Adsorption performance and mechanism of polyethyleneimine cross-linked bentonite for Cr(VI) in aqueous solution
-
摘要:
为提高膨润土的吸附容量,通过交联反应将聚乙烯亚胺(PEI)引入3-氨丙基三乙氧基硅烷(APTES)改性膨润土(APTES/Bent)表面制备得到PEI交联膨润土(PEI-APTES/Bent-4),并采用FTIR、XRD和SEM等手段对其进行表征分析。以水中Cr(Ⅵ)为吸附对象,考察了PEI-APTES/Bent-4的吸附性能,探究了吸附机制和回收利用性。结果表明:PEI成功接枝于膨润土表面,其丰富的活性基团极大地促进了六价铬的去除。吸附最佳pH为2,随pH值增加吸附量降低。PEI-APTES/Bent-4对Cr(Ⅵ)的吸附符合Langmuir等温模型和拟二级动力学模型,吸附过程为化学吸附和单层吸附,在313 K时最大理论吸附量达137.50 mg·g−1。热力学研究表明该吸附为自发吸热过程。结合吸附实验、FTIR和XPS分析推测得出PEI-APTES/Bent-4对Cr(Ⅵ)的吸附机制主要为静电作用、还原和螯合。经6次循环后吸附剂仍保持较好的吸附性能。PEI-APTES/Bent-4去除水中Cr(Ⅵ)具有较大的应用前景。
Abstract:In order to improve the adsorption capacity of bentonite, polyethyleneimine (PEI) was introduced onto the surface of 3-aminopropyltriethoxysilane (APTES)-modified bentonite (APTES/Bent) by crosslinking reaction to prepare PEI-crosslinked bentonite (PEI-APTES/Bent-4), which was characterised by FTIR, XRD and SEM. Taking Cr(VI) in water as the adsorption target, the adsorption performance of PEI-APTES/Bent-4 was investigated, and its adsorption mechanism and recyclability were explored. The results showed that PEI was successfully grafted onto the surface of bentonite, and the abundant active groups of PEI dramatically promoted the removal of Cr(VI). The optimum pH for adsorption was 2, and the adsorption capacity decreased with increasing pH. The adsorption of Cr(VI) by PEI-APTES/Bent-4 conformed to the Langmuir isotherm model and pseudo-second-order kinetic model, and the adsorption process was chemical adsorption and monolayer adsorption. The maximum theoretical adsorption capacity reached 137.50 mg·g−1 at 313 K. Thermodynamic studies indicated that the adsorption was a spontaneous endothermic process. Based on the adsorption experiments, FTIR and XPS analysis, it is speculated that the adsorption mechanism of PEI-APTES/Bent-4 for Cr(VI) is mainly electrostatic interaction, reduction, and chelation. After six cycles, the adsorbent still maintained good adsorption performance. PEI-APTES/Bent-4 has broad application prospects for the removal of Cr(VI) from water.
-
Keywords:
- polyethyleneimine /
- bentonite /
- Cr(VI) /
- cross-linked /
- adsorption
-
钨被认为是核聚变堆中面向等离子体第一壁的候选材料[1-3],但其存在韧脆转变温度高、再结晶温度低、抗高温氧化性能差、辐照脆化、高热负荷开裂熔化、等离子刻蚀严重等缺点。这些缺点导致纯W无法完全满足未来核聚变堆服役的环境需求,因此,探索开发新型W基材料是推进核聚变反应堆应用的关键之一。
难熔高熵合金(Refractory high-entropy alloys,RHEAs)[4]是以等摩尔比或近等摩尔比混合多种高熔点元素,如Hf、Nb、Ta、Mo、W 等前过渡族金属元素,具有一些传统合金无法比拟的优异性能,如高强度、高硬度、高耐磨性能、高温稳定性、抗氧化性能及抗辐照性能等[5-10]。因此,难熔高熵合金在核聚变堆中具有潜在的应用前景。
2019年,El-Atwani等[11]采用磁控溅射法制备了纳米晶WTaCrV高熵合金薄膜,该合金薄膜具有高硬度和杰出的抗辐照性能。Waseem等[12]采用机械混粉和放电等离子体烧结技术制备出块体WTaCrVTix高熵合金,研究了Ti对高熵合金组织结构的影响,发现Ti有助于BCC固溶体的形成,当Ti含量为7at%时,合金的压缩强度和硬度分别为
2069 MPa和714 HV。由于W、Ta、V、Cr和Ti元素具有低中子活化特性,可用于核聚变堆的中子辐照环境中,因此,WTaCrVTi系高熵合金开始引起研究者的关注。WTaCrVTi合金的制备一般采用磁控溅射[11, 13-14]、电弧熔炼法[15-18]和粉末冶金法[19-21]。由于合金含有多种组元,制备过程中易发生元素偏析和富集,不仅造成合金中固溶体的各组元比例偏离等原子比,而且存在大量富Ta相、富Cr相、Laves相和氧化物等,合金的组织结构不均匀。Y是一种常用的合金元素,烧结过程中它能促使其他高熔点金属元素的扩散,改善钨合金的组织结构[22]。另外,Y与O有更高的反应活性,可形成Y2O3颗粒, Y2O3弥散分布在基体中,钉扎在晶界处,可抑制晶粒长大[23]。
因此,为了进一步改善WTaCrVTi合金的组织结构,本文采用机械合金化(Mechanical alloying,MA)结合放电等离子体烧结(Spark plasma sintering,SPS)制备了WTaCrVTi6Yx高熵合金,研究了Y含量对合金的组织结构和力学性能的影响。
1. 实验材料及方法
实验原材料为W粉(纯度99.98%,1~5 μm,阿拉丁),Ta粉(纯度99.9%,~45 μm,阿拉丁),Cr粉(纯度 99.5%,≥45 μm,阿拉丁),V粉(纯度 99.5%,≥45 μm,阿拉丁),Ti粉(纯度99.99%,≥38 μm,阿拉丁)和Y粉(纯度99.9%,阿拉丁)。采用文献[3]的MA和SPS工艺制备高熵合金。按照W23.5−x/4Ta23.5−x/4Cr23.5−x/4V23.5−x/4Ti6Yx(x=0at%、2at%、4at%、6at%)计量比称量,在QM-3 SP4型(南京南大仪器有限公司)行星球磨机上进行球磨,转速为250 r/min,球磨时间为40 h。将球磨后的粉末在日本 SinterLand 的 LABOX-1575型放电等离子体烧结炉中进行烧结,以100℃/min的速率升温至
1500 ℃,在50 MPa压力下保温10 min,然后随炉冷却。最终获得圆柱形烧结试样,直径为20 mm,厚度为4 mm。为了便于叙述,不同Y含量的粉末或合金记为Yx (x表示Y元素原子比)。将圆柱体试样的上下两面依次采用30 μm、15 μm金刚石砂纸打磨,然后采用线切割技术将烧结样品切割为直径4 mm、高6 mm的圆柱形压缩试样。
采用X射线衍射仪(XRD,X'pert PRO,荷兰)分析材料的物相组成,管电压40 kV,管电流30 mA,射线源为Cu-Kα;采用扫描电子显微镜(SEM,Nova NanoSEM 450,荷兰)、能谱仪(EDS)及电子探针X射线显微分析仪(EPMA,JXA-8530F PLUS,日本)分别检测材料的显微结构和成分。
采用万能材料试验机(Universal Material Tester,Zwick Z020,德国)测试合金的压缩强度;采用维氏硬度仪(Wilson Hardness,430SVD,美国)测定合金的硬度,负载为15 kg,保压时间 10 s,测量5次后取平均值。
合金块体使用 ZY-300Z型密度天平(扬州正艺试验机械有限公司)测量样品的实际密度ρa,其原理是通过阿基米德排水法测量。利用无序固溶体的理论密度公式(1)计算高熵合金的理论密度ρtheor,公式如下:
ρtheor=∑CiAi∑CiAi/ρi (1) 式中:ρi为组元的密度;Ai为组元的原子质量;Ci为组元的含量。
根据下式计算合金的相对密度Rc:
Rc=ρaρtheor×100% (2) 2. 结果与讨论
2.1 不同Y含量的合金粉末的显微结构
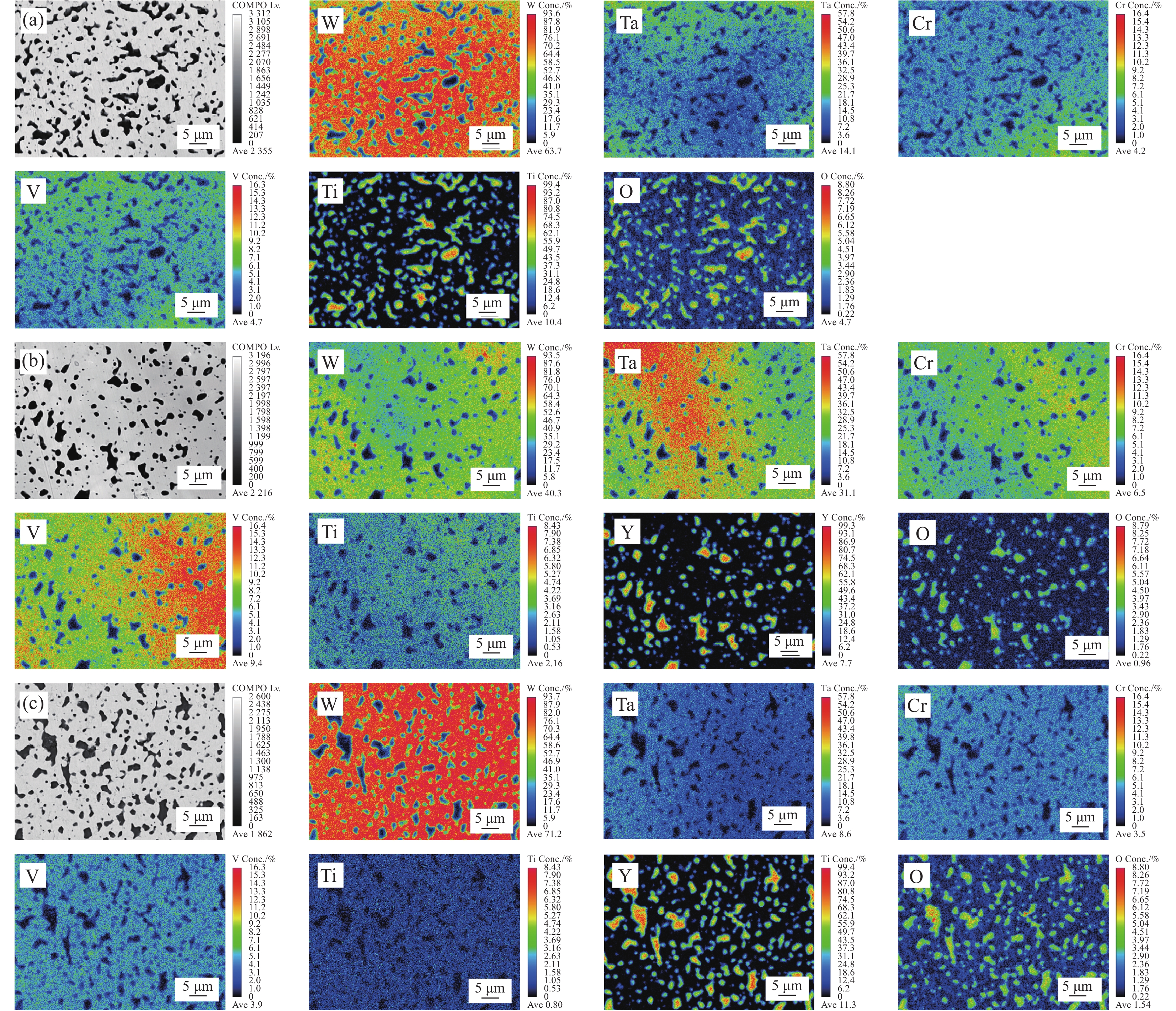
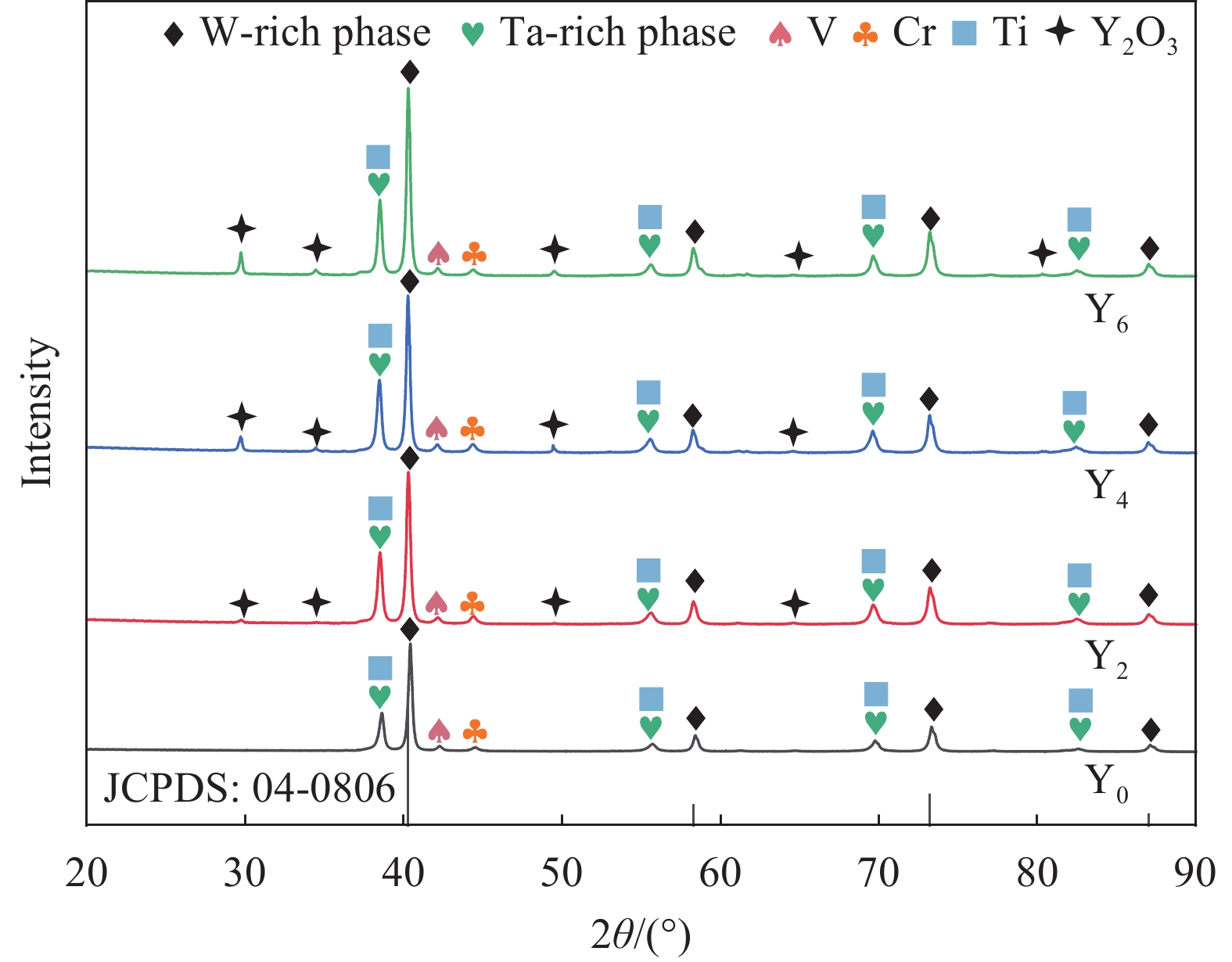
图1是不同Y含量的合金粉末的XRD图谱。从图谱中可观测到W、Ta、Cr、V、Ti各组元的衍射峰,在添加Y的粉末中还观察到了Y2O3的衍射峰。相较Ti、Cr等元素,Y对O元素的亲和力最大。因此,在球磨过程中,Y会与粉末中残留的O2优先反应形成稳定的Y2O3,并随着Y含量的增加,Y2O3衍射峰的强度明显增加。
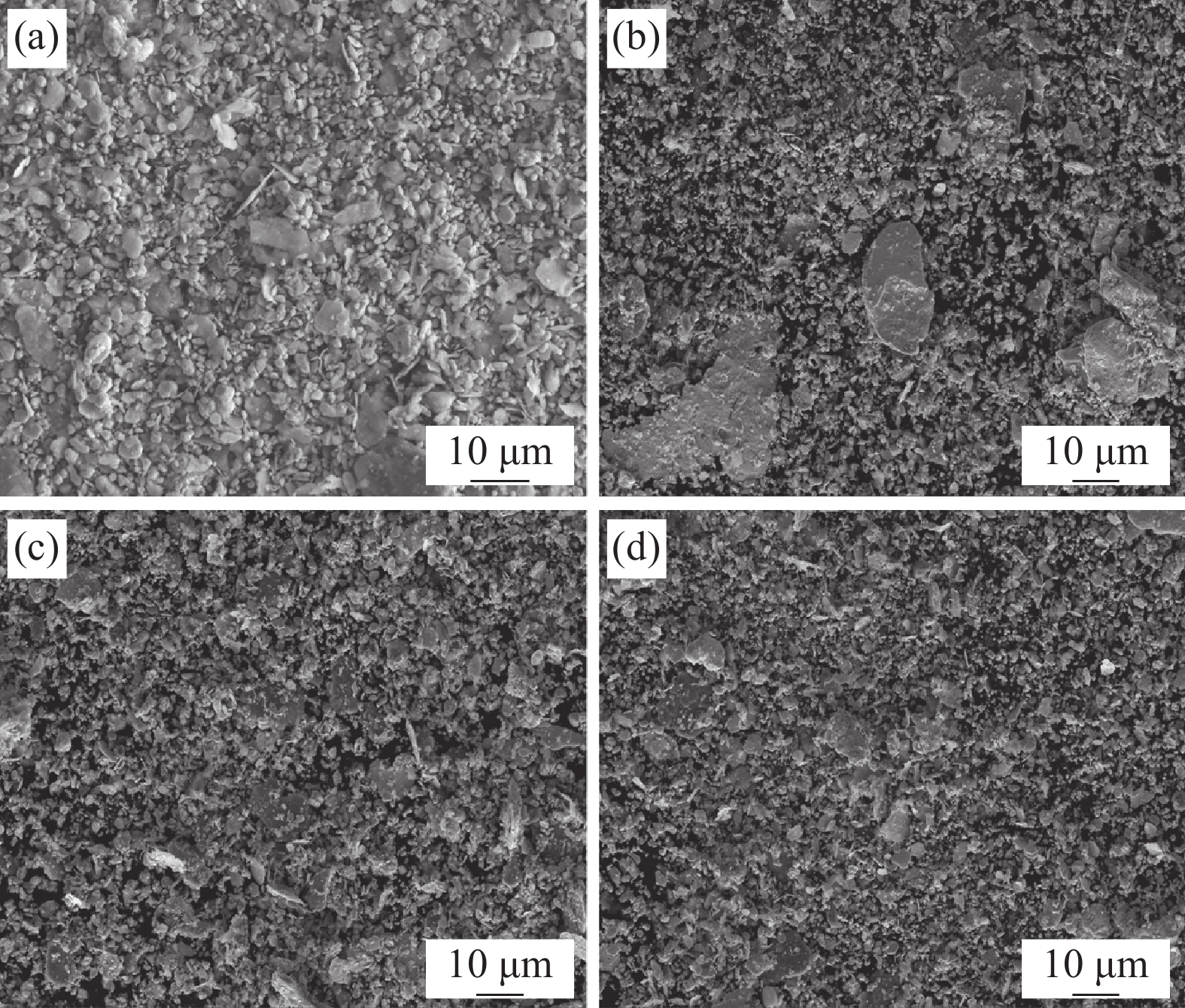
图2为合金粉末的背散射SEM图像。随着Y含量的增加,合金粉末的形态由不规则片状逐渐变为小尺寸的等轴颗粒,形态和尺寸趋于均匀。这表明Y的添加可促进合金粉末颗粒的细化。这是由于Y在高能球磨过程中,可与各元素固溶,从而引起粉末颗粒硬化,降低粉末的塑性变形能力,使粉末破碎为小尺寸的细颗粒。添加的Y含量越多,Y固溶的越多,粉末细化效果越显著[24]。合金粉末颗粒的细化使其具有更大的表面积和更高的反应活性,可以促进烧结组织的致密化和均匀化。
2.2 不同Y含量的合金的组织结构
将不同Y含量的合金粉末在
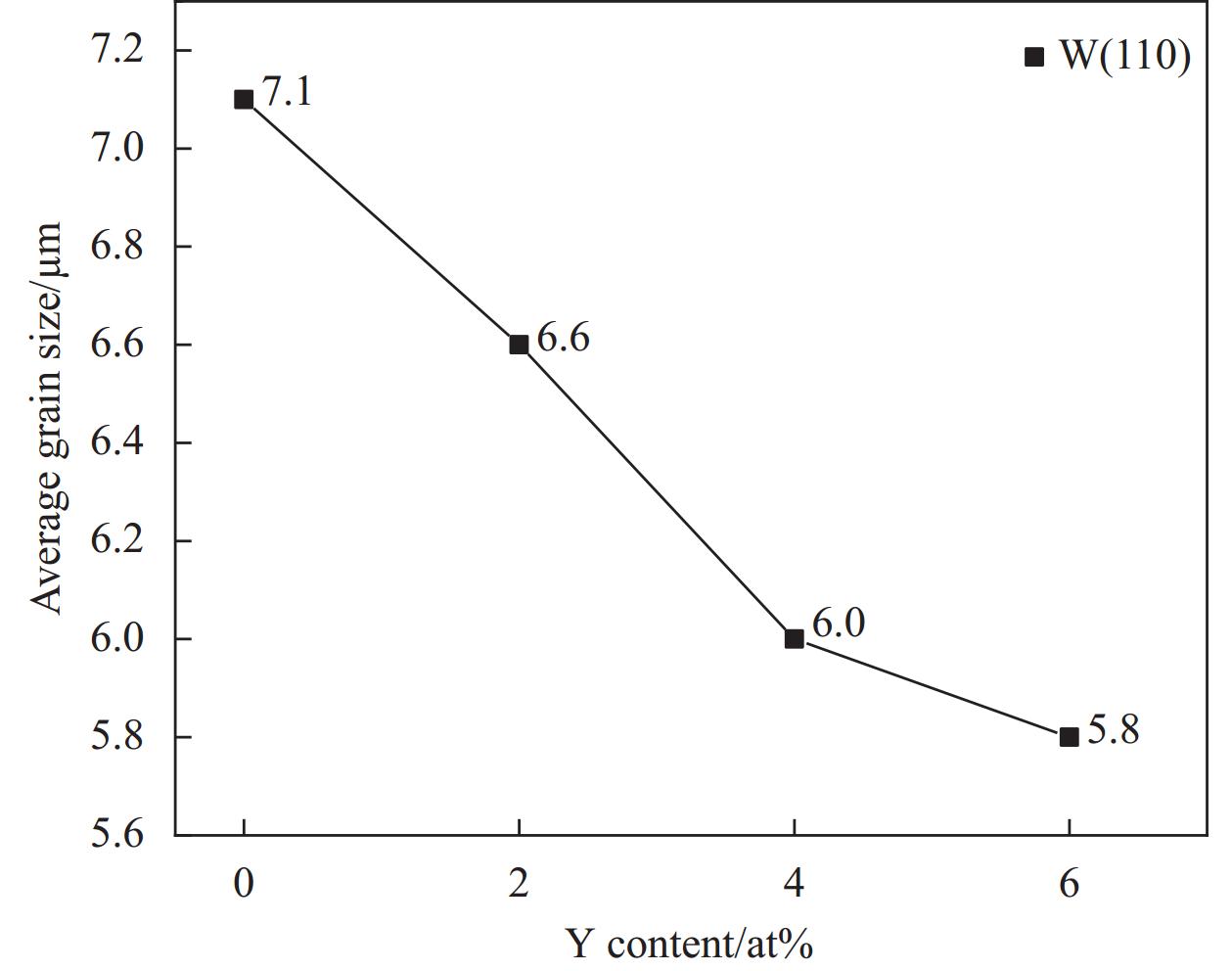
1500 ℃进行SPS烧结,得到块体合金。图3是不同Y含量的WTaCrVTi6Yx高熵合金的XRD图谱。所有合金均包含BCC相和FCC相,Y0、Y2和Y4合金中还观察到Laves相。随着Y含量的增加,合金中的FCC相和Laves相的衍射峰的数量和强度逐渐下降。另外,添加Y的Y2、Y4和Y6合金中观察到了Y2O3衍射峰。根据谢乐公式(3)[25]计算出各合金中BCC相的平均晶粒尺寸,如图4所示。可见,随着Y含量增加,BCC相的平均晶粒尺寸降低,表明Y元素可细化合金的组织结构。
d=0.89λW2θcosθ(hkl) (3) 式中:d为平均晶粒尺寸;λ为X射线衍射束波长(Cu靶,
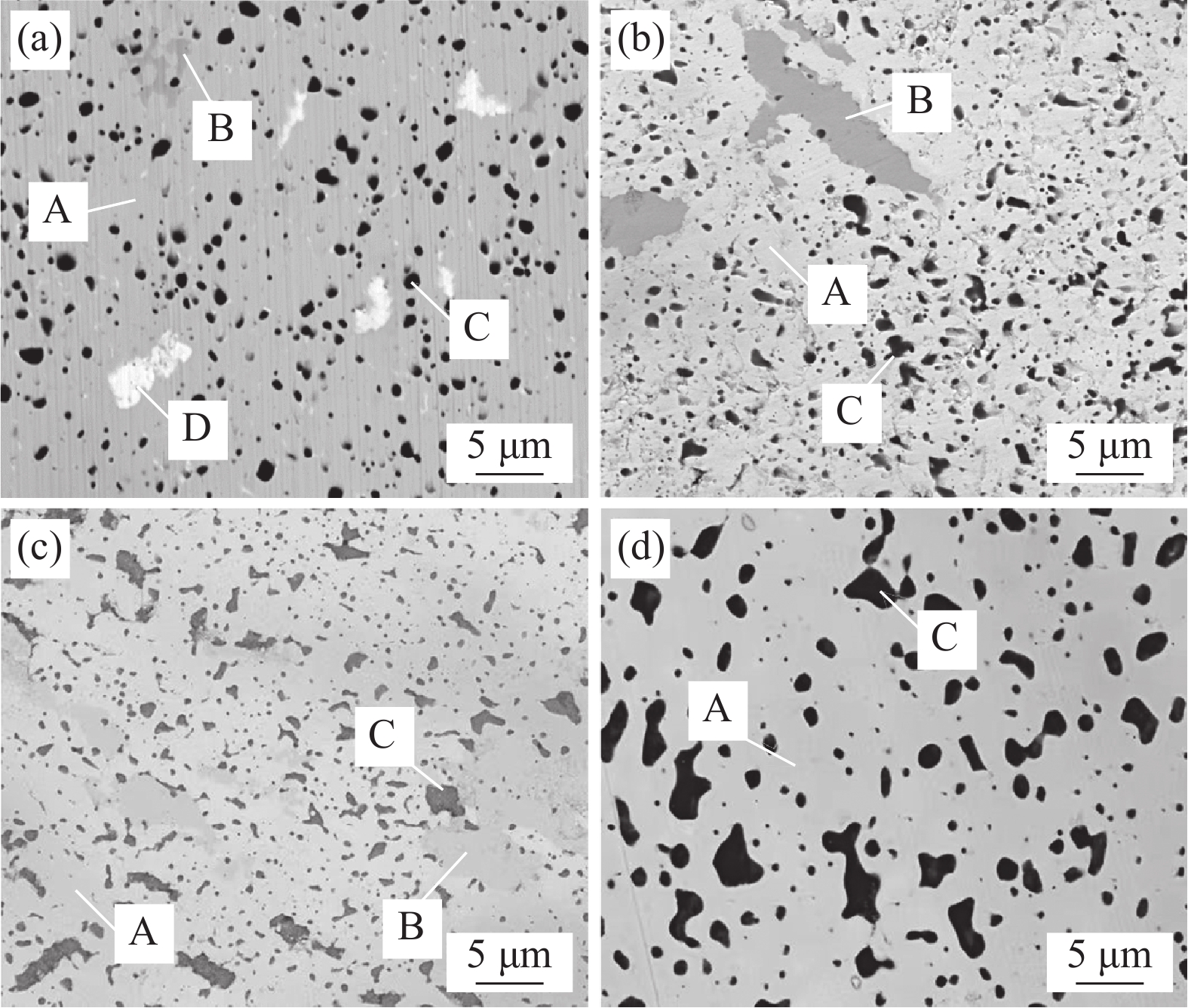
0.15406 nm);θ为衍射角;W2θ为半峰宽。图5是不同Y含量的WTaCrVTi6Yx高熵合金的背散射SEM图像。Y0合金包含A、B、C、D相;Y2和Y4合金包含A、B、C相,未观察到D相;Y6合金主要包含A和C相。对不同Y含量的合金的各个相进行EDS分析,结果如表1所示。结合XRD,可知A为BCC结构的WTaCrVTi固溶体;B为Laves相,富含Ta、Cr、V元素;C为 TiO或Ti/Y混合氧化物或Y2O3;D为富Ta相。
表 1 WTaCrVTi6Yx高熵合金中各个相的元素含量Table 1. Elemental content of individual phases in WTaCrVTi6Yx high-entropy alloysAlloy Area Element content/at% Phase W Ta Cr V Ti Y O Y0 A 24.49 24.36 24.54 24.55 2.06 0 0 Solid solution B 9.16 26.94 41.57 17.99 0.79 0 3.55 Laves C 1.08 1.42 2.98 6.81 38.11 0 49.60 TiO D 1.95 91.68 0.89 1.91 0.47 0 3.10 Ta Y2 A 43.90 25.29 14.90 12.56 3.35 0 0 Solid solution B 8.47 34.01 36.47 18.93 2.12 0 0 Laves C 0 0 0 0 17.49 17.40 65.11 (Ti/Y)-O Y4 A 30.84 28.21 19.27 16.26 5.42 0 0 Solid solution B 8.13 33.75 39.25 15.07 3.79 0 0 Laves C 0 0 0 0 0 38.86 61.14 Y2O3 Y6 A 25.12 24.94 22.03 23.66 4.25 0 0 Solid solution C 0 0 0 0 0 39.14 60.85 Y2O3 Y0合金中,BCC结构的固溶体连续分布,为基体,固溶体的W、Ta、Cr、V的原子比趋于等原子比,黑色TiO颗粒均匀分布在基体中,平均颗粒尺寸为(1.08±0.38) μm,Laves相和富Ta相零星分布在基体中。在含Cr的RHEAs中经常观察到Laves相的形成[26-28],这是由于Cr和其他元素之间的原子半径差异大,有利于AB2型Laves相的形成,如TaCr2。另外,Ti的添加,还可能促进TaV2、VTa2和TiCr2[12]等Laves相的形成。因此,Laves相富含Ta、Cr、V元素。Y2合金中,固溶体中的W、Ta、Cr、V的原子比偏离等原子比,Cr和V的含量偏少,黑色颗粒为TiO和Y2O3的混合颗粒,局部出现团聚现象,Laves相呈不规则形状,富Ta相消失。Y4合金的组织结构与Y2合金相似,但固溶体中的Cr和V的含量相对Y2合金有所增加,黑色颗粒主要为Y2O3。Y6合金主要包含BCC结构的固溶体和黑色Y2O3颗粒,未观察到明显的Laves相,固溶体的W、Ta、Cr、V的原子比趋于1。Ti和Y具有良好的亲氧性,在烧结过程中,它们会与粉末中残留的O反应生成黑色TiO或Y2O3。但Y的亲氧能力高于Ti,因此,有Y存在且充足的情况下,合金的氧化物颗粒以Y2O3为主。另外,含Y合金的BCC相中的Ti含量(3.35at%~5.42at%)均高于未含Y合金(2.06at%),表明Y的添加有助于各元素的互溶,从而促进BCC结构固溶体的形成。
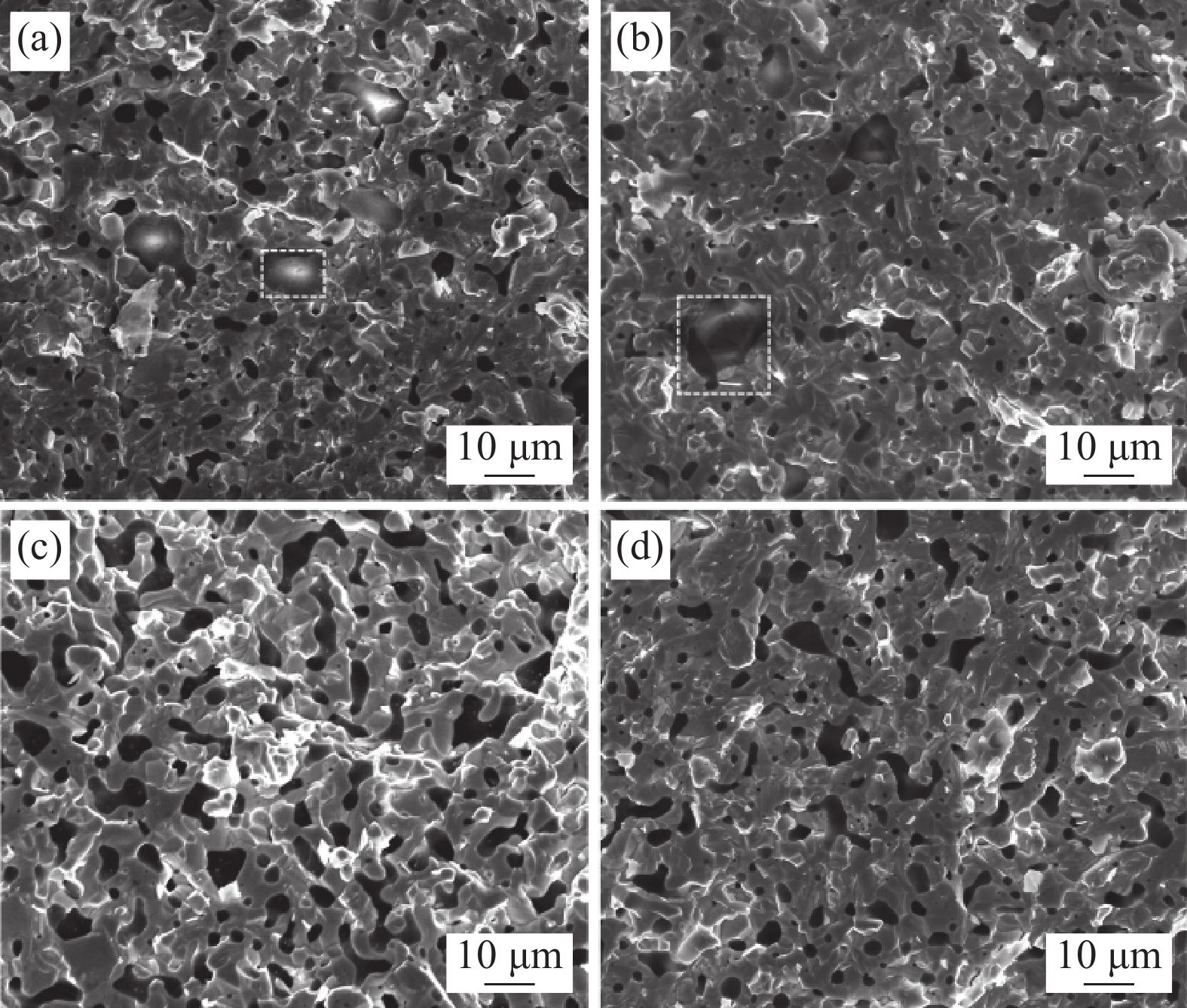
图6是不同Y含量的WTaCrVTi6Yx高熵合金断口的SEM图像。BCC相的平均晶粒尺寸分别为6.96 μm、6.35 μm、5.80 μm、5.05 μm。这进一步验证了XRD的计算结果,表明Y元素可细化合金的BCC组织结构。这可能是由于Y与O易形成Y2O3颗粒, Y2O3弥散分布在基体中,钉扎在晶界处,抑制了晶粒长大[23]。
图7分别是Y0、Y4和Y6合金的电子探针X射线显微分析(EPMA)图像。Y0和Y4图像中, Ta、Cr、V等元素在BCC相中分布不均,Y0中,黑色颗粒主要为TiO,而Y4中的黑色颗粒主要为Y2O3。当Y的添加量达到6at%,EPMA图像表明BCC相中W、Ta、Cr、V和Ti元素分布均匀,这是由于,如前所述,Y的添加可促进合金粉末颗粒的细化,使其具有更大的表面积和更高的反应活性,可以促进烧结组织的致密化和均匀化。另外,SPS烧结温度为
1500 ℃,但实际温度一般高于该温度100~300℃,因此,低熔点的Y (熔点为1522 ℃)有可能熔化形成液相,液相可促使其他固相组分的相互扩散,有利于均匀BCC相的形成。该合金中黑色颗粒主要为Y2O3,平均尺寸约为(1.25±0.85) μm。2.3 合金的力学性能
表2为WTaCVTi6Yx合金的密度。由表可知,采用MA和SPS制备的合金致密,合金的相对密度均在99.8%~99.9%。
表 2 WTaCrVTi6Yx合金的密度Table 2. Density of the WTaCrVTi6Yx alloysAlloy Actual density/
(g·cm−3)Theoretical density/
(g·cm−3)Relative
density/%Y0 12.398 12.407 99.9 Y2 12.026 12.056 99.8 Y4 11.706 11.722 99.8 Y6 11.399 11.402 99.9 图8分别为WTaCVTi6Yx高熵合金的室温压缩强度和显微硬度。添加Y的合金的室温压缩强度和显微硬度均比未添加Y的合金的小。这可能是由于氧化物颗粒的组成、含量和分布发生变化造成的。相对含Y的合金,Y0合金的氧化钛颗粒细小,且均匀分布在基体中,起到了更好的强化作用。对于含Y合金,随着Y含量的增加,合金的室温压缩强度和显微硬度也随之增强,Y6合金的室温压缩屈服强度和硬度达到最高,分别为
2674 MPa和(848.6±9.3) HV。这是由于,随Y含量增加,一方面BCC相的晶粒尺寸减小,起到细晶强化作用,另一方面BCC相的各元素含量趋于等原子比,具有更高的混合熵效应所致。3. 结 论
(1)随着Y含量的增加,合金的晶粒尺寸减小,FCC相和Laves相的数量降低。当Y的添加量为6at%时,合金主要包含BCC结构固溶体和Y2O3颗粒,Y2O3颗粒的平均尺寸约为(1.25±0.85) μm,均匀分布在基体中,固溶体的W、Ta、Cr、V的原子比趋于1。
(2) Y6合金的室温压缩屈服强度和硬度达到最高,分别为
2674 MPa和(848.6±9.3) HV。这是由于,随Y含量增加,一方面BCC相的晶粒尺寸减小,另一方面BCC相的各元素含量趋于等原子比,合金具有更高的混合熵和晶粒细化效应。 -
1 PEI-APTES/Bent-4与其他改性膨润土的Cr(VI)吸附量比较
Adsorbent qm/mg·g-1 Ref. CTMAB/Bent 27.472 [37] AC-Fe3O4/Bent 29.32 [38] Citric acid/MBent 16.67 [39] polyacrylic acid-Al/Bent 3.125 [40] Fe3O4-PDA-SDBS/Bent 103.6 [41] Chitosan-NaOH/Bent 2.72 [42] Cetylpyridinium chloride/Bent 46.03 [43] Chitosan/Bent 16.40 [44] PEI-APTES/Bent-4 137.50 This study Notes: CTMAB—Cetyltrimethylammonium bromide; MBent—Magnetic Bentonite; AC—Activated Carbon; PDA—Polydopamine; SDBS—Sodium dodecyl benzene sulfonate. 表 1 不同吸附剂的PEI掺杂量
Table 1 PEI doping amount of different adsorbents
Adsorbent Doping mass of
APTES/Bent/gDoping mass of
PEI/gPEI-APTES/Bent-0.5 6 0.5 PEI-APTES/Bent-1 6 1 PEI-APTES/Bent-2 6 2 PEI-APTES/Bent-3 6 3 PEI-APTES/Bent-4 6 4 PEI-APTES/Bent-5 6 5 表 2 PEI-APTES/Bent-4对Cr(Ⅵ)的吸附动力学参数
Table 2 Kinetic model fitting parameters for Cr(Ⅵ) adsorption on PEI-APTES/Bent-4
Adsorbent Pseudo-first-order Pseudo-second-order qe/(mg·g−1) K1/min−1 R2 qe/(mg·g−1) K2/(g·mg−1·min−1) R2 PEI-APTES/Bent-4 78.39 0.1286 0.9898 131.06 0.0076 0.9997 Notes: K1—Quasi-first-order kinetic model constant; K2—Quasi-second-order kinetic model constant; R2—Correlation coefficient. 表 3 Langmuir和Freundlich模型参数
Table 3 Langmuir and Freundlich model parameters
T/K Langmuir Freundlich qm/(mg·g−1) KL/(L·mg−1) RL R2 KF/(mg1-(1/n)·L1/n·g−1) n R2 293 132.02 0.4046 0.0049 -0.1099 0.9869 58.98 6.475 0.8479 303 135.68 0.6558 0.0030 -0.0708 0.9627 65.12 6.893 0.8711 313 137.50 1.2208 0.0016 -0.03935 0.9565 71.11 7.468 0.8835 Notes: qm—Maximum adsorption capacity; KL—Adsorption equilibrium constant of Langmuir model; KF—Adsorption equilibrium constant of Freundlich model; n—Adsorption strength constant in the Freundlich model; RL—Separation constant. 表 4 PEI-APTES/Bent-4与其他改性膨润土Cr(VI)吸附量比较
Table 4 Comparison of Cr(VI) adsorption capacity between PEI-APTES/Bent-4 and other modified bentonite
Adsorbent Maximum adsorption capacity/(mg·g−1) Ref. CTMAB/Bent 27.472 [37] AC-Fe3O4/Bent 29.32 [38] Citric acid/MBent 16.67 [39] Polyacrylic acid-Al/Bent 3.125 [40] Fe3O4-PDA-SDBS/Bent 103.6 [41] Chitosan-NaOH/Bent 2.72 [42] Cetylpyridinium chloride/Bent 46.03 [43] Chitosan/Bent 16.40 [44] PEI-APTES/Bent-4 137.50 This study Notes: CTMAB—Cetyltrimethylammonium bromide; AC—Activated carbon; PDA—Polydopamine; MBent—Magnetic bentonite; SDBS—Sodium dodecyl benzene sulfonate. 表 5 吸附Cr(Ⅵ)的热力学参数
Table 5 Thermodynamic parameters for adsorption of Cr(Ⅵ)
T/K ΔG0/(kJ·mol−1) ΔH0/(kJ·mol−1) ΔS0/(J·mol−1·K−1) 293 −6.511 303 −7.439 23.73 103.11 313 −8.578 Notes: ∆G0—Gibbs free energy change; ∆H0—Enthalpy change; ∆S0—Entropy change. -
[1] 曾涛涛, 农海杜, 沙海超, 等. 污泥基生物炭负载纳米零价铁去除Cr(VI)的性能与机制[J]. 复合材料学报, 2023, 40(2): 1037-1049. ZENG Taotao, NONG Haidu, SHA Haichao, et al. Performance and mechanism of Cr(VI) removal by sludge-derived biochar loaded with nanoscale zero-valent iron[J]. Acta Materiae Compositae Sinica, 2023, 40(2): 1037-1049(in Chinese).
[2] ZHAO C, WANG Z H, LI X, et al. Facile fabrication of BUC-21/Bi24O31Br10 composites for enhanced photocatalytic Cr(VI) reduction under white light[J]. Chemical Engineering Journal, 2020, 389: 123431. DOI: 10.1016/j.cej.2019.123431
[3] SHAW P, MONDAL P, BANDYOPADHYAY A, et al. Environmentally relevant concentration of chromium induces nuclear deformities in erythrocytes and alters the expression of stress-responsive and apoptotic genes in brain of adult zebrafish[J]. Science of the Total Environment, 2020, 703: 135622. DOI: 10.1016/j.scitotenv.2019.135622
[4] PAN Z Z, ZHU X M, SATPATHY A, et al. Cr(VI) adsorption on engineered iron oxide nanoparticles: Exploring complexation processes and water chemistry[J]. Environmental Science & Technology, 2019, 53(20): 11913-11921.
[5] LI M H, HE J, TANG Y Q, et al. Liquid phase catalytic hydrogenation reduction of Cr(VI) using highly stable and active Pd/CNT catalysts coated by N-doped carbon[J]. Chemosphere, 2019, 217: 742-753. DOI: 10.1016/j.chemosphere.2018.11.007
[6] KONG Q P, WEI J Y, HU Y, et al. Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr(VI)[J]. Journal of Hazardous Materials, 2019, 363: 161-169. DOI: 10.1016/j.jhazmat.2018.09.084
[7] TU B Y, WEN R T, WANG K Q, et al. Efficient removal of aqueous hexavalent chromium by activated carbon derived from Bermuda grass[J]. Journal of Colloid and Interface Science, 2020, 560: 649-658. DOI: 10.1016/j.jcis.2019.10.103
[8] LIM S J, KIM T H. Combined treatment of swine wastewater by electron beam irradiation and ion-exchange biological reactor system[J]. Separation and Purification Technology, 2015, 146: 42-49. DOI: 10.1016/j.seppur.2015.03.021
[9] SUN J M, SHANG C, HUANG J C. Co-removal of hexavalent chromium through copper precipitation in synthetic wastewater[J]. Environmental Science & Technology, 2003, 37: 4281-4287.
[10] LI D, WEI Y Y, WANG Y J, et al. A two-dimensional lamellar membrane: MXene nanosheet stacks[J]. Angewandte Chemie-International Edition, 2017, 56: 1825-1829. DOI: 10.1002/anie.201609306
[11] LI Y M, GAO Y Y, ZHANG Q, et al. Flexible and free-standing pristine polypyrrole membranes with a nanotube structure for repeatable Cr(VI) ion removal[J]. Separation and Purification Technology, 2021, 258: 117981. DOI: 10.1016/j.seppur.2020.117981
[12] GAO Y, CHEN C L, TAN X L, et al. Polyaniline-modified 3D-flower-like molybdenum disulfide composite for efficient adsorption/photocatalytic reduction of Cr(VI)[J]. Journal of Colloid and Interface Science, 2016, 476: 62-70. DOI: 10.1016/j.jcis.2016.05.022
[13] WANG W J, NIU Q Y, ZENG G M, et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction[J]. Applied Catalysis B: Environment and Energy, 2020, 273: 119051. DOI: 10.1016/j.apcatb.2020.119051
[14] NOROUZIAN R S, LAKOURAJ M M. Preparation and heavy metal ion adsorption behavior of novel supermagnetic nanocomposite of hydrophilic thiacalix[4]arene self-doped polyaniline: Conductivity, isotherm, and kinetic study[J]. Advances in Polymer Technology, 2017, 36(1): 107-119. DOI: 10.1002/adv.21580
[15] SHARIFUL M I, SHARIF S B, LEE J J L, et al. Adsorption of divalent heavy metal ion by mesoporous-high surface area chitosan/poly(ethylene oxide) nanofibrous membrane[J]. Carbohydrate Polymers, 2017, 157: 57-64. DOI: 10.1016/j.carbpol.2016.09.063
[16] LI Y X, CHEN Z, SHI Y Y, et al. Function of c-type cytochromes of Shewanella xiamenensis in enhanced anaerobic bioreduction of Cr(VI) by graphene oxide and graphene oxide/polyvinyl alcohol films[J]. Journal of Hazardous Materials, 2020, 387: 122018. DOI: 10.1016/j.jhazmat.2020.122018
[17] BIN Y L, LIANG Q W, LUO H J, et al. One-step synthesis of nitrogen-functionalized graphene aerogel for efficient removal of hexavalent chromium in water[J]. Environmental Science and Pollution Research, 2023, 30: 6746-6757. DOI: 10.1007/s11356-022-22591-y
[18] XU Y L, CHEN J Y, CHEN R, et al. Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent[J]. Water Research, 2019, 160: 148-157. DOI: 10.1016/j.watres.2019.05.055
[19] NOWRUZI R, HEYDARI M, JAVANBAKHT V. Synthesis of a chitosan/polyvinylalcohol/activate carbon biocomposite for removal of hexavalent chromium from aqueous solution[J]. International Journal of Biological Macromolecules, 2020, 147: 209-216. DOI: 10.1016/j.ijbiomac.2020.01.044
[20] SULISTIYO C D, CHENG K C, SU'ANDI H J, et al. Removal of hexavalent chromium using durian in the form of rind, cellulose, and activated carbon: Comparison on adsorption performance and economic evaluation[J]. Journal of Cleaner Production, 2022, 380: 135010. DOI: 10.1016/j.jclepro.2022.135010
[21] ZHAO J X, HE J, LIU L, et al. Self-cross-linking of metal-organic framework (MOF-801) in nanocellulose aerogel for efficient adsorption of Cr(VI) in water[J]. Separation and Purification Technology, 2023, 327: 124942. DOI: 10.1016/j.seppur.2023.124942
[22] LEE J H, PARK J A, KIM H G, et al. Most suitable amino silane molecules for surface functionalization of graphene oxide toward hexavalent chromium adsorption[J]. Chemosphere, 2020, 251: 126387. DOI: 10.1016/j.chemosphere.2020.126387
[23] FATMA N A T, EVREN Y, CEKYDA O K, et al. Amino-functionalized SiO2 microbeads optimize photosynthetic performance, gene expression, ROS production and antioxidant status in chromium and copper-exposed Zea mays[J]. Journal of Environmental Chemical Engineering, 2023, 11(6): 111543. DOI: 10.1016/j.jece.2023.111543
[24] CHEN Z L, ZHANG Y N, GUO J Z, et al. Enhanced removal of Cr(VI) by polyethyleneimine-modified bamboo hydrochar[J]. Environmental Science and Pollution Research, 2023, 30: 94185-94194. DOI: 10.1007/s11356-023-29085-5
[25] LIU Y, ZHONG D J, XU Y L, et al. Performance study of phosphate removal from water using synergistic interaction between lanthanum-magnesium bimetallic organic frameworks and polyethyleneimine[J]. Journal of Molecular Liquids, 2024, 396: 124065. DOI: 10.1016/j.molliq.2024.124065
[26] 王子鸣, 赵家印, 秦凯文, 等. 功能化三维石墨烯复合气凝胶对 U(VI) 的吸附行为[J]. 复合材料学报, 2023, 40(11): 6139-6153. WANG Ziming, ZHAO Jiayin, QIN Kaiwen, et al. Adsorption behavior of U(VI) on functionalized three-dimensional graphene composite aerogel[J]. Acta Materiae Compositae Sinica, 2023, 40(11): 6139-6153(in Chinese).
[27] SU S Z, LIU Q, LIU J Y, et al. Polyethyleneimine- functionalized Luffa cylindrica for efficient uranium extraction[J]. Journal of Colloid and Interface Science, 2018, 530: 538-546. DOI: 10.1016/j.jcis.2018.03.102
[28] TANG Y L, LI M H, MU C H, et al. Ultrafast and efficient removal of anionic dyes from wastewater by polyethyleneimine-modified silica nanoparticles[J]. Chemosphere, 2019, 229: 570-579. DOI: 10.1016/j.chemosphere.2019.05.062
[29] SU J J, QIAN J, ZENG W H, et al. Effective adsorption of salvianolic acids with phenylboronic acid functionalized polyethyleneimine-intercalated montmorillonite[J]. Separation and Purification Technology, 2023, 311: 123304. DOI: 10.1016/j.seppur.2023.123304
[30] GUO D M, AN Q D, XIAO Z Y, et al. Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels[J]. Carbohydrate Polymers, 2018, 202: 306-314. DOI: 10.1016/j.carbpol.2018.08.140
[31] BO S F, LUO J M, AN Q D, et al. Efficiently selective adsorption of Pb(II) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation[J]. Journal of Cleaner Production, 2020, 250: 119585. DOI: 10.1016/j.jclepro.2019.119585
[32] YAN Y Z, NAGAPPAN S, YOO J M, et al. Polyethyleneimine-grafted polysilsesquioxane hollow spheres for the highly efficient removal of anionic dyes and selective adsorption of Cr(VI)[J]. Journal of Environmental Chemical Engineering, 2021, 9: 104814. DOI: 10.1016/j.jece.2020.104814
[33] BAO S Y, YANG W W, WANG Y J, et al. PEI grafted amino-functionalized graphene oxide nanosheets for ultrafast and high selectivity removal of Cr(VI) from aqueous solutions by adsorption combined with reduction: Behaviors and mechanisms[J]. Chemical Engineering Journal, 2020, 399: 125762. DOI: 10.1016/j.cej.2020.125762
[34] YANG C Y, JIANG J W, WU Y, et al. High removal rate and selectivity of Hg(II) ions using the magnetic composite adsorbent based on starch/polyethyleneimine[J]. Journal of Molecular Liquids, 2021, 337: 116418. DOI: 10.1016/j.molliq.2021.116418
[35] ZENG H H, WANG L, ZHANG D, et al. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent[J]. Chemical Engineering Journal, 2019, 358: 253-263. DOI: 10.1016/j.cej.2018.10.001
[36] HORRI N, SANZPEREZ El S, ARENCIBIA A, et al. Amine grafting of acid-activated bentonite for carbon dioxide capture[J]. Applied Clay Science, 2019, 180: 105195. DOI: 10.1016/j.clay.2019.105195
[37] ZHANG S Q, YANG W, CHEN R P, et al. Modified geosynthetic clay liners bentonite for barriers of Cr(VI) in contaminated soil[J]. Environmental Technology, 2022, 44(20): 31-39.
[38] YAO L, ESMAEILI H, HAGHANI M, et al. Activated carbon/bentonite/Fe3O4 as novel nanobiocomposite for high removal of Cr(VI) ions[J]. Chemical Engineering & Technology, 2021, 44(10): 1908-1918.
[39] 王迎亚, 施华珍, 张寒冰, 等. 磁性柠檬酸膨润土对六价铬吸附性能的研究[J]. 高校化学工程学报, 2017, 31(3): 726-732. DOI: 10.3969/j.issn.1003-9015.2017.03.030 WANG Yingya, SHI Huazhen, ZHANG Hanbing, et al. Research on Cr(VI) adsorption with magnetic citric acid bentonite[J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(3): 726-732(in Chinese). DOI: 10.3969/j.issn.1003-9015.2017.03.030
[40] 王爽, 郭宏飞, 赵斌, 等. 聚丙烯酸复合铝改性膨润土制备及其对Cr(VI)的吸附[J]. 过程工程学报, 2020, 20(1): 44-51. DOI: 10.12034/j.issn.1009-606X.219141 WANG Shuang, GUO Hongfei, ZHAO Bin, et al. Synthesis of the polyacrylic acid aluminum modified bentonite composite and its adsorption of Cr(VI)[J]. The Chinese Journal of Process Engineering, 2020, 20(1): 44-51(in Chinese). DOI: 10.12034/j.issn.1009-606X.219141
[41] 焦林宏, 汪永丽, 王江北, 等. 纳米磁性聚多巴胺-膨润土的制备及吸附Cr(VI)[J]. 水处理技术, 2019, 45(7): 80-84. JIAO Linhong, WANG Yongli, WANG Jiangbei, et al. Preparation of magnetic nanophase polydopamine/bentonite and their adsorption properties for Cr(VI)[J]. Technology of Water Treatment, 2019, 45(7): 80-84(in Chinese).
[42] 苏建花, 王玉军, 马秀兰, 等. 膨润土改性及对水中Cr(VI) 吸附性能的研究[J]. 华南农业大学学报, 2020, 41(1): 100-107. DOI: 10.7671/j.issn.1001-411X.201906010 SU Jianhua, WANG Yujun, MA Xiulan, et al. Bentonite modification and adsorption capacity for Cr(VI) in water[J]. Journal of South China Agricultural University, 2020, 41(1): 100-107(in Chinese). DOI: 10.7671/j.issn.1001-411X.201906010
[43] SRIKACHA N, SRIUTTHA M, NEERATANAPHAN L, et al. The improvement of natural thai bentonite modified with cationic surfactants on hexavalent chromium adsorption from an aqueous solution[J]. Adsorption Science & Technology, 2022, 2022(20): 13304-13313.
[44] YANG J B, HUANG B, LIN M Z, et al. Adsorption of hexavalent chromium from aqueous solution by a chitosan/bentonite composite: Isotherm, kinetics, and thermodynamics studies[J]. Journal of Chemical & Engineering Data, 2020, 65(5): 2751-2763.
[45] ALSHAKHS F A, GIJJAPU D R, ISLAM M A, et al. A promising palm leaves waste-derived biochar for efficient removal of tetracycline from wastewater[J]. Journal of Molecular Structure, 2024, 1296: 136846. DOI: 10.1016/j.molstruc.2023.136846
[46] MILONJIC S. A consideration of the correct calculation of thermodynamic parameters of adsorption[J]. Journal of the Serbian Chemical Society, 2007, 72: 1363-1367. DOI: 10.2298/JSC0712363M
[47] GENG J J, YIN Y W, LIANG Q W, et al. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism[J]. Chemical Engineering Journal, 2019, 361: 1497-1510. DOI: 10.1016/j.cej.2018.10.141
[48] HUANG J N, CAO Y H, WEN H J, et al. Unraveling the intrinsic enhancement of fluorine doping in the dual-doped magnetic carbon adsorbent for the environmental remediation[J]. Journal of Colloid and Interface Science, 2019, 538: 327-339. DOI: 10.1016/j.jcis.2018.12.002
[49] HE K, WANG S C, LIU Y, et al. Enhanced removal of hexavalent chromium by lignosulfonate modified zero valent iron: Reaction kinetic, performance and mechanism[J]. Science of the Total Environment, 2023, 857: 159397. DOI: 10.1016/j.scitotenv.2022.159397
[50] GUO D M, AN Q D, XIAO Z Y, et al. Polyethylenimine-functionalized cellulose aerogel beads for efficient dynamic removal of chromium(VI) from aqueous solution[J]. RSC Advances, 2017, 7: 54039. DOI: 10.1039/C7RA09940A
[51] LAI Y X, WANG F, ZHANG Y M, et al. UiO-66 derived N-doped carbon nanoparticles coated by PANI for simultaneous adsorption and reduction of hexavalent chromium from waste water[J]. Chemical Engineering Journal, 2019, 378: 122069.
[52] LI Z Y, PAN Z D, WANG Y M, et al. Mechanochemical preparation of ternary polyethyleneimine modified magnetic illite/smectite nanocomposite for removal of Cr(VI) in aqueous solution[J]. Applied Clay Science, 2020, 198: 105832. DOI: 10.1016/j.clay.2020.105832
-
其他相关附件
-
目的
膨润土是储量丰富的天然粘土矿物,作为吸附材料具有巨大的潜力。但其吸附能力有限,且由于带有负电荷,对六价铬阴离子污染物吸附性能较差。针对水体Cr(Ⅵ)污染问题,为提高膨润土吸附容量,考虑氨基官能团对Cr(Ⅵ)具有优异的去除能力,拟通过在膨润土表面引入大量氨基官能团,增加膨润土吸附位点,从而制备对Cr(Ⅵ)具有高吸附容量的膨润土基吸附材料。
方法聚乙烯亚胺(PEI)是含有大量伯胺、仲胺和叔胺基团的水溶性聚合物,对Cr(VI)具有很强的亲和力。3-氨基丙基三乙氧基硅烷(APTES)和戊二醛(GA)被用来作为桥连接将PEI嫁接与膨润土表面。首先将APTES接枝与酸改性活化膨润土(Bent)表面制得APTES改性膨润土(APTES/Bent),APTES既可以为Cr(VI)吸附位点,也可为PEI的引入嫁接点;再利用GA,通过席夫碱反应将PEI引入膨润土表面制得PEI交联膨润土(PEI-APTES/Bent-4)。对Bent、APTES/Bent和PEI-APTES/Bent-4进行分析表征,采用FTIR测定材料表面官能团变化,采用XRD测定材料晶体结构变化,采用SEM-EDS进行形貌和元素分析,采用TGA测量材料的质量损失,采用XPS分析材料吸附前后元素组成和价态。开展吸附实验,测量不同PEI负载量对吸附量的影响;测量pH值对PEI-APTES/Bent-4吸附Cr(VI)的影响;测定接触时间对吸附量的影响,利用准一级、准二级动力学模型进行拟合;测定293 K、303 K和313 K下的吸附等温线,利用Langmuir和Freundlich模型拟合数据;进行吸附热力学计算,得出热力学参数吉布斯能量变化(ΔG)、焓变化(ΔH)和熵变化(ΔS)。进行脱附再生吸附实验,分析吸附材料的循环利用性能。
结果材料的FTIR、XRD、TGA和SEM-EDS分析表征结果表明,发生了席夫碱反应,PEI交联后,PEI-APTES/Bent-4表面引入了更多的氨基,热重损失由10.7%增大为41%,PEI未进入膨润土层间,N元素峰在EDS图谱中更加明显。吸附实验表明,相比APTES/Bent,经PEI交联后,膨润土表面吸附位点增加,对Cr(VI)的吸附容量得到了显著提升。pH值对吸附影响较大,吸附最佳pH值为2,酸性条件下材料Zeta电位为正,在pH<2时,Cr(Ⅵ)主要存在形式包含H2CrO4,不利于静电吸附,pH增大,质子化氨基数量减少,吸附位点减少,吸附量降低。准二级动力学模型相关系数更高,更接近实验吸附结果。Langmuir模型相关系数更高,理论吸附量更接近实验结果,分离系数(0<RL<1),吸附为有利过程。热力学计算得出Δ为负值,Δ为正值。PEI-APTES/Bent-4经6次再生后,对Cr(VI)的吸附量降为原来的74.58%。吸附前后的XPS全扫描图谱证明铬被吸附到PEI-APTES/Bent-4上,Cr2p高分辨率XPS光谱表明吸附后Cr(VI)与Cr(Ⅲ)的存在,吸附涉及吸附和还原过程。
结论利用APTES和GA可成功将PEI交联于膨润土表面,制得聚乙烯亚胺交联膨润土(PEI-APTES/Bent-4); PEI-APTES/Bent-4有更多的氨基官能团,吸附容量显著增加;吸附最佳pH值为2,吸附主要是化学吸附过程,为单分子层吸附,其理论最大吸附量达137.50 mg·g;吸附过程为自发的吸热过程。PEI-APTES/Bent-4具有良好的循环利用性。吸附机制主要为静电吸引、还原和螯合,Cr(Ⅵ)首先通过静电引力吸附与吸附材料表面,随后部分还原为Cr(Ⅲ),Cr(Ⅲ)通过螯合固定在吸附剂表面。
-
针对水体中有毒六价铬阴离子,以膨润土为原料,制备高吸附容量的改性膨润土吸附剂。膨润土是典型的天然粘土矿物,储量丰富。由于天然膨润土直接吸附Cr(Ⅵ)效果较差。为提高吸附容量,在前期3-氨丙基三乙氧基硅烷改性膨润土(APTES/Bent)的基础上,利用含有大量氨基的聚乙烯亚胺(PEI)作进一步交联改性。以戊二醛为交联剂,通过席夫碱反应,继续交联PEI,从而制备高吸附容量膨润土吸附剂PEI-APTES/Bent-4。该方法所制备的膨润土吸附材料相比利用其他方法制备的聚乙烯亚胺改性膨润土、APTES/Bent和其他改性膨润土吸附剂具有更高的吸附容量(理论吸附量可达137.50 mg/g)。
PEI-APTES/Bent-4与其他改性膨润土的Cr(VI)吸附量比较
Adsorbent qm/mg·g-1 Ref. Notes: CTMAB—Cetyltrimethylammonium bromide; MBent—Magnetic Bentonite; AC—Activated Carbon; PDA—Polydopamine; SDBS—Sodium dodecyl benzene sulfonate. CTMAB/Bent 27.472 [37] AC-Fe3O4/Bent 29.32 [38] Citric acid/MBent 16.67 [39] polyacrylic acid-Al/Bent 3.125 [40] Fe3O4-PDA-SDBS/Bent 103.6 [41] Chitosan-NaOH/Bent 2.72 [42] Cetylpyridinium chloride/Bent 46.03 [43] Chitosan/Bent 16.40 [44] PEI-APTES/Bent-4 137.50 This study 不同样品对Cr(Ⅵ)的吸附量

PEI-APTES/Bent-4对Cr(Ⅵ)的吸附机制主要为静电吸引、还原和螯合,Cr(Ⅵ)首先通过静电吸引吸附在PEI-APTES/Bent-4上,随后部分Cr(Ⅵ)还原为Cr(Ⅲ)螯合在吸附剂表面。
PEI-APTES/Bent-4吸附Cr(Ⅵ)的机制图





 下载:
下载: