Preparation of graphene-carbonyl iron powder wire and analysis of its wave absorption performance
-
摘要: 为了提高单一磁性吸波材料的吸波性能,以聚乳酸(PLA)作为基体材料,将磁性材料羰基铁粉(CIP)与石墨烯(RGO)进行复合,制备RGO-CIP/PLA复合材料。通过TG、XRD等多种测试手段对复合材料的结构、形貌等进行表征。同时使用矢量网络分析仪对复合材料的电磁参数进行测试,计算出不同厚度的吸波性能,研究了RGO的添加量对RGO-CIP/PLA复合材料的吸波性能影响。结果表明:当RGO质量分数为4wt%,CIP质量分数为20wt%时,RGO-CIP/PLA复合材料吸波性能最优;吸收厚度为3 mm时,达到了−27.25 dB最小的RL值,同时其吸收带宽为2.922 GHz (7.227~10.149 GHz)。同时,随着其吸收厚度的增加,有效吸收带宽(RL<−10 dB)会移动至较低的频带。Abstract: In order to improve the microwave absorbing properties of single magnetic absorbing material, polylactic acid (PLA) was used as the matrix material, and the magnetic material carbonyl iron powder (CIP) as well as reduced graphene oxide (RGO) were compounded to prepare RGO and CIP/PLA composites. The structure and morphology of the composites were characterized by TG, XRD and other testing methods. Meanwhile, the electromagnetic parameters of the composites were measured by vector network analyzer, and the microwave absorbing properties of different thickness were calculated. The influence of RGO addition on the microwave absorbing properties of RGO and CIP/PLA composites was studied. The results show that when the graphene content is 4wt% and the carbonyl iron powder content is 20wt%, the RGO-CIP/PLA composite has the best absorbing performance. When the absorption thickness is 3 mm, the minimum RL value of −27.25 dB is reached, and at the same time its absorption bandwidth is 2.922 GHz (7.227-10.149 GHz). At the same time, as its absorption thickness increases, the effective absorption bandwidth (RL<−10 dB) can move to a lower frequency band.
-
聚对苯二甲酸乙二醇酯(PET)是全球用量最大的高分子材料之一,其纤维制品俗称涤纶,是全球第一大化纤品种[1]。但PET属于易燃材料,且其燃烧时还伴有严重的熔滴现象,极易导致火灾蔓延和二次伤害,这为PET的应用带来了极大的安全隐患,也使其在军事、工业等诸多领域的应用受到限制[2]。目前PET阻燃改性的主流方法是引入含磷阻燃剂,但含磷聚酯的阻燃性主要是通过熔体滴落带走燃烧区域的热量和火焰来实现的[3-4],未能解决PET的熔滴问题。因此,同时赋予PET阻燃和抗熔滴特性是目前PET阻燃改性面临的一大难点。
相关研究表明,提高聚合物高温下的熔体黏度和炭化能力是实现阻燃和抗滴落的关键[5-6]。但由于PET分子链的线性结构,其在高温下具有较低的熔融黏度,这一特性在赋予其可纺性的同时,也导致其在燃烧过程中表现出严重的熔滴行为,并难以形成连续的炭层[7]。在PET中引入可交联结构单元是提高熔体黏度的一个有效途径[6, 8]。例如,Wu等[9]合成了一种芳香族席夫碱5-(亚苄基-氨基)-间苯二甲酸二甲酯,并将其用作PET的共聚单体;研究结果显示,芳族席夫碱可以在PET的熔融温度和分解温度之间形成稳定的交联网络,并在燃烧中进一步转变为致密的炭层,因而使得PET共聚酯显示出优异的自熄和抗滴落行为。但尽管上述共聚酯表现出较为理想的阻燃性和抗滴落性能,问题仍然存在。除了工艺复杂、成本高、工业化困难之外,共聚法往往会破坏分子链的规整性,从而损害PET的力学性能和可纺性。
近年来,碳基阻燃剂作为一种新型阻燃剂表现出巨大的发展潜力。研究表明,碳纳米管[10]、富勒烯[11]、纳米炭黑[12]、石墨烯[13]、碳微球[14]等碳基阻燃剂在改善聚合物的成炭质量、降低热释放速率、提高热稳定性等方面表现突出。与传统阻燃剂相比,往往少量碳基阻燃剂的引入即可显著提高聚合物的阻燃性,除此之外,引入碳基阻燃剂还能不同程度地改善聚合物的力学、热学以及电学等性能[15]。其中,碳纳米球(Carbon nanospheres,CNSs)具有无卤环保、粒径小、热稳定性高等优势,能满足PET高温加工和熔融纺丝的理论要求,但迄今为止,将碳基阻燃剂应用于PET的研究较少。
基于此,本文以CNSs为基体,在其表面接枝芳香席夫碱4-苯亚甲基氨基苯酚(4-phenyl-methyleneamino-phenol,BA)制备了一种新型碳纳米球基复合阻燃剂(CNSs-BA),旨在将CNSs和BA的优势有机结合,使阻燃剂同时具备“智能自交联”特性,从而在燃烧时在PET基体中形成三维交联网络,进而改善熔滴。重点研究了CNSs-BA/PET复合材料的阻燃性及其阻燃机制。
1. 实验材料及方法
1.1 原材料
蒸馏水,使用XY-ZL-20型蒸馏水器自制。碳纳米球(CNSs),纯度99.99%,宁波金雷纳米材料科技有限公司;30%过氧化氢,优级纯,上海沃凯生物技术有限公司;硫酸,分析纯,华东医药股份有限公司;二氯甲烷(DCM),分析纯,华东医药股份有限公司;4-苯亚甲基氨基苯酚(BA),纯度98%,东京化成工业株式会社;4-二甲氨基吡啶(DMAP),纯度99%,阿拉丁试剂(上海)有限公司;无水乙醇,分析纯,嘉兴市甬宏化工有限公司;PET切片,半消光型SD500,中国石化仪征化纤股份有限公司。
1.2 CNSs-BA阻燃剂的制备
首先采用酸化法[16]制备羧基化碳纳米球(CNSs-COOH);在DCM中加入适量氯化钙,常温振荡12 h以去除其中的水分,使用时用吸管吸取上层液体用。在三口烧瓶中加入一定量的无水DCM (50~100 mL)作为反应溶剂。搅拌状态下加入3 g CNSs-COOH,再加入1 g BA和DMAP (作为碱性催化剂,DMAP的用量为BA用量的1/10),升温至30℃,之后在搅拌状态下反应2 h,反应完成后抽滤除去液体。将反应所得固体依次用乙醇和蒸馏水洗涤,100℃下干燥6 h后研磨均匀,即得CNSs-BA阻燃剂,CNSs-BA的制备示意图见图1。
1.3 CNSs-BA/PET复合材料的制备
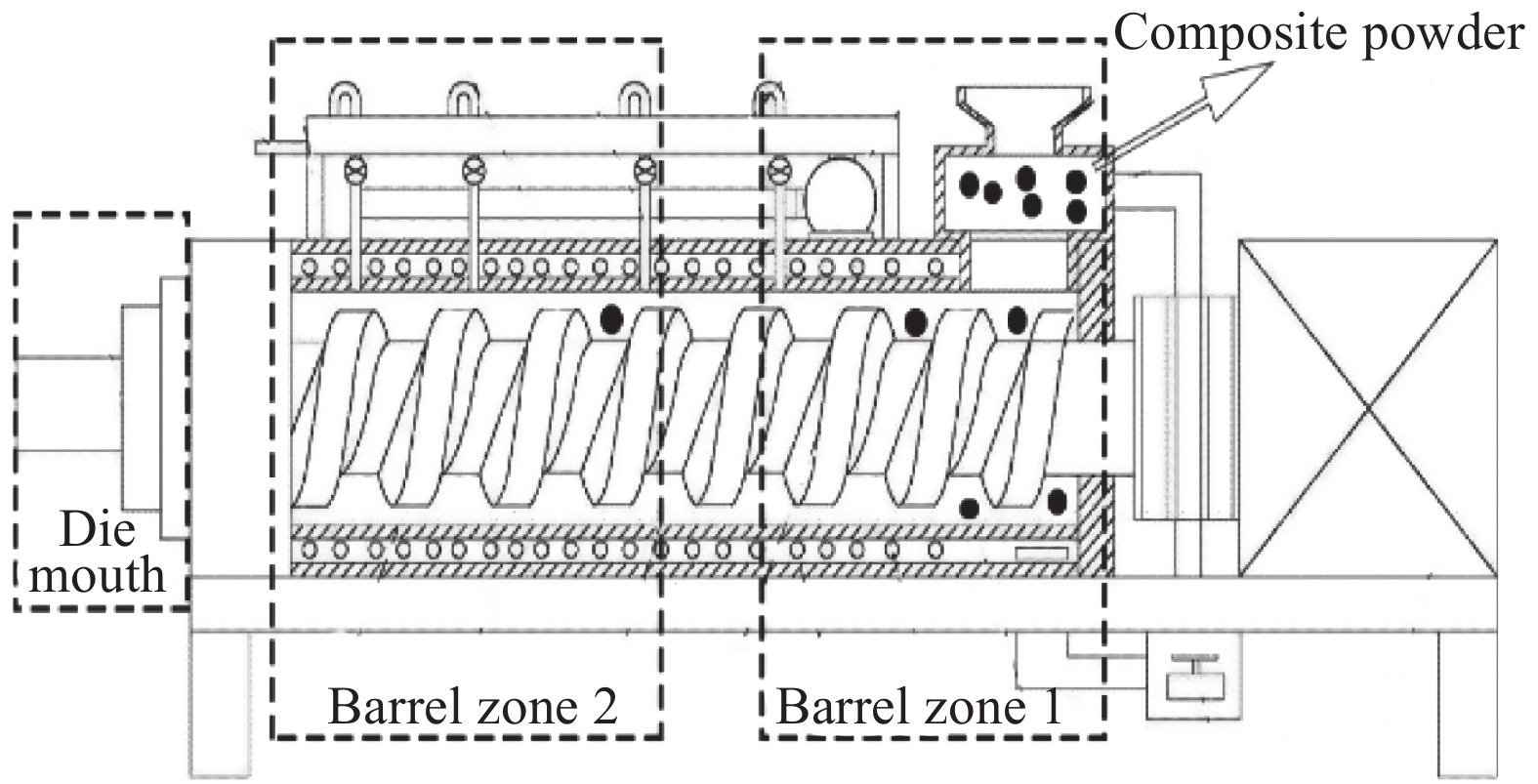
首先将纯PET切片和制备的CNSs-BA阻燃剂分别在120℃下真空干燥12 h后冷却至室温备用,然后分别按CNSs-BA占PET的质量分数为0.5wt%、1.0wt%、2.0wt%、3.0wt% 的比例将CNSs-BA与PET切片混合均匀后喂入BP-8188型转矩流变仪(东莞市宝品精密仪器有限公司)中,转矩流变仪各区的温度依次为150、255、273、275℃,转速为35~60 r/min,熔体依次经过熔融共混、挤出、切粒工序得到CNSs-BA/PET复合材料。
1.4 测试与表征
CNSs-BA阻燃剂的测试与表征:用JSM-6510LA型场发射扫描电镜(SEM,日本电子株式会社)和EM420型透射电子显微镜(赛默飞世尔科技)观察阻燃剂的微观形貌,加速电压3 kV 和20 kV。用Perkin Elmer Frontier型傅里叶变换红外光谱仪(FTIR),溴化钾压片法分析测定阻燃剂表面性质和化学结构,光谱记录范围
4000 ~400 cm−1。用Perkin Elmer TG4000型热重分析仪(TG),在N2气氛下测试阻燃剂的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。CNSs-BA/PET复合材料的测试与表征:用TM606 数显氧指数测试仪(青岛睿新杰仪器有限公司),按照GB/T 2406.2—2009[17]测试PET及其阻燃复合材料的极限氧指数(LOI),样条尺寸为120 mm×6.5 mm×3 mm。用CZF-5水平垂直燃烧仪(沧州冀路试验仪器有限公司),按照 GB/T 2408—2008[18]判定PET及其阻燃复合材料的UL-94垂直燃烧等级,样品尺寸为130 mm×13 mm×3 mm。用C-1087型锥形量热仪(英国FTT),按照ISO 5660-1: 2015[19]测试PET及其阻燃复合材料的燃烧热释放(HRR)等参数,样品尺寸:100 mm×100 mm×3 mm,辐射照度50 kW/m2。用Perkin Elmer TG4000型热重分析仪(TG),在N2气氛下测试PET及其阻燃复合材料(阻燃剂含量2wt%)的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。用Netzsch STA449F5型同步热分析仪(TG-DSC)研究PET及其阻燃复合材料(阻燃剂含量2.0wt%)的交联行为,氩气做保护气,空气气氛,气体流速20 mL/min,升温速率为10℃/min,测试温度范围为30~800℃。用气相Agilent 6980N色谱仪,Agilent 5975质谱仪,采用HP-5MS色谱柱对PET及其阻燃复合材料(阻燃剂含量2.0wt%)进行裂解-气相色谱-质谱联用(Py-GC-MS)测试。裂解条件:裂解温度750℃,时间20 s,升温速率200℃/s。色谱条件:柱温在50℃保持5 min,然后以10℃/min升温至260℃,在260℃保持10 min;进样温度220℃,传输温度280℃,He做载气,流量1.0 mL/min;裂解产物经色谱柱分离后进入质谱仪,电子能量为70 eV。
残炭的测试与表征:采用SEM观察PET及其阻燃复合材料(阻燃剂含量2.0wt%)燃烧后残炭的形貌,并用其配套的EDS能谱设备对残炭进行元素分析。采用TG在N2气氛下测试残炭的热稳定性,气体流速20 mL/min,程序设定为:30℃恒温1 min后以20℃/min的升温速率升温至800℃。
2. 结果与讨论
2.1 CNSs-BA的形貌结构和热稳定性
图2为原CNSs (图2(a))和CNSs-BA (图2(b))的SEM、TEM和EDS能谱图。可知:CNSs和CNSs-BA二者均呈规则的球形颗粒状。不同的是,原始CNSs表面光滑,平均粒径约45 nm。而经BA接枝后,CNSs-BA的表面变得粗糙,平均粒径增大到50 nm左右,由EDS谱图可知:纯CNSs中的主要成分为C元素,与CNSs相比,CNSs-BA的表面增加了N元素,源自其表面接枝的BA中的氨基。
图3是CNSs和CNSs-BA的红外图谱。对比CNSs和CNSs-BA的FTIR曲线可知,在CNSs-BA的红外曲线中,
3330 cm−1和3380 cm−1处对应N—H的伸缩振动峰,2925 cm−1和2850 cm−1处为亚甲基的伸缩振动峰,1450 cm−1处的特征峰是芳环骨架的伸缩振动峰,1269 cm−1处的特征峰是酯基C(O)—O的伸缩振动峰,1045 cm−1处的特征峰是芳环上1, 4位取代的振动峰,以上特征峰源自CMSs表面接枝的BA。图4为CNSs和CNSs-BA的TG曲线。可知:纯CNSs的初始分解温度(Tonset,定义为热失重5wt%时的温度)大于800℃,经BA接枝后Tonset降低到483.1℃,该温度远高于PET的加工温度和热分解温度。纯CNSs和CNSs-BA的最高热分解速率的温度(Tmax)分别为158.4℃和177.4℃,说明CNSs经BA接枝后热分解速率减慢。CNSs在30~800℃之间表现出3个较为明显的失重阶段,338.7℃之前对应CNSs中的少量结晶水和无定形碳的分解,338.7~544.1℃之间对应CNSs主体的热分解,544.1℃之后对应热分解产物的再分解。而CNSs-BA可划分为4个失重阶段:前两个阶段分别对应结晶水、无定形碳、小分子产物的分解以及CNSs-BA主体的分解,值得注意的是,544.1℃之后,CNSs-BA出现一个较为明显的失重峰,而CNSs的DTG曲线上并无该峰,该失重峰的出现证明CNSs表面接枝的BA在第二阶段(PET的熔融温度和分解温度之间)形成了一个较为稳定的交联网络结构,这将十分有助于燃烧时保护炭层的形成。
2.2 复合材料的阻燃性能
表1是CNSs-BA/PET复合材料的LOI和UL-94垂直燃烧测试结果。可知,与CNSs/PET相比,CNSs-BA/PET复合材料的LOI进一步提高,二者LOI规律变化一致,即随着阻燃剂含量的增大,LOI先提高后降低,当CNSs-BA含量为2.0wt%时,CNSs-BA/PET的LOI指数达到最大值28.1%,此时与纯PET相比,CNSs-BA/PET的LOI提高了33.8%。UL-94垂直燃烧测试结果表明,CNSs-BA/PET复合材料的抗熔滴性能较CNSs/PET也有明显提高,两次施加火焰后的余焰时间明显缩短,当CNSs-BA的添加量超过2.0wt%时,CNSs-BA/PET复合材料的阻燃等级可达到V-0级。
表 1 复合材料的极限氧指数(LOI)和UL-94垂直燃烧测试结果Table 1. Limiting oxygen index (LOI) and UL-94 vertical burning test results of compositesSample Flame retardant content/wt% LOI/% UL-94 vertical combustion test results t1/s t2/s t3/s Ignite cotton? Rate PET — 21.0 Burn out — — Yes NR CNSs/PET 0.5 23.2 2.6 2.5 0 Yes V-2 1.0 25.0 2.4 2.4 0 Yes V-2 2.0 26.2 2.4 2.8 0 Yes V-2 3.0 24.6 3.1 2.2 0 Yes V-2 CNSs-BA/PET 0.5 24.0 1.5 2.3 0 Yes V-2 1.0 26.9 1.2 2.1 0 Yes V-2 2.0 28.1 0.5 2.2 0 No V-0 3.0 27.5 0.6 1.9 0 No V-0 Notes: PET—Polyethylene terephthalate; t1—Afterglow time after the first application of flame; t2—Afterglow time after the second application of flame; t2—Afterglow time; NR—No rate. 锥形量热仪测试结果见图5和表2。热释放速率(HRR)是表征材料火灾危险性的主要依据。结合图5和表2可知,纯PET被点燃后热释放速率急剧增大,其峰值热释放速率(pk-HRR)为810.45 kW/m2,总热释放(THR)为150.27 MJ/m2。与之相比,CNSs-BA/PET的THR与之接近,但HRR曲线却明显变平缓。值得注意的是,CNSs-BA/PET的HRR曲线表现出两个明显的热释放阶段,即在热释放速率达到峰值之后又出现了一个较为平缓的放热平台(当CNSs-BA含量为0.5wt%时表现为放热峰),这意味着CNSs-BA/PET在燃烧过程中的热释放受到抑制,这是由燃烧时炭层的形成或可燃气体减少导致的[20]。此外,表2表明,与CNSs/PET相比,CNSs-BA/PET复合材料的pk-HRR进一步降低。当CNSs-BA 含量为2.0wt%时,CNSs-BA/PET的pk-HRR最小,为435 kW/m2,该值与相同阻燃剂含量的CNSs/PET相比降低了7.6%,较纯PET降低了46.3%,说明CNSs经BA接枝后对PET的燃烧抑制作用进一步增强,阻燃效果进一步提高。
表 2 复合材料的锥形量热仪测试数据Table 2. Data of cone calorimeter test of compositesSample FR content/wt% TTI/s Time to pk-HRR/s pk-HRR/(kW·m−2) THR/(MJ·m−2) PET 0 47 104 810.45 150.27 CNSs/PET 0.5 44 34 528.96 151.64 1 34 34 503.44 148.03 2 40 41 470.72 146.06 3 30 29 501.49 143.19 CNSs-BA/PET 0.5 35 39 485.54 146.54 1 31 55 469.98 156.04 2 34 39 435.00 146.54 3 30 39 466.05 156.69 Notes: TTI—Time to ignition; pk-HRR—Peak heat release rate; FR—Flame retardant. 2.3 复合材料的阻燃机制研究
2.3.1 阻燃复合材料的热重分析
为了研究阻燃剂的引入对PET的热降解行为的影响,对PET、CNSs/PET和CNSs-BA/PET在氮气气氛下的TG-DTG曲线作了对比分析,并计算了CNSs/PET和CNSs-BA/PET在500℃时残炭量的理论值,如图6和表3所示。由图6可知:在氮气气氛下,PET、CNSs/PET和CNSs-BA/PET三者的TG曲线和DTG曲线基本重合,说明加入少量(2.0wt%)的CNSs和CNSs-BA均不会对PET的无氧降解行为造成明显影响。由表4可知,PET、CNSs/PET和CNSs-BA/PET三者的Tonset和Tmax均较为接近,但三者在高温(500℃)下的残余质量有所不同。经计算发现CNSs/PET和CNSs-BA/PET二者在高温下残炭量的实际值(CR500℃,exp)均大于理论值(CR500℃,cal),这说明阻燃剂CNSs和CNSs-BA对PET均有促进成炭作用。其中,CNSs-BA/PET在500℃下残炭量的实际值与理论值的差值(∆CR500℃)大于CNSs/PET,这说明CNSs经BA接枝后对PET的促进成炭作用加强。聚合物在高温下形成的残炭越多,燃烧时发生热分解的部分就越少[21],这便是阻燃复合材料热释放速率降低的主要原因之一。
表 3 CNSs、CNSs-BA以及PET、CNSs/PET、CNSs-BA/PET在氮气气氛下的TG-DTG数据Table 3. TG-DTG data of CNSs, CNSs-BA, PET, CNSs/PET and CNSs-BA/PET under nitrogen atmosphereSample Tonset/℃ Tmax/℃ CR500℃/% ∆CR500℃/%c exp.a/cal.b CNSs >800 — 96.88/— — CNSs-BA 476.4 — 94.92/— — PET 379.1 419.1 10.09/— — CNSs/PET 380.1 421.4 13.97/11.52 2.45 CNSs-BA/PET 382.0 420.1 15.68/11.79 3.89 Notes: a CR500℃,exp. is the experimental value of char residue; b CR500℃,cal. is the calculated value of char residue; c ∆CR500℃=CR500℃,exp.−CR500℃,cal.. 表 4 PET、CNSs/PET和CNSs-BA/PET在空气气氛下的TG-DTG数据Table 4. TG-DTG data of PET, CNSs/PET and CNSs-BA/PET under air atmosphereSample Tonset/℃ Tmax-1/℃ Tmax-2/℃ PET 397.3 433.4 585.1 CNSs/PET 359.1 438.4 567.5 CNSs-BA/PET 391.0 439.7 563.3 Notes: Tmax-1—Maximum weightlessness temperature in the first stage; Tmax-2—Maximum weightlessness temperature of the second stage. 2.3.2 残炭分析
对纯PET、CNSs/PET和CNSs-BA/PET锥形量热仪测试后的残炭做了SEM和TG分析以进一步研究阻燃机制。
炭层的形貌结构和稳定性对于提高聚合物的阻燃性能至关重要,有效的炭层可通过阻止聚合物内部与可燃气体、氧气的接触来实现阻燃目的。图7为纯PET、CNSs/PET和CNSs-BA/PET炭层的SEM图像。可见,纯PET燃烧生成的炭层稀薄空且松散,表面存在大量气体逸出形成的气孔,显然这种形貌的炭层无法形成有效的屏障作用。与纯PET相比,CNSs/PET的炭层的致密性明显提高,气孔明显变小,意味着炭层有效性的提高。值得注意的是,与CNSs/PET相比,CNSs-BA/PET炭层的致密性和连续性得到了进一步改善,表面气孔也明显变少和变小,另外还存在大量鼓起的未破裂气泡,这种形貌的炭层在燃烧时一方面能有效地阻隔热量的传递,另一方面还能有效地阻隔PET燃烧降解生成的气态可燃物的逸出,起到隔热和隔氧的作用[22-23]。除此之外,CNSs-BA受热分解生成的CO2、氨气、氮气等难燃性气体能够稀释燃烧区域可燃气体的浓度,抑制燃烧的发展,这便是CNSs-BA/PET阻燃性提高的重要原因。
图8是PET、CNSs/PET和CNSs-BA/PET炭层的TG曲线。可知,纯PET炭层的Tonset较低,为215.08℃,其中100℃前失重为4.36wt%,这主要是由于纯PET的炭层结构松散、孔洞较多,容易吸收水分和储存小分子气体所致,其800℃时的残余质量为87.2wt%。在整个升温过程中,纯PET的炭层表现出3个失重阶段,第一个失重阶段发生在100℃之前,主要对应炭层中贮存的水分以及气态小分子的降解;第二个失重阶段发生在100~530℃之间,对应炭层主体部分的降解;第三个失重阶段发生在530℃之后,对应炭层热降解产物的再降解。与之相比,CNSs/PET炭层的Tonset提高到615.37℃,800℃时的残余质量提高到89.1wt%,这主要是由于CNSs/PET的炭层的致密性提高所致,其TG曲线基本保持了纯PET炭层的3个失重阶段。与PET和CNSs/PET的炭层相比,CNSs-BA/PET炭层的Tonset提高到800℃以上,意味着炭层在燃烧时能耐受更高的温度,从而更持久有效地起到凝聚相阻燃作用。值得注意的是,其炭层在热分解过程中只有一个较为明显的失重平台,并未像PET和的CNSs/PET的炭层一样经历3个失重阶段,说明阻燃剂CNSs-BA能使PET燃烧生成结构稳定的炭层,该炭层在燃烧过程中能耐受较高的火焰温度,从而对内部的基体起到持久有效的保护作用。
2.3.3 交联行为分析
聚合物的交联直接影响其热性能、流变性、成炭性、熔滴和自熄行为,并有助于聚合物的芳香化或炭化[24]。图9是PET、CNSs/PET和CNSs-BA/PET在热氧降解过程中的TG-DSC曲线,相关数据见表4。由图9可以看出,PET、CNSs/PET和CNSs-BA/PET在空气中均有两个失重阶段,说明PET及其复合材料发生的是两步降解反应[25]。第一个失重阶段是PET的主要失重阶段,发生在360~470℃之间。第二个失重阶段发生在470~590℃之间,该阶段对应第一个降解阶段生成的降解产物的进一步降解。值得注意的是,纯PET的Tonset为397.3℃,而PET的燃点通常在420℃左右,这说明PET在燃烧之前,首先会发生一定程度的降解并生成一些可燃性的气体或挥发性产物,以此来维持燃烧的进行。与纯PET相比,在第一个失重阶段,CNSs/PET和CNSs-BA/PET的热失重曲线稍向低温方向移动,但二者在第一个降解阶段结束时的剩余质量却大于PET,且该阶段的最大失重率所对应温度(Tmax-1)大于PET,说明阻燃剂的存在使PET的主体降解提前,但在该阶段却重组生成了热稳定性较高的物质。DSC曲线表明,CNSs-BA/PET在熔融峰和分解峰之间出现了明显的放热峰,该峰是PET的交联峰[26],而在PET和CNSs/PET的DSC曲线上交联峰却不明显,这说明CNSs经BA接枝改性后促进了PET的交联,这是由于阻燃剂表面接枝的芳香族席夫碱(BA)可以在PET的熔融温度和分解温度之间形成稳定的交联网络。
2.3.4 高温裂解产物分析
裂解-气相色谱-质谱联用(Py-GC-MS)是目前研究聚合物高温裂解产物的常用方法[27]。为了研究阻燃剂的引入对PET的热裂解行为及其高温裂解产物的影响,对PET、CNSs/PET和CNSs-BA/PET做了Py-GC-MS分析,三者的高温裂解产物对比见表5。可知,与纯PET的裂解产物相比,CNSs/PET和CNSs-BA/PET的裂解产物中都包含更多的杂环、稠环、共轭芳环类化合物,这些裂解产物具有较高的热稳定性,是难燃性的保护炭层形成的物质基础[28]。而CNSs-BA/PET的裂解产物中出现了诸如二甲基胺、偶氮苯等含氮产物,这是由于阻燃剂CNSs-BA表面接枝的苯亚甲基氨基苯酚所致。另外,与PET和CNSs/PET相比,CNSs-BA/PET的裂解产物中菲、萘、苊、芴等稠环芳烃类以及联苯类产物明显增多,佐证了CNSs-BA促进了PET降解过程中的交联,该交联一方面通过增大熔体黏度改善了熔滴现象,另一方面提高了炭层的致密性和热稳定性,这就是CNSs-BA/PET阻燃性和抗熔滴性提高的主要原因。
表 5 PET、CNSs/PET和CNSs-BA/PET裂解产物Table 5. Pyrolysis products of PET, CNSs/PET and CNSs-BA/PETPyrolysis products which found only in PET Tetrahydropyran; 2,2-dimethylpropanal; 4,8,12-trimethyl-tridecanoic acid methyl ester; 2,2-dimethoxybutane; 2-methyl-1,5-hexadien-3-yne; 1,6-heptadiyne; p-xylene; Decane; Methyl benzoate; Dodecylethyl ketone; 1-(3-methylphenyl)benzyl(2-methyl-1-methylenepropylidene); 4-methylphenyl-1-pentyn-3-ol phenol; Dimethyl 1,3-benzenedicarboxylate; Vinylmethyl terephthalate; Diphenylacetylene; Biphenyl-4-ylacetophenone; 1-(5,5-dimethyl-1,3-dioxocyclohexan-2-ylidene)-2-(N-ethylbenzothiazol-2-ylidene)-ethanes; Phthalic acid 4-formylphenyl ester; o-tertiaryl tricyclic [8.2.2.2(4,7)]hexadeca-2,4,6,8,10,12,13,15-octene; 4-(diethylaminomethyl)-2,5-dimethylphenol Pyrolysis products which found only in CNSs/PET Phenol; 1,2-dihydro-indene; 1-(4-methylphenyl)-ethanone; Stilbene; 1H-cyclopropyl[l]phenanthrene; Dihydro-p-terphenyl; 1-naphthol; Fluorene-9-methanol; 2-ethyl-1,1'-biphenyl; 1,1-diphenylethene; 4-(2-benzoyl-5-phenyl-3-thienyl)-1,2-dihydrophenanthrene; 2-phenylnaphthalenyl benzoate; 1,1-dihydro-2-phenylnaphthalenyl benzoate; 3-chlorobenzylnonyl; 1-(2,5-dimethylphenyl)ethanone; 1-(2,5-dimethylphenethyl) ethanone; Dimethyl-1H-indene; Diethylmalonic acid; 3-chlorobenzylnonyl ester Pyrolysis products which found only in CNSs-BA/PET 1,5-hexadiyne; Dimethylamine; Nitrous oxide; 1,1'-(1,4-phenylene)bis-acetophenone; 2-methylindene; Azobenzene; Benzene; (1-methyl-2-cyclopropen-1-yl)-2-methylindene; Stilbene; Ethylketone; 1-(3,4-dimethylphenyl); 1-(4-methylphenyl); 1-ethenyl-4-methylbenzene; Dibenzofuran; 2-naphthol; 4-hydroxy-1,2,3,4-tetrahydrophenanthrene; 9,10-dihydrophenanthrene; Benzopropiophenone; Fluorene; 4-vinylbiphenyl; 1,2,3,4-tetrahydrofil; 9,10-dihydrofil; 4-vinylbiphenyl; 1,4-vinylbiphenyl; Phenylacetone; 1,3,5-cycloheptatriene; 2-phenylnaphthalene; 1-acrylbenzene; 2-methylnaphthalene; 4-(2-benzoyl-5-phenyl-3-thienyl)-methylbenzoic acid; 1,3-dimethyl-1H-indene; Tricyclohexen-8-ol; Hexaethylcyclohexane; 9-phenyl-9-fluorenol; Ethylene oxide; Methoxyphenyltricyclohexadecen-5-ylmethanol; 4-benzylbiphenyl; Tritylbenzene; 9-phenylanthracene; 3-(1-phenylethoxy)-3H-isobenzofuran-1-one; 4-phenyl-3,4-dihydroisoquinoline; Oxetane; 2-phenyl; 3-phenylethynyl; Tetraphenyl; 1-[4-(2-phenylethenyl)phenyl]-ethanone; Acenaphthene; 1,2,3,5-tetraisopropyl-cyclohexane; 6,9-dimethoxy-phenazine-1-carboxylic acid; [1,1'-biphenyl]-4-yl-phenylmethanone; 1,1':4',1''-3'-methyltriphenylene Pyrolysis products which found both in PET and CNSs/PET Acetophenone; Benzoic acid; Biphenyl; 2-methyl-1,1'-biphenyl; 1,1'-(1,4-phenylene)bisacetophenone; p-terphenyl Pyrolysis products which found both in PET and CNSs-BA/PET Styrene; Acetophenone; Benzoic acid; Biphenyl; 2-ethyl-1,1'-biphenyl; Benzophenone; 9H-fluoren-9-one; p-terphenyl Pyrolysis products which found both in CNSs/PET and CNSs-BA/PET Benzene; Biphenyl; Acetophenone; Naphthalene; Toluene; Phenanthrene; Indene; 6,6-diphenylfulvene; p-terphenyl; Methylstyrene; Biphenylacetophenone; 4-ethylbiphenyl; Diphenylmethane Pyrolysis products found in PET, CNS/PET and CNSs-BA/PET Acetophenone; Benzoic acid; Biphenyl; p-terphenyl 2.4 复合材料的力学性能
图10为PET阻燃复合材料的抗拉强度和断裂伸长率图。可知,随着阻燃剂含量的增加,CNSs/PET和CNSs-BA/PET复合材料的抗拉强度和断裂伸长率均呈下降趋势。尤其是当阻燃剂含量超过2.0wt%时,PET复合材料的抗拉强度和断裂伸长率大幅度下降。这是由于高含量的阻燃剂在PET基体中形成了较大的团聚体,破坏了基体的连续性,阻碍了应力的传递所致,后续研究中应重点关注材料力学性能的改善。
3. 结 论
(1)为同时改善聚对苯二甲酸乙二醇酯(PET)的阻燃性和抗熔滴性,在碳纳米球表面接枝4-苯亚甲基氨基苯酚制备了一种新型碳基复合阻燃剂(CNSs-BA)。CNSs-BA为粒径约50 nm的球形颗粒,热稳定性良好。
(2) CNSs-BA的引入可显著提高PET的阻燃性和抗熔滴性。当CNSs-BA添加量为2.0wt%时,CNSs-BA/PET复合材料的极限氧指数(LOI)从PET的21.0%提高至28.1%,阻燃等级达到V-0级,热释放速率峰值降低了46.3%。
(3) CNSs-BA/PET表现出典型的凝聚相阻燃机制。CNSs-BA的引入能促进PET成炭,CNSs-BA/PET的高温残炭量(CR500℃)比PET提高了55.4%,且成炭量的实际值大于理论值。与纯PET的炭层相比,CNSs-BA/PET燃烧生成的炭层的致密性、连续性以及热稳定性都显著提高。这是由于CNSs-BA的引入促进了PET的高温交联,使其高温降解生成了更多的难燃性焦炭物质。
(4)本文为碳基阻燃剂的发展提供了重要理论补充,对开发无卤、阻燃、抗熔滴的PET材料具有一定的指导意义。
-
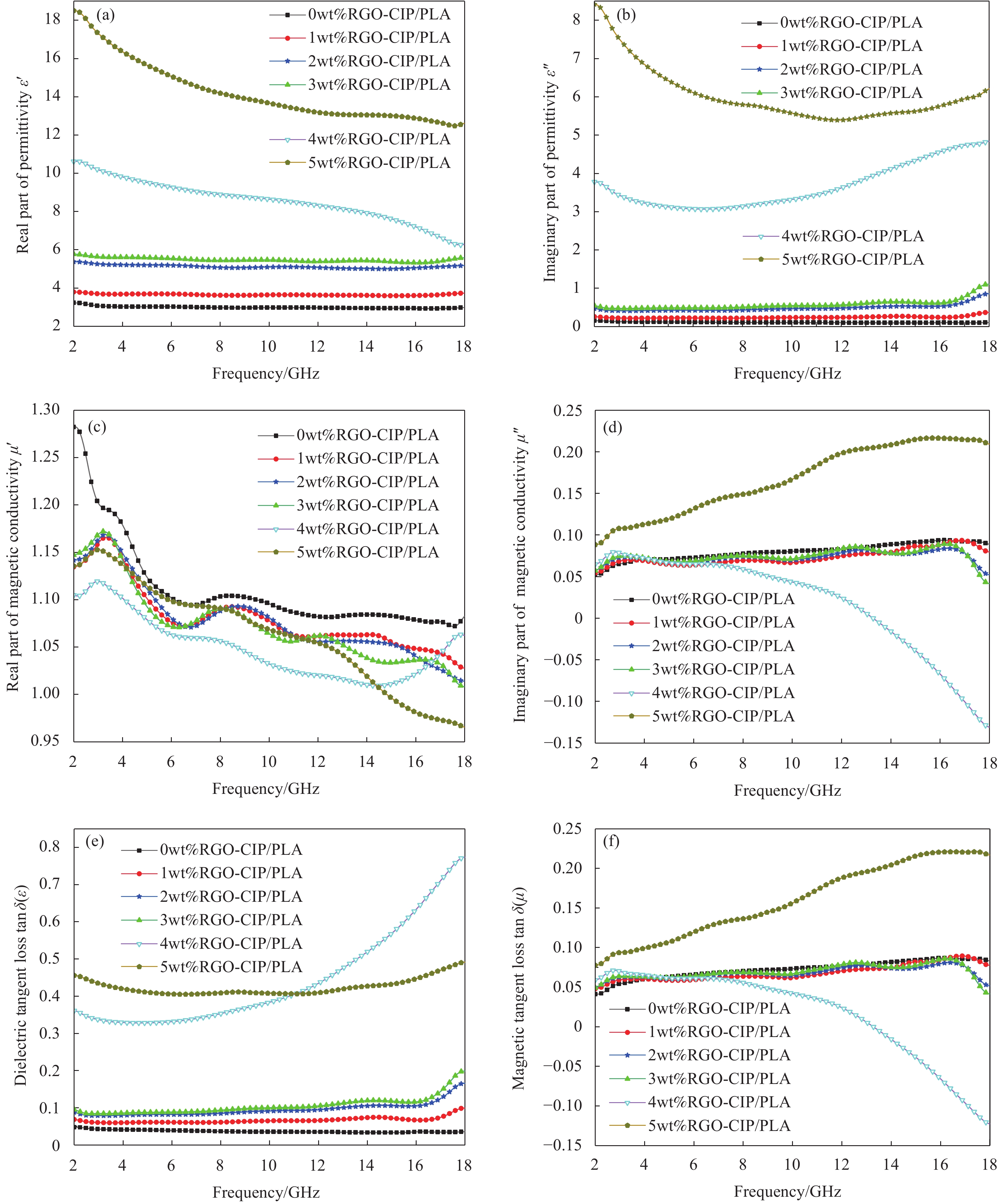
图 7 不同RGO含量同轴环的电磁参数:在2~18 GHz频率范围内,介电常数的实部(a)、虚部(b);磁导率的实部(c)、虚部(d);介电损耗角正切(e)和磁损耗角正切(f)
Figure 7. Electromagnetic parameters of coaxial rings with different RGO contents: In the frequency range of 2-18 GHz, real part (a) and imaginary part (b) of the complex permittivity; Real part (c) and imaginary part (d) of the complex magnetic conductivity; Dielectric loss tangent (e) and magnetic loss tangent (f)
图 10 不同RGO含量的RGO-CIP/PLA复合材料的三维微波反射损耗图:(a) 0wt% RGO;(b) 1wt% RGO;(c) 2wt% RGO;(d) 3wt%RGO;(e) 4wt% RGO;(f) 5wt% RGO
Figure 10. Three-dimensional microwave reflection loss diagrams of RGO-CIP/PLA composites with different RGO contents: (a) 0wt% RGO; (b) 1wt% RGO; (c) 2wt% RGO; (d) 3wt% RGO; (e) 4wt% RGO; (f) 5wt% RGO
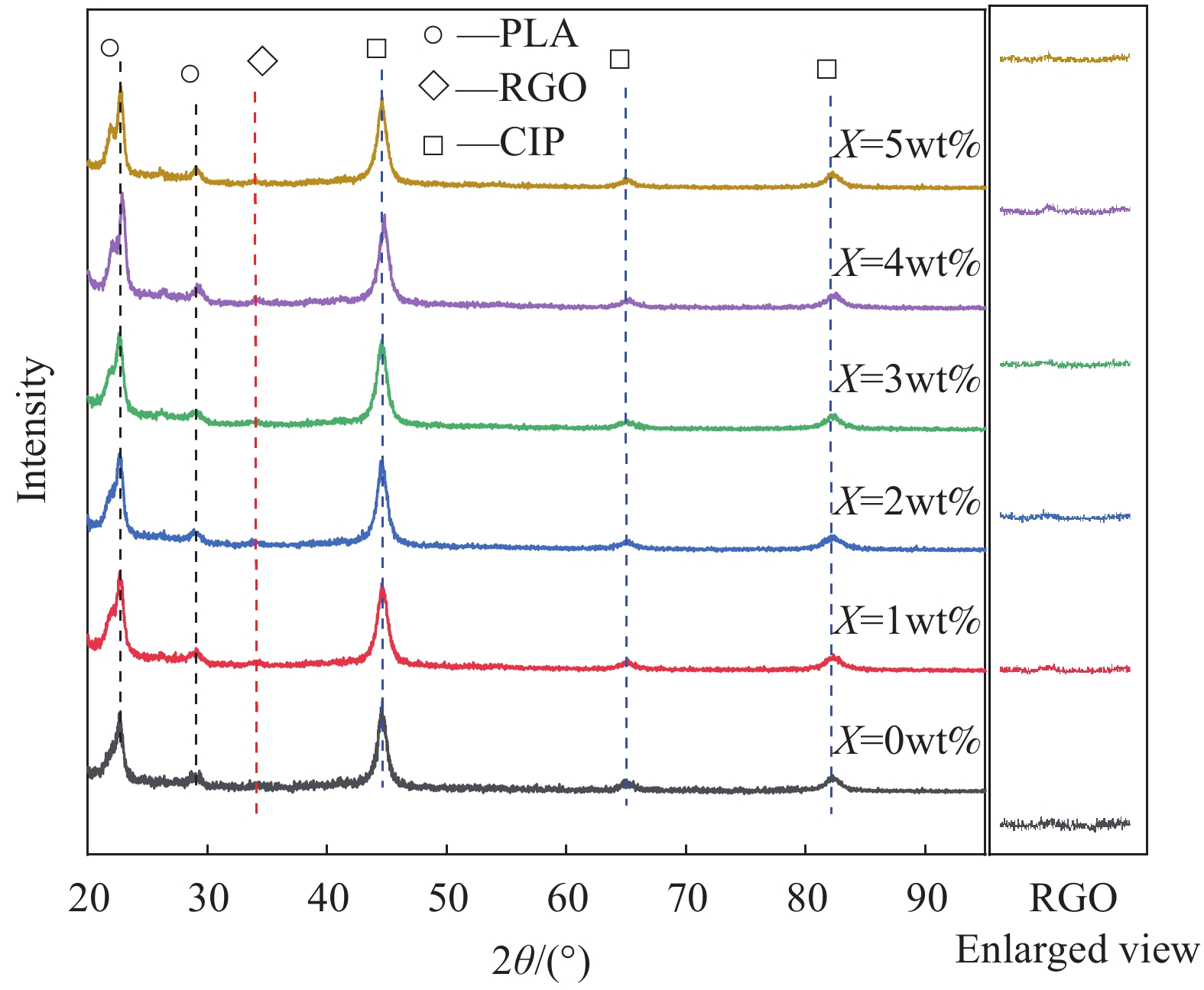
表 1 RGO-CIP/ PLA复合粉末的组成
Table 1 Composition of RGO-CIP/PLA composite powder
Sample number Mass fraction/wt% RGO CIP PLA 0wt%RGO-CIP/PLA 0 20 80 1wt%RGO-CIP/PLA 1 20 79 2wt%RGO-CIP/PLA 2 20 78 3wt%RGO-CIP/PLA 3 20 77 4wt%RGO-CIP/PLA 4 20 76 5wt%RGO-CIP/PLA 5 20 75 Notes: RGO—Reduced graphene oxide; CIP—Carbonyl iron powder; PLA— Polylactic acid. 表 2 RGO-CIP/PLA复合粉末DSC曲线数据
Table 2 DSC curve datas of RGO-CIP/PLA composite powder
Sample number RGO
content/
wt%Tm/℃ Tc/℃ Tg/℃ 0wt%RGO-CIP/PLA 0 113.79 97.98 82.86 1wt%RGO-CIP/PLA 1 112.1 100.38 81.67 2wt%RGO-CIP/PLA 2 112.6 98.99 79.69 3wt%RGO-CIP/PLA 3 111.9 99.30 82.59 4wt%RGO-CIP/PLA 4 112.1 99.65 83.52 5wt%RGO-CIP/PLA 5 112.6 101.24 82.59 Notes: Tm—The melting temperature of the composite material; Tc—The crystallization temperature of the composite material; Tg—The glass transition temperature of the composite material. 表 3 熔融沉积3D (FDM3D)打印参数
Table 3 FDM3D printing parameters
Item Printing parameters Nozzle 1 temperature/℃ 150 Nozzle 2 temperature/℃ 0 Panel temperature/℃ 40 表 4 RGO-CIP/PLA复合材料与其他复合吸波材料的性能比较
Table 4 Performance comparisons of RGO-CIP/PLA composite materials and other composite absorbing materials
-
[1] CHENG Y, ZHU W D, LU X F, et al. Recent progress of electros pun nanofibrous materials for electromagnetic interference shielding[J]. Composites Communications,2021,27:100823.
[2] ZHANG Z, CHEN X, WANG Z, et al. Carbonyl iron/graphite microspheres with good impedance matching for ultra-broadband and highly efficient electromagnetic absorption[J]. Optical Materials Express,2018,8(11):3319-3331. DOI: 10.1364/OME.8.003319
[3] MA J, SHU J, CAO W, et al. A green fabrication and variable temperature electromagnetic properties for thermal stable microwave absorption towards flower-like Co3O4@ rGO/SiO2 composites[J]. Composites Part B: Engineering,2019,166:187-195. DOI: 10.1016/j.compositesb.2018.11.119
[4] YE F, SONG Q, ZHANG Z, et al. Direct growth of edge-rich graphene with tunable dielectric properties in porous Si3N4 ceramic for broadband high-performance microwave absorption[J]. Advanced Functional Materials,2018,28(17):1707205. DOI: 10.1002/adfm.201707205
[5] MA X T, JIANG Z P, WANG F S, et al. Numerical study of thermal effect in silicone rubber filled with carbonyl iron powder under microwave radiation[J]. Journal of Materials Science,2021,56:10264-10281.
[6] GONG M J, DONG Y Q, HUANG J J, et al. The enhanced magnetic properties of FeSiCr powder cores composited with carbonyl iron powder[J]. Journal of Materials Science: Materials in Electronics,2021,32(7):8829-8836.
[7] JING H X, LI Q L, YE Y, et al. Preparationand microwave absorbing properties of Fe(CO)5/BaTiO3 composites[J]. Journal of Materials Engineering,2015,43(7):38-42.
[8] DING G X, CHEN C X, TAI H X, et al. Structural characterization and microwave absorbing performance of CuFe2O4/RGO composites[J]. Journal of Solid State Chemistry,2021,297:122051.
[9] WANG X, SHU J C, HE X M, et al. Green approach to conductive PEDOT: PSS decorating magnetic-graphene to recover conductivity for highly efficient absorption[J]. ACS Sustainable Chemistry & Engineering,2018,6(11):14017-14025. DOI: 10.1021/acssuschemeng.8b02534
[10] ZHANG M M, ZHANG J W, LV X Y, et al. How to exhibit the efficient electromagnetic wave absorbing performance of rgo aerogels: Less might be better[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(7): 5496-5500.
[11] ZHANG M M, ZHANG J W, LV X Y, et al. How to exhibit the efficient electromagnetic wave absorbing performance of RGO aerogels: Less might be better[J]. Journal of Materials Science: Materials in Electronics,2018,29(7):5496-5500.
[12] GAO Y, GAO X, LI J, et al. Microwave absorbing and mechanical properties of alternating multilayer carbonyl iron powder-poly(vinylchloride) composites[J]. Journal of Applied Polymer Science,2017,135(12):45846.
[13] LIU X G, WU N D, CUI C Y, et al. Facilepreparation of carbon-coated Mg nanocapsulesas light microwave absorber[J]. Materials Letters,2015,149:12-14.
[14] PRAVEEN C, BINOD K K, SANTANU D, et al. Theoretical circuit modeling of tetra bands DNG metamaterial by transmission line theory with very small frequency[J]. Journal of Computational Electronics,2021,20:1439-1451.
[15] WANG Y, LUO F, ZHOU W C, et al. Dielectric and electromagnetic wave absorbing properties of TiC/epoxy composites in the GHz range[J]. Ceramics International,2014,3(64):10749-10754.
[16] ZHANG Z Q, WEI J Q, YANG W F, et al. Effect of shape of sendust particles on their electromagnetic properties within 0.1-18 GHz range[J]. Physica B,2011,406:3896-3900.
[17] 杜捷. 氧化石墨烯/聚脲复合材料制备与性能研究[D]. 广州: 暨南大学, 2020. DU Jie. Preparation and properties of graphene oxide/polyurea composite materials[D]. Guangzhou: Jinan University, 2020(in Chinese).
[18] WANG X X, MA T, SHU J C, et al. Confinedly tailoring Fe3O4 clusters-NG to tune electromagnetic parameters and microwave absorption with broadened bandwidth[J]. Chemical Engineering Journal,2018,332:321-330. DOI: 10.1016/j.cej.2017.09.101
[19] LI Y, SUN N, LIU J, et al. Multifunctional BiFeO3 composites: Absorption attenuation dominated effective electromagnetic interferenceshielding and electromagnetic absorption induced by multiple dielectric and magnetic relaxations[J]. Composites Science and Technology,2018,159:240-250. DOI: 10.1016/j.compscitech.2018.02.014
[20] YUAN Z F, ZHAO H, ZHOU J. Design and preparation of the epoxy resin based sandwich composite with broad-band wave-absorbing properties in C-band[J]. Rare Metal Materials and Engineering,2020,49(11):3782-3789.
[21] 范明远. 羰基铁粉/热塑性树脂复合材料的制备及其吸波性能研究[D]. 上海: 东华大学, 2019. FAN Mingyuan. Preparation of carbonyl iron powder/thermoplastic resin composite and its microwave absorbing properties[D]. Shanghai: Donghua University, 2019(in Chinese).
[22] 景红霞, 李巧玲, 叶云, 等. 纳米Fe3O4及Fe3O4-SrFe12O19吸波复合材料的制备及性能[J]. 复合材料学报, 2013, 30(1):130-134. JING Hongxia, LI Qiaoling, YE Yun, et al. Preparation and properties of nano-Fe3O4 and Fe3O4-SrFe12O19 absorbing composite materials[J]. Acta Materiae Compositae Sinica,2013,30(1):130-134(in Chinese).
[23] 吴海华, 胡正浪, 李雨恬, 等. 铁镍合金/聚乳酸复合材料的熔融沉积成形制备及其电磁吸收性能和力学性能[J]. 复合材料学报, 2022, 39(1): 158-168. WU Haihua, HU Zhenglang, LI Yutian, et al. Electromagnetic absorption properties and mechanical properties of Fe-Ni alloy/polylactic acid composites fabricated by fused deposition modeling[J]. Acta Materiae Compositae Sinica, 2022, 39(1): 158-168(in Chinese).
[24] 黄海波, 沈勇, 杨明荣, 等. 海胆状MnO2/RGO复合材料的制备及吸波性能研究[J]. 现代化工, 2018, 38(6):154-157+159. HUANG Haibo, SHEN Yong, YANG Mingrong, et al. Study on preparation and microwave absorbin g properties of sea urchin-like MnO2/RGO composite materials[J]. Modern Engineering,2018,38(6):154-157+159(in Chinese).
[25] 黄琪惠, 张豹山, 唐东明, 等. 石墨烯-Fe@Fe3O4纳米复合材料的制备及其电磁性能研究[J]. 无机化学学报, 2012, 28(10):2077-2082. HUANG Qihui, ZHANG Baoshan, TANG Dongming, et al. Preparation and electromagnetic properties of graphene-Fe@Fe3O4 nanocomposites[J]. Chinese Journal of Inorganic Chemistry,2012,28(10):2077-2082(in Chinese).
[26] ZUO Y X, YAO Z J, LIN H Y, et al. Digital light processing 3D printing of graphene/carbonyl iron/polymethyl methacrylate nanocomposites for efficient microwave absorption[J]. Composites Part B: Engineering,2019,179:107533.
[27] NING M, LI J, KUANG B, et al. One-step fabrication of N-doped CNTs encapsulating M nanoparticles(M=Fe, Co, Ni) for efficient microwave absorption[J]. Applied Surface Science,2018,447:244-253. DOI: 10.1016/j.apsusc.2018.03.242
[28] LIU P, NG HONG V M, YAO Z, et al. Facile synthesis and hierarchical assembly of flowerlike NiO2 structures with enhanced dielectric and microwave absorption properties[J]. ACS Applied Materials & Interfaces,2017,9(19):16404-16416. DOI: 10.1021/acsami.7b02597
[29] WEI X, GEN Y, SONG B, et al. Construction of caterpillar-like hierarchically structured Co/MnO2/CNTs derived from MnO2/ZIF-8@ZIF-67 for electromagnetic wave absorption[J]. Carbon,2021,3(173):521-527.
-





 下载:
下载: