Preparation and electrochemical properties of MOF/polypyrrole/graphene ternary composite aerogel
-
摘要: 具有丰富多孔结构的金属有机框架(MOF)差的导电性限制了其在超级电容器电极材料中的实际应用。将MOF晶体材料内嵌于石墨烯(GE)气凝胶三维网络结构中,协同聚吡咯(PPy)的共轭长链构筑3D分级多孔结构的同时可实现导电PPy的高水平及稳定掺杂,进一步提高复合气凝胶的超电容性能。首先以Co(NO3)2·6H2O和Ni(NO3)2·6H2O为金属源,均苯三甲酸 (H3BTC)为有机配体,水热制备得到单金属Co-MOF、Ni-MOF和双金属CoNi-MOF晶体材料;然后将MOF晶体材料与吡咯(Py)、氧化石墨烯(GO)通过一步水热法制备得到MOF/PPy/GE三元复合气凝胶,采用SEM、TEM、FTIR、XRD、Raman和XPS等技术对复合材料的形貌、化学结构、掺杂结构进行表征。结果表明:双金属CoNi-MOF材料更易内嵌于气凝胶三维网络结构中,与石墨烯片层和PPy共轭长链共同构筑稳定的三维多孔网络结构,可有效抑制GE片层的堆积。电化学测试结果表明,CoNi-MOF/GE/PPy复合气凝胶(GPMOF-CoNi)比电容可达447 F/g,且循环10000圈电容保持率高达97%,表现出良好的超电容特性。Abstract: The poor electrical conductivity of metal organic frameworks (MOF) with rich porous structures limits their practical application in supercapacitor electrode materials. With MOF crystalline material and polypyrrole (PPy) chains embedded in the 3D network structure of graphene (GE) aerogel, 3D hierarchical porous structure can be successfully constructed. Simultaneously, high level and stable doping of conductive PPy, which can further improve the supercapacitive performance of the composite aerogel. Co-MOF, Ni-MOF and bimetallic CoNi-MOF were obtained by hydrothermal method with Co(NO3)2·6H2O and Ni(NO3)2·6H2O as metal source and trimesic acid (H3BTC) as organic ligand. Then MOF/PPy/GE ternary composite aerogels were prepared with MOF crystal material pyrrole (Py) and graphene oxide (GO) by one-step hydrothermal method. The composite morphology, chemical structure, and doping structures were characterized by SEM, TEM, FTIR, XRD, Raman and XPS techniques. The results show that the bimetallic CoNi-MOF material is more easily embedded in the three-dimensional network structure of the composite aerogel, and could effectively suppress the accumulation of GE layers by constructing a stable three-dimensional porous network structure together with graphene layers and PPy conjugated long chains. The electrochemical test results show that the specific capacitance of the CoNi-MOF/GE/PPy composite aerogel (GPMOF-CoNi) can reach 447 F/g, and the capacitance retention rate of 10000 cycles is up to 97%, showing good supercapacitive performance.
-
Keywords:
- supercapacitor /
- metal-organic framework /
- polypyrrole /
- graphene /
- aerogel
-
-
图 5 所得复合气凝胶样品的 N2 吸脱附等温线 (a)、孔径分布曲线 (b)、 FTIR 图谱 (c)、XRD图谱(d)和Raman图谱 (e)
dV/dD—Pore volume
Figure 5. N2 adsorption and desorption isotherms of the resulting composite aerogel sample (a), pore size distributions (b), FTIR spectra (c), XRD patterns (d) and Raman spectra (e) of the obtained composite aerogel
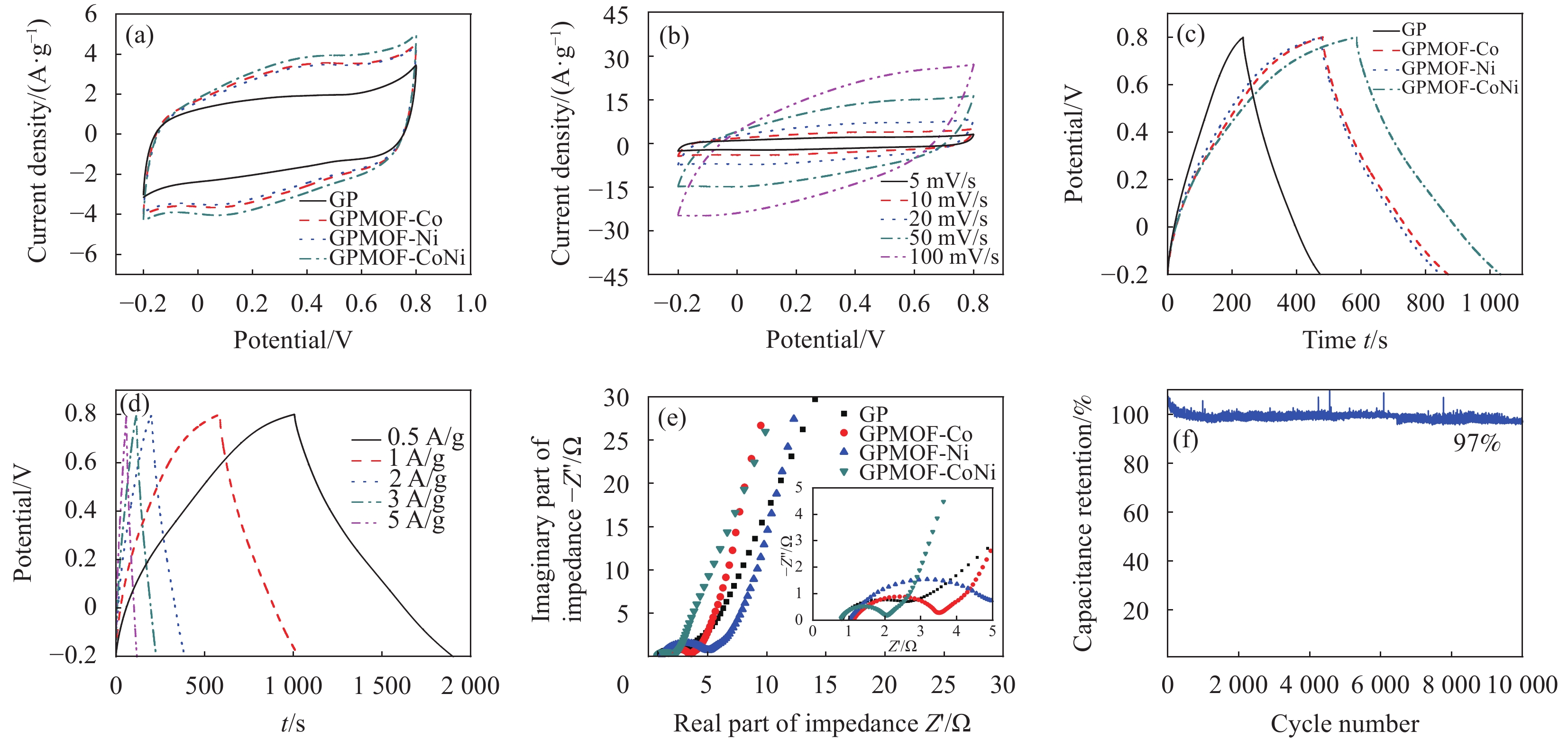
图 9 (a) GP、GPMOF-Co、GPMOF-Ni、GPMOF-CoNi的CV曲线(10 mV/s);(b) GPMOF-CoNi的CV曲线;(c) GP、GPMOF-Co、GPMOF-Ni、GPMOF-CoNi的GCD (1 A/g);(d) GPMOF-CoNi的GCD; (e) GP、GPMOF-Co、GPMOF-Ni、GPMOF-CoNi的EIS;(f) GPMOF-CoNi的10000圈循环稳定性测试(10 A/g)
Figure 9. (a) CV (10 mV/s) of GP, GPMOF-Co, GPMOF-Ni, GPMOF-CoNi; (b) CV of GPMOF-CoNi; (c) GCD of GP, GPMOF-Co, GPMOF-Ni, GPMOF-CoNi (1 A/g); (d) GCD of GPMOF-CoNi; (e) EIS of GP, GPMOF-Co, GPMOF-Ni, GPMOF-CoNi; (f) 10000 cycle stability test of GPMOF-CoNi (10 A/g)
表 1 Ni-MOF/石墨烯/聚吡咯(GPMOF-Ni)复合气凝胶的原材料配比
Table 1 Ratios of the raw materials used in the Ni-MOF/graphene/polypyrrole (GPMOF-Ni) composite aerogels
Sample Py/mg GO/mg Ni-MOF/mg GP 140 56 0 10 GPMOF-Ni 140 56 10 20 GPMOF-Ni 140 56 20 40 GPMOF-Ni 140 56 40 60 GPMOF-Ni 140 56 60 Notes: Py—Pyrrole; GO—Graphene oxide; GP—GO/PPy. 表 2 GPMOF复合气凝胶的原材料配比
Table 2 Ratios of the raw materials used in the GPMOFs composite aerogels
Sample Py/mg GO/mg MOF/mg GP 140 56 0 GPMOF-Co 140 56 20 GPMOF-Ni 140 56 20 GPMOF-CoNi 140 56 20 Notes: GP, GPMOF-Co, GPMOF-Ni and GPMOF-CoNi—Variety of MOF in the prepared composite aerogel as Co-MOF, Ni-MOF, CoNi-MOF, respectively. 表 3 复合气凝胶的BET比表面积SBET、孔体积Vtotal和平均孔径参数
Table 3 BET specific surface area SBET, pore volume Vtotal and average pore diameter parameters of composite aerogel
SampleSBET/
(m2·g−1)Vtotal/
(cm3·g−1)Average pore diameter/nm GP 14.047 0.026 3.663 GPMOF-Co 15.633 0.028 3.820 GPMOF-Ni 14.417 0.027 3.794 GPMOF-CoNi 43.513 0.063 1.614 Notes: SBET—Total area of the sample per unit mass; Vtotal—Total pore volume per unit mass of the sample. -
[1] 魏帅, 李朝霞, 孟淑娟, 等. Fe2O3/氮掺杂生物质碳复合材料制备及其在超级电容器中的应用[J]. 复合材料学报, 2023, 40(10):5736-5749. WEI Shuai, LI Zhaoxia, MENG Shujuan, et al. Preparation of Fe2O3/nitrogen-doped biomass carbon composites and their application in supercapacitors[J]. Acta Materiae Compositae Sinica,2023,40(10):5736-5749(in Chinese).
[2] ZHENG S S, LI X R, YAN B Y, et al. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage[J]. Advanced Energy Materials,2017,7(18):1602733. DOI: 10.1002/aenm.201602733
[3] DONG Y F, WU Z S, REN W C, et al. Graphene: A promising 2D material for electrochemical energy storage[J]. Science Bulletin,2017,62(10):724-740. DOI: 10.1016/j.scib.2017.04.010
[4] CAO X H, TAN C L, SINDORO M, et al. Hybrid micro-nano-structures derived from metal-organic frameworks: Preparation and applications in energy storage and conversion[J]. Chemical Society Reviews,2017,46(10):2660-2677. DOI: 10.1039/C6CS00426A
[5] PERSHAANAA M, BASHIR S, RAMESH S, et al. Every bite of supercap: A brief review on construction and enhancement of supercapacitor[J]. Journal of Energy Storage,2022,50:104599. DOI: 10.1016/j.est.2022.104599
[6] HUANG Y, LI H F, WANG Z F, et al. Nanostructured polypyrrole as a flexible electrode material of supercapacitor[J]. Nano Energy,2016,22:422-438. DOI: 10.1016/j.nanoen.2016.02.047
[7] HAMRA A A B, LIM H N, HUANG N M, et al. Microwave exfoliated graphene-based materials for flexible solid-state supercapacitor[J]. Journal of Molecular Structure,2020,1220:128710. DOI: 10.1016/j.molstruc.2020.128710
[8] CHIAM S L, LIM H N, FOO C Y, et al. How did nickel cobaltite reinforced carbon microfibre symmetrical supercapacitor fare against a commercial supercapacitor?[J]. Electrochimica Acta,2017,246:1141-1146. DOI: 10.1016/j.electacta.2017.06.132
[9] FOO C Y, LIM H N, MAHDI M A, et al. High-performance supercapacitor based on three-dimensional hierarchical rGO/nickel cobaltite nanostructures as electrode materials[J]. The Journal of Physical Chemistry C,2016,120(38):21202-21210. DOI: 10.1021/acs.jpcc.6b05930
[10] ZHAO Y H, HE X Y, CHEN R R, et al. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures[J]. Chemical Engineering Journal,2018,352:29-38. DOI: 10.1016/j.cej.2018.06.181
[11] CHEE W K, LIM H N, HARRISON I, et al. Performance of flexible and binderless polypyrrole/graphene oxide/zinc oxide supercapacitor electrode in a symmetrical two-electrode configuration[J]. Electrochimica Acta,2015,157:88-94. DOI: 10.1016/j.electacta.2015.01.080
[12] HAMRA A A B, LIM H N, HAFIZ S M, et al. Performance stability of solid-state polypyrrole-reduced graphene oxide-modified carbon bundle fiber for supercapacitor application[J]. Electrochimica Acta,2018,285:9-15. DOI: 10.1016/j.electacta.2018.07.212
[13] KORKMAZ S, KARIPER İ A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications[J]. Journal of Energy Storage,2020,27:101038. DOI: 10.1016/j.est.2019.101038
[14] XU J, SHU R W, WAN Z L, et al. Construction of three-dimensional hierarchical porous nitrogen-doped reduced graphene oxide/hollow cobalt ferrite composite aerogels toward highly efficient electromagnetic wave absorption[J]. Journal of Materials Science & Technology,2023,132:193-200.
[15] DENG L L, SHU R W, ZHANG J B. Fabrication of ultralight nitrogen-doped reduced graphene oxide/nickel ferrite composite foams with three-dimensional porous network structure as ultrathin and high-performance microwave absorbers[J]. Journal of Colloid and Interface Science,2022,614:110-119. DOI: 10.1016/j.jcis.2022.01.104
[16] LEE S P, ALI G A M, HEGAZY H H, et al. Optimizing reduced graphene oxide aerogel for a supercapacitor[J]. Energy & Fuels,2021,35(5):4559-4569.
[17] ZHANG Q Q, WANG Y, ZHANG B Q, et al. 3D superelastic graphene aerogel-nanosheet hybrid hierarchical nanostructures as high-performance supercapacitor electrodes[J]. Carbon,2018,127:449-458. DOI: 10.1016/j.carbon.2017.11.037
[18] LE Q B, VARGUN E, FEI H J, et al. Effect of PANI and PPy on electrochemical performance of rGO/ZnMn2O4 aerogels as electrodes for supercapacitors[J]. Journal of Electronic Materials,2020,49(8):4697-4706. DOI: 10.1007/s11664-020-08198-4
[19] HE Y B, BAI Y L, YANG X F, et al. Holey graphene/polypyrrole nanoparticle hybrid aerogels with three-dimensional hierarchical porous structure for high performance supercapacitor[J]. Journal of Power Sources,2016,317:10-18. DOI: 10.1016/j.jpowsour.2016.03.089
[20] ZHANG Y M, CHEN Z, ZHANG D D, et al. Diversified applications of polypyrrole/graphene aerogel in supercapacitors and three-dimensional electrode system[J]. Materials Letters,2018,227:158-160. DOI: 10.1016/j.matlet.2018.04.113
[21] LI G, CAI H R, LI X L, et al. Construction of hierarchical NiCo2O4@Ni-MOF hybrid arrays on carbon cloth as superior battery-type electrodes for flexible solid-state hybrid supercapacitors[J]. ACS Applied Materials & Interfaces,2019,11(41):37675-37684.
[22] 李文青, 王艺锟, 王全璐, 等. 5-磺基水杨酸掺杂聚吡咯/ZIF67复合材料的超电容性能[J]. 复合材料学报, 2022, 39(12):5788-5800. LI Wenqing, WANG Yikun, WANG Quanlu, et al. Supercapacitive performances of 5-sulfosalicylic acid doped polypyrrole/ZIF67 composites[J]. Acta Materiae Compo-sitae Sinica,2022,39(12):5788-5800(in Chinese).
[23] JIANG X B, LI G N, LU D P, et al. Hybrid control mechanism of crystal morphology modification for ternary solution treatment via membrane assisted crystallization[J]. Crystal Growth & Design,2018,18(2):934-943.
[24] YADAV H, SINHA N, KUMAR B. New geometrical modeling to study crystal morphology[J]. Crystal Growth & Design,2016,16(8):4559-4566.
[25] HE Q M, RUI K, CHEN C H, et al. Interconnected CoFe2O4-polypyrrole nanotubes as anode materials for high performance sodium ion batteries[J]. ACS Applied Materials & Interfaces,2017,9(42):36927-36935.
[26] LIM S P, PANDIKUMAR A, LIM Y S, et al. In-situ electrochemically deposited polypyrrole nanoparticles incorporated reduced graphene oxide as an efficient counter electrode for platinum-free dye-sensitized solar cells[J]. Scientific Reports,2014,4(1):5305. DOI: 10.1038/srep05305
[27] YANG J, WANG X, LI B, et al. Novel iron/cobalt-containing polypyrrole hydrogel-derived trifunctional electrocatalyst for self-powered overall water splitting[J]. Advanced Functional Materials,2017,27(17):1606497. DOI: 10.1002/adfm.201606497
[28] RAJESH M, RAJ C J, KIM B C, et al. Supercapacitive studies on electropolymerized natural organic phosphate doped polypyrrole thin films[J]. Electrochimica Acta,2016,220:373-383. DOI: 10.1016/j.electacta.2016.10.118
[29] JIAO Y, CHEN G, CHEN D H, et al. Bimetal-organic framework assisted polymerization of pyrrole involving air oxidant to prepare composite electrodes for portable energy storage[J]. Journal of Materials Chemistry A,2017,5(45):23744-23752. DOI: 10.1039/C7TA07464F
[30] CHI Y, YANG W P, XING Y C, et al. Ni/Co bimetallic organic framework nanosheet assemblies for high-performance electrochemical energy storage[J]. Nanoscale,2020,12(19):10685-10692. DOI: 10.1039/D0NR02016H
[31] WANG J, XU Y L, YAN F, et al. Template-free prepared micro/nanostructured polypyrrole with ultrafast charging/discharging rate and long cycle life[J]. Journal of Power Sources,2011,196(4):2373-2379. DOI: 10.1016/j.jpowsour.2010.10.066
[32] SHI K Y, ZHITOMIRSKY I. Polypyrrole nanofiber-carbon nanotube electrodes for supercapacitors with high mass loading obtained using an organic dye as a co-dispersant[J]. Journal of Materials Chemistry A,2013,1(38):11614-11622. DOI: 10.1039/c3ta12466e
-
期刊类型引用(2)
1. 李滋阳,王思佳,邓文举. 陶瓷颗粒增强金属基复合材料研究进展. 轻工科技. 2021(04): 41-44 .  百度学术
百度学术
2. 陈亚楠,金云学,牛牧野,陈洪美,杜文栋. Ni_3Al(Cr)合金室温干滑动摩擦磨损性能研究. 江苏科技大学学报(自然科学版). 2019(05): 18-26 .  百度学术
百度学术
其他类型引用(4)
-
目的
具有丰富多孔结构的金属有机框架(MOF)差的导电性限制了其在超级电容器电极材料中的实际应用。将MOF晶体材料内嵌于石墨烯(GE)气凝胶三维网络结构中,协同聚吡咯(PPy)的共轭长链构筑3D分级多孔结构的同时可实现导电PPy的高水平及稳定掺杂,进一步提高复合气凝胶的超电容性能。
方法首先以聚乙烯吡咯烷酮(PVP)为模板制备具有纳米花状结构Co-MOF、Ni-MOF以及CoNi-MOF,然后将其原位引入氧化石墨烯(GO)、吡咯(Py)体系于水热条件下制备复合水凝胶,冷冻干燥后得到三元复合气凝胶。采用扫描电子显微镜(SEM)、傅立叶红外光谱仪(FTIR)、拉曼光谱仪(Raman)、X射线衍射仪(XRD)、X射线光电子能谱仪(XPS)、透射电子显微镜(TEM)、比表面积分析仪(BET)等仪器对样品进行表征测试;采用电化学工作站、蓝电电池测试系统对三电极、二电极体系进行循环伏安(CV)、恒电流充放电(GCD)、电化学交流阻抗(EIS)及循环寿命等电化学性能测试。
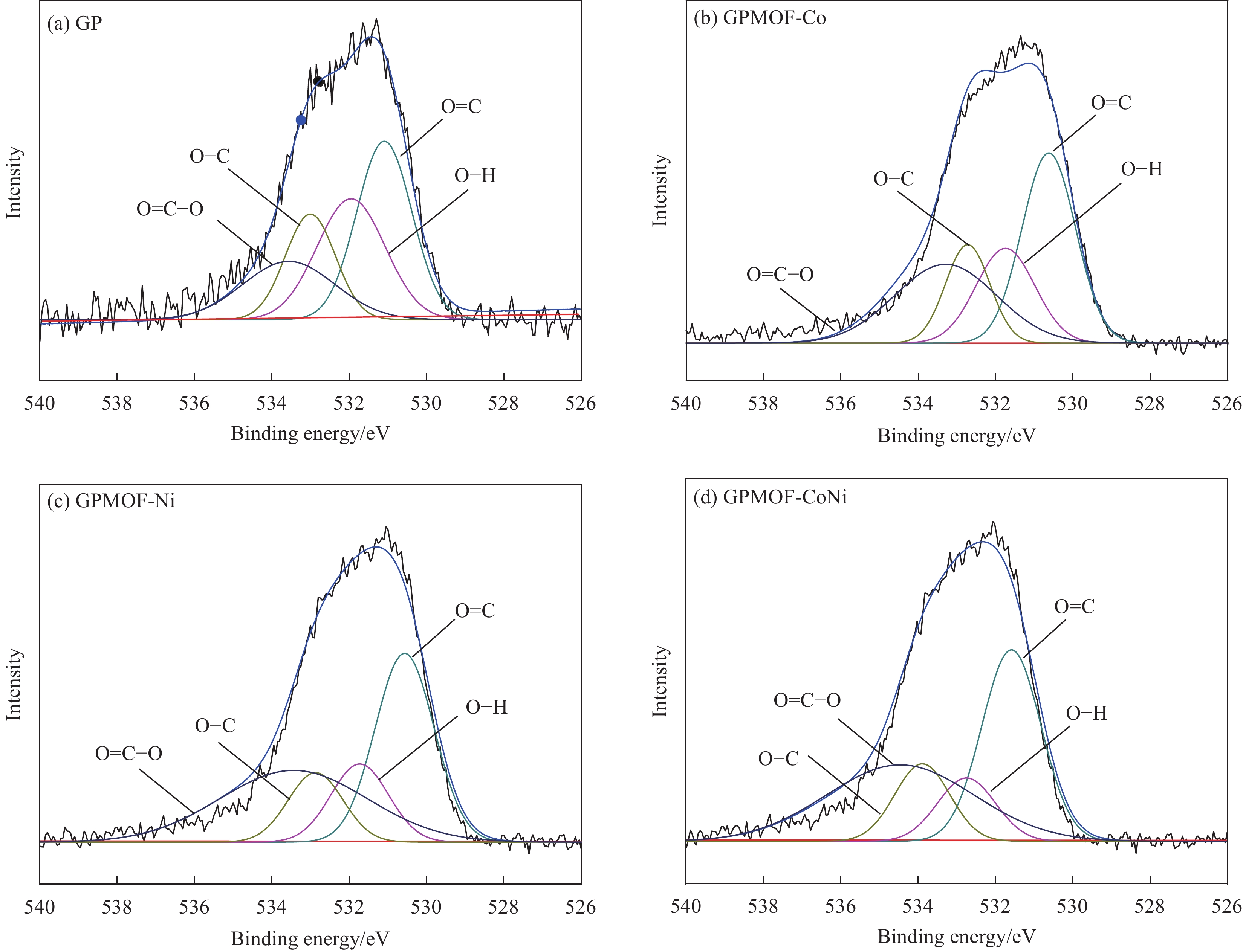
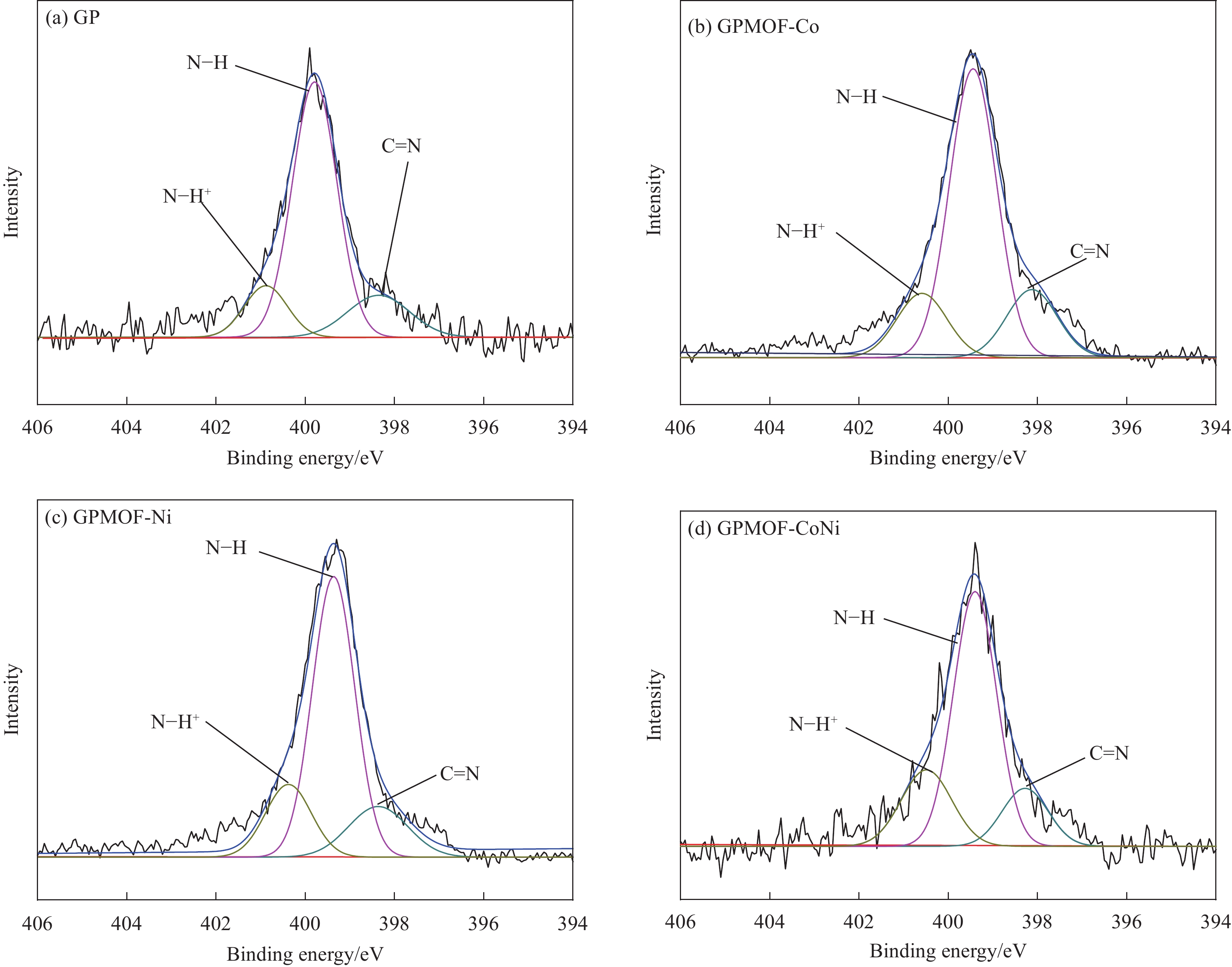
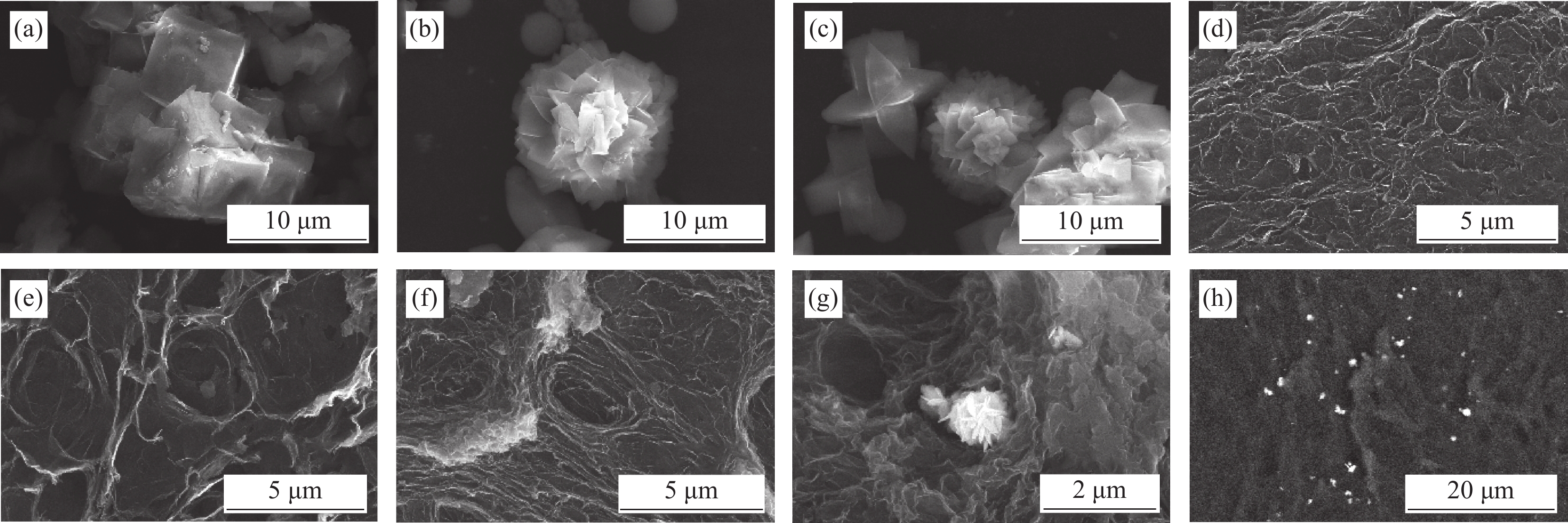
结果①复合气凝胶材料的形貌:复合材料的SEM形貌分析表明,Co-MOF为不规则的立方体结构,Ni-MOF则为微米片状组装的菜花状结构,而CoNi-MOF则为花状/杨桃形复合结构。GE/PPy(GP)二元复合气凝胶材料中石墨烯片层呈现褶皱结构,片层相互堆叠,孔径较小;MOF晶体的引入后内嵌于3D网络结构内部,减少了石墨烯片层的再堆积。复合材料的TEM形貌分析表明,复合气凝胶以GE和PPy复合片层为主,MOF散落在复合片层结构中形成三元复合气凝胶。 ②复合气凝胶材料的化学及掺杂结构分析:FTIR谱图中,复合气凝胶材料中PPy特征峰发生红移,归因于MOF配体中苯环结构的与吡咯环形成π-π共轭效应。由Raman谱图可见,MOF引入后I/I减小,表明MOF有利于复合其凝胶中石墨烯片层更有序、均匀的排列。XPS谱图进一步证实PPy的成功聚合及GO的还原,MOF的引入有助于提高复合气凝胶材料中PPy的掺杂水平,进而提高复合材料的电化学性能。③复合气凝胶材料的结晶结构分析:XRD谱图中,复合气凝胶材料中GO衍射特征峰完全消失,表明复合气凝胶材料中的GO被还原为GE;复合气凝胶中出现PPy的特征峰,表明PPy成功聚合;引入不同类型MOF后,PPy特征峰较二元气凝胶变得更加尖锐,表明MOF的引入有利于PPy分子链的规整排列。三元复合气凝胶中未检测到 MOF的特征峰,这可能是由于MOF被GE片层及PPy包覆所致。④复合气凝胶的电化学性能:引入MOF后,复合材料的比电容得以明显提高,引入双金属CoNi-MOF时,复合材料EIS曲线斜率最接近90°,表现出理想的超电容特性。其在1 A/g的电流密度下的比电容可达447 F/g,在5 A/g的高电流密度下,比电容为288.5 F/g ,电容保持率为64.5 %。在电流密度10 A/g下循环10,000圈后,电容保持率约为97 %,表现出优异的循环稳定性。⑤GPMOF-CoNi复合气凝胶材料的合成机理: 双金属CoNi-MOF的未配位-COOH因可与GO、Py形成多重氢键及共轭效应,三维结构的MOF嵌于石墨烯片层于与聚吡咯分子之间,抑制石墨烯片层的堆叠和团聚同时,增大了气凝胶材料的比表面积,有利于电解液离子运输和电子扩散。另一方面,PPy的共轭长链将2D石墨烯片与3D纳米MOF相连接,充当电荷传输的桥梁,可以有效提高MOF材料的电导率,增强复合凝胶界面的法拉第反应,最后,Co/Ni-MOF未配位的-COOH协同RGO剩余-COOH对PPy的掺杂作用可有效提高复合气凝胶材料的电化学性能。
结论利用简便的一步水热法制备得到GPMOF-CoNi复合气凝胶。复合材料中MOF晶体材料内嵌在气凝胶三维网络结构中,PPy的共轭长链与二维石墨烯片协同三维MOF有利于构筑稳定的三维分级多孔结构。三维结构的MOF的引入可抑制石墨烯片层的堆叠和团聚,PPy的共轭长链可链接2D石墨烯片与3D MOF,MOF及RGO羧基的协效掺杂可有效提高复合气凝胶的电化学性能。GPMOF-CoNi气凝胶材料三电极比电容可达447 F/g;经过10000次充/放电循环后,比电容保持率高达97 %,表现出良好的循环稳定性。
-
超级电容器(Supercapacitors;SCs)作为一种新型储能器件,具有高功率密度、长循环寿命、宽工作温度等优点。石墨烯/导电聚合物复合气凝胶电极材料因方便构筑分级多孔结构、有效结合双电层及法拉第赝电容而备受关注。然而,如何在构筑稳定的3D分级多孔结构同时实现导电聚合物的高水平及稳定掺杂,从而进一步提高电极材料的电化学性能,仍需进一步探讨。
本文首先以聚乙烯吡咯烷酮(PVP)为模板制备具有纳米花状结构Co-MOF、Ni-MOF以及CoNi-MOF,然后将其原位引入氧化石墨烯(GO)、吡咯(Py)体系于水热条件下制备复合水凝胶,冷冻干燥后得到三元复合气凝胶。双金属CoNi-MOF与GO、Py之间的多重氢键及共轭效应,有利于构筑稳定的三维多孔结构。PPy的共轭长链作为连接2D石墨烯片与3D MOF的桥梁,可有效提高电荷传输; Co/Ni-MOF与RGO的-COOH可实现对PPy的高水平掺杂。电化学测试结果表明,复合气凝胶(GPMOF-CoNi)比电容可达447 F/g,且循环10000圈电容保持率高达97%,表现出良好的超电容特性。
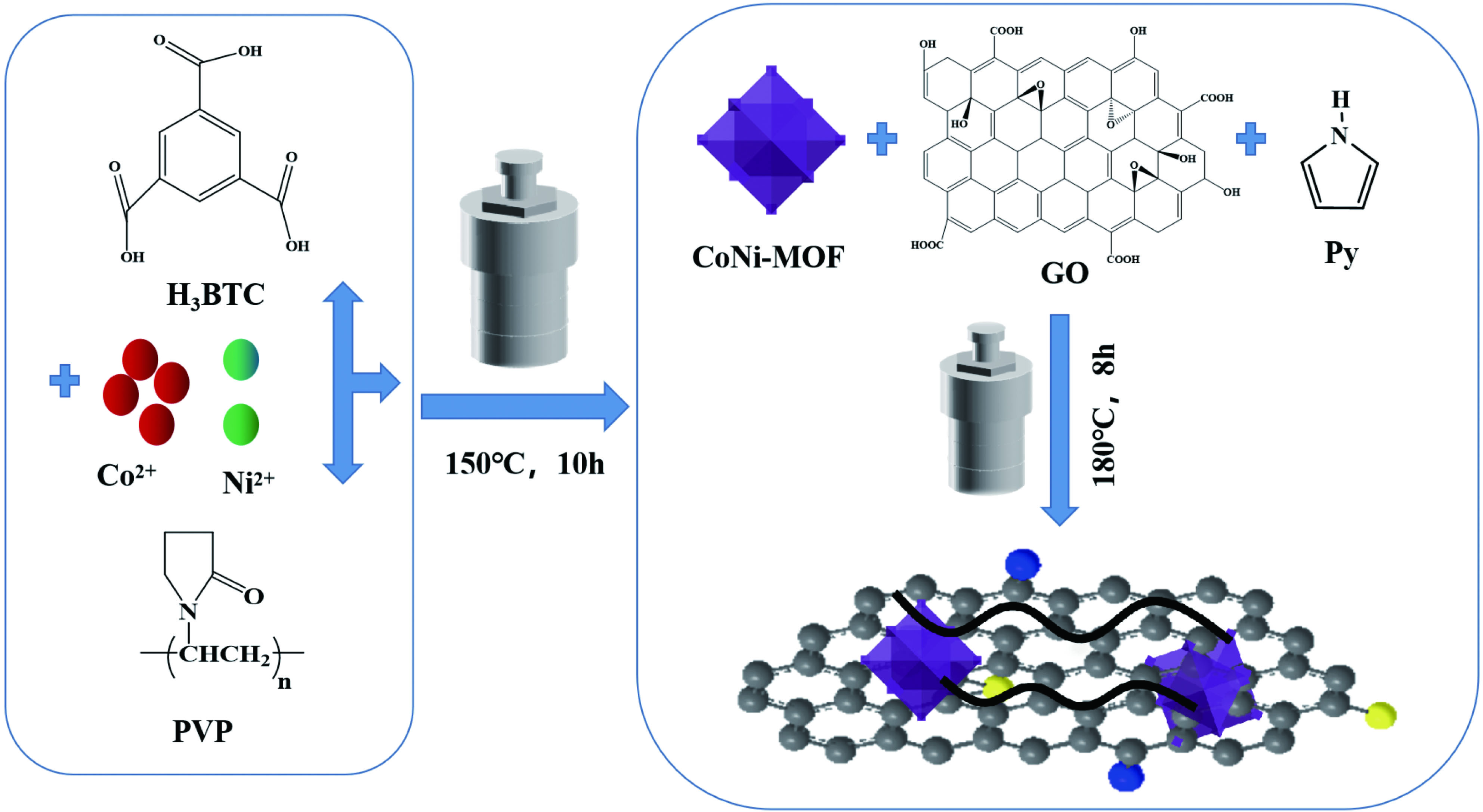
GPMOF-CoNi合成机理图




 下载:
下载: