Research progress of PEO-based composite solid-state electrolytes for lithium batteries
-
摘要:
固态锂离子电池能量密度高、安全性强,是突破电池技术瓶颈的关键,受到了学术界和工业界的广泛关注。固态电解质是固态电池的核心,其中聚氧化乙烯(PEO)基聚合物固态电解质在改善电极界面相容性方面具有优势,是最有潜力的电解质材料之一。本文系统阐述了PEO与无机填料间的协同作用及其对复合固态电解质的离子传输性和界面相容性的影响机制。首先对PEO基复合固态电解质做出概述,并探讨离子传输相关机制,然后分别综述了PEO-惰性填料和PEO-活性填料复合固态电解质体系的设计、制备、性能及机制,最后对复合固态聚合物电解质的未来发展和优化设计做出展望。
-
关键词:
- 固态锂电池 /
- 聚氧化乙烯 /
- 复合固态聚合物电解质 /
- 离子传输机制 /
- 无机填料
Abstract:Featured with high energy density and sound safety, solid-state lithium-ion battery is the key to breaking through the bottleneck shackling battery technology, gathering wide attention from academia and industry. Solid-state electrolytes are the core of solid-state batteries, among which polyethylene oxide (PEO) polymer solid-state electrolyte excels at improving electrode interface compatibility and is one of the most potential electrolyte materials. This paper systematically elucidated the synergistic effect of PEO with inorganic modified fillers and its influence on ion transport and interfacial compatibility of composite solid-state electrolytes. Following an overview of the PEO-based composite solid-state electrolyte, the mechanism of ion transport was discussed. Then, the design, preparation, performance, and mechanism of PEO-inert filler and PEO-active filler composite solid-state electrolyte systems were reviewed respectively. Finally, an outlook on the future development and optimization design of composite solid-state polymer electrolytes was presented.
-
随着电子器件功率密度的增加和工作环境的日益复杂,导热材料的界面接触性差,应力集中或分布不均都会导致材料的结构损伤。裂纹和间隙都会加剧声子散射,阻碍热流高效传递,造成热量聚集和局部温度过热,影响器件使用寿命和可靠性[1]。在这种情况下,如果热界面材料具有自修复性能,可以修复裂纹并恢复其原始功能,保证声子传导的良好通道和材料的结构稳定性,这对导热材料的发展和应用前景来说具有重要意义。
自修复材料作为一种新型智能材料,通过封装愈合剂或结合动态键在损伤发生后能恢复其基本性能,在电子、能源、环境和医学等各个领域具有广阔的应用前景[2]。聚合物的自愈能力主要由分子的可逆相互作用、化学键的重构及链的运动或动态交换构建的,其中包括动态共价键[3-5]、氢键[6-8]、离子相互作用[9-10]和π-π堆积[11-12]等。氢键由于其可逆性、方向性和修复速率快被广泛用于自愈材料的合成中[13]。而由于软硬链段的微相分离结构和大量氢键的存在,聚脲材料具有广泛的结构和性能可调性[14]。其性能优异,具备高韧性、抗冲击性、耐腐蚀和快速聚合等特点,是最有前途的自修复材料之一[15]。
基于动态交联网络的聚合物不仅具有自修复性,还兼具可回收或再加工能力,贴合循环经济和环境可持续发展的主题[16]。Yu等[17]构筑了一种新的共聚亚胺网络,拉伸强度最高可达63.7 MPa,且具有出色的自我修复能力和可回收性,有效解决了机械鲁棒性和动态性之间的平衡;Wan等[18]通过“含羞草”仿生策略制备新型动态聚酰亚胺材料,具有良好的降解效率、优异的可回收性。复合材料经多次循环后,碳纤维无损回收率高达100%。
设计自修复导热复合材料的有效策略是将导热填料引入自修复聚合物基体之中。导热填料的掺入会显著影响聚合物基体的各种性能,包括导热性、机械性和自愈性等[19]。Wang 等[20]以氮化硼纳米片(BNNS)和液态金属(LM)作为功能填料,嵌入具有自修复功能的聚(脲-氨基甲酸酯)弹性体(PUAUE)中,赋予了材料许多理想特性,如高导热性、电绝缘性、高韧性和室温自愈性等。具有“黑金”之称的石墨烯具有大的比表面积、高强度、导热 (热导率TC>
5000 W·m−1·K−1)导电性良好、化学性能稳定,由于这些特性常用作制备功能性复合材料[21]。Yu等[22]利用分子间的高密度氢键相互作用,引入褶皱石墨烯为导热填料,得到了兼具高回弹、高导热、强界面黏附性、快速自愈合的导热复合材料。材料的拉伸强度和导热性能的修复效率分别为100%和98.65%。基于以上考虑,本文通过席夫碱反应引入动态亚胺键,同时基于聚醚胺分子链的柔韧性,脲基氢键和动态亚胺键的协同作用,设计并合成了兼具自修复功能的双动态网络构筑自修复聚脲(D-PUA)柔性膜。其中,氢键位点构建了具有类似共价交联网络的鲁棒性和稳定性,同时和亚胺键的多重协同也保证了聚脲体系的动态可逆[23]。随后以D-PUA为聚合物基体,电剥离的石墨烯(GNP)为导热填料,通过简单的机械共混制备具有导热性、自修复性和可回收性的GNP/D-PUA复合材料。
1. 实验材料及方法
1.1 原材料
异佛尔酮二异氰酸酯(IPDI,99%),麦克林生化科技有限公司;聚醚胺 D-2000 (数均分子量Mn~
2000 )、聚醚胺 D-400 (Mn~400)、对苯二甲醛(TA,≥99%),阿拉丁生化科技有限公司;N, N-二甲基甲酰胺(DMF,99.8%),安徽泽生科技有限公司;石墨箔(工业级),北京晶龙特碳科技有限公司;硫酸铵(96%),天津市东丽区天大化学试剂厂。1.2 动态聚脲柔性膜的制备
控制异氰酸根(—NCO)、氨基(—NH2)和醛基(—CHO)的比例为1∶1.1∶0.1,通过改变不同分子量聚醚胺的摩尔比来调控聚脲的力学性能。以D-400和D-2000为10∶1的D-PUA合成为例,自修复D-PUA的制备步骤如下:首先取
0.8891 g(4 mmol) IPDI,将其溶解于2 mL无水DMF中,然后将1.6 g (4 mmol) D-400和0.8 g (0.4 mmol) D-2000分别溶解在3 mL无水DMF中,并与IPDI溶液混合,于40℃磁力搅拌4 h。随后,向溶液中加入0.0537 g (0.4 mmol)对苯二甲醛,升温至70℃继续反应24 h,反应完成后将高分子溶液浇注在聚四氟乙烯模具中,室温下抽真空去除气泡,60℃真空干燥24 h得透明微黄的D-PUA柔性膜,反应过程如图1 所示。下文若不做特殊说明,讨论的都是D-400/D-2000为10∶1的D-PUA。![]() 图 1 (a) 自修复聚脲(D-PUA)的合成路线图;(b) D-PUA薄膜的制备过程示意图;(c) D-PUA双动态网络结构示意图(包含氢键和动态亚胺键)IPDI—Isophorone diisocyanate; D-400, D-2000—PolyetheramineFigure 1. (a) Synthetic route of self-healing polyurea (D-PUA); (b) Schematic demonstration of the preparation process of the D-PUA films; (c) D-PUA dual dynamic network structure diagram, including hydrogen bonds and dynamic imine bonds
图 1 (a) 自修复聚脲(D-PUA)的合成路线图;(b) D-PUA薄膜的制备过程示意图;(c) D-PUA双动态网络结构示意图(包含氢键和动态亚胺键)IPDI—Isophorone diisocyanate; D-400, D-2000—PolyetheramineFigure 1. (a) Synthetic route of self-healing polyurea (D-PUA); (b) Schematic demonstration of the preparation process of the D-PUA films; (c) D-PUA dual dynamic network structure diagram, including hydrogen bonds and dynamic imine bonds同时制备了不含亚胺键的聚脲柔性膜(PUA)作为对比样,其具体制备过程如下:首先取
0.9782 g (4.4 mmol) IPDI,将其溶解于2 mL无水DMF中,然后将1.6 g (4 mmol) D-400和0.8 g (0.4 mmol) D-2000分别溶解在3 mL无水DMF中,并与IPDI溶液混合,于40℃磁力搅拌12 h。反应完成后将高分子溶液浇注在聚四氟乙烯模具中,室温下抽真空去除气泡,60℃真空干燥24 h得透明的PUA柔性膜。1.3 电剥离石墨烯(GNP)的制备
用精密线性稳压稳流电源(TN-XXZ02,国充充电科技江苏股份有限公司),石墨箔片作为阳极,铂片作为阴极,0.1 mol/L的硫酸铵水溶液作为电解液,电压恒定为15 V,对石墨箔进行电化学剥离,产物用去离子水洗去过量的硫酸铵,最后冷冻干燥得GNP。
1.4 石墨烯/聚脲(GNP/D-PUA)复合材料的制备
称取一定量的GNP在无水DMF中超声分散12 h得GNP分散液,随后将GNP分散液滴加到由IPDI、D-400、D-2000和对苯二甲醛合成聚脲高分子溶液(D-400∶D-2000=10∶1)中,机械搅拌24 h至混合均匀,在真空干燥箱中0.5 h,去除气泡,将得到的GNP/D-PUA溶液浇注在聚四氟乙烯模板上,60℃下真空干燥24 h,得到GNP/D-PUA导热复合材料。按GNP在复合材料中所占的质量分数,将样品标记为GNPx/D-PUA(x=2.5、5、7.5、10和12.5),如表1所示。
表 1 石墨烯(GNP)/D-PUA的样品命名Table 1. Sample naming of graphene (GNP)/D-PUASample Mass of GNP/mg GNP content/wt% GNP2.5/D-PUA 87.4 2.5 GNP5/D-PUA 179.3 5 GNP7.5/D-PUA 276.2 7.5 GNP10/D-PUA 378.5 10 GNP12.5/D-PUA 486.7 12.5 1.5 材料测试与表征
1.5.1 结构表征
1H NMR 测试在德国Bruker Avance II 核磁共振波谱仪上进行,所用溶剂为氘代氯仿,四甲基硅烷(TMS)作为内标物。采用傅里叶红外光谱仪(VERTEX 80,德国布鲁克公司)测试产物的全反射红外光谱(ATR-FTIR)分析其化学结构,波数范围为
4000 ~500 cm−1。变温红外光谱是在 Bruker IFS 66 v/s傅里叶红外光谱仪上进行的,光谱分辨率为4 cm−1,谱图采集范围为4000 ~500 cm−1。1.5.2 力学性能
采用万能拉力机(Instron 5967,美国英斯特朗公司)根据 GB/T 528—2009[24]标准测试材料力学性能,加载速度为 250 mm/min。
1.5.3 自修复和可回收性能
划痕修复的测试方法为:用手术刀在复合膜上划出深度一致的划痕,将受损膜放在偏光显微镜上进行加热修复。
修复效率的测试方法为:将原始试样从中间切开,两个受损末端浸入水中30 s,然后充分接触在相应温度下进行修复,修复后重新测试力学性能。拉伸强度和断裂韧性(应力-应变曲线与横轴的积分面积)的修复效率按下式进行计算:
η=σhσ0×100% (1) 其中,σ0和σh分别为原始试样和修复后试样的拉伸强度和断裂韧性。
材料重塑回收的方法为将复合膜剪碎后在 80℃、~10 MPa压力下热压15 min。
1.5.4 断面的形貌表征
采用扫描电子显微镜(SEM,S4800,日立高新技术(上海)国际贸易有限公司)对液氮脆断的样品断面形貌进行表征。
1.5.5 导热性能
采用导热系数测试仪(Hot Disk,瑞典凯戈纳斯仪器商贸(上海)有限公司),配备7577探头,采用平板模块测试。
2. 结果与讨论
2.1 D-PUA的分子结构表征
制备D-PUA的过程如图1(a)所示,IPDI 的异氰酸根与D-400、D-2000两端的氨基反应生成氨基封端的大分子聚脲。随后与对苯二甲醛在较温和的环境下进一步反应合成氢键和动态亚胺键协同作用的D-PUA,其微观组成和结构如图1(b)、图1(c)所示。
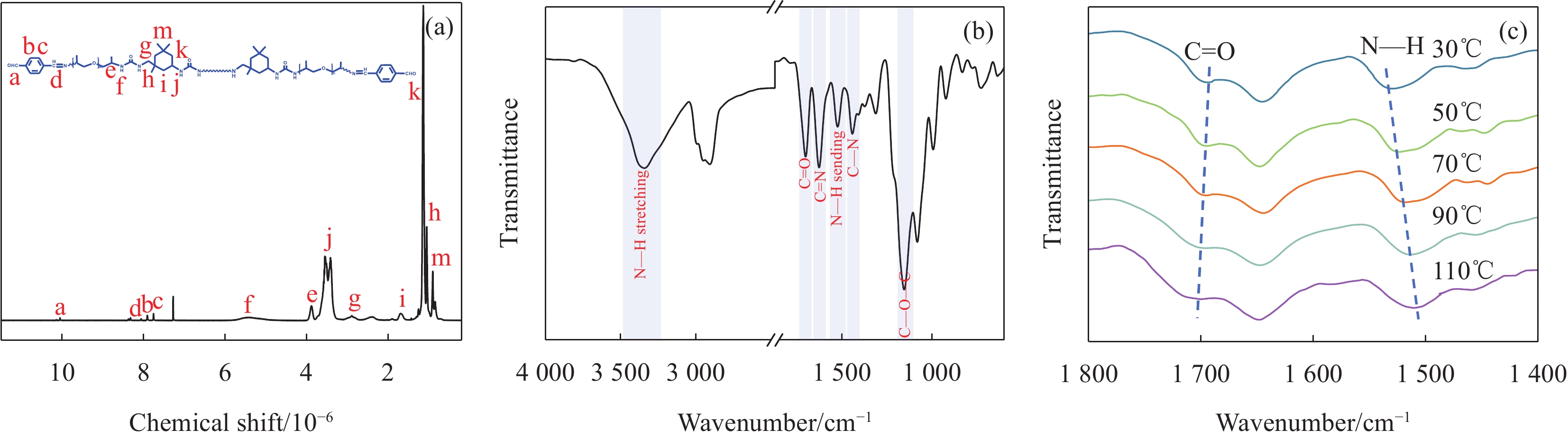
为了验证聚合物的合成,首先利用1H NMR谱图(图2(a))分析产物的化学结构。其中,化学位移5.10×10−6~5.69×10−6是NH—CO—NH的氢质子峰。化学位移8.07×10−6对应C=N的氢质子峰。化学位移7.76×10−6、7.91×10−6为苯环的特征峰。化学位移0.85×10−6~1.14×10−6、1.67×10−6和3.54×10−6属于 IPDI中甲基、亚甲基和环己烷的氢质子峰。化学位移为10.05×10−6是醛基的氢质子峰,这是由于高分子反应不完全,残余少量醛基没有反应。1H NMR结果表明D-PUA的成功合成[25-27]。
通过ATR-FTIR图谱进一步证明D-PUA的成功制备。如图2(b)所示,在
1150 和1438 cm−1分别出现 C—O—C和C—N的特征峰。1690 cm−1 C=O的伸缩振动吸收峰、3336 cm−1 N—H伸缩振动吸收峰和1520 cm−1 N—H弯曲振动吸收峰,均属于NH—CO—NH的特征峰。1625 cm−1出现的C=N特征峰,代表醛基和氨基经席夫碱反应生成亚胺键,ATR-FTIR的结果表明D-PUA中存在脲基和亚胺键,可以形成氢键和亚胺键协同作用的双动态网络[28-29]。氢键是赋予材料自愈性的重要因素。D-PUA的原位变温红外显示(图2(c)),随着温度从30℃升高至110℃,NH—CO—NH中N—H弯曲振动吸收峰从
1528 cm−1移动到1508 cm−1,C=O吸收峰从1694 cm−1蓝移至1702 cm−1,这些变化证明了体系中氢键的存在[30]。2.2 D-PUA的力学、自修复性能
聚醚胺D-400与D-2000相比分子量较小,分子链短,与异氰酸酯反应后形成的脲基氢键更加紧密[31]。因此,可以通过调节D-400和D-2000的摩尔比,来研究氢键密度对D-PUA力学性能的影响。如图3(a)所示,D-PUA的拉伸强度随着D-400/D-2000摩尔比的增加而提高。当D-400/D-2000为10∶1时,D-PUA的拉伸强度和断裂伸长率分别为(11.7±0.7) MPa和(895.9±1.6)%。D-400的占比越高,材料内的氢键密度越高,从而增强D-PUA 的拉伸强度。与已报道的动态聚脲弹性体相比[32-33],D-PUA在拉伸强度和韧性上有较大的优势。此外,在氢键密度相同的条件下,含亚胺键的D-PUA其拉伸强度和断裂伸长率皆高于不含亚胺键的PUA。说明亚胺键的引入可以进一步优化聚脲的力学性能。
D-PUA还具有良好的弹性,可通过循环拉伸实验对其回弹性进行研究。将材料拉至400%的形变,加载第一个循环后出现明显的滞后圈(图3(b)),根据滞后环的面积可以计算出消耗的能量约5.2 MJ/m3,这部分损耗能是由于拉伸时材料内部动态相互作用破坏,分子间的相互作用使链段运动受阻而产生的[34]。当第一个拉伸循环结束后立即进行第二次循环,滞后圈明显变小,能量耗散减少为3.9 MJ/m3。这主要是由于分子间的动态氢键破裂后,无法在短时间内立即重建[35]。
但当材料室温静置20 min后,D-PUA的拉伸循环曲线几乎与原曲线重合,滞后圈恢复到原来的大小。D-PUA的回弹性可通过图3(c)所见,应力卸载后伸长的材料发生弯曲。当室温静置一定时间后,材料又能自动恢复到原来的状态。上述结果说明D-PUA具有良好的回弹性,具有制备多功能柔性复合材料的潜力。
除了具有良好的回弹性外,由于氢键和亚胺键的动态特性,D-PUA在温度和水的刺激下还具有高效的自修复性能。从划痕测试(图4(a))可以看到,60℃时D-PUA在8 min内可以快速使损伤愈合,而PUA(无亚胺键)的划痕愈合在同一温度下需要更久的时间(15 min)。这说明氢键和亚胺键对自修复的协同作用优于单一的氢键。D-PUA的这种自愈过程类似于生物有机体中伤口和割伤的自然愈合[36]。为了量化修复效率,用手术刀将长条形试样规则地切割成两部分,将被切末端浸入水中30 s后使其充分接触,并在60℃烘箱中进行修复。将修复好的试样进行拉伸试验,通过拉伸强度和断裂韧性的修复效率评估其修复能力。图4(c)、图4(d)分别描绘了不同愈合时间后膜的应力-应变曲线和断裂韧性的修复效率。显然,愈合是随着时间的延长而进行的,修复72 h后D-PUA的拉伸强度和断裂韧性的修复效率分别为84.62%和80.36%。图4(b)更加直观的说明了D-PUA的自修复性能,修复72 h后蓝色部分(亚甲基蓝染色)和透明部分重新修复在一起。就分子结构而言(图4(e)),多重氢键和亚胺键可以加速分子链的运动和重排,从而实现结构愈合[33-37]。
![]() 图 4 (a) 聚脲(PUA)和D-PUA划痕自修复的光学显微镜图像;(b) 染色和未染色D-PUA样品在60℃下修复72 h的数码照片;(c) D-PUA切断后在60℃下不同愈合时间的应力-应变曲线;(d) D-PUA切断后在60℃下不同愈合时间的韧性及修复效率;(e) D-PUA自修复机制图Figure 4. (a) Optical microscope images of polyurea (PUA) and D-PUA scratch self-healing; (b) Digital photos of dyed and undyed D-PUA samples repaired at 60℃ for 72 h; (c) Stress-strain curves of D-PUA after cutting at different healing time at 60℃; (d) Toughness and repair efficiency of D-PUA after cutting at different healing time at 60℃; (e) Self-healing mechanism diagram of D-PUA
图 4 (a) 聚脲(PUA)和D-PUA划痕自修复的光学显微镜图像;(b) 染色和未染色D-PUA样品在60℃下修复72 h的数码照片;(c) D-PUA切断后在60℃下不同愈合时间的应力-应变曲线;(d) D-PUA切断后在60℃下不同愈合时间的韧性及修复效率;(e) D-PUA自修复机制图Figure 4. (a) Optical microscope images of polyurea (PUA) and D-PUA scratch self-healing; (b) Digital photos of dyed and undyed D-PUA samples repaired at 60℃ for 72 h; (c) Stress-strain curves of D-PUA after cutting at different healing time at 60℃; (d) Toughness and repair efficiency of D-PUA after cutting at different healing time at 60℃; (e) Self-healing mechanism diagram of D-PUA2.3 GNP/D-PUA的力学、自修复和再加工性能
图5(a)为复合材料的应力-应变曲线,随着GNP含量的增加,复合材料的拉伸强度先提高后降低,断裂伸长率显著降低。这主要是由于适量GNP基于自身的刚度可以起到补强效果[38]。GNP含量过高时,与基体的相容性变差,在受力过程中易出现裂纹,从而导致断裂,显著降低复合材料的拉伸强度和断裂伸长率。综合考虑材料的力学、导热和自修复性能,选择GNP10/D-PUA采用偏光显微镜观察不同温度下划痕的愈合过程,如图5(b)所示。GNP10/D-PUA在60℃下修复60 min仍存在明显的划痕,但升温至90℃修复60 min后划痕几乎完全消失。将其切断后在60℃修复72 h后,膜的应力和断裂韧性的修复效率分别为83.94%和61.07%,如图5(c)、图5(d)所示,显示出一定的自修复能力。
![]() 图 5 (a) 具有不同质量分数石墨烯(GNP)复合材料的应力-应变曲线;(b) GNP10/D-PUA划痕自修复的光学显微镜图像;(c) GNP10/D-PUA切断后在90℃下不同愈合时间的应力-应变曲线;(d) GNP10/D-PUA切断后在90℃下不同愈合时间的韧性及修复效率Figure 5. (a) Stress-strain curves of composites with different mass fractions graphene (GNP); (b) Optical microscope images of GNP10/D-PUA scratch self-healing; (c) Stress-strain curves of GNP10/D-PUA after cutting at different healing time at 90℃; (d) Toughness and repair efficiency of GNP10/D-PUA after cutting at different healing time at 90℃
图 5 (a) 具有不同质量分数石墨烯(GNP)复合材料的应力-应变曲线;(b) GNP10/D-PUA划痕自修复的光学显微镜图像;(c) GNP10/D-PUA切断后在90℃下不同愈合时间的应力-应变曲线;(d) GNP10/D-PUA切断后在90℃下不同愈合时间的韧性及修复效率Figure 5. (a) Stress-strain curves of composites with different mass fractions graphene (GNP); (b) Optical microscope images of GNP10/D-PUA scratch self-healing; (c) Stress-strain curves of GNP10/D-PUA after cutting at different healing time at 90℃; (d) Toughness and repair efficiency of GNP10/D-PUA after cutting at different healing time at 90℃进一步考察GNP的负载量对D-PUA复合材料划痕修复情况的影响,结果如图6所示。随着GNP负载量的增加,复合材料的划痕修复速率逐渐降低。当GNP负载量为12.5wt%时,即使在 90℃下修复75 min划痕仍未被修复。说明GNP的加入阻碍了基体的分子链运动和动态键的重组,使复合材料的自修复性能有不同程度的下降。
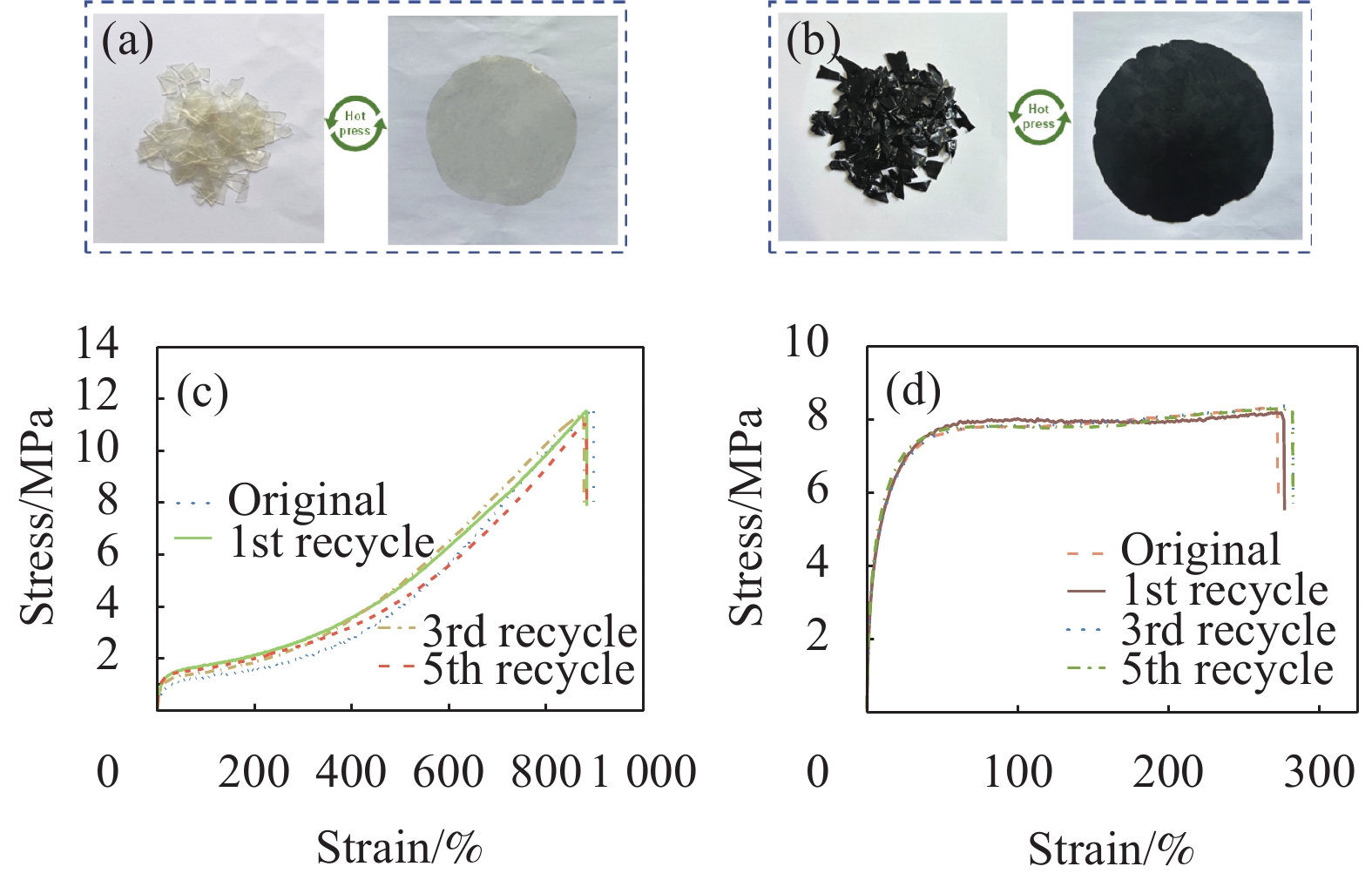
得益于氢键和动态亚胺键的高度动态性,D-PUA和GNP/D-PUA在温和的条件下都可以实现热再加工,从而具有可持续性。如图7(a)、图7(b)所示,将材料剪碎后施加一定压力和温度(80℃,~10 MPa)以加速分子链迁移并促进动态键的重建[39],15 min后可重新形成均一无缺陷的复合膜。重新测试回收膜的力学性能,其力学性能如图7(c)、图7(d)所示,拉伸强度及断裂伸长率的回复率分别在 95.16%和 97.63%以上。这样的过程可以重复5次循环,表明复合材料具有优异的可再加工性能,有望于制备易于再加工的高性能聚合物复合材料,减少电子垃圾,并提高塑料回收的效率[40]。
2.4 GNP/D-PUA的导热性能
石墨烯作为填料在聚脲基体中的分布对材料的导热性能有很大的影响,为了更加直观地对复合材料的形貌及GNP的分布情况进行清晰的观察,分别对D-PUA和GNP10/D-PUA的断面进行扫描分析。如图8所示,本征型D-PUA截面平整且光滑,在10wt%GNP 填充量下,GNP片层之间均匀分布,相互接触且无明显孔隙,有利于导热通路的构筑[41]。
由于石墨烯自身的良好导热性,复合材料的导热系数随填料负载量的增加而增加(图9(a))。填料含量较低时,在复合材料内部很难形成有效的导热路径。含量增加后,GNP之间的距离减小,更易形成导热网络,使热导率迅速增加[42]。
![]() 图 9 (a) 不同填料含量的GNP/D-PUA平面内导热系数;(b) GNP/D-PUA 的传热机制图;(c) 放置在加热板边缘的GNP/D-PUA复合材料的热红外图像; GNP/D-PUA在散热器的LED间通电前后的红外热像图(d)和不同时间点对应的表面温度(e)Figure 9. (a) In-plane thermal conductivity of GNP/D-PUA with different stuffing contents; (b) Heat transfer mechanism diagram of GNP/D-PUA composite; (c) Thermal infrared images of GNP/D-PUA composites placed on the edge of a heating plate; Infrared thermal images of GNP/D-PUA before and after power is applied between the LED of the radiator (d) and corresponding surface temperature at different time points (e)
图 9 (a) 不同填料含量的GNP/D-PUA平面内导热系数;(b) GNP/D-PUA 的传热机制图;(c) 放置在加热板边缘的GNP/D-PUA复合材料的热红外图像; GNP/D-PUA在散热器的LED间通电前后的红外热像图(d)和不同时间点对应的表面温度(e)Figure 9. (a) In-plane thermal conductivity of GNP/D-PUA with different stuffing contents; (b) Heat transfer mechanism diagram of GNP/D-PUA composite; (c) Thermal infrared images of GNP/D-PUA composites placed on the edge of a heating plate; Infrared thermal images of GNP/D-PUA before and after power is applied between the LED of the radiator (d) and corresponding surface temperature at different time points (e)图9(b)为GNP/D-PUA的导热机制图,GNP均匀分散在D-PUA基体中,片层之间相互连接以构筑导热通路。填料含量为10wt%时,面内导热系数为2.57 W·m−1·K−1,相对于本征膜提升了571%,优于之前所报道的自修复导热材料[41, 43]。为了可视化传热行为,采用红外热成像仪,记录了复合膜沿面内热传递的情况(图9(c)),结果表明GNP10/D-PUA的传热速率最快。
此外,模拟实际热界面材料的应用场景,将复合膜放置在LED小灯泡和散热器之间,并记录其红外热成像图(图9(d))。图9(e)为通电-断电过程中小灯泡表面温度随时间的变化图,75 s时GNP10/D-PUA的表面温度比D-PUA低12.6℃,表明 GNP10/D-PUA的散热效果最佳。以上结果证明了GNP10/D-PUA具有良好的传热和散热能力,有望应用于未来电子器件的散热部件。
复合材料经重塑再加工后的导热性也是自修复导热材料的一个重要性能,将GNP10/D-PUA剪碎热压(80℃,10 MPa)后再测试其面内热导率,结果如图10所示。GNP10/D-PUA在5次重塑后面内热导率最高可达2.16 W·m−1·K−1,回复率均在80.93%以上,说明复合材料重塑后的导热网络也得到了一定的恢复,具备可重复加工性。
3. 结 论
本文通过简单的合成工艺制备了具有双动态网络的自修复聚脲(D-PUA)。为了平衡自修复和导热性,选择石墨烯(GNP)为填料,主要探究石墨烯的加入对D-PUA自修复性能、导热性能和可回收性能的影响。得出的主要结论如下:
(1) D-PUA具有良好的回弹性和自修复性能。将D-PUA膜拉伸至一定形变,撤去应力并静置20 min后,可以恢复到原始形状。此外,D-PUA被完全切断后,60℃愈合72 h,断裂韧性的修复效率为80.36%;
(2)引入GNP后,GNP/D-PUA复合膜仍有自修复性能,且显著提升了D-PUA的导热性能。GNP添加量为10wt%时,90℃也可修复损伤,切开后愈合72 h,断裂韧性的修复效率为61.07%。GNP10/D-PUA的面内导热系数达到2.57 W·m−1·K−1,较本征型D-PUA提升了571%;
(3) GNP/D-PUA具有可回收性。复合膜经多次热压循环,重塑后力学性能基本不变且面内热导率的回复率均在80.93%以上,实现了高效回收和可持续发展。
总体而言,制备的GNP/D-PUA导热复合材料有望应用于柔性电子器件如可穿戴设备、导热皮肤、柔性传感器、柔性电路板和智能医疗等领域。
-
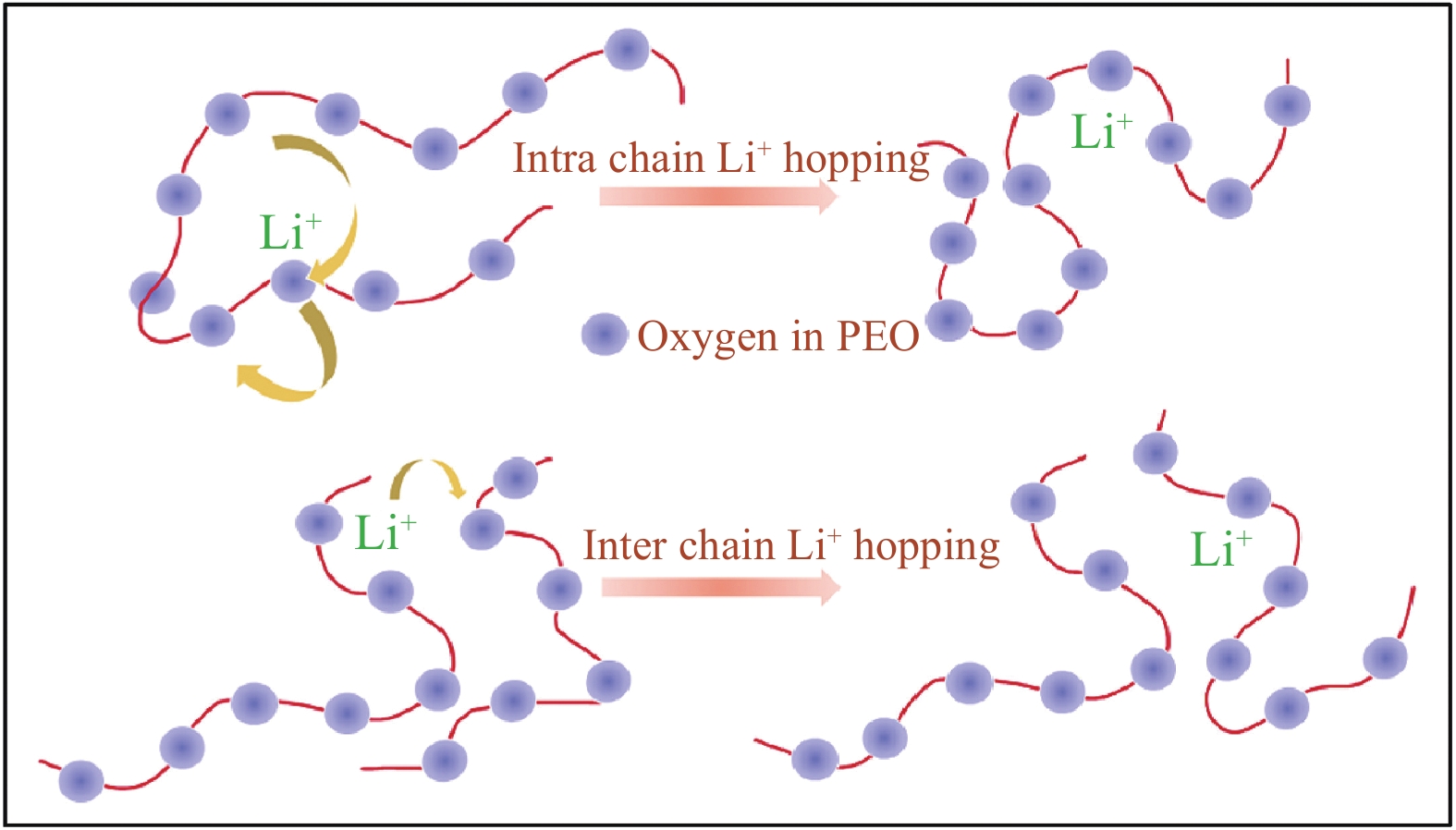
图 3 (a) Li+在聚氧化乙烯(PEO)相中、PEO相和PEO/陶瓷界面、PEO相和陶瓷相及PEO/陶瓷界面的传导途径示意图[45];(b) 锂离子在Li7La3Zr2O12 (LLZO) (5wt%) -PEO (LiTFSI)、LLZO (20wt%) -PEO (LiTFSI) 和LLZO (50wt%) -PEO (LiTFSI)复合电解质中的路径示意图[46];(c) 纳米颗粒(NPs)、无序纳米线(NWs)和排列NWs在复合聚合物电解质中的锂离子传导途径[48];(d) 填料、聚合物和锂盐之间的路易斯酸碱相互作用示意图[36]
EO—Ether oxygen
Figure 3. (a) Schematic illustration of the Li+ conduction pathways in the polyoxyethylene (PEO) phase, in the PEO phase and at PEO/ceramic interface, and in the PEO phase and ceramic phase and at PEO/ceramic interface[45]; (b) Schematic of Li-ion pathways within Li7La3Zr2O12 (LLZO) (5wt%)-PEO (LiTFSI), LLZO (20wt%)-PEO (LiTFSI) and LLZO (50wt%)-PEO (LiTFSI) composite electrolytes[46]; (c) Li-ion conduction pathways in composite polymer electrolytes with nanoparticles (NPs), random nanowires (NWs) and aligned NWs[48]; (d) Illustration of Lewis acid-base interaction between fillers, polymer, and Li salt[36]
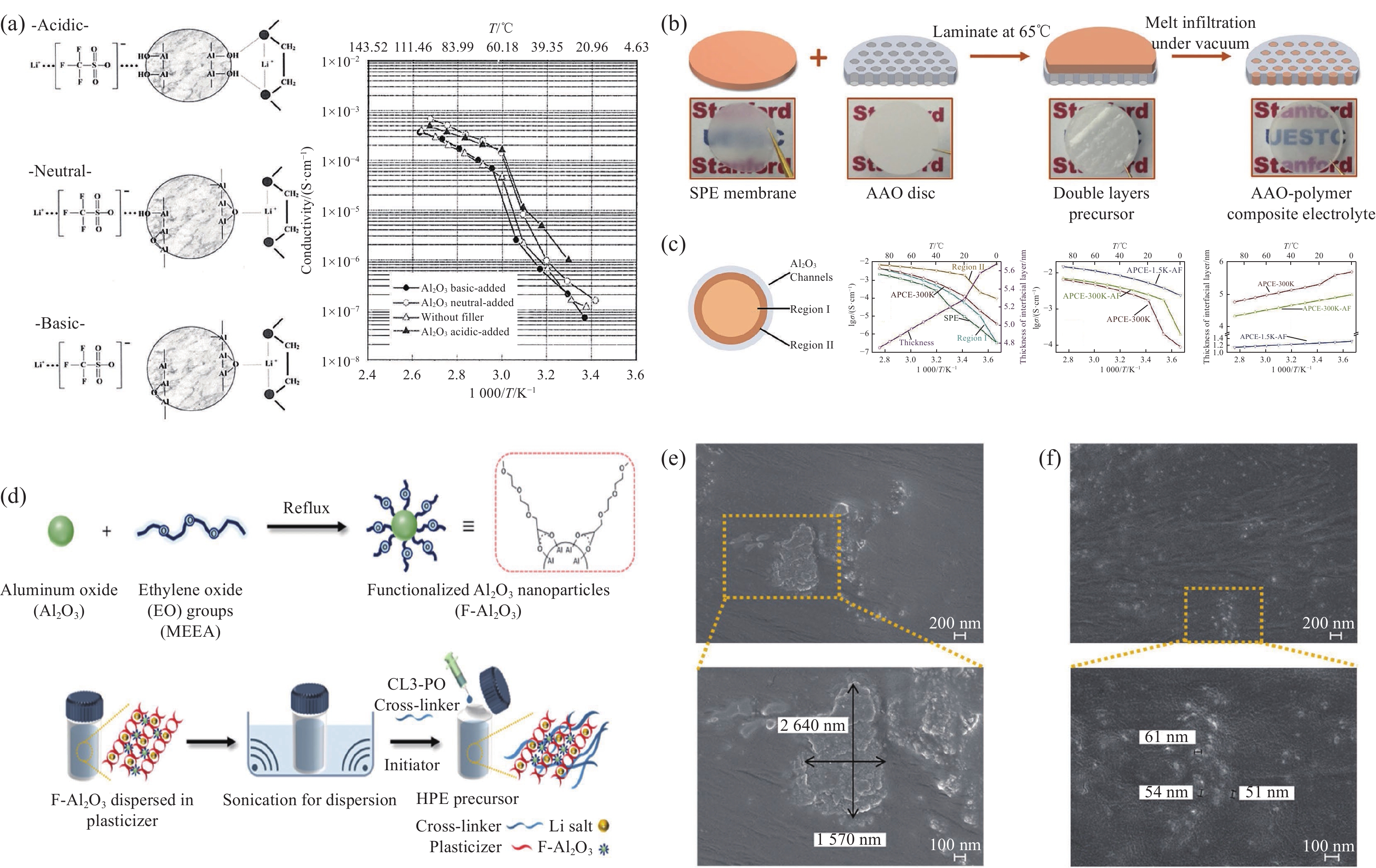
图 4 (a) 3种不同类型Al2O3与PEO表面相互作用和Al2O3-PEO复合固态聚合物电解质(CSPEs)的电导率图[56];(b) 阳极氧化铝(AAO)-聚合物复合电解质制备工艺示意图[58];(c) AAO圆盘中单个纳米通道中聚合物电解质示意图、各组分的离子电导率、界面离子电导率及AAO-聚合物复合电解质(APCE)界面层厚度[58];(d) 功能化Al2O3 (F-Al2O3)的合成及其制备杂化聚合物电解质(HPE)[59];分散在硅片上PEO薄膜中颗粒的SEM图像: (e) Al2O3;(f) F-Al2O3[59]
T—Temperature; APCE-1.5K-AF—It was prepared by infiltrating polymer electrolyte based on poly(ethylene glycol) (MW 1500) and LiTFSI into AlF3 modified AAO; APCE-300K-AF—The AAO-polymer composite electrolyte with AlF3 coated AAO discs and PEO (MW 300 000); APCE-300K—The AAO-polymer composite electrolyte without AlF3 coating AAO discs and PEO (MW 300 000); MW—Molecular weight; CL3-linker—Cross-linker; HPE—Hybrid polymer electrolyte
Figure 4. (a) Surface interactions between three diferent type Al2O3 and PEO and conductivity plots of Al2O3-PEO composite solid polymer electrolyte (CSPEs)[56]; (b) Schematics of fabrication procedures of anodized aluminum oxide (AAO)-polymer composite electrolyte[58]; (c) Schematics of polymer electrolyte in individual nanochannel of the AAO disc, the ionic conductivities of each component, the interface ion conductivities, and the thickness of interfacial layer of AAO-polymer composite electrolyte (APCE), respectively[58]; (d) Synthesis of functionalized Al2O3 (F-Al2O3) and preparation of hybrid polymer electrolyte (HPE) using F-Al2O3[59]; SEM images of the particles dispersed in PEO film on a Si wafer: (e) Al2O3; (f) F-Al2O3[59]
图 5 (a) PEO链与单分散超细SiO2(MUSiO2)的原位水解过程及相互作用机制示意图[60];(b) SiO2-Li2SO4-PEO CSPEs的制备工艺示意图及由Li2SO4衍生的SiO2纤维CSPEs实现Li+快速传导的原理图[61];(c) 原位水解制备CSPEs的示意图[53];(d) 不同成分聚合物电解质的离子电导率[64];(e) PEO-LiTFSI电解质和PEO-LiTFSI-3wt%TiO2@PDA复合电解质的DSC曲线[64];(f) CPEs中缺氧TiO2表面相互作用示意图[65];TiO2样品的XPS光谱(g)和Ti2p的高分辨率光谱(h)[66]
TEOS—Tetraethyl orthosilicate; PVA—Poly(vinyl alcohol); LiTFSI—Lithium bis(trifluoromethanesulfonyl)imide; DC—Direct current; PDA—Polydopamine; Tg—Glass transition temperature; Tm—Crystalline melting temperature
Figure 5. (a) Schematic figures showing the procedure of in situ hydrolysis and interaction mechanisms among PEO chains and monodisperse ultrafine SiO2 (MUSiO2)[60]; (b) Schematic diagram of preparation process of SiO2/Li2SO4/PEO CSPEs and schematics of fast Li+ conduction enabled by the Li2SO4-derived SiO2 fibers CSPEs [61]; (c) Schematic diagram of CPSEs prepared by in situ hydrolysis[53]; (d) Ionic conductivities of the polymer electrolytes with different compositions[64]; (e) DSC curves of the PEO-LiTFSI electrolyte and PEO-LiTFSI-3wt%TiO2@PDA composite electrolyte[64]; (f) Schematic illustrations of surface interaction of oxygen-deficient TiO2 in CPEs[65]; XPS survey spectra of the TiO2 sample (g) and high-resolution spectra of the Ti2p (h)[66]
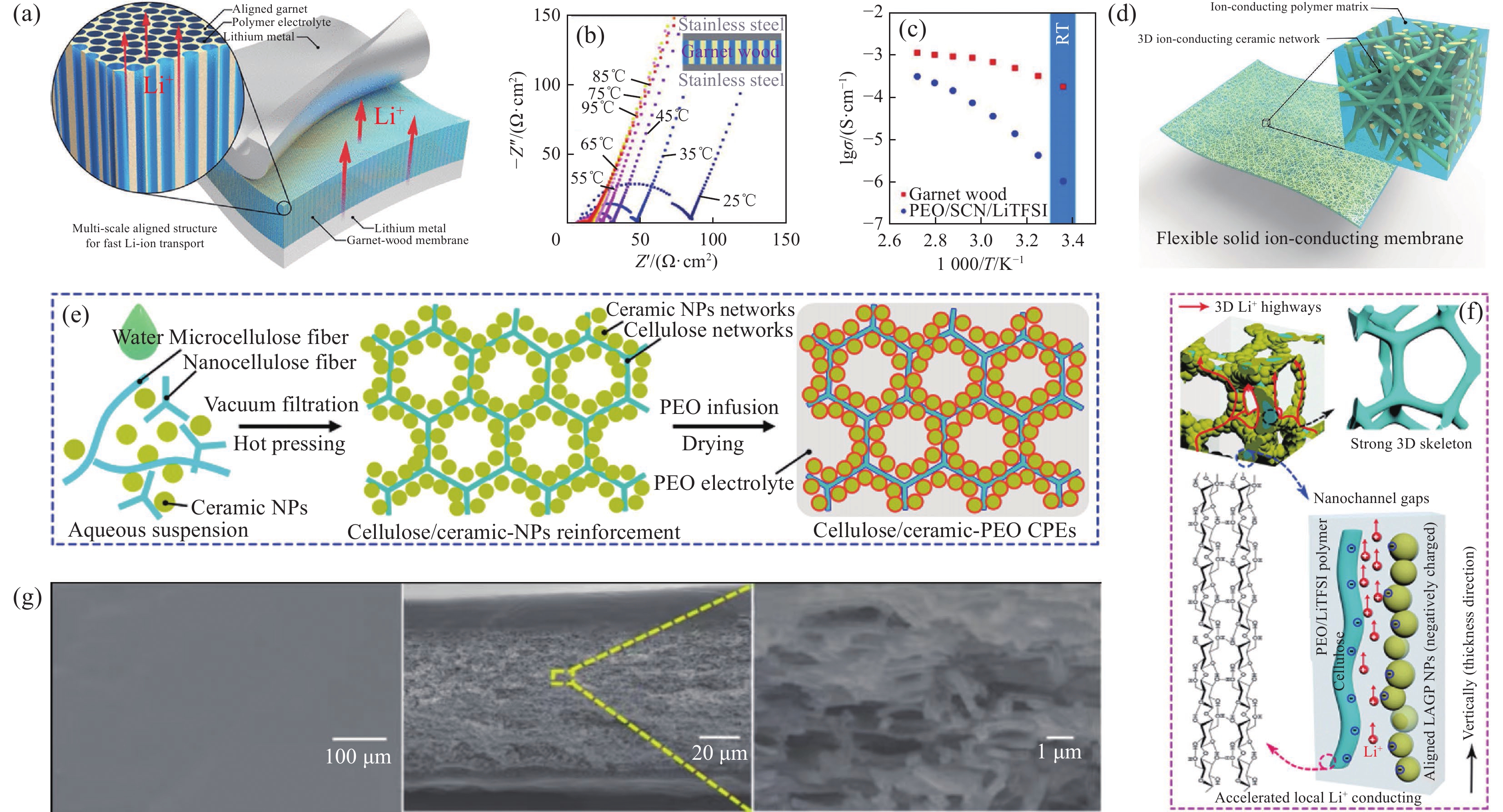
图 6 (a) 锂对称电池中多尺度排列的介孔石榴石Li6.4La3Zr2Al0.2O12 (LLZO)膜与聚合物电解质结合示意图[80];(b) 石榴石-木膜阻抗随温度升高而下降的Nyquist图(插图为测试单元的结构)[80];(c) 石榴石木和PEO聚合物电解质在不同温度下的离子电导率对比示意图[80];(d) 以陶瓷石榴石纳米纤维为增强层、锂离子导电聚合物为基体的复合固态电解质示意图[82];(e) 纤维素衍生的CPEs的制备工艺示意图[87];(f) 纤维素/陶瓷增强CPEs中Li+导电机制示意图[87];(g) PEO|PEO-钙钛矿|PEO复合电解质的表面SEM图像、横截面SEM图像及横截面形貌详细视图[89]
–Z''—Imaginary part of the impedance; Z'—Real part of the impedance; σ—Ionic conductivity; SCN—Succinonitrile; RT—Room temperature; NP—Nanoparticle; LAGP— Li1.5Al0.5Ge1.5(PO4)3
Figure 6. (a) Schematic of multi-scale aligned mesoporous garnet Li6.4La3Zr2Al0.2O12 (LLZO) membrane incorporated with polymer electrolyte in a lithium symmetric cell[80]; (b) Nyquist plot showing the decrease in the impedance of the garnet-wood membrane with increasing temperature (The inset schematic shows the structure of the testing cell)[80]; (c) Comparison of the ionic conductivity of the garnet-wood and PEO based polymer electrolyte at different temperatures[80]; (d) Schematic of the composite solid electrolyte, where ceramic garnet nanofibers function as the reinforcement and lithium-ion conducting polymer functions as the matrix[82]; (e) Schematic illustration of fabrication procedures for the cellulose derived CPEs[87]; (f) Schematic of proposed Li+ conducting mechanism in the cellulose/ceramic reinforced CPEs[87]; (g) SEM image of the surface, cross-sectional SEM image and detailed view of the cross-section morphology of the PEO|PEO-perovskite|PEO composite electrolyte[89]
表 1 聚氧化乙烯(PEO)基复合固态电解质文献报道汇总
Table 1 Summary of literature reports on polyethylene oxide (PEO)-based composite solid electrolyte
Polymer matrix Lithium salt Filler Ionic conductivity/(S·cm−1) ESW (vs. Li/Li+)/V tLi+ Ref. PEO LiTFSI Al2O3 5.82×10−4 (RT) — — [58] PEO LiTFSI Al2O3 9.6×10–4 (25℃) 5 0.81 [96] PEO LiTFSI SiO2 1.8×10–4 (30℃) 5.3 0.42 [53] PEO LiClO4 SiO2 1.2×10–3 (60℃) >5.5 — [97] PEO LiClO4 SiO2 1.1×10–4 (30℃) >4.8 — [98] PEO LiClO4 TiO2 1×10–5 (30℃) 5 0.3 [99] PEO LiTFSI TiO2@PDA 4.36×10–4 (55℃) 5 0.19 [64] PEO LiTFSI Ti3+-TiO2 1×10–4 (30℃) 5.5 0.36 [66] PEO LiBF4 ZrO2 4.4×10–4 (80℃) — 0.68 [71] PEO LiCF3SO3 ZrO2 1.38×10–4 (RT) — — [73] PEO LiTFSI LLZTO 1.9×10–4 (40℃) 5.1 0.67 [100] PEO LiTFSI LLZTO 3.03×10–4 (55℃) 4.5 0.117 [101] PEO LiTFSI LLZTO 5.6×10–4 (60℃) 4.75 0.46 [102] PEO LiTFSI LLZO 5.5×10–4 (30℃) >5.7 0.21 [103] PEO LiTFSI LLZO 2.39×10–4 (25℃) >5.5 — [104] PEO LiTFSI LATP 4×10–4 (60℃) 4.7 — [105] PEO LiTFSI LATP 3.61×10–4 (60℃) 4.8 — [76] PEO LiTFSI LAGP 6.76×10–4 (60℃) 5.3 0.378 [106] PEO LiTFSI LAGP 1.6×10–5 (20℃) — — [107] PEO LiTFSI LLTO 2.04×10–4 (25℃) 4.7 — [108] PEO LiTFSI LLTO 2.3×10–4 (RT) 4.5 — [109] PEO LiTFSI LLTO 1.8×10–4 (RT) 4.5 0.33 [110] PEO LiTFSI LGPS 1.21×10–3 (80℃) 5.7 0.26 [94] PEG-PEO LiTFSI LGPS 9.83×10–4 (RT) — 0.68 [95] Notes: ESW—Electrochemical stability window; tLi+—Lithium-ion transference number; RT—Room temperature; PDA—Polydopamine; LLZTO—Li6.4La3Zr1.4Ta0.6O12/Li6.75La3Zr1.75Ta0.25O12; LLZO—Li7La3Zr2O12; LATP—Li1.4Al0.4Ti1.6(PO4)3; LAGP—Li1.5Al0.5Ge1.5(PO4)3; LLTO—Li0.33La0.557TiO3; LGPS—Li10GeP2S12; PEG—Polyethylene glycol. -
[1] JIN B. Research on performance evaluation of green supply chain of automobile enterprises under the background of carbon peak and carbon neutralization[J]. Energy Reports, 2021, 7: 594-604. DOI: 10.1016/j.egyr.2021.10.002
[2] HU Y, XIE X, LI W, et al. Recent progress of polymer electrolytes for solid-state lithium batteries[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(4): 1253-1277.
[3] YANG X, LIU J, PEI N, et al. The critical role of fillers in composite polymer electrolytes for lithium battery[J]. Nano-Micro Letters, 2023, 15(74): 1-37. DOI: 10.1007/s40820-023-01051-3
[4] CHEN X, LI X, LUO L, et al. Practical application of all-solid-state lithium batteries based on high-voltage cathodes: Challenges and progress[J]. Advanced Energy Materials, 2023, 13(35): 2301230. DOI: 10.1002/aenm.202301230
[5] XIAO Z, LONG T, SONG L, et al. Research progress of polymer-inorganic filler solid composite electrolyte for lithium-ion batteries[J]. Ionics, 2021, 28: 15-26.
[6] CHEN S, DAI F, CAI M. Opportunities and challenges of high-energy lithium metal batteries for electric vehicle applications[J]. ACS Energy Letters, 2020, 5(10): 3140-3151. DOI: 10.1021/acsenergylett.0c01545
[7] XI G, XIAO M, WANG S, et al. Polymer-based solid electrolytes: Material selection, design, and application[J]. Advanced Functional Materials, 2021, 31(9): 2007598. DOI: 10.1002/adfm.202007598
[8] TANG S, GUO W, FU Y. Advances in composite polymer electrolytes for lithium batteries and beyond[J]. Advanced Energy Materials, 2021, 11(2): 2000802. DOI: 10.1002/aenm.202000802
[9] YANG Y, ZHOU H, XIE J, et al. Organic fast ion-conductor with ordered Li-ion conductive nano-pathways and high ionic conductivity for electrochemical energy storage[J]. Journal of Energy Chemistry, 2022, 66: 647-656. DOI: 10.1016/j.jechem.2021.09.011
[10] NGUYEN A G, PARK C J. Insights into tailoring composite solid polymer electrolytes for solid-state lithium batteries[J]. Journal of Membrane Science, 2023, 675: 121552. DOI: 10.1016/j.memsci.2023.121552
[11] FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18(12): 1278-1291. DOI: 10.1038/s41563-019-0431-3
[12] FAN P, LIU H, MAROSZ V, et al. High performance composite polymer electrolytes for lithium-ion batteries[J]. Advanced Functional Materials, 2021, 31(23): 2101380. DOI: 10.1002/adfm.202101380
[13] HOU W, OU Y, LIU K. Progress on high voltage PEO-based polymer solid electrolytes in lithium batteries[J]. Chemical Research in Chinese Universities, 2022, 38(3): 735-743. DOI: 10.1007/s40242-022-2065-2
[14] SUN J, LIU C, LIU H, et al. Advances in ordered architecture design of composite solid electrolytes for solid-state lithium batteries[J]. The Chemical Record, 2023, 23(6): e202300044. DOI: 10.1002/tcr.202300044
[15] MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 1-16.
[16] MEYER W H. Polymer electrolytes for lithium-ion batteries[J]. Advanced Materials, 1998, 10(6): 439-448. DOI: 10.1002/(SICI)1521-4095(199804)10:6<439::AID-ADMA439>3.0.CO;2-I
[17] LIU X, BI Z, WAN Y, et al. Composition regulation of polyacrylonitrile-based polymer electrolytes enabling dual-interfacially stable solid-state lithium batteries[J]. Journal of Colloid and Interface Science, 2024, 665: 582-591. DOI: 10.1016/j.jcis.2024.03.166
[18] ZHANG W, KOVERGA V, LIU S, et al. Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries[J]. Nature Energy, 2024, 9(4): 386-400.
[19] WU Y, LI Y, WANG Y, et al. Advances and prospects of PVDF based polymer electrolytes[J]. Journal of Energy Chemistry, 2022, 64: 62-84. DOI: 10.1016/j.jechem.2021.04.007
[20] LI Z, ZHANG S, JIANG Z, et al. Deep eutectic solvent-immobilized PVDF-HFP eutectogel as solid electrolyte for safe lithium metal battery[J]. Materials Chemistry and Physics, 2021, 267: 124701. DOI: 10.1016/j.matchemphys.2021.124701
[21] SU X, XU X P, JI Z Q, et al. Polyethylene oxide-based composite solid electrolytes for lithium batteries: Current progress, low-temperature and high-voltage limitations, and prospects[J]. Electrochemical Energy Reviews, 2024, 7(2): 1-38. DOI: 10.1007/s41918-023-00204-7
[22] ZHANG D, LI L, WU X, et al. Research progress and application of PEO-based solid state polymer composite electrolytes[J]. Frontiers in Energy Research, 2021, 9: 726738. DOI: 10.3389/fenrg.2021.726738
[23] FENG J, WANG L, CHEN Y, et al. PEO based polymer-ceramic hybrid solid electrolytes: A review[J]. Nano Convergence, 2021, 8: 1-12. DOI: 10.1186/s40580-020-00251-6
[24] 周伟东, 黄秋, 谢晓新, 等. 固态锂电池聚合物电解质研究进展[J]. 储能科学与技术, 2022, 11(6): 1788-1805. ZHOU Weidong, HUANG Qiu, XIE Xiaoxin, et al. Research progress of polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Science and Technology, 2022, 11(6): 1788-1805(in Chinese).
[25] SZCZĘSNA-CHRZAN A, MARCZEWSKI M, SYZDEK J, et al. Lithium polymer electrolytes for novel batteries application: The review perspective[J]. Applied Physics A, 2022, 129(37): 1-20.
[26] WANG H, SHENG L, YASIN G, et al. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2020, 33: 188-215. DOI: 10.1016/j.ensm.2020.08.014
[27] DING Z, LI J, LI J, et al. Review-interfaces: Key issue to be solved for all solid-state lithium battery technologies[J]. Journal of the Electrochemical Society, 2020, 167(7): 070541. DOI: 10.1149/1945-7111/ab7f84
[28] ZHENG Y, LI X, LI C Y. A novel de-coupling solid polymer electrolyte via semi-interpenetrating network for lithium metal battery[J]. Energy Storage Materials, 2020, 29: 42-51. DOI: 10.1016/j.ensm.2020.04.002
[29] LIU S, LIU W, BA D, et al. Filler-integrated composite polymer electrolyte for solid-state lithium batteries[J]. Advanced Materials, 2022, 35(2): 2110423.
[30] ROLLO-WALKER G, MALIC N, WANG X, et al. Development and progression of polymer electrolytes for batteries: Influence of structure and chemistry[J]. Polymers, 2021, 13(23): 4127. DOI: 10.3390/polym13234127
[31] YUE L, MA J, ZHANG J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries[J]. Energy Storage Materials, 2016, 5: 139-164. DOI: 10.1016/j.ensm.2016.07.003
[32] KUNDU S, EIN-ELI Y. A review on design considerations in polymer and polymer composite solid-state electrolytes for solid Li batteries[J]. Journal of Power Sources, 2023, 553: 232267. DOI: 10.1016/j.jpowsour.2022.232267
[33] MARZANTOWICZ M, DYGAS J R, KROK F, et al. Influence of crystalline complexes on electrical properties of PEO: LiTFSI electrolyte[J]. Electrochimica Acta, 2007, 53(4): 1518-1526. DOI: 10.1016/j.electacta.2007.03.032
[34] MARZANTOWICZ M, DYGAS J R, KROK F, et al. Crystalline phases, morphology and conductivity of PEO: LiTFSI electrolytes in the eutectic region[J]. Journal of Power Sources, 2006, 159(1): 420-430. DOI: 10.1016/j.jpowsour.2006.02.044
[35] GRUNDISH N S, GOODENOUGH J B, KHANI H. Designing composite polymer electrolytes for all-solid-state lithium batteries[J]. Current Opinion in Electrochemistry, 2021, 30: 100828. DOI: 10.1016/j.coelec.2021.100828
[36] ZHOU Q, MA J, DONG S, et al. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries[J]. Advanced Materials, 2019, 31(50): 1902029. DOI: 10.1002/adma.201902029
[37] WU N, CHIEN P H, QIAN Y, et al. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte[J]. Angewandte Chemie International Edition, 2020, 59(10): 4131-4137. DOI: 10.1002/anie.201914478
[38] ROJAEE R, CAVALLO S, MOGURAMPELLY S, et al. Highly-cyclable room-temperature phosphorene polymer electrolyte composites for Li metal batteries[J]. Advanced Functional Materials, 2020, 30(32): 1910749. DOI: 10.1002/adfm.201910749
[39] JEON Y M, KIM S, LEE M, et al. Polymer-clay nanocomposite solid-state electrolyte with selective cation transport boosting and retarded lithium dendrite formation[J]. Advanced Energy Materials, 2020, 10(47): 2003114. DOI: 10.1002/aenm.202003114
[40] XU S, SUN Z, SUN C, et al. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature[J]. Advanced Functional Materials, 2020, 30(51): 2007172. DOI: 10.1002/adfm.202007172
[41] LIU M, CHENG Z, GANAPATHY S, et al. Tandem interface and bulk Li-ion transport in a hybrid solid electrolyte with microsized active filler[J]. ACS Energy Letters, 2019, 4(9): 2336-2342. DOI: 10.1021/acsenergylett.9b01371
[42] 宋鑫, 高志浩, 骆林, 等. 全固态锂电池有机-无机复合电解质研究进展[J]. 复合材料学报, 2023, 40(4): 1857-1878. SONG Xin, GAO Zhihao, LUO Lin, et al. Research progress of organic-inorganic composite electrolytes for all-solid-state lithium batteries[J]. Acta Materiae Compositae Sinica, 2023, 40(4): 1857-1878(in Chinese).
[43] YANG T, WANG C, ZHANG W, et al. A critical review on composite solid electrolytes for lithium batteries: Design strategies and interface engineering[J]. Journal of Energy Chemistry, 2023, 84: 189-209. DOI: 10.1016/j.jechem.2023.05.011
[44] CHEN H, ZHENG M, QIAN S, et al. Functional additives for solid polymer electrolytes in flexible and high-energy-density solid-state lithium-ion batteries[J]. Carbon Energy, 2021, 3(6): 929-956. DOI: 10.1002/cey2.146
[45] SU Y, XU F, ZHANG X, et al. Rational design of high-performance PEO/ceramic composite solid electrolytes for lithium metal batteries[J]. Nano-Micro Letters, 2023, 15(82): 1-35. DOI: 10.1007/s40820-023-01055-z
[46] ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4113-4120.
[47] SHI C, SONG J, ZHANG Y, et al. Revealing the mechanisms of lithium-ion transport and conduction in composite solid polymer electrolytes[J]. Cell Reports Physical Science, 2023, 4(3): 101321.
[48] LIU W, LEE S W, LIN D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires[J]. Nature Energy, 2017, 2(5): 1-7.
[49] SHEN Z, CHENG Y, SUN S, et al. The critical role of inorganic nanofillers in solid polymer composite electrolyte for Li+ transportation[J]. Carbon Energy, 2021, 3(3): 482-508. DOI: 10.1002/cey2.108
[50] SEN S, TREVISANELLO E, NIEMÖLLER E, et al. The role of polymers in lithium solid-state batteries with inorganic solid electrolytes[J]. Journal of Materials Chemistry A, 2021, 9(35): 18701-18732. DOI: 10.1039/D1TA02796D
[51] ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23): 8790-8839. DOI: 10.1039/D0CS00305K
[52] LI J, JING M X, LI R, et al. Al2O3 fiber-reinforced polymer solid electrolyte films with excellent lithium-ion transport properties for high-voltage solid-state lithium batteries[J]. ACS Applied Polymer Materials, 2022, 4(10): 7144-7151. DOI: 10.1021/acsapm.2c01034
[53] WANG C, YANG T, ZHANG W, et al. Hydrogen bonding enhanced SiO2/PEO composite electrolytes for solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2022, 10(7): 3400-3408. DOI: 10.1039/D1TA10607D
[54] HUA S, LI J L, JING M X, et al. Effects of surface lithiated TiO2 nanorods on room-temperature properties of polymer solid electrolytes[J]. International Journal of Energy Research, 2020, 44(8): 6452-6462. DOI: 10.1002/er.5379
[55] WIECZOREK W, SUCH K, WYCIŚLIK H, et al. Modifications of crystalline structure of PEO polymer electrolytes with ceramic additives[J]. Solid State Ionics, 1989, 36(3-4): 255-257. DOI: 10.1016/0167-2738(89)90185-9
[56] CROCE F, PERSI L, SCROSATI B, et al. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes[J]. Electrochimica Acta, 2001, 46(16): 2457-2461. DOI: 10.1016/S0013-4686(01)00458-3
[57] PARK C H, KIM D W, PRAKASH J, et al. Electrochemical stability and conductivity enhancement of composite polymer electrolytes[J]. Solid State Ionics, 2003, 159(1-2): 111-119. DOI: 10.1016/S0167-2738(03)00025-0
[58] ZHANG X, XIE J, SHI F, et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity[J]. Nano Letters, 2018, 18(6): 3829-3838. DOI: 10.1021/acs.nanolett.8b01111
[59] BAE H W, SUK J, PARK H S, et al. Incorporating ethylene oxide functionalized inorganic particles to solid polymer electrolytes for enhanced mechanical stability and electrochemical performance[J]. Advanced Energy and Sustainability Research, 2023, 4(3): 2200125. DOI: 10.1002/aesr.202200125
[60] LIN D, LIU W, LIU Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide)[J]. Nano Letters, 2016, 16(1): 459-465. DOI: 10.1021/acs.nanolett.5b04117
[61] YU J, WANG C, LI S, et al. Li+-containing, continuous silica nanofibers for high Li+ conductivity in composite polymer electrolyte[J]. Small, 2019, 15(44): 1902729. DOI: 10.1002/smll.201902729
[62] CHUNG S, WANG Y, PERSI L, et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides[J]. Journal of Power Sources, 2001, 97: 644-648.
[63] WIECZOREK W, FLORJANCZYK Z, STEVENS J. Composite polyether based solid electrolytes[J]. Electrochimica Acta, 1995, 40(13-14): 2251-2258. DOI: 10.1016/0013-4686(95)00172-B
[64] ZHAO E, GUO Y, ZHANG A, et al. Polydopamine coated TiO2 nanofiber fillers for polyethylene oxide hybrid electrolytes for efficient and durable all solid state lithium ion batteries[J]. Nanoscale, 2022, 14(3): 890-897. DOI: 10.1039/D1NR06636F
[65] LUO B, WANG W, WANG Q, et al. Facilitating ionic conductivity and interfacial stability via oxygen vacancies-enriched TiO2 microrods for composite polymer electrolytes[J]. Chemical Engineering Journal, 2023, 460: 141329. DOI: 10.1016/j.cej.2023.141329
[66] LI C, HUANG Y, CHEN C, et al. High-performance polymer electrolyte membrane modified with isocyanate-grafted Ti3+ doped TiO2 nanowires for lithium batteries[J]. Applied Surface Science, 2021, 563: 150248. DOI: 10.1016/j.apsusc.2021.150248
[67] BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte[J]. Angewandte Chemie International Edition, 2018, 57(8): 2096-2100. DOI: 10.1002/anie.201710841
[68] BAE J, LI Y, ZHAO F, et al. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries[J]. Energy Storage Materials, 2018, 15: 46-52. DOI: 10.1016/j.ensm.2018.03.016
[69] CHEN W, XIONG X, ZENG R, et al. Enhancing the interfacial ionic transport via in situ 3D composite polymer electrolytes for solid-state lithium batteries[J]. ACS Applied Energy Materials, 2020, 3(7): 7200-7207. DOI: 10.1021/acsaem.0c01269
[70] XU H M, JING M X, LI J, et al. Safety-enhanced flexible polypropylene oxide-ZrO2 composite solid electrolyte film with high room-temperature ionic conductivity[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(33): 11118-11126.
[71] CROCE F, SETTIMI L, SCROSATI B. Superacid ZrO2-added, composite polymer electrolytes with improved transport properties[J]. Electrochemistry Communications, 2006, 8(2): 364-368. DOI: 10.1016/j.elecom.2005.12.002
[72] DAM T, TRIPATHY S N, PALUCH M, et al. Investigations of relaxation dynamics and observation of nearly constant loss phenomena in PEO20-LiCF3SO3-ZrO2 based polymer nano-composite electrolyte[J]. Electrochimica Acta, 2016, 202: 147-156. DOI: 10.1016/j.electacta.2016.03.134
[73] MOHD YASIN S M, JOHAN M R. Thermal, structural and morphology studies of PEO-LiCF3SO3-DBP-ZrO2 nanocomposite polymer electrolytes[J]. Malaysian Nano-An International Journal, 2021, 1(1): 1-17. DOI: 10.22452/mnij.vol1no1.1
[74] XU L, LI J, SHUAI H, et al. Recent advances of composite electrolytes for solid-state Li batteries[J]. Journal of Energy Chemistry, 2022, 67: 524-548. DOI: 10.1016/j.jechem.2021.10.038
[75] LI J, ZHU K, YAO Z, et al. A promising composite solid electrolyte incorporating LLZO into PEO/PVDF matrix for all-solid-state lithium-ion batteries[J]. Ionics, 2020, 26: 1101-1108. DOI: 10.1007/s11581-019-03320-x
[76] LIU L, CHU L, JIANG B, et al. Li1.4Al0.4Ti1.6(PO4)3 nanoparticle-reinforced solid polymer electrolytes for all-solid-state lithium batteries[J]. Solid State Ionics, 2019, 331: 89-95. DOI: 10.1016/j.ssi.2019.01.007
[77] DAEMS K, YADAV P, DERMENCI K B, et al. Advances in inorganic, polymer and composite electrolytes: Mechanisms of lithium-ion transport and pathways to enhanced performance[J]. Renewable and Sustainable Energy Reviews, 2024, 191: 114136. DOI: 10.1016/j.rser.2023.114136
[78] 国洪瑶, 吴晓萌, 吴勇民, 等. 无机填料在复合固态电解质中的作用机制研究进展[J]. 材料导报, 2023, 37(S1): 9-16. GUO Hongyao, WU Xiaomeng, WU Yongmin, et al. Research progress on the mechanism of action of inorganic fillers in composite solid electrolytes[J]. Materials Reports, 2023, 37(S1): 9-16(in Chinese).
[79] LIU Y, XU B, ZHANG W, et al. Composition modulation and structure design of inorganic-in-polymer composite solid electrolytes for advanced lithium batteries[J]. Small, 2019, 16(15): 1902813.
[80] DAI J, FU K, GONG Y, et al. Flexible solid-state electrolyte with aligned nanostructures derived from wood[J]. ACS Materials Letters, 2019, 1(3): 354-361. DOI: 10.1021/acsmaterialslett.9b00189
[81] LIU M, GUAN X, LIU H, et al. Composite solid electrolytes containing single-ion lithium polymer grafted garnet for dendrite-free, long-life all-solid-state lithium metal batteries[J]. Chemical Engineering Journal, 2022, 445: 136436. DOI: 10.1016/j.cej.2022.136436
[82] FU K, GONG Y, DAI J, et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries[J]. Proceedings of the National Academy of Sciences, 2016, 113(26): 7094-7099. DOI: 10.1073/pnas.1600422113
[83] XIAO W, WANG J, FAN L, et al. Recent advances in Li1+xAlxTi2-x(PO4)3 solid-state electrolyte for safe lithium batteries[J]. Energy Storage Materials, 2019, 19: 379-400. DOI: 10.1016/j.ensm.2018.10.012
[84] KOTOBUKI M, KOISHI M. Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a sol-gel route using various Al sources[J]. Ceramics International, 2013, 39(4): 4645-4649. DOI: 10.1016/j.ceramint.2012.10.206
[85] ZHAO E, GUO Y, XIN Y, et al. Enhanced electrochemical properties and interfacial stability of poly(ethylene oxide) solid electrolyte incorporating nanostructured Li1.3Al0.3Ti1.7(PO4)3 fillers for all solid state lithium ion batteries[J]. International Journal of Energy Research, 2020, 45(5): 6876-6887.
[86] WANG G, LIU H, LIANG Y, et al. Composite polymer electrolyte with three-dimensional ion transport channels constructed by NaCl template for solid-state lithium metal batteries[J]. Energy Storage Materials, 2022, 45: 1212-1219. DOI: 10.1016/j.ensm.2021.11.021
[87] WANG C, HUANG D, LI S, et al. Three-dimensional-percolated ceramic nanoparticles along natural-cellulose-derived hierarchical networks for high Li+ conductivity and mechanical strength[J]. Nano Letters, 2020, 20(10): 7397-7404. DOI: 10.1021/acs.nanolett.0c02721
[88] 黄永浩, 朱霨亚, 廖友好, 等. 金属锂电池用复合固体电解质的研究进展[J]. 电池, 2023, 53(1): 93-97. HUANG Yonghao, ZHU Weiya, LIAO Youhao, et al. Research progress in composite solid electrolytes for metal lithium metal battery[J]. Batteries, 2023, 53(1): 93-97(in Chinese).
[89] LIU K, ZHANG R, SUN J, et al. Polyoxyethylene (PEO)|PEO-perovskite| PEO composite electrolyte for all-solid-state lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 46930-46937.
[90] ZHU P, YAN C, DIRICAN M, et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2018, 6(10): 4279-4285. DOI: 10.1039/C7TA10517G
[91] TENG Y, GUO J, WANG Y, et al. 3D perovskite LLTO nanotubers networks for enhanced Li+ conductivity in composite solid electrolytes[J]. Journal of Materials Science: Materials in Electronics, 2022, 33(33): 25342-25354. DOI: 10.1007/s10854-022-09240-3
[92] KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10(9): 682-686. DOI: 10.1038/nmat3066
[93] LI M, KOLEK M, FRERICHS J E, et al. Investigation of polymer/ceramic composite solid electrolyte system: The case of PEO/LGPS composite electrolytes[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(34): 11314-11322.
[94] ZHAO Y, WU C, PENG G, et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries[J]. Journal of Power Sources, 2016, 301: 47-53. DOI: 10.1016/j.jpowsour.2015.09.111
[95] PAN K, ZHANG L, QIAN W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. DOI: 10.1002/adma.202000399
[96] SHI Y, FAN Z, DING B, et al. Atomic-scale Al2O3 modified PEO-based composite polymer electrolyte for durable solid-state Li-S batteries[J]. Journal of Electroanalytical Chemistry, 2021, 881: 114916. DOI: 10.1016/j.jelechem.2020.114916
[97] LIN D, LIU W, LIU Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide)[J]. Nano Letters, 2015, 16(1): 459-465.
[98] XU Z, YANG T, CHU X, et al. Strong lewis acid-base and weak hydrogen bond synergistically enhancing ionic conductivity of poly(ethylene oxide)@SiO2 electrolytes for a high rate capability Li-metal battery[J]. ACS Applied Materials & Interfaces, 2020, 12(9): 10341-10349.
[99] CROCE F, APPETECCHI G, PERSI L, et al. Nanocomposite polymer electrolytes for lithium batteries[J]. Nature, 1998, 394(6692): 456-458. DOI: 10.1038/28818
[100] KHAN K, HANIF M B, XIN H, et al. PEO-based solid composite polymer electrolyte for high capacity retention all-solid-state lithium metal battery[J]. Small, 2024, 20(4): 2305772. DOI: 10.1002/smll.202305772
[101] ZHUANG H, MA W, XIE J, et al. Solvent-free synthesis of PEO/garnet composite electrolyte for high-safety all-solid-state lithium batteries[J]. Journal of Alloys and Compounds, 2021, 860: 157915. DOI: 10.1016/j.jallcom.2020.157915
[102] ZHANG J, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. DOI: 10.1016/j.nanoen.2016.09.002
[103] CHEN F, YANG D, ZHA W, et al. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries[J]. Electrochimica Acta, 2017, 258: 1106-1114. DOI: 10.1016/j.electacta.2017.11.164
[104] WAN Z, LEI D, YANG W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials, 2019, 29(1): 1805301. DOI: 10.1002/adfm.201805301
[105] MA F, LIU Y, DU X, et al. Hybrid solid electrolyte with the combination of LATP ceramic and PEO polymer by a solvent-free procedure[J]. Solid State Ionics, 2024, 405: 116450. DOI: 10.1016/j.ssi.2023.116450
[106] ZHAO Y, HUANG Z, CHEN S, et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries[J]. Solid State Ionics, 2016, 295: 65-71. DOI: 10.1016/j.ssi.2016.07.013
[107] PIANA G, BELLA F, GEOBALDO F, et al. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, "one pot" preparation[J]. Journal of Energy Storage, 2019, 26: 100947. DOI: 10.1016/j.est.2019.100947
[108] LIU C, WANG J, KOU W, et al. A flexible, ion-conducting solid electrolyte with vertically bicontinuous transfer channels toward high performance all-solid-state lithium batteries[J]. Chemical Engineering Journal, 2021, 404: 126517. DOI: 10.1016/j.cej.2020.126517
[109] ZHU P, YAN C, ZHU J, et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries[J]. Energy Storage Materials, 2019, 17: 220-225. DOI: 10.1016/j.ensm.2018.11.009
[110] WANG X, ZHANG Y, ZHANG X, et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24791-24798.
-
目的
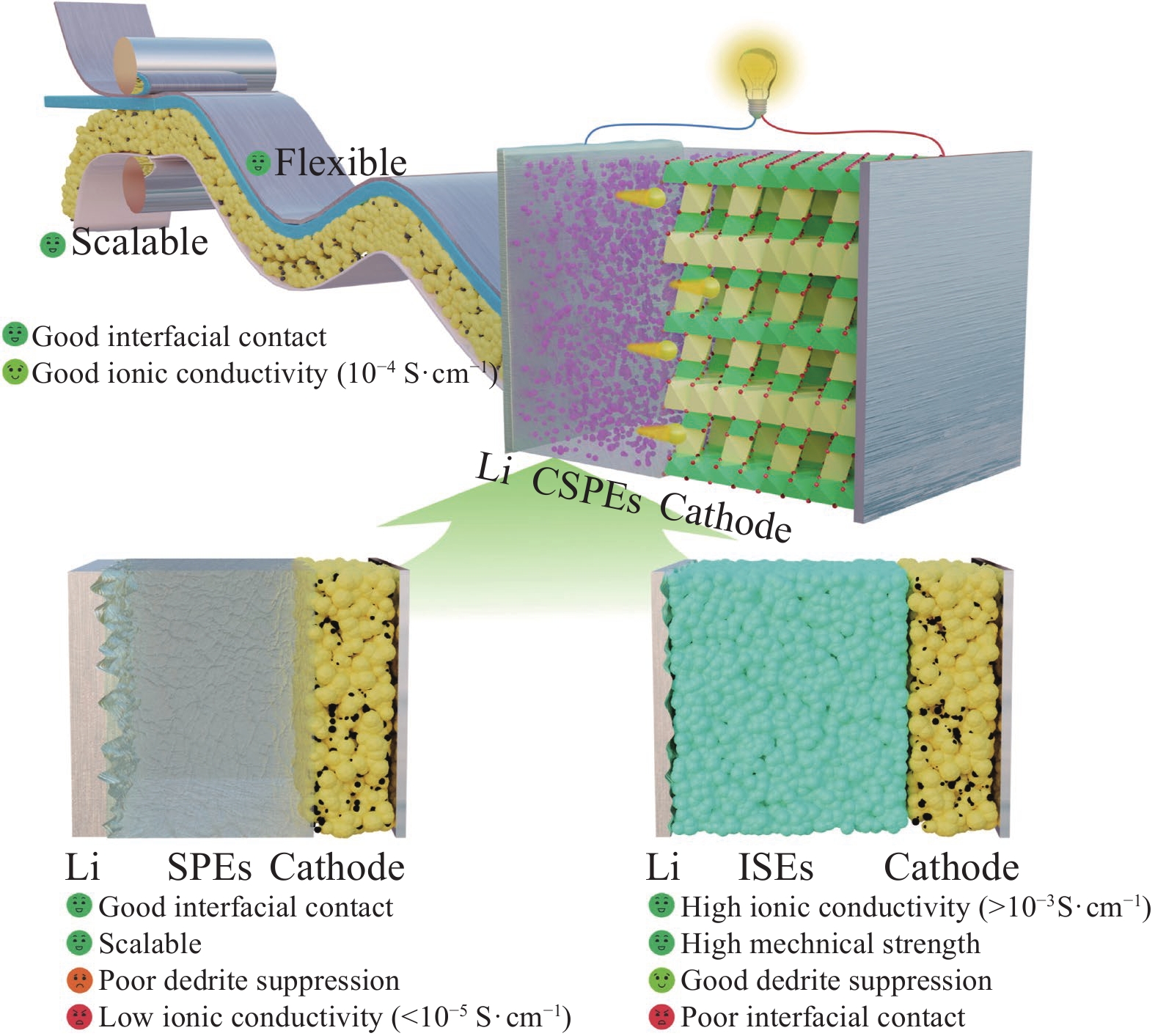
固态锂离子电池能量密度高、安全性强,是突破电池技术瓶颈的关键,受到了学术界和工业界的广泛关注。固态电解质是固态电池的核心,对提升整体电池性能具有决定性影响力。而复合固态聚合物电解质(CSPEs)在兼顾固态电解质实际应用需求的同时,更有可能突破单一类型固态电解质所面临的局限,成为未来高性能、高安全储能系统中的优选方案。聚氧化乙烯(PEO)基聚合物固态电解质在改善电极界面相容性方面具有优势,是最有潜力的电解质材料之一。鉴于此,本文全面综述了近年来关于PEO基复合固态电解质体系的前沿研究成果,主要聚焦于填料-聚合物和填料-锂盐的相互作用,最后,对CSPEs的未来发展和优化设计做出展望。
方法通过搜集整理并分析现有文献,对PEO基复合固态电解质做出概述,并探讨离子传输相关机制,总结了PEO-惰性填料和PEO-活性填料复合固态电解质体系的设计、制备、性能及机制。将惰性填料添加到PEO基体中,可以增加PEO的非晶相,降低玻璃化转变温度,此外,通过其表面化学基团与锂盐阳离子/阴离子间较强的路易斯酸碱相互作用,可以促进锂盐解离和离子输运,增强CSPEs的电化学稳定性;而活性填料不仅保留了惰性填料的一般功能,还可以在活性填料区域和填料-聚合物界面构建额外高效的Li输运通道参与离子传导过程,提高离子电导率。
结果从协同效应角度综述了无机填料在PEO基复合固态电解质的研究进展,系统阐述了PEO与无机填料间的协同作用,及其对复合固态电解质的离子传输性和界面相容性的影响机制,并对当前CSPEs领域的相关进展进行总结,针对该领域现存挑战提出见解,同时对未来发展方向做出分析与展望。
结论探索PEO基复合固态电解质依然存在挑战,未来研究可从以下几方面进行深入研究:(1)目前室温下CSPEs的离子电导率仅为10 S·cm左右,无法满足实际应用所需的10 S·cm标准。这一问题可通过增加填料的表面缺陷改变聚合物/锂盐的相互作用、Li的局部环境以及Li传输的活化能进一步改善;(2)CSPEs中离子传输的具体机制尚未阐明,这直接阻碍了离子电导率水平的进一步提升。将理论计算与先进表征技术相结合,特别是原位测试技术,通过研究Li在CSPEs内的局部结构环境和动力学行为,验证实验结果并阐明离子传导增强机制有望解决这一难题;(3)无机填料-聚合物相互作用的有待深入研究。惰性填料(如AlO、SiO、TiO等)和活性填料(如LLZTO、LLZO、LATP等)种类对CSPEs离子电导率和锂离子迁移数的影响仍缺乏系统研究,明确这些相关机制虽充满挑战,但对优选适用于CSPEs的无机填料具有重大意义。随着研究不断深入,CSPEs有望实现应用,这对突破电池能量密度和安全性瓶颈具有极为重要的意义。





 下载:
下载: