Removal of Cr(VI) from water by modified attapulgite adsorbent
-
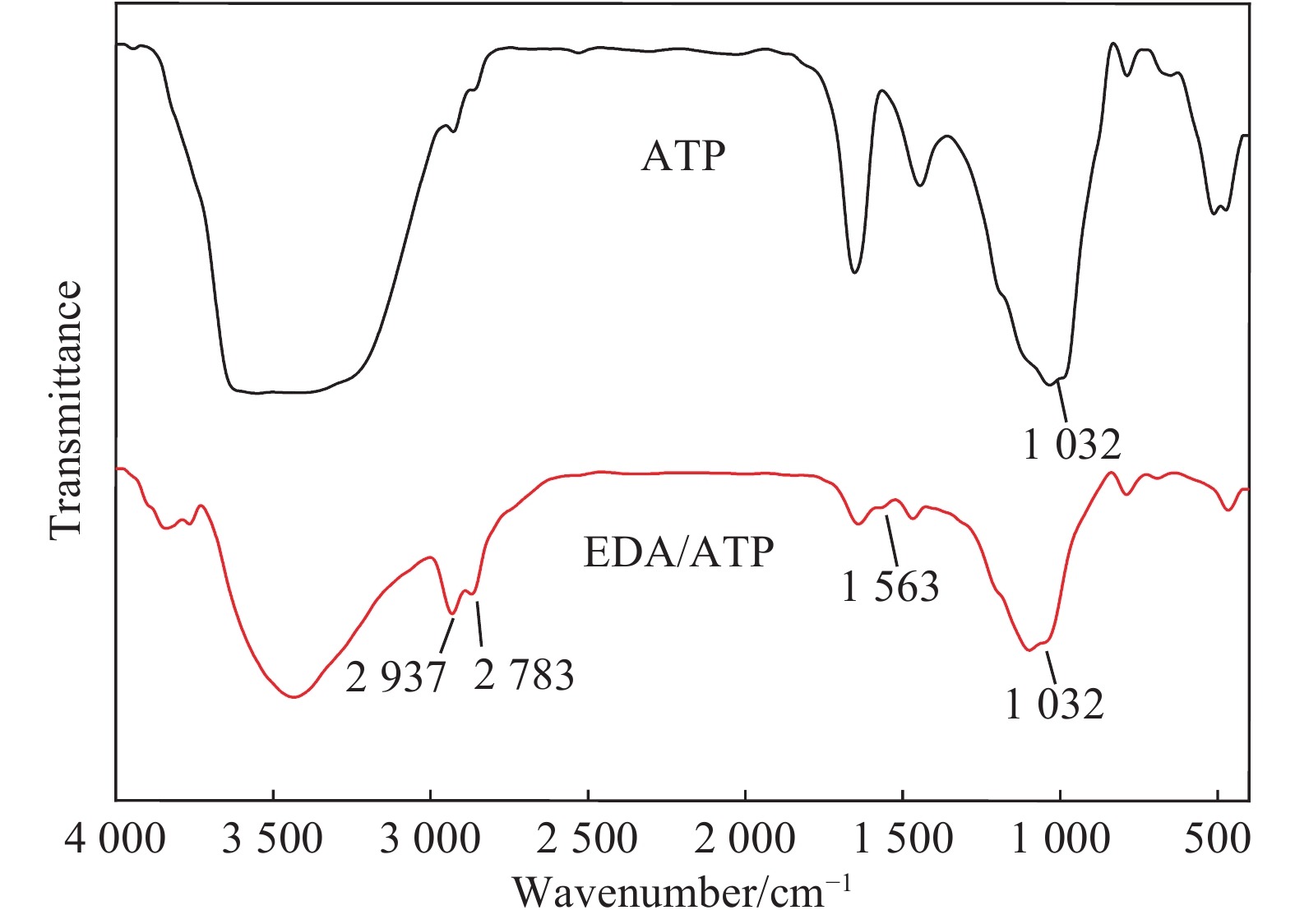
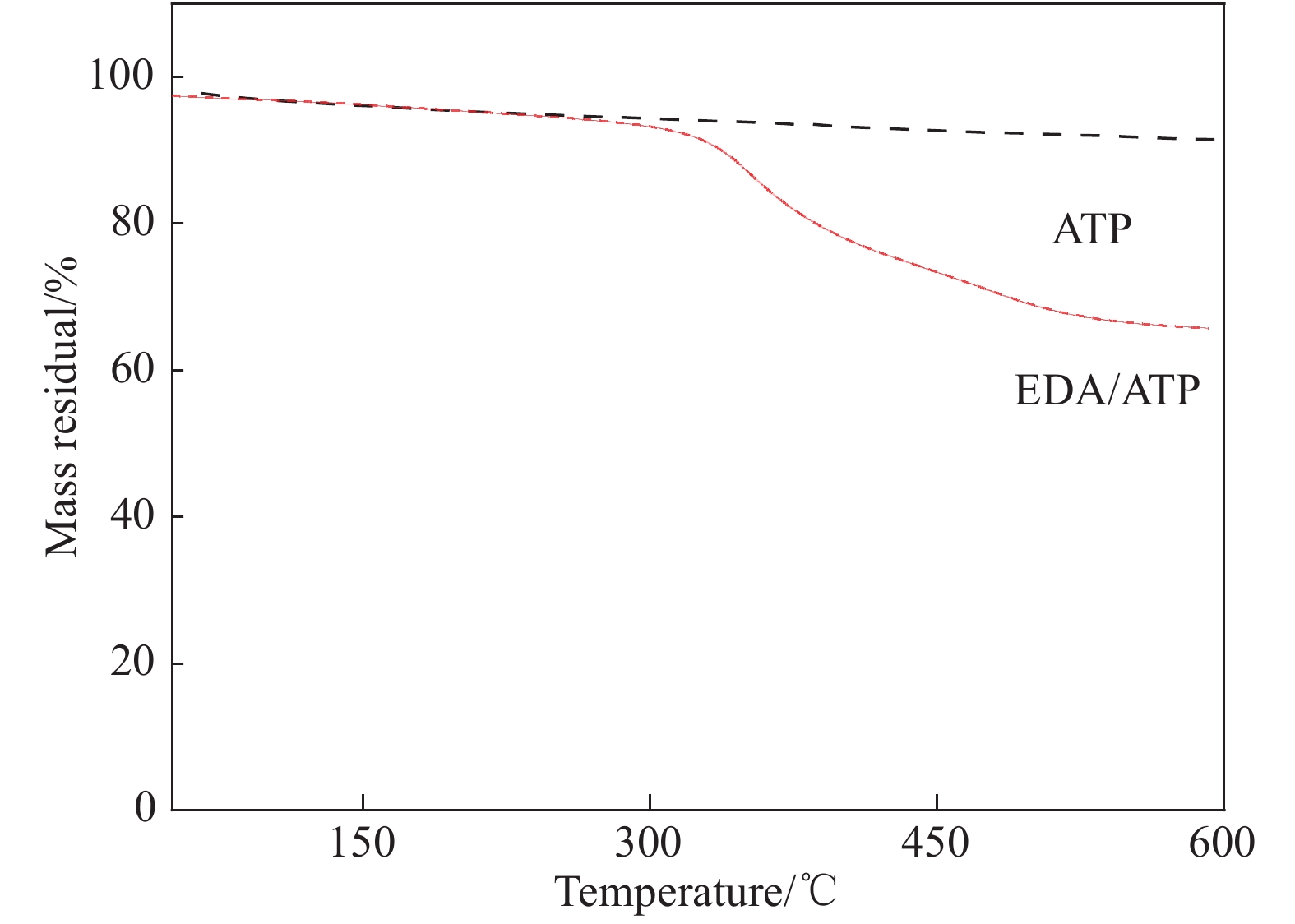
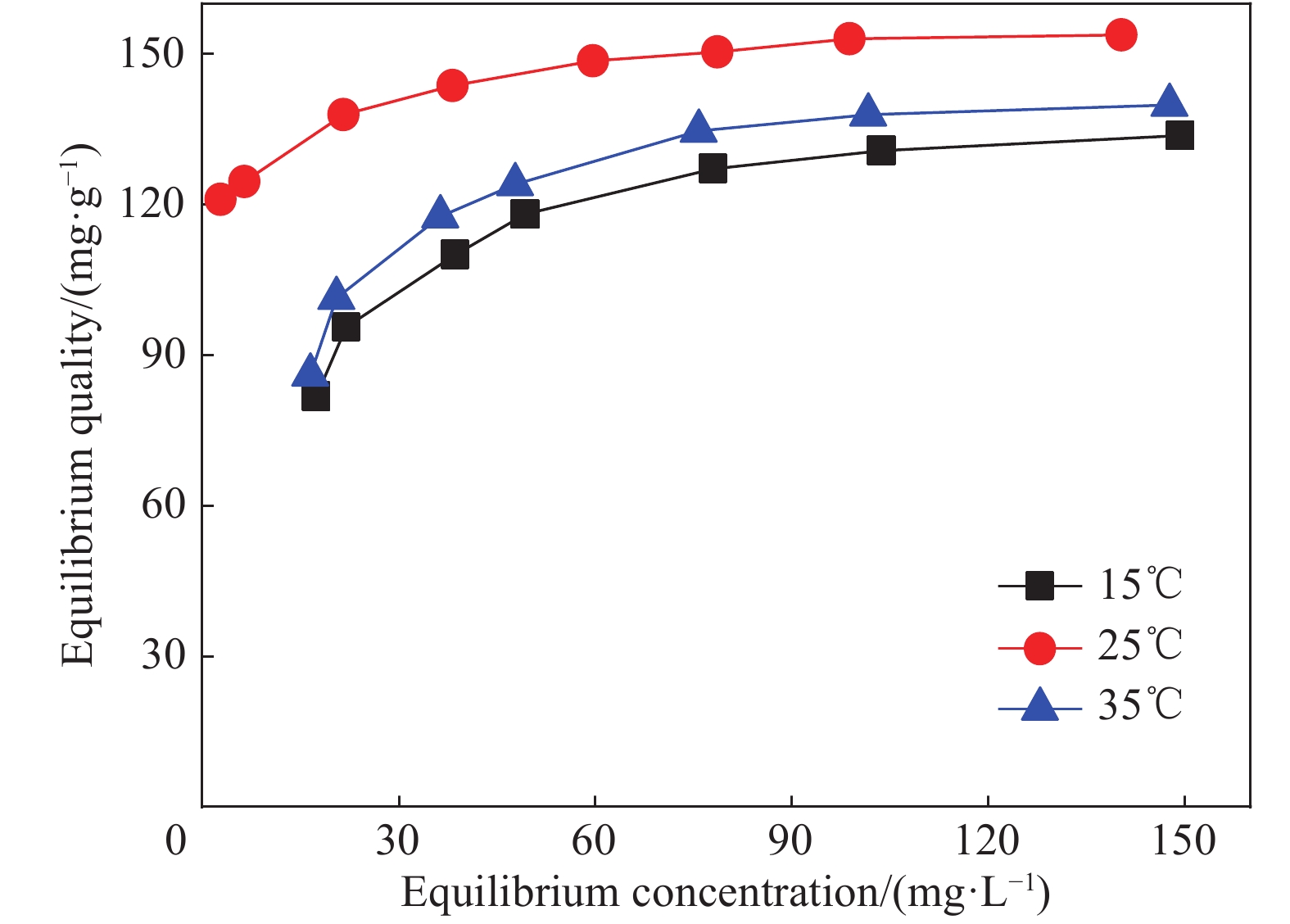
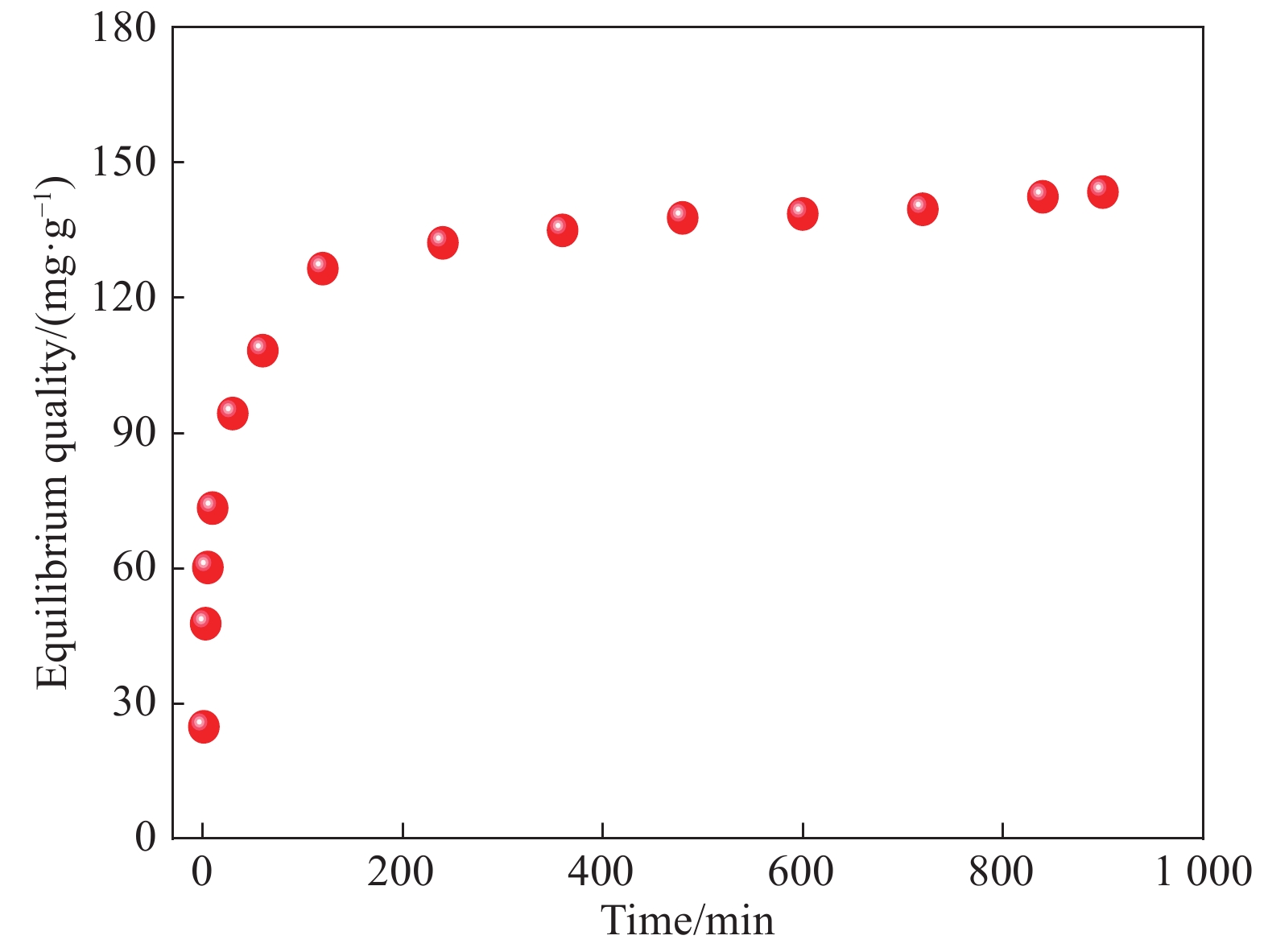
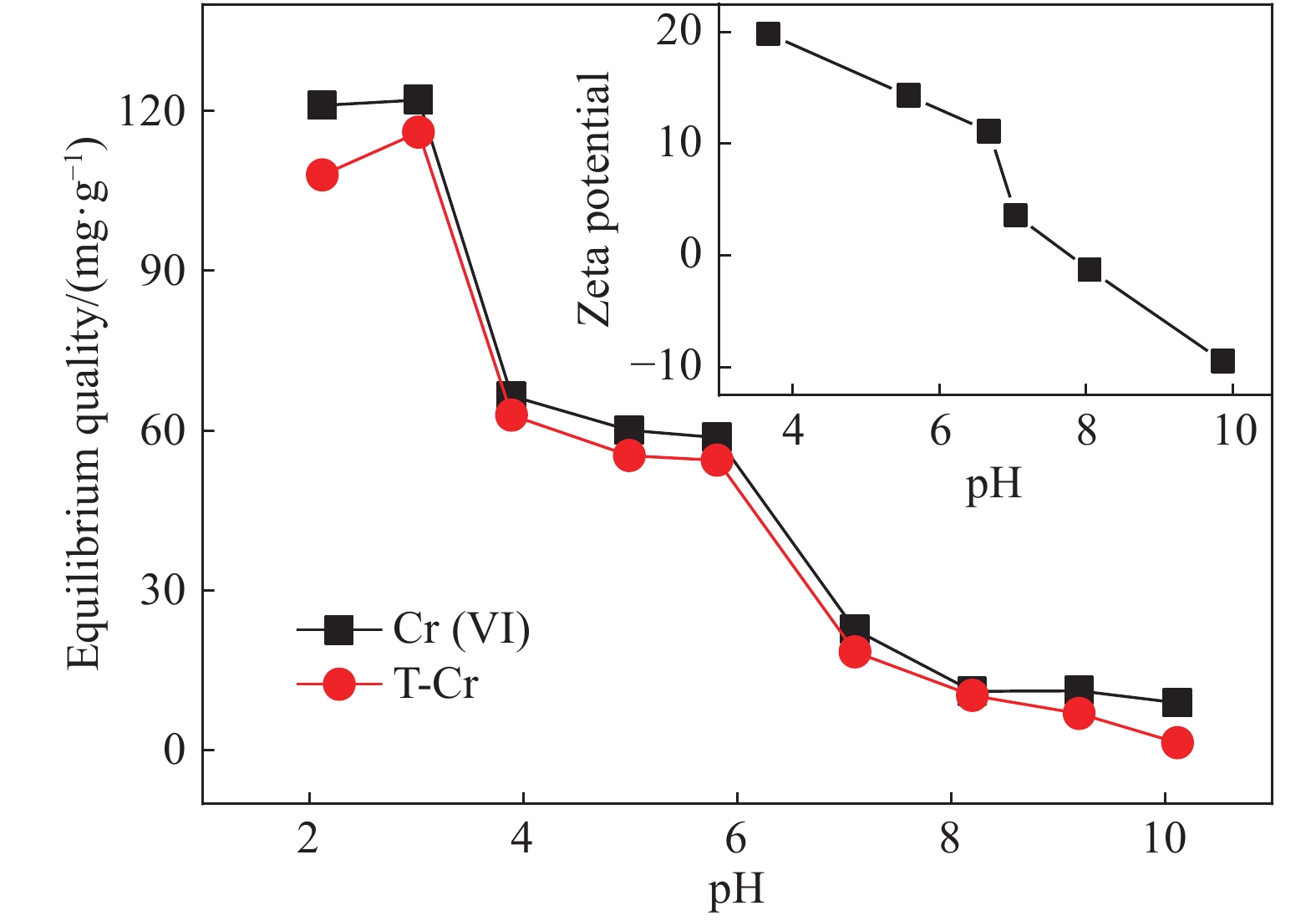
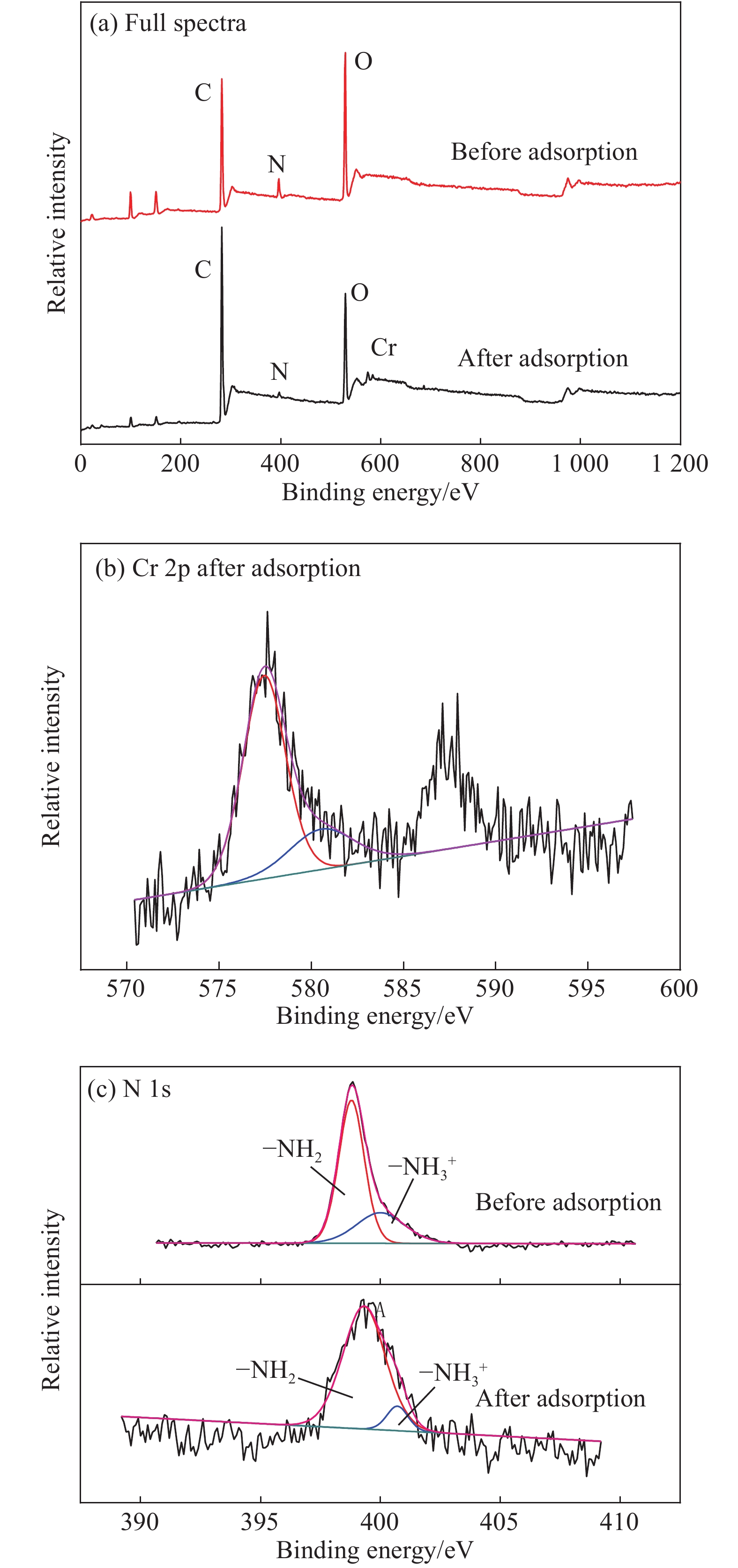
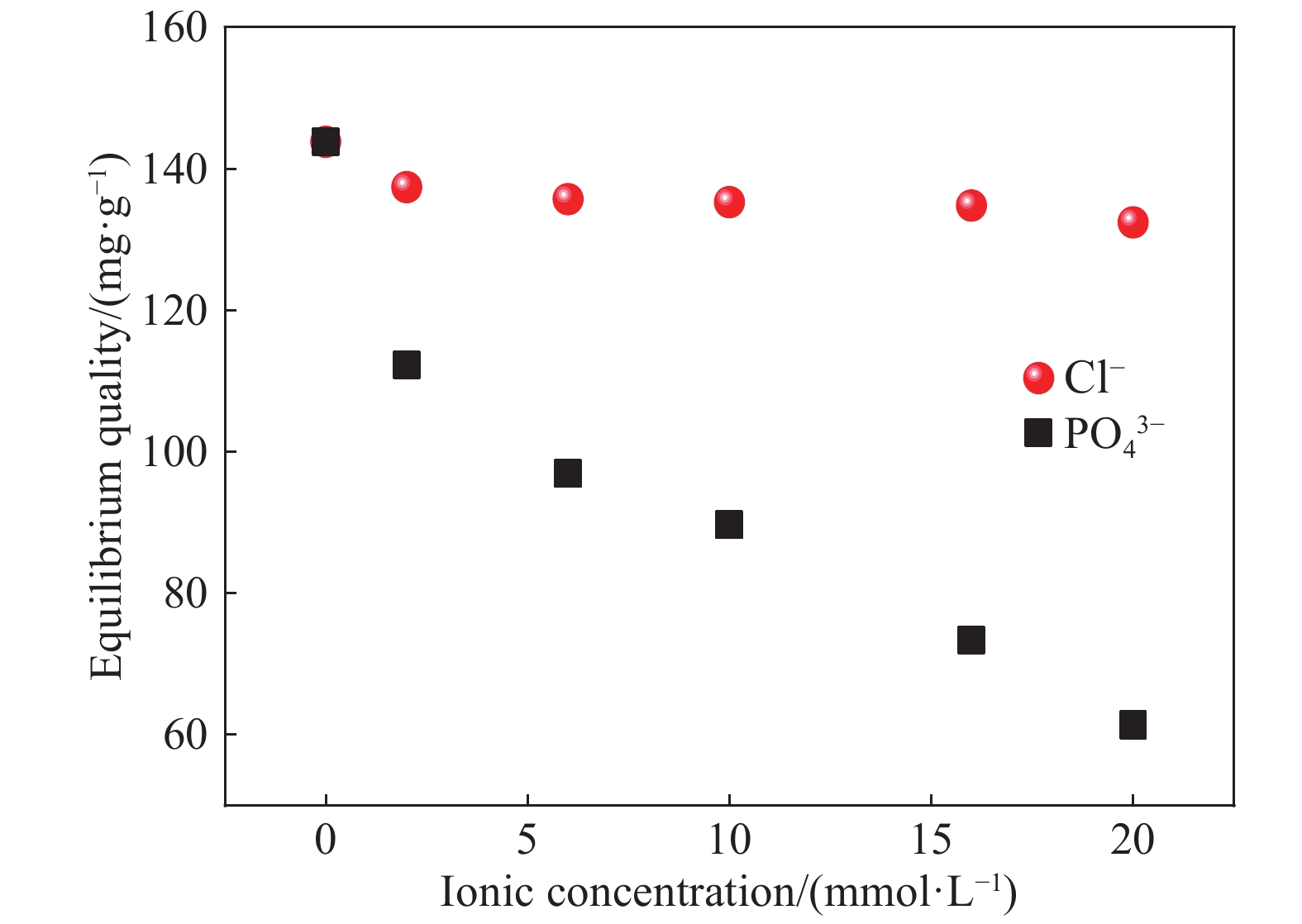
摘要: 以凹凸棒土为载体,合成了乙二胺(EDA)改性凹凸棒土(ATP)吸附剂EDA/ATP复合材料。采用FTIR、TGA对吸附剂进行表征,同时将其应用于对水中Cr(VI)的吸附,研究了溶液初始浓度、吸附时间、溶液pH、Cl−与PO43−阴离子浓度对吸附的影响。FTIR和TGA结果表明乙二胺已成功接枝到凹凸棒土表面。吸附实验表明,25℃时EDA/ATP复合材料对Cr(VI)的最大吸附容量为153.78 mg·g−1,吸附在800~900 min内达到平衡,吸附符合Freundlich吸附等温模型和拟二级动力学模型;在初始溶液pH为2~10条件下,随着pH的增加,吸附量先增加再降低,pH为3时,吸附量最大;Cl−对吸附影响较小,PO43−对吸附的影响较大,当PO43−浓度达到20 mmol·L−1时,Cr(VI)最大吸附量下降了83 mg·g−1;实验表明EDA/ATP可作为一种潜在处理水中Cr(VI)的吸附剂。Abstract: Ethylenediamine modified attapulgite(EDA/ATP) adsorbent was synthesized by using clay-based adsorbent ATP as carrier. The EDA/ATP composite adsorbent was characterized by FTIR and TGA. The adsorbent was applied to the adsorption of Cr(VI) in aqueous. The effects of initial concentration of Cr(VI) solution, adsorption time, solution pH and the anion concentration Cl− and PO43− on the adsorption of Cr(VI) were studied. The FTIR and TGA results show that EDA has been successfully grafted onto the surface of ATP. The adsorption experimental results show that at 25℃ the maximum adsorption capacity of EDA/ATP composite for Cr(VI) is 153.78 mg·g−1, and the adsorption reaches equilibrium within 800~900 min. The adsorption experimental data conform to Freundlich adsorption isotherm model and the pseudo-second-order kinetics. The initial pH of solution is ranging from 2 to 10. With the increase of pH, the adsorption capacity first increases and then decreases. When the pH is 3, the adsorption capacity reaches the maximum adsorption amount. PO43− has greater effect on the adsorption than Cl−, and the maximum adsorption capacity decreases by 83 mg·g−1 when the concentration of PO43− is 20 mmol·L−1. The experimental results show that EDA/ATP composite can be used as a potential adsorbent for Cr(VI) in water treatment.
-
Keywords:
- attapulgite /
- ethylenediamine /
- modification /
- adsorption /
- Cr(VI) /
- water treatment
-
碳化钨颗粒增强钢铁基(WCP/Fe)复合材料因兼顾金属基体的良好韧性与陶瓷增强颗粒的高强度、高硬度、高模量而广泛应用于机械制造、能源开发、交通运输等领域,但基体与增强颗粒间热物理性能差异过大使复合材料在激冷激热环境下服役时产生热应力,诱发界面处裂纹的萌生与扩展[1-4]。而将WC充分溶解,W在熔体中均匀扩散形成合金化复合层能有效解决该问题,故研究WC/Fe复合材料中W扩散均匀性十分必要[5-7]。
蜂窝结构因形状连续、比强度高等特性而对复合材料的综合性能产生重要影响[8-10]。Magotteaux公司发明X-win蜂窝结构ZTAP/Fe复合材料技术,制造的磨辊使用寿命提高两倍以上[11];WU等[12]从模拟与实验角度出发,揭示预制体孔径与孔距作为蜂窝结构重要参数对复合材料力学性能的影响;SONG等[13]成功制备蜂窝结构还原氧化石墨烯增强环氧树脂(rGH/EP)复合材料,电磁屏蔽效能与导电性明显提升。但对蜂窝预制体结构与元素扩散均匀性间的关联机制仍研究较少。
本文采用真空消失模铸渗(V-EPC)工艺制备WC/Fe复合材料[14-15],选取孔径孔距比相同孔径不同的蜂窝预制体,并将W质量分数最高与最低的预制体原孔壁与原孔心处W质量分数作为W扩散均匀性的判断条件[16-17]。表征复合层的显微组织、物相组成、元素分布,并检测预制体原孔壁与原孔心处W质量分数、硬度及复合层耐磨性,揭示其孔径对W质量分数分布的影响规律。通过求解非稳态扩散方程得解析解,对预制体孔内熔体凝固时的热物理场进行有限元模拟,并通过二次开发程序对其原孔内W质量分数分布进行数值模拟,揭示其孔径对W扩散均匀性的影响机制;提出W扩散均匀性与复合层耐磨性间的关联机制,为工程应用提供理论依据。
1. 实验材料与方法
为避免铸渗时W粉因粒径过小而大量烧损,选取铸造WC颗粒(WC/W2CP)为合金化提供W;表1为WC/Fe复合材料中预制体的成分组成,配置预制体粉末300 g,与5wt%水玻璃粘结剂均匀混合;表2为WC/Fe复合材料中预制体的结构参数,填充到预制体孔径孔距比相同孔径不同的蜂窝模具内,其轮廓为50 mm×100 mm×6 mm;采用CO2硬化与微波烧结方法,得最终预制体。
表3为WC/Fe复合材料中基体的成分组成。配置基体并采用中频感应炉熔炼20 kg。图1为WC/Fe复合材料的制备过程,采用V-EPC工艺成型,浇注温度为1 500℃,型腔负压为0.05 MPa。
表 1 WC/Fe复合材料中预制体的成分Table 1. Composition of preform in WC/Fe compositesComposition Mass fraction/wt% Size/μm WC 40 150-200 Ni60 30 60-90 FeCr55C6.0 30 150-200 表 2 WC/Fe复合材料中预制体的结构参数Table 2. Structure parameters of preform in WC/Fe compositesDiameter R/mm Distance d/mm Number n 3 6 63 6 12 16 9 18 7 表 3 WC/Fe复合材料中基体的成分Table 3. Composition of matrix in WC/Fe compositesComposition C Cr Mn Si Fe Mass fraction/wt% 1.2-1.3 18.0-20.0 0.4-0.6 1.0-1.2 Balance 采用尼康MA200型OM表征复合层显微组织,并统计预制体原孔壁与原孔心处平均晶粒尺寸分布。采用岛津7000S/L型XRD、牛津仪器Ultim Extreme型EDS面扫描表征复合层物相组成、元素分布。采用牛津仪器Ultim Extreme型EDS点扫描、上海光学仪器厂HX1000型显微硬度计表征复合层预制体原孔壁与原孔心处元素质量分数、硬度。采用广州试验仪器厂MS-5E型三体磨料磨损机表征复合层耐磨性,载荷为2 kg、转速为40 r/min、预磨时间为30 min、磨粒粒径为200~550 μm,并采用蔡司EVO18型SEM表征预制体原孔心处磨损形貌。采用COMSOL Multiphysics 5.4有限元模拟预制体孔内熔体凝固时的热物理场。采用MATLAB R2015b通过二次开发程序数值模拟预制体原孔内W质量分数分布。
2. 结果与分析
2.1 WC/Fe复合材料复合层的显微组织与元素分布
图2为不同预制体孔径下WC/Fe复合材料复合层的显微组织。熔体填充预制体孔洞,WC高温下分解,W由其孔壁扩散至孔心处,形成复合层。图3为不同预制体孔径下WC/Fe复合材料复合层原孔内的平均晶粒尺寸分布,随预制体孔径增加,其原孔壁处晶粒尺寸基本不变,而在其原孔心处先减小后增大。
图4为不同预制体孔径下WC/Fe复合材料复合层原孔内的平均晶粒尺寸分布。表明复合层中均形成W2C、WC、Ni17W3、Fe3W3C、(Fe,Cr)3C。根据W-C相图,WC/W2CP中WC分解形成W2C、C,而C扩散到熔体中使(Fe,Cr)3C增多;根据Fe-W-C相图,熔体与W2C发生包晶反应形成Fe3W3C;WC、Ni60分解使W、Ni质量分数增加,形成镍钨化合物Ni17W3。
根据Fe-Cr-C相图[18-19],推测熔体(Cr:18.0wt%~20.0wt%,C:1.2wt%~1.3wt%)为典型亚共晶成分合金(Cr:11.0wt%~30.0wt%,C:<2.8wt%),凝固时先析出一次奥氏体枝晶,待温度降至共晶点发生共晶转变形成共晶奥氏体与二次碳化物的混合共晶组织,而复合层为网状形貌的M3C型碳化物[7,20]。图5为不同预制体孔径下WC/Fe复合材料复合层的元素分布。Cr分布在共晶奥氏体与二次碳化物中,W、Ni弥散分布在一次奥氏体枝晶内。
图6为不同预制体孔径下WC/Fe复合材料复合层原孔内的W质量分数分布。其原孔壁处W质量分数较高,而在其原孔心处较低。与晶粒尺寸的变化相反,随预制体孔径增加,其原孔壁处W质量分数基本不变,而在其原孔心处先增大后减小。为进一步表征W扩散均匀性,通过计算得不同预制体孔径下W扩散均匀性分别为84.1%、88.7%、86.9%,表明W扩散均匀性随其孔径增加而先增大后减小,W扩散均匀性的表达式为
δ=1−2√3πR2C(0,tmax (1) 式中:δ为W扩散均匀性;C(0,tmax)、C(R/2,tmax)分别为预制体原孔壁、原孔心处W质量分数。
2.2 WC/Fe复合材料复合层的硬度分布与耐磨性
图7为不同预制体孔径下WC/Fe复合材料复合层原孔内的硬度分布。其原孔壁处硬度较高,而在其原孔心处较低。与W质量分数的变化相同,随预制体孔径增加,其原孔壁处硬度基本不变,而在其原孔心处先增大后减小。图8为不同预制体孔径下WC/Fe复合材料复合层原孔心处的磨损形貌。R=3 mm时其原孔心处犁沟最明显,R=9 mm时次之,R=6 mm时最不明显。图9为不同预制体孔径下WC/Fe复合材料复合层的磨损量。表明复合层耐磨性随其孔径增加而先增大后减小。
2.3 WC/Fe复合材料复合层的W扩散均匀性机制
为进一步探究影响W扩散均匀性的因素,对预制体原孔内W质量分数分布进行数值模拟。将W扩散区看作受固液界面移动驱动的半无限大物体,且扩散时间近似为预制体孔内熔体凝固时间[21-22]。下式为W扩散区边界条件的表达式:
\left\{ \begin{array}{l} C\left( {x,t} \right) = {C_{{\rm{SL}}}},x = x\left( t \right) \\ C\left( {x,t} \right) = {C_{{\rm{LS}}}},x < x\left( t \right) \end{array}\right. (2) 式中,CSL、CLS分别为固液界面固、液相侧W质量分数。根据Arrhenius方程,W质量分数分布为
\left\{ \begin{array}{l} C\left( {x,t} \right) = \dfrac{{{C_{{\rm{SL}}}}}}{{{\rm{erf}}\left( k \right) - 1}}\left( {{\rm{erf}}\left( {\dfrac{x}{{2\sqrt {Dt} }}} \right) + 1} \right) \\ D = {D_0}{\rm{exp}}\left( { - \dfrac{{{E_{\rm{A}}}}}{{{k_{\rm{B}}}T}}} \right) \end{array} \right. (3) 式中:x为W扩散距离;t为W扩散时间;T为W扩散温度;D为W扩散系数;k为常数;EA为W元素扩散激活能;kB为玻尔兹曼常数[22]。W扩散总时间为
{t_{{\rm{max}}}} = \frac{{{R^2}}}{{64{k^2}D}} (4) 通过解析解得W扩散过程同时受温度与固液界面移动影响。故先采用有限元模拟软件COMSOL Multiphysics 5.4模拟预制体孔内熔体凝固时的温度场与相场,表4为预制体孔内熔体凝固时热物理场模拟的参数设置。图10为预制体孔内熔体凝固时热物理场的有限元模拟。发现固液界面明显存在,且其左侧温度较高,熔体为液相,而其右侧温度较低,熔体为固相,即其孔壁处熔体先凝固,且固液界面移动驱动W扩散。此外,随预制体孔径增加,其孔内熔体高温区增多,使其平均温度增高,W扩散系数受W扩散温度影响,扩散温度越高扩散系数越大,其原孔内W质量分数分布曲线斜率绝对值越大;图11为不同预制体孔径下WC/Fe复合材料复合层原孔内W质量分数分布的数值模拟。再将该有限元模拟结果代入数学分析软件MATLAB R2015b的二次开发程序中进行数值模拟,最终得其原孔内W质量分数分布曲线,发现其原孔内W质量分数为W扩散距离的单调递减函数。因预制体孔径孔距比相同且W扩散区为受固液界面移动驱动的半无限大物体,故设置W初始质量分数相同。随预制体孔径增加,其原孔内W质量分数分布曲线斜率绝对值增大,故R=3 mm时其原孔心处W质量分数较R=6 mm时低,但R=6 mm时W扩散距离较R=9 mm时短,故其原孔心处W质量分数R=6 mm时最高,R=9 mm时次之,R=3 mm时最低,即该数值模拟与实验结果相符。
表 4 预制体孔内熔体凝固时热物理场模拟的参数设置Table 4. Parameters setting of thermal physical field simulation when internal matrix of preform solidifiesPhase Density/(kg·m−3) Thermal conductivity/(W·m−1·K−1) Heat capacity/(J·kg−1·K−1) Fe(s) 8 500 200 400 Fe(l) 7 800 450 550 Inlet temperature/°C Melting temperature/°C Temperature transition half width/K Surface emissivity 1 500 1 100 50 0.8 Specific heat/(J·kg−1·K−1) Solidification latent heat/(kJ·kg−1) Heat transfer coefficient/(W·m−2·K−1) 60 200 800 预制体孔径较大时,其孔内熔体较多,温度也较高。一方面扩散时间较长,有利于W扩散;另一方面扩散距离较长,不利于W扩散均匀,使预制体原孔心处W质量分数降低。同理,预制体孔径较小时,扩散距离虽短,但扩散时间较短,使W扩散不充分,其原孔心处W质量分数较低,故W扩散均匀性也较低;故预制体孔径适中时,因兼顾扩散距离与扩散时间而使W扩散均匀性最高。综上所述,W扩散过程同时受扩散距离与扩散时间的影响。
2.4 WC/Fe复合材料复合层的耐磨性与W扩散均匀性间关联机制
亚共晶Fe-Cr-C系合金中含大量低硬度、高韧性的一次奥氏体,硬度、耐磨性较低,而W弥散分布在一次奥氏体枝晶内形成M6C型碳化物Fe3W3C,细化晶粒使复合层冲击韧性未明显降低,且引入硬质相使其硬度明显提高[20,23],一定范围内也提高其耐磨性[24]。W扩散均匀性越高,预制体原孔心处W质量分数越高,形成硬质相越多,硬度也越高,最终提高复合层耐磨性。
3. 结 论
采用真空消失模铸渗(V-EPC)工艺制备WC/Fe复合材料,选取预制体孔径孔距比相同孔径不同的蜂窝预制体,并将其原孔壁与原孔心处W质量分数作为W扩散均匀性的判断条件,得如下结论。
(1) WC高温下分解,W由预制体孔壁至孔心处扩散,形成弥散分布的硬质相Fe3W3C。
(2)预制体原孔壁与原孔心处W质量分数与硬度相差随孔径增加而先增大后减小,复合层耐磨性的变化亦然。
(3) W扩散均匀性同时受扩散距离与扩散时间的影响。预制体孔径较小时,扩散距离虽短,但扩散时间较短,不利于W扩散;预制体孔径较大时,扩散时间虽长,但扩散距离增长,仍不利于W扩散;预制体孔径适中时,因兼顾扩散距离与扩散时间,利于W扩散。
(4)耐磨性与W扩散均匀性间存在关联,W扩散均匀性越高,预制体原孔心处W质量分数越高,形成硬质相越多,硬度也越高,一定范围内复合层耐磨性也越高。
-
表 1 EDA/ATP复合材料吸附剂对Cr(VI)的吸附等温线拟合参数
Table 1 Adsorption isotherm fitting parameters of Cr(VI) by EDA/ATP composite adsorbent
Temperature/℃ Equation parameters of Langmuir Equation parameters of Freundlich qm/(mg·L−1) b/(L·mg−1) R2 n Kf R2 15 130.55 0.87 0.866 17.17 100.27 0.990 25 149.25 0.76 0.818 14.81 111.84 0.989 35 136.05 1.06 0.825 30.21 104.40 0.993 Notes:qm—Theoretical maximum adsorption capacity;b—Affinity coefficient;R2—Determination coefficient;n—Adsorption intensity;Kf—Freundlich constants related to adsorption capacity. 表 2 EDA/ATP复合材料吸附剂对Cr(VI)的拟一级、拟二级动力学参数
Table 2 Simulated parameters of Cr(VI) adsorption by EDA/ATP composite using pseudo-first-order and pseudo-second-order kinetics
Adsorbent qe/(mg·g−1) Pseudo-first-order kinetics equation Pseudo-second-order kinetics equation k1/ [g· (mg·min)−1] qcal/ (mg·g−1) R2 k2/[g· (mg·min)−1] qcal/ (mg·g−1) R2 EDA/ATP 127.85 1.38×10−3 105.12 0.837 2.26×10−4 147.06 0.998 Notes: qe—Equilibrium adsorption capacity;qcal—Maximum theoretical adsorption capacity calculated by the corresponding kinetic equation;k1, k2—Pseudo-first-order kinetic and pseudo-second-order kinetic equation constants, respectively. -
[1] EGODAWATTE S, DATT A, BURNS E, et al. Chemical insight into the adsorption of chromium(Ⅲ) on iron oxide/mesoporous silica nanocomposites[J]. Langmuir,2015,31(27):7553-7562. DOI: 10.1021/acs.langmuir.5b01483
[2] GUAN X., CHEN Y, FAN H. Stepwise deprotonation of magnetite-supported gallic acid modulates oxidation state and adsorption-assisted translocation of hexavalent chromium[J]. ACS Applied Materials & Interfaces,2017,9(18):15525-15532.
[3] KUMAR A, PAUL P, SANNA K N. Bio-nanomaterial scaffolds for effective removal of fluoride, chromium and dye[J]. ACS Sustainable Chemistry & Engineering,2016,5(1):895-903.
[4] GOPALAKANNAN V, VISWANATHAN N. Development of nano-hydroxyapatite embedded gelatin biocomposite for effective Cr(VI) removal[J]. Industrial & Engineering Chemistry Research,2015,54(50):12561-12569.
[5] 林国庆, 李文娟. 海蒿子和三价铁溶液绿色制备纳米铁及其去除六价铬的实验研究[J]. 中国海洋大学学报(自然科学版), 2018, 48(S2):127-133. LIN G Q, LI W J. Green synthesis of iron nanoparticles using sargassum pallidum with ferric iron solution and its experimental study on hexavalent chromium removal[J]. Periodical of Ocean University of China,2018,48(S2):127-133(in Chinese).
[6] QIU B, XU C, SUN D, et al. Polyaniline coated ethyl cellulose with improved hexavalent chromium removal[J]. Acs Sustainable Chemistry & Engineering,2014,2(8):2070-2080.
[7] 任阳民, 梁宏, 邱阳, 等. 脉冲电解技术处理含铬废水实验研究[J]. 四川理工学院学报(自然科学版), 2017, 30(3):1-5. REN Y M, LIANG H, QIU Y, et al. Study on treatment of chromium-containing waste water by pulse-electrolysis technique[J]. Journal of Sichuan University of Science & Engineering (Natural Science Edition),2017,30(3):1-5(in Chinese).
[8] LIU S, MISHRA S B, ZHANG Y, et al. Uptake of hexavalent chromium in electroplating wastewater by hydrothermally treated and functionalized sand and its sustainable reutilization for glass production[J]. Acs Sustainable Chemistry,2017,5(2):1509-1516. DOI: 10.1021/acssuschemeng.6b02185
[9] ABUBAKR A H, GURMAN S J, MURPHY L M, et al. Remediation of chromium(VI) by a methane-oxidizing bacterium[J]. Environmental Science & Technology,2010,44(1):400-405.
[10] WANG T, CHEN Y, MA J, et al. Attapulgite nanoparticles-modified monolithic column for hydrophilic in-tube solid-phase microextraction of cyromazine and melamine[J]. Analytical Chemistry,2016,88(3):1535-1541. DOI: 10.1021/acs.analchem.5b03478
[11] 陈浩, 赵杰. 凹凸棒与酸化凹凸棒对Pb(Ⅱ)和Zn(Ⅱ)的选择吸附性差异[J]. 材料工程, 2008(10):154-157. DOI: 10.3969/j.issn.1001-4381.2008.10.039 CHEN H, ZHAO J. The difference of selective adsorption between palygorskite and acid-activated palygorskite for Pb(Ⅱ) and Zn(Ⅱ)[J]. Journal of Materials Engineering,2008(10):154-157(in Chinese). DOI: 10.3969/j.issn.1001-4381.2008.10.039
[12] 陈泳, 郝蓉蓉, 王洁琼. 酸改性聚吡咯/凹凸棒复合材料对胭脂红染料的吸附[J]. 化工新型材料, 2018, 46(4):148-151. CHEN Y, HAO R R, WANG J Q. Adsorption of carmine onto acid modified polypyrrole/attapulgite composite[J]. New Chemical Materials,2018,46(4):148-151(in Chinese).
[13] YI X, SHAO D, LU X, et al. Spectroscopic investigation of enhanced adsorption of U(VI) and Eu(Ⅲ) on magnetic attapulgite in binary system[J]. Industrial & Engineering Chemistry Research,2018,57(22):7533-7543.
[14] WANG Y, FENG Y, ZHANG X F, et al. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals[J]. Journal of Colloid and Interface Science,2018,514:190-198. DOI: 10.1016/j.jcis.2017.12.035
[15] CHEN L F, LIANG H W, LU Y, et al. Synthesis of an attapulgite clay@carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water[J]. Langmuir,2011,27(14):8998-9004. DOI: 10.1021/la2017165
[16] LI B, LI W, ZHANG Q, et al. Attapulgite as natural catalyst for glucose isomerization to fructose in water[J]. Catalysis Communications,2017,99:20-24. DOI: 10.1016/j.catcom.2017.05.011
[17] ZANG Z, HU Z, LI Z, et al. Synthesis, characterization and application of ethylenediamine-modified multiwalled carbon nano tubes for selective solid-phase extraction and preconcentration of metal ions[J]. Journal of Hazardous Materials,2009,172(2):958-963.
[18] WANG S, WANG J, ZHANG W, et al. Ethylenediamine modified graphene and its chemically responsive supramolecular hydrogels[J]. Industrial & Engineering Chemistry Research,2014,53(33):13205-13209.
[19] 黄京晶, 陈宏, 刘江, 等. 改性水葫芦粉对水体中Hg2+的吸附[J]. 环境工程学报, 2017, 11(2):798-804. DOI: 10.12030/j.cjee.201509164 HUANG J J, CHEN H, LIU J. Adsorption of Hg2+ from aqueous solution by modified eichhornia crassipes powder[J]. Chinese Journal of Environmental Engineering,2017,11(2):798-804(in Chinese). DOI: 10.12030/j.cjee.201509164
[20] XING Y, CHEN X, WANG D, et al. Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater[J]. Environmental Science & Technology,2007,41(4):1439-1443.
[21] 赵永纲. 氨基功能化纳米Fe3O4磁性高分子复合材料的合成、表征及其对废水中Cr(VI)的吸附研究[D]. 杭州: 浙江大学, 2010. ZHAO Y G. Synthesis and characterization of amino-functionalized nano-Fe3O4 magnetic polymer composites and their ad sorption of Cr(VI) in wastewater[D]. Hangzhou: Zhejiang University, 2010(in Chinese).
[22] 鲁秀国, 黄燕梅, 曹禹楠. 氨基改性核桃壳对废水中Cr(VI)的静态吸附研究[J]. 离子交换与吸附, 2014, 30(6):491-498. LU X G, HUANG Y M, CAO Y N. Static adsorption of Cr(VI) in simulated wastewater by amino modified walnut shells[J]. Ion Exchange and Adsorption,2014,30(6):491-498(in Chinese).
[23] JUN W, ZHANG H, HE P, et al. Cr(VI) removal from aqueous solution by dried activated sludge biomass[J]. Journal of Hazardous Materials,2010,176(1):697-703.
[24] BAE S, SIHN Y, KYUNG D, et al. Molecular identification of Cr(VI) removal mechanism on vivianite surface[J]. Environmental Science & Technology,2018,52(18):10647-10650.
-
期刊类型引用(0)
其他类型引用(1)
-




 下载:
下载: