10): 7144-7151. DOI: 10.1021/acsapm.2c01034
[53] WANG C, YANG T, ZHANG W, et al. Hydrogen bonding enhanced SiO2/PEO composite electrolytes for solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2022, 10(7): 3400-3408. DOI: 10.1039/D1TA10607D
[54] HUA S, LI J L, JING M X, et al. Effects of surface lithiated TiO2 nanorods on room-temperature properties of polymer solid electrolytes[J]. International Journal of Energy Research, 2020, 44(8): 6452-6462. DOI: 10.1002/er.5379
[55] WIECZOREK W, SUCH K, WYCIŚLIK H, et al. Modifications of crystalline structure of PEO polymer electrolytes with ceramic additives[J]. Solid State Ionics, 1989, 36(3-4): 255-257. DOI: 10.1016/0167-2738(89)90185-9
[56] CROCE F, PERSI L, SCROSATI B, et al. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes[J]. Electrochimica Acta, 2001, 46(16): 2457-2461. DOI: 10.1016/S0013-4686(01)00458-3
[57] PARK C H, KIM D W, PRAKASH J, et al. Electrochemical stability and conductivity enhancement of composite polymer electrolytes[J]. Solid State Ionics, 2003, 159(1-2): 111-119. DOI: 10.1016/S0167-2738(03)00025-0
[58] ZHANG X, XIE J, SHI F, et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity[J]. Nano Letters, 2018, 18(6): 3829-3838. DOI: 10.1021/acs.nanolett.8b01111
[59] BAE H W, SUK J, PARK H S, et al. Incorporating ethylene oxide functionalized inorganic particles to solid polymer electrolytes for enhanced mechanical stability and electrochemical performance[J]. Advanced Energy and Sustainability Research, 2023, 4(3): 2200125. DOI: 10.1002/aesr.202200125
[60] LIN D, LIU W, LIU Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide)[J]. Nano Letters, 2016, 16(1): 459-465. DOI: 10.1021/acs.nanolett.5b04117
[61] YU J, WANG C, LI S, et al. Li+-containing, continuous silica nanofibers for high Li+ conductivity in composite polymer electrolyte[J]. Small, 2019, 15(44): 1902729. DOI: 10.1002/smll.201902729
[62] CHUNG S, WANG Y, PERSI L, et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides[J]. Journal of Power Sources, 2001, 97: 644-648.
[63] WIECZOREK W, FLORJANCZYK Z, STEVENS J. Composite polyether based solid electrolytes[J]. Electrochimica Acta, 1995, 40(13-14): 2251-2258. DOI: 10.1016/0013-4686(95)00172-B
[64] ZHAO E, GUO Y, ZHANG A, et al. Polydopamine coated TiO2 nanofiber fillers for polyethylene oxide hybrid electrolytes for efficient and durable all solid state lithium ion batteries[J]. Nanoscale, 2022, 14(3): 890-897. DOI: 10.1039/D1NR06636F
[65] LUO B, WANG W, WANG Q, et al. Facilitating ionic conductivity and interfacial stability via oxygen vacancies-enriched TiO2 microrods for composite polymer electrolytes[J]. Chemical Engineering Journal, 2023, 460: 141329. DOI: 10.1016/j.cej.2023.141329
[66] LI C, HUANG Y, CHEN C, et al. High-performance polymer electrolyte membrane modified with isocyanate-grafted Ti3+ doped TiO2 nanowires for lithium batteries[J]. Applied Surface Science, 2021, 563: 150248. DOI: 10.1016/j.apsusc.2021.150248
[67] BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte[J]. Angewandte Chemie International Edition, 2018, 57(8): 2096-2100. DOI: 10.1002/anie.201710841
[68] BAE J, LI Y, ZHAO F, et al. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries[J]. Energy Storage Materials, 2018, 15: 46-52. DOI: 10.1016/j.ensm.2018.03.016
[69] CHEN W, XIONG X, ZENG R, et al. Enhancing the interfacial ionic transport via in situ 3D composite polymer electrolytes for solid-state lithium batteries[J]. ACS Applied Energy Materials, 2020, 3(7): 7200-7207. DOI: 10.1021/acsaem.0c01269
[70] XU H M, JING M X, LI J, et al. Safety-enhanced flexible polypropylene oxide-ZrO2 composite solid electrolyte film with high room-temperature ionic conductivity[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(33): 11118-11126.
[71] CROCE F, SETTIMI L, SCROSATI B. Superacid ZrO2-added, composite polymer electrolytes with improved transport properties[J]. Electrochemistry Communications, 2006, 8(2): 364-368. DOI: 10.1016/j.elecom.2005.12.002
[72] DAM T, TRIPATHY S N, PALUCH M, et al. Investigations of relaxation dynamics and observation of nearly constant loss phenomena in PEO20-LiCF3SO3-ZrO2 based polymer nano-composite electrolyte[J]. Electrochimica Acta, 2016, 202: 147-156. DOI: 10.1016/j.electacta.2016.03.134
[73] MOHD YASIN S M, JOHAN M R. Thermal, structural and morphology studies of PEO-LiCF3SO3-DBP-ZrO2 nanocomposite polymer electrolytes[J]. Malaysian Nano-An International Journal, 2021, 1(1): 1-17. DOI: 10.22452/mnij.vol1no1.1
[74] XU L, LI J, SHUAI H, et al. Recent advances of composite electrolytes for solid-state Li batteries[J]. Journal of Energy Chemistry, 2022, 67: 524-548. DOI: 10.1016/j.jechem.2021.10.038
[75] LI J, ZHU K, YAO Z, et al. A promising composite solid electrolyte incorporating LLZO into PEO/PVDF matrix for all-solid-state lithium-ion batteries[J]. Ionics, 2020, 26: 1101-1108. DOI: 10.1007/s11581-019-03320-x
[76] LIU L, CHU L, JIANG B, et al. Li1.4Al0.4Ti1.6(PO4)3 nanoparticle-reinforced solid polymer electrolytes for all-solid-state lithium batteries[J]. Solid State Ionics, 2019, 331: 89-95. DOI: 10.1016/j.ssi.2019.01.007
[77] DAEMS K, YADAV P, DERMENCI K B, et al. Advances in inorganic, polymer and composite electrolytes: Mechanisms of lithium-ion transport and pathways to enhanced performance[J]. Renewable and Sustainable Energy Reviews, 2024, 191: 114136. DOI: 10.1016/j.rser.2023.114136
[78] 国洪瑶, 吴晓萌, 吴勇民, 等. 无机填料在复合固态电解质中的作用机制研究进展[J]. 材料导报, 2023, 37(S1): 9-16. GUO Hongyao, WU Xiaomeng, WU Yongmin, et al. Research progress on the mechanism of action of inorganic fillers in composite solid electrolytes[J]. Materials Reports, 2023, 37(S1): 9-16(in Chinese).
[79] LIU Y, XU B, ZHANG W, et al. Composition modulation and structure design of inorganic-in-polymer composite solid electrolytes for advanced lithium batteries[J]. Small, 2019, 16(15): 1902813.
[80] DAI J, FU K, GONG Y, et al. Flexible solid-state electrolyte with aligned nanostructures derived from wood[J]. ACS Materials Letters, 2019, 1(3): 354-361. DOI: 10.1021/acsmaterialslett.9b00189
[81] LIU M, GUAN X, LIU H, et al. Composite solid electrolytes containing single-ion lithium polymer grafted garnet for dendrite-free, long-life all-solid-state lithium metal batteries[J]. Chemical Engineering Journal, 2022, 445: 136436. DOI: 10.1016/j.cej.2022.136436
[82] FU K, GONG Y, DAI J, et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries[J]. Proceedings of the National Academy of Sciences, 2016, 113(26): 7094-7099. DOI: 10.1073/pnas.1600422113
[83] XIAO W, WANG J, FAN L, et al. Recent advances in Li1+xAlxTi2-x(PO4)3 solid-state electrolyte for safe lithium batteries[J]. Energy Storage Materials, 2019, 19: 379-400. DOI: 10.1016/j.ensm.2018.10.012
[84] KOTOBUKI M, KOISHI M. Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a sol-gel route using various Al sources[J]. Ceramics International, 2013, 39(4): 4645-4649. DOI: 10.1016/j.ceramint.2012.10.206
[85] ZHAO E, GUO Y, XIN Y, et al. Enhanced electrochemical properties and interfacial stability of poly(ethylene oxide) solid electrolyte incorporating nanostructured Li1.3Al0.3Ti1.7(PO4)3 fillers for all solid state lithium ion batteries[J]. International Journal of Energy Research, 2020, 45(5): 6876-6887.
[86] WANG G, LIU H, LIANG Y, et al. Composite polymer electrolyte with three-dimensional ion transport channels constructed by NaCl template for solid-state lithium metal batteries[J]. Energy Storage Materials, 2022, 45: 1212-1219. DOI: 10.1016/j.ensm.2021.11.021
[87] WANG C, HUANG D, LI S, et al. Three-dimensional-percolated ceramic nanoparticles along natural-cellulose-derived hierarchical networks for high Li+ conductivity and mechanical strength[J]. Nano Letters, 2020, 20(10): 7397-7404. DOI: 10.1021/acs.nanolett.0c02721
[88] 黄永浩, 朱霨亚, 廖友好, 等. 金属锂电池用复合固体电解质的研究进展[J]. 电池, 2023, 53(1): 93-97. HUANG Yonghao, ZHU Weiya, LIAO Youhao, et al. Research progress in composite solid electrolytes for metal lithium metal battery[J]. Batteries, 2023, 53(1): 93-97(in Chinese).
[89] LIU K, ZHANG R, SUN J, et al. Polyoxyethylene (PEO)|PEO-perovskite| PEO composite electrolyte for all-solid-state lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 46930-46937.
[90] ZHU P, YAN C, DIRICAN M, et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2018, 6(10): 4279-4285. DOI: 10.1039/C7TA10517G
[91] TENG Y, GUO J, WANG Y, et al. 3D perovskite LLTO nanotubers networks for enhanced Li+ conductivity in composite solid electrolytes[J]. Journal of Materials Science: Materials in Electronics, 2022, 33(33): 25342-25354. DOI: 10.1007/s10854-022-09240-3
[92] KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10(9): 682-686. DOI: 10.1038/nmat3066
[93] LI M, KOLEK M, FRERICHS J E, et al. Investigation of polymer/ceramic composite solid electrolyte system: The case of PEO/LGPS composite electrolytes[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(34): 11314-11322.
[94] ZHAO Y, WU C, PENG G, et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries[J]. Journal of Power Sources, 2016, 301: 47-53. DOI: 10.1016/j.jpowsour.2015.09.111
[95] PAN K, ZHANG L, QIAN W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. DOI: 10.1002/adma.202000399
[96] SHI Y, FAN Z, DING B, et al. Atomic-scale Al2O3 modified PEO-based composite polymer electrolyte for durable solid-state Li-S batteries[J]. Journal of Electroanalytical Chemistry, 2021, 881: 114916. DOI: 10.1016/j.jelechem.2020.114916
[97] LIN D, LIU W, LIU Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide)[J]. Nano Letters, 2015, 16(1): 459-465.
[98] XU Z, YANG T, CHU X, et al. Strong lewis acid-base and weak hydrogen bond synergistically enhancing ionic conductivity of poly(ethylene oxide)@SiO2 electrolytes for a high rate capability Li-metal battery[J]. ACS Applied Materials & Interfaces, 2020, 12(9): 10341-10349.
[99] CROCE F, APPETECCHI G, PERSI L, et al. Nanocomposite polymer electrolytes for lithium batteries[J]. Nature, 1998, 394(6692): 456-458. DOI: 10.1038/28818
[100] KHAN K, HANIF M B, XIN H, et al. PEO-based solid composite polymer electrolyte for high capacity retention all-solid-state lithium metal battery[J]. Small, 2024, 20(4): 2305772. DOI: 10.1002/smll.202305772
[101] ZHUANG H, MA W, XIE J, et al. Solvent-free synthesis of PEO/garnet composite electrolyte for high-safety all-solid-state lithium batteries[J]. Journal of Alloys and Compounds, 2021, 860: 157915. DOI: 10.1016/j.jallcom.2020.157915
[102] ZHANG J, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. DOI: 10.1016/j.nanoen.2016.09.002
[103] CHEN F, YANG D, ZHA W, et al. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries[J]. Electrochimica Acta, 2017, 258: 1106-1114. DOI: 10.1016/j.electacta.2017.11.164
[104] WAN Z, LEI D, YANG W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials, 2019, 29(1): 1805301. DOI: 10.1002/adfm.201805301
[105] MA F, LIU Y, DU X, et al. Hybrid solid electrolyte with the combination of LATP ceramic and PEO polymer by a solvent-free procedure[J]. Solid State Ionics, 2024, 405: 116450. DOI: 10.1016/j.ssi.2023.116450
[106] ZHAO Y, HUANG Z, CHEN S, et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries[J]. Solid State Ionics, 2016, 295: 65-71. DOI: 10.1016/j.ssi.2016.07.013
[107] PIANA G, BELLA F, GEOBALDO F, et al. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, "one pot" preparation[J]. Journal of Energy Storage, 2019, 26: 100947. DOI: 10.1016/j.est.2019.100947
[108] LIU C, WANG J, KOU W, et al. A flexible, ion-conducting solid electrolyte with vertically bicontinuous transfer channels toward high performance all-solid-state lithium batteries[J]. Chemical Engineering Journal, 2021, 404: 126517. DOI: 10.1016/j.cej.2020.126517
[109] ZHU P, YAN C, ZHU J, et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries[J]. Energy Storage Materials, 2019, 17: 220-225. DOI: 10.1016/j.ensm.2018.11.009
[110] WANG X, ZHANG Y, ZHANG X, et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24791-24798.
-
目的
固态锂离子电池能量密度高、安全性强,是突破电池技术瓶颈的关键,受到了学术界和工业界的广泛关注。固态电解质是固态电池的核心,对提升整体电池性能具有决定性影响力。而复合固态聚合物电解质(CSPEs)在兼顾固态电解质实际应用需求的同时,更有可能突破单一类型固态电解质所面临的局限,成为未来高性能、高安全储能系统中的优选方案。聚氧化乙烯(PEO)基聚合物固态电解质在改善电极界面相容性方面具有优势,是最有潜力的电解质材料之一。鉴于此,本文全面综述了近年来关于PEO基复合固态电解质体系的前沿研究成果,主要聚焦于填料-聚合物和填料-锂盐的相互作用,最后,对CSPEs的未来发展和优化设计做出展望。
方法通过搜集整理并分析现有文献,对PEO基复合固态电解质做出概述,并探讨离子传输相关机制,总结了PEO-惰性填料和PEO-活性填料复合固态电解质体系的设计、制备、性能及机制。将惰性填料添加到PEO基体中,可以增加PEO的非晶相,降低玻璃化转变温度,此外,通过其表面化学基团与锂盐阳离子/阴离子间较强的路易斯酸碱相互作用,可以促进锂盐解离和离子输运,增强CSPEs的电化学稳定性;而活性填料不仅保留了惰性填料的一般功能,还可以在活性填料区域和填料-聚合物界面构建额外高效的Li输运通道参与离子传导过程,提高离子电导率。
结果从协同效应角度综述了无机填料在PEO基复合固态电解质的研究进展,系统阐述了PEO与无机填料间的协同作用,及其对复合固态电解质的离子传输性和界面相容性的影响机制,并对当前CSPEs领域的相关进展进行总结,针对该领域现存挑战提出见解,同时对未来发展方向做出分析与展望。
结论探索PEO基复合固态电解质依然存在挑战,未来研究可从以下几方面进行深入研究:(1)目前室温下CSPEs的离子电导率仅为10 S·cm左右,无法满足实际应用所需的10 S·cm标准。这一问题可通过增加填料的表面缺陷改变聚合物/锂盐的相互作用、Li的局部环境以及Li传输的活化能进一步改善;(2)CSPEs中离子传输的具体机制尚未阐明,这直接阻碍了离子电导率水平的进一步提升。将理论计算与先进表征技术相结合,特别是原位测试技术,通过研究Li在CSPEs内的局部结构环境和动力学行为,验证实验结果并阐明离子传导增强机制有望解决这一难题;(3)无机填料-聚合物相互作用的有待深入研究。惰性填料(如AlO、SiO、TiO等)和活性填料(如LLZTO、LLZO、LATP等)种类对CSPEs离子电导率和锂离子迁移数的影响仍缺乏系统研究,明确这些相关机制虽充满挑战,但对优选适用于CSPEs的无机填料具有重大意义。随着研究不断深入,CSPEs有望实现应用,这对突破电池能量密度和安全性瓶颈具有极为重要的意义。




 下载:
下载: