Application of porous conductive gel PAM/CNTs-PEG in zinc-air batteries
-
摘要: 为实现锌-空气电池工业化生产,优化其空气扩散电极性能,使其有利于气体的扩散,并形成更多的三相界面。导电水凝胶是由导电材料和交联聚合物网络组成,聚合物网络提供支架,而导电材料赋予水凝胶良好的导电性。多孔的结构可以给气体更多的扩散通路,也有利于催化层的负载,形成更多的三相界面。本文采用聚丙烯酰胺基水凝胶,以聚乙二醇-2000 (PEG2000)为制孔剂,合成多孔聚丙烯酰胺/碳纳米管-聚乙二醇(PAM/CNTs-PEG)导电水凝胶。将制备的PAM/CNTs-PEG导电水凝胶浸泡于乙醇溶液中,可形成不同数量的介孔。本文研究了不同浸泡时间对于多孔PAM/CNTs-PEG导电水凝胶在柔性锌空电池中的性能影响。实验结果表明:乙醇浸泡5 h的导电凝胶电化学性能最好。当电压从1 mA/cm2 时的1.23 V到5 mA/cm2时的1.11 V,仅衰减了0.12 V。8.5 mA/cm2时产生最大功率密度为77.35 mW/cm2,且放电时具有1104.85 mA·h/g的高克容量,远高于其他导电凝胶。且有较好的导电性和应变灵敏度,可应用于传感等领域。Abstract: In order to realize the industrial production of zinc-air battery, the performance of its air diffusion electrode is optimized to make it conducive to the diffusion of gas and to form more three-phase interfaces. The conductive hydrogel is composed of a conductive material and a cross-linked polymer network, the polymer network provides the scaffold, and the conductive material gives the hydrogel good electrical conductivity. The porous structure can give more diffusion paths to the gas, and is also conducive to the load of the catalytic layer, forming more three-phase interfaces. In this paper, polyacrylamide hydrogel was used to synthesize porous polyacrylamide/carbon nanotubes-polyethylene glycol (PAM/CNTs-PEG) conductive hydrogel with polyethylene glycol 2000 (PEG2000) as pore-making agent. The prepared PAM/CNTs-PEG conductive hydrogels were immersed in ethanol solution to form different numbers of mesopores. The effect of different immersion time on the properties of porous PAM/CNTs-PEG conductive hydrogels in flexible zinc-air cells was studied. The experimental results show that the electrochemistry performance of the conductive gel soaked in ethanol for 5 h is the best. When the voltage changes from 1.23 V at 1 mA/cm2 to 1.11 V at 5 mA/cm2, the attenuation is only 0.12 V. The maximum power density is 77.35 mW/cm2 at 8.5 mA/cm2, and the high gram capacity of 1104.85 mA·h/g at discharge is much higher than that of other conductive gels. It has good electrical conductivity and strain sensitivity and can be used in sensing and other fields.
-
齿轮和轴类零件常被用于高速、振动、摩擦磨损等恶劣工况,易产生断裂和磨损等失效[1-2]。此类零件多采用20 CrMnTi低碳钢材料,为提高零件使用寿命,使用电沉积方法在零件表面制备镀层增强零件性能已成为常用方法。Ni-P合金镀层具有良好的耐磨、耐腐蚀性能,还具有较高的硬度,因此被广泛应用于化工、汽车和机械等行业[3-5]。随着行业的进步与发展,Ni-P镀层已难以满足复杂特殊的使用环境,向镀液中添加纳米颗粒针对性的提升镀层性能已成为研究的热门方向[6]。目前一元纳米颗粒复合电沉积技术已比较成熟,如添加Al2O3、WC、SiC等硬质颗粒提升复合镀层的硬度及耐磨损性能[7-10],添加具有自润滑特性的BN(h)、MoS2、PTFE等降低复合镀层的摩擦系数[11-13],添加部分纳米颗粒还可以提高复合镀层的耐腐蚀性能和抗高温氧化性能[14-18]。
近年来部分学者已经对二元纳米颗粒复合镀层进行研究,徐义库[19]等通过脉冲电沉积法制备Ni-Mo-SiC-TiN复合镀层,两种纳米颗粒均匀的分散在Ni-Mo基体中显著提升了镀层的耐磨和耐腐蚀性;张银[20]等使用电沉积法制备不同浓度配比的 Ni-Co-P-BN(h)-Al2O3复合镀层,结果表明二元纳米颗粒掺杂配比会对纳米复合镀层表面产生巨大影响,且二元纳米颗粒复合镀层的耐磨性优于一元纳米颗粒复合涂层;王浩鑫[21]采用单脉冲电沉积制备Ni-TiC-GO复合镀层,该镀层具有优秀的减摩擦和耐磨性能。目前,研究对于二元纳米复合电沉积技术尤其是电沉积Ni-P-WC-BN(h)二元纳米复合镀层的表面结构和减磨耐磨性能的研究未见报道。
因此本文采用超声-脉冲电沉积法制备不同浓度BN(h)纳米颗粒的Ni-P-WC-BN(h)二元纳米颗粒复合镀层,与Ni-P、Ni-P-WC复合镀层对比,探究BN(h)质量浓度对复合镀层组织结构和减磨耐磨性能的影响
1. 实 验
1.1 基材预处理
采用20 CrMnTi钢为基体,其尺寸为40 mm×16 mm×12 mm。表面使用320#、800#、
1200 #、2000#金相砂纸打磨→抛光→去离子水超声清洗→电净除油→去离子水超声清洗并吹干→强活化→去离子水超声清洗并吹干→弱活化→去离子水超声清洗并吹干待用。1.2 实验条件
试验采用单因素实验法,首先通过预实验确定电沉积工艺参数、基础镀液成分(表1所示)和WC颗粒的最优浓度(30 g/L,纯度99.9%,平均粒度为50 nm)。实验装置如图1所示,工艺参数:电流密度3 A/dm2,脉冲频率2 kHz,占空比0.8,镀液温度50℃,电镀时间60 min,超声功率210 W,搅拌速率150 r/min,;阳极为纯Ni板。
表 1 电沉积Ni-P镀液配方Table 1. Formulation of electrodeposition Ni-P plating solutionElement Concentration/(g·L−1) NiSO4·6H2O 230 NiCl2·6H2O 30 H3PO3 5 NaH2PO2·H2O 8 NaC6H8O7·H2O 80 H3BO3 30 C₁₂H₂₅NaSO₄ 0.1 SC(NH₂)₂ 0.02 C₇H₅NO₃S 1 以上述内容为基础向镀液中分别添加15 g/L、20 g/L、25 g/L、30 g/L的BN(h)纳米颗粒(纯度99.9%,平均粒度为200 nm)。探究BN(h)添加量对Ni-P-WC-BN(h)复合镀层表面形貌、组织成分、显微硬度及耐磨性能的影响。
1.3 测试方法
试样制备完成后,使用线切割将样品切割为10 mm×10 mm×6 mm的测试试样进行下一步测试。使用Quanta FEG250扫描电子显微镜、能谱仪X Flash Detector 5030 BRUKER)对试样表面的镀层形貌和元素分布进行观察。采用Rigaku SmartLab SE型X射线衍射仪对复合镀层的物相结构进行分析。采用斯特尔显微硬度仪(Struers)对镀层的显微硬度进行测试,实验载荷
1000 g,加载时间10 s,在不同位置测量五次取平均值。采用CFT-I型综合材料表面性能测试仪(兰州中科凯华科技)对复合镀层的摩擦系数进行测试,磨件为Si3N4对磨球(直径4 mm,表面粗糙度Ra=0.06 μm,硬度为1400 -1700 HV1),加载载荷100 g,往复次数200次/min,往复行程4 mm,时长30 min。采用日本Keyence VK-X1000激光显微镜拍摄磨痕处形貌及磨痕截面轮廓并计算镀层磨损体积损失。2. 结果与讨论
2.1 复合镀层的微观形貌及元素分布
图2展示了不同镀液配方复合镀层的表面微观形貌,(a)图Ni-P镀层表面呈胞状结构,胞状结构的直径较大且分布不均匀。在镀液中加入WC纳米颗粒后,(b)图Ni-P-WC复合镀层表面和(a) Ni-P镀层相比粗糙,这是由于部分WC团聚被Ni-P镀层包裹在镀层内部,镀层表面产生了单元凸起,改变了阳极和阴极的间距,凸起部分受到电场力较大优先生长因此形成不平整的胞状物结构[22]。(c1)到(c4)图为在镀液中进一步添加15 g~30 g/L的BN(h)纳米颗粒的Ni-P-WC-BN(h)复合镀层表面的微观形貌,与Ni-P-WC复合镀层相比,随着BN(h)含量的增加,镀层表面平整度略有提高,究其原因,一方面两颗粒协同作用在一定程度上减小了团聚[20],另一方面还可能与BN(h)颗粒具有自润滑特性,层与层之间靠爱德华力连接易产生滑动有关[23]。但BN(h)浓度超过某一极值,镀液中纳米颗粒浓度过高从而使纳米颗粒团聚,阴极附近导电性下降从而使沉积效率降低。
![]() 图 2 复合镀层表面SEM图像 (a) Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 2. SEM image of composite plated surface (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)
图 2 复合镀层表面SEM图像 (a) Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 2. SEM image of composite plated surface (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)图3为不同镀液配方复合镀层的截面形貌及元素分布。WC纳米颗粒的加入后镀层厚度有所增加,但镀层内部存在裂痕等缺陷;加入BN(h)纳米颗粒后,镀层平整性略有提高且镀层内部缺陷减少,镀层厚度先增加后减小,在BN(h)浓度为25 g/L时最厚达80.0 μm。随着BN(h)浓度进一步增加,镀液中纳米颗粒浓度过高导致阴极导电性下降从而降低沉积效率,镀层厚度减小。
![]() 图 3 复合镀层截面SEM图像及截面元素分布 (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 3. SEM image of composite plating cross-section and section element distribution (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)
图 3 复合镀层截面SEM图像及截面元素分布 (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 3. SEM image of composite plating cross-section and section element distribution (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)由图中元素分布可以看出,WC和BN(h)纳米镶嵌在镀层中,镀层和基体的界面交界处存在约50 μm的中间区,该区域元素相互渗透,有助于改善镀层和基体的结合。
图4为不同镀镀液配方复合镀层表面元素分布,从元素分布图中可以看出Ni、P、W、B均匀分散在镀层中,并无明显团聚现象,这说明在该工艺参数下WC纳米颗粒和BN(h)纳米颗粒在镀层中分散效果较好。
![]() 图 4 复合镀层表面元素分布 (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 4. Composite plating surface element distribution (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)
图 4 复合镀层表面元素分布 (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h) (20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)Figure 4. Composite plating surface element distribution (a)Ni-P,(b)Ni-P-WC,(c1)Ni-P-WC-BN(h)(15 g/L),(c2) Ni-P-WC-BN(h)(20 g/L),(c3) Ni-P-WC-BN(h)(25 g/L),(c4) Ni-P-WC-BN(h)(30 g/L)2.2 复合镀层的EDS能谱及相结构
图5为二元纳米颗粒掺杂下Ni-P-WC-BN(h)复合镀层的EDS能谱图,从图中可以看出,所制备的二元纳米复合镀层表面均含有Ni、P、W、C、B等元素,各元素含量随着BN(h)浓度的变化而变化。随着BN(h)添加量从15 g/L增加到30 g/L,B元素质量分数呈先波动增大后减小趋势,在BN(h)浓度为25 g/L时B元素的质量分数达到最大值0.85%;镀层表面W元素质量分数先减小后增大,与B元素呈相反趋势;镀层中P元素质量分数逐渐减小。这说明通过超声-脉冲电沉积法制备了Ni-P-WC-BN(h)二元纳米复合镀层。Ni元素的质量分数随着BN(h)浓度的提升而增大,这是因为镀液中加入WC和BN(h)两种纳米颗粒后,不同纳米颗粒相互作用,减小团聚,镀层表面吸附的颗粒数目增加,为Ni原子提供更好的成核条件[23],从而增大Ni元素的沉积量。
图6所示为不同复合镀层的XRD图谱,镀层在2θ为44.507°、51.846°、76.370°的位置上出现了的Ni峰,分别对应(111)、(200)、(220)晶面,与Ni-P-WC复合镀层相比,随着BN(h)浓度的增加,Ni-P-WC-BN(h)复合镀层的三个晶面方向出现了明显的结晶取向,且生长晶面以(111)面为主,这是由于Ni的结晶取向同时受到生长速度、方向的影响外还受到晶体竞争模式的影响[24];Ni-P-WC复合镀层的衍射图谱中,2θ为31.474°、35.626°、48.266°的位置出现了WC的特征峰,这说明WC颗粒成功沉积在复合镀层中;在Ni-P-WC-BN(h)复合镀层的衍射图谱中并没有表现出明显的BN(h)峰,但是可以检测到BN(h),结合图5中B元素的质量分数可知这可能是BN(h)颗粒沉积量较少所致。
表2为不同配方镀层中Ni(111)元素的晶粒尺寸数据,其中FWHM为半幅宽,D为镀层中垂直于晶面的晶粒尺寸。随着BN(h)浓度的提升,Ni(111)的晶粒尺寸先减小后增大,最小晶粒尺寸为8.9 nm,这是因为BN(h)对晶粒有一定的细化作用[25],合适浓度的BN(h)可以减小晶粒尺寸,但是BN(h)浓度过高会使晶粒尺寸增大。
表 2 不同复合镀层中Ni(111)元素的晶粒尺寸Table 2. Grain size of Ni(111) elements in different composite coatingsType of plating 2θ/(°) FWHM Diameter/nm Ni-P 44.480 0.941 9.2 Ni-P-WC 44.670 0.848 10.2 Ni-P-WC-BN(h)(15 g/L) 44.480 0.894 9.7 Ni-P-WC-BN(h)(20 g/L) 44.340 0.908 9.5 Ni-P-WC-BN(h)(25 g/L) 44.660 0.968 8.9 Ni-P-WC-BN(h)(30 g/L) 44.710 0.677 12.8 2.3 复合镀层的硬度
不同复合镀层的显微硬度测试结果如图7所示,从图中可知,Ni-P镀层硬度在700 HV1左右,添加WC纳米颗粒后镀层的显微硬度为
1141 HV1,这是因为WC在镀层中弥散分布,对镀层起到弥散强化作用[26],根据晶界强化原理,晶粒越细小镀层的显微硬度越高,WC纳米颗粒自身硬度较高且可以使镀层晶粒细化从而提高硬度。加入BN(h)后,随着BN(h)浓度从15 g/L到30 g/L的过程中,Ni-P-WC-BN(h)复合镀层硬度呈先增大后减小的变化趋势,在BN(h)浓度为25 g/L时Ni-P-WC-BN(h)复合镀层的硬度达到最大值115 6HV1,硬度最大值与Ni-P-WC复合镀层相当。分析认为, 纳米粒子在沉积过程中分为弱吸附和强吸附[27],随着镀液中纳米颗粒浓度增加,更多的颗粒发生强吸附作用,从而使镀层中纳米颗粒的含量增加从而使镀层硬度提升,但当其添加量过大时镀层易产生析氢现象[28],使镀层厚度下降,因此硬度下降。2.4 复合镀层的减磨耐磨性能
不同配方复合镀层的摩擦系数如图8所示,各复合镀层的摩擦系数均存在明显的摩擦系数急剧上升后趋于平稳的“磨合”阶段[29]。分析认为随着磨损试验的进行镀层表面的磨屑不断在磨痕处堆积,最终在压应力的作用下产生塑性变形使摩擦阻力增大,从而导致摩擦系数增大。
相同条件下,未添加纳米颗粒的Ni-P镀层的摩擦系数较大。摩擦系数和复合镀层中的硬质颗粒的尺寸、均匀性和数量有关[30],添加WC纳米颗粒后,纳米WC嵌入镀层,使晶粒细化并致密镀层组织,且WC自身硬度较高,因此可以减小摩擦时的接触面积从而降低摩擦系数。在Ni-P-WC镀层基础上添加BN(h)颗粒,随着BN(h)浓度增加,Ni-P-WC-BN(h)复合镀层摩擦系数呈先减小后增大的变化趋势。
摩擦磨损实验结束后,各镀层的磨损体积如表3所示,由数据可以看出Ni-P镀层磨损体积最大;加入WC纳米颗粒后镀层的磨损体积显著减小;加入BN(h)纳米颗粒后,随着浓度的提升,复合镀层的磨损量先减小后增大,Ni-P-WC-BN(h)(25 g/L)复合镀层的磨损体积最小,与Ni-P-WC复合镀层相当,显著优于其他镀层。将磨损体积与图5硬度测试结果对比,可以得出镀层磨损量和镀层硬度呈负相关。
表 3 镀层磨损体积Table 3. Plating wear volumeType of plating Wear volume /μm3 Ni-P 459553 Ni-P-WC 51945 Ni-P-WC-BN(h)(15 g/L) 256137 Ni-P-WC-BN(h)(20 g/L) 135765 Ni-P-WC-BN(h)(25 g/L) 53587 Ni-P-WC-BN(h)(30 g/L) 102966 各镀层磨痕形貌、轮廓线如图9所示,不同区域元素分布如表4所示。由磨痕形貌与图3镀层厚度对比及磨痕元素分布可知,磨损区域均不含Fe元素,摩擦磨损试验过程中镀层并未磨穿。Ni-P镀层磨痕与其他镀层磨痕相比较为明显,且磨痕处呈明显的黏着现象和犁沟状形貌,取磨痕中部长度为310 μm的截面轮廓线可知磨损过程中Ni-P复合镀层的磨损量较大,Ni-P涂层的硬度较低,易产生剥落现象,磨痕处含有少量Si元素,这说明Si3N4对磨球在摩擦磨损试验中摩擦热的作用下转移到镀层表面产生黏着磨损现象。镀层中加入WC纳米颗粒后,Ni-P-WC复合镀层磨痕表面无明显的犁沟形貌,这是因为WC颗粒在镀层中起到弥散强化作用,大幅度提升了复合镀层的硬度,减小了摩擦的接触面积,在磨损过程中,镀层先产生塑形形变,当形变量过大时部分WC颗粒脱落,形成镀层、WC粉末、摩擦副之间的三体磨损[31],此过程中摩擦热大幅增加导致氧化磨损,涂层的氧含量大幅提高。
![]() 图 9 复合镀层磨痕SEM(左)、磨痕形貌(中)、磨痕轮廓线(右):(a)Ni-P (b)Ni-P-WC (c)Ni-P-WC-BN(h)(15 g/L) (d)Ni-P-WC-BN(h)(20 g/L) (e)Ni-P-WC-BN(h)(25 g/L) (f)Ni-P-WC-BN(h)(30 g/L)Figure 9. SEM of composite plating wear marks (left), wear mark morphology (center), and wear mark contour lines (right): (a) Ni-P (b) Ni-P-WC (c) Ni-P-WC-BN(h)(15 g/L) (d) Ni-P-WC-BN(h)(20 g/L) (e) Ni-P-WC-BN(h)(25 g/L) (f) Ni-P-WC-BN(h)(30 g/L)表 4 磨痕元素分布Table 4. Distribution of abrasion elements
图 9 复合镀层磨痕SEM(左)、磨痕形貌(中)、磨痕轮廓线(右):(a)Ni-P (b)Ni-P-WC (c)Ni-P-WC-BN(h)(15 g/L) (d)Ni-P-WC-BN(h)(20 g/L) (e)Ni-P-WC-BN(h)(25 g/L) (f)Ni-P-WC-BN(h)(30 g/L)Figure 9. SEM of composite plating wear marks (left), wear mark morphology (center), and wear mark contour lines (right): (a) Ni-P (b) Ni-P-WC (c) Ni-P-WC-BN(h)(15 g/L) (d) Ni-P-WC-BN(h)(20 g/L) (e) Ni-P-WC-BN(h)(25 g/L) (f) Ni-P-WC-BN(h)(30 g/L)表 4 磨痕元素分布Table 4. Distribution of abrasion elementsArea Atomic fraction of an element at% Ni O P Si W B Au 1 75.01 14.08 5.15 2.71 — — 2.34 2 90.1 — 6.4 — — — 3.49 3 23.16 70.39 1.49 — 2.87 — 1.28 4 84.64 2.48 3.43 — 6.14 — 3.31 5 56.59 31.00 3.36 1.35 5.41 0.35 1.93 6 63.12 4.00 3.58 — 3.13 23.59 2.60 7 37.49 53.33 2.07 1.16 3.40 0.76 1.79 8 37.68 52.95 2.56 1.06 4.05 0.2 1.50 9 66.25 9.97 4.38 0.01 4.05 14.04 1.29 10 72.42 15.68 3.59 0.15 5.63 0.13 2.42 11 58.76 7.96 3.03 0.11 4.56 20.69 4.88 12 46.65 27.96 1.23 4.89 4.47 8.99 5.82 BN(h)纳米颗粒加入后磨痕形貌如图9(c)-(f)所示,BN(h)添加量为15 g/L时,对比图8中摩擦系数曲线及磨痕处轮廓线可以发现Ni-P-WC-BN(h)(15 g/L)复合镀层发生了较为严重的剥落现象,剥落的镀层在磨痕处随摩擦副一起在镀层表面摩擦,从而导致摩擦系数短时内上升,随着摩擦实验的进行,剥落的镀层被压应力压碎,摩擦系数也逐渐减小到该涂层的正常水平;BN(h)添加量为20 g/L时,镀层的剥落现象大幅减少,磨痕处的Si元素含量增加,镀层存在黏着磨损现象,此时摩擦系数略小于Ni-P-WC复合镀层,但是由于BN(h)沉积量较少,因此减磨能力有限;BN(h)添加量为25 g/L时,镀层基本无剥落现象,磨痕较为平整,这是因为BN(h)微粒在镀层中和WC共沉积,呈弥散分布,由于BN(h)为六方结构,层与层之间靠范德华力连接,在摩擦中易产生滑动,一方面滑动的BN(h)可以在镀层和对磨球间形成固体润滑膜减小摩擦,另一方面BN(h)可以在摩擦过程中填补磨痕处的缺陷,使磨痕更加平整,此时摩擦形式为磨料磨损,伴随极微的黏着磨损;BN(h)添加量为30 g/L时,磨痕呈现较为明显的犁沟状并伴随着严重的黏着现象,此时纳米浓度颗粒浓度过高,电沉积效率下降,镀层硬度和厚度减小,镀层中Si元素含量大幅提高,镀层主要磨损形式为黏着磨损。
3. 结 论
(1)不同纳米颗粒的质量浓度对纳米复合镀层表面的微观形貌和物相结构有重要影响,Ni-P镀层表面呈现明显的胞状结构;加入WC纳米颗粒后,Ni-P-WC复合镀层表面呈不平整“菜花”状形貌;加入BN(h)纳米颗粒后,Ni-P-WC-BN(h)复合镀层微观形貌无明显变化,镀层平均晶粒尺寸先减小后增大,合适浓度的BN(h)对晶粒有一定的细化作用。
(2)试验范围内,纳米颗粒的添加可以有效的提升复合镀层的显微硬度和厚度。Ni-P-WC和Ni-P-WC-BN(h)(25 g/L)复合镀层硬度最大,平均硬度达到
1150 HV1,纳米颗粒浓度过低或者过高均会降低镀层的显微硬度。(3)试验范围内,Ni-P镀层在摩擦磨损实验中存在较为严重的黏着和剥落现象且磨损量较大;加入WC纳米颗粒后,镀层无黏着现象,磨损形式为磨料磨损和氧化磨损,此时摩擦系数较小且磨损量较低;进一步加入BN(h)纳米颗粒后,随着BN(h)浓度的提升,镀层的摩擦系数和磨损量先减小后增大,在BN(h)质量浓度为25 g/L时镀层的摩擦系数最低,在保证镀态高硬度的同时,摩擦系数较Ni-P-WC复合镀层降低22.6%,这说明二元纳米颗粒掺杂发挥了协同生长的优势,具有更好的减磨耐磨性能。
-
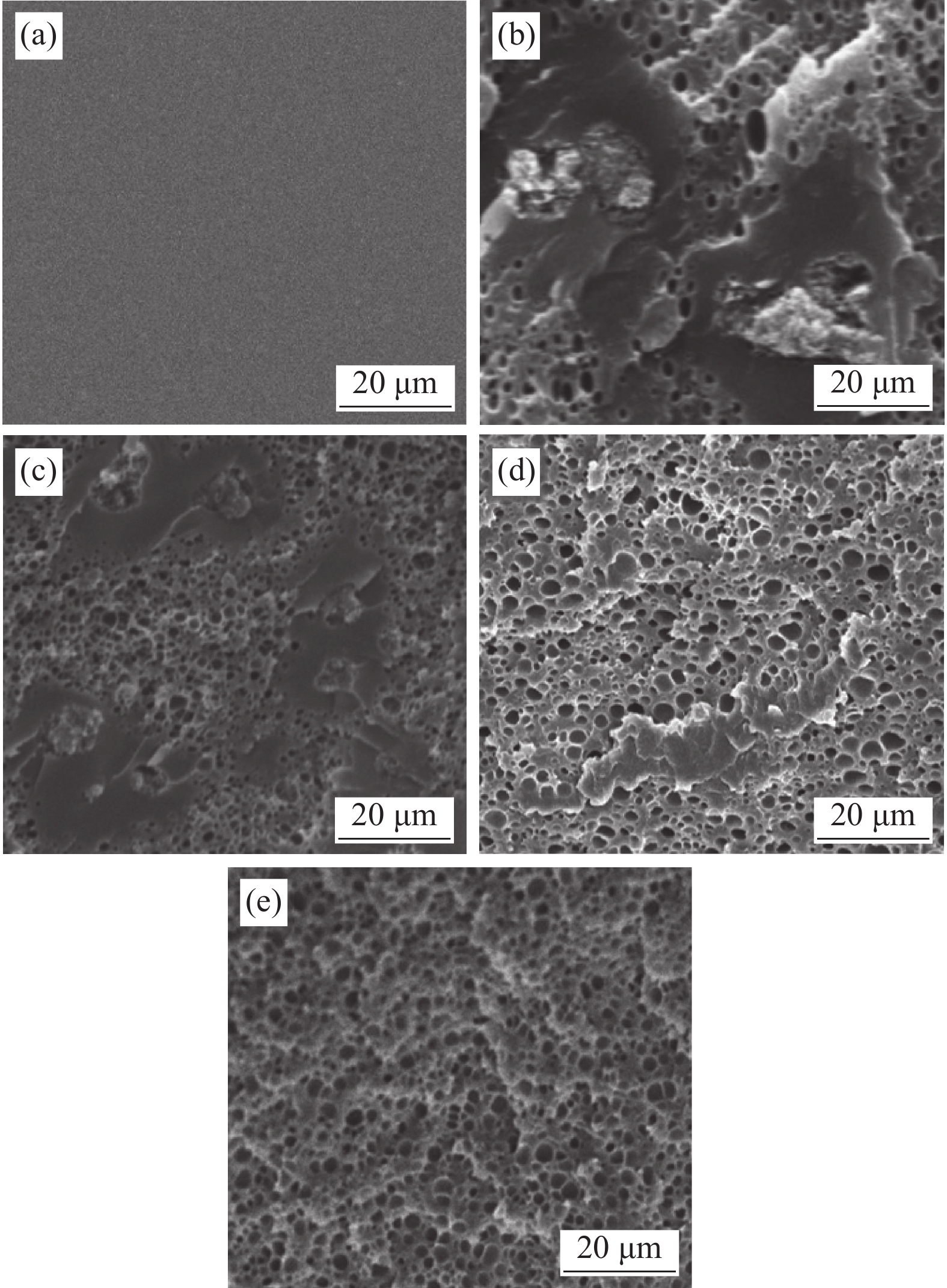
表 1 未加PEG及加入PEG不同乙醇浸泡时间的PAM/CNTs-PEG导电水凝胶的孔隙率
Table 1 Porosity of PAM/CNTs-PEG conducting hydrogels without PEG and with PEG in different ethanol soaking time
Sample Pore volume/
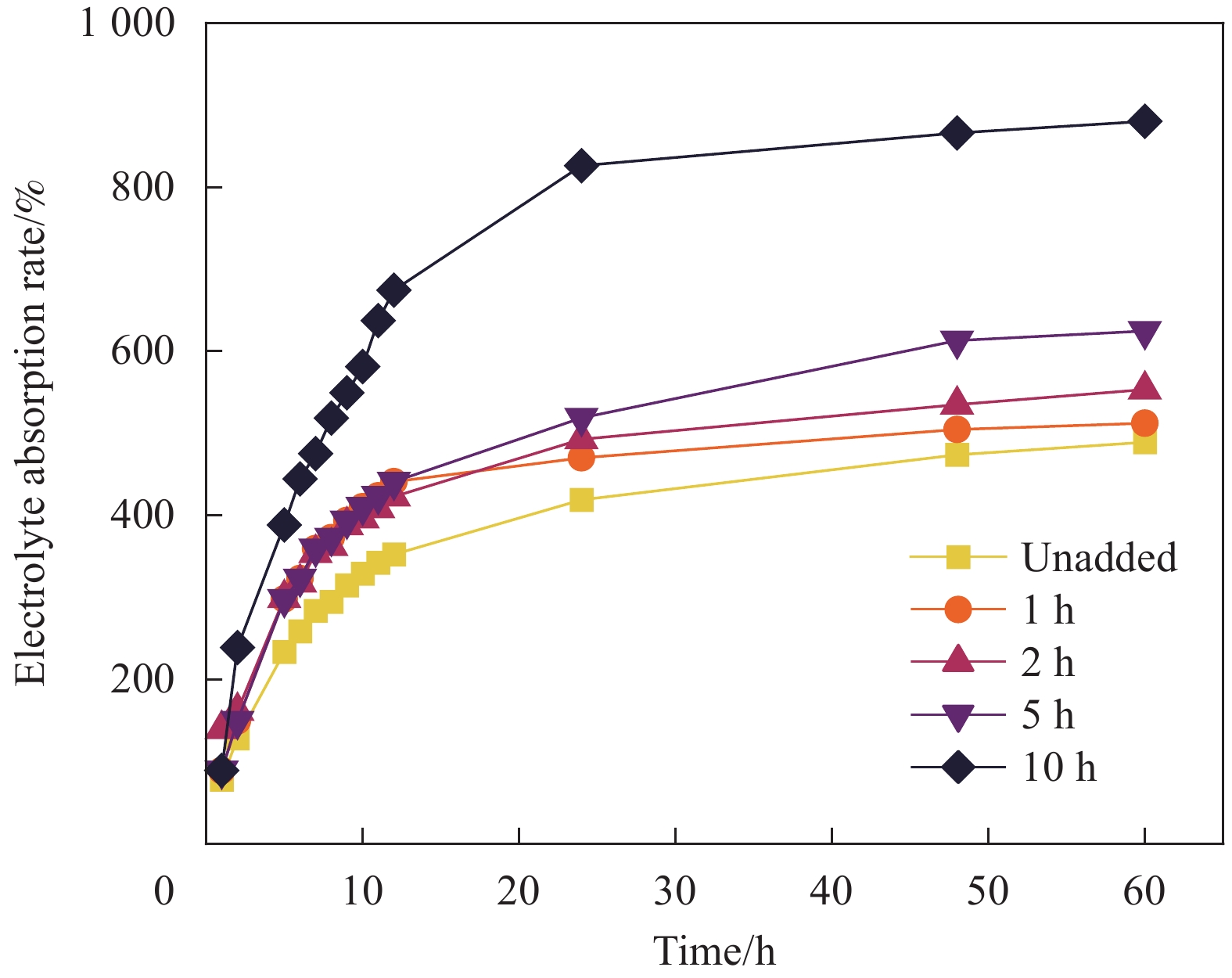
(mL·g−1)Density ρ/(g·cm−3) Porosity/% Unadded 0.0070 1.2129 3.96 1 h 0.0065 1.3217 6.50 2 h 0.0374 1.2364 27.35 5 h 0.2194 1.0754 65.71 10 h 4.7320 1.0206 95.89 -
[1] GÖHLERT T, SILES P F, PÄSSLER T, et al. Ultra-thin all-solid-state micro-supercapacitors with exceptional performance and device flexibility[J]. Nano Energy,2017,33:387-392. DOI: 10.1016/j.nanoen.2017.01.054
[2] ZHU T, NI Y, BIESOLD G M, et al. Recent advances in conductive hydrogels: Classifications, properties, and applications[J]. Chemical Society Reviews,2023,52(2):473-509. DOI: 10.1039/D2CS00173J
[3] SONG Y, NIU L, MA P, et al. Rapid preparation of anti-freezing conductive hydrogels for flexible strain sensors and supercapacitors[J]. ACS Applied Materials & Interfaces,2023,15(7):10006-10017.
[4] NISHIDE H, OYAIZU K. Toward flexible batteries[J]. Science,2008,319(5864):737-738. DOI: 10.1126/science.1151831
[5] WANG J, WU H, GAO D, et al. High-density iron nanoparticles encapsulated within nitrogen-doped carbon nanoshell as efficient oxygen electrocatalyst for zinc-air battery[J]. Nano Energy,2015,13:387-396. DOI: 10.1016/j.nanoen.2015.02.025
[6] HAN X, WU X, ZHONG C, et al. NiCo2S4 nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries[J]. Nano Energy,2017,31:541-550. DOI: 10.1016/j.nanoen.2016.12.008
[7] FU J, CANO Z P, PARK M G, et al. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives[J]. Advanced Materials,2017,29(7):1604685. DOI: 10.1002/adma.201604685
[8] YOO H D, MARKEVICH E, SALITRA G, et al. On the challenge of developing advanced technologies for electrochemical energy storage and conversion[J]. Materials Today,2014,17(3):110-121. DOI: 10.1016/j.mattod.2014.02.014
[9] AASEN D, CLARK M, IVEY D G. A gas diffusion layer impregnated with Mn3O4-decorated N-doped carbon nanotubes for the oxygen reduction reaction in zinc-air batteries[J]. Batteries & Supercaps,2019,2(10):882-893.
[10] WANG R, CHEN Z, HU N, et al. Nanocarbon-based electrocatalysts for rechargeable aqueous Li/Zn-air batteries[J]. ChemElectroChem,2018,5(14):1745-1763. DOI: 10.1002/celc.201800141
[11] ZHU Y, LIN L, CHEN Y, et al. Extreme temperature-tolerant conductive gel with antibacterial activity for flexible dual-response sensors[J]. ACS Applied Materials & Interfaces,2020,12(50):56470-56479.
[12] WEI J, XIAO P, CHEN T. Water-resistant conductive gels toward underwater wearable sensing[J]. Advanced Materials, 2023, 35(42): 2211758.
[13] CHEN L, ZHANG W, DONG Y, et al. Polyaniline/poly(acrylamide-co-sodium acrylate) porous conductive hydrogels with high stretchability by freeze-thaw-shrink treatment for flexible electrodes[J]. Macromolecular Materials and Engineering,2020,305(3):1900737. DOI: 10.1002/mame.201900737
[14] WANG S, GUAN S, ZHU Z, et al. Hyaluronic acid doped-poly(3, 4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration[J]. Materials Science and Engineering: C,2017,71:308-316. DOI: 10.1016/j.msec.2016.10.029
[15] LI Z H, LIU W T, LI Z Y, et al. Swelling and thermal properties of porous PNIPAM/PEG hydrogels prepared by radiation polymerization[J]. Nuclear Science and Techniques,2013,24(2):17-24.
[16] 李健昱, 徐朝阳, 周欢, 等. 多孔PVA/CNFs复合水凝胶的制备与性能[J]. 包装工程, 2016, 37(15):56-60. DOI: 10.19554/j.cnki.1001-3563.2016.15.012 LI Jianyu, XU Chaoyang, ZHOU Huan, et al. Preparation and properties of porous PVA/CNFs composite hydrogel[J]. Packaging Engineering,2016,37(15):56-60(in Chinese). DOI: 10.19554/j.cnki.1001-3563.2016.15.012
[17] 张思雨. 基于MXene导电水凝胶的制备及性能研究[D]. 西宁: 青海师范大学, 2022. ZHANG Siyu. Preparation and properties of conductive hydrogel based on MXene [D]. Xining: Qinghai Normal University, 2022(in Chinese).
[18] SHI Y, YU G H. Designing hierarchically nanostructured conductive polymer gels for electrochemical energy storage and conversion[J]. Chemistry of Materials,2016,28(8):2466-2477. DOI: 10.1021/acs.chemmater.5b04879
[19] 石奋玲, 杜莹, 逯帅帅, 等. 基于甜菜碱的两性离子导电水凝胶的合成及性能[J]. 印染, 2023, 49(1):49-52. SHI Fenling, DU Ying, LU Shuaishuai, et al. Synthesis and properties of zwitterionic conductive hydrogel based on betaine[J]. Dyeing and Finishing,2023,49(1):49-52(in Chinese).
[20] 汪锋. 可拉伸导电水凝胶设计及其制备方法与储能器件应用研究[D]. 南京: 南京邮电大学, 2022. WANG Feng. Design and preparation of stretchable conductive hydrogel and its application to energy storage devices[D]. Nanjing: Nanjing University of Posts and Telecommunications, 2022(in Chinese).
[21] 魏垂高, 周丽娜, 张宇彪, 等. 基于导电水凝胶的柔性电子皮肤传感器研究[J]. 电子元件与材料, 2022, 41(11):1149-1157. DOI: 10.14106/j.cnki.1001-2028.2022.0609 WEI Chuigao, ZHOU Lina, ZHANG Yubiao, et al. Research on flexible electronic skin sensor based on conductive hydrogel[J]. Electronic Components & Materials,2022,41(11):1149-1157(in Chinese). DOI: 10.14106/j.cnki.1001-2028.2022.0609
[22] 李媛. 基于中性凝胶聚合物电解质的柔性锌空气电池研究[D]. 天津: 天津大学, 2019. LI Yuan. Research on flexible zinc-air battery based on neutral gel polymer electrolyte[D]. Tianjin: Tianjin University, 2019(in Chinese).
[23] 程时富. 多孔炭材料的制备及其电化学性能[D]. 上海: 华东师范大学, 2020. CHENG Shifu. Preparation and electrochemical properties of porous carbon materials[D]. Shanghai: East China Normal University, 2020(in Chinese).
[24] 陈东方, 裴普成, 宋鑫, 等. 锌空燃料电池电化学阻抗等效电路模型[J]. 清华大学学报(自然科学版), 2020, 60(2):139-146. DOI: 10.16511/j.cnki.qhdxxb.2019.22.042 CHEN Dongfang, PEI Pucheng, SONG Xin, et al. Electrochemical impedance equivalent circuit model for zinc-air fuel cells[J]. Journal of Tsinghua University (Science and Technology),2020,60(2):139-146(in Chinese). DOI: 10.16511/j.cnki.qhdxxb.2019.22.042
[25] MA M, SHANG Y, SHEN H, et al. Highly transparent conductive ionohydrogel for all-climate wireless human-motion sensor[J]. Chemical Engineering Journal,2021,420:129865. DOI: 10.1016/j.cej.2021.129865
-
期刊类型引用(12)
1. 贾宝惠,任鹏,宋挺,崔开心,肖海建. 湿热环境下端径比对复合材料螺栓连接结构静力拉伸失效的影响. 材料导报. 2024(05): 246-252 .  百度学术
百度学术
2. 张宇,郭盼盼,熊婕,黄峰,王波. 循环湿热环境对树脂基复合材料弯曲性能的影响. 科学咨询(科技·管理). 2024(02): 135-138 .  百度学术
百度学术
3. 牛存洋,寿文凯,顾海萍,孔德良. 以价值观为导向的生态学课程思政教学设计——以种群生活史对策为例. 科学咨询(教育科研). 2024(03): 134-137 .  百度学术
百度学术
4. 刘鸿森,黄凯,黄金钊,韩晓剑,逯浩,骆杨,张莉,果立成. 考虑温度效应的复合材料紧固结构面外拉脱性能和失效机制. 复合材料学报. 2024(09): 4778-4790 .  本站查看
本站查看
5. 王慧敏,任亮,范微微,陈阳,孙丽. 基于锥体结构复合材料制品布带缠绕成型关键工艺参数优化. 宇航材料工艺. 2024(05): 87-92 .  百度学术
百度学术
6. 樊俊铃,马国庆,焦婷,陈曾美,韩啸. 温度和湿度对碳纤维增强复合材料老化影响研究综述. 航空科学技术. 2023(09): 1-13 .  百度学术
百度学术
7. 刘宋婧,冯宇,张腾,毕亚萍,张铁军. 航空复合材料加筋板湿热环境下吸湿性能. 航空动力学报. 2023(09): 2231-2240 .  百度学术
百度学术
8. 王静,程健,肖存勇,贾松,任荣,熊需海. 先进聚合物基复合材料超声焊接研究进展. 高分子材料科学与工程. 2023(09): 166-173 .  百度学术
百度学术
9. 蒋平,吕太勇,吴丽华,José Pérez-Rigueiro,胡梦蕾,徐丽萍,黄诗怡,王安萍,郭聪. 形变导致的蜘蛛大壶状腺丝力学行为的记忆与变异. 材料导报. 2023(23): 237-245 .  百度学术
百度学术
10. 吴志猛. 聚四氟乙烯芳纶1313纤维树脂基复合材料的摩擦学性能研究. 化学工程与装备. 2022(09): 40-41+44 .  百度学术
百度学术
11. 王志平,陈灏,路鹏程. 电-湿耦合作用下碳纤维增强树脂基复合材料损伤机制. 中国塑料. 2022(10): 39-45 .  百度学术
百度学术
12. 史超帆,陈叔平,王洋,金树峰,于洋,何远新,杨帅,熊珍艳,史庆智. 纤维方向对环氧树脂/玻纤复合材料导热性能影响. 工程塑料应用. 2022(11): 108-116 .  百度学术
百度学术
其他类型引用(9)
-
-
锌-空气电池要实行工业化生产,其核心部分是空气扩散电极,如果优化其性能,就有望使其有利于气体的扩散,并形成更多的三相界面,更有利于电极的电化学反应。导电水凝胶是由导电材料和交联聚合物网络组成,聚合物网络提供支架,而导电材料赋予水凝胶良好的导电性。多孔的结构可以给气体更多的扩散通路,也有利于催化层的负载,形成更多的三相界面。
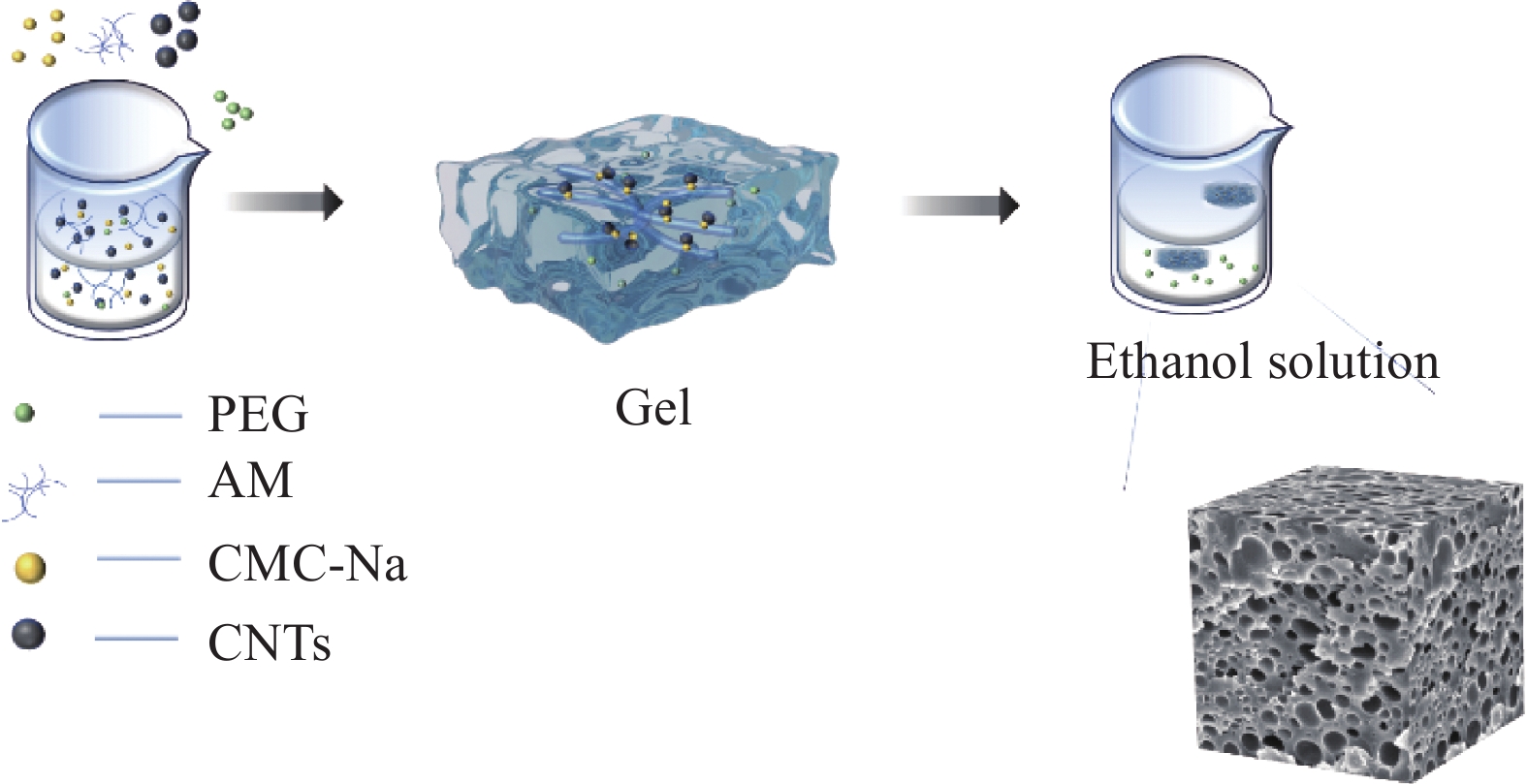
采用聚丙烯酰胺基水凝胶,以聚乙二醇-2000(PEG2000)为制孔剂,采用原位聚合合成多孔PAM/CNTs-PEG导电水凝胶。羧甲基纤维素钠(CMC-Na)为黏结剂,将PAM和CNTs黏连。聚乙二醇可溶于乙醇,将制备的PAM/CNTs-PEG导电水凝胶浸泡于乙醇溶液中,可形成不同数量的介孔。本文研究了不同浸泡时间对于多孔PAM/CNTs-PEG导电水凝胶在柔性锌空电池中的性能影响。
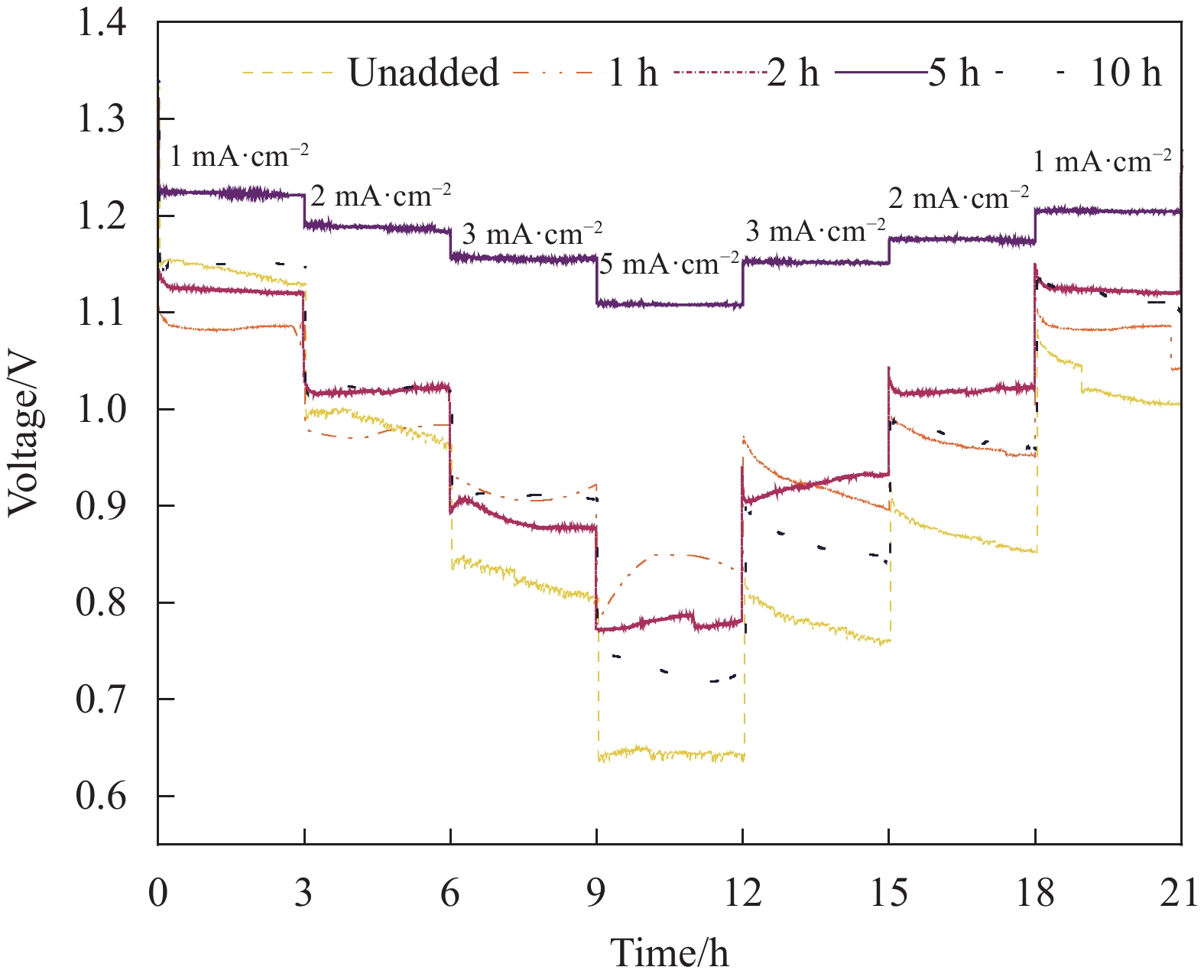
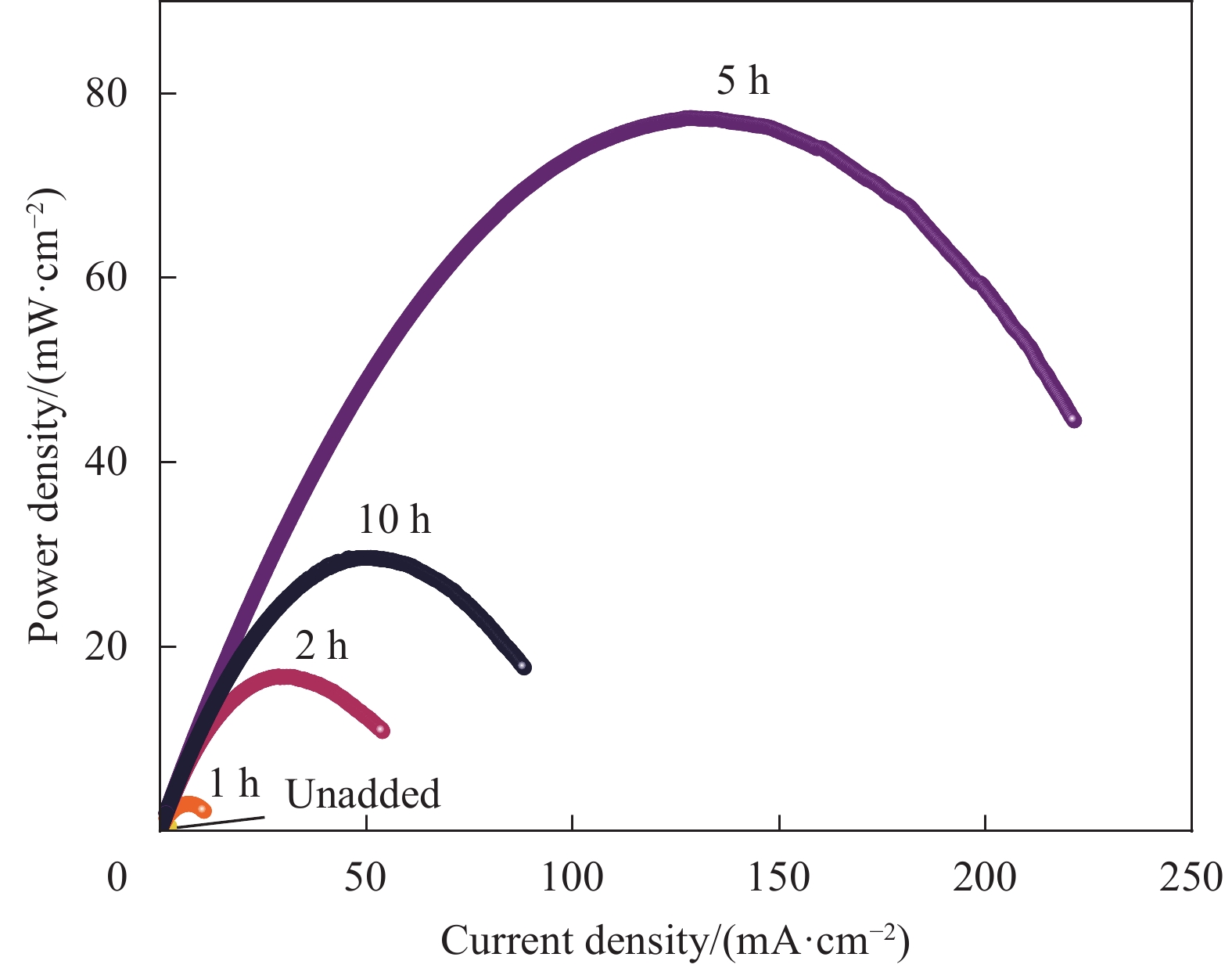
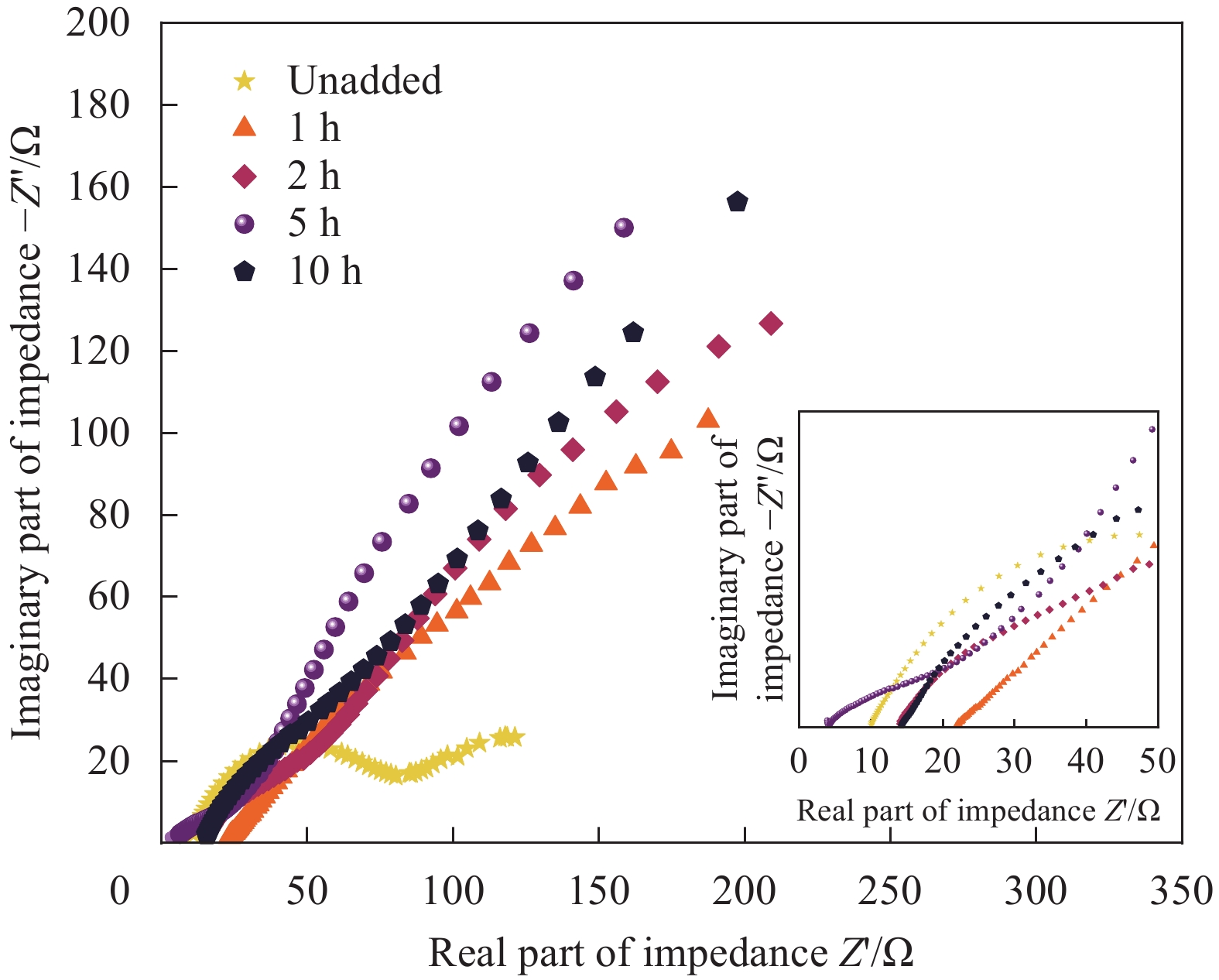
实验结果表明:这种多孔结构提高了氧气的进入量,提供了更多的三相界面,大大改善了空气阴极的电化学性能。当电压从1 mA/cm2时的1.23 V到5 mA/cm2时的1.11 V,仅衰减了0.12 V,且有高度的可逆性及稳定的放电窗口。浸泡乙醇5 h的导电凝胶在128.50 mA/cm2时产生最大的峰值功率密度77.35 mW/cm2。且该导电水凝胶有较好的导电性和较高的应变灵敏度,可以满足监测人体日常运动的需要。
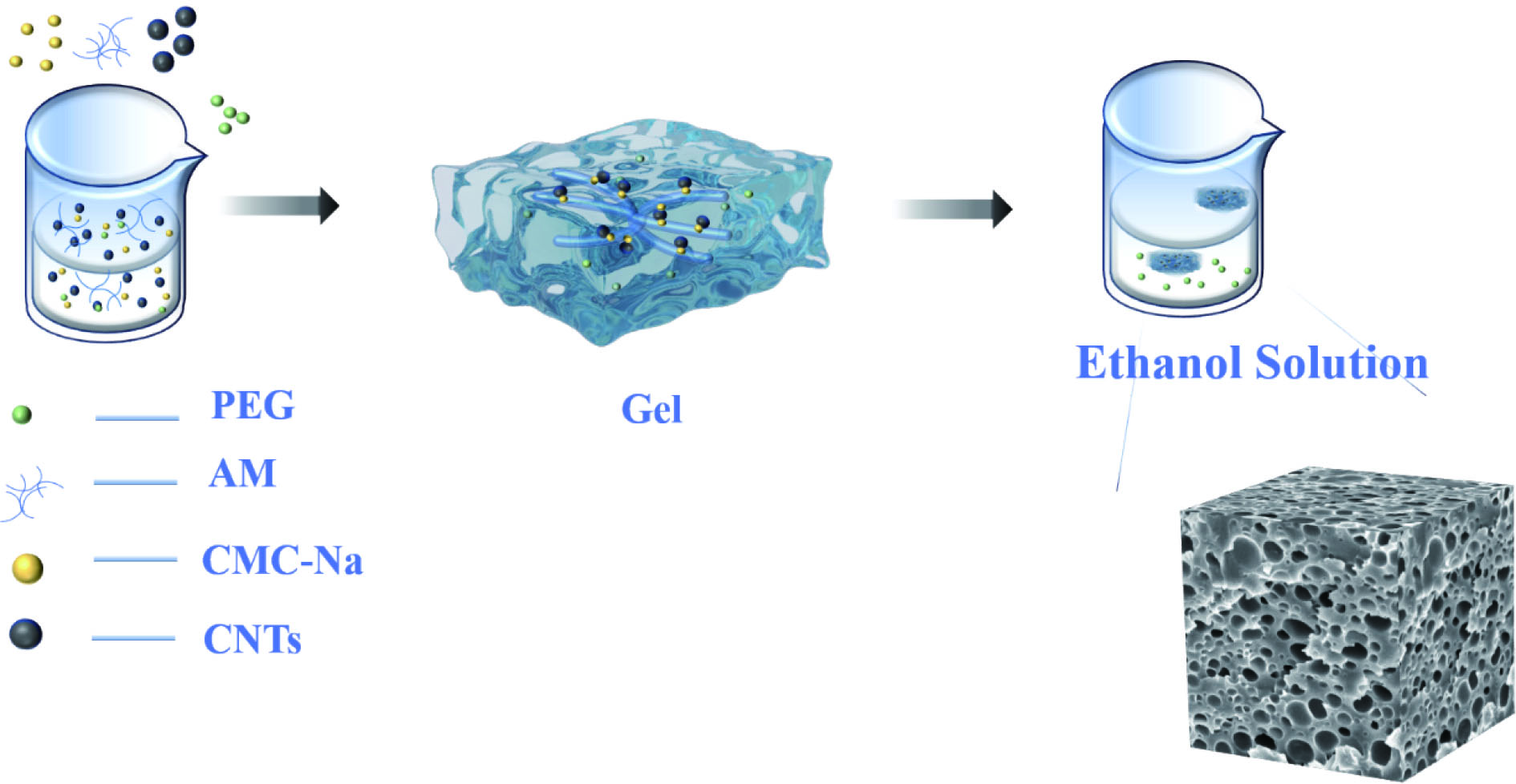
Schematic diagram of the preparation of PAM/CNTs-PEG conductive gel




 下载:
下载: