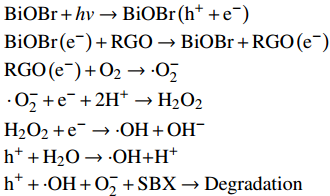Preparation of recyclable BiOBr/graphene hydrogel composite and its photodegradation of sodium butyl xanthate
-
摘要: 本文利用水热法合成BiOBr/石墨烯(BiOBr/RGO)水凝胶复合材料,采用XRD、SEM等手段表征复合材料的组成、形貌特征,并探究了BiOBr/RGO水凝胶复合材料对正丁基钠黄药的降解性能。结果表明,成功制备出有利于回收利用的三维宏观BiOBr/RGO水凝胶复合材料;50 mL浓度为25 mg/L的正丁基钠黄药溶液,降解时间为85 min时,10 mg BiOBr/RGO水凝胶复合材料(BiOBr质量分数为92wt%)对黄药的降解率可达96.69%,而纯BiOBr降解率仅为44.84%。总之,RGO的引入可以提升BiOBr的光催化性能,且宏观材料有利于回收再利用。Abstract: The BiOBr/graphene (BiOBr/RGO) hydrogel composites were prepared via hydrothermal method in this study. The composition and morphology of materials were characterized by XRD and SEM. Photocatalytic properties of the BiOBr/RGO hydrogel composites on sodium n-butyl xanthate were systematically investigated, respectively. The results show that three dimensional macroscopical BiOBr/RGO hydrogel composites are successfully prepared, which is beneficial to recycling. When initial concentration of 50 mL sodium n-butyl xanthate is 25 mg·L−1, degradation time is 85 min, and the dosage of photocatalyst is 10 mg. The degradation efficiency of the BiOBr/RGO hydrogel composite (mass fraction of BiOBr is 92wt%) for sodium n-butyl xanthate can reach 96.69%, and that of BiOBr is merely 44.84%. In all, the introduction of RGO can improve the photocatalytic performance of BiOBr, and macro material is favorable for recycling.
-
Keywords:
- BiOBr /
- graphene hydrogel composite /
- photocatalysis /
- sodium butyl xanthate /
- recyclable
-
随着工业的发展,采矿规模逐渐扩大,浮选药剂的使用量也随之增加。而黄药是最常用、最有效的硫化矿捕收剂,其不仅能使目标矿物疏水,且易于被气泡附着。但由于工厂现有的废水处理系统设计不当,尾矿中残留的黄药及其分解产物往往造成严重的环境污染[1-5]。因此,研究黄药的处理方法对矿区环境保护具有积极意义。
近几十年来,光催化技术作为环境修复的一项绿色、可持续的技术,受到广泛关注[6-8]。与其他光催化剂相比,半导体TiO2[9-13]因其独特的带隙结构,且具有毒性小、成本低、光催化活性高和光稳定性好等优点,已作为光催化剂应用于废水处理。但TiO2在可见光范围内存在吸收性能差、光能利用率低或光生载流子复合率高、难以回收利用、易于造成二次污染等缺点,限制了其在实际中的应用。BiOBr是一种多组分金属卤氧化物家族的三元化合物,具有独特的电学性能、磁性能、光学性能和发光性能[14-17],且具有约2.9 eV的带隙,在可见光-近红外光谱范围内,吸收系数高,是一种具有实际应用价值的光催化剂[6]。目前,已有一些学者研究了BiOBr对污染物的光降解性能。Deng等[18]合成了对有机污染物具有良好光降解性能的介孔TiO2-BiOBr微球。Zhang 等[19]制备了对内分泌干扰物具有优良光降解性能的BiOBr@SiO2@Fe3O4光催化剂。Song等[20]利用水热法合成了新型可见光诱导的Zn掺杂BiOBr分层纳米结构。与纯BiOBr相比,在可见光下其对罗丹明B的降解效率明显增强。这些相关研究表明,BiOBr在有机污染物处理方面虽然具有很大潜力,但其仍然存在光生载流子复合率高、难以回收利用等问题。石墨烯水凝胶是通过π-π堆叠和氢键作用将石墨烯片层保持在一起,其不仅继承了石墨烯的电荷迁移率高、导电性良好和高比表面积等优点,且具有3D多孔网络和发达的互穿孔结构,更有利于光生载流子的迁移和分离,且易于回收利用[21]。因此,将光催化剂与石墨烯水凝胶复合,以期获得光生载流子复合率低、光催化活性高及易于循环利用的水处理材料。
本研究采用一步水热法合成3D宏观BiOBr/石墨烯(BiOBr/RGO)水凝胶复合材料。充分发挥了BiOBr和RGO水凝胶的优点,并以丁基钠黄药(SBX)为目标污染物,评估了BiOBr/RGO水凝胶复合材料的光催化性能,探讨了其光降解污染物的机制。
1. 实验材料及方法
1.1 原材料
五水合硝酸铋(Bi(NO3)3·5H2O)、乙二醇 ((CH2OH)2)、十六烷基三甲基溴化铵(CTAB)、乙二胺四乙酸二钠 (EDTA-2Na)、异丙醇 ((CH3)2CHOH)、正丁基钠黄药 (SBX),分析纯,国药集团。实验用水为蒸馏水。
1.2 BiOBr/石墨烯(RGO)水凝胶复合材料的制备
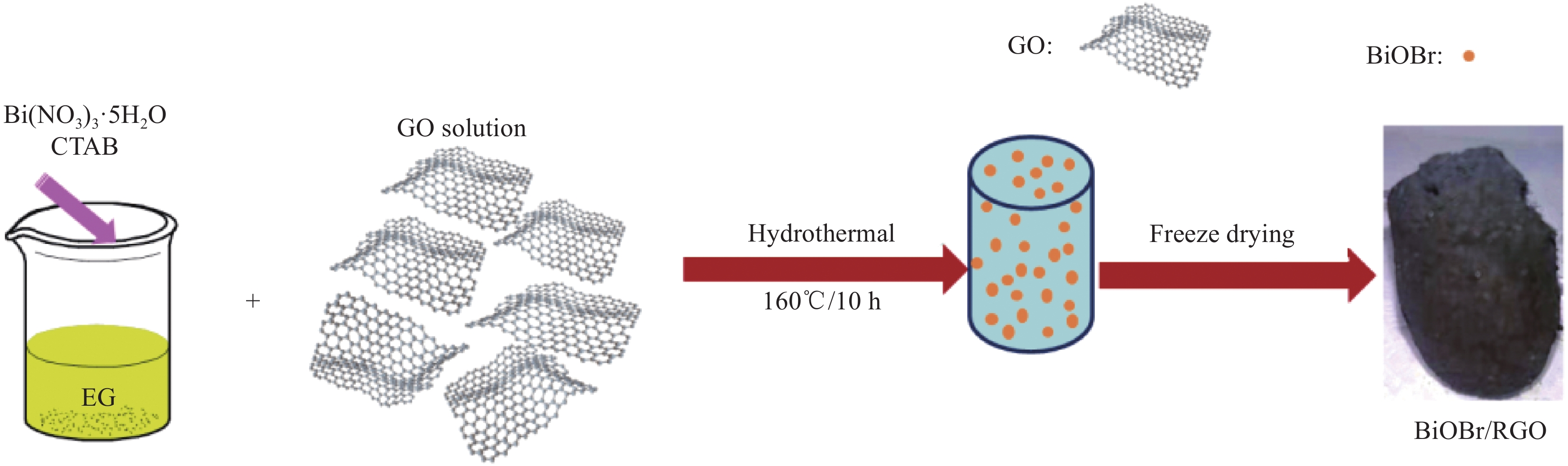
采用Hummers法制备氧化石墨烯(GO)。利用一步水热法及冷冻干燥合成BiOBr/RGO水凝胶复合材料。制备过程如图1所示,CTAB (0.75 mmol)和Bi(NO3)3·5H2O (0.75 mmol) 在40 mL (CH2OH)2溶液中完全溶解,加入20 mL 5 mol/L GO溶液,超声30 min。将混合物转移至100 mL反应釜中,在160℃下反应10 h,用去离子水和乙醇对产物进行多次洗涤,冷冻干燥24 h,得到BiOBr/RGO水凝胶复合材料。通过改变Bi(NO3)3·5H2O与CTAB摩尔比合成一系列不同BiOBr质量分数的BiOBr/RGO水凝胶复合材料(98wt%BiOBr/RGO、95wt%BiOBr/RGO、92wt%BiOBr/RGO、90wt%BiOBr/RGO),材料名称及含量如表1所示。
表 1 BiOBr/RGO水凝胶复合材料的名称及含量Table 1. Name and content of BiOBr/RGO hydrogel compositesComposite name Mass fraction of BiOBr/wt% Mass fraction of RGO/wt% 98wt%BiOBr/RGO 98 2 95wt%BiOBr/RGO 95 5 92wt%BiOBr/RGO 92 8 90wt%BiOBr/RGO 90 10 BiOBr的制备过程与BiOBr/RGO水凝胶复合材料一样,只是制备过程中不加入GO。
1.3 光催化实验
以SBX为目标污染物,将10 mg样品加入50 mL SBX溶液(25 mg/L)中,先暗反应40 min以达到吸附-解吸平衡。然后在300 W氙灯下进行光催化反应,每隔一定时间取2 mL溶液进行离心分离,并用紫外分光光度计(722G,上海仪电分析仪器有限公司)在301 nm波长下测量吸光度,并测定SBX溶液的浓度。根据公式R (%)=C/C0 (其中,C为残留SBX的浓度(mg/L),C0为SBX的光反应初始浓度(mg/L)),可以得出光降解效率R。
采用XRD (X射线衍射-6100,荷兰)分析RGO和BiOBr/RGO水凝胶复合材料的晶体结构;采用FESEM (日立SU-8010,日本)观察BiOBr、RGO和92wt%BiOBr/RGO水凝胶复合材料的形貌;利用XPS (Alpha,Thermo Fisher Scientific,美国)对复合材料的化学元素组成进行分析;利用比表面分析仪(BET,F-Sorb 2400-BET,中国)表征材料的比表面积和孔径分布;利用紫外-可见漫反射光谱仪(UV-vis-DRS,Cary 3000,美国瓦里安)研究BiOBr和92wt%BiOBr/RGO水凝胶复合材料的光学性能;利用电化学工作站(CHI-660D,中国)测试瞬态光电流响应和电化学阻抗谱(EIS),研究BiOBr和92wt%BiOBr/RGO水凝胶复合材料的电荷转移电阻。光催化反应结束后,对3D结构水凝胶分离和再生,此过程无需使用复杂的过滤系统。将回收的水凝胶材料先用无水乙醇和水分别洗涤3次,再进行光催化实验,如此循环6次,利用红外光谱对每次循环后的水凝胶材料表面官能团进行分析。
2. 结果与讨论
2.1 BiOBr/RGO水凝胶复合材料的微观结构及形貌
2.1.1 BiOBr/RGO水凝胶复合材料的微观结构
图2为GO、RGO和BiOBr/RGO水凝胶复合材料的XRD图谱。由图2(a)可知,2θ=10.25°处出现尖锐的衍射峰,对应GO的(001)晶面。由图2(b)可知,RGO在10.25°处无衍射峰,在25.92°处存在一个特征峰,说明在160℃/10 h水热处理后,GO被还原成RGO[22-23]。BiOBr/RGO水凝胶复合材料在2θ=11.871°、25.02°、31.75°、32.741°、40.831°、46.851°和58.681°处均出现衍射峰,可归属于四方相BiOBr (JCPDS file Card No. 09-0393)的衍射峰。由于BiOBr峰强度远高于RGO的峰强度,因此,BiOBr/RGO水凝胶复合材料中RGO的衍射峰不明显。结果表明,RGO的引入未影响BiOBr的晶格结构,成功制备了BiOBr/RGO水凝胶复合材料。
2.1.2 BiOBr/RGO水凝胶复合材料的微观形貌
图3为BiOBr、RGO和BiOBr/RGO水凝胶复合材料的FESEM图像。由图3(a)和图3(b)可知,BiOBr是由多个纳米片形成的直径约为2~6 μm的3D花球状结构。由图3(c)可以看到,RGO水凝胶不仅继承了GO的大比表面积,还具有多孔的3D结构,孔径为几微米。由图3(d)可以看到,大量BiOBr微球分布在石墨烯层上,说明BiOBr和RGO成功复合,且RGO丰富的多孔结构为BiOBr/RGO水凝胶复合材料提供了高的比表面积。
2.1.3 BiOBr/RGO水凝胶复合材料的化学元素组成
图4为RGO和BiOBr/RGO水凝胶复合材料的XPS图谱。由图4(a)可知,可以明显观察到C1s、O1s、Bi4f、Br3d存在于BiOBr/RGO水凝胶复合材料中;C1s和O1s存在于RGO中,说明制备的材料是纯相,与XRD的分析结果一致。由图4(b)可知,RGO的三个C1s拟合峰分别在284.82 eV (C—C、C=C)、286.53 eV (C—OH)和288.88 eV (O=C—OH、C=O)处。BiOBr/RGO水凝胶复合材料的三个C1s拟合峰分别在284.8 eV (C—C、C=C)、286.5 eV (C—OH)和288.7 eV (O=C—OH、C=O)处,说明在BiOBr/RGO水凝胶复合材料中仍存在C=O和O=C—OH官能团。但与RGO相比,BiOBr/RGO水凝胶复合材料C1s的三个峰结合能均发生负移。由图4(c)和图4(d)可观察到,在159.72 eV、165.056 eV、69.90 eV、68.80 eV处的峰分别归于BiOBr的Bi4f7/2、Bi4f5/2、Br3d3/2和Br3d5/2,但与文献[18]中纯BiOBr相比,结合能均发生正移。总之,BiOBr与RGO复合后,C1s三个峰的结合能均发生负移,Bi4f7/2、Bi4f5/2、Br3d3/2和Br3d5/2的结合能发生正移,说明BiOBr/RGO水凝胶复合材料中BiOBr与RGO之间存在一定的化学作用。
由于RGO的还原程度会影响材料的光吸收性能[24]。基于图4(b)中BiOBr/RGO水凝胶复合材料C1s 的XPS图谱,利用公式[25] (O—C)%=(AC—O+AO—C=O)/(AC—O+AO—C=O+AC—C)计算O—C的含量,来确定RGO的还原程度。其中,AC—C、AC—O和AO—C=O分别是C—C、C—OH和O=C—OH/C=O的XPS图谱峰面积。计算得到O—C的含量为20.21%。根据文献,GO中C—O含量为55%。说明BiOBr/RGO水凝胶复合材料中RGO还原程度较好,对光的吸收性能优于GO。
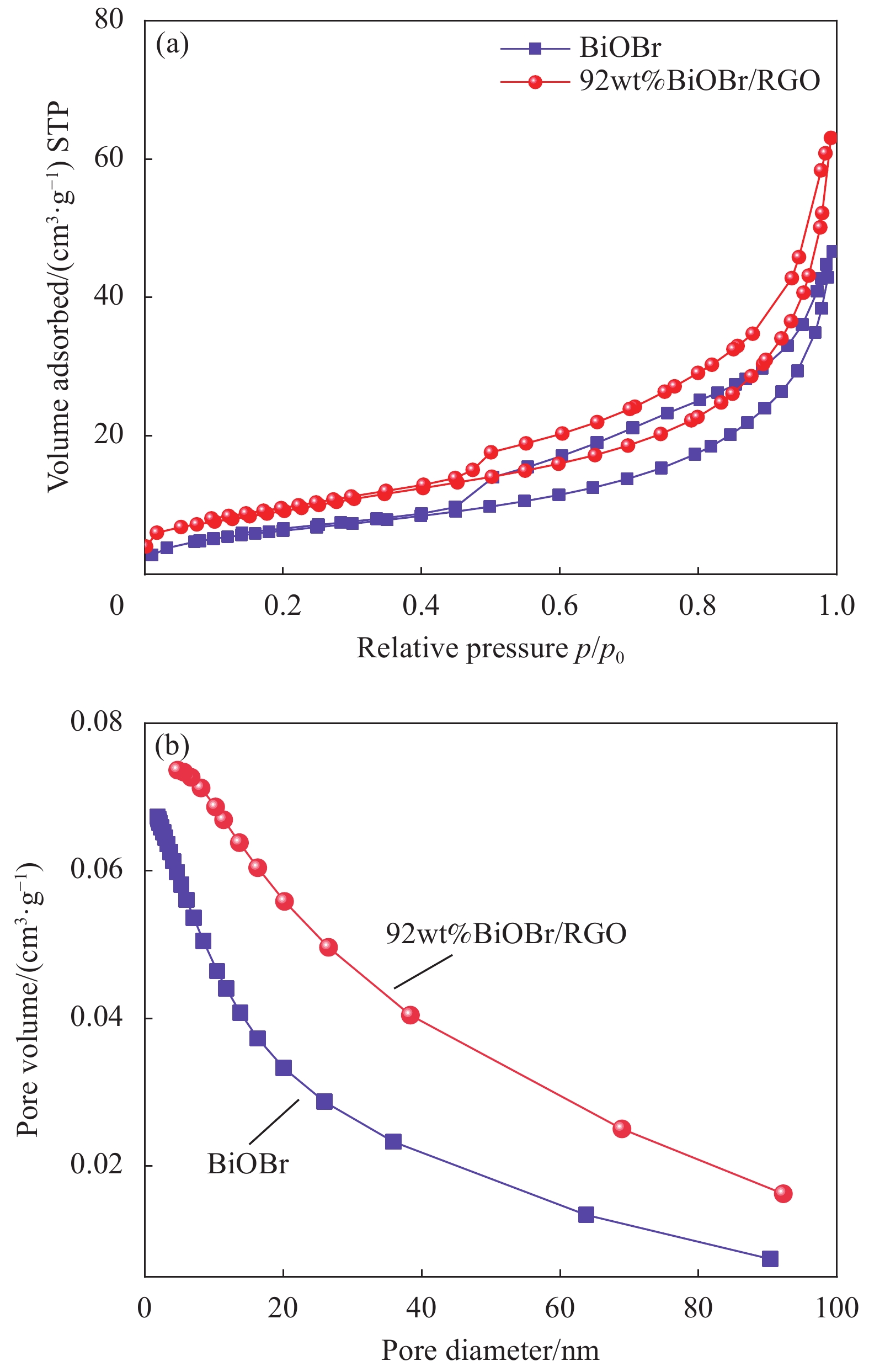
2.1.4 BiOBr/RGO水凝胶复合材料的比表面积和孔径分布
图5为BiOBr和BiOBr/RGO水凝胶复合材料的N2吸附-脱附热力学曲线及孔径分布曲线。可知,BiOBr和BiOBr/RGO水凝胶复合材料均有典型的介孔结构。BiOBr的比表面积为23.62 m²/g,孔隙体积为0.0709 cm³/g,平均孔径为12.005 nm。BiOBr/RGO水凝胶复合材料的比表面积为35.31 m²/g,孔隙体积为0.0907 cm³/g,平均孔径为16.48 nm。说明RGO的引入不仅提高了BiOBr/RGO水凝胶复合材料的比表面积,且提高了其孔径尺寸。
2.2 BiOBr/RGO水凝胶复合材料的光学性能
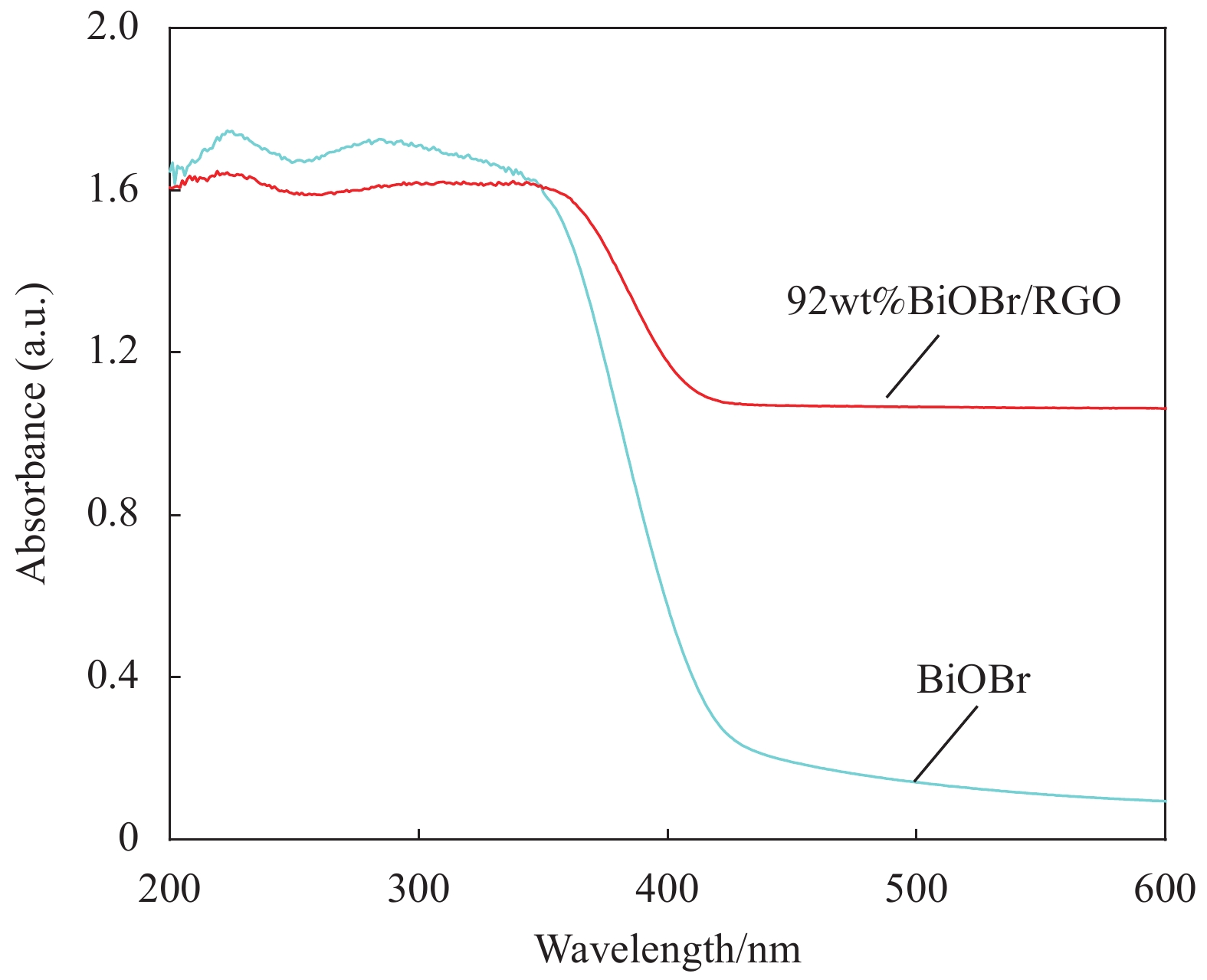
图6为BiOBr和BiOBr/RGO水凝胶复合材料的紫外-可见光漫反射图谱。可知,BiOBr的光吸收边缘在450 nm左右。掺杂RGO后,BiOBr/RGO水凝胶复合材料在可见光区的光吸收强度比BiOBr高。结果表明,BiOBr/RGO水凝胶复合材料具有良好的光吸收性能,引入RGO可提高BiOBr光催化活性。
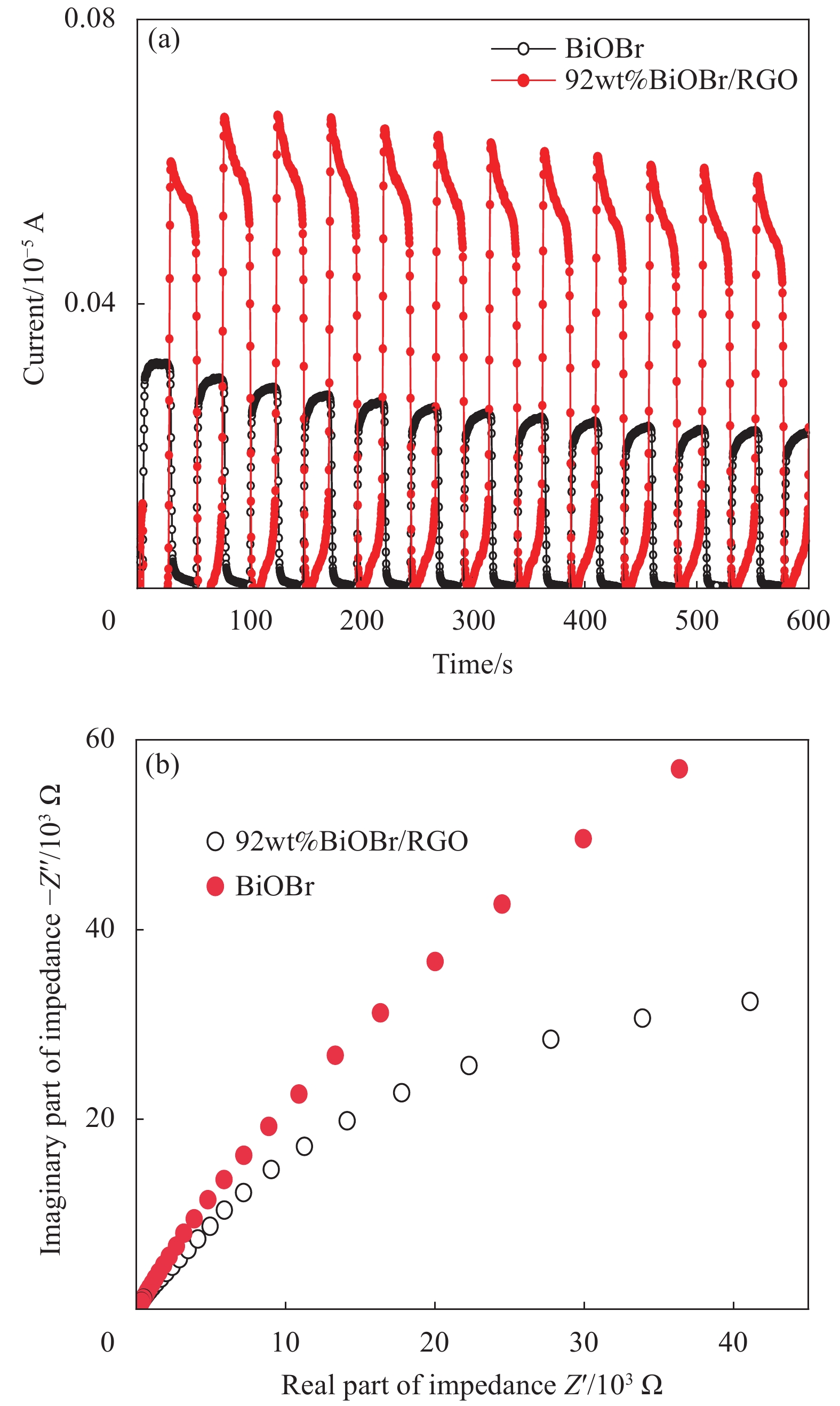
半导体材料中电子和空穴的分离可以产生光电流,说明高光电流的光生电子-空穴对的分离效率高[26]。图7为BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下的光电流瞬态响应和电化学阻抗图谱。由图7(a)可知,BiOBr和BiOBr/RGO水凝胶复合材料瞬态光电流响应在周期内是稳定的。BiOBr的光电流响应为0.31×10–6 A,而BiOBr/RGO水凝胶复合材料的瞬态光电流响应(0.75×10–6 A)是BiOBr的2.41倍。由图7(b)可知,BiOBr/RGO水凝胶复合材料的电化学阻抗图谱弧半径小于BiOBr,说明BiOBr/RGO水凝胶复合材料电化学阻抗图谱较BiOBr具有更低的电荷转移电阻。结果表明,引入RGO可以改善光催化体系中电子和空穴的分离效率,提高BiOBr/RGO水凝胶复合材料的光催化性能。
2.3 BiOBr/RGO水凝胶复合材料的光催化降解性能
2.3.1 RGO添加量对BiOBr/RGO光催化性能的影响
图8为在暗反应条件下BiOBr和BiOBr/RGO水凝胶复合材料对SBX的吸附性能。可知,RGO的引入提高了BiOBr/RGO水凝胶复合材料对SBX的吸附率,且随着RGO含量的增加,吸附率不断增大,可有效阻止SBX的自降解,且在40 min后,BiOBr及BiOBr/RGO水凝胶复合材料对SBX达到吸附-解吸平衡,因此光催化的暗反应时间为40 min。
![]() 图 8 BiOBr和BiOBr/RGO水凝胶复合材料对正丁基钠黄药(SBX)的吸附性能对比(SBX溶液体积为50 mL,10 mg样品)Figure 8. Comparison of adsorption properties of BiOBr and BiOBr/RGO hydrogel composites for sodium n-butyl xanthate (SBX) (Volume of SBX is 50 mL, the dosages of all samples is 10 mgC0—Initial concentration of SBX solution (25 mg/L)
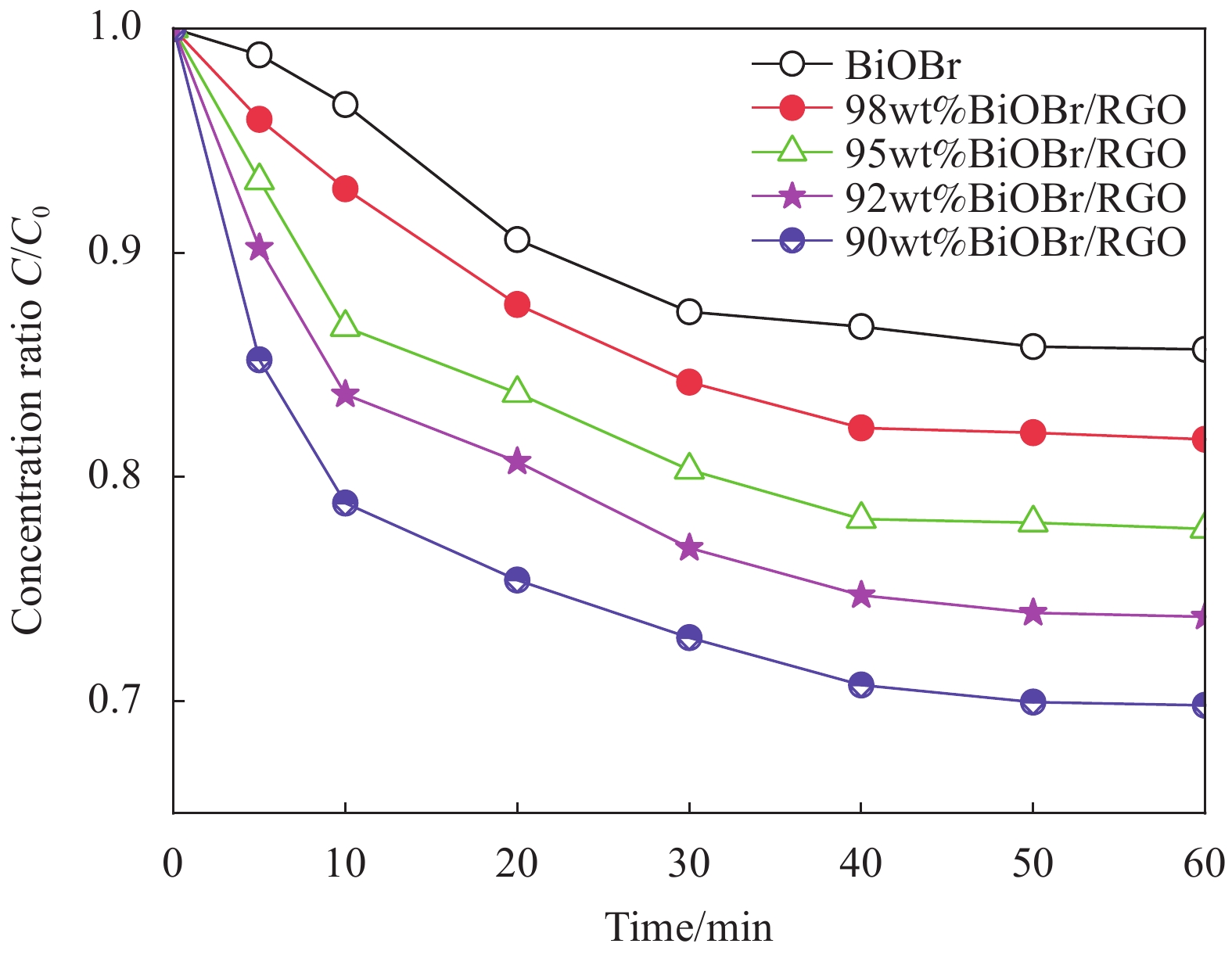
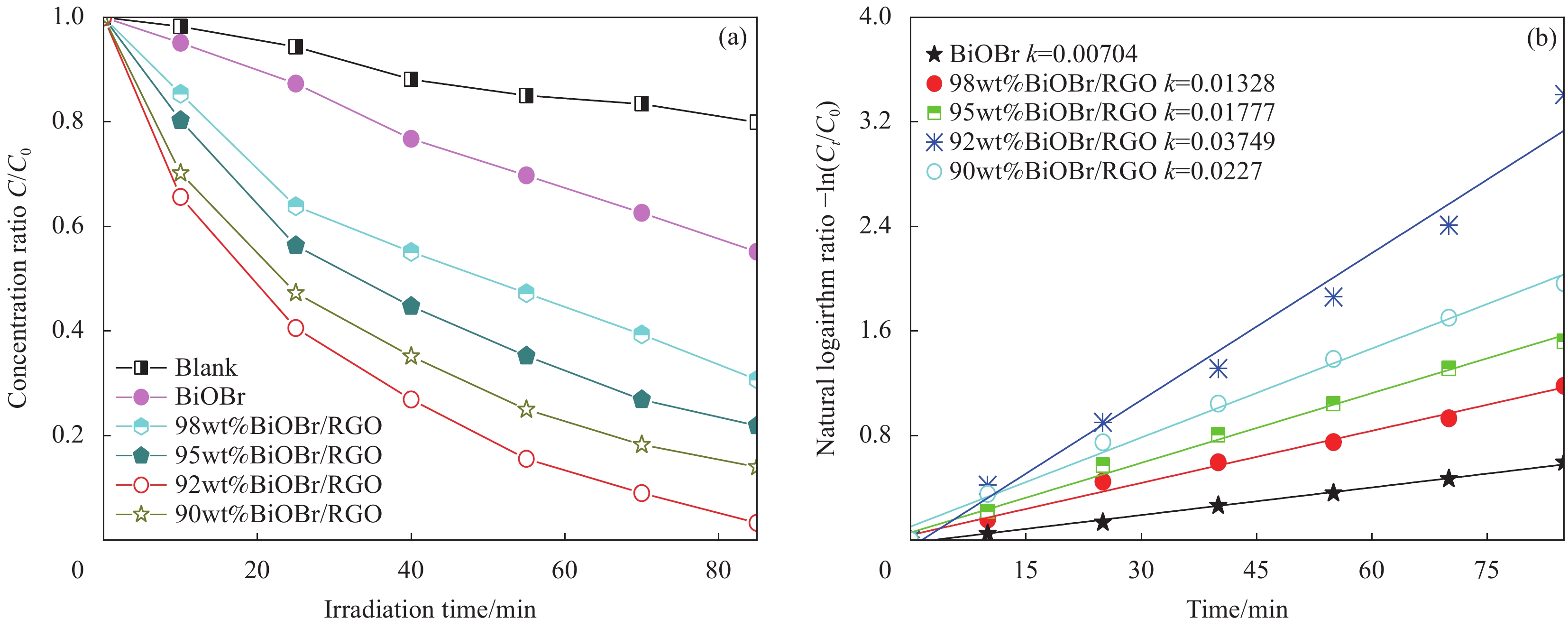
图 8 BiOBr和BiOBr/RGO水凝胶复合材料对正丁基钠黄药(SBX)的吸附性能对比(SBX溶液体积为50 mL,10 mg样品)Figure 8. Comparison of adsorption properties of BiOBr and BiOBr/RGO hydrogel composites for sodium n-butyl xanthate (SBX) (Volume of SBX is 50 mL, the dosages of all samples is 10 mgC0—Initial concentration of SBX solution (25 mg/L)图9为BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下对SBX光降解性能及光降解动力学。由图9(a)可知,在不添加光催化剂的情况下,SBX的降解可忽略。经BiOBr、98wt%BiOBr/RGO、95wt%BiOBr/RGO、92wt%BiOBr/RGO和90wt%BiOBr/RGO水凝胶复合材料处理85 min后,SBX的降解率分别为44.84%、69.30%、78.11%、96.69%和85.99%。即92wt%BiOBr/RGO水凝胶复合材料对SBX的降解效率是BiOBr的2.16倍。RGO的掺入提高了BiOBr的光催化活性。但90wt%BiOBr/RGO水凝胶复合材料的光降解性能明显低于92wt%BiOBr/RGO水凝胶复合材料,这是由于过多引入RGO使BiOBr的光吸收性能变弱。同时,利用准一级反应模型(ln(C/C0)=kt[24-25](k是反应速率常数)探讨光降解的动力学过程。由图9(b)可知,BiOBr、98wt%BiOBr/RGO、95wt%BiOBr/RGO、92wt%BiOBr/RGO和90wt%BiOBr/RGO水凝胶复合材料的k值分别为0.00704、0.01328、0.01777、0.03749和0.0227。其中,92wt%BiOBr/RGO水凝胶复合材料的k值最高,是BiOBr的5.33倍。进一步说明RGO在提高BiOBr光催化活性方面起重要作用。
![]() 图 9 BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下对SBX光降解性能(a)及光降解动力学(b)(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)Figure 9. Photocatalytic activities under 300 W xenon lamp (a) and kinetic curves of photocatalytic degradation (b) for SBX of BiOBr and BiOBr/RGO hydrogel composites (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)k—First-order kinetics constant
图 9 BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下对SBX光降解性能(a)及光降解动力学(b)(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)Figure 9. Photocatalytic activities under 300 W xenon lamp (a) and kinetic curves of photocatalytic degradation (b) for SBX of BiOBr and BiOBr/RGO hydrogel composites (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)k—First-order kinetics constant2.3.2 BiOBr/RGO水凝胶复合材料的循环利用次数
图10为BiOBr/RGO水凝胶复合材料在循环使用前和后的FTIR图谱。可知,原始BiOBr/RGO水凝胶复合材料的吸收峰在3414 cm−1、1572 cm−1、1209 cm−1和512 cm−1处分别对应O—H、C=C、C—O和Bi—O伸缩振动峰,而O—H、C=C和C—O均来自RGO,说明BiOBr与RGO成功复合,与XRD结果一致。另外,每次循环利用后的BiOBr/RGO水凝胶复合材料和原始水凝胶复合材料的红外吸收峰均未发生改变。表明用水和无水乙醇可以完全洗涤掉BiOBr/RGO水凝胶复合材料上吸附的SBX,不会影响测试结果,且经6次循环后,BiOBr/RGO水凝胶复合材料的结构未发生改变。
图11为BiOBr/RGO水凝胶复合材料过滤后循环使用的残余率及在300 W氙灯下的循环利用性能。可知,每次循环,BiOBr/RGO水凝胶复合材料都会造成一定的质量损失,6次循环后,BiOBr/RGO水凝胶复合材料仍存留86.35%,且对SBX的去除率仍在69%以上。虽然BiOBr/RGO水凝胶复合材料在循环利用过程中存在质量损失,但仍残余在80%以上,降解率仍可达60%以上,结合红外分析,说明BiOBr/RGO水凝胶复合材料的稳定性较好。
2.3.3 BiOBr/RGO水凝胶复合材料的光降解机制
羟基自由基、空穴和超氧阴离子自由基是光催化降解有机污染物的三个物种。乙二胺四乙酸二钠(EDTA-2Na)、异丙醇和对苯醌分别为h+、·OH自由基和超氧阴离子自由基的捕获剂。图12为在300 W氙灯下不同捕获剂对BiOBr/RGO水凝胶复合材料光催化性能的影响。可知,添加异丙醇对SBX的降解效率影响不大;加入对苯醌后,光催化活性略有下降,约有56.99%的SBX残留在溶液中。但加入EDTA-2Na后,光降解85 min后,73.26% SBX仍残留在光催化反应溶液中。结果说明,相比光生羟基自由基和超氧阴离子自由基,h+对SBX的降解起主要作用。光生电子主要通过下式[27-28]:
![]() 图 12 在300 W氙灯下不同捕获剂对BiOBr/RGO水凝胶复合材料光催化性能的影响(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)Figure 12. Effect of photodegradation on BiOBr/RGO hydrogel composite in presence of different scavengers under 300 W xenon lamp irradiation (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)EDTA-2Na—Ethylenediaminetetraacetic acid disodium salt
图 12 在300 W氙灯下不同捕获剂对BiOBr/RGO水凝胶复合材料光催化性能的影响(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)Figure 12. Effect of photodegradation on BiOBr/RGO hydrogel composite in presence of different scavengers under 300 W xenon lamp irradiation (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)EDTA-2Na—Ethylenediaminetetraacetic acid disodium saltBiOBr+hv→BiOBr(h++e−)BiOBr(e−)+RGO→BiOBr+RGO(e−)RGO(e−)+O2→⋅O−2⋅O−2+e−+2H+→H2O2H2O2+e−→⋅OH+OH−h++H2O→⋅OH+H+h++⋅OH+O−2+SBX→Degradation 产生·OH。其中,光生电子产生的一部分超氧阴离子参与降解SBX,剩余的超氧阴离子捕获电子产生羟基自由基,因此光生电子产生·OH的能力比h+弱。说明光催化剂溶液中的·OH主要是光生电子产生的。也是加入异丙醇对SBX的降解效率影响不大的原因。因此,BiOBr/RGO水凝胶复合材料降解SBX的主要路线和次要路线分别是h+的直接氧化和电子产生的超氧阴离子与羟基自由基的氧化。
图13为BiOBr/RGO水凝胶复合材料对SBX的光降解机制。可知,在300 W氙灯下,BiOBr被激发产生电子和空穴。BiOBr价带上的h+直接氧化污染物,而BiOBr导带上的电子由于RGO的高电导率发生快速转移,促进电子与空穴的分离,增强光催化活性。另外,电子与复合材料表面吸附的O2反应生成了·O2−,进一步产生H2O2,H2O2再进一步捕获电子产生·OH,·OH进而氧化污染物。最后,SBX被·OH、·O2−和h+联合作用而降解。
3. 结 论
采用水热法成功合成了宏观BiOBr/石墨烯(RGO)水凝胶复合材料,考察了BiOBr/RGO水凝胶复合材料对SBX的降解性能,发现RGO的引入不仅提高了BiOBr的光催化活性,且有利于催化剂的回收利用。
(1) BiOBr/RGO水凝胶复合材料的宏观3D结构利于光生载流子迁移和分离,且易于回收利用。
(2)在一定条件下,当BiOBr质量分数为92wt%时,BiOBr/RGO水凝胶复合材料对SBX降解率可达96%以上,是BiOBr降解率的2.16倍。
(3)经过6次循环使用后,BiOBr质量分数为92wt%的BiOBr/RGO水凝胶复合材料对SBX的去除率仍在69%以上。
-
图 8 BiOBr和BiOBr/RGO水凝胶复合材料对正丁基钠黄药(SBX)的吸附性能对比(SBX溶液体积为50 mL,10 mg样品)
Figure 8. Comparison of adsorption properties of BiOBr and BiOBr/RGO hydrogel composites for sodium n-butyl xanthate (SBX) (Volume of SBX is 50 mL, the dosages of all samples is 10 mg
C0—Initial concentration of SBX solution (25 mg/L)
图 9 BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下对SBX光降解性能(a)及光降解动力学(b)(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)
Figure 9. Photocatalytic activities under 300 W xenon lamp (a) and kinetic curves of photocatalytic degradation (b) for SBX of BiOBr and BiOBr/RGO hydrogel composites (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)
k—First-order kinetics constant
图 12 在300 W氙灯下不同捕获剂对BiOBr/RGO水凝胶复合材料光催化性能的影响(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)
Figure 12. Effect of photodegradation on BiOBr/RGO hydrogel composite in presence of different scavengers under 300 W xenon lamp irradiation (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)
EDTA-2Na—Ethylenediaminetetraacetic acid disodium salt
表 1 BiOBr/RGO水凝胶复合材料的名称及含量
Table 1 Name and content of BiOBr/RGO hydrogel composites
Composite name Mass fraction of BiOBr/wt% Mass fraction of RGO/wt% 98wt%BiOBr/RGO 98 2 95wt%BiOBr/RGO 95 5 92wt%BiOBr/RGO 92 8 90wt%BiOBr/RGO 90 10 -
[1] AMROLLAHI A, MASSINAEI M, MOGHADDAM A Z. Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles[J]. Minerals Engineering,2019,134:142-155. DOI: 10.1016/j.mineng.2019.01.031
[2] KIANINIA Y, KHALESI M R, SEYEDHAKIMI A, et al. Flotation of mercury from the tailings of the Agh-Darreh gold processing plant, Iran[J]. Journal of the Southern African Institute of Mining & Metallurgy,2017,117(1):83-88.
[3] BOUJEMAA D, YASSINE T, RACHID H, et al. Recovery of residual silver-bearing minerals from low-grade tailings by froth flotation: The case of zgounder mine, morocco[J]. Minerals,2018,8(7):273. DOI: 10.3390/min8070273
[4] GRANO S R, JOHNSON N W, RALSTON J. Control of the solution interaction of metabisulphite and ethyl xanthate in the flotation of the Hilton ore of Mount Isa Mines Limited, Australia[J]. Minerals Engineering,1997,10(1):17-39. DOI: 10.1016/S0892-6875(96)00129-X
[5] HIDALGO P, GUTZ I G R. Determination of low concentrations of the flotation reagent ethyl xanthate by sampled DC polarography and flow injection with amperometric detection[J]. Talanta,2001,54(2):403-409. DOI: 10.1016/S0039-9140(01)00311-3
[6] VEZIROGLU S, OBERMANN A L, ULLRICH M, et al. Photodeposition of Au nanoclusters for enhanced photocatalytic dye degradation over TiO2 thin film[J]. ACS Applied Materials & Interfaces,2020,12(13):14983-14992.
[7] BHATKHANDE D S, PANGARKAR V G, BEENACKERS A A C M. Photocatalytic degradation for environmental applications: A review[J]. Journal of Chemical Technology & Biotechnology,2002,77(1):102-116.
[8] TURCHI C S, OLLIS D F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack[J]. Journal of Catalysis,1990,122(1):178-192. DOI: 10.1016/0021-9517(90)90269-P
[9] LIU Z Y, MIAO Y E, LIU M k, et al. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance[J]. Journal of Colloid and Interface Science,2014,424:49-55. DOI: 10.1016/j.jcis.2014.03.009
[10] 李健, 闫龙, 潘盼盼, 等. 活性炭负载ZnS/TiO2光催化剂的制备及其性能研究[J]. 非金属矿, 2019, 42(2):76-79. DOI: 10.3969/j.issn.1000-8098.2019.02.021 LI J, YAN L, PAN P P, et al. Preparation and properties of ZnS/TiO2 photocatalyst supported by activated carbon[J]. Non-Metallic Mines,2019,42(2):76-79(in Chinese). DOI: 10.3969/j.issn.1000-8098.2019.02.021
[11] 周琪, 钟永辉, 陈星, 等. 石墨烯/纳米TiO2复合材料的制备及其光催化性能[J]. 复合材料学报, 2014, 31(2):255-262. ZHOU Q, ZHONG Y H, CHEN X, et al. Preparation of reduced graphene oxide/nano TiO2 composites by two-step hydrothermal method and their photocatalytic properties[J]. Acta Materiae Compositae Sinica,2014,31(2):255-262(in Chinese).
[12] 陈苗, 敖卫, 王如意, 等. 氮掺杂TiO2中空复合微球的制备及可见光光催化性能[J]. 复合材料学报, 2015, 32(3):918-923. CHEN M, AO W, WANG R Y, et al. Preparation and visible-light photocatalytic property of nitrogen-doped TiO2 hollow composite microspheres[J]. Acta Materiae Compositae Sinica,2015,32(3):918-923(in Chinese).
[13] HIROMI Y, MASARU H, JUNKO M, et al. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2[J]. Catalysis Today,2003,84(3):191-196.
[14] QU X, LIU M, LI L, et al. BiOBr flakes decoration and structural modification for CdTe/TiO2 spheres: Towards water decontamination under simulated light irradiation[J]. Materials Science in Semiconductor Processing,2019,93:331-338. DOI: 10.1016/j.mssp.2019.01.006
[15] JIANG G, LI X, WEI Z, et al. Growth of N-doped BiOBr nanosheets on carbon fibers for photocatalytic degradation of organic pollutants under visible light irradiation[J]. Powder Technology,2014,260:84-89. DOI: 10.1016/j.powtec.2014.04.005
[16] TIAN H, LI J, GE M, et al. Removal of bisphenol A by mesoporous BiOBr under simulated solar light irradiation[J]. Catalysis Science & Technology,2012,2(11):23-51.
[17] FU J, TIAN Y, CHANG B, et al. BiOBr-carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism[J]. Journal of Materials Chemistry,2012,22(39):21159-21166. DOI: 10.1039/c2jm34778d
[18] DENG W, PAN F, BATCHELOR B, et al. Mesoporous TiO2-BiOBr microspheres with tailorable adsorption capacities for photodegradation of organic water pollutants: Probing adsorption-photocatalysis synergy by combining experiments and kinetic modeling[J]. Environmental Science: Water Research & Technology,2019,5(4):769-781.
[19] ZHANG L, WANG W, SUN S, et al. Elimination of BPA endocrine disruptor by magnetic BiOBr@SiO2@Fe3O4 photocatalyst[J]. Applied Catalysis B: Environmental,2014,148-149:164-169. DOI: 10.1016/j.apcatb.2013.10.053
[20] SONG X C, ZHENG Y F, YIN H Y, et al. The solvothermal synthesis and enhanced photocatalytic activity of Zn2+ doped BiOBr hierarchical nanostructures[J]. New Journal of Chemistry,2016,40(1):130-135. DOI: 10.1039/C5NJ01282A
[21] LIU W, CAI J, LI Z. Self-assembly of semiconductor nanoparticles/reduced graphene oxide (RGO) composite aerogels for enhanced photocatalytic performance and facile recycling in aqueous photocatalysis[J]. ACS Sustainable Chemistry & Engineering,2015,3(2):277-282.
[22] HE Y R, LI S C, LI X L, et al. Graphene (rGO) hydrogel: A promising material for facile removal of uranium from aqueous solution[J]. Chemical Engineering Journal,2018,338:333-340. DOI: 10.1016/j.cej.2018.01.037
[23] PRASITTHIKUN T, WU X, SATO T, et al. Synthesis and photocatalytic activity of visible-light responsive BiOBr/GO composites[J]. Key Engineering Materials,2017,751:807-812. DOI: 10.4028/www.scientific.net/KEM.751.807
[24] 杨旭宇, 王贤保, 李静, 等. 氧化石墨烯的可控还原及结构表征[J]. 高等学校化学学报, 2012, 33(9):1902-1907. DOI: 10.3969/j.issn.0251-0790.2012.09.005 YANG X Y, WANG X B, LI J, et al. Controllable reduction and structural characterizations of graphene oxides[J]. Chemical Journal of Chinese Universities Chinese,2012,33(9):1902-1907(in Chinese). DOI: 10.3969/j.issn.0251-0790.2012.09.005
[25] CHEN Y, GE H, WEI L, et al. Reduction degree of reduced graphene oxide (RGO) dependence of photocatalytic hydrogen evolution performance over RGO/ZnIn2S4 nanocomposites[J]. Catalysis Science & Technology,2013,3(7):1712-1717.
[26] LI X, WANG L, ZHANG L, et al. A facile route to the synthesis of magnetically separable BiOBr/NiFe2O4 composites with enhanced photocatalytic performance[J]. Applied Surface Science,2017,419(15):586-594.
[27] 盘雨凝, 帅欢, 李男, 等. 纳米TiO2/硅藻土复合材料制备及亚甲基蓝降解[J]. 非金属矿, 2019, 42(5):98-100. PAN Y N, SHUAI H, LI N, et al. Study on preparation of TiO2/diatomite composite materials and its photocatalytic of methylene blue[J]. Non-Metallic Mines,2019,42(5):98-100(in Chinese).
[28] CHEN F, AN W, LIU L, et al. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy[J]. Applied Catalysis B: Environmental,2017,217:65-80. DOI: 10.1016/j.apcatb.2017.05.078
-
期刊类型引用(7)
1. 梁华霖,宋超,解正坤. 黄药降解类光催化材料制备研究现状. 山西大同大学学报(自然科学版). 2024(06): 105-111 .  百度学术
百度学术
2. 高生旺,赵星鹏,陆迦勒,崔娟,王国英,张青. Ag-BiOBr/WO_3复合材料的制备及强化磺胺异噁唑去除性能. 复合材料学报. 2023(03): 1455-1467 .  本站查看
本站查看
3. 王丽苹,阮玉娴,李仁星,余莹,陈琳,姜帅. Fe-MILs系可见光响应复合材料在光诱导反应中的应用研究进展. 化学通报. 2023(12): 1409-1425 .  百度学术
百度学术
4. 梁锐,李明阳,高翔鹏,于先坤,童雄,龙红明. 选矿废水中残留黄药光催化处理及降解效率改进方式研究进展. 过程工程学报. 2022(01): 1-13 .  百度学术
百度学术
5. 葛奉娟,朱捷,陈艳,徐艳. 制备方法对BiOBr形貌及其可见光降解活性的研究. 能源与环境. 2021(01): 97-99+101 .  百度学术
百度学术
6. 杜春艳,宋佳豪,谭诗杨,阳露,张卓,余关龙. 石墨烯桥联的ZnO/Ag_3PO_4复合材料的制备及其对环丙沙星的降解性能. 复合材料学报. 2021(07): 2254-2264 .  本站查看
本站查看
7. 陈星玉,张雪乔,鲁李李,陈彩霞,向洪瑗,郭梦圆. GQDs/BiOBr复合光催化剂可见光降解磺胺废水的研究. 天然气化工(C1化学与化工). 2021(S1): 74-81 .  百度学术
百度学术
其他类型引用(6)
-




 下载:
下载: