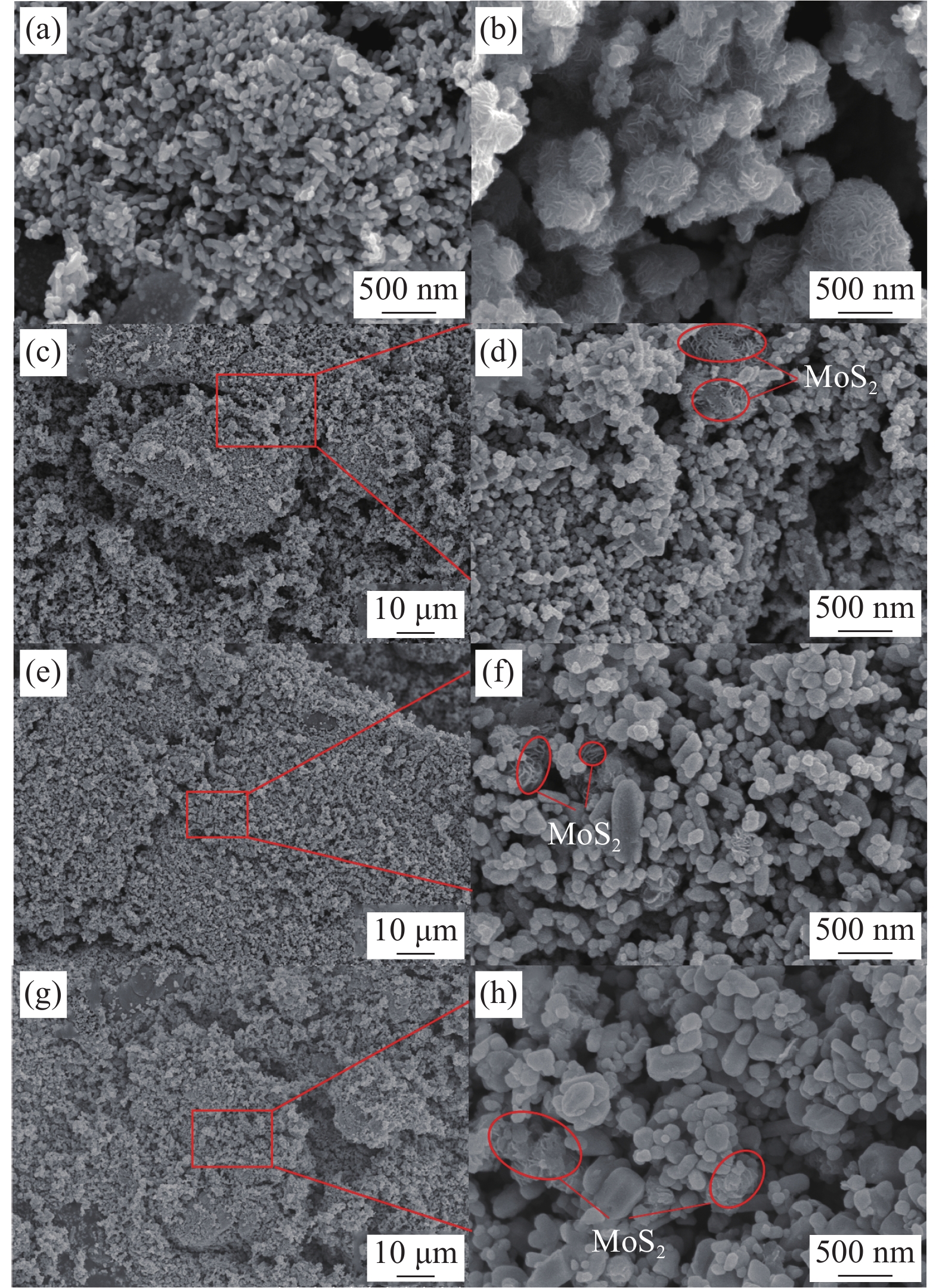
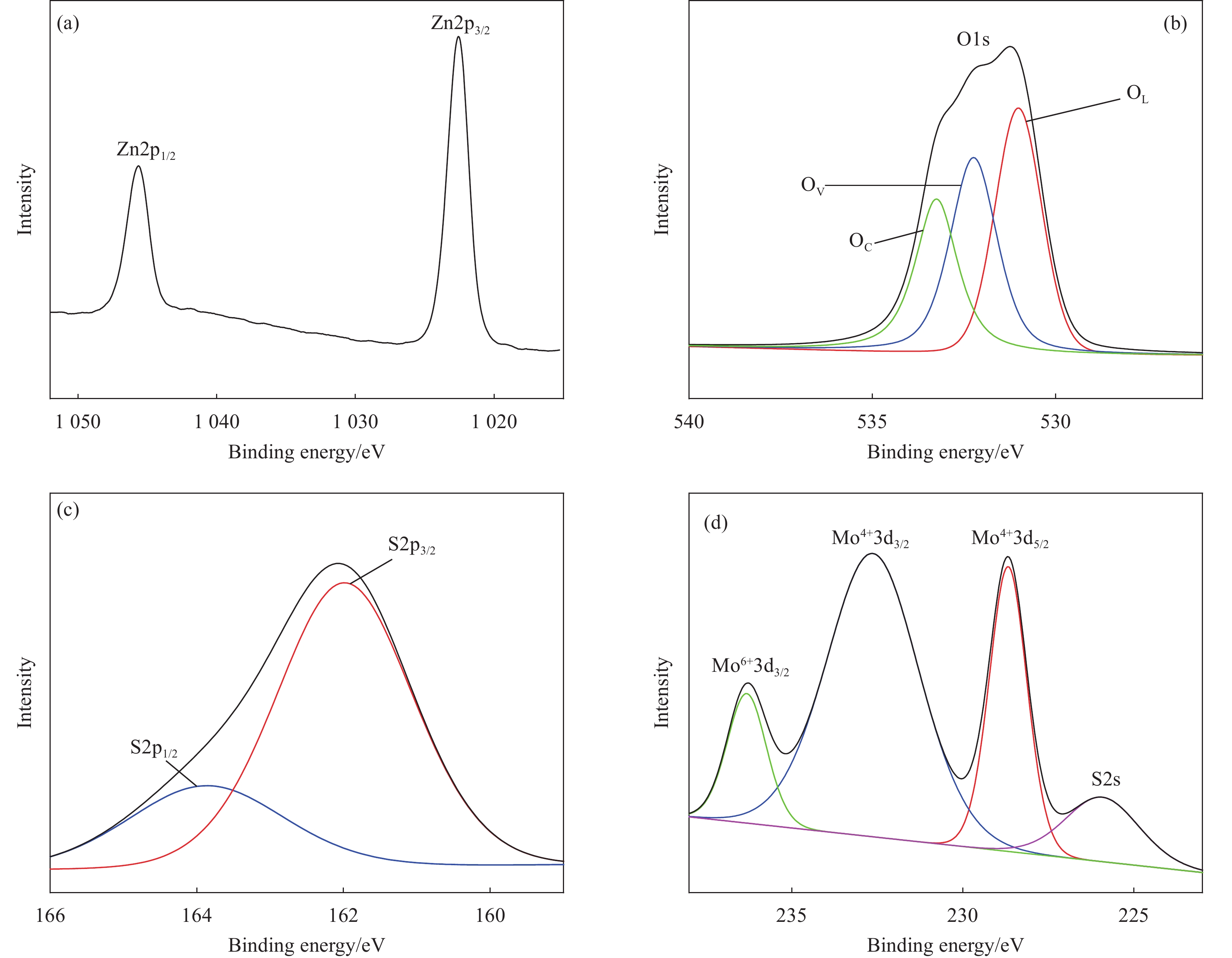
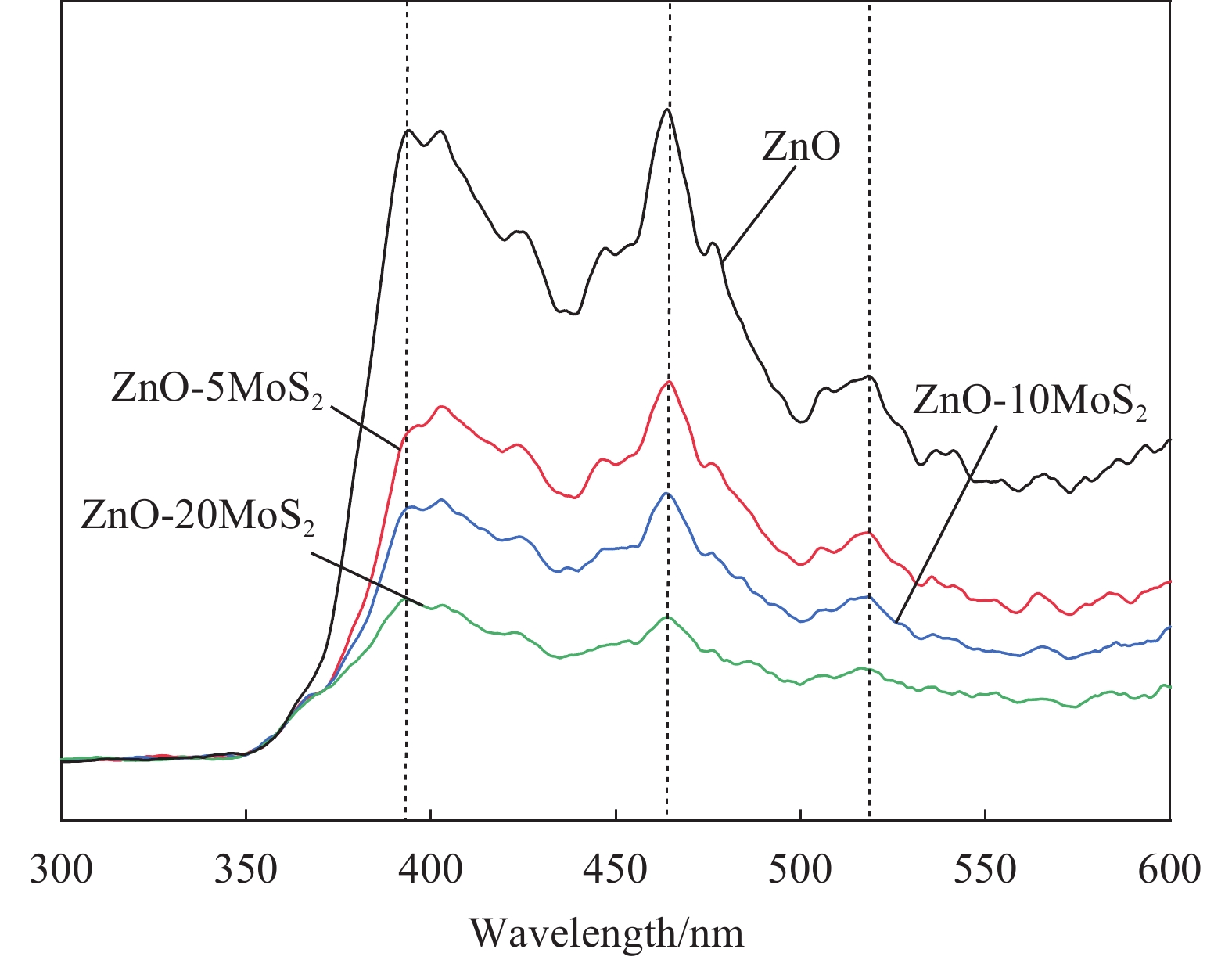
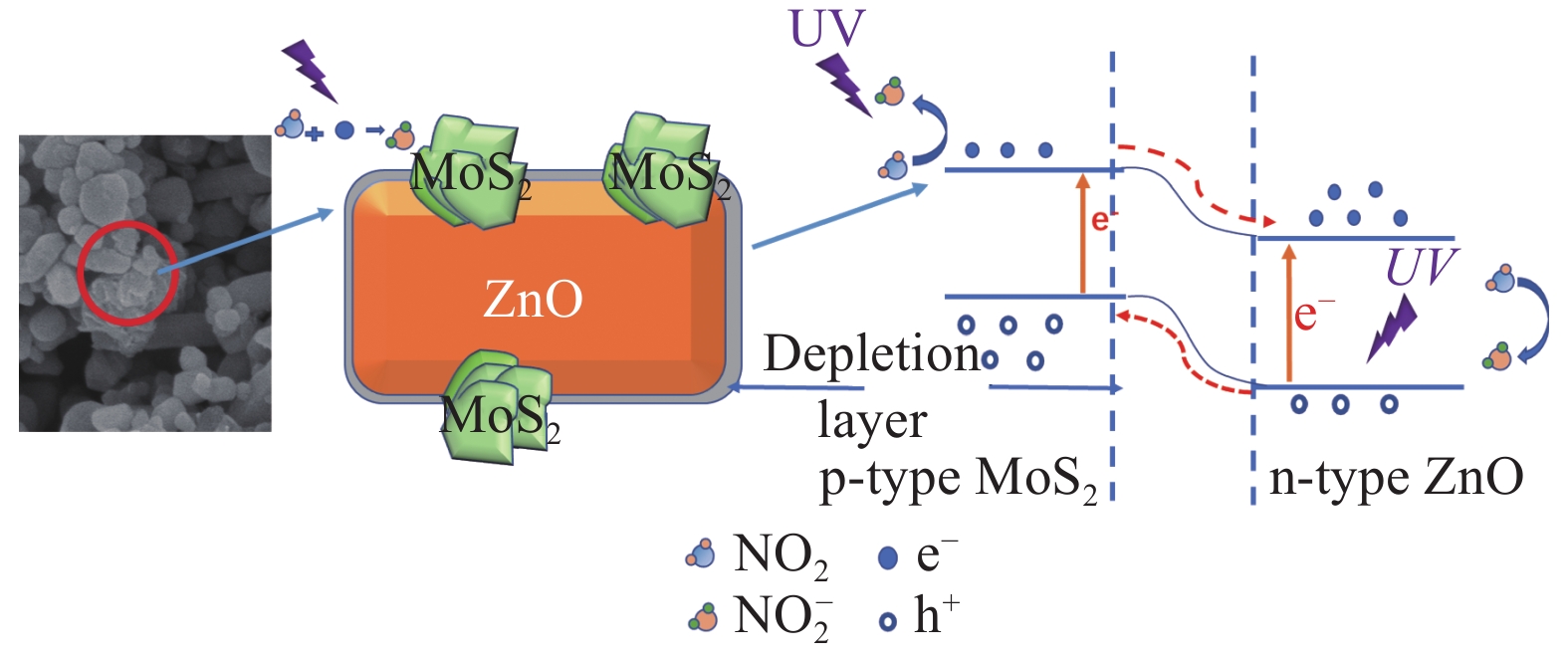
ZnO-MoS2 nano-composites with excellent light-activated NO2 gas sensitivity and MB photocatalytic degradation efficiency
-
摘要: 实现对有毒、有害气体的有效监测和对有机污染物的快速降解,对于减少大气污染和水污染所带来的危害至关重要。本研究采用超声复合方法将溶胶凝胶法制备的ZnO和水热法制备的MoS2复合到一起,成功制备了ZnO-MoS2纳米复合材料。采用XRD、SEM、TEM、XPS等手段对材料结构、形貌和表面化学组分进行表征。结果表明,多层片状MoS2均匀负载到了ZnO纳米颗粒当中,复合材料具有较好的结晶性和丰富的表面缺陷。利用紫外-可见(UV-vis)漫反射光谱、光致发光光谱(PL)和表面光电压(SPV)对材料的光电性能进行了测试。结果表明,ZnO与MoS2的复合在提升光利用率的同时,能够促进光生载流子的更有效分离。以NO2作为目标气体的室温紫外光辅助气敏测试表明,本方法制备的ZnO-MoS2气体传感器具有良好的灵敏度、恢复性、稳定性和选择性,可在室温下实现对低浓度NO2的有效响应,MoS2复合量为5wt%的ZnO-MoS2传感器对0.47 mg/m3 NO2的响应值为19.6%。同时,气敏性能研究还发现空气中O2分子在材料表面的吸附会对传感器的气敏性能产生较大的影响,ZnO-MoS2传感器在无氧条件下对NO2具有更高的气敏响应。此外,在模拟太阳光下进行的光催化降解亚甲基蓝(MB)的实验表明,依靠吸附和光催化降解的共同作用,ZnO-MoS2复合材料能够在40 min内实现水溶液当中较高浓度MB (15 mg/L)的快速清除,MoS2复合量为10wt%的ZnO-MoS2样品的反应速率常数达到了0.032 min−1。对机制的分析表明,MoS2较好的吸附性和复合所导致的光生载流子分离率的提升是ZnO-MoS2复合材料气敏和光催化性能提升的关键。Abstract: Effective monitoring of toxic and harmful gases and rapid degradation of organic pollutants are essential to reduce the hazards of air and water pollution. In this study, the MoS2 nanosheets prepared by hydrothermal method were coupled into the ZnO nanoparticles prepared by sol-gel method to form ZnO-MoS2 nano-composites via a facile ultrasonic chemical route. The structure, morphology and surface chemical component of synthesized materials were characterized by XRD, SEM, TEM and XPS. The characterizations show that multilayer MoS2 nanosheets are well dispersed among ZnO nanoparticles, and ZnO-MoS2 composites have good crystallinity and abundant surface defects. The photoelectric properties were explored by UV-vis diffuse reflectance spectrum, photoluminescence spectra (PL) and surface photovoltage spectra (SPV). The results reveal that the formation of ZnO-MoS2 heterostructure improves the utilization of light and promotes the effective separation of photo-carriers. The UV light-activated gas sensitivity test using NO2 as the target gas preformed at room temperature saw that the prepared ZnO-MoS2 gas sensor exhibited excellent gas sensing properties with good sensitivity, recoverability, stability and selectivity, which could effectively respond to low concentration NO2. The response of the optimized ZnO-MoS2 sensor with 5wt%MoS2 to 0.47 mg/m3 NO2 reached 19.6%. Meanwhile, the gas sensing performance was found to be greatly influenced by the adsorption of O2 molecule on the surface of the materials, and ZnO-MoS2 gas sensor possessed much higher gas sensitivity to NO2 under oxygen free conditions. In addition, the photocatalytic degradation of methylene blue (MB) under simulated sunlight reveal that the ZnO-MoS2 composites can rapidly remove the high concentration of MB (15 mg/L) in aqueous solution within 40 min by combined action of adsorption and photocatalysis, thereinto, the ZnO-MoS2 sample with 10wt%MoS2 shows a reaction rate constant as high as 0.032 min−1. Mechanism analysis shows that the improvement of gas sensing and photocatalytic performance of ZnO-MoS2 composites mainly attribute to the better absorbability of MoS2 and the promotion of photocarrier separation rate caused by combination.
-
Keywords:
- ZnO-MoS2 /
- photocatalysis /
- NO2 gas sensing /
- heterojunction /
- nanocomposite /
- degradation /
- methylene blue
-
近年来,柔性压力传感器凭借其轻量、柔韧、生物相容等特点在生物医疗[1]、电子皮肤[2]、人机交互[3]、柔性机器人[4]等领域具有广泛的应用前景。根据传感原理,柔性压力传感器主要分为电阻式[5]、电容式[6]、压电式[7]、摩擦电式[8]4种。而柔性电容式压力传感器因其结构简单、信号稳定,且能与静态力测量兼容及低功耗优点,得到了研究人员的广泛关注[9]。但在电容式柔性压力传感器的性能优化研究中存在一个共性问题,即高灵敏度与宽检测范围之间的制约,如何解决两者之间的矛盾仍是亟需解决的瓶颈问题。
目前,研究人员在介电层结构方面主要通过设计介电层的表面微结构或介电层体相多孔结构,来提高柔性电容压力传感器的灵敏度和检测范围。一般,在介电层表面设计金字塔形[10]、半球形[11]、荷叶表面乳突[12]等微纳结构。但制作这些微纳结构一般需要光刻[13]、3D打印[14]和仿生模板复刻[15]等技术,存在工艺复杂、成本高、耗时长等缺陷。并且由于表面微纳结构在压力作用下的变形易快速达到饱和状态,只能提高一部分检测量程。而介电层的体相多孔结构,由于本身就存在孔隙,在压力作用下先后发生孔隙减小、孔壁接触和孔壁进一步挤压过程,延缓了形变达到饱和状态的过程,从而提高了检测量程。因此相较于表面微纳结构,多孔结构在传感器的设计中存在显著优势。而在介电层材料方面,研究人员主要提出在介电层中添加高介电常数、低介电损耗填料的方法,以形成复合介电层,提高介电层的有效介电常数,从而提高灵敏度和检测范围。通常,可以分为导电填料(炭黑(CB)[16]、碳纳米管(CNTs)[17]、石墨烯(GO)[18]等),压电填料(聚偏氟乙烯(PVDF)[19]、钛酸钡(BTO)[20]等)和磁性填料(金属镍(Ni)[21]等)。
本文针对高灵敏度、宽检测范围和制作成本不能兼顾的问题,利用模板组装法制备出了一种多孔结构的电容式柔性压力传感器。首先,在基底材料上选择了具有价格低廉、质量轻、弹性好、孔隙率高、比表面积大等特点的聚氨酯(PU)海绵。其次,在填料的选择上,分别选择了导电填料炭黑(Carbon black,CB)和压电填料钛酸钡(BaTiO3,BTO),由于CB不仅具有良好的附着能力,还可以改善压力下介电常数的变化,从而提高传感器的性能[22]。而选择BTO,则是因其具有高介电常数和低介电损耗特性。两者都可以利用范德华力和静电引力附着在PU骨架上。最后,利用PU海绵现有的多孔结构作为模板,采用超声浸渍涂覆的方法将CB和BTO附着在海绵骨架上,从而制备出高的有效介电常数及低介电损耗的CB-BTO/PU海绵体。并以此为介电层组装成电容式柔性压力传感器。同时,还对该压力传感器进行了性能测试和应用范围的研究,解决了在大量程范围压力信号检测中测量量程与灵敏度之间的矛盾。
1. 实验材料及方法
1.1 CB-BTO/PU海绵柔性电容式压力传感器的制备
前处理:将聚氨酯海绵(PU,大城好五金店,优质高密度)剪切成10 mm×10 mm×3 mm的立方体,用无水乙醇(Ethanol absolute,太仓新太酒精)与去离子水(浙江南岱实业)交替清洗2次,每次10 min,以除去PU海绵表面的杂质,并在恒温培养箱中干燥1 h,以待后用。
CB-BTO/PU海绵柔性电容式压力传感器的制备过程:将炭黑(CB,美国CABOT,粉末,~15 nm)与钛酸钡(BTO,麦克林,99.9%metals basis,粉末,~100 nm)按照质量比为2.5∶100、5∶100、7.5∶100、10∶100分别加入一定量无水乙醇中,用磁力搅拌器(ZNCL-BS,山东元创仪器)进行搅拌,30 min后搅拌停止,分别往各悬浮液中加入前处理后的PU海绵,随后在超声波细胞破碎仪(LC-JY98-IIIDN,上海力辰邦西仪器科技)中进行超声分散,超声过程中产生的局部高温和超声波,其一可大幅度地减弱CB和BTO纳米颗粒之间的作用力,防止纳米颗粒团聚;其二是让悬浮液中的纳米颗粒不断地无规则运动,均匀地分散在PU海绵内部。超声1 h后,将附着有CB与BTO的PU海绵放入80℃的恒温培养箱(XMTA-600,余姚市科洋仪表)中干燥1 h,随后将干燥完成的PU海绵进行机械压缩20 min,以达到海绵的老化处理和将附着不牢的纳米颗粒去除,当压缩过程中海绵不再脱落纳米颗粒和用纸擦拭海绵表面不再出现灰色污渍时,证明得到结构稳定的CB-BTO/PU海绵三维复合材料。最后在CB-BTO/PU海绵三维复合材料的两端贴附铜箔电极,并用聚对苯二甲酸乙二醇酯(Polyethlene terephthalate,PET)薄膜封装得到CB-BTO/PU海绵柔性电容式压力传感器。具体制备流程如图1所示。
1.2 表征方法
采用扫描电子显微镜(SEM,日立SU8020)对CB-BTO/PU复合材料的形貌进行表征。用能谱分析仪(EDS,美国edax)探究CB-BTO/PU复合材料中的碳(C)、钛(Ti)和钡(Ba)元素的分布。将CB-BTO/PU海绵固定在样品台上,然后放入真空镀膜仪内喷金,喷金结束后将样品置于SEM扫描电镜下,观察海绵断面形貌。电子加速电压为5.0 kV,工作距离为13.6 mm,上下探头同时成像;然后用扫描电镜上配置的EDS对海绵上的元素成分和含量进行观察分析。
压力传感器的传感性能测试主要通过万能试验机(ZQ-950B,东莞市智取精密仪器)、LCR测试仪(TH2830,上海双旭电子)等完成。将传感器放置到万能试验机的下端压头平台上,通过控制上端压头的位移,以10 mm/min的恒定速度将0~300 kPa压力施加于传感器表面上;将传感器电极板引出的导线连接LCR测试仪(工作电压1 V),使用远端接口模式连接到PC,实时测量传感器的电容信号。
2. 结果与讨论
2.1 CB-BTO/PU海绵传感器的表征与分析
图2(a)为PU海绵浸渍前后实物图,浸渍前PU海绵骨架清晰,孔隙分布均匀;浸渍后PU海绵由淡黄色转为黑灰色,这是CB与BTO有效沉积的结果。将制备的CB-BTO/PU海绵完全压缩,当撤去外力之后,仍能恢复到初始状态,展现了该材料具有很好的柔韧性和弹性,如图2(b)、图2(c)所示。图2(d)~图2(f)为CB-BTO/PU海绵骨架的断面SEM图像。可以看出,PU海绵骨架结构明显,孔隙清晰,且随着图像的放大,能看到CB与BTO(呈颗粒状,少量团聚)均匀地附着在PU海绵骨架上。从EDS能谱分析表明,Ba (图2(g))、Ti (图2(h))、C (图2(i))3种元素在PU海绵均匀分布,再一次验证了CB与BTO在PU海绵骨架上分布均匀。
![]() 图 2 CB-BTO/PU海绵的形貌与结构表征:(a)浸渍前后PU海绵实物图;((b), (c)) PU海绵压缩的初始状态和压缩状态图;((d)~(f)) PU海绵断面SEM图像;((g)~(i)) Ba元素、Ti元素、C元素的EDS分布图Figure 2. Morphology and structure characterization of CB-BTO/PU sponge: (a) Physical diagram of PU sponge before and after impregnation; ((b), (c)) Initial compression state and compression state diagrams of PU sponge respectively; ((d)-(f)) SEM images of PU sponge sections; ((g)-(i)) EDS distribution diagrams of Ba element, Ti element and C element respectively
图 2 CB-BTO/PU海绵的形貌与结构表征:(a)浸渍前后PU海绵实物图;((b), (c)) PU海绵压缩的初始状态和压缩状态图;((d)~(f)) PU海绵断面SEM图像;((g)~(i)) Ba元素、Ti元素、C元素的EDS分布图Figure 2. Morphology and structure characterization of CB-BTO/PU sponge: (a) Physical diagram of PU sponge before and after impregnation; ((b), (c)) Initial compression state and compression state diagrams of PU sponge respectively; ((d)-(f)) SEM images of PU sponge sections; ((g)-(i)) EDS distribution diagrams of Ba element, Ti element and C element respectively2.2 CB-BTO/PU海绵传感器的传感机制与性能分析
CB-BTO/PU 海绵压力传感器可视为平行板电容器[23],在外界压力作用下,平行板电容器的两极板之间的相对距离和介电层的相对介电常数都发生了改变,从而引起了电容的变化。传感器的电容的计算公式为
C=ε0εrAd (1) 其中:C是电容器的电容;ε0是真空介电常数;εr是介电层的相对介电常数;A是上下电极板的有效重叠表面积;d是上下两电极之间的距离。
CB-BTO/PU 海绵压力传感器的传感机制为:在没有外界压力作用的初始状态下,PU海绵骨架表面附着CB和BTO,空气充满了骨架空隙,介电层具有较低的相对介电常数εr。而当受到外界压力作用以后,介电层被压缩,上下两电极之间的距离d减小,并且PU海绵微孔开始闭合,介电层中的空气被排出,εr增加,导致电容C随着压力的增加而不断增大。
对于CB-BTO/PU 海绵介电层来说,可以根据一般的Lichtenecker 混合规则[24],求出有效的相对介电常数。有效相对介电常数的计算公式为
lnεr=Vairlnεair+VCBlnεCB+VBTOlnεBTO+VPUlnεPU (2) 其中:Vair、VCB、VBTO与VPU分别为复合材料中空气、炭黑、钛酸钡与聚氨酯海绵所占的体积比;εair、εCB、εBTO与εPU分别为空气、炭黑、钛酸钡与聚氨酯海绵的介电常数。
由上式可知,当介电层受力被压缩时,空气被逐渐排出,其他三相所占的体积比逐渐增大,介电层的相对介电常数也逐渐增大,因此电容传感器的电容才能逐渐增大。
通过制备CB与BTO不同质量比的CB-BTO/PU 海绵传感器,进行传感性能测试分析,从而对传感器进行工艺参数优化。
不同配比的传感器性能也不尽相同,而质量比为mCB:mBTO=5:100的CB-BTO/PU海绵传感性能是最好的,如图3所示。从图上可知,随着CB的含量增大,传感器的灵敏度逐渐增大然后逐渐减小并趋于一致。笔者认为,一方面,CB表面具有大量的羟基、羰基、酸基和吸附水分子等官能团[25],而PU海绵中除了氨基甲酸酯官能团外,还可含有醚、酯、脲、缩二脲、脲基甲酸酯等基团[26]。这些官能团可以使CB和BTO附着在PU海绵上,并且随着CB的含量增加,附着在PU海绵上的CB与BTO也越来越多,而CB作为导电填料既可以强化BTO在PU海绵上的附着能力,还能在PU海绵上形成零星微电容,提高复合材料的介电常数,进而提高器件性能。但当CB含量超过某阈值后,虽然能让更多的BTO附着在PU海绵上,但CB附着在PU海绵上的含量也会逐渐增大,随着外界压力的作用下,CB之间相互接近逐渐形成渗流和遂穿,从而形成部分导电通路降低电容,这与田玉玉等[22]分析的导电填料在介电层中的作用结果一致。另一方面,PU海绵的空隙是有限的,而随着填料的增加,附着在PU海绵上的填料逐渐变缓,并趋于一致。上述原因解释了为什么会出现随着CB含量的增加,传感器的灵敏度会先增加后减小并趋于一致的结果。
灵敏度是评价压力传感器对压力变化敏感程度的重要性能指标,电容式压力传感器的灵敏度公式为
S=(C−C0)/C0ΔP=ΔC/C0ΔP (3) 其中: ΔC是施加压力后的电容变化量;C0是不施加压力时的初始电容值;ΔP是压力变化量;ΔC/C0为电容信号的相对变化量;C是压缩过程中传感器的实时电容。
通过上述不同配比的介电层组装的传感器的压力-电容响应曲线,可以看出,CB与BTO质量比为5∶100的海绵介电层的传感器灵敏度是最高。从图4 可以看出,CB-BTO/PU 海绵压力传感器在较小的压力范围内(0~10 kPa)的灵敏度平均为
0.6311 kPa−1,而随着压力的不断增加,在10~140 kPa范围内传感器的平均灵敏度有所增加,达到了0.7911 kPa−1。在这两个压力范围内灵敏度快速增加的原因是,越来越大的压力,使介电层不断压缩,两电极板的相对距离逐渐变小,而海绵介电层中的空气也逐渐排出,使介电层的相对介电常数迅速变大,这两方面都能增大电容的变化量,因此灵敏度在这两个压力范围内快速提升。在较大的压力范围内(140~300 kPa)的平均灵敏度为0.1395 kPa−1,相较于前两个压力范围的灵敏度,较大的压力范围内(140~300 kPa)的灵敏度有所降低,但也达到了0.1395 kPa−1,这是由于随着压力的增大,介电层的变化趋于饱和,灵敏度也随之下降。线性度值越小,表明拟合的曲线与实测的曲线之间的偏差越小[27]。如图4所示,对压力传感器进行了线性度分析,结果表明,在0~10 kPa、10~140 kPa、140~300 kPa这3个压力范围内的线性度分别为5.3%、1.4%、0.8%。该结果也从侧面证明了传感器在3个压力范围的灵敏度拟合更加可靠和准确。
综上所述,从灵敏度与线性度可以得出,CB-BTO/PU 海绵压力传感器在宽的压力范围内能保持高的灵敏度,具备良好的传感性能。
此外,与近年来相关领域文献中报道的柔性电容式压力传感器性能比较如表1所示[26, 28-30]。
表 1 CB-BTO/PU传感器与文献报道性能比较Table 1. Performance comparison between CB-BTO/PU sensor and literature reportMaterials Sensitivity Detection range/kPa Ref. TiO2@PU 0.93 kPa−1 (0-0.37 kPa)
0.079 kPa−1 (0.37-2.83 kPa)
0.02 kPa−1 (2.83-10 kPa)0-10 [26] CCTO@PU 0.73 kPa−1 (0-1.6 kPa)
0.135 kPa−1 (1.6-22.8 kPa)
0.026 kPa−1 (22.8-100 kPa)0-100 [28]
GO/CNTs@TPU0.05777 kPa−1 (0-5 kPa)
0.33213 kPa−1 (5-60 kPa)0-60 [29]
GNPs/MWCNTs/SR/PS0.062 kPa−1 (0-0.3 kPa)
0.033 kPa−1 (0.3-4.5 kPa)0-4.5 [30] CB-BTO/PU 0.6311 kPa−1 (0-10 kPa)0.7911 kPa−1 (10-140 kPa)0.1395 kPa−1 (140-300 kPa)0-300 This work Notes: CCTO—Calcium copper titanate; GO—Graphene oxide; CNTs—Carbon nanotube; TPU—Thermoplastic polyurethane; GNPs—Graphene nanosheets; MWCNTs—Carboxyl-functionalized multiwalled carbon nanotubes; SR—Silicone rubber; PS—Commercial polyurethane sponge. 对于传感器性能而言,传感器的响应时间/恢复时间、最小压力检测极限、稳定性和耐久性等性能指标也是评估传感器的重要参数。如图5(a)所示,通过在传感器加/卸载100 g砝码测量传感器的响应与恢复时间。结果表明,响应时间与恢复时间分别为0.375 s、0.125 s,响应时间略长是由于PU海绵被快速压缩形变后在短时间内仍然会持续形变才能到达最终的稳定状态,而当卸载砝码时海绵却能快速恢复原状,因此恢复时间较短。但响应时间和恢复时间都接近于人体对压力的响应时间400 ms[31],因此该传感器在人体运动监测具备一定的可行性。如图5(b)所示,为了检测传感器的最小压力测量极限,将一颗质量为0.25 g (~24.5 Pa)的小磁子放置在传感器表面,通过观察加载和卸载前后的电容变化,检测出传感器的最低压力极限。根据Zang等[32]对压力范围的分类方法,该传感器的最低检测限处于微压范围内(1 Pa~1 kPa),表明了传感器在微小压力检测方面具有潜在的应用价值。稳定性是评估压力传感器维持稳定工作的重要参数。如图5(c)所示,为了评估该传感器的输出可靠性、稳定性和重复性,分别在不同的压力下对传感器进行了响应特性测试,电容变化随着压力的增加而增加,且能在压力撤销以后恢复到初始值,这说明了该传感器具有良好的分辨率及测压的应用潜力。此外,还对传感器进行了在200 kPa压力下的
2500 次循环响应恢复测试。如图5(d)所示,在上千次的测试中,传感器的电容变化率幅值无明显变化,电容变化率曲线波形保持了良好的一致,证明了该传感器具有良好的重复性和稳定性。还与近年来相关领域文献中报道的柔性传感器耐久性进行了比较,如表2所示[33-35]。2.3 CB-BTO/PU 海绵压力传感器的应用研究
通过上述对传感器的一系列力学传感性能检测,验证了基于CB-BTO/PU海绵柔性电容式传感器具有高灵敏度、宽检测范围等较优异的传感性能。在此基础上,对传感器的应用方面进行了拓展。首先,是对传感器在小压力范围内的应用,将传感器安装在鼠标上如图6(a)所示,通过对鼠标的单击快慢和双击时的力度变化,输出不同的电容信号。结果显示,对鼠标的单击力度的不相同,所输出的信号峰值也不相同,并且随着对鼠标的单击速度加快,所得到的电容信号也会越来越密集;而双击鼠标时,则会得到连续输出的信号。
![]() 图 6 CB-BTO/PU海绵压力传感器在各种变形信号监测中的应用:(a)手指点击鼠标上传感器的响应(0~5 kPa);(b)指关节弯曲的传感器响应(0~7 kPa);(c)抓取不同质量玻璃杯的传感器响应(0~40 kPa);(d)足底压力和步态监测的传感器响应(0~110 kPa)Figure 6. Application of CB-BTO/PU sponge pressure sensor in monitoring various deformation signals: (a) Response of sensor when finger clicks the mouse (0-5 kPa); (b) Sensor response of knuckle bending (0-7 kPa); (c) Sensor responses for grabbing glasses with different qualities (0-40 kPa); (d) Sensor response of plantar pressure and gait monitoring (0-110 kPa)
图 6 CB-BTO/PU海绵压力传感器在各种变形信号监测中的应用:(a)手指点击鼠标上传感器的响应(0~5 kPa);(b)指关节弯曲的传感器响应(0~7 kPa);(c)抓取不同质量玻璃杯的传感器响应(0~40 kPa);(d)足底压力和步态监测的传感器响应(0~110 kPa)Figure 6. Application of CB-BTO/PU sponge pressure sensor in monitoring various deformation signals: (a) Response of sensor when finger clicks the mouse (0-5 kPa); (b) Sensor response of knuckle bending (0-7 kPa); (c) Sensor responses for grabbing glasses with different qualities (0-40 kPa); (d) Sensor response of plantar pressure and gait monitoring (0-110 kPa)该传感器还可以监测人体的小规模活动,如图6(b)所示,将传感器安装在手指的关节处,监测手指关节在不同弯曲角度时的电容相对变化值。当手指关节的弯曲角度变大时,对传感器的压缩形变也越来越大,而传感器所输出的电容信号值也不断变大,因此通过传感器的电容相对变化,可以精确地区分手指的弯曲程度。
除了传感器在上述小压力范围内的应用以外,该传感器还能应用于较大的压力场景,如图6(c)所示,通过拿起和放下侧面安装有传感器的玻璃杯(重600 g),传感器的电容幅值也会产生相应的变化,而随着玻璃杯中水的质量不断增加(50 g、100 g、150 g、200 g、250 g、300 g),其电容的相对变化也不断变大,并且当玻璃杯被拿起和放下时,传感器都能产生稳定的信号。在图6(d)中,将传感器连接到鞋底,用于检测足底压力和步态监测。当落脚时,传感器被压缩,电容值立即增大;而当抬脚时,传感器被释放恢复原状,电容值立即恢复初始值,表明了该传感器具有良好的稳定性及快速响应的特性。
以上的4种不同压力范围的应用检测无不都验证了该传感器在人机交互、电子皮肤、运动监测等领域应用的巨大潜力。
3. 结 论
本工作利用模板组装的方法制备了炭黑(CB)-钛酸钡(BTO)/聚氨酯(PU)海绵型电容式柔性压力传感器。得出以下结论:
(1)通过对制备的CB-BTO/PU海绵骨架进行SEM、EDS表征,结果表明CB与BTO均匀地浸渍涂覆在PU海绵骨架上。从而使该传感器的介电层融合了PU海绵的低弹性模量和CB-BTO的高介电常数等特性,显著地增强了电容变化,使传感器拥有了良好的传感性能;
(2)通过控制CB和BTO的质量比可改善海绵介电层的传感性能,最佳的配比为mCB:mBTO=5:100。该配比所制备的电容传感器,兼具了高灵敏度与宽的检测范围,在0~10 kPa、10~140 kPa与140~300 kPa的灵敏度分别为
0.6311 kPa−1、0.7911 kPa−1与0.1395 kPa−1。同时,传感器还展示出较快的响应与恢复时间(<0.375 s)及低的检测限(~0.25 g),并且还具有良好的分辨率和长久的使用寿命(>2500 次);(3)本工作被成功地验证可用于在人机交互、电子皮肤、运动监测等领域的巨大潜力,并为低成本、大规模商业化制备柔性压力传感器提供了可能。
-
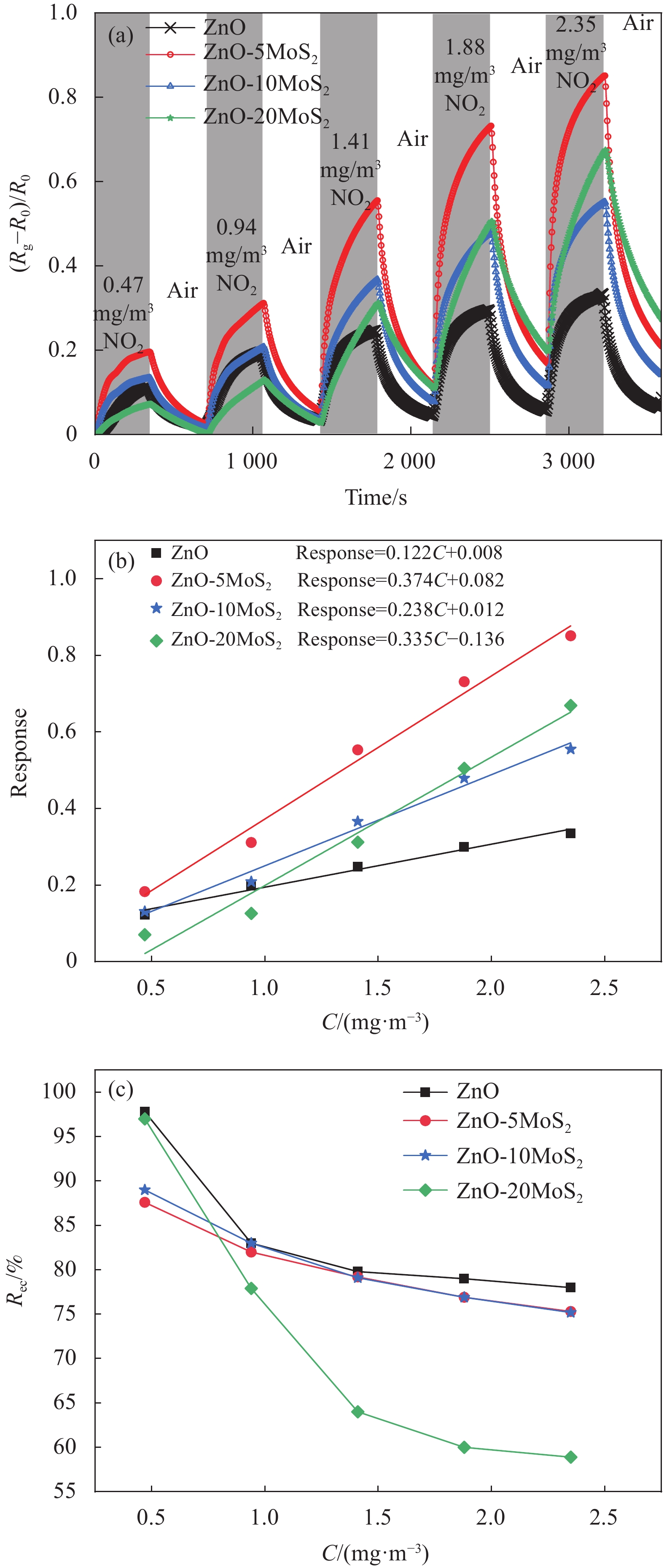
图 9 (a) 室温紫外光照射下,ZnO和ZnO-MoS2传感器对0.47~2.35 mg/m3浓度NO2的动态响应曲线(干燥空气作为背景气体);(b) 4组传感器的响应-浓度曲线;(c) 4组传感器恢复率与浓度之间的关系
Figure 9. (a) Time-dependent response curves of ZnO, and ZnO-MoS2 sensors to 0.47-2.35 mg/m3 NO2 at room temperature with the irradiation of UV light (Dry air as background gas); (b) Response-concentration curves of four sensors; (c) Recovery rate-concentration plots of four sensors
Rg—Measuring the resistance; R0—Initial resistance; Rec—Percentage of recovery rate; Response—Response intensity; C—Concentration
图 10 (a) 室温紫外光照射下,ZnO和ZnO-MoS2传感器对2.35 mg/m3浓度NO2的5次重复动态响应曲线(干燥空气作为背景气体);(b) ZnO-5MoS2气体传感器对不同气体的选择性测试
Figure 10. (a) Repeated time-dependent response curves of ZnO and ZnO-MoS2 sensors to 2.35 mg/m3 NO2 in five cycles at room temperature with the irradiation of UV light (Dry air as background gas); (b) Selectivity test of ZnO-5MoS2 gas sensor for different gases
图 12 (a) 氮气作为背景气体四种传感器对0.47~2.35mg/m3 NO2的动态响应曲线;(b) ZnO-5MoS2气体传感器分别在空气与氮气作为背景气体时对0.47~2.35mg/m3的动态响应曲线
Figure 12. (a) Time-dependent response curves of the four sensors to 0.47~2.35 mg/m3 NO2 with nitrogen as background gas; (b) Time-dependent response curves of ZnO-5MoS2 gas sensor to 0.47~2.35 mg/m3 NO2 with air and nitrogen as background gas respectively
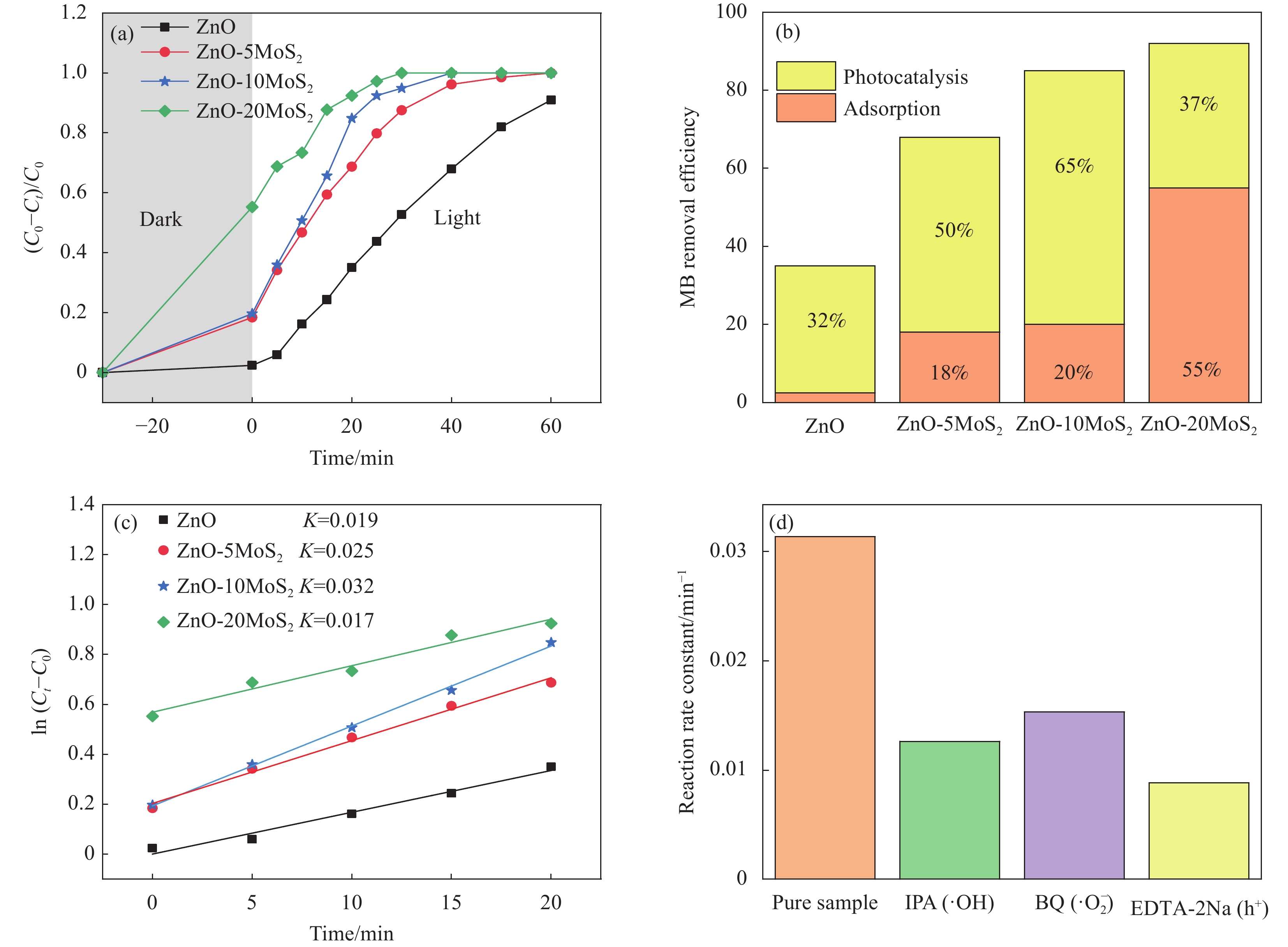
图 13 (a) ZnO及ZnO-MoS2样品在暗环境中吸附和在模拟太阳光照射下光催化降解亚甲基蓝(MB)的曲线;(b) 光照20 min时4种样品对于MB的清除效率;(c) 光照前20 min 4种样品降解MB的反应速率常数;(d) 模拟太阳光照射下添加不同牺牲剂后ZnO-10MoS2样品降解MB的反应速率常数
Figure 13. (a) Dark adsorption and photocatalytic degradation of methylene blue (MB) with the ZnO and ZnO-MoS2 samples under simulated sunlight irradiation; (b) MB removal efficiency for four samples after 20 min irradiation; (c) Reaction rate constants of four samples for the first 20 min of irradiation; (d) Reaction rate constants of ZnO-10MoS2 for photodegradation of MB with different sacrificial agents under the simulated sunlight irradiation
IPA—Isopropyl alcohol; EDTA-2Na—Edetate disodium; BQ—Benzoquinone; K—Reaction rate constant (min−1); C0—Initial concentration; Ct—Concentration at time t
表 1 ZnO-MoS2 样品成分配比
Table 1 Composition proportion of ZnO-MoS2 samples
Sample Mass of ZnO/g Mass of MoS2/g ZnO-5MoS2 0.95 0.05 ZnO-10MoS2 0.90 0.10 ZnO-20MoS2 0.80 0.20 表 2 不同复合材料的NO2气敏性能
Table 2 NO2 gas sensing performance of different composite materials
Sensor materials Gas concentration/(mg·m−3) Operation temperature/℃ Response Ref. ZnO-MoS2 NWs 94 200 31.2% [17] Ag-Fe2O3-MoS2 1.88 120 70.8% [34] MoS2-SnS2 9.4 25 60% [35] Au-MoS2 4.7 25 30% [36] CuO-ZnO 188 150 96% [37] ZnO-RGO 9.4 25 7% [38] ZnO-5MoS2 2.35 25 85.1% This work Notes: NWs—Nanowires; RGO—Reduced graphene oxide. 表 3 不同ZnO基材料光催化降解MB对比
Table 3 Comparison of photocatalytic efficiency of ZnO based composites for the degradation of MB
-
[1] EVANS A E, MATEO-SAGASTA J, QADI R M, et al. Agricultural water pollution: Key knowledge gaps and research needs[J]. Current Opinion in Environmental Sustainability,2019,36:20-27. DOI: 10.1016/j.cosust.2018.10.003
[2] MANNUCCI P M, HARARI S, MARTINE-LLI I, et al. Effects on health of air pollution: A narrative review[J]. Internal and Emergency Medicine,2015,10(6):657-662.
[3] MEHDI AGHAEI S, AASI A, PANCHA-PAKESAN B, et al. Experimental and theoretical advances in MXene-based gas sensors[J]. ACS Omega,2021,6(4):2450-2461. DOI: 10.1021/acsomega.0c05766
[4] SHINDHAL T, RAKHOLIYA P, VARJANI S, et al. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater[J]. Bioengi-neered,2021,12(1):70-87. DOI: 10.1080/21655979.2020.1863034
[5] SONG Z, ZHANG J, JIANG J. Morphological evolution, luminescence properties and a high-sensitivity ethanol gas sensor based on 3 D flower-like MoS2–ZnO micro/nanosphere arrays[J]. Ceramics International,2020,46(5):6634-6640.
[6] YAO C, WU L, LI H, et al. WS2 coating and Au nanoparticle decoration of ZnO nanorods for improving light-activated NO2 sensing[J]. Applied Surface Science, 2022, 584: 152508.
[7] WANG Z, ZHANG T, ZHAO C, et al. Anchoring ultrafine Pd nanoparticles and SnO2 nanoparticles on reduced graphene oxide for high-performance room temperature NO2 sensing[J]. Journal of Colloid and Interface Science, 2018, 514(2): 599–608.
[8] 陈奕桦, 胡俊俊, 丁同悦, 等. CeO2/ZnO 复合光催化剂制备及其可见光催化性能[J]. 复合材料学报, 2021, 38(9):3008-3015. CHEN Yihua, HU Junjun, DING Tongyue, et al. Preparation and visible light catalytic performance of CeO2/ZnO composite photocatalyst[J]. Acta Materiae Compositae Sinica,2021,38(9):3008-3015(in Chinese).
[9] SCHÜTT F, POSTICA V, ADELUNG R, et al. Single and networked ZnO-CNT hybrid tetrapods for selective room-temperature high-performance ammonia sensors[J]. ACS Applied Materials and Interfaces, 2017, 9(27): 23107-23118.
[10] GOKTAS S, GOKTAS A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review[J]. Journal of Alloys and Compounds,2021,863:158734.
[11] GENG X, ZHANG C, DEBLIQUY M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination[J]. Ceramics International,2016,42(4):4845-4852. DOI: 10.1016/j.ceramint.2015.11.170
[12] 王儒杰, 余锡孟, 王芳芳, 等. 竹炭基铈掺杂氧化锌制备及催化降解亚甲基蓝[J]. 复合材料学报, 2021, 38(6): 1896-1910. WANG Rujie, YU Ximeng, WANG Fangfang, et al. Preparation of carbon supported cerium doped zinc oxide composite material and its photocatalytic propertiesstudy in degradation of methylene blue dye[J]. Acta Materiae Compositae Sinica, 2021, 38(6): 1896-1910(in Chinese).
[13] LI Y, CHEN H, WANG L, et al. KNbO3/ZnO heterojunction harvesting ultrasonic mechanical energy and solar energy to efficiently degrade methyl orange[J]. Ultrasonics Sonochemistry,2021,78:105754.
[14] QAMAR M A, SHAHID S, JAVED M, et al. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photocatalytic and antibacterial activity[J]. Journal of Photochemistry and Photobiology A: Chemistry,2020,401:112776.
[15] KRISHNAN U, KAUR M, SINGH K, et al. A synoptic review of MoS2: Synthesis to applications[J]. Superlattices and Microstructures,2019,128:274-297. DOI: 10.1016/j.spmi.2019.02.005
[16] GAO X, YAO Y, MENG X. Recent development on BN-based photocatalysis: A review[J]. Materials Science in Semiconductor Processing,2020,120:1052-56.
[17] ZHAO S, WANG G, LIAO J, et al. Vertically aligned MoS2/ZnO nanowires nanostructures with highly enhanced NO2 sensing activities[J]. Applied Surface Science,2018,456(2):808-816.
[18] WANG S, CHEN W, LI J, et al. Low working temperature of ZnO-MoS2 nanocomposites for delaying aging with good acetylene gas-sensing properties[J]. Nanomaterials, 2020, 10(10): 1902.
[19] BENAVENTE E, DURÁN F, SOTOMAYORTORRES C, et al. Heterostructured layered hybrid ZnO/MoS2 nanosheets with enhanced visible light photocatalytic activity[J]. Journal of Physics and Chemistry of Solids,2018,113:119-124. DOI: 10.1016/j.jpcs.2017.10.027
[20] FU Y, REN Z, WU J, et al. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances[J]. Applied Catalysis B: Environmental,2021,285(1):119785.
[21] SELVARAJ R, KALIMUTHU K R, KALIMUTHU V. A type-II MoS2/ZnO heterostructure with enhanced photocatalytic activity[J]. Materials Letters,2019,243:183-186.
[22] HUNGE Y M, YADAV A A, MATHE V L. Ultrasound assisted synthesis of WO3-ZnO nanocomposites for brilliant blue dye degradation[J]. Ultrasonics Sonochemistry,2018,45:116-122.
[23] MA Q, HAN X M, LV K, et al. Ultrasound-enhanced preparation and photocatalytic properties of graphene-ZnO nanorod composite[J]. Separation and Purification Technology,2020,259(1):118-131.
[24] DONG H, LI J, CHEN M, et al. High-throughput production of ZnO-MoS2-graphene hetero-structures for highly efficient photocatalytic hydrogen evolution[J]. Materials, 2019, 12(14): 2233.
[25] KUMAR V, SHUKLA R K, SHAKYA J. Effect of ultraviolet irradiation on photo-physical and surface electronic properties of MoS2[J]. Journal of Nanoscience and Nanotechnology,2020,20(10):6500-6504. DOI: 10.1166/jnn.2020.18581
[26] WANG J, DENG J, LI Y, et al. ZnO nanocrystal-coated MoS2 nanosheets with enhanced ultraviolet light gas sensitive activity studied by surface photovoltage technique[J]. Ceramics International,2020,46(8):11427-11431.
[27] ZHANG G, LANG J, ZHANG Q, et al. Defects driven photoluminescence property of Sm-doped ZnO porous nanosheets via a hydrothermal approach[J]. Journal of Materials Science: Materials in Electronics,2018,29(19):16534-16542. DOI: 10.1007/s10854-018-9747-z
[28] YE Z, TAI H, XIE T, et al. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide[J]. Sensors and Actuators, B: Chemical,2016,223:149-156. DOI: 10.1016/j.snb.2015.09.102
[29] LIU Y, ZHANG Q, XU M, et al. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation[J]. Applied Surface Science,2019,476:632-640. DOI: 10.1016/j.apsusc.2019.01.137
[30] GAO X, WEN Y, QU D, et al. Interference effect of alcohol on Nessler’s reagent in photocatalytic nitrogen fixation[J]. ACS Sustainable Chemistry and Engineering,2018,6(4):5342-5348. DOI: 10.1021/acssuschemeng.8b00110
[31] GE L, HAN C, XIAO X, et al. Synthesis and characterization of composite visible light active photocatalysts MoS2-g-C3N4 with enhanced hydrogen evolution activity[J]. International Journal of Hydrogen Energy,2013,38(17):6960-6969.
[32] LUO K, ZHANG Q, YUAN H, et al. Facile synthesis of Ag/Zn1-xCuxO nanoparticle compound photocatalyst for highefficiency photocatalytic degradation: Insights into the synergies and antagonisms between Cu and Ag[J]. Ceramics International, 2021, 47(1): 48-56.
[33] KO K Y, SONG J G, KIM Y, et al. Improvement of gas-sensing performance of large-area tungsten disulfide nanosheets by surface functionalization[J]. ACS Nano,2016,10(10):9287-9296.
[34] YIN M, WANG Y, YU L, et al. Ag nanoparticles-modified Fe2O3@MoS2 core-shell micro/nanocomposites for high-performance NO2 gas detection at low temperature[J]. Journal of Alloys and Compounds,2020,829:154471. DOI: 10.1016/j.jallcom.2020.154471
[35] LIU J B, HU J Y, LIU C, et al. Mechanically exfoliated MoS2 nanosheets decorated with SnS2 nanoparticles for high-stability gas sensors at room temperature[J]. Rare Metals, Nonferrous Metals Society of China,2021,40(6):1536-1544.
[36] ZHOU Y, ZOU C, LIN X, et al. UV light activated NO2 gas sensing based on Au nanoparticles decorated few-layer MoS2 thin film at room temperature[J]. Applied Physics Letters,2018,113(8):2-7.
[37] NAVALE Y H, NAVALE S T, CHOUGULE M A, et al. NO2 gas sensing properties of heterostructural CuO nanoparticles/ZnO nanorods[J]. Journal of Materials Science: Materials in Electronics,2021,32(13):18178-18191. DOI: 10.1007/s10854-021-06360-0
[38] ZHANG L, ZHANG J, HUANG Y, et al. Hexagonal ZnO nanoplates/graphene composites with excellent sensing performance to NO2 at room temperature[J]. Applied Surface Science, 2021, 537: 147785.
[39] KAUR M, UMAR A, MEHTA S K, et al. Rapid solar-light driven superior photocatalytic degradation of methylene blue using Mo-S2-ZnO heterostructure nanorods photocatalyst[J]. Materials,2018,11(11):2254. DOI: 10.3390/ma11112254
[40] LI Q, ZHANG N, YANG Y, et al. High efficiency photocatalysis for pollutant degradation with MoS2/C3N4 heterostructures[J]. Langmuir,2014,30(29):8965-8972.
[41] LV T, PAN L, LIU X, et al. Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite syntheied via microwave-assisted reaction[J]. Journal of Alloys and Compounds,2011,509(41):10086-10091. DOI: 10.1016/j.jallcom.2011.08.045
[42] WANG Y, WANG F, HE J. Controlled fabricaion and photocatalytic properties of a three-dimensional ZnO nanowire/reduced graphene oxide/CdS heterostructure on carbon cloth[J]. Nanoscale,2013,5(22):11291-11297. DOI: 10.1039/c3nr03969b
-
期刊类型引用(1)
1. 曾鹏程,肖书平,杨柳,谈灵操,徐百平. 较宽压强响应范围和较高灵敏度的聚丙烯基电容式压力传感器的研制. 机电工程技术. 2025(03): 58-63 .  百度学术
百度学术
其他类型引用(3)
-





 下载:
下载: